Abstract
Hyponatremia is the most common electrolyte disturbance seen in clinical practice, affecting up to 30% of acute hospital admissions, and is associated with significant adverse clinical outcomes. Acute or severe symptomatic hyponatremia carries a high risk of neurological morbidity and mortality. In contrast, chronic hyponatremia is associated with significant morbidity including increased risk of falls, osteoporosis, fractures, gait instability, and cognitive decline; prolonged hospital admissions; and etiology-specific increase in mortality. In this Approach to the Patient, we review and compare the current recommendations, guidelines, and literature for diagnosis and treatment options for both acute and chronic hyponatremia, illustrated by 2 case studies. Particular focus is concentrated on the diagnosis and management of the syndrome of inappropriate antidiuresis. An understanding of the pathophysiology of hyponatremia, along with a synthesis of the duration of hyponatremia, biochemical severity, symptomatology, and blood volume status, forms the structure to guide the appropriate and timely management of hyponatremia. We present 2 illustrative cases that represent common presentations with hyponatremia and discuss the approach to management of these and other causes of hyponatremia.
Keywords: hyponatremia, SIAD, tolvaptan, fluid restriction, hypertonic saline
Case 1
A 55-year-old woman presented to the emergency department accompanied by a family member, who volunteered that she had reported headaches and nausea the previous evening and this morning had vomited and appeared confused. She had recently commenced a selective serotonin reuptake inhibitor (SSRI). On examination, the patient was drowsy and clinically euvolemic with no evidence of trauma or infection. Her weight was 49 kg (body mass index 19 kg/m2), and collateral history revealed a history of alcohol excess. Laboratory investigations were as follows: urea 2.1 mmol/L, creatinine 63 μmol/L, sodium 113 mmol/L, and potassium 4.4 mmol/L. Samples were sent for urine osmolality, urinary sodium, thyroid function, and serum cortisol concentration.
Case 2
A 72-year-old man was referred complaining of a persistent cough, occasional blood-streaked sputum, and unintentional weight loss. Initial biochemistry was as follows: urea 3 mmol/L, creatinine 87 μmol/L, sodium 124 mmol/L, and potassium 4.8 mmol/L. On clinical examination, he was clinically euvolemic. Paired urine sodium and osmolality were 46 mmol/L and 340 mOsm/kg, respectively. Morning serum cortisol concentration was 487 nmol/L (17.7 µg/dL), and he was biochemically euthyroid. Imaging studies detected a suspicious lung lesion, and histology of a computed tomography–guided biopsy confirmed a diagnosis of small-cell lung cancer.
Prevalence of Hyponatremia
Hyponatremia is the most commonly encountered electrolyte disturbance in clinical practice (1). In a hospitalized patient cohort, the reported incidence of hyponatremia is 15% to 30% (2-4); however, severe hyponatremia (<125 mmol/L) is less common and is reported in 0.5% to 3% of hospitalized patients (2, 5).
Physiology of Salt and Water Balance
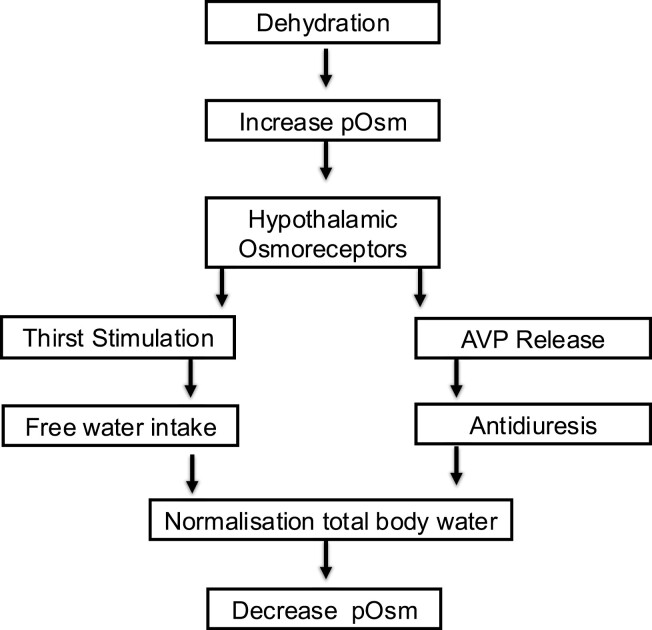
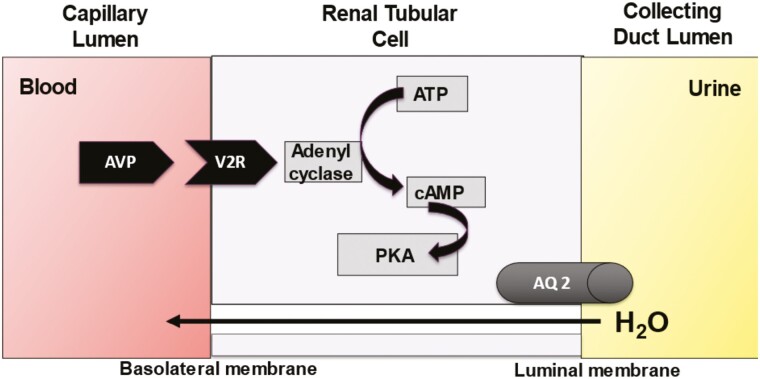
Plasma sodium concentration is the principle determinant of plasma osmolality (6), and both plasma sodium and osmolality are maintained within a narrow physiological range by osmotically regulated arginine vasopressin (AVP) secretion and the sensation of thirst (7) (Fig. 1). A change in plasma osmolality is detected by specialized neurones in the circumventricular organs of the anterior hypothalamus. AVP is synthesized in the paraventricular and supraoptic nuclei of the hypothalamus and is transported as a prohormone, in conjunction with copeptin and neurophysin, to the posterior pituitary where it is stored in nerve termini in secretory granules. Elevation of plasma osmolality is the principal physiological stimulus that causes cleavage of the prohormone and release of AVP and copeptin into the systemic circulation (8). AVP binding to the vasopressin 2 receptor on the cell surface of the renal collecting ducts leads to intracellular generation of aquaporin 2 (AQ-2) and translocation of preformed AQ-2 to the luminal membrane of the collecting duct, where AQ-2 is inserted to form a water channel. This renders the membrane permeable to free water, promoting reabsorption of water from the renal tubules and reducing renal free water loss (9) (Fig. 2). Coincidentally, rising plasma osmolality stimulates thirst perception, which starts at a similar osmolar threshold to the release of AVP (10), driving water intake. The combination of free water intake and the antidiuretic effect of AVP activity results in an increase in plasma free water and a reduction in plasma osmolality and sodium concentration.
Figure 1.
Water homeostasis. Abbreviations: AVP, arginine vasopressin; pOsm, plasma osmolality.
Adapted from Hannon et al (8).
Figure 2.
Renal response to vasopressin. Abbreviations: AQ-2, aquaporin 2 channel; ATP, adenosine triphosphate; AVP, arginine vasopressin; cAMP, cyclic adenosine monophosphate; PKA, protein kinase A; V2R, vasopressin receptor type 2.
Mineralocorticoid and glucocorticoid activity will also influence salt and water homeostasis (11). Aldosterone action in the renal distal tubules and collecting ducts promotes expression of apical epithelial sodium channels and the Na+/K+/ATPase, resulting in increased reabsorption of both sodium and water (12). Cortisol also plays an integral role in the regulation of free water excretion (13).
Hyponatremia is almost always associated with hypotonicity, or hypo-osmolality (plasma osmolality < 280 mOsm/kg) (14). However, there are 2 circumstances where plasma sodium concentration and plasma osmolality diverge: pseudohyponatremia and isotonic/hypertonic hyponatremia.
Pseudohyponatremia is an artifactual decrease in plasma sodium concentration due to displacement of free water in plasma by very high concentrations of lipids or proteins, which interfere with the accurate measurement of sodium; however, plasma osmolality remains normal (15). Isotonic/hypertonic hyponatremia occurs when unmeasured solutes other than sodium (such as glucose or mannitol) are present and contribute to plasma osmolality (7). An important clinical example of this occurs with significant hyperglycemia, which in addition to glucose-mediated osmotic diuresis, can be associated with either iso- or hypertonic hyponatremia, and therefore the effect of glucose should be corrected for in this setting (16). If the rate of fall in glucose exceeds the rate of plasma sodium increase—for example, during treatment with intravenous insulin in patients with significant hyperglycemia—then plasma osmolality can fall quickly, with the risk of associated cerebral edema. Therefore, care should be taken to control the rate of glucose lowering (17).
Etiology of Hyponatremia
The etiology of hypotonic hyponatremia can be divided into 3 main groups based on the clinical volume status of the patient. Hyponatremia can also be further classified by acuity of onset, biochemical severity, and the presence and severity of associated symptoms, which will be discussed in detail later in this review (Table 1).
Table 1.
Classification of hyponatremia
| Biochemical | Symptoms |
|---|---|
| Mild 130-135mmol/ | Mild |
| Moderate 125-129 mmol/L | Moderate |
| Profound/Severe < 125 mmol/L | Severe |
| Etiology | Acuity of onset |
| Hypovolemic | Acute < 48 hours |
| Euvolemic | Chronic > 48 hours |
| Hypervolemic |
Hypovolemic Hyponatremia
Hypovolemic hyponatremia occurs due to a loss of both total body water and plasma sodium. Reduction in circulating blood volume stimulates baroregulated AVP secretion despite hypotonicity, which, combined with sodium losses (renal or nonrenal), leads to a greater sodium loss relative to total body water losses (11). Thiazide diuretic use is an important cause of hypovolemic hyponatremia, causing both renal sodium loss and hypotension (stimulating baroregulated AVP secretion) (18). Thiazide-induced hyponatremia is frequently accompanied by hypokalemia (19). Other renal sodium losses include mineralocorticoid deficiency, salt-wasting nephropathies, and, rarely, cerebral salt wasting (18). Nonrenal sodium losses include gastrointestinal loss due to vomiting or diarrhea and transdermal loss.
Cerebral salt-wasting syndrome
Cerebral salt-wasting syndrome (CSWS) was originally described in 1950 by Peters et al, who described hyponatremia and a natriuresis observed in a neurosurgical setting (20). However, the degree to which CSWS contributes to hyponatremia has remained controversial (21-23). Reported prevalence of CSWS varies significantly (23), which may be due to the challenge in distinguishing CSWS from syndrome of inappropriate antidiuresis (SIAD) and in the inherent challenges in accurate volume status assessment (21, 23-25). Table 2 highlights the differences between CSWS and SIAD (Table 2). Two studies performed in our center concluded that CSWS is a rare cause of hyponatremia in a neurosurgical setting (24, 25).
Table 2.
Differentiating the syndrome of inappropriate antidiuresis from cerebral salt-wasting syndrome
| SIAD | CSWS | |
|---|---|---|
| Plasma Na | Low | Low |
| Blood urea | Low | Elevated |
| BP | Normal | Low/ postural drop |
| Urine volume | Low | High |
| Urine Na | >40 mmol/L | >>40 mmol/L |
| CVP | Normal | Low |
Abbreviations: BP, blood pressure; CSWS, cerebral salt-wasting syndrome; CVP, central venous pressure; Na, sodium; SIAD, syndrome of inappropriate antidiuresis.
Euvolemic Hyponatremia
Euvolemic hyponatremia is the commonest form of hyponatremia in hospitalized patients (11). Total body sodium remains unchanged; however, a relative increase in total body water, not appreciable on clinical examination, results in a dilutional hyponatremia (26). This can occur due to excess free water intake in the context of impaired free water excretion or, less commonly, low solute intake (18). While the majority of euvolemic hyponatremia is caused by SIAD, careful clinical assessment is essential to assess for other causes of euvolemic hyponatremia (eg, glucocorticoid deficiency, which is frequently overlooked, and hypothyroidism, which is an extremely rare cause of hyponatremia).
Syndrome of inappropriate antidiuresis
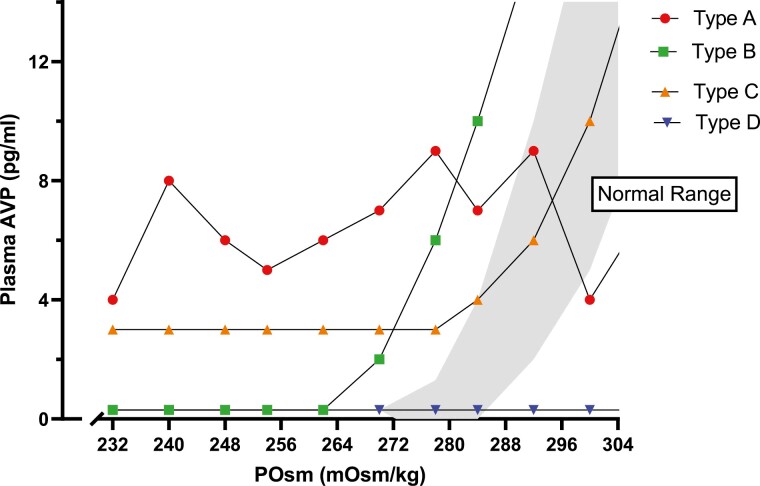
SIAD was first described in the 1950s in 2 patients with lung cancer (27). Since then, it has been described in association with a wide range of disorders (28, 29) (Table 3). The diagnostic criteria first described by Bartter and Schwartz remains largely unchanged and must be fulfilled before a diagnosis of SIAD can be made (Table 4) (30). SIAD is the most common cause of hyponatremia in hospitalized patients, accounting for up to 46% of cases of hyponatremia in an unselected population (31). There are 4 subtypes of SIAD (types A, B, C, and D), which can be differentiated by measuring the AVP (or copeptin) response to an osmotic stimulus (32, 33) (Fig. 3). Types A and B SIAD are the most common subtypes encountered; however, the delineation of the subtype of SIAD is rarely required in clinical practice.
Table 3.
Causes of syndrome of inappropriate antidiuresis
| Causes of SIAD |
|---|
| Malignancy Lung cancer (small cell carcinoma, non-small cell, mesothelioma, thymoma) CNS tumors Head and neck tumors Hematological (leukemia, lymphoma) Urological (bladder, urethral, prostate) Gastrointestinal (duodenal, pancreatic) |
| Central nervous system Trauma Vascular insult (SAH, SDH, stroke) Infection (meningitis, encephalitis, abscess) Inflammation (GBS, SLE, MS) |
| Medications Chemotherapy (vincristine, cyclophosphamide) Psychiatric (SSRI, TCA, clozapine, phenothiazines) Other: omeprazole, nicotine, oxytocin, clofibrate, carbamazepine |
| Respiratory Infectious: pneumonia, Sars-CoV-2 pneumonitis, TB, empyema Mechanical: COPD, acute respiratory failure, positive pressure ventilation |
| Other Nausea Pain Prolonged exercise Surgery Activating mutations of V2 receptor |
| Idiopathic |
Abbreviations: CNS, central nervous system; COPD, chronic obstructive pulmonary disease; GBS, Guillain-Barre syndrome; MS, multiple sclerosis; SAH, subarachnoid hemorrhage; SIAD, syndrome of inappropriate antidiuresis; SLE, systemic lupus erythematosus; SSRI, selective serotonin reuptake inhibitors; TCA, tricyclic antidepressants; V2 receptor vasopressin-2 receptor.
Table 4.
Criteria for the diagnosis of syndrome of inappropriate antidiuresis
| Criteria |
|---|
| 1. Hypotonic hyponatremia (plasma osmolality < 275 mOsm/kg) |
| 2. Evidence of inappropriate AVP activity relative to plasma osmolality (Urine osmolality > 100 mOsm/kg) |
| 3. Euvolemic state on clinical examination |
| 4. Urinary sodium concentration > 30 mmol/L (normal salt and water intake) |
| 5. Absence of recent diuretic use |
| 6. Normal renal function |
| 7. Exclusion of alternative diagnosis (glucocorticoid deficiency, hypothyroidism) |
Figure 3.
Classification of the syndrome of inappropriate antidiuresis (types A-D) according to pattern of vasopressin (AVP) secretion (shaded area represents normal AVP response to rising plasma osmolality (POsm).
Glucocorticoid deficiency
Glucocorticoid deficiency is associated with an inappropriately elevated AVP concentration relative to plasma osmolality and a reduction in effective renal free water clearance, resulting in an increase in total body water (35). Glucocorticoid-deficient animal models demonstrate increased expression of AQ-2 and higher AVP concentration following a free water load than those with intact adrenal function (13, 35, 36), which reverses once glucocorticoid replacement has been initiated (37, 38). Primary adrenal insufficiency classically presents with hypovolemic hyponatremia due to a combination of both glucocorticoid and mineralocorticoid deficiency (39). Hyponatremia due to secondary adrenal insufficiency is typically euvolemic (as the renin-angiotensin-aldosterone system remain intact) and indistinguishable clinically from SIAD (31, 37, 40, 41). In a large, single-center prospective study of euvolemic hyponatremia, 4% of patients initially classified as SIAD were found to have undiagnosed adrenocorticotropin deficiency (31).
Thyroid-stimulating hormone deficiency
The diagnostic criteria for SIAD require the exclusion of hypothyroidism; however, in clinical practice, hyponatremia due to hypothyroidism is extremely rare and only seen in patients with profound hypothyroidism (42).
Exercise-induced hyponatremia
Exercise-induced hyponatremia is defined as hyponatremia that develops during or within 24 hours of exercise (43) and is typically associated with long-distance and endurance sports (43-47). Exercise is a nonosmotic stimulus of AVP secretion (48, 49), and when large quantities of hypotonic fluids are ingested, acute hyponatremia may develop (46), which can be fatal if untreated (50). Consequently, fluid intake according to thirst is currently recommended to prevent exercise-induced hyponatremia (43, 51). This area has been extensively reviewed by Hew-Butler et al (50, 51).
High water and low solute intake
If large volumes of fluid are consumed with relatively low solute intake, fluid intake may exceed the renal capacity to excrete free water, expanding total plasma free water relative to total body sodium (7). This phenomenon is observed in those who consume large volumes of beer (beer potomania) (52, 53) or, occasionally, in those with hypotonic fluid consumption in combination with low protein diets (54). Patients with primary polydipsia may also present with hyponatremia if water intake exceeds renal capacity for excretion despite maximal suppression of AVP activity (7). A recent study by Sailer et al describing the characteristics of patients with severe hyponatremia due to primary polydipsia reported an additional nonosmotic stimulus for AVP was found in all cases, the most common cause being medication (55).
Hypervolemic Hyponatremia
Hypervolemic hyponatremia is seen in cardiac, liver, and renal failure and results from an expansion of both total body water and sodium, with a relatively greater increase in free water than sodium (26). A fall in mean arterial pressure stimulates baroregulated AVP secretion and activation of the renin-angiotensin-aldosterone system (with the development of secondary hyperaldosteronism), resulting in a relative excess of total body water and a reduction in both plasma osmolality and sodium concentration (7, 11, 56). The presence of hyponatremia is associated with a poor prognosis in patients with heart failure (57, 58), chronic kidney disease (59, 60), and decompensated liver disease (61, 62).
Morbidity and Mortality Associated With Hyponatremia
Mortality in Acute Hyponatremia
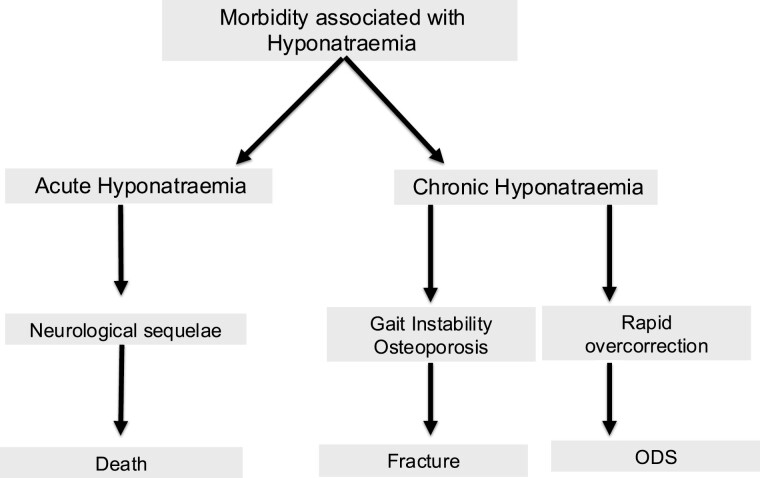
Acute hyponatremia is associated with significant morbidity and mortality (Fig. 4). The reduction in plasma osmolality that accompanies acute, severe hyponatremia results in osmotic movement of water from the low osmolality plasma into the normal osmolality brain before intracellular electrolytes and organic osmolytes can be extruded as an adaptive response (63). This results in cerebral edema (25), with associated increased intracranial pressure causing neurological compromise and ultimately brainstem herniation (26, 64). Acute or severe symptomatic hyponatremia carries a high mortality rate if untreated (65).
Figure 4.
Morbidity associated with both acute severe and chronic hyponatremia and its treatment. Abbreviation: ODS, osmotic demyelination syndrome.
Adapted from Ellison et al, Adrogué et al, Verbalis et al, and Sterns et al (14, 29, 78, 115).
Mortality in Chronic Hyponatremia
Chronic hyponatremia is associated with an increased in-hospital mortality risk (66-70) compared to normonatremic controls, which persists up to 1 year following discharge (71). The mortality risk associated with hyponatremia varies, depending on the underlying etiology. A prospective, single-center study carried out in our institution reported that SIAD was associated with an increased mortality risk compared with normonatremic controls. The mortality risk was even greater in patients with hypervolemic or hypovolemic hyponatremia (68). Whether hyponatremia plays a causative role in increased mortality risk or is merely a marker of severity of underlying disease is not clear (72-74). However, the impact of hyponatremia on organ dysfunction has also been postulated as a contributory factor in the observed increased mortality (73, 74).
Morbidity in Chronic Hyponatremia
Chronic hyponatremia is associated with significant morbidity including increased admissions to intensive care (66), prolonged length of stay (24, 75), and increased readmissions to hospital (4, 76), as well as cognitive dysfunction, gait instability, and fractures (77, 78). In addition, treatment resulting in rapid correction of chronic hyponatremia can result in osmotic demyelination syndrome (ODS) (14).
Osmotic demyelination syndrome
ODS, also known as central pontine myelinolysis, is a potentially devastating condition due to demyelination of pontine and extrapontine neurones, which can result in serious neurological dysfunction, seizures, and death (29). ODS can occur following rapid correction of hyponatremia of chronic duration (>48 hours) (79-82), if the rate of correction of plasma sodium exceeds the rate of recovery of lost intracellular solutes (83). The loss of intracellular solutes, which forms the adaptive response to a fall in plasma osmolality to prevent cerebral edema, leaves astrocytes vulnerable to injury, and therefore a rapid rise in plasma osmolality represents an osmotic stress, causing astrocyte apoptosis, damage to the blood-brain barrier, and demyelination (14, 84). To reduce the risk of ODS, both the US and European recommendations/guidelines for the management of hyponatremia advise setting maximal limits to the daily increases in plasma sodium concentration and intervening with hypotonic fluids and parenteral desmopressin to relower sodium if the rate of correction exceeds safe limits (7, 18). This is discussed later in this review in more detail.
Neurocognitive function and chronic hyponatremia
Chronic, mild-to-moderate hyponatremia has been associated with impairment in cognition, attention, and cognitive decline compared to normonatremic controls in various patient populations (85-88). There is evidence to suggest that the correction of hyponatremia is associated with an improvement in neurocognitive functioning (77, 89, 90).
Osteoporosis, falls, and fracture risk
Hyponatremia is a recognized independent risk factor for gait instability and osteoporosis, leading to falls and fracture occurrence (91-93). A recent study found a 3-fold increase in falls risk in patients with mild hyponatremia in an emergency geriatric assessment unit (94). Hyponatremia is also associated with increased risk of both osteoporosis and fragility fractures, the risk of which increases with severity and chronicity of hyponatremia (95). In an animal model of chronic hyponatremia, Verbalis et al demonstrated a reduction in bone mineral density of up to 30% compared to normonatremic controls and loss of both cortical and trabecular bone (78). Analysis of data from the NHANES III survey comparing bone mineral density at the femoral neck and hip between patients with hyponatremia with normonatremic controls found that hyponatremia was associated with an increased risk of osteoporosis (78).
Clinical Approach to Hyponatremia
In recent years, there have been several international guidelines/ recommendations regarding the management of hyponatremia. There are currently 2 sets of international clinical practice guidelines/recommendations on the evaluation, diagnosis, and management of hyponatremia, which are mostly widely cited and utilized in clinical practice (7, 18). Verbalis et al published a US-based expert panel in 2013 (7), and Spasovski et al published a clinical practice guideline in 2014 by the European Society of Endocrinology, European Society of Intensive Care Medicine, European Renal Association, and European Dialysis and Transplant Association (18).
These manuscripts have many similarities and agreements but also some areas in which they differ, and we will refer to both when discussing the clinical approach to the patient with hyponatremia.
When evaluating a patient with hyponatremia, there are 4 important points that should be considered (Table 1):
The presence of symptoms suggestive of cerebral edema
The estimated duration of hyponatremia
The biochemical severity of hyponatremia
A clinical assessment of the patient’s volume status to help elucidate the underlying etiology of hyponatremia
The combined careful evaluation of each of these points will guide appropriate and timely management.
Presence of Symptoms
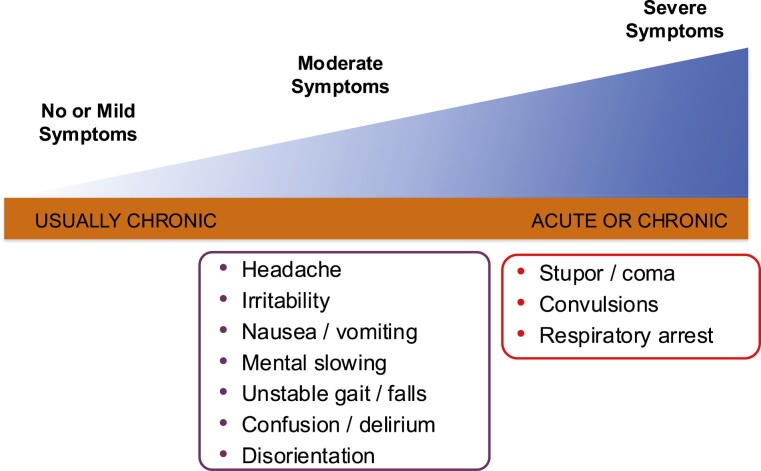
Documentation of symptoms is an essential step when approaching a patient with hyponatremia. The presence of moderate or severe symptoms indicates the presence of cerebral edema and demands immediate intervention. Clinical manifestation of hyponatremia varies from asymptomatic cases to moderate symptoms such as headaches, nausea, and confusion, and, ultimately, to severe symptoms include vomiting, depressed level of consciousness, abnormal somnolence, seizure, coma, and death (Fig. 5) (7, 64).
Figure 5.
Spectrum of symptoms associated with hyponatremia.
Chronicity
The definition of acute and chronic hyponatremia is defined as hyponatremia being present for <48 hours or >48 hours, respectively. The rapidity of onset of hyponatremia is important for 2 reasons. First, acute hyponatremia (<48 hours) is more likely to present with severe neurological symptoms due to cerebral edema requiring emergency treatment (65, 96). Second, the safe rate of correction of hyponatremia depends on duration, in that patients with acute hyponatremia have less risk of osmotic demyelination (7). An acute fall in both plasma sodium concentration and osmolality creates an osmotic gradient between plasma and the brain, resulting in cerebral edema, raised intracranial pressure and, ultimately, a risk of brainstem herniation and death (14, 18). If the fall in plasma osmolality is more gradual (>48 hours), the brain adapts to its hypotonic environment by extruding ions and organic osmolytes to maintain a normal cell volume, resulting in more modest symptoms (7, 14). However, this adaptive process leaves astrocytes vulnerable to injury if plasma osmolality rises rapidly (14), and therefore overrapid correction of chronic hyponatremia is associated with the risk of ODS (81, 97), and extreme care must be taken to limit correction of chronic hyponatremia to within safe threshold limits.
Biochemical Severity
Most guidelines and papers arbitrarily define mild hyponatremia as 130 to 135 mmol/L and moderate hyponatremia as 125 to 129 mmol/L (18). However, guidelines differ slightly regarding the biochemical definition of severe hyponatremia. Verbalis et al define “severe” hyponatremia as plasma sodium concentration ≤ 120 mmol/L (7), while Spasovski et al define “profound” hyponatremia as plasma sodium concentration < 125mmol/L (18). It is important to stress that while symptoms are more common among those with severe or profound biochemical hyponatremia, severity of symptoms does not always correlate with biochemical severity. Other factors, such as coexistent brain injury or cerebral edema, additionally impact on neurological sequelae (7, 98), while the most important determinant is speed of onset of hyponatremia.
Clinical Volume Status
The clinical assessment of volume status is a crucial step in the identification of the etiology of hyponatremia. However, it is the least accurate aspect of the diagnostic process with a sensitivity and specificity < 50% (99, 100). It can be particularly challenging to distinguish euvolemia from subtle volume depletion. Therefore, if sodium concentration either fails to improve or worsens in response to treatment based on initial volume assessment, it is worthwhile reassessing the original diagnosis (7).
Defining the Etiology of Hyponatremia
The spectrum of diseases causing hyponatremia is wide and hyponatremia is often multifactorial. A detailed clinical history and careful clinical examination is essential. As the clinical assessment of volume status has a limited sensitivity and specificity (99, 101), the measurement of both urinary osmolality and urine sodium concentration can improve diagnostic accuracy (7, 18, 102). Laboratory investigations should also include an assessment of renal function, and in the case of euvolemic hyponatremia, both adrenal insufficiency and hypothyroidism must be excluded before making a diagnosis of SIAD (Table 4).
Urinary Sodium Concentration
Urinary sodium concentration (UNa) reflects renal mineralocorticoid activity and therefore indirectly reflects effective circulating volume (98). In the absence of diuretic use, UNa < 20 mmol/L implies the effect of aldosterone to promote renal sodium reabsorption. This suggests hypovolemic hyponatremia with extrarenal solute loss or hypervolemic hyponatremia, with secondary hyperaldosteronism. Elevated urine sodium concentration (UNa > 30 mmol/L) indicates diuretic use, euvolemic hyponatremia, or hypovolemia with renal solute loss (Table 5). It should also be noted that patients with low solute intake may have a low UNa despite being euvolemic.
Table 5.
Etiology of hyponatremia using urinary sodium concentration and clinical volume assessment
| UNa < 30 mmol/L | UNa > 30 mmol/L | |
|---|---|---|
| Hypovolemia | Extrarenal sodium loss Vomiting Diarrhoea Burns Pancreatitis |
Renal sodium loss Primary adrenal insufficiency Salt-wasting nephropathy Cerebral salt wasting Diuretic use |
| Euvolemia | Primary polydipsia Beer potomania(UOsm ≤ 100 mOsm/kg) Low solute intake Hypotonic fluid replacement |
SIAD Secondary adrenal insufficiency(UOsm > 100 mOsm/kg) |
| Hypervolaemia | Cirrhosis Nephrotic syndrome Renal failure Heart failure |
Diuretic use |
Abbreviations: UNa, urinary sodium concentration; UOsm urine osmolality.
Adapted from Smith et al (11).
Urine Osmolality
Urine osmolality directly reflects plasma AVP activity, making it a useful and readily available biomarker (98). Physiologically, when plasma osmolality falls below the osmotic threshold for AVP secretion (approximately 284 mOsm/kg) (10), AVP secretion is suppressed, resulting in maximally dilute urine (UOsm < 100 mOsm/kg). Measurements of UOsm > 100 mOsm/kg in the setting of euvolemic hyponatremia therefore indicates AVP activity that is inappropriate to the plasma osmolality (Table 3) (7). UOsm is also elevated in hypovolemic states due to the renal actions of baroregulated AVP secretion. A low urine osmolality is a good indicator of excess fluid intake leading to dilutional hyponatremia and can therefore predict rapid overcorrection of hyponatremia once fluid intake is curtailed (103). An elevated urinary osmolality (>500 mOsm/kg) has also been shown to be predictive of failure of fluid restriction (FR) to work in patients with SIAD (7).
Fraction Excretion of Uric Acid
Fractional excretion of uric acid (FEUA) can be useful in patients treated with diuretics, where UNa may be difficult to interpret. Fenske et al reported that in patients not treated with diuretics, both UNa and FEUA performed equally well in identifying SIAD; however, in diuretic use, FEUA was superior (104). The same study reported that FEUA > 12% had a 100% positive predictive value for SIAD, and conversely, FEUA < 8% has a negative predictive value for SIAD of 100%.
Copeptin and AVP Measurement
AVP is derived from provasopressin, a precursor peptide that is enzymatically cleaved in the neurosecretory granules of the posterior pituitary to yield AVP, copeptin, and neurophysin. There is no role for the measurement of plasma AVP concentrations in the differential diagnosis of hyponatremia. Plasma AVP concentrations have been shown to be elevated in all causes of hyponatremia, and the assay results take too long to come back to be of clinical diagnostic value.
As copeptin is cosecreted with AVP, it has been suggested as an alternative biomarker for plasma AVP concentrations (105) and has shown to be a useful surrogate marker in the diagnosis of polyuric-polydipsic states during osmoregulatory studies. However, use of copeptin in the diagnostic evaluation of hyponatremia is limited (106,107); as with AVP, there is significant overlap in copeptin concentrations in all other forms of hyponatremia (108,109).
Treatment of Acute or Severe Symptomatic Hyponatremia
Acute or severe symptomatic hyponatremia is a medical emergency requiring urgent, lifesaving treatment that should ideally be delivered in a monitored critical care setting. Hypertonic saline is the treatment of choice for acute or severe symptomatic hyponatremia to reverse cerebral edema and prevent brainstem herniation (7, 18). Hypertonic saline can be administered as either an intravenous bolus or an intravenous infusion. Both the US and European recommendations/guidelines currently recommend bolus therapy in acute or severe symptomatic hyponatremia, although they differ slightly in their advice regarding administration (Table 6) (7, 18). The US recommendations suggest the administration of 100 mL 3% hypertonic saline over 10 minutes (repeated up to 2 times if required) with the aim of achieving a 4- to 6-mmol/L rise in plasma sodium concentration. Where the duration of hyponatremia is clearly known to be <24 to 48 hours, the US recommendations state that the rate of correction does not need to be restricted; however, where there is any doubt regarding duration, correction limits should be managed as per chronic hyponatremia guidance (7). The European guideline’s diagnostic algorithm focuses on the presence and severity of symptoms and recommends 150 mL bolus 3% hypertonic saline to be administered over 20 minutes for severe symptoms and giving a second bolus while checking a repeat plasma sodium concentration, with the aim of achieving a 5 mmol/L rise within the first hour of treatment. The European guidelines also recommend treatment with a single bolus of 150 mL 3% hypertonic saline if moderately severe symptoms are present, with the target of achieving a 5 mmol/L rise in sodium concentration within 24 hours; however, the US recommendations suggest a weight-based hypertonic saline infusion for mild-moderate symptoms at low risk of brainstem herniation (Table 6) (7).
Table 6.
Comparison of the management of acute or symptomatic hyponatremia between the US and European recommendations/guidelines
| US Recommendations | European Guidelines | |
|---|---|---|
| Severe symptoms | 100 mL 3% HS | 150 mL 3% HS |
| Duration | 10 minutes | 20 minutes |
| Target Na+ rise | 4-6 mmol/L over 4 hours | 5 mmol/L within 1 hour |
| Boluses, max, n | 3 | 3 |
| Mild-moderate symptoms | 0.5-2 mL/kg/hour 3% HS infusion | Single bolus 150 mL 3% HS |
| Maximal Na+ rise (24 hours) | Acute (<24-48 hours) No restriction Chronic (>48 hours) 8-12 mmol/L per 24 hours (6-8 mmol/L per 24 hours if high ODS risk) |
10 mmol/L per 24 hours in initial 24 hours 8 mmol/L per 24 hours thereafter |
| Management of excessive correction | Replace water losses (PO/IV) Parenteral desmopressin Consider high-dose glucocorticoid Consider relowering Na+ to target sodium |
Stop treatment 10 mL/kg IV 5% dextrose over 1 houra Consider parenteral desmopressina |
Abbreviations: HS, hypertonic saline; Na+, plasma sodium concentration; ODS, osmotic demyelination syndrome; PO/IV, intravenous/enteral.
aExpert consultation advised.
Garrahy et al reported the findings of a study comparing the clinical and biochemical outcomes for patients with severe symptomatic SIAD treated with hypertonic saline bolus therapy (as per US recommendations) to patients who received continuous hypertonic saline infusion and reported that bolus therapy achieved a greater initial rise in plasma sodium concentration [6 mmol/L (CI 2-11) vs 3 mmol/L (CI 1-4), P < 0.0001] and improvement in neurological status [median change in Glasgow Coma Scale (GCS) 3 vs 1, P < 0.0001] within the first 6 hours of treatment compared to continuous infusion (110). The beneficial early rise in plasma sodium in the bolus therapy group was not accompanied by osmotic demyelination, and plasma sodium at 24 hours were similar in both groups (110). Baek et al published the results of the SALSA trial, a randomized control trial of bolus (as per European guidelines) vs continuous infusion of hypertonic saline for the treatment of symptomatic hyponatremia. This study included 178 patients with symptomatic hyponatremia of heterogenous etiology, including volume depletion, adrenal insufficiency, and thiazide use but excluding primary polydipsia. It concluded that patients treated with bolus hypertonic saline therapy were more likely to achieve an early target plasma sodium rise within 1 hour than those treated with continuous infusion; however, this study did not observe a difference in improvement in symptoms between the 2 groups. It is worth noting that the mean pretreatment GCS score in the SALSA trial was 14, and only 25% participants had severe symptomatic hyponatremia, compared to the cohort studied by Garrahy et al, who had a median pretreatment GCS score of 12.
Garrahy et al reported that patients who received 100 mL 3% hypertonic saline bolus therapy (as per US recommendations) (7) were more likely to require treatment with dextrose or desmopressin to prevent overcorrection of serum sodium concentration than those who received continuous infusion therapy, particularly in patients who received a third bolus. Reassuringly, there was no difference in serum sodium concentration between the 2 groups 24 hours after treatment (110). Conversely, the SALSA trial reported a greater incidence of relowering treatment in the continuous infusion arm compared to the bolus therapy arm (41.4% vs 57.1%, P = 0.04) and a higher incidence of overcorrection in the continuous infusion arm compared with bolus therapy (24.2% vs 17.2%); however, this was not statistically significant (111). Overall, the incidence of need for relowering treatment was greater in the SALSA trial compared to Garrahy et al (49.4% vs 10%). This may relate to relatively large total volumes (>500 mL) of hypertonic saline administered to both arms in the SALSA group or due to less frequent plasma sodium monitoring in the SALSA trial (every 6 hours) compared to the protocol used by Garrahy et al (every 2 hours) (112). A recent real-world observational study by Chifu et al reported a high rate of overcorrection (approximately 47%) in those receiving boluses of 150 mL 3% hypertonic saline in line with the European guidelines, particularly in patients with severe symptomatic hyponatremia, and suggested that the use of a lower bolus volume or fewer repeated boluses may reduce the risk of overcorrection (113).
Treatment of Chronic or Asymptomatic Hyponatremia
In the absence of moderate-severe symptoms, chronic hyponatremia management is dependent on the underlying etiology, and recommended treatment is grouped according to volume status (Table 7). The rate of correction is an important consideration for all patients with either unknown or prolonged duration (>48 hours) of hyponatremia. The European guidelines recommend a limit of 10 mmol/L rise in plasma sodium for the first 24 hours of treatment and 8 mmol/L/day thereafter (18). The US recommendations suggest a more conservative target of 4 to 8 mmol/L/day rise in plasma sodium concentration, with a maximal limit of 8 to 12 mmol/L/day.
Table 7.
Comparison of the recommendations for management of chronic, asymptomatic hyponatremia between the US and European recommendations/guidelines
| US recommendations | European guidelines | |
|---|---|---|
| Hypovolemic hyponatremia | Replace volume deficit | Replace volume deficit |
| SIAD | Advise: 1. Fluid restriction 2. Vaptan therapy |
Advise: 1. Fluid restriction 2. Urea or NaCL/loop diuresis 3. Vaptan therapy Mild/moderate: not recommended Profound: recommend against |
| Hypervolemic hyponatremia | Fluid restriction ±Loop diuresis HF/nephrotic syndrome: consider vaptan Cirrhosis: vaptan only in exceptional circumstances |
Fluid restriction Recommend against vaptan use |
Abbreviations: HF, heart failure; NaCL, sodium chloride; SIAD, syndrome of inappropriate antidiuresis; Vaptan, vasopressin 2 receptor antagonist.
Patients with chronic liver disease, hypokalemia, alcohol excess, and malnutrition are at particularly high risk of ODS, and therefore the US recommendations suggest a slower rate of correction in this patient group, with a maximal limit of correction of 4 to 8 mmol/L/day (7) (Table 6). It is important to recognize that the previously noted cutoffs are limits, not targets, for rise in sodium concentrations and apply to all etiologies of hyponatremia.
Where the rate of correction exceeds these thresholds, both recommendations/guidelines advise intervening to limit further rise and to consider relowering plasma sodium concentration to within original target limits, particularly if starting plasma sodium concentration is <120 mmol/L (Table 6) (7). Oral fluids or intravenous dextrose can be given to replace urinary free water losses, and the administration of parenteral desmopressin will prevent further urinary losses (Table 6). The combination of desmopressin and replacement of free water losses can be used either to stop a further increase plasma sodium concentration or to relower to the target plasma sodium concentration. When relowering plasma sodium, desmopressin is administered, and hypotonic fluids (eg, oral free water or parenteral 5% dextrose) are given until sodium returns to target value (maximum allowed increase during the timeframe). Plasma sodium concentration should be checked hourly during this process (7). The US recommendations suggest considering high-dose glucocorticoids such as dexamethasone to reduce demyelination risk if overcorrection occurs (113), although this intervention has not yet been proven to reduce demyelination risk in humans (7).
Hypovolemic Hyponatremia
Hypovolemic hyponatremia will respond to volume expansion, and both guidelines recommend the use of intravenous isotonic fluids (Table 7). Restoration of circulating plasma volume will suppress baroreceptor-mediated AVP secretion, and therefore patients should be carefully monitored for a rapid increase in urinary output (>100 mL/hour) in response to intravenous fluids, which may herald a rapid rise and potential overcorrection of plasma sodium concentration (7, 18). Care should be taken in thiazide-induced hyponatremia, as the combination of thiazide discontinuation, correction of hypokalemia, and volume expansion can result in a rapid rise in plasma sodium concentration (7). Hyponatremia due to primary adrenal insufficiency will respond to high-dose glucocorticoid replacement, but as patients are frequently profoundly volume deplete, they also require large volumes of isotonic fluid resuscitation (114). In these patients, the combination of high doses of glucocorticoid therapy and large volumes of isotonic saline may lead to a rapid correction of sodium, and close monitoring (and appropriate action to prevent overcorrection) is required. Treatment targets for the rate of rise of plasma sodium concentration are equally important in hypovolemic hyponatremia as other etiologies.
Euvolemic Hyponatremia
When hyponatremia is caused by adrenocorticotropin deficiency, adequate steroid replacement is often sufficient to restore clearance of free water and eunatremia (115). If glucocorticoid deficiency is suspected, steroid replacement should be commenced without delay. It is important to note that osmotic demyelination has been reported very occasionally after steroid replacement, so careful monitoring should always be maintained.
If the underlying cause of SIAD is either transient or reversible (eg, due to pneumonia or if culpable medication is discontinued), further management beyond treatment of the cause may not be necessary (116). However, where SIAD is chronic or the precipitant is not readily reversible, further SIAD-specific intervention is necessary.
Fluid Restriction
FR has been the mainstay of treatment of SIAD in clinical practice for many years (117). It is recommended as first-line therapy in both the US and European recommendations/guidelines (7, 18). FR should achieve a negative balance of >500 mL/day to be clinically effective in raising plasma sodium concentration. Despite its position as the first-line therapy, until recently there was a paucity of data to support the efficacy of FR in patients with chronic SIAD. Observational data from the Hyponatremia Registry study, which reports the results of clinical practice, suggested that FR is of limited benefit in routine treatment of SIAD, achieving elevation in plasma sodium of only 2 mmol/L in the first day, which was not statistically different from no treatment at all (118). In a prospective randomized control trial of FR (1000 mL/day) vs no specific treatment, in chronic SIAD Garrahy et al reported a benefit in favor of FR, which was associated with a modest, but significantly greater median plasma sodium rise at 3 days compared with no treatment [3 mmol/L (interquartile range 2-4 mmol/L) vs 1 mmol/L (interquartile range 0-1 mmol/L), P = 0.04], with no reported safety concerns (119). Patients treated with FR were also more likely to achieve a plasma sodium concentration ≥ 130 mmol/L than those who received no treatment (119). Two recent studies have examined the combination of FR with additional measures to potentially improve efficacy. The EFFUSE-FLUID trial compared FR alone to FR with frusemide and FR with frusemide and oral sodium in hospitalized patients with SIAD (pNa+ < 130 mmol/L) and found no difference between the groups (120). Refardt et al combined FR with the use of empagliflozin, a sodium-glucose cotransporter 2 inhibitor, to induce an osmotic diuresis and enhance clearance of free water and compared it to FR alone in hospitalized patients with SIAD. Their data showed a significantly greater rise in the FR-empagliflozin group than FR alone at day 4 (10 vs 7mmol/L, P = 0.04) (121). Both studies reported a better-than-expected response to FR; however, it should be noted that in both studies, patients with potentially reversible and self-limiting causes of SIAD were included (infection, medications, and nausea), which may have contributed to the observed improvement in plasma sodium during this study (116, 122).
There are several factors that have been suggested to predict the response to FR in SIAD. A high urine osmolality (UOsm > 500mOsm/kg) or low 24-hour urine volume (<1500 mL) predict poor response to FR, as they reflect higher plasma AVP activity (7). In addition, the Furst equation (urine sodium + potassium concentration/ plasma sodium concentration) > 1 suggests FR alone is unlikely to be effective (123). Cuesta et al reported that up to 60% patients treated with FR have at least 1 feature that would predict poor response to FR, and the authors postulate that this may explain the relatively modest benefit for FR reported (124).
Vasopressin receptor antagonists
Vasopressin receptor antagonists (vaptans) inhibit the activity of AVP by competitively binding to the V2 receptors of the distal collecting ducts in the kidney. They therefore offer a targeted therapy for AVP-mediated hyponatremia (125, 126).
The use of tolvaptan, an oral, selective V2-receptor antagonist, was shown to be an effective treatment for both SIAD and hypervolemic hyponatremia in the SALT 1 and 2 trials. Patients on tolvaptan (≥15 mg/day) were more 2× as likely to achieve a normal sodium by day 30 compared with placebo (127), an effect that was sustained throughout the duration of the open-label extension study, SALTWATER, but disappeared within a week when treatment was discontinued (127, 128). The main reported side effects of thirst, dry mouth, and polyuria (127) are not unexpected considering the drug’s mechanism of action, and overall tolvaptan appears to be a safe and well-tolerated therapy when used under specialist supervision (125). Since the publication of the SALT trials, several studies have suggested that the use of 15 mg tolvaptan as a starting dose may be too high (129, 130), and in practice, many physicians use lower doses when initiating therapy with similar efficacy (130-133). Overcorrection is reported even in doses as low as 3.75 mg, and therefore clinicians should be vigilant to this risk, particularly in patients with an initial sodium < 125 mmol/L (133-135). Vaptan therapy should be initiated in an hospital setting to facilitate close monitoring of plasma sodium concentration (every 6-8 hours) to prevent overcorrection (7, 136). It is crucial in the prevention of overcorrection that patients should be allowed to drink to thirst, and fluid balance should be monitored closely as a negative fluid balance is associated with increased risk of overcorrection (133).
Conivaptan is a dual vasopressin (V1a and V2) receptor antagonist, which is licensed by the US Food and Drug Administration for the treatment of hospitalized patients with euvolemic and hypervolemic hyponatremia (7) and has been shown to be a safe and well-tolerated treatment for euvolemic and hypervolemic hyponatremia in both its oral and parenteral form (137-140).
Vaptan therapy is recommended as a second-line treatment (following failure of FR) for asymptomatic SIAD by the US recommendations, although it should not be instituted immediately after cessation of hypertonic saline. Vaptans are not recommended by the European guidelines (18).
Urea
Urea is recommended as a second-line option to treat SIAD in the European guidelines, as an inexpensive method to increase solute intake (18). A recent study in patients with SIAD used a spot urine sample to identify patients with low solute intake and low diuresis and proposed that this subgroup of patients may benefit from oral solute administration instead of FR (141). Urea has been reported as beneficial in both hospitalized and outpatient cohorts in several nonrandomized, retrospective European studies (142-145), and since the introduction of Ure-Na™ to the US market, a retrospective study also suggests it is a safe and well-tolerated treatment (146).
Demeclocycline
Demeclocycline is a tetracycline antibiotic that can be used treat SIAD by counteracting AVP activity by inducing nephrogenic diabetes insipidus (147, 148). However, the unpredictable onset of action and potential nephrotoxicity and photosensitivity limit its use in clinical practice, and the European guidelines recommend against its use in hyponatremia (18). A recent meta-analysis failed to find sufficient good-quality scientific evidence to support the continued use of demeclocycline in SIAD (149).
Loop diuresis
The efficacy of loop diuresis as monotherapy is limited by the natriuresis, which accompanies the diuresis, and the stimulation of AVP (96). The European guidelines suggest a combination therapy of low-dose loop diuretics with oral sodium as an alternative second-line agent to oral urea (18). However, data from the EFFUSE-FLUID trial suggested that this approach was no more effective in hospitalized patients than FR alone and was associated with higher rates of adverse events including hypokalemia and acute kidney injury (120).
Hypervolemic Hyponatremia
Treatment of hypervolemic hyponatremia is typically directed at the underlying cause and involves the use of dietary salt restriction, diuretics, and inhibition of the renin-angiotensin-aldosterone system (angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, and mineralocorticoid receptor antagonists) (7). Vaptan therapy has been trialed in both heart and liver failure. The European guidelines currently recommend against the use of vaptans in hypervolemic hyponatremia; however, the US recommendations suggest that vaptan therapy be considered where hyponatremia is limiting use of diuretics in heart failure or nephrotic syndrome with preserved renal function. Both guidelines currently recommend against vaptan use in cirrhosis (7).
Hyponatremia and COVID-19
Hyponatremia is reported in between 10% and 30% of all cases of COVID-19 admitted to acute hospital care (150-152). Hyponatremia is associated with adverse outcomes in COVID-19 patients, including increased requirement for mechanical ventilation, admission to critical care, and increased mortality (151-154). Recently updated guidance from the European Society of Endocrinology suggests that hyponatremia in patients with COVID-19 should be treated in accordance with existing guidelines (155).
Back to the Cases
Case 1
Due to the presence of severe symptoms suggestive of cerebral edema in conjunction with severe biochemical hyponatremia, emergency treatment was administered with a bolus of 100 mL over 10 minutes of hypertonic saline in an intensive care setting. After a second bolus of hypertonic saline was administered, plasma sodium concentration rose from 113 mmol/L to 118 mmol/L, with a corresponding improvement in neurological status.
She was deemed to be at increased risk of ODS due to her history of alcohol excess and malnutrition, and therefore the target rise in plasma sodium was set at 4 to 6 mmol/L/day, with a maximum rate of 8 mmol/L/day. An increase in urine output was noted, and repeat bloods taken 4 hours later showed a plasma sodium 121 mmol/L. Subcutaneous desmopressin (1 μg) was administered to stop further urinary free water losses, and intravenous 5% dextrose was commenced at 3 mL/kg/hour. Bloods were taken hourly until plasma sodium returned to 119 mmol/L, and intravenous dextrose was continued to replace urinary losses to maintain plasma sodium < 119 mmol/L for the next 24 hours. Her SSRI medication was discontinued, and plasma sodium returned to 136 mmol/L over several days with careful monitoring and the requirement of intermittent dextrose infusions to prevent overcorrection.
Case 2
The diagnosis is SIAD in the context of newly diagnosed small cell lung cancer. He was commenced on a 1000 mL/day FR, and after his sodium improved to 132 mmol/L, he was discharged from hospital.
He represented several months later following several falls. Despite carefully adhering to FR, his repeat biochemistry was as follows: urea 2.7 mmol/L, creatinine 84 μmol/L, sodium 122 mmol/L, potassium 4.6 mmol/L, UNa+ 39 mmol/L, and UOsm 573 mOsm/kg. He remained clinically euvolemic. Imaging revealed new metastatic disease, and he was admitted to hospital to commence 7.5 mg tolvaptan therapy. FR was discontinued, and he was encouraged to drink according to thirst. His urinary output increased, and his plasma sodium rose to 125 mmol/L after 6 hours and 129 mmol/L after 12 hours, at which point intravenous 5% dextrose was commenced to replace any further urinary free water losses and limit further rise in plasma sodium. Over the next 3 days, plasma sodium rose to 133 mmol/L and remained stable on alternate-day administration of 7.5 mg tolvaptan.
Conclusion
Hyponatremia is the most commonly encountered electrolyte disturbance in clinical practice and is associated with significant morbidity and mortality in both the acute and chronic setting. A clinical evaluation to establish the chronicity of hyponatremia, the presence or absence of symptoms suggestive of cerebral irritation, and a clinical volume assessment and biochemical severity, aided by urinary osmolality and sodium concentration, are imperative to guide timely and effective management of hyponatremia. The presence of severe symptoms suggestive of cerebral edema is a medical emergency, and bolus therapy with 3% hypertonic saline should be administered without delay to prevent neurological complications and death. Chronic or asymptomatic hyponatremia management will depend on the underlying cause, and close monitoring is required to prevent rapid overcorrection of plasma sodium concentration.
Contributor Information
Julie Martin-Grace, Academic Department of Endocrinology, Beaumont Hospital and Royal College of Surgeons in Ireland, Dublin, Ireland.
Maria Tomkins, Academic Department of Endocrinology, Beaumont Hospital and Royal College of Surgeons in Ireland, Dublin, Ireland.
Michael W O’Reilly, Academic Department of Endocrinology, Beaumont Hospital and Royal College of Surgeons in Ireland, Dublin, Ireland.
Chris J Thompson, Academic Department of Endocrinology, Beaumont Hospital and Royal College of Surgeons in Ireland, Dublin, Ireland.
Mark Sherlock, Academic Department of Endocrinology, Beaumont Hospital and Royal College of Surgeons in Ireland, Dublin, Ireland.
Funding
J.M.G. receives funding from her fellowship on the Royal College of Surgeons in Ireland (RCSI)/Beacon Hospital Strategic Academic Recruitment (StAR) programme, and the Irish Endocrine Society Clinical Science Award. M.T. is an Irish Clinical Academic Training (ICAT) programme fellow, funded by the Health Research Board (HRB) and Wellcome Trust, Grant Number 203930/B/16/Z. M.W.O.R. is funded by a HRB Emerging Clinician Scientist Award (ECSA-2020-001).
Disclosures
The authors have nothing to declare.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study
References
- 1. Kumar S, Berl T. Sodium. Lancet. 1998;352(9123):220-228. doi: 10.1016/s0140-6736(97)12169-9 [DOI] [PubMed] [Google Scholar]
- 2. Hoorn EJ, Lindemans J, Zietse R. Development of severe hyponatraemia in hospitalized patients: treatment-related risk factors and inadequate management. Nephrol Dial Transplant. 2006;21(1):70-76. doi: 10.1093/ndt/gfi082 [DOI] [PubMed] [Google Scholar]
- 3. Natkunam A, Shek CC, Swaminathan R. Hyponatremia in a hospital population. J Med. 1991;22(2):83-96. [PubMed] [Google Scholar]
- 4. Lu H, Vollenweider P, Kissling S, Marques-Vidal P. Prevalence and description of hyponatremia in a Swiss tertiary care hospital: an observational retrospective study. Front Med (Lausanne). 2020;7:512. doi: 10.3389/fmed.2020.00512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kennedy PG, Mitchell DM, Hoffbrand BI. Severe hyponatraemia in hospital inpatients. Brit Med J. 1978;2(6147):1251-1253. doi: 10.1136/bmj.2.6147.1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Edelman IS, Leibman J, O’Meara MP, Birkenfeld LW. Interrelations between serum sodium concentration, serum osmolarity and total exchangeable sodium, total exchangeable potassium and total body water. J Clin Invest. 1958;37(9):1236-1256. doi: 10.1172/jci103712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126(suppl 110):S1-S42. doi: 10.1016/j.amjmed.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 8. Hannon MJ, Finucane FM, Sherlock M, Agha A, Thompson CJ. Disorders of water homeostasis in neurosurgical patients. J Clin Endocrinol Metab. 2012;97(5):1423-1433. doi: 10.1210/jc.2011-3201 [DOI] [PubMed] [Google Scholar]
- 9. Knoers NV, van Os CH. The clinical importance of the urinary excretion of aquaporin-2. N Engl J Med. 1995;332(23):1575-1576. doi: 10.1056/nejm199506083322310 [DOI] [PubMed] [Google Scholar]
- 10. Thompson CJ, Bland J, Burd J, Baylis PH. The osmotic thresholds for thirst and vasopressin release are similar in healthy man. Clin Sci (Lond). 1986;71(6):651-656. doi: 10.1042/cs0710651 [DOI] [PubMed] [Google Scholar]
- 11. Smith DM, McKenna K, Thompson CJ. Hyponatraemia. Clin Endocrinol (Oxf). 2000;52(6):667-678. doi: 10.1046/j.1365-2265.2000.01027.x [DOI] [PubMed] [Google Scholar]
- 12. White PC. Disorders of aldosterone biosynthesis and action. N Engl J Med. 1994;331(4):250-258. doi: 10.1056/NEJM199407283310408 [DOI] [PubMed] [Google Scholar]
- 13. Linas SL, Berl T, Robertson GL, Aisenbrey GA, Schrier RW, Anderson RJ. Role of vasopressin in the impaired water excretion of glucocorticoid deficiency. Kidney Int. 1980;18(1):58-67. doi: 10.1038/ki.1980.110 [DOI] [PubMed] [Google Scholar]
- 14. Sterns RH. Disorders of plasma sodium—causes, consequences, and correction. N Engl J Med. 2014;372(1):55-65. doi: 10.1056/NEJMra1404489 [DOI] [PubMed] [Google Scholar]
- 15. Turchin A, Seifter JL, Seely EW. Mind the gap. N Engl J Med. 2003;349(15):1465-1469. doi: 10.1056/NEJMcps031078 [DOI] [PubMed] [Google Scholar]
- 16. Hillier TA, Abbott RD, Barrett EJ. Hyponatremia: evaluating the correction factor for hyperglycemia. Am J Med. 1999;106(4):399-403. doi: 10.1016/s0002-9343(99)00055-8 [DOI] [PubMed] [Google Scholar]
- 17. Hoorn EJ, Carlotti AP, Costa LA, et al. Preventing a drop in effective plasma osmolality to minimize the likelihood of cerebral edema during treatment of children with diabetic ketoacidosis. J Pediatr. 2007;150(5):467-473. doi: 10.1016/j.jpeds.2006.11.062 [DOI] [PubMed] [Google Scholar]
- 18. Spasovski G, Vanholder R, Allolio B, et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Eur J Endocrinol. 2014;170(3):G1-G47. doi: 10.1530/EJE-13-1020 [DOI] [PubMed] [Google Scholar]
- 19. Sonnenblick M, Friedlander Y, Rosin AJ. Diuretic-induced severe hyponatremia: review and analysis of 129 reported patients. Chest. 1993;103(2):601-606. doi: 10.1378/chest.103.2.601 [DOI] [PubMed] [Google Scholar]
- 20. Peters JP, Welt LG, Sims EA, Orloff J, Needham J. A salt-wasting syndrome associated with cerebral disease. Trans Assoc Am Physicians. 1950;63:57-64. [PubMed] [Google Scholar]
- 21. Sterns RH, Silver SM. Cerebral salt wasting versus SIADH: what difference? J Am Soc Nephrol.2008;19(2):194-196. doi: 10.1681/asn.2007101118 [DOI] [PubMed] [Google Scholar]
- 22. Singh S, Bohn D, Carlotti AP, Cusimano M, Rutka JT, Halperin ML. Cerebral salt wasting: truths, fallacies, theories, and challenges. Crit Care Med. 2002;30(11):2575-2579. doi: 10.1097/00003246-200211000-00028 [DOI] [PubMed] [Google Scholar]
- 23. Verbalis JG. The curious story of cerebral salt wasting: fact or fiction? Clin J Am Soc Nephrol. 2020;15(11):1666-1668. doi: 10.2215/cjn.00070120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sherlock M, O’Sullivan E, Agha A, et al. The incidence and pathophysiology of hyponatraemia after subarachnoid haemorrhage. Clin Endocrinol (Oxf). 2006;64(3):250-254. doi: 10.1111/j.1365-2265.2006.02432.x [DOI] [PubMed] [Google Scholar]
- 25. Hannon MJ, Behan LA, O’Brien MM, et al. Hyponatremia following mild/moderate subarachnoid hemorrhage is due to SIAD and glucocorticoid deficiency and not cerebral salt wasting. J Clin Endocrinol Metab. 2014;99(1):291-298. doi: 10.1210/jc.2013-3032 [DOI] [PubMed] [Google Scholar]
- 26. Thompson C, Hoorn EJ. Hyponatraemia: an overview of frequency, clinical presentation and complications. Best Pract Res Clin Endocrinol Metab. 2012;26(suppl 1):S1-S6. doi: 10.1016/s1521-690x(12)00019-x [DOI] [PubMed] [Google Scholar]
- 27. Schwartz WB, Bennett W, Curelop S, Bartter FC. A syndrome of renal sodium loss and hyponatremia probably resulting from inappropriate secretion of antidiuretic hormone. Am J Med. 1957;23(4):529-542. doi: 10.1016/0002-9343(57)90224-3 [DOI] [PubMed] [Google Scholar]
- 28. Shepshelovich D, Leibovitch C, Klein A, et al. The syndrome of inappropriate antidiuretic hormone secretion: distribution and characterization according to etiologies. Eur J Intern Med. 2015;26(10):819-824. doi: 10.1016/j.ejim.2015.10.020 [DOI] [PubMed] [Google Scholar]
- 29. Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342(21):1581-1589. doi: 10.1056/nejm200005253422107 [DOI] [PubMed] [Google Scholar]
- 30. Bartter FC, Schwartz WB. The syndrome of inappropriate secretion of antidiuretic hormone. Am J Med. 1967;42(5):790-806. doi: 10.1016/0002-9343(67)90096-4 [DOI] [PubMed] [Google Scholar]
- 31. Cuesta M, Garrahy A, Slattery D, et al. The contribution of undiagnosed adrenal insufficiency to euvolaemic hyponatraemia: results of a large prospective single-centre study. Clin Endocrinol (Oxf). 2016;85(6):836-844. doi: 10.1111/cen.13128 [DOI] [PubMed] [Google Scholar]
- 32. Robertson GL, Aycinena P, Zerbe RL. Neurogenic disorders of osmoregulation. Am J Med. 1982;72(2):339-353. doi: 10.1016/0002-9343(82)90825-7 [DOI] [PubMed] [Google Scholar]
- 33. Fenske WK, Christ-Crain M, Hörning A, et al. A copeptin-based classification of the osmoregulatory defects in the syndrome of inappropriate antidiuresis. J Am Soc Nephrol. 2014;25(10):2376-2383. doi: 10.1681/ASN.2013080895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Melmed S, Auchus R, Goldfine A, Koenig R, Rosen C.. Williams Textbook of Endocrinology. 14th ed. Elsevier: Philadelphia, USA; 2020. [Google Scholar]
- 35. Saito T, Ishikawa SE, Ando F, Higashiyama M, Nagasaka S, Sasaki S. Vasopressin-dependent upregulation of aquaporin-2 gene expression in glucocorticoid-deficient rats. Am J Physiol Renal Physiol. 2000;279(3):F502-F508. doi: 10.1152/ajprenal.2000.279.3.F502 [DOI] [PubMed] [Google Scholar]
- 36. Boykin J, DeTorrenté A, Erickson A, Robertson G, Schrier RW. Role of plasma vasopressin in impaired water excretion of glucocorticoid deficiency. J Clin Invest. 1978;62(4):738-744. doi: 10.1172/jci109184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oelkers W. Hyponatremia and inappropriate secretion of vasopressin (antidiuretic hormone) in patients with hypopituitarism. N Engl J Med. 1989;321(8):492-496. doi: 10.1056/nejm198908243210802 [DOI] [PubMed] [Google Scholar]
- 38. Garrahy A, Thompson CJ. Hyponatremia and glucocorticoid deficiency. Front Horm Res. 2019;52:80-92. doi: 10.1159/000493239 [DOI] [PubMed] [Google Scholar]
- 39. Martin-Grace J, Dineen R, Sherlock M, Thompson CJ. Adrenal insufficiency: physiology, clinical presentation and diagnostic challenges. Clin Chim Acta. 2020;505:78-91. doi: 10.1016/j.cca.2020.01.029 [DOI] [PubMed] [Google Scholar]
- 40. Hannon AM, Hunter S, Smith D, et al. Clinical features and autoimmune associations in patients presenting with idiopathic isolated ACTH deficiency. Clin Endocrinol (Oxf). 2018;88(3):491-497. doi: 10.1111/cen.13536 [DOI] [PubMed] [Google Scholar]
- 41. Kamoi K, Tamura T, Tanaka K, Ishibashi M, Yamaji T. Hyponatremia and osmoregulation of thirst and vasopressin secretion in patients with adrenal insufficiency. J Clin Endocrinol Metab. 1993;77(6):1584-1588. doi: 10.1210/jcem.77.6.8263145 [DOI] [PubMed] [Google Scholar]
- 42. Liamis G, Filippatos TD, Liontos A, Elisaf MS. Management of endocrine disease: hypothyroidism-associated hyponatremia: mechanisms, implications and treatment. Eur J Endocrinol. 2017;176(1):R15-R20. doi: 10.1530/eje-16-0493 [DOI] [PubMed] [Google Scholar]
- 43. Hew-Butler T, Rosner MH, Fowkes-Godek S, et al. Statement of the Third International Exercise-Associated Hyponatremia Consensus Development Conference, Carlsbad, California, 2015. Clin J Sport Med. 2015;25(4):303-320. doi: 10.1097/jsm.0000000000000221 [DOI] [PubMed] [Google Scholar]
- 44. Martinez-Cano JP, Cortes-Castillo V, Martinez-Villa J, Ramos JC, Uribe JP. Dysnatremia among runners in a half marathon performed under warm and humid conditions. BMJ Open Sport Exerc Med. 2018;4(1):e000351-e000351. doi: 10.1136/bmjsem-2018-000351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hew TD, Chorley JN, Cianca JC, Divine JG. The incidence, risk factors, and clinical manifestations of hyponatremia in marathon runners Clin J Sport Med. 2003;13(1):41-47. doi: 10.1097/00042752-200301000-00008 [DOI] [PubMed] [Google Scholar]
- 46. Almond CSD, Shin AY, Fortescue EB, et al. Hyponatremia among runners in the Boston Marathon. N Engl J Med. 2005;352(15):1550-1556. doi: 10.1056/NEJMoa043901 [DOI] [PubMed] [Google Scholar]
- 47. Knechtle B, Chlíbková D, Papadopoulou S, Mantzorou M, Rosemann T, Nikolaidis PT. Exercise-associated hyponatremia in endurance and ultra-endurance performance-aspects of sex, race location, ambient temperature, sports discipline, and length of performance: a narrative review. Medicina. 2019;55(9):537. doi: 10.3390/medicina55090537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Popovic M, Timper K, Seelig E, et al. Exercise upregulates copeptin levels which is not regulated by interleukin-1. PLoS One. 2019;14(5):e0217800. doi: 10.1371/journal.pone.0217800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hew-Butler T, Jordaan E, Stuempfle KJ, et al. Osmotic and nonosmotic regulation of arginine vasopressin during prolonged endurance exercise. J Clin Endocrinol Metab. 2008;93(6):2072-2078. doi: 10.1210/jc.2007-2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hew-Butler T, Loi V, Pani A, Rosner MH. Exercise-associated hyponatremia: 2017 update. Front Med (Lausanne). 2017;4:21-21. doi: 10.3389/fmed.2017.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hew-Butler T. Exercise-associated hyponatremia. Front Horm Res. 2019;52:178-189. doi: 10.1159/000493247 [DOI] [PubMed] [Google Scholar]
- 52. Sanghvi SR, Kellerman PS, Nanovic L. Beer potomania: an unusual cause of hyponatremia at high risk of complications from rapid correction. Am J Kidney Dis. 2007;50(4):673-680. doi: 10.1053/j.ajkd.2007.07.015 [DOI] [PubMed] [Google Scholar]
- 53. Fenves AZ, Thomas S, Knochel JP. Beer potomania: two cases and review of the literature. Clin Nephrol. 1996;45(1):61-64. [PubMed] [Google Scholar]
- 54. Thaler SM, Teitelbaum I, Berl T.“Beer potomania” in non-beer drinkers: effect of low dietary solute intake. Am J Kidney Dis. 1998;31(6):1028-1031. doi: 10.1053/ajkd.1998.v31.pm9631849 [DOI] [PubMed] [Google Scholar]
- 55. Sailer CO, Winzeler B, Nigro N, et al. Characteristics and outcomes of patients with profound hyponatraemia due to primary polydipsia. Clin Endocrinol (Oxf). 2017;87(5):492-499. doi: 10.1111/cen.13384 [DOI] [PubMed] [Google Scholar]
- 56. Schrier RW, Fassett RG. Pathogenesis of sodium and water retention in cardiac failure. Ren Fail. 1998;20(6):773-781. doi: 10.3109/08860229809045175 [DOI] [PubMed] [Google Scholar]
- 57. Balling L, Schou M, Videbæk L, Hildebrandt P, Wiggers H, Gustafsson F. Prevalence and prognostic significance of hyponatraemia in outpatients with chronic heart failure. Eur J Heart Fail. 2011;13(9):968-973. doi: 10.1093/eurjhf/hfr086 [DOI] [PubMed] [Google Scholar]
- 58. Rusinaru D, Tribouilloy C, Berry C, et al. Relationship of serum sodium concentration to mortality in a wide spectrum of heart failure patients with preserved and with reduced ejection fraction: an individual patient data meta-analysis(†): Meta-Analysis Global Group in Chronic Heart Failure (MAGGIC). Eur J Heart Fail. 2012;14(10):1139-1146. doi: 10.1093/eurjhf/hfs099 [DOI] [PubMed] [Google Scholar]
- 59. Lim LM, Tsai N-C, Lin M-Y, et al. Hyponatremia is associated with fluid imbalance and adverse renal outcome in chronic kidney disease patients treated with diuretics. Sci Rep. 2016;6(1):36817. doi: 10.1038/srep36817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kovesdy CP. Significance of hypo- and hypernatremia in chronic kidney disease. Nephrol Dial Transplant. 2012;27(3):891-898. doi: 10.1093/ndt/gfs038 [DOI] [PubMed] [Google Scholar]
- 61. Barber K, Madden S, Allen J, Collett D, Neuberger J, Gimson A. Elective liver transplant list mortality: development of a United Kingdom end-stage liver disease score. Transplantation. 2011;92(4):469-476. doi: 10.1097/TP.0b013e318225db4d [DOI] [PubMed] [Google Scholar]
- 62. Huo TI, Wang YW, Yang YY, et al. Model for end-stage liver disease score to serum sodium ratio index as a prognostic predictor and its correlation with portal pressure in patients with liver cirrhosis. Liver Int. 2007;27(4):498-506. doi: 10.1111/j.1478-3231.2007.01445.x [DOI] [PubMed] [Google Scholar]
- 63. Gullans SR, Verbalis JG. Control of brain volume during hyperosmolar and hypoosmolar conditions. Annu Rev Med. 1993;44:289-301. doi: 10.1146/annurev.me.44.020193.001445 [DOI] [PubMed] [Google Scholar]
- 64. Arieff AI. Hyponatremia, convulsions, respiratory arrest, and permanent brain damage after elective surgery in healthy women. N Engl J Med. 1986;314(24):1529-1535. doi: 10.1056/nejm198606123142401 [DOI] [PubMed] [Google Scholar]
- 65. Arieff AI, Llach F, Massry SG. Neurological manifestations and morbidity of hyponatremia: correlation with brain water and electrolytes. Medicine. 1976;55(2):121-129. doi: 10.1097/00005792-197603000-00002 [DOI] [PubMed] [Google Scholar]
- 66. Beukhof CM, Hoorn EJ, Lindemans J, Zietse R. Novel risk factors for hospital-acquired hyponatraemia: a matched case-control study. Clin Endocrinol (Oxf). 2007;66(3):367-372. doi: 10.1111/j.1365-2265.2007.02741.x [DOI] [PubMed] [Google Scholar]
- 67. Corona G, Giuliani C, Parenti G, et al. Moderate hyponatremia is associated with increased risk of mortality: evidence from a meta-analysis. PLoS One. 2013;8(12):e80451. doi: 10.1371/journal.pone.0080451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cuesta M, Garrahy A, Slattery D, et al. Mortality rates are lower in SIAD, than in hypervolaemic or hypovolaemic hyponatraemia: results of a prospective observational study. Clin Endocrinol (Oxf). 2017;87(4):400-406. doi: 10.1111/cen.13388 [DOI] [PubMed] [Google Scholar]
- 69. Girardeau Y, Jannot AS, Chatellier G, Saint-Jean O. Association between borderline dysnatremia and mortality insight into a new data mining approach. BMC Med Inform Decis Mak. 2017;17(1):152. doi: 10.1186/s12911-017-0549-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Thorpe O, Cuesta M, Fitzgerald C, et al. Active management of hyponatraemia and mortality in older hospitalised patients compared with younger patients: results of a prospective cohort study. Age Ageing. 2021;50(4):1144-1150. doi: 10.1093/ageing/afaa248 [DOI] [PubMed] [Google Scholar]
- 71. Holland-Bill L, Christiansen CF, Heide-Jørgensen U, et al. Hyponatremia and mortality risk: a Danish cohort study of 279 508 acutely hospitalized patients. Eur J Endocrinol. 2015;173(1):71-81. doi: 10.1530/eje-15-0111 [DOI] [PubMed] [Google Scholar]
- 72. Chawla A, Sterns RH, Nigwekar SU, Cappuccio JD. Mortality and serum sodium: do patients die from or with hyponatremia? Clin J Am Soc Nephrol. 2011;6(5):960-965. doi: 10.2215/cjn.10101110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hoorn EJ, Zietse R. Hyponatremia and mortality: how innocent is the bystander? Clin J Am Soc Nephrol. 2011;6(5):951-953. doi: 10.2215/cjn.01210211 [DOI] [PubMed] [Google Scholar]
- 74. Hoorn EJ, Zietse R. Hyponatremia and mortality: moving beyond associations. Am J Kidney Dis. 2013;62(1):139-149. doi: 10.1053/j.ajkd.2012.09.019 [DOI] [PubMed] [Google Scholar]
- 75. Gill G, Huda B, Boyd A, et al. Characteristics and mortality of severe hyponatraemia—a hospital-based study. Clin Endocrinol (Oxf). 2006;65(2):246-249. doi: 10.1111/j.1365-2265.2006.02583.x [DOI] [PubMed] [Google Scholar]
- 76. Kutz A, Ebrahimi F, Aghlmandi S, et al. Risk of adverse clinical outcomes in hyponatremic adult patients hospitalized for acute medical conditions: a population-based cohort study. J Clin Endocrinol Metab. 2020;105(11):3428-3436. doi: 10.1210/clinem/dgaa547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Refardt J, Kling B, Krausert K, et al. Impact of chronic hyponatremia on neurocognitive and neuromuscular function. Eur J Clin Invest. 2018;48(11):e13022. doi: 10.1111/eci.13022 [DOI] [PubMed] [Google Scholar]
- 78. Verbalis JG, Barsony J, Sugimura Y, et al. Hyponatremia-induced osteoporosis. J Bone Min Res. 2010;25(3):554-563. doi: 10.1359/jbmr.090827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kleinschmidt-DeMasters BK, Norenberg MD. Rapid correction of hyponatremia causes demyelination: relation to central pontine myelinolysis. Science. 1981;211(4486):1068-1070. doi: 10.1126/science.7466381 [DOI] [PubMed] [Google Scholar]
- 80. Sterns RH, Thomas DJ, Herndon RM. Brain dehydration and neurologic deterioration after rapid correction of hyponatremia. Kidney Int. 1989;35(1):69-75. doi: 10.1038/ki.1989.9 [DOI] [PubMed] [Google Scholar]
- 81. Sterns RH, Riggs JE, Schochet SS Jr. Osmotic demyelination syndrome following correction of hyponatremia. N Engl J Med. 1986;314(24):1535-1542. doi: 10.1056/nejm198606123142402 [DOI] [PubMed] [Google Scholar]
- 82. Verbalis JG, Martinez AJ, Rutarosky D, Wttao MD. Neurological and neuropathological sequelae of correction of chronic hyponatremia. Kidney Int. 1991;39(6):1274-1282. doi: 10.1038/ki.1991.161 [DOI] [PubMed] [Google Scholar]
- 83. Sterns RH. Adverse consequences of overly-rapid correction of hyponatremia. Front Horm Res. 2019;52:130-142. doi: 10.1159/000493243 [DOI] [PubMed] [Google Scholar]
- 84. Gankam Kengne F, Nicaise C, Soupart A, et al. Astrocytes are an early target in osmotic demyelination syndrome. J Am Soc Nephrol. 2011;22(10):1834-1845. doi: 10.1681/asn.2010111127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Suárez V, Norello D, Sen E, et al. Impairment of neurocognitive functioning, motor performance, and mood stability in hospitalized patients with euvolemic moderate and profound hyponatremia. Am J Med. 2020;133(8):986-993.e5. doi: 10.1016/j.amjmed.2019.12.056 [DOI] [PubMed] [Google Scholar]
- 86. Xu R, Pi HC, Xiong ZY, et al. Hyponatremia and cognitive impairment in patients treated with peritoneal dialysis. Clin J Am Soc Nephrol. 2015;10(10):1806-1813. doi: 10.2215/cjn.02240215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gosch M, Joosten-Gstrein B, Heppner HJ, Lechleitner M. Hyponatremia in geriatric inhospital patients: effects on results of a comprehensive geriatric assessment. Gerontology. 2012;58(5):430-440. doi: 10.1159/000339100 [DOI] [PubMed] [Google Scholar]
- 88. Nowak KL, Yaffe K, Orwoll ES, et al. Serum sodium and cognition in older community-dwelling men. Clin J Am Soc Nephrol. 2018;13(3):366-374. doi: 10.2215/cjn.07400717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ahluwalia V, Heuman DM, Feldman G, et al. Correction of hyponatraemia improves cognition, quality of life, and brain oedema in cirrhosis. J Hepatol. 2015;62(1):75-82. doi: 10.1016/j.jhep.2014.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Watson H, Guevara M, Vilstrup H, Ginès P. Improvement of hyponatremia in cirrhosis is associated with improved complex information processing. J Gastroenterol Hepatol. 2019;34(11):1999-2003. doi: 10.1111/jgh.14683 [DOI] [PubMed] [Google Scholar]
- 91. Kinsella S, Moran S, Sullivan MO, Molloy MG, Eustace JA. Hyponatremia independent of osteoporosis is associated with fracture occurrence. Clin J Am Soc Nephrol. 2010;5(2):275-280. doi: 10.2215/cjn.06120809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119(1):71 e1-71.e8. doi: 10.1016/j.amjmed.2005.09.026 [DOI] [PubMed] [Google Scholar]
- 93. Renneboog B, Sattar L, Decaux G. Attention and postural balance are much more affected in older than in younger adults with mild or moderate chronic hyponatremia. Eur J Intern Med. 2017;41:e25-e26. doi: 10.1016/j.ejim.2017.02.008 [DOI] [PubMed] [Google Scholar]
- 94. Boyer S, Gayot C, Bimou C, et al. Prevalence of mild hyponatremia and its association with falls in older adults admitted to an emergency geriatric medicine unit (the MUPA unit). BMC Geriatr. 2019;19(1):265. doi: 10.1186/s12877-019-1282-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Usala RL, Fernandez SJ, Mete M, et al. Hyponatremia is associated with increased osteoporosis and bone fractures in a large US health system population. J Clin Endocrinol Metab. 2015;100(8):3021-3031. doi: 10.1210/jc.2015-1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Dineen R, Thompson CJ, Sherlock M. Hyponatraemia - presentations and management. Clin Med (Lond). 2017;17(3):263-269. doi: 10.7861/clinmedicine.17-3-263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Singh TD, Fugate JE, Rabinstein AA. Central pontine and extrapontine myelinolysis: a systematic review. Eur J Neurol. 2014;21(12):1443-1450. doi: 10.1111/ene.12571 [DOI] [PubMed] [Google Scholar]
- 98. Hoorn EJ, Zietse R. Diagnosis and treatment of hyponatremia: compilation of the guidelines. J Am Soc Nephrol. 2017;28(5):1340-1349. doi: 10.1681/asn.2016101139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Chung H-M, Kluge R, Schrier RW, Anderson RJ. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med. 1987;83(5):905-908. doi: 10.1016/0002-9343(87)90649-8 [DOI] [PubMed] [Google Scholar]
- 100. McGee S, Abernethy WB 3rd, Simel DL. The rational clinical examination: is this patient hypovolemic? JAMA. 1999;281(11):1022-1029. doi: 10.1001/jama.281.11.1022 [DOI] [PubMed] [Google Scholar]
- 101. Hoorn EJ, Halperin ML, Zietse R. Diagnostic approach to a patient with hyponatraemia: traditional versus physiology-based options. QJM. 2005;98(7):529-540. doi: 10.1093/qjmed/hci081 [DOI] [PubMed] [Google Scholar]
- 102. Fenske W, Maier SKG, Blechschmidt A, Allolio B, Störk S. Utility and limitations of the traditional diagnostic approach to hyponatremia: a diagnostic study. Am J Med. 2010;123(7):652-657. doi: 10.1016/j.amjmed.2010.01.013 [DOI] [PubMed] [Google Scholar]
- 103. Chifu I, Gerstl A, Lengenfelder B, et al. Treatment of symptomatic hyponatremia with hypertonic saline: a real-life observational study. Eur J Endocrinol. 2021;184(5):647-655. doi: 10.1530/eje-20-1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Fenske W, Störk S, Koschker AC, et al. Value of fractional uric acid excretion in differential diagnosis of hyponatremic patients on diuretics. J Clin Endocrinol Metab. 2008;93(8):2991-2997. doi: 10.1210/jc.2008-0330 [DOI] [PubMed] [Google Scholar]
- 105. Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006;52(1):112-119. doi: 10.1373/clinchem.2005.060038 [DOI] [PubMed] [Google Scholar]
- 106. Refardt J, Winzeler B, Christ-Crain M. Copeptin and its role in the diagnosis of diabetes insipidus and the syndrome of inappropriate antidiuresis. Clin Endocrinol (Oxf). 2019;91(1):22-32. doi: 10.1111/cen.13991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Christ-Crain M, Fenske W. Copeptin in the diagnosis of vasopressin-dependent disorders of fluid homeostasis. Nat Rev Endocrinol. 2016;12(3):168-176. doi: 10.1038/nrendo.2015.224 [DOI] [PubMed] [Google Scholar]
- 108. Fenske W, Störk S, Blechschmidt A, Maier SG, Morgenthaler NG, Allolio B. Copeptin in the differential diagnosis of hyponatremia. J Clin Endocrinol Metab. 2009;94(1):123-129. doi: 10.1210/jc.2008-1426 [DOI] [PubMed] [Google Scholar]
- 109. Christ-Crain M. Vasopressin and copeptin in health and disease. Rev Endocr Metab Disord. 2019;20(3):283-294. doi: 10.1007/s11154-019-09509-9 [DOI] [PubMed] [Google Scholar]
- 110. Garrahy A, Dineen R, Hannon AM, et al. Continuous versus bolus infusion of hypertonic saline in the treatment of symptomatic hyponatremia caused by SIAD. J Clin Endocrinol Metab. 2019;104(9):3595-3602. doi: 10.1210/jc.2019-00044 [DOI] [PubMed] [Google Scholar]
- 111. Baek SH, Jo YH, Ahn S, et al. Risk of overcorrection in rapid intermittent bolus vs slow continuous infusion therapies of hypertonic saline for patients with symptomatic hyponatremia: the SALSA randomized clinical trial. JAMA Intern Med. 2021;181(1):81-92. doi: 10.1001/jamainternmed.2020.5519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Donato AA, Lloyd BJ. In hyponatremia, rapid intermittent bolus vs. slow continuous infusion of hypertonic saline did not differ for overcorrection of serum sodium. Ann Intern Med. 2021;174(3):JC33Jc33. doi: 10.7326/acpj202103160-033 [DOI] [PubMed] [Google Scholar]
- 113. Sugimura Y, Murase T, Takefuji S, et al. Protective effect of dexamethasone on osmotic-induced demyelination in rats. Exp Neurol. 2005;192(1):178-183. doi: 10.1016/j.expneurol.2004.10.018 [DOI] [PubMed] [Google Scholar]
- 114. Arlt W, Society for Endocrinology Clinical Committee. Society for Endocrinology endocrine emergency guidance: emergency management of acute adrenal insufficiency (adrenal crisis) in adult patients. Endocr Connect. 2016;5(5):G1-G3. doi: 10.1530/EC-16-0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Garrahy A, Thompson CJ. Glucocorticoid deficiency and syndrome of inappropriate antidiuresis: an underdiagnosed association? Ann Clin Biochem. 2018;55(1):4-6. doi: 10.1177/0004563217743776 [DOI] [PubMed] [Google Scholar]
- 116. Cuesta M, Slattery D, Goulden EL, et al. Hyponatraemia in patients with community-acquired pneumonia; prevalence and aetiology, and natural history of SIAD. Clin Endocrinol (Oxf). 2019;90(5):744-752. doi: 10.1111/cen.13937 [DOI] [PubMed] [Google Scholar]
- 117. Ellison DH, Berl T. Clinical practice: the syndrome of inappropriate antidiuresis. N Engl J Med. 2007;356(20):2064-2072. doi: 10.1056/NEJMcp066837 [DOI] [PubMed] [Google Scholar]
- 118. Greenberg A, Verbalis JG, Amin AN, et al. Current treatment practice and outcomes. Report of the Hyponatremia Registry. Kidney Int. 2015;88(1):167-177. doi: 10.1038/ki.2015.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Garrahy A, Galloway I, Hannon AM, et al. Fluid restriction therapy for chronic SIAD: results of a prospective randomized controlled trial. J Clin Endocrinol Metab. 2020;105(12):e4360–e4369. doi 10.1210/clinem/dgaa619 [DOI] [PubMed] [Google Scholar]
- 120. Krisanapan P, Vongsanim S, Pin-On P, Ruengorn C, Noppakun K. Efficacy of furosemide, oral sodium chloride, and fluid restriction for treatment of syndrome of inappropriate antidiuresis (SIAD): an open-label randomized controlled study (the EFFUSE-FLUID trial). Am J Kidney Dis. 2020;76(2):203-212. doi: 10.1053/j.ajkd.2019.11.012 [DOI] [PubMed] [Google Scholar]
- 121. Refardt J, Imber C, Sailer CO, et al. A randomized trial of empagliflozin to increase plasma sodium levels in patients with the syndrome of inappropriate antidiuresis. J Am Soc Nephrol. 2020;31(3):615-624. doi: 10.1681/asn.2019090944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Garrahy A, Sherlock M, Thompson CJ. Treatment outcomes in syndrome of inappropriate antidiuresis: improvements in hyponatremia may reflect successful treatment or resolution of the underlying cause. Am J Kidney Dis. 2020;76(4):599. doi: 10.1053/j.ajkd.2020.04.011 [DOI] [PubMed] [Google Scholar]
- 123. Furst H, Hallows KR, Post J, et al. The urine/plasma electrolyte ratio: a predictive guide to water restriction. Am J Med Sci. 2000;319(4):240-244. doi: 10.1097/00000441-200004000-00007 [DOI] [PubMed] [Google Scholar]
- 124. Cuesta M, Ortolá A, Garrahy A, et al. Predictors of failure to respond to fluid restriction in SIAD in clinical practice; time to re-evaluate clinical guidelines? QJM. 2017;110(8):489-492. doi: 10.1093/qjmed/hcx036 [DOI] [PubMed] [Google Scholar]
- 125. Peri A. Management of hyponatremia: causes, clinical aspects, differential diagnosis and treatment. Expert Rev Endocrinol Metab. 2019;14(1):13-21. doi: 10.1080/17446651.2019.1556095 [DOI] [PubMed] [Google Scholar]
- 126. Ohnishi A, Orita Y, Okahara R, et al. Potent aquaretic agent. a novel nonpeptide selective vasopressin 2 antagonist (OPC-31260) in men. J Clin Invest. 1993;92(6):2653-2659. doi: 10.1172/JCI116881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355(20):2099-2112. doi: 10.1056/NEJMoa065181 [DOI] [PubMed] [Google Scholar]
- 128. Berl T, Quittnat-Pelletier F, Verbalis JG, et al. Oral tolvaptan is safe and effective in chronic hyponatremia. J Am Soc Nephrol. 2010;21(4):705-712. doi: 10.1681/asn.2009080857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Sterns RH. Tolvaptan for the syndrome of inappropriate secretion of antidiuretic hormone: is the dose too high? Am J Kidney Dis. 2018;71(6):763-765. doi: 10.1053/j.ajkd.2018.02.355 [DOI] [PubMed] [Google Scholar]
- 130. Hanna RM, Velez JC, Rastogi A, et al. Equivalent efficacy and decreased rate of overcorrection in patients with syndrome of inappropriate secretion of antidiuretic hormone given very low-dose tolvaptan. Kidney Med. 2020;2(1):20-28. doi: 10.1016/j.xkme.2019.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Pose-Reino A, Runkle de la Vega I, de Jong-Laird A, Kabra M, Lindner U. Real-World, Non-Interventional, Retrospective Study (SAMPLE) of tolvaptan in patients with hyponatraemia secondary to the syndrome of inappropriate antidiuretic hormone secretion. Adv Ther. 2021;38(2):1055-1067. doi: 10.1007/s12325-020-01560-2 [DOI] [PubMed] [Google Scholar]
- 132. Harbeck B, Lindner U, Haas CS. Low-dose tolvaptan for the treatment of hyponatremia in the syndrome of inappropriate ADH secretion (SIADH). Endocrine. 2016;53(3):872-873. doi: 10.1007/s12020-016-0912-y [DOI] [PubMed] [Google Scholar]
- 133. Shoaf SE, Bricmont P, Dandurand A. Low-dose tolvaptan PK/PD: comparison of patients with hyponatremia due to syndrome of inappropriate antidiuretic hormone secretion to healthy adults. Eur J Clin Pharmacol. 2017;73(11):1399-1408. doi: 10.1007/s00228-017-2302-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Tzoulis P, Waung JA, Bagkeris E, et al. Real-life experience of tolvaptan use in the treatment of severe hyponatraemia due to syndrome of inappropriate antidiuretic hormone secretion. Clin Endocrinol. 2016;84(4):620-626. doi: 10.1111/cen.12943 [DOI] [PubMed] [Google Scholar]
- 135. Morris JH, Bohm NM, Nemecek BD, et al. Rapidity of correction of hyponatremia due to syndrome of inappropriate secretion of antidiuretic hormone following tolvaptan. Am J Kidney Dis. 2018;71(6):772-782. doi: 10.1053/j.ajkd.2017.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Peri A. Clinical review: the use of vaptans in clinical endocrinology. J Clin Endocrinol Metab. 2013;98(4):1321-1332. doi: 10.1210/jc.2012-4082 [DOI] [PubMed] [Google Scholar]
- 137. Zeltser D, Rosansky S, van Rensburg H, Verbalis JG, Smith N. Assessment of the efficacy and safety of intravenous conivaptan in euvolemic and hypervolemic hyponatremia. Am J Nephrol. 2007;27(5):447-457. doi: 10.1159/000106456 [DOI] [PubMed] [Google Scholar]
- 138. Ghali JK, Koren MJ, Taylor JR, et al. Efficacy and safety of oral conivaptan: a V1A/V2 vasopressin receptor antagonist, assessed in a randomized, placebo-controlled trial in patients with euvolemic or hypervolemic hyponatremia. J Clin Endocrinol Metab. 2006;91(6):2145-2152. doi: 10.1210/jc.2005-2287 [DOI] [PubMed] [Google Scholar]
- 139. Verbalis JG, Zeltser D, Smith N, Barve A, Andoh M. Assessment of the efficacy and safety of intravenous conivaptan in patients with euvolaemic hyponatraemia: subgroup analysis of a randomized, controlled study. Clin Endocrinol. 2008;69(1):159-168. doi: 10.1111/j.1365-2265.2007.03149.x [DOI] [PubMed] [Google Scholar]
- 140. Velez JCQ, Dopson SJ, Sanders DS, Delay TA, Arthur JM. Intravenous conivaptan for the treatment of hyponatraemia caused by the syndrome of inappropriate secretion of antidiuretic hormone in hospitalized patients: a single-centre experience. Nephrol Dial Transplant. 2010;25(5):1524-1531. doi: 10.1093/ndt/gfp731 [DOI] [PubMed] [Google Scholar]
- 141. Decaux G, Musch W. Estimated daily urine volume and solute excretion from spot urine samples to guide the therapy of hyponatremia in SIADH. J Clin Med. 2019;8(10):1511. doi: 10.3390/jcm8101511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Berkman K, Haigh K, Li L, et al. Investigation and management of moderate to severe inpatient hyponatraemia in an Australian tertiary hospital. BMC Endocr Dis. 2018;18(1):93. doi: 10.1186/s12902-018-0320-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Nervo A, D’Angelo V, Rosso D, et al. Urea in cancer patients with chronic SIAD-induced hyponatremia: old drug, new evidence. Clin Endocrinol (Oxf). 2019;90(6):842-848. doi: 10.1111/cen.13966 [DOI] [PubMed] [Google Scholar]
- 144. Soupart A, Coffernils M, Couturier B, Gankam-Kengne F, Decaux G. Efficacy and tolerance of urea compared with vaptans for long-term treatment of patients with SIADH. Clin J Am Soc Nephrol. 2012;7(5):742-747. doi: 10.2215/cjn.06990711 [DOI] [PubMed] [Google Scholar]
- 145. Decaux G, Genette F. Urea for long-term treatment of syndrome of inappropriate secretion of antidiuretic hormone. Brit J Med (Clin Res Ed). 1981;283(6299):1081-1083. doi: 10.1136/bmj.283.6299.1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Rondon-Berrios H, Tandukar S, Mor MK, et al. Urea for the treatment of hyponatremia. Clin J Am Soc Nephrol. 2018;13(11):1627-1632. doi: 10.2215/cjn.04020318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Padfield PL, Hodsman GP, Morton JJ. Demeclocycline in the treatment of the syndrome of inappropriate antidiuretic hormone release: with measurement of plasma ADH. Postgrad Med J. 1978;54(635):623-627. doi: 10.1136/pgmj.54.635.623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Cherrill DA, Stote RM, Birge JR, Singer I. Demeclocycline treatment in the syndrome of inappropriate antidiuretic hormone secretion. Ann Intern Med. 1975;83(5):654-656. doi: 10.7326/0003-4819-83-5-654 [DOI] [PubMed] [Google Scholar]
- 149. Miell J, Dhanjal P, Jamookeeah C. Evidence for the use of demeclocycline in the treatment of hyponatraemia secondary to SIADH: a systematic review. Int J Clin Pract. 2015;69(12):1396-1417. doi: 10.1111/ijcp.12713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Hu W, lv X, Li C, et al. Disorders of sodium balance and its clinical implications in COVID-19 patients: a multicenter retrospective study. Intern Emerg Med. 2021;16(4):853-862. doi: 10.1007/s11739-020-02515-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Atila C, Sailer CO, Bassetti S, et al. Prevalence and outcome of dysnatremia in patients with COVID-19 compared to controls. Eur J Endocrinol. 2021;184(3):409-418. doi: 10.1530/eje-20-1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Berni A, Malandrino D, Corona G, et al. Serum sodium alterations in SARS CoV-2 (COVID-19) infection: impact on patient outcome. Eur J Endocrinol. 2021;185(1):137-144. doi: 10.1530/eje-20-1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. De Carvalho H, Letellier T, Karakachoff M, et al. Hyponatremia is associated with poor outcome in COVID-19. J Nephrol. 2021;34(4):991-998. doi: 10.1007/s40620-021-01036-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Ruiz-Sánchez JG, Núñez-Gil IJ, Cuesta M, et al. Prognostic impact of hyponatremia and hypernatremia in COVID-19 pneumonia. a HOPE-COVID-19 (Health Outcome Predictive Evaluation for COVID-19) Registry analysis. Front Endocrinol (Lausanne). 2020;11:599255. doi: 10.3389/fendo.2020.599255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Christ-Crain M, Hoorn EJ, Sherlock M, Thompson CJ, Wass J. Endocrinology in the time of COVID-19—2021 updates: the management of diabetes insipidus and hyponatraemia. Eur J Endocrinol. 2021;185(4):G35-G42. doi: 10.1530/eje-21-0596 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study