Abstract
Chemotherapy-induced cognitive impairment (CICI) has emerged as a significant medical problem without therapeutic options. Using the platinum-based chemotherapy cisplatin to model CICI, we revealed robust elevations in the adenosine A2A receptor (A2AR) and its downstream effectors, cAMP and CREB, by cisplatin in the adult mouse hippocampus, a critical brain structure for learning and memory. Notably, A2AR inhibition by the Food and Drug Administration–approved A2AR antagonist KW-6002 prevented cisplatin-induced impairments in neural progenitor proliferation and dendrite morphogenesis of adult-born neurons, while improving memory and anxiety-like behavior, without affecting tumor growth or cisplatin’s antitumor activity. Collectively, our study identifies A2AR signaling as a key pathway that can be therapeutically targeted to prevent cisplatin-induced cognitive impairments.
Keywords: chemotherapy-induced cognitive impairment, chemobrain, adenosine A2A receptor, KW-6002, adult neurogenesis
Chemotherapy has improved survival for millions of cancer patients. However, patients report dysfunctional learning, memory, and mood during and following chemotherapy. Chemotherapy-induced cognitive impairment (CICI or chemobrain) can affect up to 60% of cancer survivors, negatively impacting quality of life (1, 2). There are no treatments due to a lack of understanding of pathophysiological mechanisms driving CICI.
Using cisplatin to model CICI (3), we performed RNA sequencing in the mouse hippocampus to identify novel molecular contributors and found profound up-regulation of the adenosine A2A receptor (A2AR) by cisplatin (Fig. 1A), potentially representing a mechanism for cisplatin-induced neurotoxicity. Adenosine maintains central nervous system homeostasis through G protein-coupled (A1R, A2AR, A2BR, A3R) adenosine receptor neurotransmission (4). Notably, clinical studies report high levels of A2AR expression in normal aging and Alzheimer’s disease (5, 6), while selective pharmacological or optogenetic A2AR activation impairs memory and synaptic plasticity (7, 8). Conversely, selective pharmacological or genetic A2AR inhibition improves synaptic plasticity, learning, and memory in aging and Alzheimer’s disease models (5, 9, 10), thus suggesting that increased levels of A2AR may contribute to cognitive impairments. Given the similarities between increased A2AR expression in neurodegeneration and in our CICI mouse model, we sought to determine whether A2AR inhibition therapeutically prevented CICI.
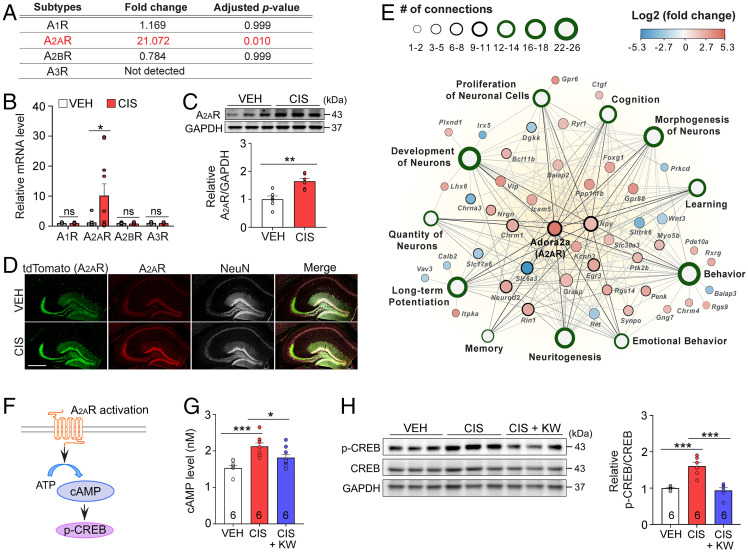
Fig. 1.
Cisplatin elevates adenosine A2AR expression. (A–C) RNA sequencing (A) of adenosine family receptors in the adult mouse hippocampus treated with cisplatin (CIS) or vehicle (VEH). Increased A2AR expression by qRT-PCR (B) and Western blot (C). (D) Confocal micrographs showing neuronal A2AR expression in the hippocampus from A2A-Cre;Ai9tdTomato mice. (Scale bar: 400 µm.) (E) Ingenuity Pathway Analysis network mapped A2AR with enriched gene pathways. (F) Hypothesized mechanism of cisplatin-induced neurotoxicity. (G and H) KW-6002 attenuates cisplatin-induced elevation of cAMP (G) and CREB phosphorylation (H). Results reported in mean ± SEM *P < 0.05, **P < 0.01, ***P < 0.001, using two-tailed Student’s t test (B and C) or one-way ANOVA, Dunnett’s post hoc (G and H). n = 3 mice per group (A and E), 10 mice per group (B), 6 mice per group (C), and 6 mice per group (G and H). ns, not statistically significant.
Results and Discussion
RNA sequencing from the adult mouse hippocampus identified 85 up-regulated and 24 down-regulated genes after cisplatin administration (log2 fold change ±2.0; adjusted P value ≤ 0.05; Fig. 1A and Dataset S1). The A2AR gene was among the top five genes increased by cisplatin without affecting other adenosine receptor subtypes. qRT-PCR and Western blot confirmed increased A2AR expression (Fig. 1B and C). To visualize cell type-specific A2AR expression, we utilized A2AR-Cre mice with Ai9 mice expressing tdTomato (A2A-Cre;Ai9-mice) and immunohistochemistry with A2AR-specific antibodies, finding that cisplatin preferentially up-regulated A2AR in hippocampal neurons (Fig. 1D). Ingenuity Pathway Analysis further revealed that increased A2AR mapped to biological pathways associated with long-term potentiation, learning, memory, and cognition, as well as neuronal development, morphogenesis, and proliferation (Fig. 1E), suggesting that A2AR dysregulation by cisplatin may underlie cisplatin-induced cognitive impairment. Mechanistically, downstream of the A2AR, cisplatin significantly increased cAMP production and CREB phosphorylation (Fig. 1F–H). Remarkably, these effects were abolished by the Food and Drug Administration–approved selective A2AR antagonist KW-6002, indicating that cisplatin-mediated cAMP–CREB activation depends on A2AR signaling. Although the underlying mechanisms linking cisplatin to increased A2AR density are unknown, one possibility may be epigenetically through A2AR gene regulation (11).
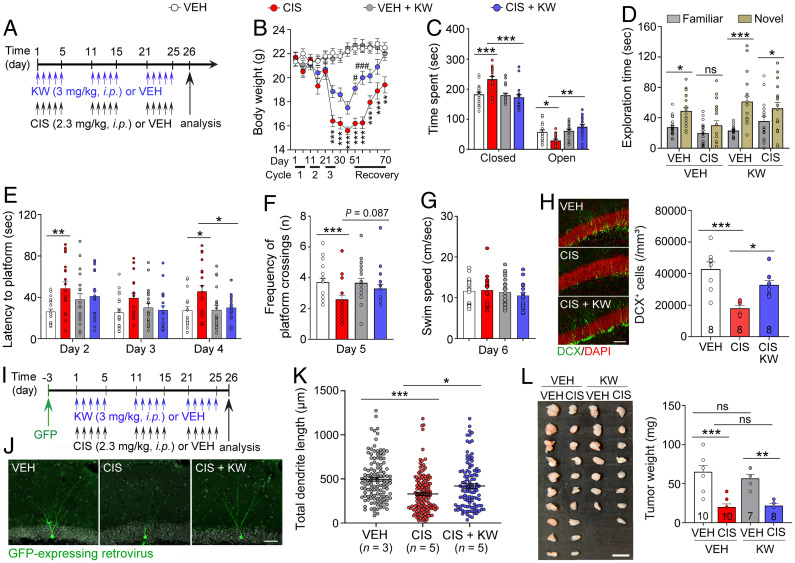
Next, to test whether A2AR inhibition could prevent cisplatin-induced cognitive impairments, adult female mice were pretreated with KW-6002 (3 mg/kg, intraperitoneally [i.p.]) followed by cisplatin or vehicle administration 2 h later (Fig. 2A). We found that cisplatin reduced body weight, while KW-6002 pretreatment in cisplatin-treated mice promoted faster body-weight recoveries relative to vehicle (Fig. 2B), suggesting that KW-6002 protected against cisplatin-induced body-weight loss. Elevated plus maze assessment found that cisplatin-treated mice spent more time in the closed arms and less time in the open arms, while KW-6002 pretreatment reversed this in cisplatin-treated mice, suggesting that KW-6002 prevents cisplatin-induced anxiety-like behavior (Fig. 2C). We then examined novel object recognition memory. Replacement of a familiar object with a novel object (day 3; Fig. 2D) showed that cisplatin decreased novel object exploration, while KW-6002 pretreatment significantly increased novel object exploration in cisplatin-treated mice, suggesting that A2AR inhibition by KW-6002 prevents cisplatin-induced memory deficits. Using the Morris water maze (MWM), we found that in comparison to vehicle alone, cisplatin-treated mice displayed longer platform escape latencies, suggesting that cisplatin impairs learning (Fig. 2E). KW-6002 pretreatment significantly prevented cisplatin-induced learning impairments, as these mice exhibited decreased platform escape latencies on day 4 (Fig. 2E). During probe testing (day 5; Fig. 2F), in comparison to vehicle-alone, cisplatin-treated mice had significantly lower platform crossing frequencies, while KW-6002 pretreatment increased crossings when compared to cisplatin alone. Although not statistically significant, it suggests KW-6002 provides a modest improvement in spatial memory. There were no swim speed differences (day 6) between the treatment groups (Fig. 2G), indicating that poor MWM performance was due to cisplatin-induced cognitive detriments and not impaired physicality.
Fig. 2.
KW-6002 prevents cisplatin-induced impairments in cognition and neurogenesis without affecting tumor growth. (A) Timeline of cisplatin (CIS) or KW-6002 (KW) treatment. (B) Body-weight measurement. (C) Time spent in the closed vs. open arm of the elevated plus maze. (D) Novel object recognition. Time exploring between the familiar vs. novel object on day 3. (E) MWM hidden-platform escape latencies. (F) Target zone platform crossing frequency during MWM probe trial. (G) MWM swim-speed during day 6. (H) Representative images showing KW-6002 preventative neuroprotection against cisplatin-induced reduction in DCX+ newborn neurons (green). DAPI, red. (Scale bar: 100 µm.) (I) Schematic of GFP+ retrovirus injections and administration of cisplatin or KW-6002. (J) Representative images of GFP+ adult-born neuron and (K) quantitative analysis of total dendrite length. DAPI, gray. Circles represent an individual dendrite. (Scale bar: 50 µm.) (L) Tumor weight comparison of SCID mice MCF-7 xenograft tumors. (Scale bar: 1 cm.) Results reported in mean ± SEM *P < 0.05, **P < 0.01, ***P < 0.001, using repeated measures (RM) two-way ANOVA, Tukey’s post hoc (B), two-way ANOVA, Tukey’s post hoc (C), two-way ANOVA, Bonferroni’s post hoc (D), RM two-way ANOVA, Dunnett’s post hoc (E), one-way ANOVA, Dunnett’s post hoc (F and G), one-way ANOVA, Tukey’s post hoc (H, K, and L). n = 9 or 10 mice per group (B), 19 or 20 mice per group (C–G), 8 mice per group (H), 3 to 5 mice per group (K), and 7 to 10 mice per group (L). ns, not statistically significant.
We previously demonstrated that cisplatin impairs adult hippocampal neurogenesis, a process that generates adult-born neurons, leading to cognitive impairment (3). Consequently, immunostaining with the immature neuron marker doublecortin (DCX+) revealed that although cisplatin suppressed DCX+ expression, KW-6002 significantly attenuated cisplatin-induced decreases of DCX+ neurons in the hippocampal dentate gyrus (Fig. 2H). Additionally, using green fluorescent protein (GFP) retroviral expression to visualize adult-born dendrite morphogenesis (12), we found that while cisplatin decreased total dendrite length, KW-6002 significantly prevented cisplatin-induced dendrite length reductions (Fig. 2I–K). Collectively, these results suggest KW-6002 confers neuroprotection against cisplatin-induced neurogenesis defects in the adult mouse hippocampus. However, further studies are needed to determine whether the effect of KW-6002 on memory mechanistically depends on hippocampal A2AR.
To ensure that KW-6002 did not have aberrant effects on tumor growth or impair cisplatin’s antitumor activity, adult severe combined immunodeficiency (SCID) female mice were implanted with estrogen receptor-positive (MCF-7) breast cancer cell lines followed by KW-6002 and cisplatin administration. KW-6002 neither promoted tumor growth nor interrupted the antitumor activity of cisplatin (Fig. 2L), thus ensuring the safety profile of KW-6002.
Our study indicates that A2AR signaling dysregulation plays a critical role in cisplatin-induced cognitive impairment. Notably, KW-6002–mediated A2AR antagonism significantly attenuates cisplatin-induced impairments in neurogenesis. However, while we only tested neurogenesis as a potential mechanism mediating CICI, other cellular mechanisms such as synaptic plasticity may contribute to cognitive impairments (13). Given that CICI severely hinders quality of life in ∼14 million cancer patients, targeting the adenosine A2AR may represent a novel, safe therapeutic strategy for cisplatin-induced cognitive impairments, thus improving quality of life in cancer survivors.
Materials and Methods
Unless otherwise specified, cisplatin (2.3 mg/kg i.p.) and KW-6002 (3 mg/kg i.p.) administration was performed on 3- to 4-mo-old female C57BL/6J mice. Biochemical, including RNA-sequencing and pathway analysis, as well as immunohistology and behavior testing was performed 24 h following three or four cycles of treatment. Memory (MWM, novel object recognition) and anxiety (elevated plus maze) testing was performed according to standard protocols as previously described (3). Detailed extended methods are provided in SI Appendix.
Mouse experiments were conducted in accordance with NIH guidance on the care and use of laboratory animals. All procedures were approved by the Mayo Clinic Institutional Animal Care and Use Committee (IACUC protocol nos. A00005043 and A00004190).
Supplementary Material
Acknowledgments
We thank Dr. Jiang-Fan Chen for providing A2AR-Cre mice. This work was supported by NIH (R01CA242158 and R01AG058560), Regenerative Medicine Minnesota (RMM091718DS005), and a Rutgers Cancer Institute of New Jersey (CINJ) survivorship award to M.-H.J. Support to A.O. was provided by the American Association for Cancer Research-Bosarge Family Foundation-Waun Ki Hong Scholar Regenerative Cancer Medicine Award (19-40-60-OLIV) and the Rutgers CINJ Pediatric Cancer and Blood Disorders Research Center.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2206415119/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information.
References
- 1.Lange M., et al. , Cancer-related cognitive impairment: An update on state of the art, detection, and management strategies in cancer survivors. Ann. Oncol. 30, 1925–1940 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koppelmans V., et al. , Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J. Clin. Oncol. 30, 1080–1086 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Yoo K. H., et al. , Nicotinamide mononucleotide prevents cisplatin-induced cognitive impairments. Cancer Res. 81, 3727–3737 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J. F., Adenosine receptor control of cognition in normal and disease. Int. Rev. Neurobiol. 119, 257–307 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Temido-Ferreira M., et al. , Age-related shift in LTD is dependent on neuronal adenosine A2A receptors interplay with mGluR5 and NMDA receptors. Mol. Psychiatry 25, 1876–1900 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orr A. G., et al. , Astrocytic adenosine receptor A2A and Gs-coupled signaling regulate memory. Nat. Neurosci. 18, 423–434 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li P., et al. , Optogenetic activation of intracellular adenosine A2A receptor signaling in the hippocampus is sufficient to trigger CREB phosphorylation and impair memory. Mol. Psychiatry 20, 1481 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Li X. C., et al. , Blockade of adenosine A2A receptor alleviates cognitive dysfunction after chronic exposure to intermittent hypoxia in mice. Exp. Neurol. 350, 113929 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Li Z., et al. , The corticostriatal adenosine A2A receptor controls maintenance and retrieval of spatial working memory. Biol. Psychiatry 83, 530–541 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Laurent C., et al. , A2A adenosine receptor deletion is protective in a mouse model of Tauopathy. Mol. Psychiatry 21, 149 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Buira S. P., et al. , DNA methylation and Yin Yang-1 repress adenosine A2A receptor levels in human brain. J. Neurochem. 115, 283–295 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Jang M. H., et al. , Secreted frizzled-related protein 3 regulates activity-dependent adult hippocampal neurogenesis. Cell Stem Cell 12, 215–223 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunha R. A., How does adenosine control neuronal dysfunction and neurodegeneration? J. Neurochem. 139, 1019–1055 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.