Abstract
The degradation of long-chain n-alkylbenzenes and n-alkylcyclohexanes by Alcanivorax sp. strain MBIC 4326 was investigated. The alkyl side chain of these compounds was mainly processed by β-oxidation. In the degradation of n-alkylcyclohexanes, cyclohexanecarboxylic acid was formed as an intermediate. This compound was further transformed to benzoic acid via 1-cyclohexene-1-carboxylic acid.
Crude oil is a complex mixture containing a homologous series of alkylated hydrocarbons, such as n-alkylcyclohexanes, n-alkylcyclopentanes, and n-alkylbenzenes (7, 10, 14, 16–19, 22, 24–28, 30). These hydrocarbons are degraded by a number of fungi and bacteria (1–3, 6, 8, 11–13, 20, 21, 23, 29). Generally, the terminal methyl group of a long n-alkyl side chain of these compounds is initially oxidized to a carboxylic group, which is followed by classical β-oxidation to form carboxylic or acetic acid derivatives, depending on whether there is an odd or even number of carbons in the alkyl side chain (3, 29). In Acinetobacter lwoffii, for example, n-dodecylbenzene was completely degraded via phenylacetic acid and homogentisic acid, while n-tridecylbenzene was transformed via 3-phenylpropionic acid to trans-cinnamic acid, which was the dead-end product (1).
We investigated the degradation of n-alkylbenzenes and n-alkylcyclohexanes in Alcanivorax sp. strain MBIC 4326. This strain was isolated from Kamaishi Bay seawater and closely related to Alcanivorax borkumensis, the type strain of the genus Alcanivorax (31). This strain grew on BSM medium (8) supplemented with 1 g of n-octadecylcyclohexane (compound 1 in Fig. 1), n-nonadecylcyclohexane (compound 2), n-undecylbenzene (compound b in Fig. 2), or n-hexadecylbenzene (compound a) per liter as the sole source of carbon and energy. These cultures were acidified to pH 2 with concentrated hydrochloric acid and extracted three times with an equal volume of dichloromethane. The combined extracts were evaporated in a rotary evaporator to dryness under reduced pressure. The residues of each dichloromethane extract were methylated with a boron trifluoride-methanol solution (Supelco) prior to an analysis by gas chromatography-mass spectrometry (GC-MS). The GC-MS analysis was performed as described previously (8, 9).
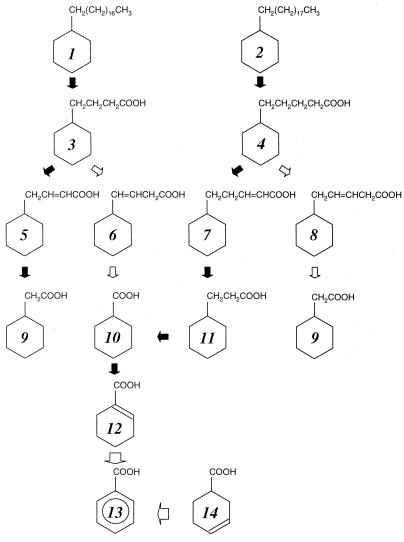
FIG. 1.
Proposed pathways for the biodegradation of n-alkylcyclohexanes by Alcanivorax sp. strain MBIC 4326. β-Oxidation routes shown to be major metabolic routes are indicated by solid arrows, while minor routes are indicated by open arrows. Novel metabolic routes are indicated by larger open arrows. Compound numbers are defined in the text.
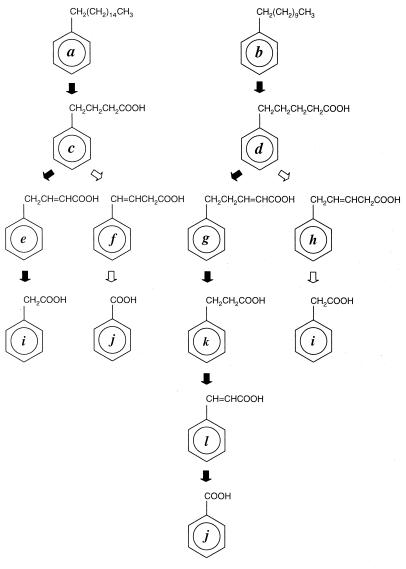
FIG. 2.
Proposed pathway(s) for the biodegradation of n-alkylbenzenes by Alcanivorax sp. strain MBIC 4326. β-Oxidation routes shown to be major metabolic routes are indicated by solid arrows, while minor routes are indicated by open arrows. Trace amounts of 3-phenylpropanoic acid (k) and trans-cinnamic acid (l) were formed from n-hexadecylbenzene. This route is not indicated by an arrow. Compound letters are defined in the text.
Both cyclohexanecarboxylic acid (compound 10) and cyclohexaneacetic acid (compound 9) accumulated in the cultures grown on n-octadecylcyclohexane (compound 1) and n-nonadecylcyclohexane (compound 2), indicating that these n-alkylcyclohexanes were transformed by following more than a single pathway (Table 1). One pathway might be involved in the oxidation of the terminal methyl group of the alkyl side chain to a carboxylic group, with subsequent β-oxidation yielding cyclohexaneacetic acid (compound 9) from n-octadecylcyclohexane and cyclohexanecarboxylic acid (compound 10) from n-nonadecylcyclohexane (compound 2). The formation of cyclohexaneacetic acid (compound 9) from n-nonadecylcyclohexane (compound 2) and of cyclohexanecarboxylic acid (compound 10) from n-octadecylcyclohexane (compound 1) (Table 1) cannot be explained by β-oxidation. This point is discussed later in this paper.
TABLE 1.
GC-MS data for the metabolitesa formed from n-alkylcyclohexanes and n-alkylbenzenes by Alcanivorax sp. strain MBIC 4326
| Compound sourceb and metabolite no. | Amt (μmol)c | Retention time (min) | m/z of major ion peaks (%)d | Suggested structuree |
|---|---|---|---|---|
| n-Octadecylcyclohexane | 51 | 8.9 | 142 (M+) (30), 127 (9), 113 (22), 111 (20), 110 (26), 101 (16), 100 (7), 88 (8), 87 (100), 84 (5), 83 (73), 82 (31), 81 (18), 79 (6), 75 (9), 74 (55), 69 (17), 68 (28) | Methyl cyclohexanecarboxylate |
| 83 | 11.2 | 156 (M+) (2), 125 (20), 113 (5), 97 (13), 83 (9), 82 (11), 81 (17), 79 (7), 76 (7), 75 (68), 74 (100), 68 (5) | Methyl cyclohexaneacetate | |
| 3.1 | 10.8 | 140 (M+) (31), 109 (22), 108 (24), 82 (8), 81 (100), 80 (73), 79 (64), 77 (16), 67 (5) | Methyl 1-cyclohexene-1-carboxylate | |
| 0.02 | 9.7 | 136 (M+) (58), 135 (6), 106 (14), 105 (100), 92 (6), 78 (9), 77 (81), 75 (4), 74 (6) | Methyl benzoate | |
| n-Nonadecylcyclohexane | 86 | 8.9 | Same as the results described above | Methyl cyclohexanecarboxylate |
| 42 | 11.2 | Same as the results described above | Methyl cyclohexaneacetate | |
| 4.7 | 10.8 | Same as the results described above | Methyl 1-cyclohexene-1-carboxylate | |
| 1.1 | 9.7 | Same as the results described above | Methyl benzoate | |
| n-Undecylbenzene | 190 | 9.7 | Same as the results described above | Methyl benzoate |
| 4.6 | 11.9 | 150 (M+) (71), 119 (6), 118 (5), 92 (22), 91 (100), 89 (14), 65 (41), 64 (5) | Phenylacetic acid, methyl ester | |
| 0.9 | 14.2 | 164 (M+) (24), 133 (8), 107 (4), 105 (33), 104 (100), 103 (13), 92 (5), 91 (69), 79 (12), 78 (13), 77 (18), 65 (12) | 3-Phenylpropanoic acid, methyl ester | |
| 5.0 | 16.7 | 162 (M+) (43), 161 (24), 132 (9), 131 (100), 104 (7), 103 (60), 102 (14), 78 (4), 77 (40), 76 (7), 75 (4), 74 (4), 65 (4) | trans-Cinnamic acid, methyl ester | |
| n-Hexadecylbenzene | ||||
| 4 | 14 | 9.7 | Same as the results described above | |
| 5 | 130 | 11.8 | Same as the results described above | |
| 6 | 0.7 | 14.2 | Same as the results described above | |
| 7 | 0.4 | 16.7 | Same as the results described above | |
| 8 | 3.2 | 16.8 | 178 (M+) (16), 162 (7), 161 (5), 147 (21), 146 (25), 131 (17), 117 (5), 115 (5), 105 (30), 104 (100), 103 (17), 92 (6), 91 (64), 89 (4), 78 (8), 77 (15), 75 (5), 74 (68), 65 (23) | 4-Phenylbutanoic acid, methyl ester |
| 9 | 0.8 | 17.2 | 176 (M+) (28), 145 (5), 134 (9), 118 (9), 117 (100), 116 (13), 115 (51), 91 (17), 65 (7) | 4-Phenyl-2-butenoic acid, methyl esterf |
| 10 | 9.4 | 18.6 | 176 (M+) (32), 161 (4), 144 (5), 134 (10), 118 (11), 117 (100), 116 (14), 115 (49), 91 (17), 89 (4), 65 (6) | 4-Phenyl-3-butenoic acid, methyl ester |
Analyses were performed after methylation with trifluoro boron-methanol.
Substrate concentration was as follows: 1 g/liter in 50 ml of medium contains 149, 143, 215, and 165 μmol of n-octadecylcyclohexane, n-nonadecylcyclohexane, n-undecylbenzene, and n-hexadecylbenzene, respectively.
Based on the total ion current response of the mass-selective detector relative to the authentic standards.
The ion abundance percentages are shown in parentheses.
Identification was based on the match of mass spectra (fragmentation and peak intensity) and capillary GC retention times with data for authentic samples, except for data described in footnote f, which indicated a fragmentation match higher than 85%.
High degree of match to the library (NIST and WILEY installed in Shimadzu GCMS-QP5050A9).
Trace amounts of 1-cyclohexene-1-carboxylic acid (compound 12) and benzoic acid (compound 13) were also detected in the cultures grown on n-octadecylcyclohexane (compound 1) and n-nonadecylcyclohexane (compound 2) (Table 1), indicating that cyclohexanecarboxylic acid (compound 10) was further metabolized.
Alcanivorax sp. strain MBIC 4326 did not grow on 4-cyclohexylbutanoic acid (compound 3) and 3-cyclohexylpentanoic acid (compound 4) as the sole sources of carbon and energy. Thus, the strain was grown on n-hexadecane (0.5 g/liter) in BSM medium for 5 days. One gram of 4-cyclohexylbutanoic acid (compound 3) or 3-cyclohexylpentanoic acid (compound 4) per liter was subsequently added, and the cultivation was continued for different periods of time. The major product of the 4-cyclohexylbutanoic acid degradation was cyclohexaneacetic acid (compound 9), which could have been formed by β-oxidation (Table 2). 4-Cyclohexyl-2-butenoic acid (compound 5) detected in a trace amount could also have been formed by β-oxidation, while the formation of other intermediates, 4-cyclohexyl-3-butenoic acid (compound 6) and cyclohexanecarboxylic acid (compound 10), suggested the low probability of another type of degradation. The same conclusion was obtained by examining the biodegradation of 5-cyclohexylpentanoic acid (compound 4). The detection of cyclohexanecarboxylic acid (compound 10) as the major product and 3-cyclohexylpropionic acid (compound 11) and 5-cyclohexyl-2-pentenoic acid (compound 7) as minor products supported the β-oxidation pathway, while the formation of cyclohexaneacetic acid (compound 9) and 5-cyclohexyl-3-pentenoic acid (compound 8) suggested another type of degradation.
TABLE 2.
Biodegradation of cyclohexyl- and phenylalkanoic acids by Alcanivorax sp. strain MBIC 4326
| Source compounda | Metabolite(s) | Amt of metabolites (μmol) with time:
|
|||
|---|---|---|---|---|---|
| 8 h | 1 day | 3 days | 5 days | ||
| 4-Cyclohexanebutanoic acid | Cyclohexanecarboxylic acid | Trace | 0.13 | 0.38 | |
| Cyclohexaneacetic acid | 2.6 | 4.1 | 15 | 16 | |
| 1-Cyclohexene-1-carboxylic acid | 0.03 | 0.12 | |||
| 4-Cyclohexane-2-butenoic acid | Trace | Trace | Trace | ||
| 4-Cyclohexane-3-butenoic acid | Trace | Trace | |||
| 4-Phenylbutanoic acid | Benzoic acid | 0.05 | 0.11 | 0.25 | 0.42 |
| Phenylacetic acid | 4.3 | 10 | 26 | 28 | |
| 4-Phenyl-2-butenoic acid | 0.60 | 1.7 | 1.9 | 2.3 | |
| 4-Phenyl-3-butenoic acid | 8.9 | 17 | 25 | 29 | |
| 5-Cyclohexanepentanoic acid | Cyclohexanecarboxylic acid | 0.17 | 0.23 | 1.4 | 44 |
| Cyclohexaneacetic acid | Trace | Trace | |||
| 3-Cyclohexanepropionic acid | Trace | 0.14 | Trace | ||
| 1-Cyclohexene-1-carboxylic acid | 0.21 | 3.6 | |||
| Benzoic acid | 0.08 | ||||
| 5-Cyclohexane-2-pentenoic acid | Trace | Trace | Trace | ||
| 5-Cyclohexane-3-pentenoic acid | Trace | Trace | |||
| 5-Phenylpentanoic acid | Benzoic acid | 1.2 | 7.5 | 31 | 43 |
| Phenylacetic acid | Trace | Trace | Trace | ||
| 3-Phenylpropionic acid | 5.2 | 21 | 4.5 | 0.25 | |
| trans-Cinnamic acid | 3.2 | 14 | 2.9 | 0.08 | |
| 5-Phenyl-2-pentenoic acid | Trace | Trace | Trace | ||
| 5-Phenyl-3-pentenoic acid | Trace | Trace | Trace | ||
| 4-Phenyl-3-butenoic acid | Benzoic acid | NDb | ND | ND | 1.8 |
| trans-Cinnamic acid | Benzoic acid | ND | ND | ND | 2.5 |
| Cyclohexanecarboxylic acid | 1-Cyclohexene-1-carboxylic acid | ND | ND | ND | 0.77 |
| Benzoic acid | ND | ND | ND | 0.06 | |
| 1-Cyclohexene-1-carboxylic acid | Benzoic acid | ND | ND | ND | 0.39 |
| 3-Cyclohexene-1-carboxylic acid | Benzoic acid | ND | ND | ND | 0.09 |
Substrate concentration was as follows: 1 g/liter in 10 ml of medium contains 59, 61, 54, 56, 62, 67, 78, 79, and 79 μmol of 4-cyclohexanebutanoic acid, 4-phenylbutanoic acid, 5-cyclohexanepentanoic acid, 5-phenylpentanoic acid, 4-phenyl-3-butenoic acid, trans-cinnamic acid, cyclohexanecarboxylic acid, 1-cyclohexene-1-carboxylic acid, and 3-cyclohexene-1-carboxylic acid, respectively.
ND, not determined.
Besides these observations, the formation of 1-cyclohexene-1-carboxylic acid (compound 12) and benzoic acid (compound 13) from 5-cyclohexylpentanoic acid (compound 4) was observed, suggesting the transformation of cyclohexanecarboxylic acid (compound 10) to these products. This was confirmed by cultivating Alcanivorax sp. strain MBIC 4326 on 0.5 g of n-hexadecane per liter in the presence of 1 g of cyclohexanecarboxylic acid (compound 10) per liter. Cyclohexanecarboxylic acid (compound 10) was converted to 1-cyclohexene-1-carboxylic acid (compound 12) and benzoic acid (compound 13) (Table 2). When benzoic acid (compound 13) or cyclohexaneacetic acid (compound 9) was used as a cosubstrate for the culture of Alcanivorax sp. strain MBIC 4326 grown on n-hexadecane, the transformation of these cosubstrates was not observed.
The metabolism of cyclohexanecarboxylic acid (compound 10) to 1-cyclohexene-1-carboxylic acid (compound 12) and pimelic acid has been observed (4, 20). In some bacteria, cyclohexanecarboxylic acid (compound 10) is transformed to 4-hydroxybenzoic acid via 4-oxocyclohexanecarboxylic acid (29). In contrast to these previous findings, the formation of benzoic acid (compound 13) from cyclohexanecarboxylic acid (compound 10) via 1-cyclohexene-1-carboxylic acid (compound 12) was demonstrated in the present study. In addition, n-hexadecane-grown cells could also transform 3-cyclohexene-1-carboxylic acid (compound 14) to benzoic acid (compound 13) (Table 2). To our knowledge, this is the first report of a pathway involving the conversion of cyclohexanecarboxylic acid (compound 10) to benzoic acid (compound 13).
In the cultures grown on n-undecylbenzene (compound b) and n-hexadecylbenzene (compound a), both benzoic acid (compound j) and phenylacetic acid (compound i) were detected (Table 1). Benzoic acid (compound j) was the major product from the n-undecylbenzene (compound b) culture, while n-hexadecylbenzene (compound a) mainly yielded phenylacetic acid (compound i). Thus, these compounds were mainly degraded by β-oxidation. Apart from these, small amounts of 3-phenylpropanoic acid (compound k) and trans-cinnamic acid (compound l) were also formed in both the n-undecylbenzene (compound b) and n-hexadecylbenzene (compound a) cultures. In addition, 4-phenylbutanoic acid (compound c) and two isomers of 4-phenylbutenoic acid (compounds e and f) were detected in the n-hexadecylbenzene-grown culture.
To investigate further, the transformation of 4-phenylbutanoic acid (compound c) and 5-phenylpentanoic acid (compound d) was examined with cultures of Alcanivorax sp. strain MBIC 4326 grown on n-hexadecane. 5-Phenylpentanoic acid (compound d) was transformed by Alcanivorax sp. strain MBIC 4326, mainly via β-oxidation, with 5-phenyl-2-pentenoic acid (compound g), 3-phenylpropionic acid (compound k), trans-cinnamic acid (compound l), and benzoic acid (compound j) detected as the β-oxidation intermediates. While trans-cinnamic acid (compound l) has been reported as the dead-end metabolite in the degradation of tridecanylbenzene by Acinetobacter lwoffii (1), this compound was found to be efficiently degraded by Alcanivorax sp. strain MBIC 4326 to benzoic acid (compound j) by classical β-oxidation pathway enzymes (Table 2). The detection of 5-phenyl-3-pentenoic acid (compound h) and phenylacetic acid (compound i), however, indicated the existence of another minor pathway for the degradation of 5-phenylpentanoic acid.
The degradation of the n-alkyl side chain by mechanisms other than β-oxidation has been suggested by a number of observations. In fungi (Beauveria, Penicillium, and Paecilomyces spp.), both carboxylic acid and acetic acid derivatives accumulated from 2-n-dodecyltetrahydrothiophene (12). Similar phenomena have been observed in various microbial degradation processes of n-alkylcyclohexanes, n-alkylbenzenes, and n-alkylbenzene sulfonic acids (3, 11, 20, 23). In the degradation of branched-chain dodecylbenzene sulfonic acid by Pseudomonas aeruginosa W51D, desulfonation was followed by complete oxidation of the alkyl side chain via 3-(4-hydroxyphenyl)propionic acid, 4-hydroxycinnamic acid, 4-hydroxyphenylacetic acid, and 4-hydroxybenzoic acid (5). n-Undecylcyclohexane and n-dodecylcyclohexane were both transformed to cyclohexaneacetic acid and cyclohexanecarboxylic acid in a marine bacterium. To explain the formation of these metabolic intermediates, the simultaneous occurrence of α- and β-oxidation has been proposed as a possible mechanism (20).
The major degradation products of 4-phenylbutanoic acid (compound c) were phenylacetic acid (compound i) and 4-phenyl-2-butenoic acid (compound e), which had certainly been formed by β-oxidation. In addition, the accumulation of the Δ3 isomer of 4-phenylbutenoic acid (compound f) was exceptionally high when 4-phenylbutanoic acid (compound c) was used as the substrate. It has been reported that one of the β-oxidation enzymes, butyryl coenzyme A (CoA) dehydrogenase, was found to possess low affinity towards 4-phenylbutyryl-CoA, resulting in the accumulation of 4-phenylbutyric acid (compound c) in the degradation of 1-phenyldodecane by Nocardia salmonicolor (23). In the present study, the accumulation of 4-phenyl-3-butenoic acid at a relatively high concentration indicates that 4-phenyl-3-butenoic acid (compound f) might have been formed either by direct Δ3-dehydrogenation of 4-phenylbutanoic acid (compound c) or from 4-phenyl-2-butenoic acid (compound l) by the action of enoyl CoA isomerase (15). 4-Phenyl-3-butenoic acid (compound f) was degraded by Alcanivorax sp. strain MBIC 4326 to benzoic acid (Table 2).
Considering the structures of the characterized metabolites, the pathways for the degradation of n-alkylcyclohexanes (Fig. 1) and n-alkylbenzenes (Fig. 2) in Alcanivorax sp. strain MBIC 4326 are proposed. The transformation may proceed in vivo in the form of CoA derivatives. Further genetic and biochemical studies are required to clarify the enzymes involved in these steps.
Acknowledgments
This work was supported by the New Energy and Industrial Technology Development Organization (NEDO) of Japan.
REFERENCES
- 1.Amund O O, Higgins I J. The degradation of 1-phenylalkanes by an oil-degrading strain of Acinetobacter lwoffii. Antonie Leeuwenhoek. 1985;51:45–56. doi: 10.1007/BF00444227. [DOI] [PubMed] [Google Scholar]
- 2.Bayona J M, Albaiges J. Selective aerobic degradation of linear alkylbenzenes by pure microbial culture. Chemosphere. 1986;15:595–598. [Google Scholar]
- 3.Beam H W, Perry J J. Microbial degradation and assimilation of n-alkyl-substituted cycloparaffins. J Bacteriol. 1974;118:394–399. doi: 10.1128/jb.118.2.394-399.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blakley E R, Papish B. The metabolism of cyclohexanecarboxylic acid and 3-cyclohexanecarboxylic acid by Pseudomonas putida. Can J Microbiol. 1982;28:1324–1329. doi: 10.1139/m82-198. [DOI] [PubMed] [Google Scholar]
- 5.Campos-Garcia J, Esteve A, Vázquez-Duhalt R, Ramos J L, Soberón-Chávez G. The branched-chain dodecylbenzene sulfonate degradation pathway of Pseudomonas aeruginosa W51D involves a novel route for degradation of the surfactant lateral alkyl chain. Appl Environ Microbiol. 1999;65:3730–3734. doi: 10.1128/aem.65.8.3730-3734.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis J B, Raymond R L. Oxidation of alkyl-substituted cyclic hydrocarbons by a nocardia during growth on n-alkanes. Appl Microbiol. 1961;9:383–388. doi: 10.1128/am.9.5.383-388.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong J, Vorkink W P, Lee M L. Origin of long-chain alkylcyclohexanes and alkylbenzenes in a coal-bed wax. Geochim Cosmochim Acta. 1993;57:837–849. [Google Scholar]
- 8.Dutta T K, Harayama S. Analysis of long-side-chain alkylaromatics in crude oil for evaluation of their fate in the environment. Environ Sci Technol. 2001;35:102–107. doi: 10.1021/es001165a. [DOI] [PubMed] [Google Scholar]
- 9.Dutta T K, Harayama S. Fate of crude oil by the combination of photooxidation and biodegradation. Environ Sci Technol. 2000;34:1500–1505. [Google Scholar]
- 10.Ellis L, Singh R K, Alexander R, Kagi R I. Geosynthesis of organic compounds. III. Formation of alkyltoluenes and alkylxylenes in sediments. Geochim Cosmochim Acta. 1995;59:5133–5140. [Google Scholar]
- 11.Fedorak P M, Westlake D W S. Fungal metabolism of n-alkylbenzenes. Appl Environ Microbiol. 1986;51:435–437. doi: 10.1128/aem.51.2.435-437.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fedorak P M, Payzant J D, Montgomery D S, Westlake D W S. Microbial degradation of n-alkyl tetrahydrothiophenes found in petroleum. Appl Environ Microbiol. 1988;54:1243–1248. doi: 10.1128/aem.54.5.1243-1248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fedorak P M, Peakman T M. Aerobic microbial metabolism of some alkylthiophenes found in petroleum. Biodegradation. 1992;2:223–236. [Google Scholar]
- 14.Fowler G M, Abolins O, Douglas A G. Monocyclic alkanes in Ordovician organic matter. Org Geochem. 1986;10:815–823. [Google Scholar]
- 15.Henke B, Girzalsky W, Berteaux-Lecellier V, Erdmann R. IDP3 encodes a peroxisomal NADP-dependent isocitrate dehydrogenase required for the β-oxidation of unsaturated fatty acids. J Biol Chem. 1998;273:3702–3711. doi: 10.1074/jbc.273.6.3702. [DOI] [PubMed] [Google Scholar]
- 16.Ingram L L, Ellis J, Crisp P T, Cook A C. Comparative study of oil shales and shale oils from the Mahogany Zones, Green River Formation (U.S.A.) and Kerosene Creek Seam, Rundle Formation (Australia). Chem Geol. 1983;38:185–212. [Google Scholar]
- 17.Kissin Y V. Catagenesis of light cycloalkanes in petroleum. Org Geochem. 1990;15:575–594. [Google Scholar]
- 18.Ostroukhov S B, Aref'yev O A, Pustil'nikova S D, Zabrodina M N, Petrov A A. C12-C30 n-alkylbenzenes in crude oils. Pet Chem USSR. 1983;23:1–12. [Google Scholar]
- 19.Radke M, Willsch H. Generation of alkylbenzenes and benzo[b]thiophenes by artificial thermal maturation of sulfur-rich coal. Fuel. 1993;72:1103–1107. [Google Scholar]
- 20.Rontani J F, Bonin P. Utilization of n-alkyl-substituted cyclohexanes by a marine Alcaligenes. Chemosphere. 1992;24:1441–1446. [Google Scholar]
- 21.Rontani J F, Bonin P, Giusti G. Mechanistic study of interactions between photo-oxidation and biodegradation of n-nonylbenzene in seawater. Mar Chem. 1987;22:1–12. [Google Scholar]
- 22.Rubinstein I, Strausz O P. Geochemistry of the thiourea adduct fraction from an Alberta petroleum. Geochim Cosmochim Acta. 1979;43:1387–1392. [Google Scholar]
- 23.Sariaslani F S, Harper D B, Higgins I J. Microbial degradation of hydrocarbons: catabolism of 1-phenyl-alkanes by Nocardia salmonicolor. Biochem J. 1974;140:31–45. doi: 10.1042/bj1400031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinninghe Damsté J S, Kock-van Dalen A C, Albrecht P A, De Leeuw J W. Identification of long-chain 1,2-di-n-alkylbenzenes in Amposta crude oil from the Terragona Basin, Spanish Mediterranean: implications for the origin and fate of alkylbenzenes. Geochim Cosmochim Acta. 1991;55:3677–3683. [Google Scholar]
- 25.Sinninghe Damsté J P, de Leeuw J W, Kock-van Dalen A C, de Zeeuw M A, de Lange F, Rijpstra W I C, Schenck P A. The occurrence and identification of series of organic sulfur compounds in oils and sediment extracts. I. A study of Rozel Point Oil (USA) Geochim Cosmochim Acta. 1987;51:2369–2391. [Google Scholar]
- 26.Sinninghe Damsté J P, Rijpstra W I C, de Leeuw J W, Schenck P A. The occurrence and identification of series of organic sulfur compounds in oils and sediment extracts. II. Their presence in samples from hypersaline and non-hypersaline palaeoenvironments and possible application as source, palaeoenvironmental and maturity indicators. Geochim Cosmochim Acta. 1989;53:1323–1341. [Google Scholar]
- 27.Solli H, Larter S R, Douglas A G. Analysis of kerogens by pyrolysis-gas-chromatography-mass-spectrometry using selective ion monitoring. III. Long-chain alkylbenzenes. In: Douglas A G, Maxwell R J, editors. Advances in organic geochemistry. Oxford, United Kingdom: Pergamon Press; 1980. pp. 591–597. [Google Scholar]
- 28.Summons R E, Powell T G. Identification of aryl isoprenoids in source rocks and crude oils: biological markers from the green sulfur bacteria. Geochim Cosmochim Acta. 1987;51:557–566. [Google Scholar]
- 29.Trudgill P W. Microbial degradation of the alicyclic ring: structural relationship and metabolic pathways. In: Gibson D T, editor. Microbial degradation of organic compounds. New York, N.Y: Marcel Dekker; 1984. pp. 131–180. [Google Scholar]
- 30.Williams J A, Dolcater D L, Torkelson B E, Winters J C. Anomalous concentrations of specific alkylaromatic and alkylparaffin components in West Texas and Michigan crude oils. Org Geochem. 1988;13:47–60. [Google Scholar]
- 31.Yakimov M M, Golyshin P N, Lang S, Moore E R, Abraham W R, Lunsdorf H, Timmis K N. Alcanivorax borkumensis gen. nov., sp. nov., a new, hydrocarbon-degrading and surfactant-producing marine bacterium. Int J Syst Bacteriol. 1998;48:339–348. doi: 10.1099/00207713-48-2-339. [DOI] [PubMed] [Google Scholar]