Abstract
Mycoplasma genitalium (MG) is an emerging sexually transmitted pathogen. It is an important cause of nongonococcal urethritis in men and is associated with cervicitis and pelvic inflammatory disease in women, putting them at risk of infertility. Multiple factors that aid pathogenesis of MG include its ability of adhesion, gliding motility, and intracellular invasion by means of the tip organelle. Through intracellular localization and antigenic variation, MG could result in treatment-resistant chronic infection. There are limited data on the prevalence of MG in Indian patients with urogenital syndromes. Recently, a high prevalence of extra genital infection with MG has been reported. Molecular assays are the major diagnostic techniques of MG infection. Antimicrobial agents such as macrolides, along with fluoroquinolones, are the treatment of choice for MG infections. The issue of drug resistance to azithromycin and fluoroquinolones in MG is rising globally. As molecular tests are becoming available for MG, both for the diagnosis and the detection of antimicrobial resistance, any patient with MG infection should then be tested for antimicrobial resistance. Consideration of MG as a cause of sexually transmitted disease in the Indian population is crucial in diagnostic algorithms and treatment strategies. The purpose of this review is to understand the prevalence of MG in different clinical scenarios, molecular mechanisms of pathogenesis, current status of antimicrobial resistance, and its impact on MG treatment.
Keywords: Antimicrobial resistance, extragenital infections, Mycoplasma genitalium, pathogenesis
Introduction
Mycoplasma genitalium (MG) is an emerging cause of sexually transmitted infection (STI) in both men and women. It is a common cause of nonchlamydial nongonococcal urethritis (NCNGU) in men and cervicitis, pelvic inflammatory diseases (PIDs), and tubal infertility in women.[1] The organism may also play a role in increasing the risk of human immunodeficiency virus (HIV) infection.
MG was first isolated in 1980 from the urethral swabs of two homosexual men. G37 and M30 were the first MG strains to be isolated.[2] MG is the smallest free living, self-replicating microbe with minimal genome (580 kb). It was the first bacteria to be fully sequenced and the first genome to be chemically synthesized.[3]
MG belongs to the class Mollicutes and family Mycoplasmataceae that colonizes the male and female reproductive tract. Multiple species of Mycoplasmataceae family are present as both commensals and pathogens of human genital tract. The pathogenic genital Mycoplasmas include MG, Mycoplasma hominis, Ureaplasma urealyticum, and Mycoplasma fermentans, whereas Mycoplasma penetrans is of doubtful pathogenicity. Mycoplasma primatum and Mycoplasma spermatophilum are the nonpathogenic genital Mycoplasmas.[4]
Remarkably, MG has a disturbing capacity to develop resistance to the major antimicrobials available against it: macrolides and fluoroquinolones. The lack of peptidoglycan in MG precludes the use of antibiotics acting on the cell wall. Although resistance in other STI pathogens such as gonococcus has increased insidiously, resistance in MG has emerged at a relatively greater speed belying its small size.[2] This review on MG focuses on the recent advances in the developments of its molecular pathogenesis and antimicrobial resistance. We surveyed PubMed literature and Google Search engine using the terms “Mycoplasma,” “MG,” and “Genital Mycoplasma.” The relevant literatures were selected to provide current perspectives of MG.
Genome of Mycoplasma Genitalium
MG has a minimal genome size of 580 kbp. The GC content is approximately 31%. MG evolved from clostridium-like Gram-positive bacteria by genomic reduction process during which it lost most of the genes including the genes for enzymes involved in amino acid synthesis, de novo nucleic acid synthesis, and synthesis of fatty acid. Hence, MG relies on the host for several metabolic growth factors. MG type strain G37 was the second bacterial genome to be fully sequenced.
Pathogenesis
Multiple factors that aid pathogenesis of MG include the ability for adhesion, gliding motility, and cell invasion. All these functions are performed by a specialized tip structure. However, the mechanisms by which MG is able to maintain infection within the stratified squamous epithelia of the vagina and ectocervix despite normal sloughing of the apical most layers remains unknown.[5]
Tip organelle
The tip organelle is a multisubunit dynamic motor[6] made of the following three parts: terminal button, segmented pair plates, and wheel complex. Each part consists of different proteins. Table 1 shows genes encoding for tip organelle proteins and their subcellular localization.[7]
Table 1.
Genes encoding for tip organelle proteins and their subcellular localization[7]
| Gene | Protein | Sub cellular localization |
|---|---|---|
| MG191 | P140 | Surface of TO |
| MG192 | P110 | Surface of TO |
| MG200 | DNAj like protein | Wheel complex |
| MG217 | P65 homolog | Terminal button |
| MG218 | HMW2 | Rod |
| MG491 | MG491 | Wheel complex |
| MG219 | MG219 | Wheel complex |
| MG312 | HMW1 | Rod |
| MG317 | HMW3 | Terminal button |
| MG318 | P32 | Terminal button |
| MG386 | P200 | Wheel complex |
TO=Tip organelle; MG=Mycoplasma genitalium; HMW=High molecular weight
Terminal button
The terminal button is the distal end of the tip organelle. It comprises P110, P140, P65, P32, and HMW1 proteins. Among these proteins, P110 and P140 are the major adhesions encoded by MG192 and MG191, respectively.[8] These two proteins are immunological determinants and important for the organism to adhere to the host epithelial cells. They are also required for the proper development of the terminal organelle. Burgos et al. showed that MG191 and MG192 mutant cells showed a loss of terminal organelle, suggesting the absolute requirement of both the P140 and P110 proteins for the proper development of such a structure.[9]
Adhesion proteins of MG and P1 protein of Mycoplasma pneumoniae exhibit homology at both DNA and protein level. At DNA level, 48% of coding sequence of adhesion genes of MG was 60%–70% homologous to the sequence of P1 adhesion gene. Figure 1 is showing clinical samples positive for MG using Mgpa1 and Mgpa3 primers for amplification. At protein level, 85% of the deduced amino acid sequence of MG adhesin exhibited 42%–74% identity with M. pneumoniae P1 protein.[10]
Figure 1.
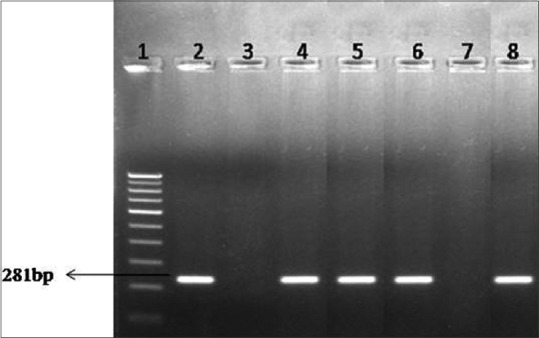
Agarose gel electrophoresis for Mycoplasma genitalium. Lane 1: 100 bp DNA ladder, Lane 2: Positive control, Lane 3: Negative control, Lane 4–6: Clinical samples-positive, Lane 7: Clinical sample-negative, Lane 8: Clinical sample-positive
The functions of P65 and HMW1 proteins include the determination of the curvature of terminal button and for assembly of the tip organelle, respectively.[9]
Segmented pair plates
They form an electron dense core that is a part of the Mycoplasma cytoskeleton. HMW3 protein is involved in the formation of terminal button and contributes to anchoring the electron dense core to the cell membrane.[7]
Wheel complex
The proximal end of the electron dense core is in contact with the wheel complex. It is connected to the cell periphery by fibrils and it is thought to be the connecting link between terminal organelle and cell body. The wheel complex contains MG219, MG200, MG386, and MG491 proteins and which have been implicated in gliding motility.[7]
Virulence Factors
Adhesion
It is an essential process for MG pathogenicity. Adhesion is the primary step in the initiation of an infection/colonization. MG adheres to the plastic and glass surfaces, epithelial cells,[11] spermatozoa,[12] and erythrocytes. Adhesion process is achieved by the major adhesion proteins P140 and P110.
Gliding motility
MG does not contain flagellum for its motility. Gliding motility of this organism is achieved with the help of the specialized tip organelle, mainly by the proteins that are forming the wheel complex (MG200, MG386, MG219, and MG491). Of these proteins, MG 200 and MG386 are specific for gliding motility.[13]
Luca observed that MG491-deficient MG showed alteration in gliding motility.[7] Burgos et al. showed that MG191 and MG192 negative mutants showed decreased MG386 protein. This observation reinforces the close connection between gliding motility and adherence machineries.[9]
Gliding motility is essential for the penetration of MG into the mucus layer covering the epithelial cells followed by adhesion to the epithelial cells and invasion.[2]
Intracellular localization
MG is a facultative intracellular pathogen. After attaching to the epithelial cells, cell entry is mediated by the tip organelle. Exact mechanism of entry of the organism is still not knsown. In a study by Mernaugh et al., attachment of MG into human lung fibroblasts was followed by the formation of cup or depression in the plasma membrane. The membrane pockets resembled clathrin-coated pits, suggesting that the Mycoplasma might adhere to and enter the cells by a site-directed, receptor-mediated event resembling cell entry by Chlamydiae.[14] Ueno et al. observed intranuclear localization of MG proteins.[15] Intracellular localization protects the organism from the both host immune system and antibiotics. It also promotes chronic and latent infection.[16,17]
Enzymes
MG lacks toxins and secreted virulence factors. However, MG186 acts as a calcium-dependent membrane-associated nuclease which degrades the host nucleic acid and provides nucleotide precursors for growth and pathogenic processes.[18]
Genetic variation
MG is able to generate a high frequency of intragenomic variation in nucleotide sequence or DNA arrangement at selected chromosomal loci (Mgpar islands and Mgpa operon) promoting random phenotypic variation as a result of constantly changing host environment.
Mgpar islands are found in the genome of MG. Mgpar islands are a family of repetitive DNA elements with homology to the MgPa adhesin gene (MgpB/MgpC).[19] As explained earlier, MG191 (MgpB) and MG192 (MgpC) encode for P140 and P110 major adhesins, respectively. Both these genes are present in the Mgpa operon. These Mgpar islands do not express protein coding sequences but are involved in genetic variation through recombination between Mgpar islands and Mgpa operon.[20]
There are two types of genetic variation; antigenic variation and phase variation.
Antigenic variation
In order to escape host immune response, MG alters its entire genetic sequence of the Mgpa adhesion gene with subsequent generation of variants that are not recognized by the host immune system on subsequent encounters.
Mechanism for antigenic variation
Antigenic variation arises when the variable region of the expression site (MgpB/MgpC) exchanges sequences through segmental recombination with ≥1 of Mgpar sites.[20] This recombination process is reciprocal. Furthermore, antigenic diversity achieved by initial recombination within Mgpar islands followed by recombination with MgpB/MgpC genes.[21]
Phase variation
In phase variation, the organisms will lose their ability to adhere to the epithelial cells due to loss of their major adhesion proteins.
Mechanism of phase variation
It arises by multiple recombination processes involving both the variable and conserved regions of expression sites (MgpB/MgpC) with Mgpar islands. This recombination results in translocation of the conserved MgpB/MgpC sequences to participating Mgpar sites, thereby leaving an incomplete Mgpa operon.[20]
Regulation of recombination
Recombination is a highly regulated process. In MG, MG428 is a positive regulator of this recombination process.[22] It co-ordinates the expression of the key genes of recombination such as recA, ruvA, ruvB, and other proteins required for recombination.[23] MG428 is considered as an alternative sigma factor since, it binds to the RNA polymerase and unique promotor sequence present upstream of the MG428-activated gene in response to external stimuli.
Clinical Scenarios
MG has potential to cause clinical disease, in both men and women but more so than women. Despite its identification nearly four decades ago, much remains unclear. While there is a clear association with NGU in men, the clinical evidence that it causes epididymo-orchitis, proctitis, reactive arthritis, and facilitates HIV transmission in men is weak, although biologically plausible. It is not known how long asymptomatic infection persists in untreated men, nor the risk of developing disease if left untreated. Although there is evidence of sexual transmission from male to female, it is unclear how often this occurs and of the risk of developing reproductive tract disease. Summary of studies showing the prevalence of MG in different population groups is mentioned in Table 2.
Table 2.
Studies from India showing prevalence of Mycoplasma genitalium in different population groups using polymerase chain reaction
| Year of study | Study population | Sample tested | Test method | Prevalence of MG (total number of sample tested), n (%) |
|---|---|---|---|---|
| Dhawan et al., 2020[80] | MSMs | FVU Rectal swab Oropharyngeal swab |
PCR | 46 (30.4) |
| Rekha et al., 2019[115] | Infertility | Peritonial fluid | PCR | Infertile 162 (6.1) Fertile 162 (0.6) |
| Saigal et al., 2016[116] | STIC | Endocervical/urethral swabs, FVU | PCR | 164 (1.2) |
| Rajkumari et al., 2015[117] | Infertility | FVU Endocervical swab Endometrium biopsy |
PCR | 100 (16) |
| Kokkayil et al., 2013[89] | Infertility | Endocervical swab | PCR | Co-infection of MG and CT in one patient |
| Ghosh et al., 2011[118] | HIV population | FVU | PCR | 100 (0) |
| Manhas et al., 2009[119] | HIV infected men with NGU | Urine | PCR | HIV positive: 70 (7.1) HIV negative: 30 (3.3) |
STIC=Sexually transmitted infection clinic; FVU=First void urine; PCR=Polymerase chain reaction; MSMs=Men who have sex with mens; MG=Mycoplasma genitalium; CT=Chlamydia trachomatis; NGU=Nongonococcal urethritis; HIV=Human immunodeficiency virus
Relationship Between Mycoplasma Genitalium and Disease in Men
Acute nongonococcal urethritis
MG was isolated initially from men with acute nongonococcal urethritis (NGU). In numerous studies, MG has been strongly and almost uniformly associated with acute NGU, in which diagnosis was done by microscopy. In one study,[24] in which the diagnosis was based on clinical symptoms and signs only, the association with MG was weaker, as those subjects were not recorded as having NGU but had microscopic evidence of disease. Overall, MG has been detected in the urethras of 15%–25% of men with symptomatic NGU, compared to about 5%–10% of those without disease. Among sexually transmitted disease (STD) clinic populations, 90% of MG-infected men have microscopic evidence of urethritis, with a complaint of discharge being more common than in NGU of other etiologies.[25] Indeed, there is evidence that MG is more closely associated with symptomatic than with asymptomatic NGU.[26,27] Furthermore, the development and the use of quantitative polymerase chain reaction (PCR) assays for MG have shown greater MG DNA loads in urine from men with NGU than in urine from those without the disease.[28,29] It is noteworthy that the association between Mycoplasma and disease is even stronger for acute NCNGU,[30,31] with the Mycoplasma being found in more than one-third of men with such disease, indicating that MG and Chlamydia trachomatis act as separate causes of the condition.
Chronic nongonococcal urethritis
Persistent or recurrent NGU following an acute attack was noted by Hooton et al.[32] to be associated with MG. Since then, MG has been found in up to 40% of men presenting with chronic disease after treatment with doxycycline.[33] Indeed, in several clinical studies,[27,34,35] a strong correlation was found between MG infection and persistent or recurrent NGU, probably due to tetracyclines and more recently, azithromycin[36] eradicating MG from only a subset of the patients.[37]
Balanoposthitis
Inflammation of the glans penis (balanitis) and inflammation of the prepuce (posthitis) frequently occur together (balanoposthitis). In one study,[38] MG was associated significantly (P = 0.01) with balanitis and/or posthitis in 114 men with acute symptomatic NGU.
Chronic prostatitis
Association of MG with chronic prostatitis is sparsely evidenced. In a study by Doble et al., MG could not be detected by PCR on ultrasound guided transperineally derived prostatic biopsy samples in fifty patients with chronic abacterial prostatitis.[39] In another study,[40] MG was detected by a PCR assay in prostatic biopsy specimens from 5 (4%) of 135 men, and in yet another study,[41] MG was detected in semen from 2 (11%) of 18 men with chronic abacterial inflammatory prostatitis, compared to 20 controls, showing insufficient evidence to suggest any significant association.
Acute epididymitis
Detection of MG in few patients during an antibiotic trial[42] indicated that MG may be a cause of acute epididymitis in some patients. An undoubted causal involvement is certainly true in the case of C. trachomatis and by analogy might well be so for MG. To firmly establish this, epididymal fluid should be examined whenever possible in addition to urine and/or urethral swabs.[43]
Diseases in Women
Nongonococcal urethritis
There is evidence for an association between MG and urethritis in women attending STD clinics.[44,45] The observations have been made mainly in Scandinavia, where examination of urethral smears from women is a part of routine STD examinations, and the numbers of these infections are few compared with those in men. It is clear that further studies are warranted, as it is not yet fully clear to what extent MG is involved in symptomatic or asymptomatic pyuria or in the so-called “urethral syndrome” (dysuria and frequency in women with apparently sterile urine).
Bacterial vaginosis and vaginitis
MG was first detected in the lower genital tract of about one-fifth of women attending an STD clinic at St. Mary's Hospital, London, United Kingdom,[46] and in cervical samples from 5 of 74 women in Copenhagen, Denmark.[47] However, unlike M. hominis, which is very strongly associated with bacterial vaginosis (BV),[48] the association of MG with BV is controversial. In some studies,[49,50] there was no evidence that MG played any part in BV, while in a one of the study,[51] the presence of MG in women was independently associated with BV, being more common in women with BV than in those without the condition. Gonococcal and chlamydial infections are not known for causing inflammation of the vagina in sexually mature women. However, aerobic vaginitis with aerobic bacteria has been described,[52] and infection of vaginal cells in vitro[17] and skin cells in balanoposthitis[38] by MG raises the intriguing question of whether it might cause vaginitis in vivo.
Cervicitis
The first evidence of an association of MG with cervicitis came from a Japanese study, reported in 1997,[53] in which MG was detected in the cervices of 5 (9%) of 57 women with cervicitis but in none of 79 women without the condition. Subsequently, the results of other studies,[30,54] to a large extent attest to MG having a significant role in causing cervicitis. In another study,[55] MG was the only genital Mycoplasma/ureaplasma regarded as causing cervicitis.
Pelvic inflammatory disease
Further evidence for MG causing PID is (i) the ability of the organisms to adhere to Fallopian tube mucosal epithelial cells in organ culture[56] and to affect the cells and cause ciliary damage,[57] (ii) the production of endometritis and salpingitis experimentally in several subhuman primate species[58,59] and hydrosalpinx formation in mice,[60] (iii) the association of tubal factor infertility with a previous infection with MG,[61] and (iv) the demonstration of MG antibody responses in one-third of women with acute PID, a finding disputed by some investigators.[62,63] In summary, the overall supportive aspects have led to the conclusion that MG is one of the causes of PID.[64] PID comprises endometritis and/or salpingitis.
Endometritis
In an early study[65] on endometritis, MG was reported to have been detected in endometrial biopsy specimens from women with clinically suspected PID. In another study,[66] MG was found to be strongly associated with acute endometritis, being detected in 9 (16%) of 58 women with histologically diagnosed endometritis, but in only one (2%) of 57 women without endometritis.
Salpingitis
There have been few studies, in which the fallopian tubes have been examined at laparoscopy. In one study,[67] MG was detected in the cervix/endometrium of 9 (7%) of 123 women with acute salpingitis but in only a single tube.
Reproductive Disease in Women
In relation to pregnancy outcome, there is evidence that MG alone or in combination with other microorganisms causes some cases of PID. As this disease damages Fallopian tubes,[57] there is a small chance that such a prior Mycoplasmal infection could be responsible for an ectopic pregnancy. However, a serological study provided no support for this.[62] In consideration of the poor pregnancy outcomes of spontaneous preterm labor (SPTL) and preterm birth (PTB), which have been shown to occur for women with BV,[68] few studies[69,70,71] suggested that MG was unlikely to be responsible for such outcomes, whereas in two other studies,[72,73] it was reported to be a significant independent risk factor for SPTL and PTB. M. hominis is considered to be responsible for some cases of maternal fever after a normal delivery or abortion,[74] but the role, if any, of MG has not been assessed.
Complications
Infertility
MG affects the motility of human spermatozoa.[12] Whether this could reduce male fertility in vivo is unknown. MG is known to cause PID, this could result in tubal damage and occlusion and subsequent infertility. Two seroepidemiological studies[61,75] have shown an association with tubal factor infertility, with 17%–22% of women having MG antibodies, compared to 4%–6% of women with normal tubes. There is a chance that women who have had a prior infection with MG could also be at a higher risk for infection by sexually transmitted organisms other than C. trachomatis, which might cause infertility.
Arthritis
Sexually acquired reactive arthritis or the less common Reiter's disease, in which conjunctivitis also develops, occurs in men who have or have recently had NGU and less often in women. MG was detected from adult with conjunctivitis which was not a part of Reiter's disease. MG was detected in the knees of 2 of 13 patients with arthritis, one of whom had Reiter's disease and another one had seronegative arthritis.[76] In addition, clinical experience indicates that reactive arthritis occurs occasionally in patients with MG genital tract infections. MG has also been reported with or without M. fermentans and C. trachomatis in 9 (35%) of 26 “deranged” temporomandibular joints considered possibly of a reactive nature.[77]
Extragenital Manifestations
Proctitis
Trends in oral and anal sex have increased over the past decades; anal intercourse has doubled over a 10-year period. Sexually transmitted proctitis is commonly caused by Neisseria gonorrhea and C. trachomatis. Proctitis is commonly observed in MSM. MG is also a cause of sexually transmitted proctitis. It is mostly an asymptomatic infection. In symptomatic patients, it manifests as rectal pain and anal discharge. However, severity of symptoms of proctitis due to MG is less as compared to C. trachomatis and Neisseria gonorrhea. Prevalence of proctitis associated with MG showed in studies done by different countries. In a study by Francis et al. from the USA, 5.4% of the rectal swabs collected from 500 MSM positive for MG.[78] Bissessor et al. from Australia observed that the prevalence of MG in MSM with HIV infection was more as compared to those without HIV (21% vs. 8%) also the load of the organism was higher in symptomatic patients as compared to asymptomatic patients.[79]
In a study from India, the prevalence of MG in MSM was 41.3% of the infected patients, anorectal infection was observed in 68.4% of cases.[80] Furthermore, there is a co-infection of MG with C. trachomatis and N. gonorrhea. Latimer et al. reported co-infection of MG with C. trachomatis and N. gonorrhea at the anorectal site ranging from 13% to 14%.[81] MG has been found in the anorectal region, but its pathogenicity in causing clinical proctitis has not been elucidated and more research is required.
Oropharyngeal infection
Oropharyngeal infection is usually asymptomatic. Prevalence of pharyngeal infection due to MG is less as compared to anorectum. Couldwell et al.[82] and Dhawan et al.[80] in their studies did not detect any MG in oropharyngeal infection. However, Jiang et al. showed a prevalence of 13.5% oropharyngeal infection.[83]
Co-infections
Co-infection of MG with other pathogens has been observed by many authors. In a study by Getman et al.,[84] a lower prevalence of co-infections of MG with other sexually transmitted organisms was seen. However, in a study by Gaydos et al.,[85] the percentage of co-infections of MG with another organism for those infected with at least one organism ranged from 30.6% for TV-infected women to 73.3% for NG-infected women. In a study by Yokoi et al.,[86] rates of co-infection with MG among men with gonococcal urethritis were shown to be low (4.1%), compared with the C. trachomatis co-infection rate (21.2%). Another study evaluated the prevalence of MG co-infection in 302 chlamydia-infected women at a STD clinic in Birmingham. Co-infection of MG was detected in 22 (7.3%).[87] In West Africa, Pépin et al.[88] showed that almost half of the infections due to MG occurred as co-infections. The prevalence of co-infection with gonococcal urethritis, C. trachomatis, and TV was 37.9%, 10.6%, and 7.6%, respectively. In a study from India, co-infection of both MG and C. trachomatis was found in 5 (10.8%) in MSMs diagnosed with urethritis[80] co-infection in patient with MG and C. trachomatis in an infertile female patient with genital tuberculosis has also been reported. Co-infections among genital Mycoplasmas have also been reported.[89] In a study by Darkahi,[90] simultaneous occurrence of MG and U. urealyticum was shown in 1.4% of women with genital infections, while triple infection of MG, U. urealyticum, and M. hominis was seen in 0.5% of patients.
Infection in Immunodeficient or Immunosuppressed Patients
About a decade ago, it was reported that more than 50% of men who had AIDS but no urethritis were MG positive. A study by Loubinoux et al.[91] failed to detect MG in urine from 54 HIV-positive patients. More recently, it was reported[92] that MG was found much more frequently at both urethral and rectal sites of HIV-positive MSM than HIV-negative MSM. MG-induced cervicitis[55] has been shown to occur more often in HIV positive than in HIV-negative women, and the Mycoplasma has been found more frequently in endometrial biopsy specimens of women who were HIV positive[65] and can persist longer in HIV-positive women.[93]
Diagnostic Tests for Mycoplasma Genitalium
Availability of nucleic acid amplification testing for MG is limited in India. Testing is currently available at some tertiary care hospitals. A summary of studies from India showing prevalence of MG in different population groups using PCR is shown in Table 2. Isolation and culturing of MG is slow, time consuming, and not feasible when there is a need to institute immediate antimicrobial therapy. Therefore, nucleic acid amplification test (NAAT) is the preferred diagnostic method where feasible.
Although research companies have quantitative PCR detection kits in the market, the United States Food and Drug Administration has not approved any of these methods for the clinical screening or detection of MG Vandepitte et al.[94] compared two commercially available kits (TIB MOLBIOL LightMix kit) and the Diagenode MG (real-time PCR kit) as well as an in-house PCR method using the Roche Diagnostics cobas z 480 analyzer[95] TIB MOLBIOL LightMix kit targeted the MG219 gene, Diagenode MG real-time PCR kit targeted the gap gene, and the in-house kit targeted the Mgpa1 adhesion protein gene. The commercial kits had a sensitivity of 92.6% and 87%, respectively, and a specificity of 100% which was concordant with the in-house kit that was >95%.
In an effort to establish a simpler and streamlined protocol for MG detection, Takanashi et al. developed a PCR test using Invader Plus technology, carrying out both the endonuclease and PCR in the same simple step.[96] This approach would require less genetic material and would be of less labor and would be time consuming. The approach was tested with first-void urine samples. The Invader Plus assay was comparable to typical hybridization microtiter PCR and was able to detect as few as 10 DNA copies per reaction.
Genotype Assays for Predicting Resistance Phenotype
Another opportunity for the detection of MG is establishing genetic markers of resistance to first-line therapy. It is currently recommended that detection of MG is followed by testing for mutations associated with macrolides and fluoroquinolone resistance in order to guide antibiotic treatment.[97]
There has been considerable recent progress in developing molecular tests to evaluate resistance mechanisms for MG that is difficult (or slow) to culture and for which resistance to frontline antibiotics is a serious concern. MG exhibits considerable resistance to fluoroquinolones and macrolides. A previously published report demonstrated excellent results for a multiplex PCR assay designed to detect MG, as well as mutations in MG 23S rRNA associated with macrolides resistance.[98] Fernández-Huerta et al. described an assay for simultaneous detection of MG and mutations to subunit A of topoisomerase IV (Par C) that lead to fluoroquinolone resistance.[99] In both studies, multiplex PCR assays were compared to Sanger sequencing. Macrolide resistance was predicted in 63% of MG clinical isolates;[98,99] fluoroquinolone resistance was predicted in 8.8% (Spanish cohort) to 23.4% (Australian cohort) of MG clinical isolates.[99] A similar approach was taken to detect macrolide resistance in M. pneumoniae in a Pennsylvania cohort,[100] wherein investigators found 7.5% of isolates were predicted to be resistant to macrolides. Summary of the laboratory studies of MG antimicrobial susceptibility and genotypic resistance testing in the literature subsequent to the report by Couldwell et al., 2015, is mentioned in Table 3.
Table 3.
Summary of the laboratory studies on Mycoplasma genitalium antimicrobial susceptibility and genotypic resistance testing in the literature subsequent to the report by Deborah L Couldwell and David A Lewis, 2015
| Reference | Study type | Population | MG DNA extracts or isolates examined | Macrolide resistance (MIC data/resistance mutations) | Fluoroquinolone resistance (MIC data/resistance mutations) | Comments |
|---|---|---|---|---|---|---|
| Huerta et al., 2020[120] | Prospective study | 95 positive specimens from 89 individuals included 8 vaginal swabs, 20 endocervical swabs, 8 urethral swabs, 25 first-void urine, and 34 rectal swabs | 90 DNA extracts | The rate of MRMM in MG among the study population was 41.8% | Not done | The ResistancePlus® MG FleXible a rapid, simple, and accurate cartridge-based assay for simultaneous detection of MG and MRMM in clinical settings |
| Pitt et al., 2020[121] | Laboratory analysis | Sexually active British general population | 66 DNA extracts | Mutations in 23 S rRNA gene were detected in 9/56 (16%) specimens, with the A2058G mutation being most common (n=7), followed by A2059G (n=1) and A2059C (n=1) | parC gene mutations associated with fluoroquinolone resistance were detected in 2/61 (4%) | Specimens with macrolide resistance were more likely to come from participants reporting a history of diagnosed bacterial STIs or recent sexual health clinic attendance |
| Martens et al., 2019[122] | Retrospe-ctive study | Tested 28,408 samples from 20,537 patients for the presence of STD organisms. Most (n=25,132) samples were provided by general practitioners, 3087 (10.9%) by hospitals, and 189 (0.7%) from other and unknown locations | 894 DNA extracts | Single-nucleotide polymorphisms A2058C, A2058G, A2058T, and A2059G in the 23S ribosomal RNA–encoding region of MG, which together account for >95% of the cases of azithromycin resistance | Not done | The rate of MRMM positivity rose from 22.7% in 2014 and 22.3% in 2015 to 44.4% in 2016 but decreased to 39.7% in 2017 |
| Sweeney et al., 2019[123] | Retrospective study | Patients with genital symptoms urine (n=280), cervicovaginal swabs (n=90), urethral swabs (n=10), anal/rectal swabs (n=60), throat swabs (n=1), and samples from unknown sites (n=6) | 447 DNA extracts | 277/447 (61%) carried strains which harbored MRDR 35/447 (8%) patient samples harbored both MRDR and QRDR mutations | 47/447 (11%) samples harbored MG strains with parC or gyrA mutations in QRDR | The levels of antibiotic resistance may differ between populations within the same state, which has implications for clinical management and treatment guidelines |
| Hokynar et al., 2018[124] | Laboratory analysis | Specimens from heterosexual population included swabs from vagina (n=30), urethra (n=8), rectum (n=1), cervix (n=31) and FVU, (n=233) | 17 DNA extracts | 4 mutation associated with macrolide resistance A2058/9G and 9 were wild type by sequence | Only one specimen contained a mutation at the QRDR area parC gene leading to fluoroquinolone resistance | Recommend testing for the MG positive samples for mutations leading to macrolide resistance but not for fluoroquinolones to guide in selecting treatment |
| Mondeja et al., 2018[125] | Retrospe-ctive study | 280 MG positive DNA extracts conserved at the Cuban National Reference Laboratory of Mycoplasma Research between 2009 and 2016 from Cuban patients with urogenital syndromes, spontaneous abortion and infertility | 280 DNA extracts | 52/64 (82%) samples were identified as A2058G/A2059G and 12/64 (19%) as A2058C/T Three new MG isolates confirmed phenotypic resistance to macrolides in a cell-culture assisted susceptibility test |
Not done | Rapid emergence and high prevalence of MRMM in MG-infected patients and confirmed the phenotypic resistance in isolates carrying MRMM |
|
| ||||||
| Le Roux et al., 2018[126] | Reterospe-ctive study | Vaginal swab samples from 100 and 104 termination of pregnancy attendees at a tertiary hospital in Pretoria, South Africa during 2012 and 2016 respectively | 13 clinical isolsates | 2 isolates had A2059G mutation in region V of the 23S rRNA gene | One a fluoroquin-olone resistance- associated mutation in the parC gene | Increase in macrolide and fluoroquinolone resistance among local MG strains |
| Braam et al., 2017[127] | Laboratory analysis | 147 women and 73 men (general population) | 220 DNA extracts | Mutation at position A2058G (n=18/46), (39%) followed by A2059G (n=16/46), (34%) A2058T (n=10/46) (21%) and A2058C (n=2/46) (5%) | Not done | Molecular methods designed to detect all macrolide resistance-associated mutations, patients infected with proven macrolide-resistant strains can be empirically treated with moxifloxacin |
| Forslund et al., 2017[128] | Retrospe-ctive study | 3167 males and 5636 women who were seeking care at diverse clinics were routinely tested for MG during 2015 | 271 clinical isolates | Macrolide associated resistance mutations in the 23S rRNA gene 8.8% and 4.2% of the isolates had point mutations of the 23S-gene at position 2072 and 2071, respectively | Not done | Relatively low rate of macrolide-resistant MG |
| Mondeja et al., 2016[129] | Laboratory analysis | 7 strains isolated from endocervical and urethral swab specimens from cuban patients | 7 DNA extracts | A2059G transition was detected in the phenotypically macrolide resistant B19 strain | No mutations detected in the QRDR of the parC gene | None |
| Kristiansen et al., 2016[130] | Laboratory analysis | 113 samples were obtained from females (92 cervical swabs, 17 urethral swabs, and 4 urine samples), and 146 were obtained from males (94 urethral swabs and 52 urine samples) | 253 DNA extracts | 109=Wild type 75=A2058G mutation 65=A2059G mutation 2=A2058T mutation 1=A2058C mutation |
Not done | 5’nuclease genotyping assay is easily interpretable and allows timely reporting of macrolide resistance in MG The assay can genotype a large proportion of samples and displays a high concordance with sequencing |
MRMM=Macrolide-resistance mediating mutations; MG=Mycoplasma genitalium; QRDR=Quinolone resistance-determining regions; MRDR=Macrolide resistance-determining region; STIs=Sexually transmitted infections; STD=Sexually transmitted disease; FVU=First void urine
Resistance Issues
M. genitalium is intrinsically resistant to cell wall-acting agents because of lack of cell wall and is sensitive to limited group of antibiotics. In addition to this, it developed resistance to most of the available antibiotics.
Azithromycin
Mechanism of resistance
Azithromycin inhibits the protein synthesis by binding to the A2058 and A2059 residues of region V of 23S rRNA in 50S ribosomal subunit, thereby inhibits the translation of mRNA and thus interfere with protein synthesis and also binds to the L4 and L22 proteins which are important for the assembly of ribosome.[101]
MG develops resistance to azithromycin by single nucleotide polymorphism at A2058G, A2058C, A2059G, and A2059C of V region. Thereby, it prevents the binding of drug. MG develops resistance also by mutation in L4 and L22 proteins.[101]
Reason for high Azithromycin resistance
Differentiation between C. trachomatis and MG cannot made clinically, because both are the cause of NGU. Infection with C. trachomatis is treated with single dose azithromycin 1 g, but this is a suboptimal dose for treating MG. Instead of eradication, it will select the resistance.
Resistance can also be explained by intranuclear localization of MG. Azithromycin is capable of entering eukaryotic cells, it primarily accumulates in the cytoplasm and only low concentrations were observed in the nucleus. This reduces the efficacy of azithromycin and selects the resistance.[101]
Cure rates with single dose azithromycin regimen decreased over the period of time which is shown in Table 4.[37,102,103,104] Azithromycin resistance rate in different countries from 2011 to 2018 varied from 5.3% to 75%,[101] Mulligan et al. in Ireland showed the highest resistant rate of 75%.[105]
Table 4.
Change in the azithromycin cure rates over a period of 10 years
Moxifloxacin
Moxifloxacin is a fourth-generation fluoroquinolone and is used as the second-line treatment against MG in most of the countries.
Mechanism of resistance
It acts by inhibiting DNA replication process by inhibiting two enzymes involved in DNA replication process. First enzyme is DNA gyrase which is encoded by gyrA and gyrB genes. Function of this enzyme is to introduce the negative supercoils, thereby unwind the DNA and initiates the replication process. Second enzyme is topoisomerase IV which is involved in the release of daughter DNA from the parent DNA and this enzyme is encoded by parC and parE gene. Mutation in Quinolone Resistance Determining Region of gyrA and parC gene is the most common mechanism for moxifloxacin resistance.
Moxifloxacin resistance was first reported from Sydney with resistance rate of 16.1%.[106] The resistance rate for moxifloxacin ranged from 5% to 47.1%, as was observed from various studies done in the different parts of the world from 2008 to 2018.[101] Maximum resistance (47.1%) was reported from Japan.[107]
Josamycin
It is a 16 membered lactone ring macrolide antibiotic. In Russia, josamycin is a first-line drug for treatment of MG infections. In other parts of the world, it is not commonly used. Not much data about josamycin are available. Guschin et al. showed the eradication rate with josamycin was 93.5% and faster and higher eradication was seen in patient with lower pretreatment load. In contrast, 50% of patients with higher load were resistant to josamycin and mutations were detected at A2059G and A2062G residues of 23S rRNA.[108]
Management Issues in the Treatment of Mycoplasma Genitalium Infections
Syndromic treatment of NGU has focused on the eradication of C. trachomatis, a well-established cause of reproductive morbidity in women, and is usually instituted at initial presentation before results of investigations to detect specific bacterial causes are made available. In most cases of sexually acquired urethritis and cervicitis, tests are only performed for N. gonorrhoeae and C. trachomatis. Few countries offer routine screening for MG and where this is performed, it typically relies on the use of in-house NAATs performed on specimens collected at either the initial visit or after failure of first-line therapy. Importantly, there are still no validated and commercially available assays for routine diagnostic testing although these may be available in the near future.
Treatment Options for Mycoplasma Genitalium Infection are Limited by Antimicrobial Resistance
Treatment of MG urogenital infection is important from the view point of transmission and complications. Due to the lack of cell wall, limited antibiotic options are available.[13] Tetracyclines, macrolides, and fluoroquinolones have activity against Mycoplasmas. Therapy for MG is indicated if detected in any genitourinary sample in symptomatic patients or as part of an epidemiological survey. Macrolides remain the mainstay of therapy in susceptible infections and have been covered under the syndromic approach for genito-urinary discharge. It achieves a good cure rate of 85%–95% in susceptible infections as single dose therapy [Table 4]. However, increasing macrolide resistance has been reported with the widespread use of azithromycin 1 g single dose without test of cure.
Azithromycin is recommended as the first-line agent for the treatment of uncomplicated MG infections (including in pregnancy). Individuals who have not received previous empirical treatment for urethritis or cervicitis with single-dose azithromycin should receive an extended oral macrolide regimen with azithromycin 500 mg on day 1, then 250 mg on days 2–5. In treatment failure or with confirmed macrolide-resistant infection, moxifloxacin is recommended. Treatment failure with moxifloxacin is uncommon. Test of cure is recommended only in those with persistent symptoms after treatment.[109]
An extended oral macrolide regimen with azithromycin or Josamycin 500 mg three times daily for 10 days drastically improves the cure rate. Macrolide resistance rates vary significantly geographically, but where azithromycin 1 g single dose is used for the treatment of NGU, it is usually found in 30%–45% of samples.[110,111,112]
Josamycin is widely used in Russia with 500 mg three times a day for 10 days but will not eradicate macrolide-resistant strains. Moxifloxacin can be used as second line therapy or for complicated cases for 7–14 days.[111] Moxifloxacin is the most commonly used second line antimicrobial. It is bactericidal and has a cure rate approaching 100% in infections with susceptible strains. However, resistance has developed with treatment failures ranged from 5% to 47.1% primarily in patients from the Asia-Pacific region.
Doxycycline in a dose of 100 mg two times daily for 14 days has a low cure rate of 30%–40% but does not increase resistance.
Emerging Treatment Options
Pristinamycin
Pristinamycin is a bactericidal streptogramin group of drug. It is a third-line treatment option for MDR strains and is effective against macrolide-susceptible MG. In a study by Bissessor et al. showed that pristinamycin was highly effective in treating macrolide- and quinolone-resistant strains.[113] The maximal recommended dose is 1 g four times a day for 10 days. Due to the high price, lack of clinical registration of drug, and patient compliance for the drug issues, this drug has not been established as a second-line drug.
Other drugs used were solithromycin, lifamulin, sitafloxacin, and spectinomycin. However, the clinical efficacy of these drugs is still under evaluation.
Newer Drug Targets
Because of rising of resistance rate to all the available antibiotics, it is essential to identify the newer drug targets. Butt et al. identified 67 nonhomologous essential proteins using comparative genomic and metabolic pathway analysis. Enzymes from Thiamine, protein, and folate biosynthetic pathways were identified. These proteins could serve as novel drug targets for MG.[114]
Conclusion
MG has emerged as a superbug and the rising resistance in this bacterium with only a few treatment options in hand is an imminent problem. Future research should look toward to developing newer antimicrobials and proper management algorithms. Monotherapy should no longer be used. Etiology-based treatment will be a definitive solution to this emerging antimicrobial resistance due to the misuse of antibiotics as a part of syndromic management130.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Gnanadurai R, Fifer H. Mycoplasma genitalium: A review. Microbiology (Reading) 2020;166:21–9. doi: 10.1099/mic.0.000830. [DOI] [PubMed] [Google Scholar]
- 2.Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: From Chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011;24:498–514. doi: 10.1128/CMR.00006-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sethi S, Zaman K, Jain N. Mycoplasma genitalium infections: Current treatment options and resistance issues. Infect Drug Resist. 2017;10:283–92. doi: 10.2147/IDR.S105469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Procop GW, Church DL, Hall GS, Janda WM, Koneman EW, Schreckenberger PC. 7th ed. Philadelphia: Walters Kluwer; 2017. Koneman’s Color Atlas and Textbook of Diagnostic Microbiology. [Google Scholar]
- 5.Dehon PM, McGowin CL. The immunopathogenesis of Mycoplasma genitalium infections in women: A narrative review. Sex Transm Dis. 2017;44:428–32. doi: 10.1097/OLQ.0000000000000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinelli L, Lalli D, García-Morales L, Ratera M, Querol E, Piñol J, et al. A major determinant for gliding motility in Mycoplasma genitalium: The interaction between the terminal organelle proteins MG200 and MG491. J Biol Chem. 2015;290:1699–711. doi: 10.1074/jbc.M114.594762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luca M. Towards the Understanding of the Terminal Organelle of Mycoplasma genitalium: Structural Insights Into the Wheel Complex. Barcelona. 2014 [Google Scholar]
- 8.Sethi S, Singh G, Samanta P, Sharma M. Mycoplasma genitalium: An emerging sexually transmitted pathogen. Indian J Med Res. 2012;136:942–55. [PMC free article] [PubMed] [Google Scholar]
- 9.Burgos R, Pich OQ, Ferrer-Navarro M, Baseman JB, Querol E, Piñol J. Mycoplasma genitalium P140 and P110 cytadhesins are reciprocally stabilized and required for cell adhesion and terminal-organelle development. J Bacteriol. 2006;188:8627–37. doi: 10.1128/JB.00978-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dallo SF, Chavoya A, Su CJ, Baseman JB. DNA and protein sequence homologies between the adhesins of Mycoplasma genitalium and Mycoplasma pneumoniae. Infect Immun. 1989;57:1059–65. doi: 10.1128/iai.57.4.1059-1065.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tully JG, Taylor-Robinson D, Rose DL, Cole RM, Bove JM. Mycoplasma genitalium, a new species from the human urogenital tract. Int J Syst Bacteriol. 1983;33:387–96. [Google Scholar]
- 12.Svenstrup HF, Fedder J, Abraham-Peskir J, Birkelund S, Christiansen G. Mycoplasma genitalium attaches to human spermatozoa. Hum Reprod. 2003;18:2103–9. doi: 10.1093/humrep/deg392. [DOI] [PubMed] [Google Scholar]
- 13.Pich OQ, Burgos R, Ferrer-Navarro M, Querol E, Piñol J. Mycoplasma genitalium mg200 and mg386 genes are involved in gliding motility but not in cytadherence. Mol Microbiol. 2006;60:1509–19. doi: 10.1111/j.1365-2958.2006.05187.x. [DOI] [PubMed] [Google Scholar]
- 14.Mernaugh GR, Dallo SF, Holt SC, Baseman JB. Properties of adhering and nonadhering populations of Mycoplasma genitalium. Clin Infect Dis. 1993;17(Suppl 1):S69–78. doi: 10.1093/clinids/17.supplement_1.s69. [DOI] [PubMed] [Google Scholar]
- 15.Ueno PM, Timenetsky J, Centonze VE, Wewer JJ, Cagle M, Stein MA, et al. Interaction of Mycoplasma genitalium with host cells: Evidence for nuclear localization. Microbiology (Reading) 2008;154:3033–41. doi: 10.1099/mic.0.2008/020735-0. [DOI] [PubMed] [Google Scholar]
- 16.Le Roux MC, Hoosen AA. Mycoplasma genitalium : A brief review. South Afr J Epidemiol Infect. 2010;25:7–10. [Google Scholar]
- 17.McGowin CL, Popov VL, Pyles RB. Intracellular Mycoplasma genitalium infection of human vaginal and cervical epithelial cells elicits distinct patterns of inflammatory cytokine secretion and provides a possible survival niche against macrophage-mediated killing. BMC Microbiol. 2009;9:139. doi: 10.1186/1471-2180-9-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L, Krishnan M, Baseman JB, Kannan TR. Molecular cloning, expression, and characterization of a Ca2+-dependent, membrane-associated nuclease of Mycoplasma genitalium. J Bacteriol. 2010;192:4876–84. doi: 10.1128/JB.00401-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterson SN, Bailey CC, Jensen JS, Borre MB, King ES, Bott KF, et al. Characterization of repetitive DNA in the Mycoplasma genitalium genome: Possible role in the generation of antigenic variation. Proc Natl Acad Sci U S A. 1995;92:11829–33. doi: 10.1073/pnas.92.25.11829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGowin CL, Totten PA. The unique microbiology and molecular pathogenesis of Mycoplasma genitalium. J Infect Dis. 2017;216:S382–8. doi: 10.1093/infdis/jix172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iverson-Cabral SL, Astete SG, Cohen CR, Totten PA. mgpB and mgpC sequence diversity in Mycoplasma genitalium is generated by segmental reciprocal recombination with repetitive chromosomal sequences. Mol Microbiol. 2007;66:55–73. doi: 10.1111/j.1365-2958.2007.05898.x. [DOI] [PubMed] [Google Scholar]
- 22.Burgos R, Totten PA. MG428 is a novel positive regulator of recombination that triggers mgpB and mgpC gene variation in Mycoplasma genitalium. Mol Microbiol. 2014;94:290–306. doi: 10.1111/mmi.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torres-Puig S, Broto A, Querol E, Piñol J, Pich OQ. A novel sigma factor reveals a unique regulon controlling cell-specific recombination in Mycoplasma genitalium. Nucleic Acids Res. 2015;43:4923–36. doi: 10.1093/nar/gkv422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor-Robinson D, Jensen JS, Fehler G, Radebe F, Ballard RC. Observations on the microbiology of urethritis in black South African men. Int J STD AIDS. 2002;13:323–5. doi: 10.1258/0956462021925144. [DOI] [PubMed] [Google Scholar]
- 25.Wetmore CM, Manhart LE, Lowens MS, Golden MR, Whittington WL, Xet-Mull AM, et al. Demographic, behavioral, and clinical characteristics of men with nongonococcal urethritis differ by etiology: A case-comparison study. Sex Transm Dis. 2011;38:180–6. doi: 10.1097/OLQ.0b013e3182040de9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Björnelius E, Lidbrink P, Jensen JS. Mycoplasma genitalium in non-gonococcal urethritis – A study in Swedish male STD patients. Int J STD AIDS. 2000;11:292–6. doi: 10.1177/095646240001100504. [DOI] [PubMed] [Google Scholar]
- 27.Horner P, Thomas B, Gilroy C, Egger M, McClure M, Taylor-Robinson D. Antibodies to Chlamydia trachomatis heat-shock protein 60 kDa and detection of Mycoplasma genitalium and Ureaplasma urealyticum are associated independently with chronic nongonococcal urethritis. Sex Transm Dis. 2003;30:129–33. doi: 10.1097/00007435-200302000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Jensen JS. Mycoplasma genitalium infections.Diagnosis, clinical aspects, and pathogenesis. Dan Med Bull. 2006;53:1–27. [PubMed] [Google Scholar]
- 29.Jensen JS, Björnelius E, Dohn B, Lidbrink P. Use of TaqMan 5’ nuclease real-time PCR for quantitative detection of Mycoplasma genitalium DNA in males with and without urethritis who were attendees at a sexually transmitted disease clinic. J Clin Microbiol. 2004;42:683–92. doi: 10.1128/JCM.42.2.683-692.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anagrius C, Loré B, Jensen JS. Mycoplasma genitalium: Prevalence, clinical significance, and transmission. Sex Transm Infect. 2005;81:458–62. doi: 10.1136/sti.2004.012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berntsson M, Löwhagen GB, Bergström T, Dubicanac L, Welinder-Olsson C, Alvengren G, et al. Viral and bacterial aetiologies of male urethritis: Findings of a high prevalence of Epstein-Barr virus. Int J STD AIDS. 2010;21:191–4. doi: 10.1258/ijsa.2009.009262. [DOI] [PubMed] [Google Scholar]
- 32.Hooton TM, Roberts MC, Roberts PL, Holmes KK, Stamm WE, Kenny GE. Prevalence of Mycoplasma genitalium determined by DNA probe in men with urethritis. Lancet. 1988;1:266–8. doi: 10.1016/s0140-6736(88)90350-9. [DOI] [PubMed] [Google Scholar]
- 33.Taylor-Robinson D, Gilroy CB, Thomas BJ, Hay PE. Mycoplasma genitalium in chronic non-gonococcal urethritis. Int J STD AIDS. 2004;15:21–5. doi: 10.1258/095646204322637209. [DOI] [PubMed] [Google Scholar]
- 34.Deguchi T, Yoshida T, Yokoi S, Ito M, Tamaki M, Ishiko H, et al. Longitudinal quantitative detection by real-time PCR of Mycoplasma genitalium in first-pass urine of men with recurrent nongonococcal urethritis. J Clin Microbiol. 2002;40:3854–6. doi: 10.1128/JCM.40.10.3854-3856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horner P, Thomas B, Gilroy CB, Egger M, Taylor-Robinson D. Role of Mycoplasma genitalium and Ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin Infect Dis. 2001;32:995–1003. doi: 10.1086/319594. [DOI] [PubMed] [Google Scholar]
- 36.Bradshaw CS, Chen MY, Fairley CK. Persistence of Mycoplasma genitalium following azithromycin therapy. PLoS One. 2008;3:e3618. doi: 10.1371/journal.pone.0003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Björnelius E, Anagrius C, Bojs G, Carlberg H, Johannisson G, Johansson E, et al. Antibiotic treatment of symptomatic Mycoplasma genitalium infection in Scandinavia: A controlled clinical trial. Sex Transm Infect. 2008;84:72–6. doi: 10.1136/sti.2007.027375. [DOI] [PubMed] [Google Scholar]
- 38.Horner PJ, Taylor-Robinson D. Association of Mycoplasma genitalium with balanoposthitis in men with non-gonococcal urethritis. Sex Transm Infect. 2011;87:38–40. doi: 10.1136/sti.2010.044487. [DOI] [PubMed] [Google Scholar]
- 39.Doble A, Thomas BJ, Furr PM, Walker MM, Harris JR, Witherow RO, et al. A search for infectious agents in chronic abacterial prostatitis using ultrasound guided biopsy. Br J Urol. 1989;64:297–301. doi: 10.1111/j.1464-410x.1989.tb06017.x. [DOI] [PubMed] [Google Scholar]
- 40.Krieger JN, Riley DE, Roberts MC, Berger RE. Prokaryotic DNA sequences in patients with chronic idiopathic prostatitis. J Clin Microbiol. 1996;34:3120–8. doi: 10.1128/jcm.34.12.3120-3128.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mändar R, Raukas E, Türk S, Korrovits P, Punab M. Mycoplasmas in semen of chronic prostatitis patients. Scand J Urol Nephrol. 2005;39:479–82. doi: 10.1080/00365590500199822. [DOI] [PubMed] [Google Scholar]
- 42.Eickhoff JH, Frimodt-Møller N, Walter S, Frimodt-Møller C. A double-blind, randomized, controlled multicentre study to compare the efficacy of ciprofloxacin with pivampicillin as oral therapy for epididymitis in men over 40 years of age. BJU Int. 1999;84:827–34. doi: 10.1046/j.1464-410x.1999.00252.x. [DOI] [PubMed] [Google Scholar]
- 43.Doble A, Thomas BJ, Walker MM, Harris JR, Witherow RO, Taylor-Robinson D. The role of Chlamydia trachomatis in chronic abacterial prostatitis: A study using ultrasound guided biopsy. J Urol. 1989;141:332–3. doi: 10.1016/s0022-5347(17)40758-0. [DOI] [PubMed] [Google Scholar]
- 44.Falk L, Fredlund H, Jensen JS. Signs and symptoms of urethritis and cervicitis among women with or without Mycoplasma genitalium or Chlamydia trachomatis infection. Sex Transm Infect. 2005;81:73–8. doi: 10.1136/sti.2004.010439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Högdahl M, Kihlström E. Leucocyte esterase testing of first-voided urine and urethral and cervical smears to identify Mycoplasma genitalium-infected men and women. Int J STD AIDS. 2007;18:835–8. doi: 10.1258/095646207782716983. [DOI] [PubMed] [Google Scholar]
- 46.Palmer HM, Gilroy CB, Claydon EJ, Taylor-Robinson D. Detection of Mycoplasma genitalium in the genitourinary tract of women by the polymerase chain reaction. Int J STD AIDS. 1991;2:261–3. doi: 10.1177/095646249100200407. [DOI] [PubMed] [Google Scholar]
- 47.Jensen JS, Uldum SA, Søndergård-Andersen J, Vuust J, Lind K. Polymerase chain reaction for detection of Mycoplasma genitalium in clinical samples. J Clin Microbiol. 1991;29:46–50. doi: 10.1128/jcm.29.1.46-50.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenstein IJ, Morgan DJ, Sheehan M, Lamont RF, Taylor-Robinson D. Bacterial vaginosis in pregnancy: Distribution of bacterial species in different gram-stain categories of the vaginal flora. J Med Microbiol. 1996;45:120–6. doi: 10.1099/00222615-45-2-120. [DOI] [PubMed] [Google Scholar]
- 49.Keane FE, Thomas BJ, Gilroy CB, Renton A, Taylor-Robinson D. The association of Mycoplasma hominis, Ureaplasma urealyticum and Mycoplasma genitalium with bacterial vaginosis: Observations on heterosexual women and their male partners. Int J STD AIDS. 2000;11:356–60. doi: 10.1258/0956462001916056. [DOI] [PubMed] [Google Scholar]
- 50.Lawton BA, Rose SB, Bromhead C, Gaitanos LA, MacDonald EJ, Lund KA. High prevalence of Mycoplasma genitalium in women presenting for termination of pregnancy. Contraception. 2008;77:294–8. doi: 10.1016/j.contraception.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 51.Oakeshott P, Aghaizu A, Hay P, Reid F, Kerry S, Atherton H, et al. Is Mycoplasma genitalium in women the “New Chlamydia.” A community-based prospective cohort study? Clin Infect Dis. 2010;51:1160–6. doi: 10.1086/656739. [DOI] [PubMed] [Google Scholar]
- 52.Donders GG, Van Calsteren K, Bellen G, Reybrouck R, Van den Bosch T, Riphagen I, et al. Predictive value for preterm birth of abnormal vaginal flora, bacterial vaginosis and aerobic vaginitis during the first trimester of pregnancy. BJOG. 2009;116:1315–24. doi: 10.1111/j.1471-0528.2009.02237.x. [DOI] [PubMed] [Google Scholar]
- 53.Uno M, Deguchi T, Komeda H, Hayasaki M, Iida M, Nagatani M, et al. Mycoplasma genitalium in the cervices of Japanese women. Sex Transm Dis. 1997;24:284–6. doi: 10.1097/00007435-199705000-00009. [DOI] [PubMed] [Google Scholar]
- 54.Arráiz RN, Colina Ch S, Marcucci JR, Rondón GN, Reyes SF, Bermúdez PV, et al. Mycoplasma genitalium detection and correlation with clinical manifestations in population of the Zulia State, Venezuela. Rev Chilena Infectol. 2008;25:256–61. [PubMed] [Google Scholar]
- 55.Lusk MJ, Konecny P, Naing ZW, Garden FL, Cumming RG, Rawlinson WD. Mycoplasma genitalium is associated with cervicitis and HIV infection in an urban Australian STI clinic population. Sex Transm Infect. 2011;87:107–9. doi: 10.1136/sti.2010.045138. [DOI] [PubMed] [Google Scholar]
- 56.Collier AM. Attachment of Mycoplasma genitalium to the ciliated epithelium of human fallopian tubes. In: Stanek G., editor. Recent Advances in Mycoplasmology. Stuttgart, Germany: Gustav Fischer Verlag; 1990. pp. 730–2. [Google Scholar]
- 57.Baczynska A, Funch P, Fedder J, Knudsen HJ, Birkelund S, Christiansen G. Morphology of human Fallopian tubes after infection with Mycoplasma genitalium and Mycoplasma hominis – in vitro organ culture study. Hum Reprod. 2007;22:968–79. doi: 10.1093/humrep/del455. [DOI] [PubMed] [Google Scholar]
- 58.Møller BR, Taylor-Robinson D, Furr PM, Freundt EA. Acute upper genital-tract disease in female monkeys provoked experimentally by Mycoplasma genitalium. Br J Exp Pathol. 1985;66:417–26. [PMC free article] [PubMed] [Google Scholar]
- 59.Taylor-Robinson D, Furr PM, Hetherington CM. The pathogenicity of a newly discovered human Mycoplasma (strain G37) for the genital tract of marmosets. J Hyg (Lond) 1982;89:449–55. doi: 10.1017/s0022172400071011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McGowin CL, Spagnuolo RA, Pyles RB. Mycoplasma genitalium rapidly disseminates to the upper reproductive tracts and knees of female mice following vaginal inoculation. Infect Immun. 2010;78:726–36. doi: 10.1128/IAI.00840-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clausen HF, Fedder J, Drasbek M, Nielsen PK, Toft B, Ingerslev HJ, et al. Serological investigation of Mycoplasma genitalium in infertile women. Hum Reprod. 2001;16:1866–74. doi: 10.1093/humrep/16.9.1866. [DOI] [PubMed] [Google Scholar]
- 62.Jurstrand M, Jensen JS, Magnuson A, Kamwendo F, Fredlund H. A serological study of the role of Mycoplasma genitalium in pelvic inflammatory disease and ectopic pregnancy. Sex Transm Infect. 2007;83:319–23. doi: 10.1136/sti.2007.024752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lind K, Kristensen GB. Significance of antibodies to Mycoplasma genitalium in salpingitis. Eur J Clin Microbiol. 1987;6:205–7. doi: 10.1007/BF02018216. [DOI] [PubMed] [Google Scholar]
- 64.Haggerty CL. Evidence for a role of Mycoplasma genitalium in pelvic inflammatory disease. Curr Opin Infect Dis. 2008;21:65–9. doi: 10.1097/QCO.0b013e3282f3d9ac. [DOI] [PubMed] [Google Scholar]
- 65.Irwin KL, Moorman AC, O'Sullivan MJ, Sperling R, Koestler ME, Soto I, et al. Influence of human immunodeficiency virus infection on pelvic inflammatory disease. Obstet Gynecol. 2000;95:525–34. doi: 10.1016/s0029-7844(99)00621-3. [DOI] [PubMed] [Google Scholar]
- 66.Cohen CR, Manhart LE, Bukusi EA, Astete S, Brunham RC, Holmes KK, et al. Association between Mycoplasma genitalium and acute endometritis. Lancet. 2002;359:765–6. doi: 10.1016/S0140-6736(02)07848-0. [DOI] [PubMed] [Google Scholar]
- 67.Cohen CR, Mugo NR, Astete SG, Odondo R, Manhart LE, Kiehlbauch JA, et al. Detection of Mycoplasma genitalium in women with laparoscopically diagnosed acute salpingitis. Sex Transm Infect. 2005;81:463–6. doi: 10.1136/sti.2005.015701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hay PE, Lamont RF, Taylor-Robinson D, Morgan DJ, Ison C, Pearson J. Abnormal bacterial colonisation of the genital tract and subsequent preterm delivery and late miscarriage. BMJ. 1994;308:295–8. doi: 10.1136/bmj.308.6924.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garcia P, Totten P, Paul K, Garcia P, Holmes K, Hitti J. Risk of preterm birth associated with Mycoplasma genitalium infection. Am J Obstet Gynecol. 2006;195:S53. [Google Scholar]
- 70.Kataoka S, Yamada T, Chou K, Nishida R, Morikawa M, Minami M, et al. Association between preterm birth and vaginal colonization by Mycoplasmas in early pregnancy. J Clin Microbiol. 2006;44:51–5. doi: 10.1128/JCM.44.1.51-55.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Labbé AC, Frost E, Deslandes S, Mendonça AP, Alves AC, Pépin J. Mycoplasma genitalium is not associated with adverse outcomes of pregnancy in Guinea-Bissau. Sex Transm Infect. 2002;78:289–91. doi: 10.1136/sti.78.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Edwards RK, Ferguson RJ, Reyes L, Brown M, Theriaque DW, Duff P. Assessing the relationship between preterm delivery and various microorganisms recovered from the lower genital tract. J Matern Fetal Neonatal Med. 2006;19:357–63. doi: 10.1080/00207170600712071. [DOI] [PubMed] [Google Scholar]
- 73.Hitti J, Garcia P, Totten P, Paul K, Astete S, Holmes KK. Correlates of cervical Mycoplasma genitalium and risk of preterm birth among Peruvian women. Sex Transm Dis. 2010;37:81–5. doi: 10.1097/OLQ.0b013e3181bf5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taylor-Robinson D, McCormack WM. Mycoplasmas in human genitourinary infections. In: Tully JG, Whitcomb RF, editors. The Mycoplasmas. New York, NY: Academic Press, Inc.; 1979. pp. 307–66. [Google Scholar]
- 75.Svenstrup HF, Fedder J, Kristoffersen SE, Trolle B, Birkelund S, Christiansen G. Mycoplasma genitalium, Chlamydia trachomatis, and tubal factor infertility – A prospective study. Fertil Steril. 2008;90:513–20. doi: 10.1016/j.fertnstert.2006.12.056. [DOI] [PubMed] [Google Scholar]
- 76.Taylor-Robinson D, Gilroy CB, Horowitz S, Horowitz J. Mycoplasma genitalium in the joints of two patients with arthritis. Eur J Clin Microbiol Infect Dis. 1994;13:1066–9. doi: 10.1007/BF02111830. [DOI] [PubMed] [Google Scholar]
- 77.Henry CH, Hughes CV, Gérard HC, Hudson AP, Wolford LM. Reactive arthritis: Preliminary microbiologic analysis of the human temporomandibular joint. J Oral Maxillofac Surg. 2000;58:1137–42. doi: 10.1053/joms.2000.9575. [DOI] [PubMed] [Google Scholar]
- 78.Francis SC, Kent CK, Klausner JD, Rauch L, Kohn R, Hardick A, et al. Prevalence of rectal Trichomonas vaginalis and Mycoplasma genitalium in male patients at the San Francisco STD clinic, 2005-2006. Sex Transm Dis. 2008;35:797–800. doi: 10.1097/OLQ.0b013e318177ec39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bissessor M, Tabrizi SN, Bradshaw CS, Fairley CK, Hocking JS, Garland SM, et al. The contribution of Mycoplasma genitalium to the aetiology of sexually acquired infectious proctitis in men who have sex with men. Clin Microbiol Infect. 2016;22:260–5. doi: 10.1016/j.cmi.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 80.Dhawan B, Rawre J, Dhawan N, Bhatia R, Gupta V, Khanna N. High prevalence of Mycoplasma genitalium in men who have sex with men: A cross-sectional study. Indian J Dermatol Venereol Leprol. 2020;86:195–6. doi: 10.4103/ijdvl.IJDVL_494_18. [DOI] [PubMed] [Google Scholar]
- 81.Latimer RL, Vodstrcil L, De Petra V, Fairley CK, Read TR, Williamson D, et al. Extragenital Mycoplasma genitalium infections among men who have sex with men. Sex Transm Infect. 2020;96:10–8. doi: 10.1136/sextrans-2019-054058. [DOI] [PubMed] [Google Scholar]
- 82.Couldwell DL, Jalocon D, Power M, Jeoffreys NJ, Chen SC, Lewis DA. Mycoplasma genitalium: High prevalence of resistance to macrolides and frequent anorectal infection in men who have sex with men in western Sydney. Sex Transm Infect. 2018;94:406–10. doi: 10.1136/sextrans-2017-053480. [DOI] [PubMed] [Google Scholar]
- 83.Jiang J, Liu P, Cao N. The prevalence of Mycoplasma genitalium and Chlamydia trachomatis at various anatomical sites of men who have sex with men in five cities of china. Sex Transm Infect. 2015;91:A30–1. [Google Scholar]
- 84.Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the united states. J Clin Microbiol. 2016;54:2278–83. doi: 10.1128/JCM.01053-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gaydos C, Maldeis NE, Hardick A, Hardick J, Quinn TC. Mycoplasma genitalium as a contributor to the multiple etiologies of cervicitis in women attending sexually transmitted disease clinics. Sex Transm Dis. 2009;36:598–606. doi: 10.1097/OLQ.0b013e3181b01948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yokoi S, Maeda S, Kubota Y, Tamaki M, Mizutani K, Yasuda M, et al. The role of Mycoplasma genitalium and Ureaplasma urealyticum biovar 2 in postgonococcal urethritis. Clin Infect Dis. 2007;45:866–71. doi: 10.1086/521266. [DOI] [PubMed] [Google Scholar]
- 87.Harrison SA, Olson KM, Ratliff AE, Xiao L, Van Der Pol B, Waites KB, et al. Mycoplasma genitalium coinfection in women with Chlamydia trachomatis infection. Sex Transm Dis. 2019;46:e101–4. doi: 10.1097/OLQ.0000000000001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pépin J, Sobéla F, Deslandes S, Alary M, Wegner K, Khonde N, et al. Etiology of urethral discharge in West Africa: the role of Mycoplasma genitalium and Trichomonas vaginalis. Bull World Health Organ. 2001;79:118–26. [PMC free article] [PubMed] [Google Scholar]
- 89.Kokkayil P, Rawre J, Malhotra N, Dhawan B. Co-infection of Mycoplasma genitalium and Chlamydia trachomatis in an infertile female patient with genital tuberculosis. Indian J Pathol Microbiol. 2013;56:457–9. doi: 10.4103/0377-4929.125371. [DOI] [PubMed] [Google Scholar]
- 90.Darkahi FD. Simultaneous detection of genital Mycoplasma in women with genital infections by PCR. J Biol Sci. 2009;9:804–9. [Google Scholar]
- 91.Loubinoux J, Piroux V, Guyot PY, Amiel C, Catelle A, Rihn B, et al. Genital Mycoplasmas in patients with acquired immunodeficiency syndrome. New Microbiol. 1998;21:403–5. [PubMed] [Google Scholar]
- 92.Soni S, Alexander S, Verlander N, Saunders P, Richardson D, Fisher M, et al. The prevalence of urethral and rectal Mycoplasma genitalium and its associations in men who have sex with men attending a genitourinary medicine clinic. Sex Transm Infect. 2010;86:21–4. doi: 10.1136/sti.2009.038190. [DOI] [PubMed] [Google Scholar]
- 93.Cohen CR, Nosek M, Meier A, Astete SG, Iverson-Cabral S, Mugo NR, et al. Mycoplasma genitalium infection and persistence in a cohort of female sex workers in Nairobi, Kenya. Sex Transm Dis. 2007;34:274–9. doi: 10.1097/01.olq.0000237860.61298.54. [DOI] [PubMed] [Google Scholar]
- 94.Vandepitte J, Weiss HA, Bukenya J, Kyakuwa N, Muller E, Buvé A, et al. Association between Mycoplasma genitalium infection and HIV acquisition among female sex workers in Uganda: Evidence from a nested case-control study. Sex Transm Infect. 2014;90:545–9. doi: 10.1136/sextrans-2013-051467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Le Roy C, Pereyre S, Bébéar C. Evaluation of two commercial real-time PCR assays for detection of Mycoplasma genitalium in urogenital specimens. J Clin Microbiol. 2014;52:971–3. doi: 10.1128/JCM.02567-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takanashi M, Ito S, Kaneto H, Tanahashi Y, Kitanohara M, Yanagihara A, et al. Development and clinical application of an InvaderPlus® assay for the detection of genital Mycoplasmas. J Infect Chemother. 2015;21:516–9. doi: 10.1016/j.jiac.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 97.Soni S, Horner P, Rayment M, Pinto-Sander N, Naous N, Parkhouse A, et al. British association for sexual health and HIV national guideline for the management of infection with Mycoplasma genitalium (2018) Int J STD AIDS. 2019;30:938–50. doi: 10.1177/0956462419825948. [DOI] [PubMed] [Google Scholar]
- 98.Tabrizi SN, Su J, Bradshaw CS, Fairley CK, Walker S, Tan LY, et al. Prospective Evaluation of ResistancePlus MG, a new multiplex quantitative PCR assay for detection of Mycoplasma genitalium and macrolide resistance. J Clin Microbiol. 2017;55:1915–9. doi: 10.1128/JCM.02312-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fernández-Huerta M, Bodiyabadu K, Esperalba J, Bradshaw CS, Serra-Pladevall J, Garland SM, et al. Multicenter clinical evaluation of a novel multiplex real-time PCR (qPCR) assay for detection of fluoroquinolone resistance in Mycoplasma genitalium. J Clin Microbiol. 2019;57(11):e00886–19. doi: 10.1128/JCM.00886-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Waites KB, Ratliff A, Crabb DM, Xiao L, Qin X, Selvarangan R, et al. Macrolide-resistant Mycoplasma pneumoniae in the United States as determined from a national surveillance program. J Clin Microbiol. 2019;57(11):e00968–19. doi: 10.1128/JCM.00968-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.van der Schalk TE, Braam JF, Kusters JG. Molecular basis of antimicrobial resistance in Mycoplasma genitalium. Int J Antimicrob Agents. 2020;55:105911. doi: 10.1016/j.ijantimicag.2020.105911. [DOI] [PubMed] [Google Scholar]
- 102.Twin J, Jensen JS, Bradshaw CS, Garland SM, Fairley CK, Min LY, et al. Transmission and selection of macrolide resistant Mycoplasma genitalium infections detected by rapid high resolution melt analysis. PLoS One. 2012;7:e35593. doi: 10.1371/journal.pone.0035593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bradshaw CS, Lim YM, Tabrizi SN, Twin J, Bush M. Paper #179. Sydney: 2010 Australasian Sexual Health Conference; 2010. The Effectiveness of 1 g of Azithromycin for Mycoplasma genitalium Infections: A Five Year Review. [Google Scholar]
- 104.Manhart LE, Gillespie CW, Lowens MS, Khosropour CM, Colombara DV, Golden MR, et al. Standard treatment regimens for nongonococcal urethritis have similar but declining cure rates: A randomized controlled trial. Clin Infect Dis. 2013;56:934–42. doi: 10.1093/cid/cis1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mulligan V, Lynagh Y, Clarke S, Unemo M, Crowley B. Prevalence, macrolide resistance, and fluoroquinolone resistance in Mycoplasma genitalium in men who have sex with men attending an sexually transmitted disease clinic in dublin, ireland in 2017-2018. Sex Transm Dis. 2019;46:e35–7. doi: 10.1097/OLQ.0000000000000940. [DOI] [PubMed] [Google Scholar]
- 106.Tagg KA, Jeoffreys NJ, Couldwell DL, Donald JA, Gilbert GL. Fluoroquinolone and macrolide resistance-associated mutations in Mycoplasma genitalium. J Clin Microbiol. 2013;51:2245–9. doi: 10.1128/JCM.00495-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kikuchi M, Ito S, Yasuda M, Tsuchiya T, Hatazaki K, Takanashi M, et al. Remarkable increase in fluoroquinolone-resistant Mycoplasma genitalium in Japan. J Antimicrob Chemother. 2014;69:2376–82. doi: 10.1093/jac/dku164. [DOI] [PubMed] [Google Scholar]
- 108.Guschin A, Ryzhikh P, Rumyantseva T, Gomberg M, Unemo M. Treatment efficacy, treatment failures and selection of macrolide resistance in patients with high load of Mycoplasma genitalium during treatment of male urethritis with josamycin. BMC Infect Dis. 2015;15:40. doi: 10.1186/s12879-015-0781-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bullard J, Fisher WA, Gale-Rowe M, Gemmill I, Gratrix J, Grennan T, et al. Ottawa: Public Health Agency of Canada; 2016. Section 5-1: Canadian Guidelines on Sexually Transmitted Infections — Management and Treatment of Specific Infections — Mycoplasma genitalium Infections. [Google Scholar]
- 110.Workowski KA, Bolan GA Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1–37. [PMC free article] [PubMed] [Google Scholar]
- 111.Jensen JS, Cusini M, Gomberg M, Moi H. European guideline on Mycoplasma genitalium infections. J Eur Acad Dermatol Venereol. 2016;30:1650–6. doi: 10.1111/jdv.13849. [DOI] [PubMed] [Google Scholar]
- 112.Taylor-Robinson D. Diagnosis and antimicrobial treatment of Mycoplasma genitalium infection: Sobering thoughts. Expert Rev Anti Infect Ther. 2014;12:715–22. doi: 10.1586/14787210.2014.919220. [DOI] [PubMed] [Google Scholar]
- 113.Bissessor M, Tabrizi SN, Twin J, Abdo H, Fairley CK, Chen MY, et al. Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis. 2015;60:1228–36. doi: 10.1093/cid/ciu1162. [DOI] [PubMed] [Google Scholar]
- 114.Butt AM, Tahir S, Nasrullah I, Idrees M, Lu J, Tong Y. Mycoplasma genitalium: A comparative genomics study of metabolic pathways for the identification of drug and vaccine targets. Infect Genet Evol. 2012;12:53–62. doi: 10.1016/j.meegid.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 115.Rekha S, Nooren M, Kalyan S, Mohan M, Bharti M, Monika R, et al. Occurrence of Mycoplasma genitalium in the peritoneal fluid of fertile and infertile women with detailed analysis among infertile women. Microb Pathog. 2019;129:183–6. doi: 10.1016/j.micpath.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 116.Saigal K, Dhawan B, Rawre J, Khanna N, Chaudhry R. Genital Mycoplasma and Chlamydia trachomatis infections in patients with genital tract infections attending a tertiary care hospital of North India. Indian J Pathol Microbiol. 2016;59:194–6. doi: 10.4103/0377-4929.182019. [DOI] [PubMed] [Google Scholar]
- 117.Rajkumari N, Kaur H, Roy A, Gupta N, Dhaliwal LK, Sethi S. Association of Mycoplasma genitalium with infertility in North Indian women. Indian J Sex Transm Dis AIDS. 2015;36:144–8. doi: 10.4103/2589-0557.167141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ghosh A, Dhawan B, Chaudhry R, Vajpayee M, Sreenivas V. Genital Mycoplasma & Chlamydia trachomatis infections in treatment naïve HIV-1 infected adults. Indian J Med Res. 2011;134:960–6. doi: 10.4103/0971-5916.92643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Manhas A, Sethi S, Sharma M, Wanchu A, Kanwar AJ, Kaur K, et al. Association of genital Mycoplasmas including Mycoplasma genitalium in HIV infected men with nongonococcal urethritis attending STD & HIV clinics. Indian J Med Res. 2009;129:305–10. [PubMed] [Google Scholar]
- 120.Fernández-Huerta M, Salmerón P, Silgado A, Espasa M, Pumarola T, Tulsiani-Drud S, et al. Clinical evaluation of the ResistancePlus MG FleXible test on the GeneXpert Infinity-48s instrument: A near-patient assay for simultaneous detection of Mycoplasma genitalium and macrolide resistance. Diagn Microbiol Infect Dis. 2020;97:115062. doi: 10.1016/j.diagmicrobio.2020.115062. [DOI] [PubMed] [Google Scholar]
- 121.Pitt R, Unemo M, Sonnenberg P, Alexander S, Beddows S, Cole MJ, et al. Antimicrobial resistance in Mycoplasma genitalium sampled from the British general population. Sex Transm Infect. 2020;96:464–8. doi: 10.1136/sextrans-2019-054129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Martens L, Kuster S, de Vos W, Kersten M, Berkhout H, Hagen F. Macrolide-Resistant Mycoplasma genitalium in Southeastern Region of the Netherlands, 2014-2017. Emerg Infect Dis. 2019;25:1297–303. doi: 10.3201/eid2507.181556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sweeney EL, Trembizki E, Bletchly C, Bradshaw CS, Menon A, Francis F, et al. Levels of Mycoplasma genitalium antimicrobial resistance differ by both region and gender in the state of queensland, Australia: Implications for treatment guidelines. J Clin Microbiol. 2019;57(3):e01555–18. doi: 10.1128/JCM.01555-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hokynar K, Hiltunen-Back E, Mannonen L, Puolakkainen M. Prevalence of Mycoplasma genitalium and mutations associated with macrolide and fluoroquinolone resistance in Finland. Int J STD AIDS. 2018;29:904–7. doi: 10.1177/0956462418764482. [DOI] [PubMed] [Google Scholar]
- 125.Mondeja BA, Couri J, Rodríguez NM, Blanco O, Fernández C, Jensen JS. Macrolide-resistant Mycoplasma genitalium infections in Cuban patients: an underestimated health problem. BMC Infect Dis. 2018;18:601. doi: 10.1186/s12879-018-3523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Le Roux MC, Mafunise M, de Villiers BE, Ditsele RM. Antimicrobial susceptibility of Mycoplasma genitalium isolates from Pretoria, South Africa in 2012 and 2016. South Afr J Infect Dis. 2018;33:46–9. [Google Scholar]
- 127.Braam JF, Slotboom B, Van Marm S, Severs TT, Van Maarseveen NM, Van Zwet T, et al. High prevalence of the A2058T macrolide resistance-associated mutation in Mycoplasma genitalium strains from the Netherlands. J Antimicrob Chemother. 2017;72:1529–30. doi: 10.1093/jac/dkw584. [DOI] [PubMed] [Google Scholar]
- 128.Forslund O, Hjelm M, El-Ali R, Johnsson A, Bjartling C. Mycoplasma genitalium and Macrolide resistance-associated mutations in the skåne region of southern sweden 2015. Acta Derm Venereol. 2017;97:1235–8. doi: 10.2340/00015555-2746. [DOI] [PubMed] [Google Scholar]
- 129.Mondeja BA, Rodríguez NM, Barroto B, Blanco O, Jensen JS. Antimicrobial susceptibility patterns of recent cuban Mycoplasma genitalium isolates determined by a modified cell-culture-based method. PLoS One. 2016;11:e0162924. doi: 10.1371/journal.pone.0162924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kristiansen GQ, Lisby JG, Schønning K. A 5’ nuclease genotyping assay for identification of macrolide-resistant Mycoplasma genitalium in clinical specimens. J Clin Microbiol. 2016;54:1593–7. doi: 10.1128/JCM.00012-16. [DOI] [PMC free article] [PubMed] [Google Scholar]


