Abstract
Context
Germline CDKN1B pathogenic variants result in multiple endocrine neoplasia type 4 (MEN4), an autosomal dominant hereditary tumor syndrome variably associated with primary hyperparathyroidism, pituitary adenoma, and duodenopancreatic neuroendocrine tumors.
Objective
To report the phenotype of 3 unrelated cases each with a unique germline CDKN1B variant (of which 2 are novel) and compare these cases with those described in the current literature.
Design/Methods
Three case studies, including clinical presentation, germline, and tumor genetic analysis and family history.
Setting
Two tertiary University Hospitals in Sydney, New South Wales, and 1 tertiary University Hospital in Canberra, Australian Capital Territory, Australia.
Outcome
Phenotype of the 3 cases and their kindred; molecular analysis and tumor p27kip1 immunohistochemistry.
Results
Family A: The proband developed multiglandular primary hyperparathyroidism, a microprolactinoma and a multifocal nonfunctioning duodenopancreatic neuroendocrine tumor. Family B: The proband was diagnosed with primary hyperparathyroidism from a single parathyroid adenoma. Family C: The proband was diagnosed with a nonfunctioning pituitary microadenoma and ectopic Cushing’s syndrome from an atypical thymic carcinoid tumor. Germline sequencing in each patient identified a unique variant in CDKN1B, 2 of which are novel (c.179G > A, p.Trp60*; c.475G > A, p.Asp159Asn) and 1 previously reported (c.374_375delCT, p.Ser125*).
Conclusions
Germline CDKN1B pathogenic variants cause the syndrome MEN4. The phenotype resulting from the 3 pathogenic variants described in this series highlights the heterogenous nature of this syndrome, ranging from isolated primary hyperparathyroidism to the full spectrum of endocrine manifestations. We report the first described cases of a prolactinoma and an atypical thymic carcinoid tumor in MEN4.
Keywords: multiple endocrine neoplasia type 4, MEN4, CDKN1B germline mutation, primary hyperparathyroidism, pituitary adenoma, pancreatic neuroendocrine tumor, atypical carcinoid tumor
Multiple endocrine neoplasia includes several inherited syndromes associated with tumors in multiple endocrine organs, and sometimes nonendocrine tumors. Morbidity or mortality arises through excess hormone secretion, local growth and invasion, or metastasis. Multiple endocrine neoplasia type 1 (MEN1) syndrome resulting from germline heterozygous loss-of-function mutations in the tumor suppressor gene MEN1 is well-recognized. The hallmark features of this syndrome include a high penetrance of primary hyperparathyroidism (PHPT), followed by pituitary adenoma, duodenopancreatic neuroendocrine tumors (DP-NET), and adrenal lesions.
Despite advances in genetic testing technology 5% to 25% of patients with clinically suspected MEN1 do not have a pathogenic variant within the MEN1 coding region (1). Of these patients, technical limitations of molecular testing or failure to undertake detailed interrogation of the genome may account for the inability to identify the MEN1 mutation. Sequencing of the promoter and untranslated regions and multiplex-ligand-dependent probe amplification to uncover gene deletions are required (2, 3). MEN1 phenocopies, which occur in 5-10% of cases, are an alternative explanation for not detecting an MEN1 mutation on molecular analysis (4, 5).
Panel testing is now used in clinical practice to investigate familial hypercalcemia or syndromic/familial MEN1 syndrome at initial presentation, or in MEN1-mutation-negative patients. A typical multigene panel may include molecular analysis for germline mutations in hyperparathyroidism jaw tumor syndrome (CDC73); familial hypocalciuric hypercalcemia 1, 2, and 3 (FHH1, FHH2, and FHH3 from CASR, GNA11, AP2S1); familial isolated pituitary adenoma (with AIP accounting for 20%-30% of cases (6)); multiple endocrine neoplasia type 2 (RET); and, more recently, multiple endocrine neoplasia type 4 (MEN4, CDKN1B) (7, 8).
Thakker et al estimated that 3% of MEN1 mutation–negative patients harbor loss of function mutations in the cyclin-dependent kinase inhibitor 1B gene (CDKN1B) (1). Pathogenic variants within this gene result in the autosomal dominant condition MEN4 (OMIM #610755). MEN4 syndromically mimics MEN1 with some discernible differences. Primary hyperparathyroidism occurs most commonly with a seemingly older age of onset (9). The occurrence of anterior pituitary adenoma appears similar to MEN1, but DP-NETs are rare (9). Penetrance estimates are limited by the small number of cases described in the literature. Segregation studies in 1 kindred suggested penetrance may be near complete (9); however, other studies report unaffected variant carriers (10-12). The incidence of MEN4 syndrome is reported to be 1.5% to 3.7% in patients with a MEN1 related phenotype.
CDKN1B is a tumor suppressor gene comprising of 2 exons located on chromosome 12 (12p13.1) that encodes the nuclear protein p27Kip1 (14, 15). p27Kip1 is a cyclin-dependent kinase inhibitor limiting cell-cycle progression. Antimitogenic signals drive the formation of cyclin-dependent kinase inhibitor complexes with nuclear cyclin-dependent kinases D and E blocking their catalytic activity (16, 17). This prevents the transcriptional activation of genes involved in the transition from G1 to S phase (16, 18). Disruption of p27Kip1, resulting from loss of function mutations in CDKN1B, impedes its nuclear localization and binding with cyclin-dependent kinases (18-20). This is the key molecular mechanism driving MEN4 syndrome (19).
Genetic testing first identified homozygous Cdkn1b null alleles in a model rat that spontaneously developed multiple endocrine tumors as a recessive trait (anterior pituitary adenoma, phaeochromocytoma and paraganglioma, c-cell hyperplasia, and pancreatic islet cell hyperplasia) and was initially termed MENX because of the overlap of features with MEN1 and MEN2 (21). A heterozygous germline nonsense mutation in the CDKN1B gene was identified in a patient with a GH-secreting pituitary adenoma, parathyroid adenoma, and segregation within the family (11). A second patient described soon after was diagnosed with small cell neuroendocrine cervical carcinoma, an ACTH-secreting pituitary adenoma, and primary hyperparathyroidism (22). The 11th International Workshop on MENs subsequently named this MEN1-like syndrome MEN4 (23).
We aimed to describe and expand the phenotype of MEN 4 syndrome by presenting clinical, genetic, and immunohistochemical features of 3 unrelated patients who harbor pathogenic CDKN1B germline variants.
Materials and Methods
Ethics Approval
Informed consent was obtained from each individual and the research was approved by the Human Research Ethics Committee at Prince of Wales Hospital, Royal North Shore Hospital, and St Vincent’s Hospital, Sydney, and The Canberra Hospital, Canberra.
Phenotype
Clinical information including presenting complaint, biochemical results, and radiological findings were obtained from the medical records.
Germline Sequencing
Genomic DNA was extracted from blood using Qiagen DNAeasy extraction kits (Qiagen) for all patients.
Patient A
Using the Roche/NimbleGen SeqCap EZ Choice Library, Next Generation Sequencing on a custom panel incorporating 8 familial pituitary tumor syndrome genes (AIP, CDKN1B, MEN1, PRKAR1A, SDHA, SDHB, SDHC, and SDHC) was performed using Illumina’s HiSeq 2500 platform. Depth of coverage was > 30-fold in 97% of the targeted genomic region and > 100-fold in 91%. Sequencing data were processed according to Genome Analysis Toolkit’s (GATK) best practice. Sequencing reads were aligned to the human genome via Burrows-Wheeler Alignment and Novosort. Single-nucleotide variants and small insertions/deletions were identified and annotated with HaplotypeCaller v3.3 and Ensembl Variant Effector Predictor v74, respectively. Data were filtered and prioritized using an in-house platform.
Patient B
Next-generation sequencing (NGS) was performed incorporating 4 familial hypercalcemia syndrome genes (MEN1, CDKN1B, RET, and CDC73) using targeted capture (custom Roche SeqCap EZ capture “Roche 1k Disease v.6”) and sequenced on Illumina NextSeq Sequencing System. Sequences were aligned to the human reference genome (hg19) using Burrows-Wheeler Aligner (BWA mem). Variant calling was performed using GATK. Variant annotation was performed using Variant Studio v3, Alamut v2.11, and VariantGrid. Variants with a minor allele frequency > 1% or > 0.1% in population databases (ExAC, gnomAD) were excluded from the analysis.
Patient C
Targeted NGS of coding regions and splice sites of MEN1 and CDKN1B was performed on the Illumina NextSeq500 with a targeted coverage of 700 reads/base. Seqliner v0.8 was used to generate aligned reads and call variants against the hg19 human reference genome. Copy number was analyzed using Gaffa v3.0 Targeted. PathOS v1.5 was used to annotate and transform variants to standard nomenclature and filter for rare, nonsynonymous variants within 20 bp of coding regions.
Sanger sequencing was used to confirm the identified variants within CDKN1B. Variant nomenclature is according to Human Genome Variant Society nomenclature v20.05 (24) using the reference sequence CDKN1B (NCBI:NM_0004064.4;NG_016341.1) (25). Interpretation of variant pathogenicity is according to American College of Medical Genetics (ACMG) guidelines (26).
Tumor Sequencing
Tumoral DNA was extracted from formalin-fixed paraffin embedded tumor samples using Qiagen DNAeasy blood and tumor extraction kit (Qiagen, Germany). Tumor specimens available for analysis were as follows: parathyroid adenoma, adrenal cortical adenoma, and pancreatic neuroendocrine tumor from patient A; parathyroid adenoma from patient B; and atypical thymic carcinoid tumor from patient C. DNA libraries were prepared and sequenced on a MiSeq platform using a custom hereditary endocrine tumor gene panel (TruSeq Custom Amplicon Assay, Illumina). FASTQ files were generated for each sample, and alignment of reads (banded Smith-Waterman algorithm) and variant calling (GATK) was processed by MiSeq Reporter v2.0, Illumina. Annotation of functional consequences to variant calls was performed using ANNOVAR (version2013Jul). Output was filtered to analyze relevant coding regions (CDKN1B to assess for loss of heterozygosity [LOH], MEN1, and ATRX), to remove untranslated regions and synonymous single nucleotide polymorphisms, include read depth ≥ 30 and include variants with a population variant allele frequency < 0.001 or not reported in the population database gnomAD (27). Visualization of reads was performed using IGV v2.1. The Catalogue of Somatic Mutations in Cancer was used to categorize somatic variants with regard to pathogenicity (28).
p27Kip1 Immunohistochemistry
Immunohistochemistry for p27Kip1 was performed on archived formalin-fixed paraffin embedded tissue using a commercially available mouse monoclonal antibody (RRID: AB_11190322, https://scicrunch.org/resources/Any/search?q=AB_11190322&l=AB_11190322, clone SX53G8, predilute, catalog number LS-C389523, Ventana Medical Systems, Tucson, AZ, USA) on an automated staining platform—the Ventana BenchMark Ultra (Ventana Medical Systems) using the manufacturer’s protocol.
Results
Clinical information and the outcome of molecular testing are summarized for each patient in Table 1. Detailed laboratory results are provided in Table 2.
Table 1.
Clinical features and outcome of molecular testing
| Tumor (age, y) | Family history | Germline pathogenic variant | LOH | Somatic pathogenic variant | |
|---|---|---|---|---|---|
| Patient A | Multigland PHPT; single adenoma (33 y), second adenoma (45 y) Prolactinoma (39 y) Multifocal pancreatic NET (47 y) Benign cortical adrenal adenoma (47 y) |
First-degree relative with PHPT (genetic testing not undertaken) |
CDKN1B
(c.179G > A, p.Trp60Ter) |
No | No |
| Patient B | PHPT (26 y) | No |
CDKN1B
(c.475G > A, p.Asp159Asn) |
No | ATRX (c.6406G > A, p.Asp2136Asn) |
| Patient C | Atypical thymic carcinoid tumor (31y) | Second-degree relative who underwent “neck surgery” |
CDKN1B
(c.374_375delCT, pSer125*) |
No |
MEN1
(c.974C > T, p.Pro325Leu) |
Abbreviations: LOH, loss of heterozygosity; NET, neuroendocrine tumor; PHPT, primary hyperparathyroidism.
Table 2.
Results of laboratory investigations
| Patient A | Patient B | Patient C | |
|---|---|---|---|
| PTH | 1.5 × ULN (12.4 pmol/L, rr 0.8–8.0) |
3.0 × ULN (20 pmol/L, rr 1.5-6.9) |
1.2 × ULN ( 8.8 pmol/L, rr 1.6-7.2) |
| Corrected calcium | 1.9 × ULN (2.92 mmol/L, rr 2.16-2.45) |
1.2 × ULN (3.06 mmol/L, rr < 2.55) |
Normal |
| 25OHD | Normal | Normal | 0.4 × LLN (24 nmol/L, rr > 50) |
| TSH | Normal | Normal | Normal |
| Prolactin | 3.8 × ULN (1898 mIU > L, rr 50-500) |
Normal | Normal |
| IGF-1 | 1.0 × ULN (251 µg/L, rr 93-245) |
Normal | 1.2 × ULN (49 nmol/L, rr 8-42) |
| GH | Normal | Normal | Normal |
| ACTH | Normal | Normal | 1.5 × ULN (21 pmol/L, rr < 14) |
| Cortisol | 1.3 × ULN | Normal | Normal |
| Morning (600 am) | (699 nmol/L, rr 100-535) (initial) Normal (490 nmol/L) (repeat) |
- | - |
| Midnight salivary |
1.1 × ULN (3.6 nmol/L, rr < 3.2) |
- | - |
| 24-h urinary excretion (preoperative) | 2.3 × ULN (624 nmol/L, rr < 270) |
- | 2.9 × ULN (438 nmol/24h, rr < 150) |
| 24-h urinary excretion (postoperative) | - | - | Normal |
| 1 mg DST | 1.2 × ULN (62 nmol/L, rr < 50) |
- | 5 × ULN (249 nmol/L, rr < 50) |
| DHEAS | 1.6 × ULN (11.3 µmol/L, rr 1.0-7.0) |
- | - |
| Androstenedione | Normal | - | - |
| HbA1c% | Normal | - | 1.2 × ULN (6.7%, rr < 5.6%) |
| CgA | Normal | Normal | Normal |
| Gastrin | Normal | Normal | Normal |
| 5HIAA | |||
| Preoperative | - | - | 1.5 × ULN (53 µmol/24 h, rr < 35) |
| Postoperative | - | - | Normal |
| c-peptide | - | - | 1.5 × ULN (2.3 nmol/L, rr 0.4-1.5) |
| Insulin | - | Normal | N/A |
| VIP | Normal | Normal | N/A |
| Glucagon | Normal | Normal | N/A |
| Pancreatic polypeptide | Normal | 1.4 × ULN (78.1 pmol/L, rr < 55) |
N/A |
Abbreviations: -, not performed; 5HIAA, 5-hydroxyindoleacetic acid; CgA, chromogranin A; DHEAS, dehydroepiandrosterone sulfate; DST, dexamethasone suppression test; HbA1c, glycated hemoglobin; N/A, performed but not available; rr, reference range; ULN, upper limit of normal; VIP, vasoactive intestinal peptide.
Patient A
Clinical details
The proband (II.2, Fig. 1.1) presented aged 32 years with PHPT (corrected calcium 2.92 mmol/L, normal range 2.16-2.45 mmol/L; PTH 12.4 pmol/L, normal range 0.8-8.0 pmol/L) and a left superior parathyroid adenoma was resected via minimally invasive surgery. More than a decade later, aged 45 years, a recurrence of PHPT was diagnosed (corrected calcium 2.69 mmol/L, normal range 2.1-2.6 mmol/L; PTH 104 pg/mL, normal range 15-68 pg/mL) and a second parathyroid adenoma was resected. Hypercalcemia resolved postoperatively on both occasions. End-organ complications of PHPT included osteopenia (L1-L4 T-score -0.3 SD and left femoral neck T-score -1.5 SD on dual-energy X-ray absorptiometry) and asymptomatic nonobstructive renal calculi diagnosed radiologically.
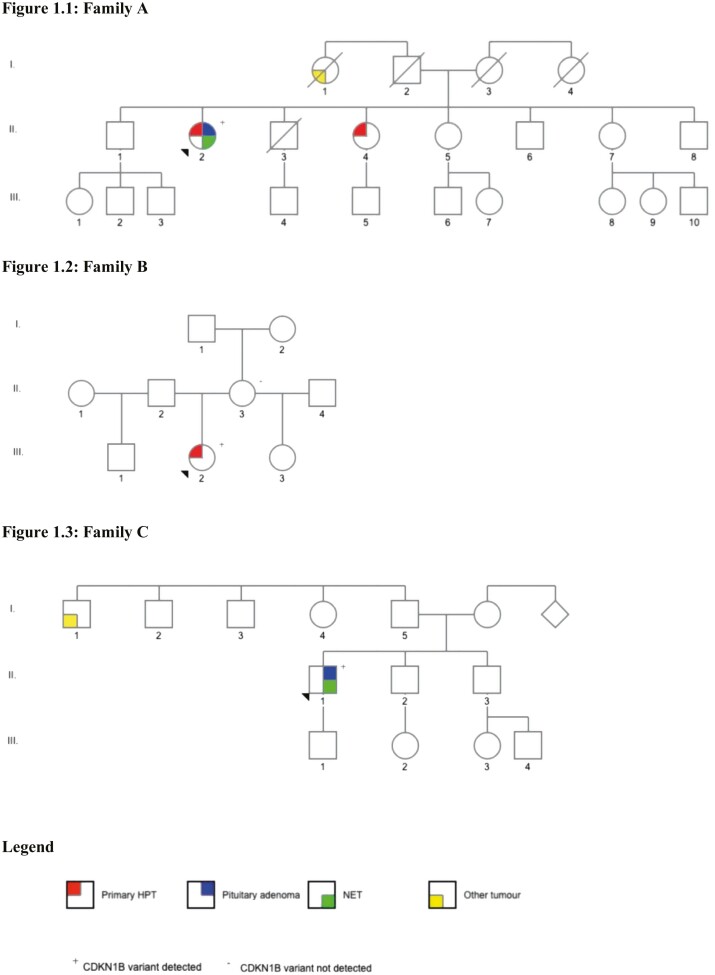
Figure 1.
Pedigrees. (1.1) Family A. (1.2) Family B. (1.3) Family C.
At age 39 years following the development of recurrent tonic-clonic seizures a cerebral 18F-fluorodeoxyglucose-positron emission tomography (PET) scan was performed to evaluate for an epileptogenic focus. This revealed increased tracer uptake in the region of the pituitary gland. Further investigation with contrast-enhanced pituitary magnetic resonance imaging (MRI) identified an 8 × 6 × 3-mm hypointense nodule in the right aspect of the adenohypophysis consistent with a pituitary microadenoma. A pituitary hormone panel found isolated hyperprolactinemia (prolactin 1898 mIU/L, normal range 50-500 mIU/L), from which she was symptomatic with oligomenorrhea. She was started on cabergoline 0.5mg weekly with normalization of prolactin within 4 months.
At age 43 years, the patient was investigated for hypercortisolemia in the context of a 16kg weight gain and plethoric facies. ACTH was normal (1.9pmol/L, normal range < 10.8pmol/L) and cortisol was elevated (699pmol/L, normal range < 539pmol/L). Midnight salivary and 24-hour urinary free cortisol (UFC) levels were both elevated (3.6nmol/L, normal range < 3.2nmol/L; and 624nmol/L, normal range < 270nmol/L, respectively). An overnight 1-mg dexamethasone suppression test failed to suppress cortisol below 50nmol/L. On repeating the 24-hour UFC, salivary cortisol and dexamethasone suppression test results were normal, raising the possibility of cyclical Cushing’s syndrome.
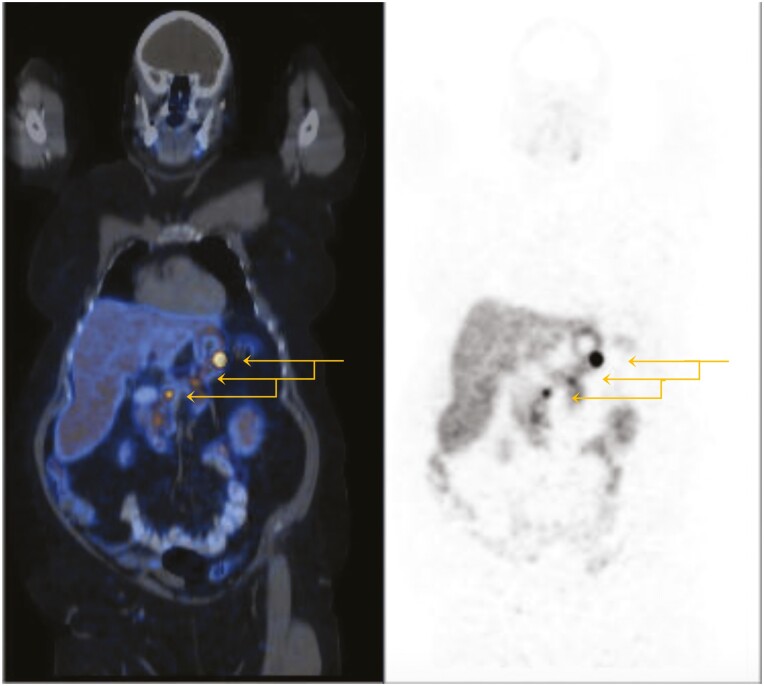
In the context of young-onset multiglandular primary hyperparathyroidism and a microprolactinoma, genetic testing was undertaken and no pathogenic variant was identified in MEN1. Based a clinical diagnosis of MEN1 syndrome, a screening MRI of the abdomen was performed; 2 adrenal lesions were found (on the left measuring 59 × 49 mm [-5HU] and on the right 26 × 21 mm [-17HU]) and multiple pancreatic lesions in the body and tail of the pancreas, with the largest measuring 15 × 13 × 10 mm. The pancreatic lesions were avid on 68Ga-DOTATATE-PET (Fig. 2). An adrenal hormone panel (plasma metanephrines, renin, aldosterone, cortisol) and a limited pancreatic hormone panel (gastrin, pancreatic polypeptide, vasoactive intestinal polypeptide) were normal.
Figure 2.
Functional imaging. 68Ga-DOTATATE-PET of patient A.
Left adrenalectomy was undertaken to remove the large left nonfunctioning adrenal nodule based on the size. There was no evidence of hypercortisolism perioperatively. Distal pancreatectomy was performed to resect the multiple pancreatic lesions. Histopathology of the left adrenal nodule showed a 53mm encapsulated tumor consistent with an adrenal cortical adenoma. Histopathology of the pancreas showed 3 well-differentiated neuroendocrine tumors measuring 2, 4, and 11mm each with Ki67 < 1%. A repeat 68Ga-DOTATATE-PET scan 12 months following surgery showed a possible recurrence at the pancreatic resection margin and new lesions in the neck and body of the pancreas. The patient underwent total pancreaticoduodenectomy; histopathology identified 2 neuroendocrine tumors measuring 13 × 12 × 12 mm in the body of pancreas and 5 × 5 × 5 mm in the neck. Ki67 of each tumor was < 1%.
Additional diagnoses include polycystic ovarian syndrome and antiphospholipid syndrome. Her family history is notable for a sister (II.4, Fig. 1.1) with a parathyroid adenoma diagnosed at age 50 years. Family members have declined predictive testing.
Germline and tumor sequencing
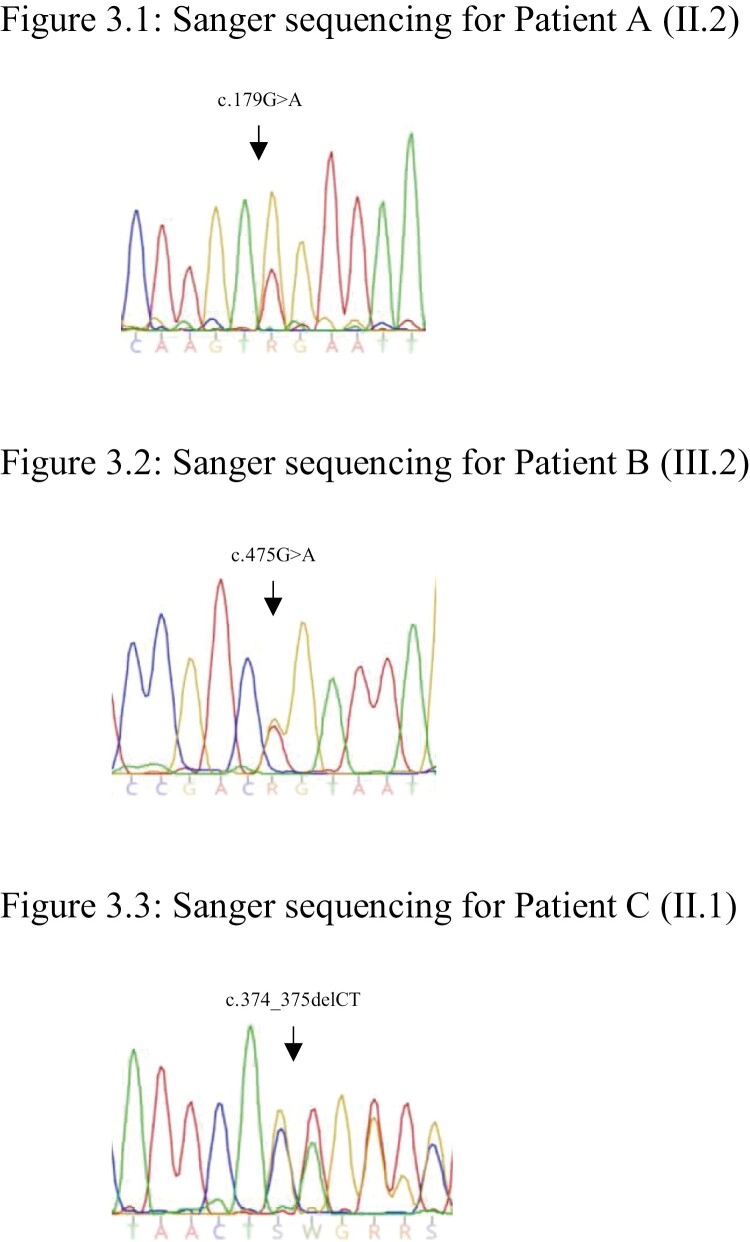
The patient was recruited into the Familial Pituitary Tumour Syndrome study, a cross-sectional study aimed at identifying germline variants in patients diagnosed with pituitary tumors ≤ 40 years of age and/or other personal/family history of endocrine neoplasia (29). Germline sequencing identified a CDKN1B (c.179G > A, p.Trp60*) nonsense variant resulting in a premature stop codon in exon 1, which is predicted to lead to nonsense-mediated decay of CDKN1B mRNA with loss of protein function (Fig. 3.1). This variant has not been reported in the scientific literature to date or in population (gnomAD v2.1.1, 1000Genomes (30)) or variant (ClinVar (31), LOVD (32), HGMD (33)) databases. In silico tools predict this variant to be pathogenic (CADD score 38). As per ACMG guidelines the variant is classified as “likely pathogenic” (PVS1, PM2).
Figure 3.
Germline CDKN1B electropherograms. (3.1) Sanger sequencing for patient A. (3.2) Sanger sequencing for patient B. (3.3) Sanger sequencing for patient C.
NGS of parathyroid adenoma, adrenal cortical adenoma, and pancreatic neuroendocrine tumor identified the same CDKN1B (c.179G > A, p.Trp60*) variant as was present in the germline. There was no LOH. No additional “pathogenic” or “likely pathogenic” variants were identified.
p27Kip1 immunohistochemistry
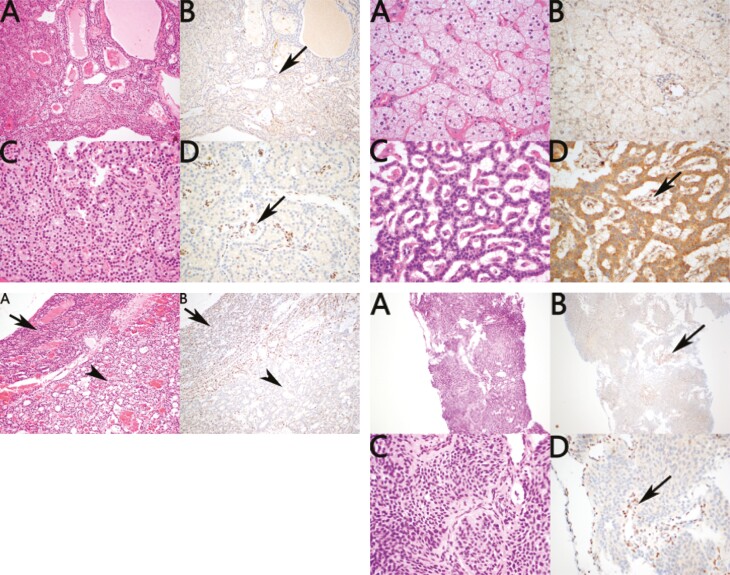
Immunohistochemistry of the resected parathyroid adenoma showed loss of staining for p27Kip1 consistent with an acquired second hit in p27Kip1 resulting in subsequent loss of expression in the neoplastic cells (Fig. 4.1). Loss of p27Kip1was also seen in the adrenal cortical adenoma but without a positive internal control limiting its significance. The pancreatic NET demonstrated loss of expression with positive expression in the internal controls in the stroma (Fig. 4.2). However, because a similar pattern of loss of expression was identified in all non-neoplastic islets from the same patient, this pattern of staining is considered noncontributory (ie, p27Kip1 loss may be a feature of islet cell differentiation).
Figure 4.
Immunohistochemistry. (4.1, top left) p27kip1 immunohistochemistry of parathyroid adenoma from patient A. (4.2, top right) p27kip1 immunohistochemistry of adrenal cortical adenoma and pancreatic neuroendocrine tumor from patient A. (4.3, bottom left) p27kip1 immunohistochemistry of parathyroid adenoma from patient B. (4.4, bottom right) p27kip1 immunohistochemistry of the thymic neuroendocrine tumor from patient C.
Patient B
Clinical details
The proband (III.2, Fig. 1.2) was diagnosed with primary hyperparathyroidism aged 26 years (peak corrected calcium 3.06mmol/L, normal range 2.5-2.55mmol/L; PTH 20pmol/L, normal range 1.5-6.9pmol/L). Parathyroid sestamibi imaging demonstrated a hyperfunctioning right inferior gland, and an equivocal focus on the left concerning for multigland disease. The highly avid gland was resected with resolution of hypercalcemia; there is no evidence of recurrence 3 years later.
The diagnosis of primary hyperparathyroidism under the age of 40 years prompted genetic testing for hereditary causes of hypercalcemia, including MEN1, MEN4, and hyperparathyroidism jaw tumor syndrome. A mutation search in MEN1 and CDC73 was negative; a pathogenic variant was identified in CDKN1B. The diagnosis of MEN4 syndrome resulted in further investigation for additional syndromic features. MRI of the pituitary did not identify a pituitary adenoma. Pituitary and pancreatic hormone measurements were normal. Imaging of the pancreas for a nonfunctioning NET has not yet been performed.
The patient has a medical history of polycystic ovary syndrome and depression. There is no known family history of endocrine or related neoplasia; however, the details of her paternal family history are unknown. Her mother (II.3, Fig. 1.2) underwent predictive testing for the CDKN1B pathogenic variant and was wild-type.
Germline and tumor sequencing
Germline sequencing identified a novel heterozygous CDKN1B (c.475G > A, p.Asp159Asn) missense variant involving the final codon of exon 1 (Fig. 3.2). This variant has not been reported in scientific literature to date and is not listed in population (gnomAD v2.1.1, 1000Genomes (30)) or variant (ClinVar (31), LOVD (32), HGMD (33)) databases. In silico tools predict the variant to remove a splice donor site (Alamut version 2.7) and to be “probably damaging” (PolyPhen) and “deleterious” (SIFT score 0.005, CADD score 29.1). Per ACMG criteria, the variant is classified “likely pathogenic” (PVS1, PM2).
NGS of parathyroid adenoma identified the identical CDKN1B (c.475G > A, pAsp159Asn) variant as was present in the germline with no LOH. A somatic ATRX (c.6406G>A, p.Asp2136Asn) missense variant was identified and has been reported in glioma (astrocytoma grade IV) and endometrioid carcinoma (34, 35). In silico analysis predicted the variant to be deleterious (SIFT score 0.002, PolyPhen 1.0, MutationTaster 1.0).
p27Kip1 immunohistochemistry
Immunohistochemistry of the single parathyroid adenoma for p27Kip1 demonstrated somewhat decreased expression in the neoplastic cells compared with non-neoplastic cells without complete absence of staining (Fig. 4.3). That is, although this pattern of staining supported the likelihood of decreased expression of p27Kip1, it was not definitive.
Patient C
Clinical details
The proband (II.1, Fig. 1.3) presented aged 31 years with an atypical thymic carcinoid tumor causing ectopic Cushing’s syndrome.
This diagnosis was made in the context of a hospital admission for severe hypertension (SBP 199/80 mm Hg) and clinical features consistent with cortisol excess (rounded facies, abdominal striae, supraclavicular/interscapular fat pads). The patient was started on 2 antihypertensive agents. Type 2 diabetes mellitus was also diagnosed and managed with metformin. Investigations into secondary causes of diabetes were performed and identified ACTH-dependent hypercortisolism with an elevated 24-hour UFC (438nmol/24 hr, normal range < 150 nmol/24 hr), lack of cortisol suppression on a 1-mg overnight dexamethasone suppression test, and an elevated ACTH (21 pmol/L, reference range < 14pmol/L). Contrast-enhanced MRI of the pituitary reported a 7mm focus of “differential low enhancement” on the right side of the pituitary suggestive of a pituitary microadenoma. However, bilateral inferior petrosal sinus sampling confirmed an ectopic source of ACTH, with central-to-peripheral plasma ACTH gradient of < 2.0 before corticotropin-releasing hormone administration and < 3.0 after corticotropin-releasing hormone.
Computed tomography imaging identified an ovoid superior mediastinal mass with a slightly irregular border measuring 66 × 45 mm that abutted the brachiocephalic vein and aortic arch. Histopathology from a CT-guided core biopsy of the superior mediastinal mass was consistent with a thymic neuroendocrine tumor. The tumor was resected via thoracotomy, which on histopathology was a 59mm atypical carcinoid tumor (pT3pN0M0). After recovering from a postoperative pleural effusion, exogenous glucocorticoid replacement was weaned, and UFC normalized. His diabetes resolved. He remains on single-agent antihypertensive treatment. Postoperative radiotherapy was administered to the mediastinum (3-dimensional conformal radiation therapy, 60 Gy/30 fractions). Now 2 years following his diagnosis, he remains without evidence of clinical, biochemical, or radiological recurrence.
The patient has no additional medical history. He reported a paternal aunt (I.1 Fig. 1.3) with a neck tumor resected between the ages of 30 and 40 years. His mother and brother have a diagnosis of hypertension. No family members have undergone predictive testing as his immediate and extended family live overseas.
Germline and tumor sequencing
Germline sequencing identified a heterozygous variant in exon 1 of CDKN1B (c.374_375delCT, p.Ser125*), with the deletion of 2 bases resulting in a premature stop codon predicted to result in nonsense-mediated decay (Fig. 3.3). This variant has not been reported in population (gnomAD v2.1.1, 1000Genomes (30)) or variant (ClinVar (31), LOVD (32), HGMD (33)) databases. The variant has been detected in an individual with multiglandular PHPT and DP-NET with complete loss of nuclear p27kip1 expression in the parathyroid adenoma on immunohistochemistry (12). Per ACMG guidelines, the variant is classified as “pathogenic” (PVS1, PS3, PM2)
NGS of the atypical thymic carcinoid tumor identified the identical CDKN1B (c.374_375delCT) variant as was detected in the germline with no LOH. A somatic MEN1 (c.974C > T, p.Pro325Leu) missense variant was detected. This variant has previously been reported in sporadic parathyroid adenoma (36). In silico analysis predicted the variant to be “deleterious” (SIFT score 0.002, PolyPhen 1.0, MutationTaster 1.0).
p27Kip1 immunohistochemistry
Immunohistochemistry of the thymic atypical carcinoid for p27Kip1 was completely negative in all neoplastic cells, with preserved diffuse strong expression in the non-neoplastic cells, which served as an internal positive control (Fig. 4.4). This pattern of staining is in keeping with an acquired second hit in p27Kip1 with subsequent loss of expression in the neoplastic cells.
Discussion
We describe three unrelated cases of MEN4 syndrome resulting from different germline CDKN1B null variants, including two novel variants. All patients developed an endocrine tumor at a young age (with one patient developing multiple endocrine tumors), prompting genetic testing for a hereditary endocrine tumor syndrome. We report the first cases of prolactinoma and a thymic carcinoid tumor in MEN4 syndrome. These three cases and their kindreds emphasize the phenotypic heterogeneity of MEN4 syndrome.
Our three cases add to more than 34 previously reported cases of MEN4 syndrome, with 21 unique mutations (9, 37-39). Of the various endocrine tumors in MEN4, parathyroid adenomas are most common (either single or multiple), followed by other endocrine tumors (pituitary and adrenal adenoma, DP-NET), and rarely nonendocrine tumors. Primary hyperparathyroidism develops in most, if not all, carriers of a CDKN1B mutation with an estimated penetrance approaching 90% to 100% (9, 19). Frederikson et al presented an extensive family segregation study with 13 individuals harboring a CDKN1B pathogenic variant; all developed primary hyperparathyroidism (9). Although this resembles MEN1 syndrome, biochemical aberrations appear milder, are often asymptomatic, and with an onset later in life (mean age of diagnosis 56 years) (9, 13). The youngest age of onset reported is 15 years (10), and patient A adds evidence that multiglandular PHPT does occur (12).
14 cases of pituitary tumors have been described in the literature, four nonfunctioning (9, 40), five with acromegaly (11, 39, 41, 42), five with Cushing’s disease (9, 22, 38), and the first described case of a prolactinoma resulting from a pathogenic CDKN1B mutation has now been identified in this series. The penetrance of pituitary adenoma is estimated to approach 30% (38). Of note, Chasseloup et al screened a predominantly pediatric cohort with Cushing’s disease and identified 3 disease-causing (pathogenic/likely pathogenic) variants in early adolescence with the youngest age of disease onset at 9 years (38).
Nine cases of DP-NET have been described in MEN4 syndrome, with the youngest age of onset at 42 years (43), of which three cases were gastrinomas and six nonfunctioning (12, 19, 43, 44). Although DP-NET may result in a high degree of morbidity and mortality in MEN1 patients, the natural progression in MEN4 has not been established.
These key features of MEN4 syndrome contrast with MEN1 syndrome whereby the penetrance of primary hyperparathyroidism approaches 100% by age 50 years with a high rate of recurrent disease (>50% by 12 years) (45, 46). The penetrance of pituitary adenoma in MEN1 is ~40% with lactotroph adenoma occurring most frequently (20%), and somatotroph, gonadotroph, and clinically nonfunctioning adenoma occurring infrequently (~5% each). Corticotroph (2%) and TSHomas rarely develop (45). DP-NET penetrance may be as high as 90% with gastrinoma occurring in 30% to 40% of patients, followed by nonfunctioning adenoma in 20% to 55% and insulinoma in 10% (45, 47).
Less frequent endocrine lesions occurring in MEN1 syndrome include adrenocortical tumors, gastric NET, bronchopulmonary NET, thymic NET, and pheochromocytoma with an estimated prevalence of 40%, 10%, 2%, 2%, and < 1%, respectively (45). Bilateral adrenal nodular hyperplasia, primary bilateral adrenocortical hyperplasia, and adrenocortical carcinoma have been described in MEN1 syndrome. In comparison, adrenal nodules (including patient A from this study) have been described in patients with MEN4 syndrome (13), and bronchial (40) and neuroendocrine cervical carcinoma have occurred in individual cases (22). Thymic NETs are already known to have a strong association with hereditary endocrine syndromes, with 25% occurring in the context of MEN1 syndrome (48, 49). MEN1-associated thymic NETs are highly lethal. Lifelong radiological screening because of the primarily nonfunctional nature of these tumors is recommended and risk-reducing transcervical thymectomy at the time of parathyroidectomy is suggested (8). Patient C is the first described case of a thymic neuroendocrine tumor in a patient in MEN4 syndrome. Whether a high mortality rate will transpire within this syndrome is yet to be seen.
The small number of cases and a paucity of segregation studies limits confidence in recommending surveillance guidelines for primary tumors. Considering our findings in the context of previously described MEN4 cases in the literature, screening for primary hyperparathyroidism biennially from age 15 years, pituitary adenoma from 10 years and DP-NET from age 30 years (10 years earlier than the youngest described case because of the high degree of morbidity associated with NETs) could be considered. MRI (to coincide with pancreatic imaging) to detect thymic NET should also be incorporated.
In our three cases, tumor testing failed to demonstrate a second somatic event in the wild-type allele, which is in keeping with the literature. Haploinsufficiency for CDKN1B appears sufficient for tumors to develop (50). Murine studies in 1998 by Fero et al first identified this, showing both p27Kip1 nullizygous and p27Kip1 heterozygous mice challenged with gamma-irradiation or a chemical carcinogen were predisposed to developing tumors in multiple tissues, including pituitary tumors (51). Following on from these findings, Teixeira et al highlighted the role of p27Kip1 in hampering aberrant somatotroph proliferation in p27Kip1 deficiency with even partial reductions in p27Kip1 augmenting pituitary oncogenesis (52). Within MEN4 syndrome, LOH has been found in a minority of tumor samples (9, 12, 22) with inconsistency between tumor types and between tumors from a single patient (44). As such, CDKN1B shares similarities with p53, NF1, and PTEN as “early generation” haplo-insufficient tumor suppressor genes (50).
Silencing of the wild-type allele by somatic mutations in alternative genes is a proposed trigger for tumor development in haploinsufficiency (19). Examples of such genes specific to endocrine tumors include other CDK family members (CDKN2B, CDKN2C, CDKN1A) and, as identified in the thymic carcinoid tumor from patient C, MEN1. ATRX was identified in the parathyroid adenoma tissue from patient B. Mutations in ATRX are associated with alternative lengthening of telomeres and have been shown to be associated with clinically aggressive behavior in SDHB mutated phaeochromocytoma and paraganglioma (53). ATRX and MEN1 genes are both involved in chromatin remodelling and have been identified as the most frequent somatic mutation in pancreatic NETs (54, 55). Expanding tumor sequencing in MEN4 syndrome may identify genotype/phenotype correlations with these other well-recognized tumor suppressor genes.
A key limitation of the study is the absence of segregation studies with only the single unaffected first-degree relative of patient B undergoing predictive testing. All three patients are in the third and fourth decades of life and potentially are yet to develop further syndromic manifestations.
Conclusion
MEN4 syndrome demonstrates the hallmark features of many hereditary endocrine cancer syndromes: tumor multifocality and phenotypic heterogeneity. Index cases with features consistent with MEN1 should be considered for molecular testing for a CDKN1B mutation either as part of panel testing for MEN1 phenocopies or if genetic testing has not identified a mutation in MEN1. Simultaneous testing of MEN1 and CDKN1B in patients with an MEN1 phenotype is increasingly applicable given improved access to NGS. As with many other rare hereditary tumor syndromes, the heterogenous pattern of disease presentation has hampered the development of syndrome-specific management guidelines. As such, the frequency of tumor specific biochemical and radiological surveillance in MEN4 syndrome is yet to be established.
Acknowledgments
The authors thank the patients and their genetic counsellors, and Steven Macaskill for laboratory technical support.
Glossary
Abbreviations
- ACMG
American College of Medical Genetics
- DP-NET
duodenopancreatic neuroendocrine tumor
- GATK
Genome Analysis Toolkit
- LOH
loss of heterozygosity
- MEN1
multiple endocrine neoplasia type 1
- MEN4
multiple endocrine neoplasia type 4
- MRI
magnetic resonance imaging
- NGS
next-generation sequencing
- PET
positron emission tomography
- PHPT
primary hyperparathyroidism
- rr
reference range
- UFC
urinary free cortisol
Contributor Information
Amanda Seabrook, Cancer Genetics Laboratory, Kolling Institute, Royal North Shore Hospital, Sydney, NSW, 2065, Australia; The University of Sydney, Sydney, NSW, 2006, Australia.
Ayanthi Wijewardene, Cancer Genetics Laboratory, Kolling Institute, Royal North Shore Hospital, Sydney, NSW, 2065, Australia; The University of Sydney, Sydney, NSW, 2006, Australia.
Sunita De Sousa, Endocrine and Metabolic Unit, Royal Adelaide Hospital, Adelaide, SA, 5000; South Australian Adult Genetics Unit, Royal Adelaide Hospital, Adelaide, SA, 5000, Australia; Adelaide Medical School, University of Adelaide, Adelaide, SA, 5000, Australia.
Tang Wong, The University of New South Wales, Sydney, NSW, 2052, Australia; The University of Western Sydney, Sydney, NSW, 2560, Australia; Department of Endocrinology, Prince of Wales Hospital, Sydney, NSW, 2064, Australia.
Nisa Sheriff, Department of Endocrinology, Hornsby Ku-ring-gai Hospital, Sydney, NSW, 2077, Australia.
Anthony J Gill, The University of Sydney, Sydney, NSW, 2006, Australia; NSW Health Pathology, Department of Anatomical Pathology, Royal North Shore Hospital, Sydney, NSW, 2064, Australia; Cancer Diagnosis and Pathology Group, Kolling Institute, Royal North Shore Hospital, Sydney, NSW, 2064, Australia.
Rakesh Iyer, Calvary Public Hospital, Canberra, ACT, 2617, Australia.
Michael Field, Familial Cancer Service, Royal North Shore Hospital, Sydney, NSW, 2065, Australia.
Catherine Luxford, Cancer Genetics Laboratory, Kolling Institute, Royal North Shore Hospital, Sydney, NSW, 2065, Australia; The University of Sydney, Sydney, NSW, 2006, Australia.
Roderick Clifton-Bligh, Cancer Genetics Laboratory, Kolling Institute, Royal North Shore Hospital, Sydney, NSW, 2065, Australia; The University of Sydney, Sydney, NSW, 2006, Australia; Department of Endocrinology, Royal North Shore Hospital, Sydney, NSW, 2064, Australia.
Ann McCormack, Hormones and Cancer Group, Garvan Institute of Medical Research, Sydney, NSW, 2010, Australia; Department of Endocrinology, St. Vincent’s Hospital, Sydney, NSW, 2010, Australia; St. Vincent’s Clinical School, Faculty of Medicine, University of New South Wales, Sydney, NSW, 2010, Australia.
Katherine Tucker, Hereditary Cancer Service, Prince of Wales Hospital, Sydney, NSW, 2064, Australia; Prince of Wales Clinical School, University of New South Wales, Sydney, NSW, 2031, Australia.
Financial Support
No grant support was used for this project.
Disclosures
The authors have no relevant conflicts to disclose.
Data Availability
All data analyzed during this study are included in this published article.
References
- 1. Thakker RV. Multiple endocrine neoplasia type 1 (MEN1) and type 4 (MEN4). Mol Cell Endocrinol. 2014;386(1-2):2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Romanet P, Mohamed A, Giraud S, et al. UMD-MEN1 database: an overview of the 370 MEN1 variants present in 1676 patients from the French population. J Clin Endocrinol Metab. 2019;104(3):753-764. [DOI] [PubMed] [Google Scholar]
- 3. Kooblall KG, Boon H, Cranston T, et al. Multiple endocrine neoplasia type 1 (MEN1) 5′UTR deletion, in MEN1 family, decreases Menin expression. J Bone Miner Res. 2021; 36(1):100-109. [DOI] [PubMed] [Google Scholar]
- 4. Burgess JR, Nord B, David R, et al. Phenotype and phenocopy: the relationship between genotype and clinical phenotype in a single large family with multiple endocrine neoplasia type 1 (MEN 1). Clin Endocrinol (Oxf). 2000;53(2):205-211. [DOI] [PubMed] [Google Scholar]
- 5. Turner JJ, Christie PT, Pearce SH, Turnpenny PD, Thakker RV. Diagnostic challenges due to phenocopies: lessons from multiple endocrine neoplasia type1 (MEN1). Hum Mutat. 2010;31(1):E1089-E1101. [DOI] [PubMed] [Google Scholar]
- 6. Beckers A, Aaltonen LA, Daly AF, Karhu A. Familial isolated pituitary adenomas (FIPA) and the pituitary adenoma predisposition due to mutations in the aryl hydrocarbon receptor interacting protein (AIP) gene. Endocr Rev. 2013;34(2):239-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Sousa SMC, Carroll RW, Henderson A, Burgess J, Clifton-Bligh RJ. A contemporary clinical approach to genetic testing for heritable hyperparathyroidism syndromes. Endocrine. 2022;75(1):23-32. [DOI] [PubMed] [Google Scholar]
- 8. Brandi ML, Agarwal SK, Perrier ND, Lines KE, Valk GD, Thakker RV. Multiple endocrine neoplasia type 1: latest insights. Endocr Rev. 2021;42(2):133-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frederiksen A, Rossing M, Hermann P, Ejersted C, Thakker RV, Frost M. Clinical features of multiple endocrine neoplasia type 4: novel pathogenic variant and review of published cases. J Clin Endocrinol Metab. 2019;104(9):3637-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elston MS, Meyer-Rochow GY, Dray M, Swarbrick M, Conaglen JV. Early onset primary hyperparathyroidism associated with a novel germline mutation in CDKN1B. Case Rep Endocrinol. 2015;2015:510985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pellegata NS, Quintanilla-Martinez L, Siggelkow H, et al. Germ-line mutations in p27 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc Natl Acad Sci U S A.. 2006;103(42):15558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tonelli F, Giudici F, Giusti F, et al. A heterozygous frameshift mutation in exon 1 of CDKN1B gene in a patient affected by MEN4 syndrome. Eur J Endocrinol. 2014;171(2):K7-K17. [DOI] [PubMed] [Google Scholar]
- 13. Alrezk R, Hannah-Shmouni F, Stratakis CA. MEN4 and CDKN1B mutations: the latest of the MEN syndromes. Endocr Relat Cancer. 2017;24(10):T195-T208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Polyak K, Kato JY, Solomon MJ, et al. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994;8(1):9-22. [DOI] [PubMed] [Google Scholar]
- 15. Hengst L, Dulic V, Slingerland JM, Lees E, Reed SI. A cell cycle-regulated inhibitor of cyclin-dependent kinases. Proc Natl Acad Sci U S A. 1994;91(12):5291-5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Polyak K, Lee MH, Erdjument-Bromage H, et al. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78(1):59-66. [DOI] [PubMed] [Google Scholar]
- 17. Russo AA, Jeffrey PD, Patten AK, Massagué J, Pavletich NP. Crystal structure of the p27Kip1 cyclin-dependent-kinase inhibitor bound to the cyclin A-Cdk2 complex. Nature. 1996;382(6589):325-331. [DOI] [PubMed] [Google Scholar]
- 18. Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008;8(4):253-267. [DOI] [PubMed] [Google Scholar]
- 19. Agarwal SK, Mateo CM, Marx SJ. Rare germline mutations in cyclin-dependent kinase inhibitor genes in multiple endocrine neoplasia type 1 and related states. J Clin Endocrinol Metab. 2009;94(5):1826-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee M, Pellegata NS. Multiple endocrine neoplasia type 4. Front Horm Res. 2013;41:63-78. [DOI] [PubMed] [Google Scholar]
- 21. Fritz A, Walch A, Piotrowska K, et al. Recessive transmission of a multiple endocrine neoplasia syndrome in the rat. Cancer Res. 2002;62(11):3048-3051. [PubMed] [Google Scholar]
- 22. Georgitsi M, Raitila A, Karhu A, et al. Germline CDKN1B/p27Kip1 mutation in multiple endocrine neoplasia. J Clin Endocrinol Metab. 2007;92(8):3321-3325. [DOI] [PubMed] [Google Scholar]
- 23. Alevizaki M, Stratakis CA. Multiple endocrine neoplasias: advances and challenges for the future. J Intern Med. 2009;266(1):1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. den Dunnen JT, Dalgleish R, Maglott DR, et al. HGVS recommendations for the description of sequence variants: 2016 update. Hum Mutat. 2016;37(6):564-569. [DOI] [PubMed] [Google Scholar]
- 25. Coordinators NR.. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2016;44(D1):D7-D19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Richards S, Aziz N, Bale S, et al. ; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karczewski KJ, Francioli LC, Tiao G, et al. ; Genome Aggregation Database Consortium. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581(7809):434-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tate JG, Bamford S, Jubb HC, et al. COSMIC: the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2019;47(D1):D941-D947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Sousa SMC, McCabe MJ, Wu K, et al. Germline variants in familial pituitary tumour syndrome genes are common in young patients and families with additional endocrine tumours. Eur J Endocrinol. 2017;176(5):635-644. [DOI] [PubMed] [Google Scholar]
- 30. 1000 Genomes Project Consortium; Auton A, Brooks LD, Durbin RM, et al. A global reference for human genetic variation. Nature. 2015;526(7571):68-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Landrum MJ, Lee JM, Benson M, et al. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018;46(D1):D1062-D1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fokkema IF, Taschner PE, Schaafsma GC, Celli J, Laros JF, den Dunnen JT. LOVD v.2.0: the next generation in gene variant databases. Hum Mutat. 2011;32(5):557-563. [DOI] [PubMed] [Google Scholar]
- 33. Stenson PD, Ball EV, Mort M, Phillips AD, Shaw K, Cooper DN. The Human Gene Mutation Database (HGMD) and its exploitation in the fields of personalized genomics and molecular evolution. Curr Protoc Bioinformatics. 2012;Chapter 1:Unit1.13. [DOI] [PubMed] [Google Scholar]
- 34. Schwartzentruber J, Korshunov A, Liu XY, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482(7384):226-231. [DOI] [PubMed] [Google Scholar]
- 35. COSMIC (Catalogue of Somatic Mutations in Cancer). 2021. [cited 2021 28th June]; https://cancer.sanger.ac.uk/cosmic.
- 36. Bergman L, Boothroyd C, Palmer J, et al. Identification of somatic mutations of the MEN1 gene in sporadic endocrine tumours. Br J Cancer. 2000;83(8):1003-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brock P, Bustamante Alvarez J, Mortazavi A, et al. Co-occurrence of multiple endocrine neoplasia type 4 and spinal neurofibromatosis: a case report. Fam Cancer. 2020;19(2):189-192. [DOI] [PubMed] [Google Scholar]
- 38. Chasseloup F, Pankratz N, Lane J, et al. Germline CDKN1B loss-of-function variants cause pediatric Cushing’s disease with or without an MEN4 phenotype. J Clin Endocrinol Metab. 2020;105(6):1983-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sambugaro S, Di Ruvo M, Ambrosio MR, et al. Early onset acromegaly associated with a novel deletion in CDKN1B 5’UTR region. Endocrine. 2015;49(1):58-64. [DOI] [PubMed] [Google Scholar]
- 40. Molatore S, Marinoni I, Lee M, et al. A novel germline CDKN1B mutation causing multiple endocrine tumors: clinical, genetic and functional characterization. Hum Mutat. 2010;31(11):E1825-E1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tichomirowa MA, Lee M, Barlier A, et al. Cyclin-dependent kinase inhibitor 1B (CDKN1B) gene variants in AIP mutation-negative familial isolated pituitary adenoma kindreds. Endocr Relat Cancer. 2012;19(3):233-241. [DOI] [PubMed] [Google Scholar]
- 42. Occhi G, Regazzo D, Trivellin G, et al. A novel mutation in the upstream open reading frame of the CDKN1B gene causes a MEN4 phenotype. PLoS Genet. 2013;9(3):e1003350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Belar O, De La Hoz C, Pérez-Nanclares G, Castaño L, Gaztambide S; Spanish MEN1 Group. Novel mutations in MEN1, CDKN1B and AIP genes in patients with multiple endocrine neoplasia type 1 syndrome in Spain. Clin Endocrinol (Oxf). 2012;76(5):719-724. [DOI] [PubMed] [Google Scholar]
- 44. Pardi E, Mariotti S, Pellegata NS, et al. Functional characterization of a CDKN1B mutation in a Sardinian kindred with multiple endocrine neoplasia type 4 (MEN4). Endocr Connect. 2015;4(1):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thakker RV, Newey PJ, Walls GV, et al. ; Endocrine Society. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab. 2012;97(9):2990-3011. [DOI] [PubMed] [Google Scholar]
- 46. Eller-Vainicher C, Chiodini I, Battista C, et al. Sporadic and MEN1-related primary hyperparathyroidism: differences in clinical expression and severity. J Bone Miner Res. 2009;24(8):1404-1410. [DOI] [PubMed] [Google Scholar]
- 47. Niederle B, Selberherr A, Bartsch DK, et al. Multiple endocrine neoplasia type 1 and the pancreas: diagnosis and treatment of functioning and non-functioning pancreatic and duodenal neuroendocrine neoplasia within the MEN1 syndrome - an International Consensus Statement. Neuroendocrinology. 2021;111(7):609-630. [DOI] [PubMed] [Google Scholar]
- 48. Gibril F, Chen YJ, Schrump DS, et al. Prospective study of thymic carcinoids in patients with multiple endocrine neoplasia type 1. J Clin Endocrinol Metab. 2003;88(3):1066-1081. [DOI] [PubMed] [Google Scholar]
- 49. Ferolla P, Falchetti A, Filosso P, et al. Thymic neuroendocrine carcinoma (carcinoid) in multiple endocrine neoplasia type 1 syndrome: the Italian series. J Clin Endocrinol Metab. 2005;90(5):2603-2609. [DOI] [PubMed] [Google Scholar]
- 50. Inoue K, Fry EA. Haploinsufficient tumor suppressor genes. Adv Med Biol. 2017;118:83-122. [PMC free article] [PubMed] [Google Scholar]
- 51. Fero ML, Randel E, Gurley KE, Roberts JM, Kemp CJ. The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature. 1998;396(6707):177-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Teixeira LT, Kiyokawa H, Peng XD, Christov KT, Frohman LA, Kineman RD. p27Kip1-deficient mice exhibit accelerated growth hormone-releasing hormone (GHRH)-induced somatotrope proliferation and adenoma formation. Oncogene. 2000;19(15):1875-1884. [DOI] [PubMed] [Google Scholar]
- 53. Fishbein L, Khare S, Wubbenhorst B, et al. Whole-exome sequencing identifies somatic ATRX mutations in pheochromocytomas and paragangliomas. Nat Commun. 2015;6:6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jiao Y, Shi C, Edil BH, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331(6021):1199-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chan CS, Laddha SV, Lewis PW, et al. ATRX, DAXX or MEN1 mutant pancreatic neuroendocrine tumors are a distinct alpha-cell signature subgroup. Nat Commun. 2018; 9(1):4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analyzed during this study are included in this published article.