Significance
The future of forests is crucial for the Earth system, yet anticipating and detecting forest change is challenging because trees are long lived, and stand development takes many decades. We suggest that it is not necessary to wait for such extended time periods to see forest changes unfold. Rather, altered dynamics can be anticipated by focusing on the reorganization phase, the short but critical period after disturbance when “the deck is reshuffled,” because early stand development pathways are often locked in for decades to centuries. We present a synthetic framework for studying forest reorganization in a changing world and propose a research agenda to better understand and predict forest change.
Keywords: forest disturbance, recovery, tree regeneration, forest structure, forest composition
Abstract
Forest ecosystems are strongly impacted by continuing climate change and increasing disturbance activity, but how forest dynamics will respond remains highly uncertain. Here, we argue that a short time window after disturbance (i.e., a discrete event that disrupts prevailing ecosystem structure and composition and releases resources) is pivotal for future forest development. Trees that establish during this reorganization phase can shape forest structure and composition for centuries, providing operational early indications of forest change. While forest change has been fruitfully studied through a lens of resilience, profound ecological changes can be masked by a resilience versus regime shift dichotomy. We present a framework for characterizing the full spectrum of change after disturbance, analyzing forest reorganization along dimensions of forest structure (number, size, and spatial arrangement of trees) and composition (identity and diversity of tree species). We propose four major pathways through which forest cover can persist but reorganize following disturbance: resilience (no change in structure and composition), restructuring (structure changes but composition does not), reassembly (composition changes but structure does not), and replacement (structure and composition both change). Regime shifts occur when vegetation structure and composition are altered so profoundly that the emerging trajectory leads to nonforest. We identify fundamental processes underpinning forest reorganization which, if disrupted, deflect ecosystems away from resilience. To understand and predict forest reorganization, assessing these processes and the traits modulating them is crucial. A new wave of experiments, measurements, and models emphasizing the reorganization phase will further the capacity to anticipate future forest dynamics.
The biosphere is undergoing rapid transformation as global climate change increasingly alters ecosystem structure and composition (1, 2). In response to more-frequent and severe disturbances, forests are becoming younger and more open (3). Furthermore, climatic extremes increasingly favor trees with lower stature (4). Forest tree species composition is shifting toward more warm-adapted and drought-tolerant species at local to global scales (5, 6). Important thresholds could be crossed as a consequence of these ongoing changes, resulting in regime shifts in the Earth system (7, 8). Understanding ongoing change and anticipating future change in ecosystems is one of the most pressing issues of contemporary ecology.
In many ecological systems, change happens in pulses that interrupt phases of relative stability. Theory suggests that ecosystems continually shift through an adaptive cycle consisting of the phases of exploitation, conservation, release, and reorganization (9). Whereas change is gradual in the exploitation and conservation phases, disturbances [here defined as discrete events, such as wildfires or disease outbreaks, that disrupt prevailing ecosystem structure and composition (10)] trigger an abrupt release of resources and catalyze change. The reorganization phase is a relatively short window of time after disturbance in which a system either renews itself (i.e., follows a development trajectory similar to the one before the disturbance) or changes to a different trajectory that leads to the emergence of an altered ecosystem. Once the ecosystem has reorganized and moves into the exploitation and conservation phases, the propensity for fundamental change [i.e., regime shifts sensu Andersen et al. (8)] declines rapidly—the system is increasingly locked into its trajectory (11, 12). This process of post-disturbance reorganization and lock-in is particularly pronounced for ecosystems dominated by sessile, long-lived species, such as trees: Individuals that establish in the first years after a disturbance often determine forest structure and composition for decades and centuries to come, frequently until another disturbance occurs. Thus, the reorganization phase is a critical window that determines the occurrence, direction, and magnitude of forest change (13, 14). Studying the patterns and processes of ecosystem reorganization can help to contextualize observed changes in the environment, answering central questions such as: is the current period of accelerated ecosystem dynamics a transient phase of turnover that will return to systems similar to those of the past, or is the current development the first step toward a fundamental regime shift? A better understanding of ecosystem reorganization can increase predictive capacity regarding long-term consequences of global change for ecosystems. Yet, a conceptual framework for studying the reorganization of ecosystems in a changing world is still missing to date.
Resilience has been the prime lens through which questions of ecosystem stability and change have been investigated. The concept of resilience was first used in an ecological context five decades ago (15), and its broad application in recent years has produced major advances in the understanding of ecosystem dynamics. Notions such as alternative stable states, critical transitions, and hysteretic behavior are now well established in ecology (8, 16–20). However, translating theoretical advances of resilience to real-word systems has been more difficult for some ecosystems than for others. In forest ecosystems, for instance, while the resilience concept is increasingly being used (21), it remains difficult to operationalize. Reasons for this difficulty include 1) the longevity of trees (the foundational organisms in forests), making regime shifts rare at time scales for which robust observational data exist; 2) time lags in forest dynamics, making it difficult to link drivers to novel responses; and 3) challenges of experimentally manipulating forests at scales on which processes of reorganization play out (i.e., landscapes and regions). As a consequence, many applications of the resilience concept in forest ecology have focused on regime shifts from forest to nonforest (22–24). These are important and ecologically significant transitions worthy of investigation. However, these transitions remain rare thus far in many parts of the world, despite an increasing rate of global change. In Europe, for instance, areas where the rate of disturbance exceeds the rate of forest recovery (indicating an increased propensity for impending regime shifts) make up only 6% of the total forest area (25). Consequently, limiting the investigation of forest responses to global change to regime shifts is not sufficient to grasp the full breadth and depth of ongoing changes. In the worst of cases, focusing solely on regime shifts could suggest resilience where forest structure and composition are changing drastically, with considerable implications for the functions and services provided by forests (26, 27). Thus, there is a pressing need to consider a broad spectrum of responses between resilience and regime shift to capture the complex nature of forest change. Such a more nuanced approach is supported by a growing body of information on changes in forest structure and composition (28–31). However, lack of a comprehensive framework for diagnosing and contrasting altered dynamics across ecosystems limits the ability to understand ongoing forest change.
Here, we describe how forest ecosystems reorganize along a spectrum from resilience to regime shift. Our specific objectives were 1) to present a framework that allows a consistent and comprehensive assessment of changing dynamics within and among forest ecosystems, focused on the reorganization phase; 2) to synthesize the processes that determine reorganization and highlight where deflections in ecosystem processes are likely to result in forest change; 3) to give examples of distinct pathways of reorganization in forests around the globe; and 4) to propose a research agenda focused on forest reorganization in a changing world.
Forests are responding to myriad drivers of global change, yet it is important to recognize that change is ubiquitous in forests even in the absence of human activity. Thus, changes that occur naturally because forests are dynamic systems need to be distinguished from altered forest dynamics that reflect ecological responses to anthropogenic changes in the Earth system. We use “forest dynamics” and “forest reorganization” (the latter when addressing forest development after disturbance in particular) to reference the natural dynamics of forest ecosystems, even in the absence of global change. In contrast, we use “forest change” or “altered forest dynamics/reorganization” to describe deflections from natural dynamics that represent new pathways in response to drivers of global change.
A Framework for Investigating Forest Reorganization in a Changing World
Forest Reorganization in Response to Global Change.
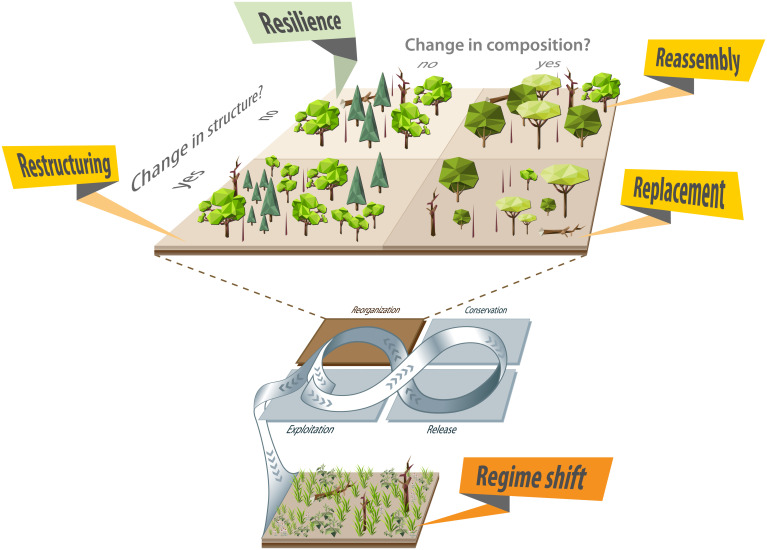
We characterize forest reorganization along dimensions of forest structure (i.e., number, size, and spatial arrangement of trees) and forest composition (i.e., the identity and diversity of the tree community). We focus on structure and composition [but not function, which is the third dimension characterizing ecosystems (32)] for two main reasons. First, forest development is largely locked in after the reorganization phase, which makes this relatively short time window after disturbance disproportionally important for long-term forest dynamics. While this lock-in occurs for forest structure and composition, it does not happen in the same way for forest function, as fluxes of carbon, water, and energy can fluctuate strongly between years. Second, while structure and composition are largely independent dimensions (e.g., forests can be dense or sparse, regardless of their composition), function depends on these two dimensions (e.g., the number, size, and identity of trees determine leaf area, which, in turn, governs carbon uptake and the transpiration of water). Focusing on forest structure and composition, we simplify the continuous variation in these dimensions to categories to illustrate distinct pathways of forest reorganization in response to global change; we refer to these as resilience, restructuring, reassembly, and replacement (Fig. 1).
Fig. 1.
Response pathways of post-disturbance forest reorganization to global change. Reorganization is one of the four phases of the adaptive cycle (sensu Holling (9)—central figure eight). The response of forest reorganization to global change can be characterized along dimensions of forest structure (number, size, and spatial arrangement of trees) and composition (identity and diversity of tree species): resilience (no change in structure and composition), restructuring (structure changes), reassembly (composition changes), and replacement (structure and composition change). Regime shifts occur when vegetation structure and composition are altered so profoundly that the emerging trajectory leads to nonforest, representing a transition to an alternative adaptive cycle.
Resilience means that the system emerging from the reorganization phase will be structurally and compositionally equivalent to the pre-disturbance system. Resilience thus describes situations in which disturbance–recovery processes remain intact relative to historical conditions. Resilience (and a loss thereof) has received considerable attention in the literature, but we here characterize three additional pathways in which forests can persist but change qualitatively after disturbance. Restructuring takes place when the number, size, and spatial arrangement of trees change in the reorganization phase but species composition remains unchanged. An example is a post-disturbance stem density at the end of the reorganization phase that is much lower than pre-disturbance stem density (13). Reassembly denotes a change in the identity and diversity of the tree community while forest structure is sustained. This happens, for example, when species of the pre-disturbance community are outcompeted by other species (12) such that stem density recovers, but the tree community composition shifts. Replacement of the previously dominant ecosystem happens if forest structure and composition are both changed in the reorganization stage. An example is the disturbance-mediated transition from a dense, conifer-dominated, closed-canopy forest to a sparse, broadleaf-dominated, open forest (5). While the ecosystem remains forested, it differs from the pre-disturbance system in both structure and composition. Beyond these four responses wherein a forest reorganizes but remains a forest, regime shifts occur when reorganization alters vegetation structure and composition so profoundly that the emerging trajectory leads to a nonforest ecosystem. In current studies, a loss of ecological resilience is most frequently described by such regime shifts (33). An example is a transition to grassland or shrubland because of the lack of viable tree propagules that can establish under emerging environmental conditions (22). We refer herein to ecological resilience (21) and use the term resilience in a strict sense (i.e., resilience of forest structure and composition) to characterize one end point of the spectrum of responses to global change (with regime shift being the other end point). When adopting a broader definition of resilience (e.g., resilience of forest cover), restructuring, reassembly, and replacement would also be considered resilient; yet we deem more nuanced categories of reorganization responses to global change important for clearly diagnosing forest change.
On Quantifying Forest Reorganization.
To describe forest reorganization responses to global change, three elements must be specified: 1) reference conditions against which to measure change (34), 2) methods to quantify change, and 3) ecological evaluations of change. A widely applied approach for establishing reference conditions is to use pre-disturbance forest structure and composition (12, 13, 35). Other reference conditions, such as old-growth structure and composition or certain desired vegetation states (e.g., in the context of management), can also be used (36). It is important to note that a single value is not sufficient to characterize reference conditions. Rather, variation around the central tendency (e.g., mean ± SD) is necessary because of intrinsic variability of ecosystems in time and across space, for example, as captured in the historical range of variability of a system (37–39). As variation in ecological variables is contingent on spatial and temporal scales of observation, it is necessary to specify these aspects explicitly. Whether forest ecosystems are found to be resilient or undergoing restructuring, reassembly, or replacement after disturbance depends on the reference conditions specified and the scales over which change is assessed.
Second, quantifying change in ecosystems requires more than the calculation of statistically significant numerical differences from reference conditions, because ecological context is critical for interpreting change. We suggest considering multiple forest attributes to evaluate reorganization trajectories, as resilience in a single indicator (e.g., tree species composition) does not necessarily equate to resilience of the ecosystem, and could even mask changes in other dimensions (e.g., forest structure). Rather than asking multiple “resilience of what” questions (40) to address this issue (and potentially introducing ambiguity in outcomes because of diverging responses), we suggest the use of the four categories introduced above to describe the response of ecosystems in comprehensive yet accessible terms. Furthermore, it can be valuable to consider both absolute and relative differences in response variables relevant for forest reorganization, as absolute and relative changes might result in widely differing signals of change, depending on the ecological context. We note that, particularly for variables of forest composition, relative values of change might not be computable, as the denominator is zero, for example, when a species establishing after disturbance was not present under reference conditions.
Third, global change responses of reorganization (sensu restructuring, reassembly, or replacement) must be distinguished from the dynamics expected under the historical range of variability, given past patterns of disturbance and succession. Stem density, for instance, can be low immediately after disturbance, then increase sharply upon the successful establishment of trees, only to decrease again from self-thinning after the canopy has closed. A key challenge is to distinguish altered stem density in response to global change (sensu a restructuring of the system) from that expected during succession. As a powerful means for increasing the signal-to-noise ratio in this context, we suggest the analysis of functional traits. Functional traits are well-defined and measurable properties of an organism that strongly influence its performance (41). Response traits (42) are of particular relevance in the context of reorganization. These include traits associated with disturbance resistance and recovery as well as traits related to environmental filters and competitive performance (see the next section, as well as refs. 43 and 44). Shifts in functional traits can help distinguish altered dynamics in response to global change from the expected successional dynamics of forest ecosystems. For instance, if the vegetation composition at the end of the reorganization phase consists of tree species that are more light demanding than the pre-disturbance reference conditions (e.g., as measured by community-weighted mean shade tolerance), this shift may reflect expected successional dynamics after disturbance (e.g., early establishment of light-demanding pioneer species that subsequently yield to more shade-tolerant species). Such a shift does not give a strong indication for reassembly in response to global change. Alternatively, if the post-disturbance community is more shade tolerant than the pre-disturbance community (which can happen, e.g., when light-demanding trees in the overstory are killed by windthrow, but shade-tolerant understory trees survive), development toward more light-demanding species is unlikely over the course of stand development, suggesting ecosystem reassembly.
Processes of Forest Reorganization.
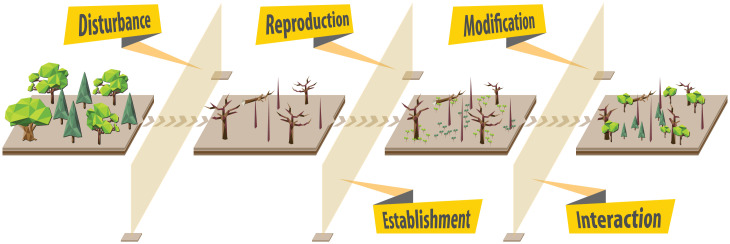
Identifying processes that underpin forest reorganization pathways (resilience, restructuring, reassembly, and replacement) is central for making accurate predictions of forest change. Building on more than 100 y of research on forest development and succession (45–49), we identified five processes that are crucial for determining pathways of forest reorganization (Fig. 2): disturbance, reproduction, establishment, modification, and interaction.
Fig. 2.
Illustration of one exemplarily pathway of forest development, highlighting the five key processes influencing forest reorganization. Disruption of any one of these five processes or shifts in respective process rates in response to global change can lead the system away from resilience, toward restructuring, reassembly, replacement, or regime shift (Fig. 1). For more details on processes and examples for their influence on reorganization pathways, see Fig. 3.
First, the disturbance event [i.e., the type of disturbance as well as its size, intensity, and severity (10, 50)] that triggers reorganization is of key importance for reorganization outcomes. The disturbance event determines the information and material legacies that are carried over from the pre-disturbance forest to the post-disturbance forest (51), and it establishes the physical template (e.g., in terms of patch sizes and microclimate) against which reorganization takes place. Beyond the individual event, the disturbance regime (i.e., the frequency and timing of disturbances as well as the distribution of the characteristics of individual disturbance events over extended spatiotemporal scales) also influences reorganization, determining the spatiotemporal context for an individual stand undergoing reorganization. For example, changes in disturbance frequency (e.g., as a result of climate change) can restructure forests (e.g., when forests regenerate only sparsely after two disturbances in close succession), lead to reassembly (e.g., when changed disturbance frequency alters the competitive balance between species), or even lead to replacement with a different forest ecosystem (e.g., when disturbance intervals become so short that regeneration of the pre-disturbance cohort fails because of immaturity risk, but new tree species establish) (Fig. 3).
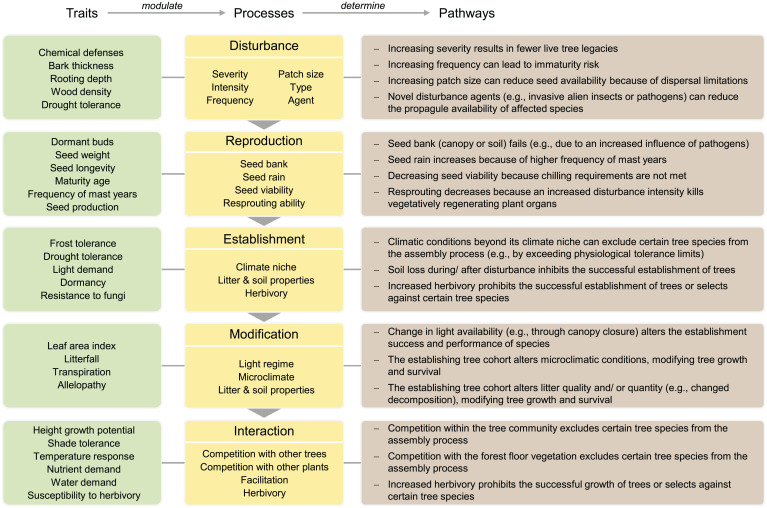
Fig. 3.
Processes of forest reorganization (compare Fig. 2) and illustrative pathways of how a disruption in these processes can lead to forest change (i.e., reorganization trajectories beyond resilience; Fig. 1). Traits give examples of plant properties that modulate processes of reorganization. The processes column gives illustrative examples of characteristics useful for quantifying and monitoring the five major processes influencing forest reorganization. Pathways describe ways in which disruption of reorganization processes can initiate trajectories leading away from resilience.
The second critical process is reproduction, including reproduction from seeds as well as resprouting. Seeds can originate from trees surviving the disturbance or from parts of the landscape that have not been disturbed. They can be produced in the years following a disturbance, or can be stored over extended periods of time pre-disturbance in the form of seed banks in the canopy or soil. Sprouts can appear from buds on roots or stem parts surviving the disturbance. Changes in reproduction can lead to restructuring (e.g., when a failing seed bank results in decreased stem densities) or reassembly (e.g., when the seed rain of a species that was present previously decreases sharply) of forest ecosystems, and can even trigger the replacement of the current forest type (e.g., when a changing frequency in mast years alters regeneration structure and composition; see Fig. 3).
Third, tree establishment is an important determinant of forest reorganization. This process entails seed germination and the early seedling survival and growth. Tree establishment is crucial for vegetation development, because of the high sensitivity of tree seedlings to abiotic and biotic stressors (52, 53). Changes in the strong climatic filters on tree establishment can lead to a reassembly of forest ecosystems, and seed predation can reduce stem densities and restructure the ecosystem. Combined changes in biotic and abiotic influences can also result in replacement (Fig. 3).
Fourth, the regenerating tree cohort increasingly modifies the environment as it grows up. For example, it modifies the light regime on the forest floor, thus influencing subsequent vegetation establishment and growth. In addition, the establishing tree cohort modulates microclimate and alters litter quality and quantity (via changed rates of litter input and decomposition). These modifications can result in restructuring (e.g., where altered microclimate improves regeneration success and increases stem density), reassembly (e.g., where allelopathic compounds accumulating in the litter prohibit the regeneration of certain species), or replacement (e.g., where altered light regimes change the survival rate of the recovering tree cohort, and thus alter forest structure and composition) (Fig. 3).
Fifth, biotic interactions also influence the outcome of forest reorganization. Plant competition for resources, including interactions among trees and between trees and other forest floor plants, is particularly important during the reorganization phase. Furthermore, interactions exist between the regenerating tree cohort and herbivores such as ungulates and rodents (with trees modulating herbivore habitat, and herbivores reducing tree biomass). Pests and pathogens can also strongly influence post-disturbance forest development. Changes in prevailing interactions can restructure forests (e.g., when high levels of herbivory reduce stem density), lead to reassembly (e.g., when a changing climate alters the competitive balance between species), or result in a trajectory toward replacement (e.g., when intense competition with forest floor vegetation reduces the success of tree regeneration and filters out certain tree species) (Fig. 3).
These five processes critically determine the response of forest reorganization to global change. Disruption of these processes relative to how they operated under reference conditions (e.g., the historical range of variability) will lead away from resilience and toward restructuring, reassembly, replacement, or regime shift. Various functional traits modulate each process (Fig. 3). Disturbance and reproduction processes are mainly influenced by resistance and recovery traits, describing the ability of trees to survive or regenerate following disturbances. For instance, tree species with thicker bark are more likely to survive fire, increasing live tree legacies. Tree species that mast (i.e., substantial interannual variability in seed production) cause variation in the quantity and quality of seed rain, which modulates the process of reproduction. Processes of establishment, modification, and interaction are influenced by traits that determine the relationship of trees with their abiotic (environmental filtering traits) and biotic (competition traits) environment. Drought tolerance can, for instance, modulate establishment success, while a higher leaf area and higher growth rate alter how critical variables like light are modified. Shade tolerance, in turn, modulates the interactions between trees in competing for light.
Forest Reorganization Responses Exemplified
Resilience.
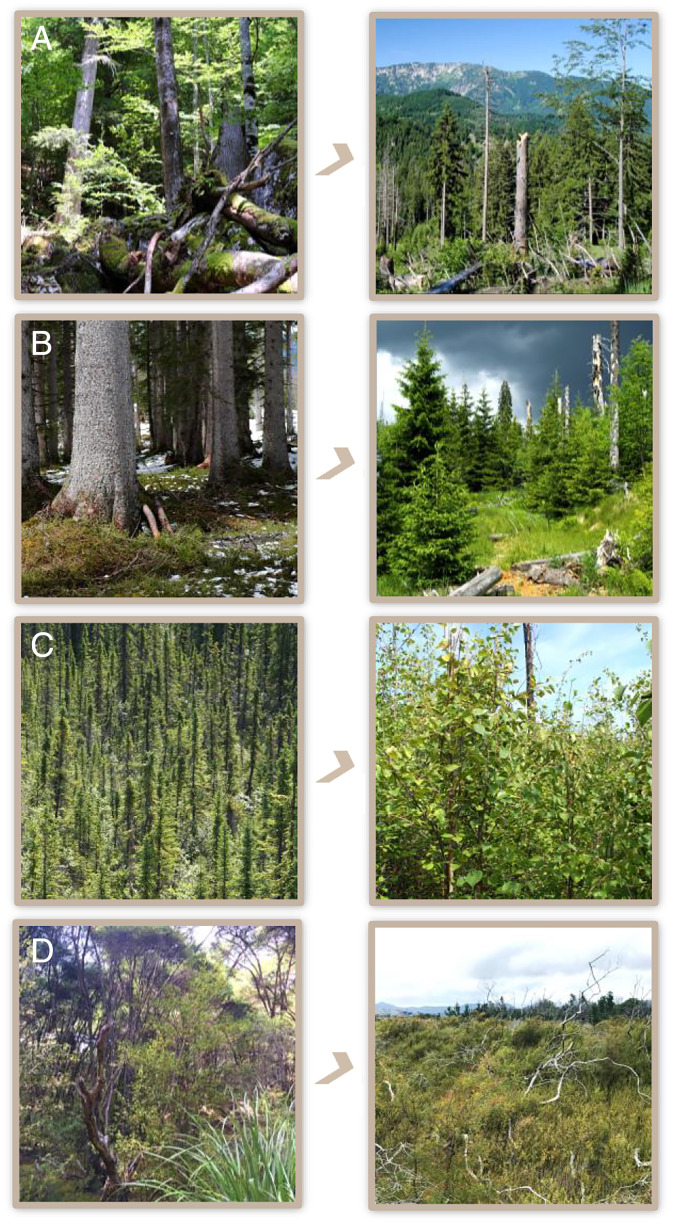
The Dürrenstein Wilderness Area, located in the Northern Front Range of the Alps in Austria, is the only International Union for Conservation of Nature (IUCN) category I protected area in the Eastern Alps. It contains one of the last primary forests of Central Europe (54), whereas other parts of the landscape have a varied history of human land use. Using a 220-y chronosequence of forest development after human disturbance, Albrich et al. (36) showed that out of six indicators of forest structure and composition investigated, five indicators returned to primary forest reference conditions within two centuries. In addition, Albrich et al. (36) investigated forest development trajectories after recent bark beetle disturbance to test whether reorganization trajectories differed between past human disturbance and more recent natural disturbance. Simulated forest development did not indicate significant changes in forest structure and composition after recent natural disturbance. The forests of the Dürrenstein Wilderness Area are thus resilient to these disturbances (Fig. 4).
Fig. 4.
Illustrative examples of the four response pathways of forest reorganization from around the globe. (A) Resilience (Dürrenstein Wilderness Area, Austria; photos by Rupert Seidl). (B) Restructuring (Bohemian Forest Ecosystem, Germany/Czechia; photos by R.S.). (C) Reassembly (black spruce forests of boreal North America; photos by Jill F. Johnstone). (D) Replacement (southern beech forests of New Zealand; photos by Monica G. Turner). See text for details.
Restructuring.
The Bohemian Forest ecosystem, situated in Central Europe at the border between Germany and Czechia, is a mid-elevation mountain range dominated by closed-canopy Norway spruce (Picea abies (L.) Karst.) forests. The area was affected by two consecutive waves of high-severity bark beetle outbreaks between 1995 and 2010, creating the single largest bark beetle patch in all of Europe (55). Intensive studies of reorganization after bark beetle disturbance using both field-based approaches and remote sensing documented swift establishment of the regenerating tree cohort. Fifteen years after disturbance, regenerating trees occupied 76% of the disturbed area, with average stem densities reaching 2,000 stems per hectare (56). The regenerating cohort consisted almost exclusively of Norway spruce, indicating no change in composition after disturbance. Yet, forest structure deviated from the homogeneous pre-disturbance state because of patchy establishment on favorable microsites (e.g., nurse logs) (57). Forest structure was highly variable, with stem densities varying by four orders of magnitude 15 y after disturbance, and structural variation persisted for at least several decades (30, 56). The development trajectory away from the structurally homogeneous pre-disturbance condition makes the Bohemian Forest ecosystem an example of ecosystem restructuring (Fig. 4).
Reassembly.
Black spruce (Picea mariana (Mill.) B.S.P.) is one of the dominant species of the current boreal forest of North America. The species has successfully self-replaced from aerial seedbanks after wildfire throughout the Holocene, indicating high resilience. A recent increase in fire severity and/or decrease in fire return interval have reduced the regeneration success of black spruce (58) and triggered a reassembly to broadleaved forests or jack pine (Pinus banksiana Lamb.). Across the North American boreal forest, Baltzer et al. (12) found that black spruce either regenerated poorly or failed to regenerate after fire on 38% of sites where it dominated before fire. Interestingly, reassembly trajectories differed between ecoregions, with broadleaved species gaining dominance over black spruce in western North America, and jack pine taking over in eastern North America. The decline in black spruce forests is an example of the reassembly of ecosystems after disturbance (Fig. 4).
Replacement.
Prior to human settlement, most of New Zealand was dominated by evergreen forests that included kauri (Agathis australis (D. Don) Loudon), conifers (Podocarpaceae, Phyllocladaceae, Cupressaceae), and southern beech (Nothofagus spp.). Today, the most extensive tracts of remaining native forests are dominated by five species of beech, all of which are obligate seeders with thin bark and shallow roots. These beech forests are extremely vulnerable to fire; however, natural ignition sources (lightning or volcanic eruptions) are scarce, and fire was infrequent throughout most of the Holocene (59). Anthropogenic ignitions increased with settlement and fundamentally altered the fire regime. Beech forest regeneration is very slow because propagule supply is spatially limited by short-distance dispersal and temporally limited due to masting (60). After fire, beech forests are thus often replaced by a short-statured open forest dominated by manuka (Leptospermum) and kanuka (Kunzea) with bracken fern (Pteridium esculentum (G. Forst.) Cockayne) and indigenous shrubs in the understory. These emerging forests have open canopies that do little to modify microclimate, thus enhancing persistence of understory vegetation. Furthermore, a positive feedback to fire has perpetuated a fire-prone, short-statured, open forest, because manuka and kanuka shoots and bark are highly flammable, and some manuka are serotinous. Leptospermum woodland has formed a quasi-permanent stable state that now occupies ∼50% of Great Barrier Island (61), and similar replacement dynamics have occurred in Patagonia (59). Such fire-driven transitions in both forest structure and composition are examples of forest replacement.
All Reorganization Responses in the Same Landscape.
We have described distinct pathways of forest change on three different continents, but all four response categories of forest reorganization can also be found within the same landscape. A prime example is the Greater Yellowstone Ecosystem (GYE) in the northern US Rocky Mountains, for which studies suggest potential for drastic vegetation changes in response to changes in climate and fire (14, 28, 62). Dominant forest types include tree species with varied fire-related traits, including thick-barked fire resisters (Douglas-fir, Pseudotsuga menziesii var. glauca (Beissn.) Mayr) and resprouters (aspen, Populus tremuloides Michx.) common at lower elevations; seed bankers (serotinous lodgepole pine, Pinus contorta var. latifolia (Engelm.) Crichfield) throughout midelevation plateaus; and fire-sensitive shade tolerants (Engelmann spruce, Picea engelmannii Parry ex Engelm), subalpine fir, (Abies lasiocarpa (Hook.) Nutt.), and nonserotinous lodgepole pines at higher elevations. The disturbance regime was dominated by infrequent, stand-replacing fires that occurred at intervals of 100 y to 300 y throughout the Holocene (63). After the historic 1988 Yellowstone fires, most forests rapidly recovered their prefire structure and composition, demonstrating remarkable resilience to extensive high-severity fire (64). However, there was also reassembly where seedlings of aspen colonized burned forests at elevations well above and locations far from their prefire distributions (65). This expansion of aspen led to stand densities similar to the prefire forest but a compositional change to lodgepole pines and aspens (66). Increased fire activity is now initiating further change. Restructuring occurred where young lodgepole pine forests reburned at high severity in <30 y, well before recovering their prefire biomass and cone production. Lodgepole pine often remained dominant, but a 95% reduction in postfire establishment converted dense forests to sparse ones (67). Furthermore, replacement was also observed in some short-interval fires where sparse lodgepole pine establishment was augmented by vigorous resprouting of aspens that had established from seed after the previous fire, leading to a pine–aspen woodland.
Taking the Pulse of Reorganizing Forests
The future of forests is crucial for the Earth system. Forests are a central component of the global climate system, taking up large amounts of carbon and altering the albedo of the Earth surface (68, 69). Forests cover just over 30% of the global land surface but harbor ∼80% of the world’s amphibian species, 75% of bird species, and 68% of mammals (70). Forests are thus crucial for addressing global climate change and biodiversity loss, two of the most pressing issues of the 21st century. That forest ecosystems will be changing in response to global change is virtually certain. Yet, what remains widely unclear is how these changes will unfold, and what they will entail.
We identified the reorganization phase as the time window crucial for understanding and predicting change in forest ecosystems. Resilience research has generated novel tools and perspectives for understanding past and future changes, including theoretical early warning signals founded in system analysis (71, 72). Yet, the applicability of these tools to complex and long-lived ecosystems such as forests has been limited. We suggest that the reorganization phase can provide a window into the future of post-disturbance forest development—the seedlings and saplings of today are the towering forest giants of the future. As forests are increasingly locked into their development trajectory after the reorganization phase, this phase holds great potential for providing a different class of early warning indicators (i.e., one rooted in ecological process understanding rather than in theoretical considerations of system dynamics). Focusing on reorganization processes (Fig. 2) is informative because disruption of these processes or shifts in their rates can lead the system away from resilience. Monitoring reorganization processes could thus provide an early indication of disturbance-mediated forest change.
Our framework provides the foundation for a new wave of monitoring, experimentation, and modeling focused on forest reorganization (Box 1). Historically, the reorganization phase has not been a primary focus of forest science and has even been called the “forgotten” phase of forest succession (73). Given the importance of the reorganization phase for the future trajectories of forests and its relevance for projecting and managing change, we maintain that this must change. Forest monitoring activities, for instance, were largely designed to quantify timber (and, more recently, carbon) stocks. As a consequence, they often fall short in adequately characterizing the reorganization phase of forest dynamics. We argue that improved monitoring of the five cardinal processes of reorganization identified here—disturbance, reproduction, establishment, modification, and interaction—is critically needed. Such enhancements will provide early indications of forest change and give managers and policy makers opportunities to counteract undesired developments as they unfold (rather than having to cope with the end points of such developments). Decisions about where and when to resist, accept, or direct change (74, 75) in forest ecosystems require this information.
Box 1.
Twelve questions for future forest reorganization research
-
1) How long is the critical reorganization window?
What influences the duration of the reorganization phase, and does it differ between ecosystems? Which indicators best capture when the critical window of reorganization has closed? Will climate change prolong or shorten the reorganization phase?
-
2) How strong is the lock-in after the reorganization phase?
Which factors strengthen or weaken the lock-in after the reorganization phase? Do ecosystems differ in the degree to which the reorganization phase determines future stand development? Will climate change increase or decrease the strength of lock-in after reorganization?
-
3) Which traits influence forest reorganization and how?
Trait-based ecology has made important leaps over recent years, yet the traits most frequently measured are effect traits (42) and not the response traits that are crucial for forest reorganization (compare Fig. 3). As relevant traits are difficult to measure (82), their specific influence remains unknown.
-
4) What is the role of intraspecific variation in traits for reorganization?
The intraspecific variation within tree populations is large, and can influence forest trajectories significantly [e.g., when intraspecific variation in resistance traits modifies disturbance severity, or when variation in prevalence of serotiny influences postfire regeneration density (10)]. Yet, it remains incompletely quantified in most analyses, and is widely ignored in simulation models (83).
-
5) What is the role of compounding and linked disturbances in reorganization?
As disturbance frequency increases, the probability of compounding and linked events also rises (84, 85). Understanding when compounding and linked disturbances will amplify or dampen the rate or magnitude of ecosystem change is incomplete. Specifically, compounding disturbances could modify the pathways of reorganization [e.g., from restructuring to replacement in the example of the Bohemian Forest given above (86)].
-
6) What is the contribution of individual processes to observed reorganization outcomes?
It remains easier to quantify the outcomes of reorganization than to identify the underlying drivers that lead to the emergence of a specific reorganization pathway. However, improved understanding of the underlying processes is needed to robustly predict change. For example, it remains unclear whether changes in different processes could lead to similar reorganization pathways, or whether alterations to each individual process result in distinct signatures of forest change.
-
7) Are trajectories of forest reorganization reversible?
Can a forest developing along a pathway of restructuring/reassembly/replacement revert back toward resilience, and, if so, under which circumstances? Do restructuring, reassembly, and replacement differ in terms of reversibility? Is there hysteresis in reorganization pathways (5)?
-
8) Is there a consistent sequence of change in the different pathways of reorganization?
Does restructuring or reassembly always precede replacement, or can systems change directly from resilience to replacement? Does restructuring or reassembly eventually lead to replacement [ratcheting down (28)], or are there stabilizing feedbacks in restructuring and reassembly pathways?
-
9) Where are thresholds for changes in forest structure and composition?
What level of change in structure and/or composition is needed to constitute a restructuring or reassembly of forest ecosystems? Are changes from the reference condition gradually (87), or are there discontinuities? Do novel feedbacks establish to stabilize the new forest state?
-
10) What are the implications of reorganization for forest functioning and ecosystem services?
How are carbon, nutrient, and water cycles affected by restructuring, reassembly, and replacement? How are important provisioning, regulating, and cultural ecosystem services affected by the different pathways of reorganization?
-
11) What are the implications of reorganization for biodiversity?
How are different taxa affected by pathways of restructuring, reassembly, and replacement? What are the effects of different forest reorganization responses across trophic levels?
-
12) To what extent can forest reorganization be managed and how?
How strong is the leverage of management to influence forest reorganization? Can restructuring, reassembly, or replacement trajectories be reverted to resilience by means of management? To what extent can forest reorganization be managed to direct forest change (75)? Which management measures result in desired outcomes of forest reorganization?
Experiments focused on forest reorganization are needed to better understand forest change and develop more robust simulation models. Experimental manipulation is a powerful means to gain insights into the effects of disturbance on future stand development, and to determine what drives reproduction and limits establishment. Furthermore, experiments are uniquely able to address the complex interplay between the next generation of trees and their abiotic and biotic environment in the form of modification and interaction. In addition to manipulative experiments, we also stress the value of natural experiments, that is, quasi-experimental studies that make use of the inherent variability in ecosystems. This is particularly relevant in the context of disturbances (76), as conditions created by disturbances are often difficult to mimic in manipulative studies. Furthermore, experiments conducted in silico (i.e., in a simulated ecosystem) complement manipulative and natural experiments, especially with regard to exploring long-term consequences over greater spatial extents. Simulation models also are the prime tools for projecting future forest development. Because a historical focus of forest modeling was on tree growth, disturbance and regeneration processes are not well specified in many models. For example, the dynamic vegetation models widely used to make inferences about the future development of forests often neglect important processes of disturbance and regeneration (33). A new wave of model development is thus needed—guided by data from improved monitoring and novel experiments—to simulate future trajectories of forest reorganization more robustly.
To understand the responses of forest reorganization to global change, joint analysis of tree mortality and regeneration processes with consideration of both forest structure and composition is needed (compare Figs. 1 and 2). In this regard, our framework extends previous approaches that have largely focused on individual components of forest change (20, 77, 78). Studying tree mortality and regeneration together will increase the inferential potential regarding future forest trajectories, given the strong interdependencies between these two demographic processes. Furthermore, a nuanced framework that incorporates both forest structure and composition is needed for a comprehensive view of forest change as well as for anticipating future forest ecosystem functioning. The dichotomy of resilient versus not resilient is insufficient to capture the intricacies of forest responses to global change and might mask profound ecological change in forests. Alterations in forest structure and composition have major implications for biodiversity and ecosystem services, and these changes might go unnoticed when applying coarse and/or dichotomous frameworks of forest change.
Forest change is inevitable, given the magnitude and rate of environmental change. We maintain that it is not necessary to wait a century or two to understand how forest change will unfold. Rather, trajectories of change can be anticipated by focusing on the reorganization phase, that is the critical period after disturbance in which “the deck is reshuffled,” determining the pathways of forest development for decades to centuries. As such, the reorganization phase is also a critical window for management, because future stand development trajectories are not yet locked in and can still be steered toward desired trajectories. The ongoing wave of disturbance in forests around the globe (79–81) offers a unique opportunity for initiating new research programs focused on forest reorganization. Such research can offer a much-needed glimpse into the future of our forests.
Acknowledgments
We thank C. Schauflinger for the design and implementation of Figs. 1 and 2. These figures use resources from Freepik.com. We are grateful to K. H. Braziunas, J. F. Johnstone, A. S. Mori, and W. Rammer for helpful comments on an earlier version of the manuscript, and to F. S. Chapin III and P. E. Higuera for their thoughtful constructive reviews. R.S. acknowledges support from the European Research Council under the European Union’s Horizon 2020 research and innovation program (Grant Agreement 101001905). M.G.T. acknowledges support from the Joint Fire Science Program (Grant No.16-3-01-4) and the University of Wisconsin Vilas Trust.
Footnotes
Reviewers: F.S.C., University of Alaska Fairbanks; and P.H., University of Montana College of Forestry and Conservation.
The authors declare no competing interest.
Data Availability
There are no data underlying this work.
References
- 1.Thomas C. D., The development of Anthropocene biotas. Philos. Trans. R. Soc. Lond. B Biol. Sci. 375, 20190113 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blowes S. A., et al. , The geography of biodiversity change in marine and terrestrial assemblages. Science 366, 339–345 (2019). [DOI] [PubMed] [Google Scholar]
- 3.McDowell N. G., et al. , Pervasive shifts in forest dynamics in a changing world. Science 368, eaaz9463 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Stovall A. E. L., Shugart H., Yang X., Tree height explains mortality risk during an intense drought. Nat. Commun. 10, 4385 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albrich K., Rammer W., Seidl R., Climate change causes critical transitions and irreversible alterations of mountain forests. Glob. Change Biol. 26, 4013–4027 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brodribb T. J., Powers J., Cochard H., Choat B., Hanging by a thread? Forests and drought. Science 368, 261–266 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Rocha J. C., Peterson G., Bodin Ö., Levin S., Cascading regime shifts within and across scales. Science 362, 1379–1383 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Andersen T., Carstensen J., Hernández-García E., Duarte C. M., Ecological thresholds and regime shifts: Approaches to identification. Trends Ecol. Evol. 24, 49–57 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Holling C. S., Gunderson L. H., “Resilience and adaptive cycles” in Panarchy. Understanding the Transformations in Human and Natural Systems, Gunderson L. H., Holling C. S., Eds. (Island, 2002), pp. 25–62. [Google Scholar]
- 10.Turner M. G., Disturbance and landscape dynamics in a changing world. Ecology 91, 2833–2849 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Johnstone J. F., Hollingsworth T. N., Chapin F. S., Mack M. C., Changes in fire regime break the legacy lock on successional trajectories in Alaskan boreal forest. Glob. Change Biol. 16, 1281–1295 (2010). [Google Scholar]
- 12.Baltzer J. L., et al. , Increasing fire and the decline of fire adapted black spruce in the boreal forest. Proc. Natl. Acad. Sci. U.S.A. 118, e2024872118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batllori E., et al. , Forest and woodland replacement patterns following drought-related mortality. Proc. Natl. Acad. Sci. U.S.A. 117, 29720–29729 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rammer W., et al. , Widespread regeneration failure in forests of Greater Yellowstone under scenarios of future climate and fire. Glob. Change Biol. 27, 4339–4351 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Holling C. S., Resilience and stability of ecological systems. Annu. Rev. Ecol. Syst. 4, 1–23 (1973). [Google Scholar]
- 16.Ratajczak Z., et al. , Abrupt change in ecological systems: Inference and diagnosis. Trends Ecol. Evol. 33, 513–526 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Beisner B. E., Haydon D. T., Cuddington K., Alternative stable states in ecology. Front. Ecol. Environ. 1, 376–382 (2003). [Google Scholar]
- 18.Reyer C. P. O., et al. , Forest resilience and tipping points at different spatio-temporal scales: Approaches and challenges. J. Ecol. 103, 5–15 (2015). [Google Scholar]
- 19.Ghazoul J., Burivalova Z., Garcia-Ulloa J., King L. A., Conceptualizing forest degradation. Trends Ecol. Evol. 30, 622–632 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Coop J. D., et al. , Wildfire-driven forest conversion in western North American landscapes. Bioscience 70, 659–673 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikinmaa L., et al. , Reviewing the use of resilience concepts in forest sciences. Curr. For. Rep. 6, 61–80 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevens-Rumann C. S., et al. , Evidence for declining forest resilience to wildfires under climate change. Ecol. Lett. 21, 243–252 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Scheffer M., Hirota M., Holmgren M., Van Nes E. H., Chapin F. S. III, Thresholds for boreal biome transitions. Proc. Natl. Acad. Sci. U.S.A. 109, 21384–21389 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirota M., Holmgren M., Van Nes E. H., Scheffer M., Global resilience of tropical forest and savanna to critical transitions. Science 334, 232–235 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Senf C., Seidl R., Post‐disturbance canopy recovery and the resilience of Europe’s forests. Glob. Ecol. Biogeogr. 31, 25–36 (2022). [Google Scholar]
- 26.Bohn F. J., Huth A., The importance of forest structure to biodiversity-productivity relationships. R. Soc. Open Sci. 4, 160521 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baeten L., et al. , Identifying the tree species compositions that maximize ecosystem functioning in European forests. J. Appl. Ecol. 56, 733–744 (2019). [Google Scholar]
- 28.Turner M. G., et al. , The magnitude, direction, and tempo of forest change in Greater Yellowstone in a warmer world with more fire. Ecol. Monogr. 92, e01485 (2022). [Google Scholar]
- 29.Whitman E., Parisien M.-A., Thompson D. K., Flannigan M. D., Short-interval wildfire and drought overwhelm boreal forest resilience. Sci. Rep. 9, 18796 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Senf C., Müller J., Seidl R., Post-disturbance recovery of forest cover and tree height differ with management in Central Europe. Landsc. Ecol. 34, 2837–2850 (2019). [Google Scholar]
- 31.Hagmann R. K., et al. , Evidence for widespread changes in the structure, composition, and fire regimes of western North American forests. Ecol. Appl. 31, e02431 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franklin J. F., et al. , “Ecological characteristics of old-growth Douglas-fir forests” (General Tech. Rep. PNW-GTR-118, Southeastern Forest Experiment Station, 1981). [Google Scholar]
- 33.Albrich K., et al. , Simulating forest resilience: A review. Glob. Ecol. Biogeogr. 29, 2082–2096 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shackelford N., Dudney J., Stueber M. M., Temperton V. M., Suding K. L., Measuring at all scales: Sourcing data for more flexible restoration references. Restor. Ecol., 10.1111/rec.13541 (2022). [DOI] [Google Scholar]
- 35.Ilisson T., Chen H. Y. H., The direct regeneration hypothesis in northern forests. J. Veg. Sci. 20, 735–744 (2009). [Google Scholar]
- 36.Albrich K., Thom D., Rammer W., Seidl R., The long way back: Development of Central European mountain forests towards old‐growth conditions after cessation of management. J. Veg. Sci. 32, e13052 (2021). [Google Scholar]
- 37.Morgan P., et al. , Historical range of variability. J. Sustain. For. 2, 87–111 (1994). [Google Scholar]
- 38.Keane R. E., Hessburg P. F., Landres P. B., Swanson F. J., The use of historical range and variability (HRV) in landscape management. For. Ecol. Manage. 258, 1025–1037 (2009). [Google Scholar]
- 39.Seidl R., Spies T. A., Peterson D. L., Stephens S. L., Hicke J. A., Searching for resilience: Addressing the impacts of changing disturbance regimes on forest ecosystem services. J. Appl. Ecol. 53, 120–129 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carpenter S., Walker B., Anderies J. M., Abel N., From metaphor to measurement: Resilience of what to what? Ecosystems (N. Y.) 4, 765–781 (2001). [Google Scholar]
- 41.McGill B. J., Enquist B. J., Weiher E., Westoby M., Rebuilding community ecology from functional traits. Trends Ecol. Evol. 21, 178–185 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Suding K. N., et al. , Scaling environmental change through the community-level: A trait-based response-and-effect framework for plants. Glob. Change Biol. 14, 1125–1140 (2008). [Google Scholar]
- 43.Laughlin D. C., Applying trait-based models to achieve functional targets for theory-driven ecological restoration. Ecol. Lett. 17, 771–784 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Aubin I., et al. , Traits to stay, traits to move: A review of functional traits to assess sensitivity and adaptive capacity of temperate and boreal trees to climate change. Environ. Rev. 24, 164–186 (2016). [Google Scholar]
- 45.Clements F. E., Plant Succession: An Analysis of the Development of Vegetation (Carnegie Institution of Washington, 1916). [Google Scholar]
- 46.MacMahon J. A., “Successional processes: Comparisons among biomes with special reference to probable role of and influences on animals” in Forest Succession: Concepts and Applications, West D. C., Shugart H. H., Botkin D. B., Eds. (Springer, 1981), pp. 277–304. [Google Scholar]
- 47.Pickett S. T. A., Cadenasso M. L., Meiners S. J., Ever since clements: From succession to vegetation dynamics and understanding to intervention. Appl. Veg. Sci. 12, 9–21 (2009). [Google Scholar]
- 48.Davis K. T., Higuera P. E., Sala A., Anticipating fire‐mediated impacts of climate change using a demographic framework. Funct. Ecol. 32, 1729–1745 (2018). [Google Scholar]
- 49.Christensen N. L., An historical perspective on forest succession and its relevance to ecosystem restoration and conservation practice in North America. For. Ecol. Manage. 330, 312–322 (2014). [Google Scholar]
- 50.Peters D. P. C., et al. , Cross-system comparisons elucidate disturbance complexities and generalities. Ecosphere 2, art81 (2011). [Google Scholar]
- 51.Johnstone J. F., et al. , Changing disturbance regimes, ecological memory, and forest resilience. Front. Ecol. Environ. 14, 369–378 (2016). [Google Scholar]
- 52.Grubb P. J., The maintenance of species-richness in plant communities: The importance of the regeneration niche. Biol. Rev. Camb. Philos. Soc. 52, 107–145 (1977). [Google Scholar]
- 53.Hansen W. D., Turner M. G., Origins of abrupt change? Postfire subalpine conifer regeneration declines nonlinearly with warming and drying. Ecol. Monogr. 89, e01340 (2019). [Google Scholar]
- 54.Splechtna B. E., Gratzer G., Black B. A., Disturbance history of a European old-growth mixed-species forest – A spatial dendro-ecological analysis. J. Veg. Sci. 16, 511 (2005). [Google Scholar]
- 55.Seidl R., et al. , Small beetle, large-scale drivers: How regional and landscape factors affect outbreaks of the European spruce bark beetle. J. Appl. Ecol. 53, 530–540 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeppenfeld T., et al. , Response of mountain Picea abies forests to stand-replacing bark beetle outbreaks: Neighbourhood effects lead to self-replacement. J. Appl. Ecol. 52, 1402–1411 (2015). [Google Scholar]
- 57.Wild J., et al. , Spatial patterns with memory: Tree regeneration after stand-replacing disturbance in Picea abies mountain forests. J. Veg. Sci. 25, 1327–1340 (2014). [Google Scholar]
- 58.Johnstone J. F., et al. , Factors shaping alternate successional trajectories in burned black spruce forests of Alaska. Ecosphere 11, e03129 (2020). [Google Scholar]
- 59.Kitzberger T., et al. , Fire–vegetation feedbacks and alternative states: Common mechanisms of temperate forest vulnerability to fire in southern South America and New Zealand. N. Z. J. Bot. 54, 247–272 (2016). [Google Scholar]
- 60.Wardle J. A., The New Zealand Beeches: Ecology, Utilisation and Management (New Zealand Forest Service, 1984). [Google Scholar]
- 61.Perry G. L. W., Ogden J., Enright N. J., Davy L. V., Vegetation patterns and trajectories in disturbed landscapes, Great Barrier Island, northern New Zealand. N. Z. J. Ecol. 34, 311–323 (2010). [Google Scholar]
- 62.Westerling A. L., Turner M. G., Smithwick E. A. H., Romme W. H., Ryan M. G., Continued warming could transform Greater Yellowstone fire regimes by mid-21st century. Proc. Natl. Acad. Sci. U.S.A. 108, 13165–13170 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Higuera P. E., Whitlock C., Gage C., Linking tree-ring and sediment-charcoal records to reconstruct fire occurrence and area burned in subalpine forests of Yellowstone National Park, USA. Holocene 21, 327–341 (2011). [Google Scholar]
- 64.Romme W. H., et al. , Twenty years after the 1988 Yellowstone fires: Lessons about disturbance and ecosystems. Ecosystems (N. Y.) 14, 1196–1215 (2011). [Google Scholar]
- 65.Turner M. G., Romme W. H., Reed R. A., Tuskan G. A., Post-fire aspen seedling recruitment across the Yellowstone (USA) landscape. Landsc. Ecol. 18, 127–140 (2003). [Google Scholar]
- 66.Hansen W. D., Romme W. H., Ba A., Turner M. G., Shifting ecological filters mediate postfire expansion of seedling aspen (Populus tremuloides) in Yellowstone. For. Ecol. Manage. 362, 218–230 (2016). [Google Scholar]
- 67.Turner M. G., Braziunas K. H., Hansen W. D., Harvey B. J., Short-interval severe fire erodes the resilience of subalpine lodgepole pine forests. Proc. Natl. Acad. Sci. U.S.A. 116, 11319–11328 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harris N. L., et al. , Global maps of twenty-first century forest carbon fluxes. Nat. Clim. Chang. 11, 234–240 (2021). [Google Scholar]
- 69.Alibakhshi S., Naimi B., Hovi A., Crowther T. W., Rautiainen M., Quantitative analysis of the links between forest structure and land surface albedo on a global scale. Remote Sens. Environ. 246, 111854 (2020). [Google Scholar]
- 70.United Nations Environment Programme; Food and Agriculture Organization;, “The state of the world’s forests 2020” (Rep. CA8642, United Nations Environment Programme, 2020). [Google Scholar]
- 71.Scheffer M., et al. , Early-warning signals for critical transitions. Nature 461, 53–59 (2009). [DOI] [PubMed] [Google Scholar]
- 72.Dakos V., Carpenter S. R., van Nes E. H., Scheffer M., Resilience indicators: Prospects and limitations for early warnings of regime shifts. Philos. Trans. R. Soc. Lond. B Biol. Sci. 370, 20130263 (2015). [Google Scholar]
- 73.Swanson M. E., et al. , The forgotten stage of forest succession: Early-successional ecosystems on forest sites. Front. Ecol. Environ. 9, 117–125 (2011). [Google Scholar]
- 74.Jackson S. T., Transformational ecology and climate change. Science 373, 1085–1086 (2021). [DOI] [PubMed] [Google Scholar]
- 75.Schuurman G. W., et al. , Navigating ecological transformation: Resist–accept–direct as a path to a new resource management paradigm. Bioscience 72, 16–29 (2022). [Google Scholar]
- 76.Lindenmayer D. B., Likens G. E., Franklin J. F., Rapid responses to facilitate ecological discoveries from major disturbances. Front. Ecol. Environ. 8, 527–532 (2010). [Google Scholar]
- 77.Frelich L. E., Reich P. B., Neighborhood effects, disturbance severity, and community stability in forest. Ecosystems (N. Y.) 2, 151–166 (1999). [Google Scholar]
- 78.Frelich L. E., Jõgiste K., Stanturf J., Jansons A., Vodde F., Are secondary forests ready for climate change? It depends on magnitude of climate change, landscape diversity and ecosystem legacies. Forests 11, 965 (2020). [Google Scholar]
- 79.Parks S. A., Abatzoglou J. T., Warmer and drier fire seasons contribute to increases in area burned at high severity in western US forests from 1985 to 2017. Geophys. Res. Lett. 47, e2020GL089858 (2020). [Google Scholar]
- 80.Senf C., Seidl R., Persistent impacts of the 2018 drought on forest disturbance regimes in Europe. Biogeosciences 18, 5223–5230 (2021). [Google Scholar]
- 81.Kharuk V. I., et al. , Climate‐driven conifer mortality in Siberia. Glob. Ecol. Biogeogr. 30, 543–556 (2021). [Google Scholar]
- 82.Yang J., Cao M., Swenson N. G., Why functional traits do not predict tree demographic rates. Trends Ecol. Evol. 33, 326–336 (2018). [DOI] [PubMed] [Google Scholar]
- 83.Berzaghi F., et al. , Towards a new generation of trait-flexible vegetation models. Trends Ecol. Evol. 35, 191–205 (2020). [DOI] [PubMed] [Google Scholar]
- 84.Buma B., Disturbance interactions: Characterization, prediction, and the potential for cascading effects. Ecosphere 6, art70 (2015). [Google Scholar]
- 85.Simard M., Romme W. H., Griffin J. M., Turner M. G., Do mountain pine beetle outbreaks change the probability of active crown fire in lodgepole pine forests? Ecol. Monogr. 81, 3–24 (2011). [Google Scholar]
- 86.Sommerfeld A., et al. , Do bark beetle outbreaks amplify or dampen future bark beetle disturbances in Central Europe? J. Ecol. 109, 737–749 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Radeloff V. C., et al. , The rise of novelty in ecosystems. Ecol. Appl. 25, 2051–2068 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no data underlying this work.