Abstract
Pressure-damaged Escherichia coli O157 cells were more acid sensitive than native cells and were impaired in pH homeostasis. However differences in acid sensitivity were not related to differences in cytoplasmic pH (pHi). Cellular β-galactosidase was more acid labile in damaged cells. Sensitization to acid may thus involve loss of protective or repair functions.
High-pressure processing of food is being increasingly investigated as a means of extending the shelf life of food while avoiding the adverse affects on flavor, color, or texture associated with thermal processing. Several pressure-treated foods, particularly fruit juices, pureés, jams, and other acidic products, are now commercially available (6, 13, 17). By combining pressure with other treatments it may be possible to extend the range of products that can be preserved by using pressure technology (5, 8).
There have been outbreaks of Escherichia coli O157 food poisoning associated with the consumption of unfermented apple juice (2, 3, 11), and it is therefore particularly important to ensure that pressure processing will eliminate this organism from fruit-based products. Natural isolates of E. coli O157 vary quite widely in pressure resistance, and some isolates can survive pressure treatment at levels of up to 700 MPa under neutral pH conditions (1, 14). However, a relatively mild pressure treatment of fruit juices (300 MPa), followed by subsequent holding, allowed substantial inactivation even of pressure-resistant mutants of E. coli (4, 10). The sensitization of cells to acid by a prior pressure treatment is an important consideration because it allows pathogens to be inactivated by much milder treatments than would be necessary for neutral foods. However, the mechanism by which pressure sensitizes bacteria to a subsequent acid challenge acid is unknown. Damage to the bacterial cell membrane is believed to be an important event, leading to the inactivation of cells by high pressure. Partial loss of the F0F1 ATPase activity and impaired ability to maintain a transmembrane pH gradient (ΔpH) has been described for Lactobacillus plantarum, and membrane leakiness following pressure treatment has been reported for several other organisms (12, 15, 16, 18). Loss of such membrane functions would be expected to impair pH homeostasis, and this might account for the increased sensitivity to acid conditions. The aim of this work was to investigate the effect of pressure treatment on the acid sensitivity of E. coli O157:H7 strain C9490, a strain previously found to be among the most acid and pressure resistant of several natural isolates (1, 7).
Bacterial strain and growth conditions.
E. coli O157:H7 strain C9490 (a clinical isolate from the Jack-in-the-Box western U.S. hamburger patty outbreak of 1993) was kindly provided by M. Doyle, University of Georgia, Griffin. It was grown to stationary phase in tryptone soya broth supplemented with yeast extract (TSBYE), as described previously (12).
Pressure treatment.
Cells from stationary-phase cultures were centrifuged at 3,000 × g for 20 min at 4°C, and the pellets were resuspended in phosphate-buffered saline (PBS), pH 7.0, to yield viable counts of about 5 × 109 CFU/ml. Cell suspensions were pressure treated in a Foodlab pressure vessel (model S-FL-850-9-W; Stanstead Fluid Power, Stanstead, United Kingdom), as described previously (12).
Measurement of acid resistance.
The cells were challenged by diluting either native (i.e., untreated) or pressure-treated cells 1:30 into TSBYE adjusted to different pH values (from 3.5 to 7) with HCl. The media were preheated to 37, 30, or 20°C by immersion in a thermostated water bath.
Viable counts.
Samples were removed at intervals, serially diluted in maximum recovery diluent (Oxoid, Basingstoke, United Kingdom), and plated onto tryptone soya agar supplemented with yeast extract and containing 0.1% sodium pyruvate. When neat, acid-treated samples were plated, an equal volume of HEPES buffer (100 mM, pH 7) was added to neutralize acid that would otherwise inhibit growth on the plates during recovery. Colonies were counted after incubating the plates at 37°C for 48 h.
Measurement of pHi.
The pHi was determined as described previously (7, 9). Every pHi value was based on the mean value of at least six measurements obtained under standard conditions.
Measurement of β-galactosidase activity.
Cells were grown in the presence of 2 mM isopropyl-β-d-thiogalactopyranoside (IPTG; Sigma-Aldrich) at 37°C for 18 h. Cells were harvested by centrifugation at 3,000 × g for 20 min at 4°C, and the pellets were resuspended in PBS (pH 7.0) to yield viable counts of about 5 × 109 CFU/ml. Suspensions of cells (450 μl) were permeabilized by the addition of 50 μl of chloroform and 25 μl of 0.1% (wt/vol) sodium dodecyl sulfate. A sample of suspension (225 μl) was incubated with 1 mM fluorescein-di-(β-d-galactopyranoside (FDG; Sigma-Aldrich) and incubated at 30°C for approximately 60 min. The reaction was stopped by dilution of the sample into PBS buffer, and the fluorescence was measured with a spectrofluorophotometer (model LS-5B; Perkin-Elmer) with the excitation wavelength set at 490 nm and the emission wavelength set at 514 nm. The slit width was 10 nm. To enable the conversion of the number of fluorescence units into the fluorescein concentration, a calibration curve was constructed using serial dilutions of 0 to 100 nM fluorescein (Sigma-Aldrich) in distilled water. Fluorescence values obtained for untreated cells were subtracted from all experimental values. The amount of fluorescein (nanomolar concentration) released per hour was considered to indicate the β-galactosidase activity.
Effect of pressure pre-treatment on acid sensitivity.
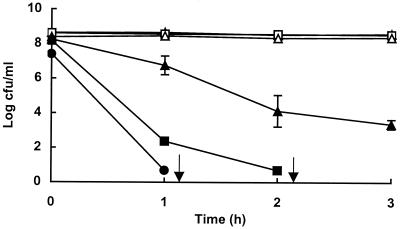
Stationary-phase cells of E. coli O157:H7 strain C9490 were pressure treated in PBS at pH 7 for 10 min at pressures of up to 500 MPa, then inoculated into TSBYE at pH 3.5 and held at 37°C for 3 h. The effect of pressure treatment on subsequent survival under acid conditions is shown in Fig. 1. Nonpressurized cells or those treated at 100 or 200 MPa showed no loss of viability at pH 3.5, whereas cells that had been exposed to pressures of 300 MPa or higher died with a rate of inactivation that increased with increasing pretreatment pressure. Following treatment at 300 and 400 MPa, more than 99.99 and 99.9999% of cells, respectively, were inactivated after 2 h of incubation in TSBYE at pH 3.5. No viable cells were recovered from suspensions treated at 500 MPa after incubation for 1 h at pH 3.5. In subsequent work, treatment for 10 min at 400 MPa was used as the standard protocol for producing pressure-damaged cells.
FIG. 1.
Effect of pressure pretreatment on acid resistance of E. coli O157:H7 strain C9490. Cells were untreated (○) or treated in PBS for 10 min at 100 MPa (□), 200 MPa (▵), 300 MPa (▴), 400 MPa (■), or 500 MPa (●) before being diluted into TSBYE, pH0 3.5, and held at 37°C for up to 3 h. Data are means ± standard deviations (error bars). Arrows indicate that viable counts were below the limit of detection (100 CFU/ml).
Loss of viability and loss of pH homeostasis.
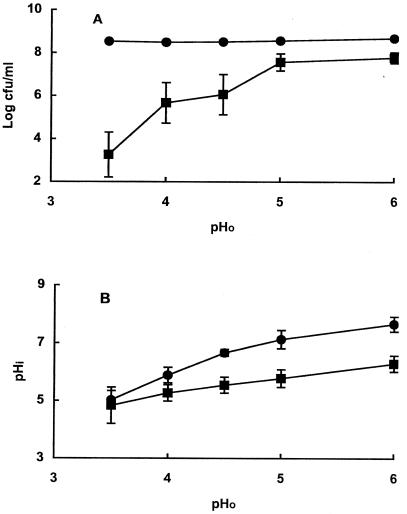
The threshold pH for inactivation was investigated by incubating pressure-damaged cells for 1 h at pH values between 3.5 and 6.0. Native cells showed no loss of viability within 1 h at any pH, whereas pressure-treated cells died at pH values of 4.5 or lower (Fig. 2A). The extent of inactivation increased as the pH of the medium decreased.
FIG. 2.
Effect of pH0 on survival (A) and pHi (B) of native (●) and pressure-damaged (■)cells. Data are means ± standard deviations (error bars).
The cytoplasmic pH values of native stationary-phase cells of strain C9490 at external pHs between 6 and 3.5 were very similar to those previously reported (7) for E. coli O157 strain 30-2C4 (Fig 2B). Pressure treatment impaired pH homeostasis, but the difference in pHi between native and pressure-damaged cells became smaller as the external pH (pH0) decreased, such that at pH0 3.5 the pHi of both cell types was similar (ca. 4.9). However, at that particular pH0, native cells were fully viable after 1 h of incubation, whereas more than 99.9% of pressure-damaged cells had died. Therefore, lowering of the internal pH does not appear to explain the death of pressure-damaged cells under acidic conditions.
Jordan et al. (7) concluded that lowering the cytoplasmic pH of stationary-phase cells of E. coli O157:H7 strain C9490 was not sufficient to cause cell death and postulated that protective functions activated when cells enter stationary phase can overcome the lowering of cytoplasmic pH. If true, this would suggest that those protective functions no longer operate in pressure-damaged cells.
Acid inactivation of β-galactosidase in native and damaged cells.
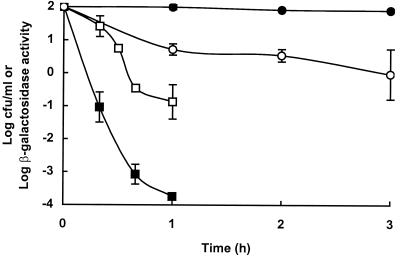
To examine the effect of pressure-damage on acid resistance of a cellular protein, we measured the loss of β-galactosidase activity and the loss of viability in native and pressure-treated cells incubated at 37°C and pH 3.5. Inactivation of β-galactosidase and cell death both occurred more rapidly in pressure-damaged cells than in native cells, but enzyme inactivation was not directly correlated with cell death (Fig. 3).
FIG. 3.
Effect of pressure pretreatment on survival and loss of β-galactosidase activity in cells incubated at pH 3.5 for varying periods of time. Data are means ± standard deviations (error bars). Symbols: ●, survival of native cells; ■, survival of pressure-damaged cells; ○, β-galactosidase activity of native cells; □, β-galactosidase activity of pressure-damaged cells.
Relationship between loss of viability and inactivation of β-galactosidase under different conditions.
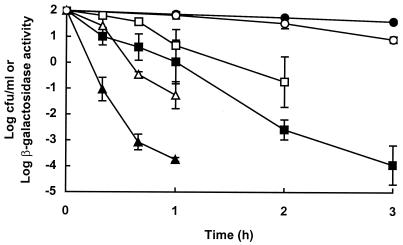
The loss of viability and the loss of β-galactosidase activity were monitored for damaged cells incubated at pH 3.5 at 20, 30, and 37°C. The rates of inactivation of β-galactosidase and loss of viability both increased as the temperature increased, but viability was affected more than loss of enzyme activity (Fig. 4). The lack of correlation between cell death and inactivation of β-galactosidase was confirmed by the following observations: (i) when pressure-damaged cells were incubated at 37°C at pH0 4.5, more than 99.99% of cells died within 3 h with no loss of β-galactosidase activity; (ii) when native cells were incubated at pH0 3.5 at 37°C, β-galactosidase activity decreased by at least 99% within 3 h, whereas no loss of viability occurred under these conditions (data not shown).
FIG. 4.
Effect of temperature on survival and β-galactosidase activity of pressure-damaged cells incubated at pH 3.5. Data are means ± standard deviations (error bars). Symbols: ●, cell survival at 20°C; ■, cell survival at 30°C; ▴, cell survival at 37°C; ○, β-galactosidase activity at 20°C; □, β-galactosidase activity at 30°C; ▵, β-galactosidase activity at 37°C.
β-galactosidase is not essential for the viability of E. coli growing in TSBYE, and we would not expect there to be a precise correspondence between inactivation of this marker enzyme and cell death. Nevertheless, the use of β-galactosidase as an activity marker allowed us to measure quantitatively the relative acid stability of a cellular component of intact and pressure-damaged cells. Our results suggests that the acid sensitivity of stationary-phase cells caused by pressure treatment may be due to the loss of protective or repair functions rather than to the loss of pH homeostasis per se.
High-pressure treatments that had little or no effect on viability effectively sensitized cells of E. coli O157:H7 strain C9490 to acid. This strain is resistant to a range of stresses, including high pressure, acid, mild heat, and osmotic and oxidative stresses (1). The combination of high pressure and acid thus provides an effective means of eliminating even this robust strain from food by a relatively mild process.
Acknowledgments
We are grateful to the Ministry of Agriculture Fisheries and Food and Food Standards Agency, London, United Kingdom, for financial support of this work and to the Spanish Ministry of Education and Science, who provided R. Pagán with a grant to carry out this investigation.
REFERENCES
- 1.Benito A, Ventoura G, Casadei M, Robinson T, Mackey B M. Variation in resistance of natural isolates of Escherichia coli O157 to high hydrostatic pressure, mild heat, and other stresses. Appl Environ Microbiol. 1999;65:1564–1569. doi: 10.1128/aem.65.4.1564-1569.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Besser R E, Lett S M, Weber J T, Doyle M P, Barret T J, Wells J G, Griffin P M. An outbreak of diarrhea and hemolytic uremic syndrome from Escherichia coli O157:H7 in fresh-pressed cider. JAMA. 1993;269:2217–2220. [PubMed] [Google Scholar]
- 3.Cody S H, Glynn M K, Farrar J A, Cairns K L, Griffin P M, Kobayashi J, Fyfe M, Hoffman R, King A S, Lewis J H, Swaminathan B, Bryant R G, Vugia D J. An outbreak of Escherichia coli O157:H7 infection from unpasteurised apple juice. Ann Int Med. 1999;130:202–209. doi: 10.7326/0003-4819-130-3-199902020-00005. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Graells C, Hauben K J A, Michiels C W. High-pressure inactivation and sublethal injury of pressure-resistant Escherichia coli mutants in fruit juices. Appl Environ Microbiol. 1998;64:1566–1568. doi: 10.1128/aem.64.4.1566-1568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hauben K J A, Wuytack E Y, Soontjens C C F, Michiels C W. High-pressure transient sensitisation of Escherichia coli to lysozyme and nisin by disruption of outer-membrane permeability. J Food Protect. 1996;59:350–355. doi: 10.4315/0362-028X-59.4.350. [DOI] [PubMed] [Google Scholar]
- 6.Hoover D. Minimally processed fruits and vegetables: reducing microbial load by nonthermal physical treatments. Food Technol. 1997;51:66–71. [Google Scholar]
- 7.Jordan S L, Glover J, Malcolm L, Thomson-Carter F M, Booth I R, Park S F. Augmentation of killing of Escherichia coli O157 by combination of lactate, ethanol, and low pH conditions. Appl Environ Microbiol. 1999;65:1308–1311. doi: 10.1128/aem.65.3.1308-1311.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalchayanand N, Sikes A, Dunne C P, Ray B. Hydrostatic pressure and electroporation have increased bactericidal efficiency in combination with bacteriocins. Appl Environ Microbiol. 1994;60:4174–4177. doi: 10.1128/aem.60.11.4174-4177.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kroll R G, Booth I R. The role of potassium transport in the generation of a pH gradient in Escherichia coli. Biochem J. 1981;198:691–698. doi: 10.1042/bj1980691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linton M, McClements J M J, Patterson M F. Survival of Escherichia coli O157:H7 during storage in pressure-treated orange juice. J Food Prot. 1999;62:1038–1040. doi: 10.4315/0362-028x-62.9.1038. [DOI] [PubMed] [Google Scholar]
- 11.McCarthy M. E. coli O157:H7 outbreak in USA traced to apple juice. Lancet. 1996;348:1299. [Google Scholar]
- 12.Pagán R, Mackey B. Relationship between membrane damage and cell death in pressure-treated Escherichia coli cells: differences between exponential- and stationary-phase cells and variation among strains. Appl Environ Microbiol. 2000;66:2829–2834. doi: 10.1128/aem.66.7.2829-2834.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patterson M. High-pressure treatment of foods. In: Robertson R K, Batt C A, Patel P D, editors. The encyclopedia of food microbiology. London, United Kingdom: Academic Press; 1999. pp. 1059–1065. [Google Scholar]
- 14.Patterson M F, Quinn M, Simpson R, Gilmour A. Sensitivity of vegetative pathogens to high hydrostatic pressure treatment in phosphate-buffered saline and foods. J Food Prot. 1995;58:524–529. doi: 10.4315/0362-028X-58.5.524. [DOI] [PubMed] [Google Scholar]
- 15.Shigehisa T, Ohmori T, Saito A, Taji S, Hayashi R. Effects of high hydrostatic pressure on characteristics of pork slurries and inactivation of microorganisms associated with meat products. Int J Food Microbiol. 1991;12:207–216. doi: 10.1016/0168-1605(91)90071-v. [DOI] [PubMed] [Google Scholar]
- 16.Smelt J P P M, Rijke A G F, Hayhurst A. Possible mechanism of high-pressure inactivation of microorganisms. High Pressure Res. 1994;12:199–203. [Google Scholar]
- 17.Thakur B R, Nelson P E. High-pressure processing and preservation of food. Food Rev Int. 1998;14:427–447. [Google Scholar]
- 18.Wouters P C, Glaasker E, Smelt J P P M. Effects of high pressure on inactivation kinetics and events related to proton efflux in Lactobacillus plantarum. Appl Environ Microbiol. 1998;64:509–514. doi: 10.1128/aem.64.2.509-514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]