Abstract
A quantitative determination method for Al3+ in immunobiological drugs using atomic absorption spectroscopy with electrothermal atomization was developed. Conditions for the preparation of aluminum hydroxide gel were selected [NaOH (5 M), heating in a boiling-water bath for 5 min followed by neutralization with conc. HNO3]. The method was validated. The analytical range of Al3+ was established in the interval 10 – 50 ig/L. The accuracy and in-laboratory precision were confirmed. The results allowed the proposed method to be considered an alternative for quantitative determination of Al3+ in immunobiological drugs.
Keywords: aluminum hydroxide gel, atomic absorption spectrometry, electrothermal atomization, immunobiological drugs
Aluminum hydroxide gel is a component of many immunobiological drugs (IBDs), which include anatoxins, vaccines against hepatitis B and A and tick encephalitis, pneumococcal vaccines, combined vaccines, and the latest generation of vaccines against coronavirus infection that are based on synthetic peptides. Aluminum hydroxide gel remains the most popular adjuvant and has a lengthy history of use and proof of its immunological efficacy despite the continuous search and implementation into practice of new substances that increase the immunogenicity of IBDs. The allowed content of aluminum hydroxide gel can vary from 0.2 to 1.7 mg/mL depending on the drug. An individual standard confirmed by pharmaceutical development data should be determined for each drug.
However, besides increased immunogenicity, aluminum hydroxide gel in a drug may cause adverse local reactions such as redness at the injection site, induration, and the possibility of developing inflammatory syndrome associated with adjuvants [1–3].
According to pharmacopoeial requirements, quantitative analysis of Al is an obligatory component of a quality assessment. Correspondence of the content of aluminum hydroxide gel or aluminum ions to the established standard is considered one of the important confirmations of the declared efficacy and safety for use of drugs containing this adjuvant.1
The traditional method for quantitative determination of aluminum hydroxide gel in IBDs is complexometric titration. 2 However, incorporation of high-technology methods with high sensitivity and specificity that ensure traceability of results and minimize operator involvement by process automation is a growing trend in quality assessment of IBDs.
Currently, methods based on atomic absorption spectroscopy are used for quantitative determination of Al3+ in IBDs. A distinctive feature of sample preparation for this method is its length (4 h and more) using concentrated acids [4–7].
Instruments for atomic absorption spectroscopy are divided according to the sample atomization method. Flame atomization reaches sample temperatures up to 2000 – 3000°C. For this, a flame of hot gases mixed with oxidants is used. This method has several drawbacks. The main ones are [8]:
low sensitivity;
problems with passage of particles through the illuminated zone;
use of flammable gases for atomization.
Electrothermal atomization employs a graphite tube furnace as the atomizer. This significantly reduces the physical and chemical limitations of the process as compared to flame atomization. For example, refractory elements and trace quantities of analyte can be analyzed. Smaller samples can be used for electrothermal atomization. This is an advantage for working with a limited amount of material or an expensive material. Also, the sensitivity of the method is increased because of the simultaneous atomization of the whole sample, after which free atoms remain in the optical path for a long time [9].
Thus, atomic absorption spectroscopy with electrothermal atomization has several advantages and is a promising direction for development of new methods.
The aim of the present work was to develop a quantitative determination method for aluminum ions in adsorbed drugs using atomic absorption spectroscopy with electrothermal atomization.
Pharmacopoeial monograph 3.3.1.0010.15 Diphtheria, tetanus and pertussis vaccine adsorbed (AKDS-vaccine). State Pharmacopoeia of the Russian Federation, XIVth Ed., Vol. 4, 2018.
Pharmacopoeial monograph 3.3.1.0026.15 Recombinant hepatitis B vaccine. State Pharmacopoeia of the Russian Federation, XIVth Ed., Vol. 4, 2018.
Pharmacopoeial monograph 3.3.1.0029.15 Cultured vaccine for prevention of hepatitis A, purified concentrated adsorbed inactivated liquid. State Pharmacopoeia of the Russian Federation, XIVth Ed., Vol. 4, 2018.
Pharmacopoeial monograph 3.3.1.0031.15 Tick encephalitis vaccine cultured purified concentrated inactivated liquid adsorbed or dry in a complex with aluminum hydroxide solvent. State Pharmacopoeia of the Russian Federation, XIVth Ed., Vol. 4, 2018.
Pharmacopoeial monograph 3.3.1.0049.15 Pertussis, diphtheria, tetanus and hepatitis B vaccine adsorbed, suspension for intramuscular injection. State Pharmacopoeia of the Russian Federation, XIVth Ed., Vol. 4, 2018.
Pharmacopoeial monograph 3.3.1.0050.15 Hepatitis B vaccine and diphtheria and tetanus anatoxin with reduced antigen content adsorbed, suspension for intramuscular injection. State Pharmacopoeia of the Russian Federation, XIVth Ed., Vol. 4, 2018.
Experimental Part
The work used SRS 7758-2000 aluminum ions (EAA Eco-analitika), nominal concentration 1000 mg/L; Al (ICP Standard, Merck), nominal concentration 1000 mg/L; aluminum hydroxide gel, PRS 42-28-423-2019, batch No. 10 [composition: aluminum hydroxide gel (nominal concentration 1660 mg/L recalculated as aluminum ions), protein (diphtheria anatoxin), trace quantities of formaldehyde, thiomersal, 91.17 ig/mL as a preservative]; aluminum hydroxide gel, PRS 42-28-333-2019, batch No. 4 [composition: aluminum hydroxide gel (nominal concentration 810 mg/L recalculated as aluminum ions), protein (diphtheria anatoxin), trace quantities of formaldehyde, thiomersal, 193.81 ig/mL as a preservative); NaOH (Sigma-Aldrich); HNO3 (Merck); H2SO4 (Sigma-Aldrich); HCl (Merck); Triton X-100 (Merck); solution for dilution.
The equipment included a GTA 120 Graphite Tube Atomizer atomic absorption spectrometer (Agilent Technologies, USA) with electrothermal atomization, an autosampler, and computerized data collection and processing; a PD 303S spectrophotometer (Apel, Japan), spectral range 340 – 1000 nm.
A method based on atomic absorption spectroscopy (AAS) with electrothermal atomization was developed. The method consisted in principle of passing radiation of a certain wavelength through a layer of atomic vapor produced using the atomizer followed by measurement of resonance line intensities due to absorption of the radiation by atoms of a certain element [10]. The aliquot volume for the analysis was 20 μL; analytical wavelength, 309.3 nm. These conditions were chosen according to the recommendations of the instrument manufacturer.
Results and Discussion
Aluminum hydroxide gel is a colloidal system with particles of various sizes. The sample must be mineralized to dissolve the aluminum.
Solutions of both acid and base can be used to dissolve Al because aluminum hydroxide gel is amphoteric.
For example, H2SO4 is used to dissolve Al for determination of Al3+ by complexometric titration. However, it is not used in atomic absorption spectroscopy with electrothermal atomization because sulfate ions can have a chemical effect on atomization of the sample and can create ionization interference, i.e., cause a so-called matrix effect.
HNO3 was present in the solution for dilution. [The instrument manufacturer recommended diluting the sample with a solution of Triton X-100 (1 mL) and conc. HNO3(5 mL) in purified H2O (1 L). This composition allowed possible noise to be neutralized.] It is also preferentially used, like HCl, for quantitative determination of Al3+ by atomic absorption spectroscopy with plasma atomization. Thus, HNO3 and HCl of various concentrations and NaOH solution were chosen for dissolution of the samples.
PRS 42-28-423-2019 was used as the sample because its composition was typical of that of biological drugs. Besides aluminum hydroxide gel, the PRS contained protein (diphtheria anatoxin) and excipients. Next, test samples of the following composition were prepared:
Sample (1 mL) + HCl (37%, 1 mL) + purified H2O (8 mL) according to the method for AAS with plasma atomization (sample with HCl).
Sample (1 mL) + HNO3 (65%, 60 μL) + purified H2O (8.94 mL) according to the method for AAS with plasma atomization (sample with HNO3).
Sample (1 mL) + NaOH (5 M, 0.3 mL) + purified H2O (8.7 mL) (sample with NaOH).
A concentration of 5 M was used to study dissolution of aluminum hydroxide by NaOH to obtain primary data. This concentration was chosen to increase the accuracy of the addition with the minimal volume of added solvent. The volume of 5M NaOH was chosen so that the mole ratio of Al3+:NaOH concentrations would be close to the Al3+:H2SO4 ratio (1:20) in the pharmacopoeial complexometric titration method [11].
-
4.
A control sample used sample (1 mL) + purified H2O (9 mL).
The test samples were stored for 30 min in two regimes, i.e., without heating and with heating for 30 min in a boiling-water bath. The degree of dissolution was evaluated in percent relative to the optical densities of samples with the reagent for dissolution to the optical density of the control sample at 540 nm [12]. The reference solution was purified H2O (Table 1). It is noteworthy that this method was used for an instrumental approximation of the different efficiencies of the proposed methods for sample dissolution. Thus, the percentages given in Tables 1 – 3 are a rough comparison and did not affect the quantitative evaluation of Al3+ by AAS and could not be considered a quantitative characteristic of the degree of dissolution with the established precision.
Table 1.
Degree of Sample Dissolution as a Function of Dissolution Reagent and Heat-Treatment Regime (n = 2)
| Solvent | Regime “without heat” | Regime “heating for 30 min” | ||
|---|---|---|---|---|
| Optical density at 540 nm | Degree of dissolution, % | Optical density at 540 nm | Degree of dissolution, % | |
| Control | 0.051 | 0.0 | 0.050 | 0.0 |
| HCl | 0.039 | 23.8 | 0.009 | 53.8 |
| HNO3 | 0.049 | 3.0 | 0.016 | 20.5 |
| NaOH | 0.045 | 10.9 | 0.008 | 59.0 |
Table 3.
Dependence of Degree of Dissolution on Reaction Mixture Heating Time (n = 2)
| Heating time, min | Optical density at 540 nm | Degree of dissolution | ||
|---|---|---|---|---|
| HCl | NaOH | HCl | NaOH | |
| 0 | 0.030 | 0.032 | 0.0 | 0.0 |
| 5 | 0.024 | 0.001 | 20.0 | 96.9 |
| 10 | 0.022 | 0.001 | 26.7 | 96.9 |
| 20 | 0.021 | 0.000 | 30.0 | 100.0 |
| 30 | 0.019 | – | 36.7 | – |
| 40 | 0.019 | – | 36.7 | – |
Table 1 presents the data used to select the reagents giving good degrees of dissolution of aluminum hydroxide gel in heating mode, i.e., HCl (53.8%) and NaOH (59.0%).
The next stage of the work was the selection of the mole ratio of the reagent for dissolution giving the maximum dissolution of the gel and aluminum hydroxide (Table 2).
Table 2.
Degree of Sample Dissolution as a Function of Mole Ratio of Dissolution Reagent and Aluminum Hydroxide (n = 2)
| Moles of dissolution reagent per mole of aluminum hydroxide | Optical density at 540 nm | Degree of dissolution | ||
|---|---|---|---|---|
| HCl | NaOH | HCl | NaOH | |
| 0 | 0.031 | 0.031 | 0.0 | 0.0 |
| 1 | 0.011 | 0.009 | 65.6 | 71.0 |
| 10 | 0.015 | 0.005 | 52.5 | 83.9 |
| 25 | 0.013 | 0.003 | 59.0 | 90.3 |
| 50 | 0.015 | 0.002 | 50.8 | 90.3 |
| 100 | 0.011 | 0.002 | 65.6 | 90.3 |
Table 2 shows that a 25-fold excess of NaOH was the mole ratio that provided the maximum gel dissolution. Greater than 90% of the gel was dissolved after heating for 30 min in a boiling-water bath. Increasing the base concentration further did not affect the result.
The sample dissolution time was also an important factor in sample preparation. Table 3 presents the degrees of gel dissolution as a function of heating time in a boiling-water bath.
Table 3 shows that heating for 5 min was required for the maximum dissolution of the gel in a sample containing NaOH. The degree of gel dissolution was ~37% after prolonged heating of samples containing HCl. This was substantially below analogous values after using base. Also, the degree of dissolution was not affected if the sample heating time with NaOH was increased.
The high sensitivity of the instrument and the dilution factor for sample preparation had to be considered for further work. Solutions of the SRS diluted to concentrations of 5, 10, 20, 40, 60, 80, and 100 μg/L were analyzed because the acceptable sample dilution depended on the working (analytical) range of the method (Fig. 1).
Fig. 1.
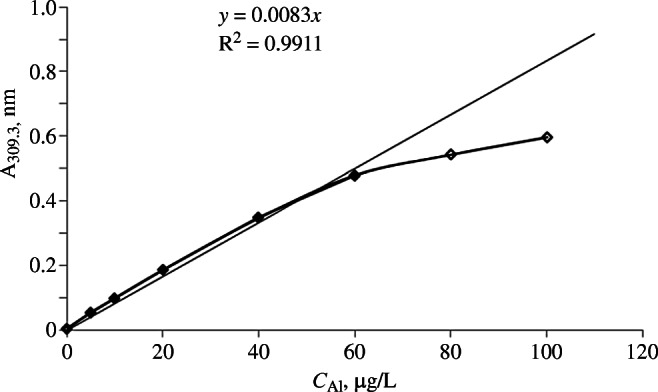
Dependence of optical density of atomic vapor (A) at 309.3 nm on Al3+ concentration (C) in solution.
Figure 1 shows that the dependence of the optical density on Al3+ concentration was linear in the range 0 – 60 μg/L.
The dependence became nonlinear at higher concentrations and could not be described by a linear regression equation. Thus, the section 0 – 60 μg/L could be considered a basis for establishing the analytical range of the method. The limits of detection and quantitation of the method did not have to be evaluated for quantitative determination, like for analysis of trace quantities of undesired impurities, because the aluminum hydroxide gel was a compound added to the drug in a rather high concentration. Thus, the analytical range of the method could be considered from 10 to 50 μg/L in concentration steps of 10 μg/L for five calibration solutions if the nominal concentration of the diluted sample after sample preparation was 30 μg/L (Fig. 2).
Fig. 2.
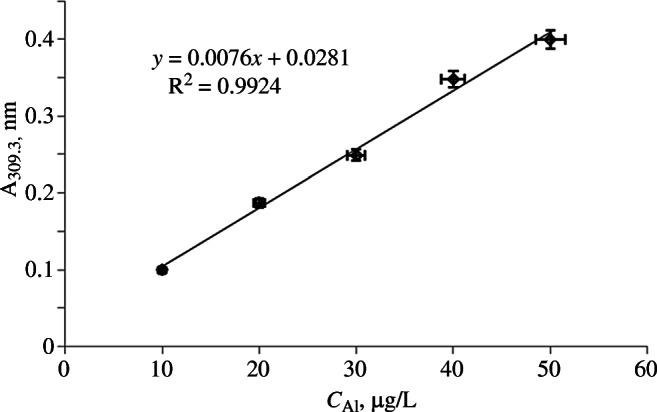
Calibration curve [dependence of optical density of atomic vapor (A) at 309.3 nm on concentration of Al3+ (C) in solution].
Considering the above, the following sample preparation procedure was proposed. Test samples containing Al3+ (0.4 mL, ~1 mg) were treated with NaOH solution (0.35 mL, 20%). The mixture was heated in a boiling-water bath for 5 min. The mixture was cooled, treated with conc. HNO3 (0.5 mL) to neutralize the excess of base, adjusted to a volume of 25 mL with purified H2O, and diluted by ~500 times (0.1 mL of sample in a 50-mL volumetric flask treated to the mark with solvent).
Calibration solutions were prepared by treating the SRS aluminum ions solution (0.4 mL) with NaOH solution (0.35 mL, 20%), heating the mixture in a boiling-water bath for 5 min, cooling, adding conc. HNO3 (0.5 mL) to neutralize the excess of base, and adjusting to 25 mL with purified H2O. Aliquots (15.6, 31.3, 46.9, 62.5, and 78.1 μL) of the resulting solution were taken and adjusted to 25 mL with the reagent for dissolution of the samples to produce solutions of concentrations 10, 20, 30, 40, and 50 μg/L.
Next, the method was validated according to GPM.1.1.0012.15 “Validation of analytical methods” [13] and GOST 5725 [14], namely, its accuracy characteristics, trueness and precision, were evaluated. The specificity of this method was determined by a selective elemental analysis method where the detection wavelength was narrowly specific for each actual element, in this instance, for Al3+.
The precision of the method was evaluated for repeatability (convergence) of the results and the in-laboratory precision. The repeatability was evaluated using a sample of PRS 42-28-333-2019 (five parallel measurements) by a single analyst during a single day. The in-laboratory precision was evaluated using samples of PRS 42-28-333-2019 (10 parallel measurements) by two analysts (five measurements each) on two days.
The accuracy of the method was evaluated by analyzing samples with various concentrations (80 – 120% of the nominal concentration, n = 6) in the analytical range of the method (Tables 4 and 5).
Table 4.
Assessment of Accuracy of Aluminum Determination Method by Atomic Absorption Spectroscopy with Electrothermal Atomization
| Sample | Nominal concentration, mg/L | Dilution, % of nominal | Theoretical concentration, μg/L | Actual concentration, μg/L | Actual concentration considering dilution, mg/L | Percent detection | Percent detection, average |
|---|---|---|---|---|---|---|---|
| 810 | 1000 | 80 | 25.6 | 28.7 | 1122.8 | 112.3 | 111.7 |
| 100 | 32.0 | 36.4 | 1138.4 | 113.8 | |||
| 120 | 38.4 | 41.9 | 1090.7 | 109.1 | |||
| ICP | 1000 | 80 | 25.6 | 28.6 | 1116.9 | 111.7 | 109.8 |
| 100 | 32.0 | 35.8 | 1118.7 | 111.9 | |||
| 120 | 38.4 | 40.7 | 1059.2 | 105.9 | |||
| PRS | 810 | 80 | 20.7 | 22.5 | 877.5 | 108.3 | 109.4 |
| 100 | 25.9 | 28.2 | 882.3 | 108.9 | |||
| 120 | 31.1 | 34.5 | 898.3 | 110.9 |
Table 5.
Validation Parameters of Aluminum Determination Method by Atomic Absorption Spectroscopy
| Parameter | Metric | Acceptance criterion | Result |
|---|---|---|---|
| Linearity | Visual evaluation of plot | Linear regression | 0.9968 |
| Correlation coefficient | R ≥ 0.985 | ||
| Analytical range | – | – | 5 – 60 μg/L |
| Repeatability | Relative standard deviation | ≤ 5.0 % | 3.14 % |
| In-laboratory precision | Relative standard deviation | ≤ 5.0 % | 4.74 % |
| Accuracy | Mean value | 85 – 115 % | 105.9 – 113.8 % |
The obtained validation data (Table 5) were evaluated according to recommendations in the SP RF [13].
Acknowledgments
The work was performed in the framework of State Task No. 056-00005-21-00 for the SCEEMP, MH of Russia, for applied scientific research (State Acct. No. NIR 121022000147-4).
CONFLICT OF INTEREST
We declare no conflict of interest requiring disclosure in this article.
Footnotes
Pharmacopoeial monograph 3.3.1.0002.15 Diphtheria and tetanus toxoids adsorbed (ADS-anatoxin). State Pharmacopoeia of the Russian Federation, XIVth Ed., Vol. 4, 2018.
Pharmacopoeial monograph 3.3.1.0003.15 Diphtheria and tetanus toxoids adsorbed with reduced antigen content (ADS-M-anatoxin). State Pharmacopoeia of the Russian Federation, XIVth Ed., Vol. 4, 2018.
Pharmacopoeial monograph 3.3.1.0004.15 Diphtheria toxoid adsorbed with reduced antigen content (AD-M-anatoxin). State Pharmacopoeia of the Russian Federation, XIVth Ed., Vol. 4, 2018.
Pharmacopoeial monograph 3.3.1.0005.15 Anatoxin staphylococcus purified adsorbed, solution for subcutaneous injection. State Pharmacopoeia of the Russian Federation, XIVth Ed., Vol. 4, 2018.
Pharmacopoeial monograph 3.3.1.0007.15 Tetanus toxoid adsorbed (AS-anatoxin). State Pharmacopoeia of the Russian Federation, XIVth Ed., Vol. 4, 2018.
Pharmacopoeial monograph 3.3.1.0008.15 Trianatoxin adsorbed. State Pharmacopoeia of the Russian Federation, XIVth Ed., Vol. 4, 2018.
Pharmacopoeial monograph 3.3.1.0009.15 Tetraanatoxin adsorbed. State Pharmacopoeia of the Russian Federation, XIVth Ed., Vol. 4, 2018.
General pharmacopoeial monograph 1.7.2.0016.15 Determination of aluminum ions in adsorbed biological drugs. State Pharmacopoeia of the Russian Federation, XIVth Ed., Vol. 2, 2018.
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 56, No. 4, pp. 53 – 58, April, 2022.
References
- 1.S. G. Radenska-Lopovok and P. Volkova, Arkh. Patol., No. 5, 56 – 62 (2018). [DOI] [PubMed]
- 2.H. Hogen Esch, Vaccine, No. 20, 34 – 39 (2002).
- 3.R. K. Gheradi and F. J. Authier, Lupus, No. 2, 184 – 189 (2012). [DOI] [PMC free article] [PubMed]
- 4.Shardlowa E, Linharta C, Connor S. J. Trace Elem. Med. Biol. 2021;66:1–7. doi: 10.1016/j.jtemb.2021.126762. [DOI] [PubMed] [Google Scholar]
- 5.May JC, Progar JJ, Chin R. J. Biol. Stand. 1984;12:175–183. doi: 10.1016/S0092-1157(84)80051-7. [DOI] [PubMed] [Google Scholar]
- 6.Mishra A, Bhalla SR, Rawat S. Biologicals. 2007;35:277–284. doi: 10.1016/j.biologicals.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Asin J, Molin J, Perez M. Vet. Pathol. 2019;56:418–428. doi: 10.1177/0300985818809142. [DOI] [PubMed] [Google Scholar]
- 8.Alemasova AS, Rokun AN, Shevchuk IA. Analytical Atomic Absorption Spectroscopy: Study Aide. Donetsk: Donetsk; 2003. pp. 16–22. [Google Scholar]
- 9.Zolotov YA. Principles of Analytical Chemistry. Moscow: Akademiya; 2012. pp. 114–116. [Google Scholar]
- 10.State Pharmacopoeia of the RF, Atomic absorption spectrometry, GPM 1.2.1.1.0005.18; https://docs.rucml.ru/feml/pharma/v14/vol1/771/
- 11.State Pharmacopoeia of the RF, Determination of aluminum ions in absorbed biological drugs, GPM 1.7.2.0016.15; https://docs.rucml.ru/feml/pharmz/v14/vol2/1103/
- 12.O. V. Fadeikina, Etalony Stand. Obraztsy, No. 2, 41 – 47 (2014).
- 13.State Pharmacopoeia of the RF, Validation of analytical methods, GPM. 1.1.0012.15; https://docs.rucml.ru/feml/pharma/v14/vol1/275/
- 14.GOST R ISO 5725-3-2002, Accuracy (trueness and precision) of measurement methods and results; https://docs.cntd.ru/document/1200029977.


