Abstract
The Established Status Epilepticus Treatment Trial was a blinded, comparative-effectiveness study of fosphenytoin, levetiracetam, and valproic acid in benzodiazepine-refractory status epilepticus. The primary outcome was clinical seizure cessation and increased responsiveness without additional anticonvulsant medications. Weight-based dosing was capped at 75 kg. Hence, patients weighing >75 kg received a lower mg/kg dose. Logistic regression models were developed in 235 adults to determine the association of weight (≤ or >75 kg, ≤ or >90 kg), sex, treatment, and weight-normalized dose with the primary outcome and solely seizure cessation. The primary outcome was achieved in 45.1% and 42.5% of those ≤75 kg and >75 kg, respectively. Using univariate analyses, the likelihood of success for those >75 kg (odds ratio [OR] = 0.9, 95% confidence interval [CI] = 0.54–1.51) or >90 kg (OR = 0.85, 95% CI = 0.42–1.66) was not statistically different compared with those ≤75 kg or ≤90 kg, respectively. Similarly, other predictors were not significantly associated with primary outcome or clinical seizure cessation. Our findings suggest that doses, capped at 75 kg, likely resulted in concentrations greater than those needed for outcome. Studies that include drug concentrations and heavier individuals are needed to confirm these findings.
Keywords: antiseizure medications, dose-response, ESETT, seizure cessation, weight-based dosing
1 |. INTRODUCTION
The Established Status Epilepticus Treatment Trial (ESETT), which completed enrollment in January 2019, was a multicenter, randomized, double-blind study to determine the best or worst second-line treatment among fosphenytoin (FOS), levetiracetam (LEV), and valproic acid (VPA) in patients with benzodiazepine-refractory status epilepticus (SE).1 The primary outcome of the study was cessation of SE at 60 minutes after the start of study drug infusion without use of additional antiseizure medication, as determined by absence of clinically apparent seizures and improved consciousness. Subjects aged ≥2 years who failed first-line treatment with benzodiazepines and continued to have seizures were included in this study.
To maintain the blind, the three drugs, FOS, LEV, and VPA, had to be administered at the same volume and infusion rate even though the drugs had different mg/kg doses.2 The FOS product label recommends a maximum dose of FOS (prodrug of phenytoin) of 20 mg phenytoin equivalents (PE)/kg and that the rate of intravenous administration should not exceed 150 mg PE per minute due to cardiovascular risks associated with rapid injection.3 Given that the ESETT protocol fixed the infusion time at 10 minutes, dosing was capped at 1500 mg PE. As a result, all patients weighing ≥75 kg received the same capped dose of FOS (20 mg/kg, maximum = 1500 mg PE). Similarly, weight-based dosing was also capped at 75 kg for LEV (60 mg/kg, maximum = 4500 mg) and VPA (40 mg/kg, maximum = 3000 mg).
Patients weighing >75 kg received a lower mg/kg dose; thus, lower drug exposure would be expected given the pharmacokinetic properties of these drugs. Therefore, we performed a secondary analysis to assess whether the odds of treatment success were lower in patients weighing >75 kg as compared to those weighing ≤75 kg. Because a primary outcome failure could be a result of one or more of the following: (1) need for an additional antiseizure medication before 60 minutes, (2) clinically apparent seizures at 60 minutes, and (3) lack of improvement in consciousness and response at 60 minutes, we also evaluated the association of weight and other predictors with clinical seizure cessation alone at 60 minutes.
2 |. MATERIALS AND METHODS
ESETT was approved by institutional review boards for all participating institutions.1 Of the 478 patients enrolled in ESETT, 48.2% of adults and 0.9% of children weighed >75 kg. Because of the low number of children receiving a fixed dose and the possibility of differing response rates within children and adults, the analyses were limited to those ≥18 years old (n = 249). Two patients were excluded because the study drug volume administered could not be determined. Among the 247 enrollments, 12 patients were enrolled more than once but only their first enrollments were used. Among the 235 unique adult patients, 132 (56.2%) failed the ESETT primary outcome. Of the 132 failures, 87 (65.9%) failed because they needed an additional antiseizure medication prior to 60 minutes, 10 (7.6%) failed due to clinically apparent seizures at 60 minutes, and 35 (26.5%) failed because they did not show an improvement in responsiveness at 60 minutes despite clinical seizure cessation.
2.1 |. ESETT primary outcome as the dependent variable
The ESETT primary outcome was expressed as binary (0 = treatment failure, 1 = treatment success) and used as the dependent variable for the following logistic regression models.
2.1.1 |. Association of weight with primary outcome using univariate and multivariate analyses
Two logistic regression models were used to test the association of weight, as a binary predictor, with primary outcome using weight cutoffs of 75 and 90 kg, respectively. A 90-kg cutoff was chosen to examine the association for higher weight individuals more rigorously. A logistic regression model also tested association of interactions of weight, sex, and treatment with the primary outcome. The model included treatment group (FOS, LEV, or VPA), sex (male or female), and weight as binary (≤ or >75 kg), with all the interaction terms (weight × treatment group × sex) as predictors of the primary outcome.
2.1.2 |. Association of weight-normalized dose and sex with primary outcome
Separate logistic regression models were built for FOS, LEV, and VPA to test the association of weight-normalized dose in mg/kg as a continuous variable, sex (male or female), and the interaction of dose and sex with the ESETT primary outcome.
2.1.3 |. Association of weight, sex, and treatment with clinical seizure cessation without additional antiseizure medication
A logistic regression model was used to test the association of weight and other predictors with clinical seizure cessation without additional antiseizure medication. Adult ESETT patients whose seizures were terminated but failed the primary outcome due to lack of improved responsiveness at 60 minutes (n = 35) were treated as successes. Clinical seizure cessation, as binary (1 = success, 0 = failure), was used as the dependent variable for this analysis. A logistic model with weight, as a binary (≤ or >75 kg), sex (male or female), and treatment group (FOS, LEV, or VPA) with all interactions (weight × treatment group × sex) as predictors was used to test their association with clinical seizure cessation.
Significance was determined as an alpha level < .05. All the analyses were conducted using R (v3.6.1), RStudio (v1.2.5001), and SAS (v9.4).
3 |. RESULTS
3.1 |. Distribution of weights
ESETT patients ≥18 years old weighed from 36 to 157 kg and weights were approximately normally distributed, with a mean of 76.7 kg and standard deviation of 18.9 kg (Figure 1). Of the 235 patients, 113 (48.1%) weighed >75 kg and received the maximum doses. The overall success rate for the primary outcome was 45.1% in those ≤75 kg versus 42.5% in those >75 kg. Baseline characteristics of the adult population by weight group (Table S1) show that male patients were more likely to weigh >75 kg (50% vs 66.4%), but all the other baseline characteristics were evenly distributed between the ≤75-kg and >75-kg groups, respectively.
FIGURE 1.
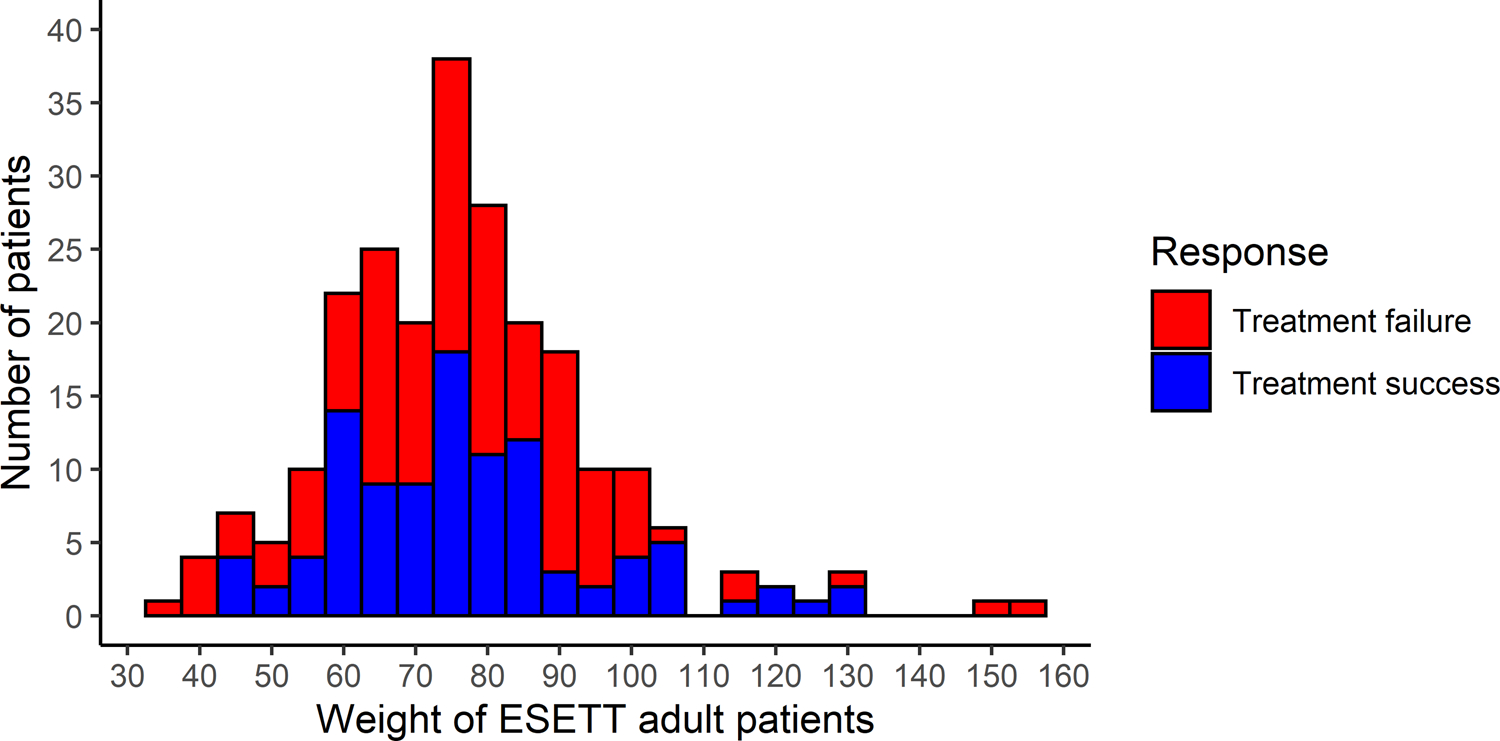
Distribution of Established Status Epilepticus Treatment Trial (ESETT) adult patient weights and the response to the treatment administered as treatment success (blue) or treatment failure (red)
3.2 |. Comparison of response rates between the weight-based dosing group and fixed dose group
The difference and 95% confidence interval (CI) in the response rates for those ≤75 kg versus those >75 kg were 3.1% (95% CI = −20.5% to 26.6%) for FOS, −1.2% (95% CI = −21.6% to 19.3%) for LEV, and 6.4% (95% CI = −16.1% to 28.9%) for VPA. None of the differences was statistically significant, as the 95% CI included 0 for each drug.
3.3 |. Association of weight and other predictors with primary outcome using univariate and multivariate analyses
3.1.1 |. Primary outcome versus weight
The odds of success were 10.1% lower (odds ratio = 0.9, 95% CI = 0.54–1.51) for those >75 kg compared to those ≤75 kg and 15.4% lower (odds ratio = 0.85, 95% CI = 0.42–1.66) for those >90 kg compared to those ≤90 kg. These differences were not significant, as the 95% CIs for the odds ratios included 1. Similarly, there was no statistically significant association with treatment success when sex, treatment group, and interaction of weight with sex and treatment group were included in the model (Table 1).
Table 1:
Logistic regression models of the probability of success using weight, treatment and sex with all interactions (weight ≤ 75 kg as reference group) (n=235)
| Weight-group | Treatment | Sex |
Using primary outcome as dependent variable Adjusted Odds Ratio (95% confidence interval) |
Using clinical seizure cessation without additional antiseizure medication as dependent variable Adjusted Odds Ratio (95% confidence interval) |
|---|---|---|---|---|
| > 75 kg | Fosphenytoin | Male | 0.71 (0.20, 2.55) | 0.56 (0.14, 2.26) |
| > 75 kg | Fosphenytoin | Female | 1.13 (0.26, 4.94) | 1.16 (0.27, 5.05) |
| > 75 kg | Levetiracetam | Male | 0.82 (0.26, 2.56) | 0.47 (0.15, 1.49) |
| > 75 kg | Levetiracetam | Female | 1.70 (0.45, 6.44) | 1.08 (0.29, 4.08) |
| > 75 kg | Valproic acid | Male | 0.83 (0.26, 2.66) | 0.72 (0.22, 2.40) |
| > 75 kg | Valproic acid | Female | 0.64 (0.14, 2.91) | 0.49 (0.11, 2.20) |
3.3.2 |. Primary outcome versus sex and weight-normalized dose
When each drug was modeled separately, the weight-normalized dose was not associated with success, nor was sex or the interaction of dose and sex (Table S2).
3.4 |. Association of weight, sex, and treatment with clinical seizure cessation without additional antiseizure medication
A total of 138 (59%) patients did not have clinically apparent seizures at 60 minutes without receiving additional antiseizure medication (regardless of whether they were responsive to verbal commands or noxious stimuli). As seen from Table 1, weight (≤ or >75 kg), sex (male or female), treatment group (FOS, LEV, or VPA), and all the interaction terms (weight × sex × treatment group) did not have a significant association with clinical seizure cessation.
4 |. DISCUSSION
The results of these secondary analyses demonstrate that the differences in response rates between the fixed dosing regimen (>75 kg) and weight-based regimen (≤75 kg) were not significant when the study drugs were grouped together or analyzed separately. The logistic regression models using the ESETT primary outcome and clinical seizure cessation without additional antiseizure medication as dependent variables also failed to find significant associations with weight, treatment, sex, or weight-normalized dose.
Fixed dosing, which is commonly used in adults, results in lower doses per body weight in heavier individuals and potentially lower drug concentrations for many drugs. Furthermore, if drug concentrations fall in the linear portion of the dose-response curve, lower drug concentrations may result in reduced efficacy. In this study, although approximately half of the ESETT adult patients received the maximum dose, the response rates between weight-based and fixed dosing regimen were similar. It is possible that weight or weight-normalized dose did not affect the primary outcome or clinical seizure cessation because the doses used in the trial resulted in drug concentrations greater than those needed for therapeutic outcome even in patients weighing >75 kg. Although this may be true, other predictors, such as drug concentration, would have been a better metric to evaluate the differences between responders and nonresponders. We know that drug concentrations can be variable in individuals receiving an identical dose.4–6 There is also evidence that the pharmacokinetics of FOS, LEV, and VPA are altered in overweight and obese patients.7–10 In particular, patients with higher body fat will likely have greater volume of distribution. However, we were not able to investigate the effect of drug concentrations or body mass index (BMI), as sufficient information was not available. Furthermore, only 18 (7.7%) patients weighed >100 kg. Thus, differences in pharmacokinetics, if any, may not have been large enough to impact the outcome.
The ESETT primary outcome was a composite and included absence of clinically apparent seizures and improved responsiveness at 60 minutes. It is possible that those who received higher doses were more likely to stop seizing but also more likely to have no improvement in responsiveness. To tease out the association of weight and other variables with clinical seizure cessation alone, we included those who failed the primary outcome only due to the lack of improved responsiveness at 60 minutes as successes, but found no significant differences between fixed and weight-based dosing. Future studies of SE will likely include electroencephalogram as a part of outcome and allow us to better understand this subgroup.
A limitation of these analyses is the small number of patients in each treatment group (~40/drug) weighing >75 kg. The wide CIs for the difference in response rates suggest that a larger sample size would be needed to confirm these findings. While these were secondary analyses, the adaptive study design was powered adequately for the primary outcomes.
5 |. CONCLUSION
Weight-based dosing used in ESETT with a 75-kg cutoff does not appear to have an impact on the primary outcome or clinical seizure cessation. It is possible that the concentrations attained were greater than those needed for therapeutic outcome. However, studies with larger sample size and additional data (drug concentrations, BMI, etc) are required to confirm our findings. Future studies that measure drug concentrations would allow exploration of exposure-response instead of dose-response relationships.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this article was supported by the National Institutes of Health, National Institutes of Neurological Disorders and Stroke under awards U01NS088034, U01NS088023, U01NS056975, U01NS059041, and R01NS099653. Clinicaltrials.gov identifier is NCT01960075. A.G.S. was supported in part by the OLSTEINS Graduate Fellowship from the College of Pharmacy, University of Minnesota. We would like to acknowledge the ESETT Data and Safety Monitoring Board. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke, National Institutes of Health, or the United States Government.
Funding information
National Institute of Neurological Disorders and Stroke, Grant/Award Number: R01NS099653, U01NS056975, U01NS059041, U01NS088023 and U01NS088034; College of Pharmacy, University of Minnesota
Footnotes
CONFLICT OF INTEREST
H.R.C. received consulting fees from BIAL Pharma UK and Sage Therapeutics; her institution has received funds for her work on other trials from UCB Pharma, Eisai Europe, Novartis, and GW Pharma. N.B.F. reports research grants awarded to the University of Virginia with N.B.F. as principal investigator from the Epilepsy Foundation, InSightech, UCB, SK Life Sciences, GW Pharmaceuticals, Neurelis, Takeda, Medtronic, and Cerebral Therapeutics. D.H.L. has served on the scientific advisory board of Bloom Science. J.C.C. received consulting fees from Neurelis Pharmaceuticals and reports funding awarded to University of Minnesota from Neurelis, holds a patent US9629797B2 on intravenous carbamazepine and intellectual property on intravenous topiramate, licensed to Ligand. The remaining authors have no conflicts of interest. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- 1.Kapur J, Elm J, Chamberlain JM, et al. Randomized trial of three anticonvulsant medications for status epilepticus. N Engl J Med. 2019;381(22):2103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cock HR, Coles LD, Elm J, et al. Lessons from the estab- lished status epilepticus treatment trial. Epilepsy Behav. 2019;2019(101):106296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CEREBYX® (fosphenytoin sodium injection)[package insert]. New York, NY: Pfizer Injectables; 2015. [Google Scholar]
- 4.Tanaka J, Kasai H, Shimizu K, Shimasaki S, Kumagai Y. Population pharmacokinetics of phenytoin after intravenous administration of fosphenytoin sodium in pediatric patients, adult patients, and healthy volunteers. Eur J Clin Pharmacol. 2013;69(3):489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uges JWF, van Huizen MD, Engelsman J, et al. Safety and pharma- cokinetics of intravenous levetiracetam infusion as add-on in status epilepticus. Epilepsia. 2009;50(3):415–21. [DOI] [PubMed] [Google Scholar]
- 6.Park H-M, Kang S-S, Lee Y-B, et al. Population pharmacokinetics of intravenous valproic acid in Korean patients. J Clin Pharm Ther. 2002;27(6):419–25. [DOI] [PubMed] [Google Scholar]
- 7.Clark SL, Leloux MR, Dierkhising RA, et al. IV fosphenytoin in obese patients. Neurol Clin Pract. 2017;7(1):45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abernethy DR, Greenblatt DJ. Phenytoin disposition in obesity: determination of loading dose. Arch Neurol. 1985;42(5):468–71. [DOI] [PubMed] [Google Scholar]
- 9.Kuranari M, Chiba S, Ashikari Y, et al. Clearance of phenytoin and valproic acid is affected by a small body weight reduction in an epileptic obese patient: a case study. J Clin Pharm Ther. 1996;21(2):83–7. [DOI] [PubMed] [Google Scholar]
- 10.Alzueta N, Ortega A, Aldaz A. Influence of sex, age, and weight on levetiracetam pharmacokinetics. Ther Drug Monit. 2018;40(5):628–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


