Abstract
Mineralization of [U-14C]methyl t-butyl ether (MTBE) to 14CO2 without accumulation of t-butyl alcohol (TBA) was observed in surface-water sediment microcosms under denitrifying conditions. Methanogenic activity and limited transformation of MTBE to TBA were observed in the absence of denitrification. Results indicate that bed sediment microorganisms can effectively degrade MTBE to nontoxic products under denitrifying conditions.
Pervasive contamination of groundwater (2, 7, 22–24) and surface-water systems (1, 4, 13, 16, 25) by the fuel oxygenate methyl t-butyl ether (MTBE) makes crucial an accurate understanding of its environmental fate. A drinking water advisory exists for MTBE for taste and odor of 20 to 40 μg/liter (26), and MTBE is classified by the U.S. Environmental Protection Agency as a possible human carcinogen (7, 26). The U.S. Geological Survey national water use summary estimated that 60% of the drinking water consumed in the continental United States comes from surface water (20). Because these systems are not easily shielded from numerous point (5, 9) and non-point (1, 2, 4, 6, 10, 13, 14, 16) sources of MTBE contamination, identifying natural sinks for MTBE in surface waters is particularly important. Although microorganisms in surface-water bed sediments can mineralize MTBE to CO2 under oxic conditions (5), geochemical constraints on oxygen transport (27) may limit the importance of aerobic MTBE mineralization in these systems (5). Evidence for anaerobic MTBE biodegradation in surface-water sediments currently is limited to a single methanogenic microcosm exhibiting incomplete transformation to the toxic product, t-butyl alcohol (TBA) (11). Such findings (5, 11) suggest that efficient anaerobic degradation of MTBE to nontoxic products may require relatively oxidizing terminal electron-accepting conditions.
Of the anaerobic terminal electron-accepting processes common to surface-water environments, denitrification is the most energetically favorable and is widely observed in both pristine and waste-impacted systems (8, 15). The ability of surface-water microorganisms to degrade MTBE under denitrifying conditions was examined in bed sediments from Charleston, S.C., and Pensacola, Fla. At the Charleston site (5), MTBE-contaminated groundwater discharges to a shallow freshwater stream containing poorly sorted sandy bed sediments. Charleston sediments contained significant concentrations of dissolved CH4 (100 ± 15 μM), SO4 (70 ± 10 μM), and sulfide (>200 μM) but no detectable O2 (method detection limit [MDL] < 2 μM), NO3 (MDL < 2 μM), Fe(II) (MDL < 1 μM), or MTBE (MDL = 2 μg/liter). Pensacola sediments were well-sorted medium sands collected from a shallow freshwater wetland with no detectable MTBE (MDL = 2 μg/liter) and no history of MTBE exposure. Pensacola sediments contained significant concentrations of dissolved NO3 (60 ± 10 μM), SO4 (2 ± 0 mM), and CH4 (100 ± 15 μM) but no detectable O2 (MDL < 2 μM) or Fe(II) (MDL < 1 μM).
MTBE mineralization was investigated using [U-14C]MTBE (5). The radiochemical composition (mean ± standard deviation [SD]) of the [U-14C]MTBE stock was evaluated in our lab by radiometric detection high-performance liquid chromatography and gas chromatography and found to be 97.4% ± 0.3% as [14C]MTBE and 2.6% ± 0.2% as [14C]TBA. The presence of TBA as a trace contaminant in commercially available MTBE is not uncommon (P. M. Bradley, unpublished results) and must be considered when evaluating the significance of TBA as an intermediate in MTBE biodegradation. Bed sediment microcosms were prepared as described previously (5) and were composed of 5 ml of saturated sediment and an atmosphere of air (oxic treatment) or helium (anoxic treatment) in 10-ml serum vials. An anoxic KNO3 solution was added to NO3-amended treatments to yield initial dissolved NO3 concentrations of 4.6 ± 0.1 mM and 7.3 ± 0.2 mM in the Charleston and Pensacola microcosms, respectively. All microcosms were amended with 0.5 μCi of [U-14C]MTBE (specific activity, 10.1 mCi/mmol) to yield initial dissolved MTBE concentrations of 17.2 ± 0.3 μM and 19.9 ± 0.2 μM in the Charleston and Pensacola treatments, respectively. Headspace concentrations were monitored periodically using radiometric detection gas chromatography combined with thermal conductivity detection. The radioactivity associated with C1 to C4 organic acids, TBA, and MTBE was assessed using radiometric detection high-performance liquid chromatography. NO3 and SO4 concentrations were determined by ion chromatography. Radiometric detectors were calibrated by liquid scintillation counting using H14CO3 and [U-14C]MTBE. The results presented below in Table 2 and Fig. 1 were corrected for losses due to sampling.
TABLE 2.
Final distribution of 14C radioactivity in anoxic bed sediment microcosms after 77 daysa
| Sediment source | Treatment | Compound |
14C distribution (%)
|
|
|---|---|---|---|---|
| Experimental | Control | |||
| Charleston | NO3 amended | MTBE | 70 ± 1 | 97 |
| TBA | NDb | 3 | ||
| CO2 | 26 ± 10 | ND | ||
| CH4 | ND | ND | ||
| Total recovery | 96 ± 10 | 100 | ||
| Unamended | MTBE | 85 ± 5 | 91 | |
| TBA | 10 ± 2 | 3 | ||
| CO2 | 3 ± 3 | ND | ||
| CH4 | 1 ± 1 | ND | ||
| Total recovery | 99 ± 5 | 94 | ||
| Pensacola | NO3 amended | MTBE | 71 ± 4 | 94 |
| TBA | 1 ± 1 | 4 | ||
| CO2 | 23 ± 5 | ND | ||
| CH4 | ND | ND | ||
| Total recovery | 95 ± 5 | 98 | ||
| Unamended | MTBE | 78 ± 4 | 101 | |
| TBA | 9 ± 2 | 4 | ||
| CO2 | 5 ± 2 | ND | ||
| CH4 | 5 ± 1 | ND | ||
| Total recovery | 97 ± 4 | 105 | ||
No radiolabeled C1 to C4 organic acids were detected in this study. For each treatment, experimental data are means ± SD for triplicate microcosms, and control data are from a single microcosm.
ND, not detected. The MDL for radiometric detection was equivalent to 1% final recovery.
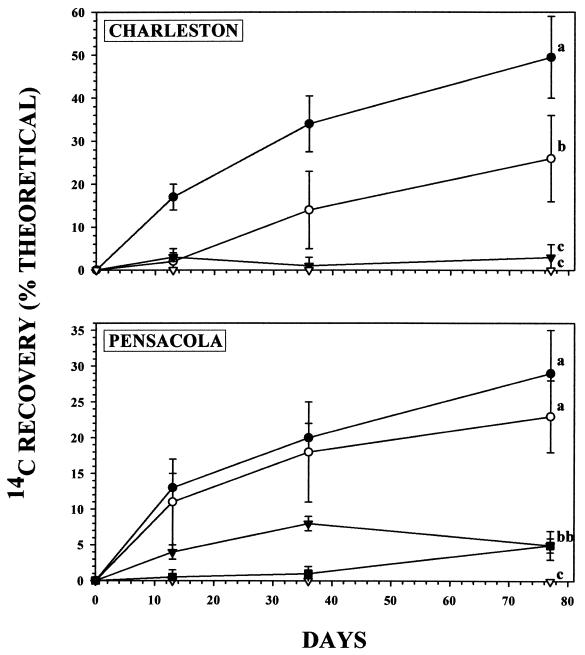
FIG. 1.
Percent mineralization of [U-14C]MTBE to 14CO2 or 14CH4 in microcosms containing bed sediments from the Charleston and Pensacola sites. The symbols indicate 14CO2 in the oxic (●), anoxic (▾), NO3-amended anoxic (○), and control (▿) treatments and 14CH4 (■) in the anoxic Pensacola treatment. Experimental data are means ± SD for triplicate microcosms, and the control data are from a single autoclaved control microcosm. For each sediment, superscript letters adjacent to final data points indicate statistically significantly different final mean 14C recoveries according to the Kruskal-Wallis one-way analysis of variance on ranks (P < 0.05).
The observed production of N2-N (Table 1) and the lack of detectable SO4 reduction or methanogenesis indicated that, under NO3-amended anoxic conditions, both sediments were dominated by denitrification. The fact that NO3-N loss and N2-N production were not statistically different confirmed that denitrification was the primary sink for NO3 under these conditions (Table 1). Under unamended anoxic conditions, production of N2-N also was observed in Pensacola sediments over the first 36 days but production was insignificant between 36 and 77 days (Table 1). SO4 reduction was substantial (42% ± 8% decrease in dissolved SO4 concentrations over 77 days), and methanogenesis (1.5 ± 0.3 nmol of CH4 · g−1 · day−1) became significant between 36 and 77 days. These observations indicate that, in the absence of added NO3, anoxic Pensacola sediments shifted from denitrifying conditions to predominantly SO4-reducing and methanogenic conditions. In contrast, under unamended anoxic conditions, the Charleston sediments were characterized by insignificant denitrification (Table 1), trace SO4-reducing activity (data not shown), and extensive methanogenesis (19 ± 6 nmol of CH4 · g−1 · day−1, expressed per gram of sediment [dry weight]).
TABLE 1.
Final amount of NO3-N consumed and N2-N produced after 77 days in anoxic microcosms containing bed sediment from the Charleston and Pensacola sitesf
| Sediment source | Anaerobic treatment | NO3-N consumeda (μmol) | N2-N producedb (μmol) |
|---|---|---|---|
| Charleston | NO3 amended | 5.4 ± 0.5 | 5.6 ± 0.8 |
| Unamended | —c | —d | |
| Pensacola | NO3 amended | 2.3 ± 1.4 | 3.2 ± 0.7 |
| Unamended | 0.1 ± 0.0 | 0.3 ± 0.2e |
No changes in dissolved NO3 concentrations were observed in sterile control microcosms.
N2-N level produced in experimental treatments minus that in sterile control microcosms.
No NO3 (MDL = 2 μM) was detected in unamended Charleston sediments.
N2-N production did not differ significantly from that observed in sterile control microcosms.
Produced in the first 36 days of incubation.
Data are means ± SD for triplicate microcosms. Transient production of N2O was observed for both sediments in NO3-amended microcosms, but no N2O was detected in any treatment at the end of the incubation period.
The microorganisms indigenous to both sediments demonstrated efficient mineralization of [U-14C]MTBE under NO3-amended, denitrifying conditions (Fig. 1). Approximately 25% of the [U-14C]MTBE radioactivity was recovered as 14CO2 in 77 days. The mineralization of [U-14C]MTBE observed in this study under NO3-amended, anoxic conditions was comparable to that observed under oxic conditions (Fig. 1). The fact that the final combined recovery of [U-14C]MTBE and 14CO2 in the experimental microcosms did not differ significantly from the final recovery of radioactivity in autoclaved control microcosms (≥98%) indicates that 14CO2 was the only significant product of MTBE biodegradation under denitrifying conditions (Table 2). [U-14C]MTBE mineralization was attributable to biological activity, because the final recovery of 14CO2 in killed control microcosms was less than 1% (Table 2). In earlier studies, the potential for anaerobic MTBE biodegradation under NO3-enriched conditions was investigated in soils (28) and aquifer sediments (3, 11) and was reported to be insignificant. In contrast, the results of this study demonstrate that microorganisms indigenous to surface-water bed sediments can mineralize MTBE under denitrifying conditions.
The results of this study also indicate that bed sediment microorganisms can mineralize TBA under denitrifying conditions (Table 2). TBA contamination of surface water is an environmental concern due to its presence in gasoline spills (7, 24), demonstrated carcinogenicity in laboratory animals, and significance as the presumptive initial intermediate in microbial degradation of MTBE (11, 17, 28). In the present study, no significant recovery of [14C]TBA was observed in NO3-amended, experimental microcosms in spite of the fact that approximately 3% of the radioactivity in the original stock was [14C]TBA (Table 2). In contrast, the recovery of radiolabel as [U-14C]MTBE and [14C]TBA in autoclaved control microcosms was the same as that observed in the original added substrate (Table 2). The results are consistent with previous reports of enhanced biodegradation of TBA in soil samples under NO3-amended conditions (28) and demonstrate that the microorganisms indigenous to surface-water bed sediments can oxidize [14C]TBA to 14CO2 under denitrifying conditions.
A number of observations indicate that NO3 availability was a primary determinant of the efficiency and the products of anaerobic [U-14C]MTBE biodegradation in this study. First, NO3 amendment stimulated denitrification (Table 1) and MTBE biodegradation (Table 2) in both sediments. Second, mineralization of [U-14C]MTBE to 14CO2 only occurred if dissolved NO3 concentrations were significant (Table 2). In NO3-amended, denitrification-dominated microcosms, 14CO2 was the sole product of [U-14C]MTBE biodegradation (Table 2). For the unamended, anoxic Pensacola sediments, the percentage of radioactivity recovered as 14CO2 increased during the initial period of denitrification (first 36 days) but decreased from 36 to 77 days as methanogenesis became significant (Fig. 1 and Table 1). The simultaneous decrease in 14CO2 and increase in 14CH4 indicate that 14CH4 was formed autotrophically at the expense of 14CO2 (Table 1 and Fig. 1). Finally, accumulation of [14C]TBA was not observed in this study under denitrifying conditions. [14C]TBA was oxidized to 14CO2 under NO3-amended conditions but increased in the absence of significant denitrifying activity (Table 2). Accumulation of TBA during biodegradation of MTBE under methanogenic conditions has been reported previously (11). The present results indicate that denitrification and anaerobic MTBE mineralization were limited by NO3 availability and suggest that MTBE mineralization was coupled to denitrification.
The demonstrated ability of naturally occurring microorganisms to degrade MTBE to CO2 under anoxic conditions without the accumulation of TBA has important implications for the fate of MTBE in groundwater and surface-water systems. Although the potential for aerobic biodegradation of MTBE to nontoxic products has been demonstrated for a number of groundwater (3, 9, 18) and surface-water sites (5), the actual contribution of this process to natural attenuation of MTBE is unclear because the onset and subsequent predominance of anoxic conditions are characteristics of hydrocarbon-contaminated waters. Consequently, engineered systems are being developed to support aerobic MTBE biodegradation under otherwise anoxic conditions (18). Unfortunately, recent groundwater and surface-water quality assessments indicate that environmental MTBE contamination is so widespread (1, 2, 4, 7, 13, 16, 22–25) that engineered solutions are realistic only for a small percentage of contaminated sites. For the remaining sites where natural attenuation would be expected to be the primary method for environmental restoration, identifying the conditions which support efficient anaerobic degradation of MTBE to nontoxic products is crucial. The demonstrated ability of bed sediment microorganisms to mineralize MTBE under denitrifying conditions indicates that anaerobic biodegradation of MTBE can be a significant contributor to the natural attenuation of MTBE in the environment. Because dissolved NO3 concentrations are typically low (<10 μM) in uncontaminated groundwater (12), denitrification-associated MTBE biodegradation would be expected to be limited except in NO3-contaminated aquifers or under engineered conditions. However, because NO3 concentrations and denitrifying activity are often substantial in natural as well as waste-impacted surface waters (8, 15, 19, 21), these results hold considerable promise for the natural attenuation of MTBE in surface-water systems. Combined with previous demonstrations of a potential for aerobic MTBE mineralization in surface waters (5), these results indicate that bed sediment microbial processes represent a potentially important sink for MTBE in oxic and anoxic surface-water environments.
REFERENCES
- 1.Baehr A L, Zapecza O S. Methyl tert-butyl ether (MTBE) and other volatile organic compounds in lakes in Byram Township, Sussex County, New Jersey, summer 1998. USGS Water Resources Investigation WRI-98–4264. U.S. West Trenton, N.J: Geological Survey; 1998. [Google Scholar]
- 2.Baehr A L, Stackelberg P E, Baker R J. Evaluation of the atmosphere as a source of volatile organic compounds in shallow groundwater. Water Resour Res. 1999;35:127–136. [Google Scholar]
- 3.Borden R C, Daniel R A, LeBrun IV L E, Davis C W. Intrinsic biodegradation of MTBE and BTEX in a gasoline-contaminated aquifer. Water Resour Res. 1997;33:1105–1115. [Google Scholar]
- 4.Boughton C J, Lico M S. Volatile organic compounds in Lake Tahoe, Nevada and California, July-September 1997. USGS Fact Sheet FS-055-98. U.S. Reston, Va: Geological Survey; 1998. [Google Scholar]
- 5.Bradley P M, Landmeyer J E, Chapelle F H. Aerobic mineralization of MTBE and tert-butyl alcohol by stream-bed-sediment microorganisms. Environ Sci Technol. 1999;33:1877–1879. [Google Scholar]
- 6.Delzer G C, Zogorski J S, Lopes T J, Bosshart R L. Occurrence of the gasoline oxygenate MTBE and BTEX compounds in urban stormwater in the United States, 1991–1995. USGS Water Resources Investigation WRI-96–4145. Rapid City, S.D: U.S. Geological Survey; 1996. [Google Scholar]
- 7.Johnson R, Pankow J, Bender D, Price C, Zogorski J. MTBE, to what extent will past releases contaminate community water supply wells? Environ Sci Technol. 2000;34:210A–217A. doi: 10.1021/es003268z. [DOI] [PubMed] [Google Scholar]
- 8.Knowles R. Denitrification. Microbiol Rev. 1982;46:43–70. doi: 10.1128/mr.46.1.43-70.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landmeyer J E, Chapelle F H, Bradley P M, Pankow J F, Church C D, Tratnyek P G. Fate of MTBE relative to benzene in a gasoline-contaminated aquifer (1993-98) Ground Water Monit Remediation. 1998;18:93–102. [Google Scholar]
- 10.Lopes T J, Dionne S G. A review of semivolatile and volatile organic compounds in highway runoff and urban stormwater. USGS Open File Report OF-98-0409. U.S. Reston, Va: Geological Survey; 1998. [Google Scholar]
- 11.Mormile M R, Liu S, Suflita J M. Anaerobic biodegradation of gasoline oxygenates: extrapolation of information to multiple sites and redox conditions. Environ Sci Technol. 1994;28:1727–1732. doi: 10.1021/es00058a026. [DOI] [PubMed] [Google Scholar]
- 12.Nolan B T, Stoner J D. Nutrients in groundwaters of the conterminous United States, 1992–1995. Environ Sci Technol. 2000;34:1156–1165. [Google Scholar]
- 13.O'Brien A K, Reiser R G, Gylling H. Spatial variability of volatile organic compounds in streams on Long Island, New York, and in New Jersey. USGS Fact Sheet FS-0194-97. U.S. West Trenton, N.J: Geological Survey; 1997. [Google Scholar]
- 14.Pankow J F, Thomson N R, Johnson R L, Baehr A L, Zogorski J S. The urban atmosphere as a non-point source for the transport of MTBE and other volatile organic compounds (VOCs) to shallow groundwater. Environ Sci Technol. 1997;31:2821–2828. [Google Scholar]
- 15.Payne W J. Denitrification. New York, N.Y: John Wiley and Sons; 1981. pp. 149–161. [Google Scholar]
- 16.Reuter J E, Allen B C, Richards R C, Pankow J F, Goldman C R, Scholl R L, Seyfried J S. Concentrations, sources, and fate of the gasoline oxygenate methyl tert-butyl ether (MTBE) in a multiple-use lake. Environ Sci Technol. 1998;32:3666–3672. [Google Scholar]
- 17.Salanitro J P, Diaz L A, Williams M P, Wisniewski H L. Isolation of a bacterial culture that degrades methyl t-butyl ether. Appl Environ Microbiol. 1994;60:2593–2596. doi: 10.1128/aem.60.7.2593-2596.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salanitro J P, Johnson P C, Spinnler G E, Maner P M, Wisniewski H L, Bruce C. Field-scale demonstration of enhanced MTBE bioremediation through aquifer bioaugmentation and oxygenation. Environ Sci Technol. 2000;34:4152–4162. [Google Scholar]
- 19.Smith R A, Alexander R B, Lanfear K J. Stream water quality in the conterminous United States—status and trends of selected indicators during the 1980's. USGS Water Supply Paper 2400. U.S. Reston, Va: Geological Survey; 1994. [Google Scholar]
- 20.Solley W B, Pierce R R, Perlman H A. Estimated use of water in the United States in 1995. USGS Circular 1200. U.S. Reston, Va: Geological Survey; 1998. [Google Scholar]
- 21.Sprent J I. The ecology of the nitrogen cycle. Cambridge, United Kingdom: Cambridge University Press; 1987. pp. 101–118. [Google Scholar]
- 22.Squillace P J, Pope D A, Price C V. Occurrence of the gasoline additive MTBE in shallow ground water in urban and agricultural areas. USGS Fact Sheet FS-114-95. U.S. Reston, Va: Geological Survey; 1995. [Google Scholar]
- 23.Squillace P J, Zogorski J S, Wilber W G, Price C V. Preliminary assessment of the occurrence and possible sources of MTBE in groundwater in the United States, 1993–1994. Environ Sci Technol. 1996;30:1721–1730. [Google Scholar]
- 24.Squillace P J, Moran M J, Lapham W W, Price C V, Clawges R M, Zogorski J S. Volatile organic compounds in untreated ambient groundwater of the United States, 1985–1995. Environ Sci Technol. 1999;33:4176–4187. [Google Scholar]
- 25.Terracciano S A, O'Brien A K. Occurrence of volatile organic compounds in streams on Long Island, New York, and New Jersey; overview of available data and reconnaissance sampling. USGS Fact Sheet FS-0063-97. U.S. West Trenton, N.J: Geological Survey; 1997. [Google Scholar]
- 26.U.S. Environmental Protection Agency. Drinking water advisory. Consumer acceptability advice and health effects analysis on methyl tertiary-butyl ether. EPA-822-F-97-009. U.S. Washington, D.C.: Environmental Protection Agency; 1997. [Google Scholar]
- 27.Woessner W W. Stream and fluvial plain ground water interactions: rescaling hydrogeologic thought. Ground Water. 2000;38:423–429. [Google Scholar]
- 28.Yeh C K, Novak J T. Anaerobic biodegradation of gasoline oxygenates in soils. Water Environ Res. 1994;66:744–752. [Google Scholar]