Abstract
Introduction:
Lichen sclerosus (LS) is a chronic dermatosis frequently located over labial, perineal, and perianal areas. The etiology is multifactorial and includes genetic, autoimmune, hormonal, and infectious aspects.
Materials and Methods:
A series of twenty genital LS patients was carried out to evaluate the signs, symptoms, complications, and affliction of quality of life.
Results:
Eighteen out of twenty patients were female between 30 and 73 years and showed smooth, glistening, and whitish plaques. The mean duration was 8.4 years. Itching and burning was the most common symptom (75%) corresponding to excoriation and fissuring of genitalia (75%). Malignancy was seen in two cases (10%). The mean Vulvar Quality of Life Index was 9.2, higher in younger patients.
Discussion:
LS is a disorder of older age group with female preponderance. It is a source of significant morbidity, and long-standing cases predispose to vulvar malignancy.
Conclusion:
To conclude, early diagnosis with proper counselling of the patient and his/her partner regarding various aspects of disease are essential for a wholesome approach.
Keywords: Genital, lichen sclerosus, malignancy, vulvar, Vulvar Quality of Life Index
Introduction
The first report of lichen sclerosus (LS) was by Hallopeau in 1887. Many names have been used for the disorder (e.g., leukoplakia, lichen albus, and LS et atrophicus [LSA]), but the International Society for the Study of Vulvovaginal Disease favors the term LS.[1] In 1975, “et atrophicans” was dropped because not all LS is histologically atrophic.
LS has a bimodal age distribution in prepubertal girls and menopausal women. Previous literature suggests a mean age of diagnosis of 52 years.[2] Women being more commonly affected than men with a 10:1 ratio. Anogenital region is the most commonly affected site with 85%–98% of cases whereas extragenital LS can be seen in 15%–20% of cases. Genital lesions may appear as a figure-of-eight pattern around the vulva and anus. In men, the glans penis and foreskin are usually affected, but not the perianal region. This can lead to phimosis and other significant genitourinary morbidities in men. Vulvar LS causes substantial discomfort (intractable itching, soreness, constipation, and dyspareunia) and morbidity (introital narrowing, burying of clitoris, and atrophied labia minora).[3]
A number of mechanisms have been proposed to explain the cause and development of LS including low estrogen levels, abnormal androgen metabolism, and autoimmunity (alopecia areata, vitiligo, thyroid disease, pernicious anemia, diabetes mellitus, and cicatricial pemphigoid). Association with human leukocyte antigen, particularly DQ7, in patients with early-onset LS is also seen. “Local factors” such as friction or rubbing result in Koebner phenomenon, triggering LS. Various studies have indicated possible roles of Borrelia burgdorferi. Coincidental presence of mycobacteria and human papillomavirus in biopsy samples has also been seen. LS is an inflammatory dermatosis, evident by its histological pattern of vacuolar interface dermatitis, composition of the inflammatory infiltrate, immunoreactant deposition at the dermal-epidermal junction, and good response to topical steroids and potent anti-inflammatory agents. The scientific rationale for this study is to propose proper guidelines for long-term follow-up in these patients due to the risk of malignancy in these patients.
Aims
To see the clinical spectrum (signs, symptoms, and complications) of patients with LS
To assess the impact of disease on quality of life (Vulvar Quality of Life Index [VQLI]) of patients.
Materials and Methods
We conducted an observational cross-sectional study comprising twenty cases presenting to the outpatient department of a tertiary care center in North India over a period of 1 year ranging between December 2018 and November 2019. Patients presenting with clinical features suggestive of genital LS were enrolled, and biopsy was done in doubtful cases. Routine investigations including hemogram, liver, and kidney function tests were done in all the patients. However, the histopathological examination was done only in selective cases. All the consecutive cases were included during the period of study. Written and informed consent was obtained from all patients prior to enrollment into the study. Relevant clinical (systemic and dermatological) examination and investigations were carried out for each patient, and findings were recorded.
Inclusion criteria
The following patients were included in our study:
All patients of both sexes between 30 and 80 years of age having LSA
Patients who were ready to sign a consent form for the study.
Exclusion criteria
The following patients were excluded from our study:
All patients with diabetes or hypertension
Uncooperative patients.
Results
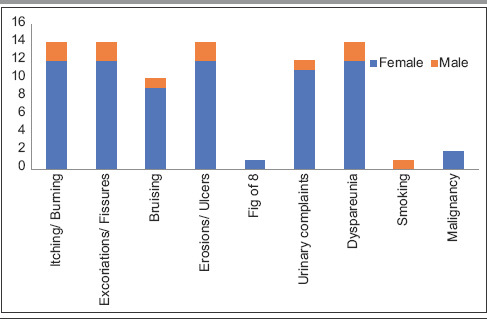
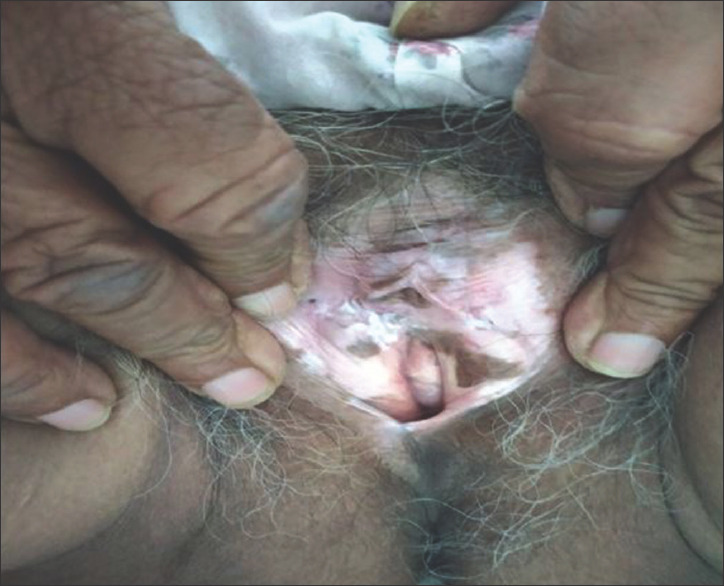
Data were collected and processed using appropriate statistical tests, and P value was calculated at the end of the study. Usual presentation was pearly white, smooth, glistening plaque over genital area with atrophic labia minora and often buried clitoris [Figure 1]. Eighteen out of twenty patients were female of ages ranging between 30 and 73 years, with a mean age of presentation being 52 years [Tables 1-3]. There was a higher incidence in 51–60-year-old females. The duration of symptoms was 8.4 years [Table 3]. Itching and burning was the most common symptom (75%) corresponding to excoriation and fissuring [Figure 2] of genitalia (75%) followed by dyspareunia/sexual difficulties (70%), erosions/ulcers (70%), urinary symptoms (60%), and genital bruising and bleeding (50%) in that order [Tables 4 and 5]. Male patient presented with phimosis [Figure 3]. Figure-of-eight pattern was present in one patient (5%). The mean VQLI score was 9.2 – maximum in 31–50 years of age group. Higher incidence of sexual difficulties in the form of raised VQLI was observed in younger age group [Table 6]. Malignancy was seen in two cases (10%), and biopsy was suggestive of well-differentiated squamous cell carcinoma (SCC) [Figure 4]; patients were referred to oncology for further management.
Figure 1.
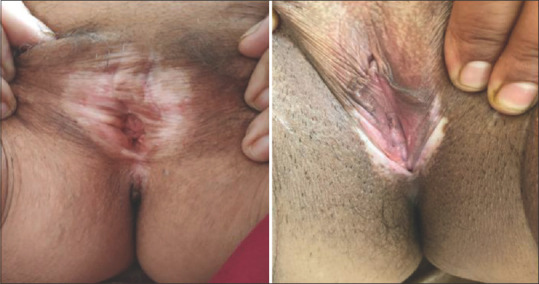
Lichen sclerosus showing burying of clitoris and atrophied labia minora
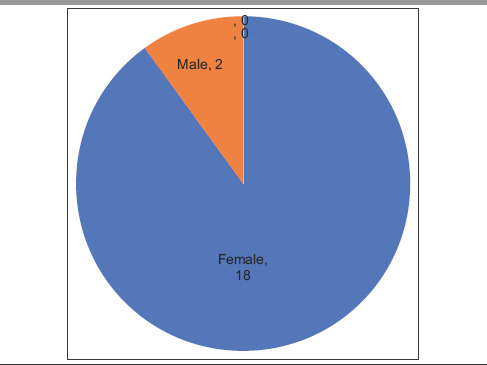
Table 1.
Gender distribution
|
Table 3.
Demographic distribution of study population (n=20)
| Sex (female:male) | 9:1 (approximate) 18:2 |
| Mean duration of illness (years) | 8.4 |
| Mean age of onset (years) | 43.6 |
| Mean age at presentation (years) | 52 |
Figure 2.
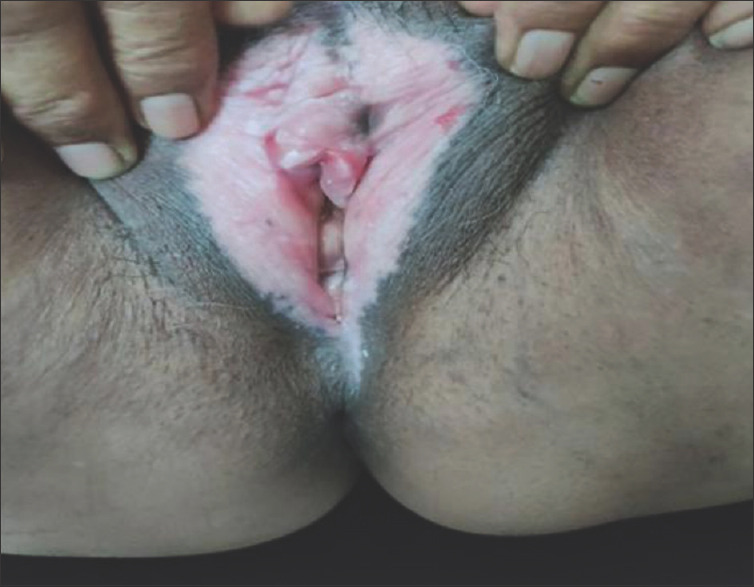
Lichen sclerosus with excoriations
Table 4.
Clinical details
| Symptoms | Number of patient, n (%) | P |
|---|---|---|
| Itching and/or burning | 15 (75) | 0.188 |
| Excoriation, fissuring | 15 (75) | 0.389 |
| Bruising | 10 (50) | 0.136 |
| Erosions, ulcers | 14 (70) | 0.329 |
| Figure-of-eight appearance | 1 (5) | 0.488 |
| Dysuria | 12 (60) | 0.514 |
| Dyspareunia/sexual difficulties | 14 (70) | 0.367 |
| Malignancy | 2 (10) | 0.247 |
| Smoking | 1 (5) | 0.051 |
Table 5.
Sex-wise distribution of clinical features
|
Figure 3.
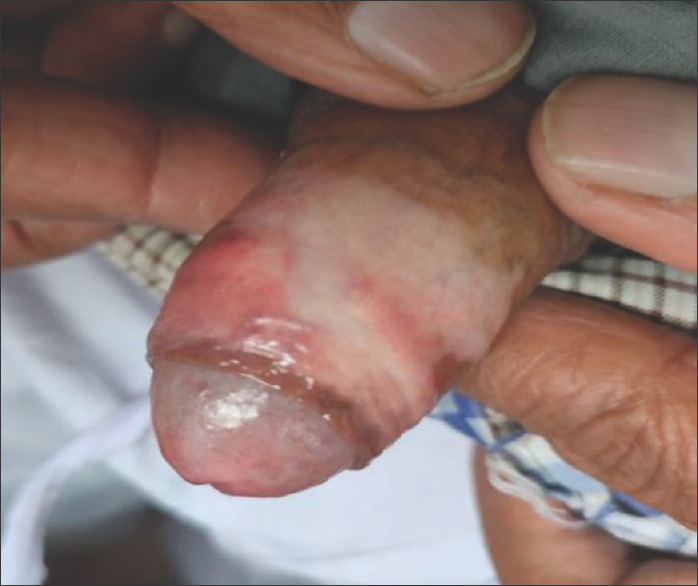
Male genital lichen sclerosus with glans and prepuce involvement leading to phimosis
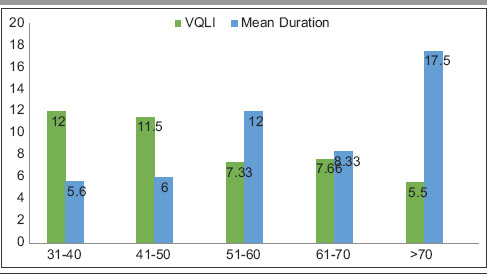
Table 6.
Age-wise correlation of vulvar quality of life index and duration
|
Figure 4.
Whitish plaque on right labia which turned out to be squamous cell carcinoma on biopsy
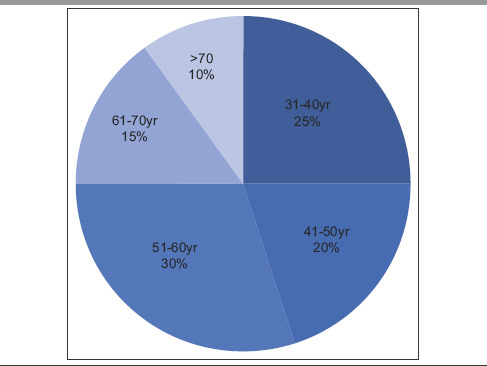
Table 2.
Age-wise distribution
|
Discussion
LS is a disorder of unknown etiology most commonly found in adult women. In this series, women were more commonly affected than men in a ratio of 9:1 which is consistent with reported ratio of 10:1–6:1.1 in previous literature [Table 4].[4,5] Both men were uncircumscribed consistent with the previous observations that male genital LS occurs more commonly in uncircumcised men.[6] Prevalence rates are difficult to establish, as the condition is underreported and also hospital-based studies cannot reflect the prevalence. The prevalence of disease (20 in 4000) in this series is in accordance to previously reported studies 1.7%–3%.[7] Most of our patients were those referred for expert opinion from periphery and other specialties in view of low or unsatisfactory response.[5] Moreover, a large number (78%) were labeled as “chronic genital candidiasis” and “eczema.”
The highest number of patients was in 51–60 years of age group and was postmenopausal.[8] However, we also observed an increased number of patients in 31–40 years and 41–50 years of age group (25% and 20%, respectively). This finding is also reflected in symptomatology where itching/burning, fissures, erosions, and dyspareunia were the most frequent symptoms reported, followed by urinary complaints such as dysuria, thinning stream, and incomplete voiding [Tables 1 and 6]. Patients in the age group of 31–50 years also reported increased VQLI scores of >10 with mean = 9.2 [Table 5]. Average VQLI score is consistent with previous studies such as by Saunderson et al. and Felmingham et al.[9,10] Our center caters primarily to rural population owing to its location, hence patients do not always seek medical attention because of fear or embarrassment and among those who seek treatment often present late with mis/undertreatment and therefore have more predilection for development of complications. Adding to the owe of the patients, physicians may fail to recognize the disorder leading to misdiagnosis and mistreatment. Without proper identification of the disease and its nature, many women end up losing hope of getting relieved of the symptoms and fail to receive counseling regarding the natural course, avoiding friction, methods for improving difficulties in sexual activities, etc. Self-medication and treatment from quacks further predispose them to secondary infections and contact dermatitis superimposed on primary pathology. This prolongs duration (in our case 8.2 years) and mean age of presentation (in our case 52 years) explaining higher incidence of SCC (10%) as compared to previous literatures reporting it between 4% and 6% (5%) [Table 1].[8,11] SCC was found in older age group (mean age: 67.5) with long-standing disease (>10 years).[12,13]
Apart from longer duration and older age of presentation, we were not able to directly correlate any other predisposing history. Furthermore, because of resource-limited setup, the oncogenic molecular analysis (p53 gene, PTEN gene, etc.) or immunohistochemical studies were not possible. Based on the above findings, we stress upon the need for long-term follow-up of all LS cases. Persistent ulcerated area which fails to respond to routine treatments may turn out to be malignant, and in such cases, serial biopsies should be done.[14] Interestingly, females of age >70 years despite longer duration of disease did not have corresponding increased VQLI, we postulate that decline in sexual activities being the reason for the same. Correlation with autoimmune disorders and infections was not observed.
Patients diagnosed with malignancies were referred to the oncology department for further management. Chances of spontaneous remission are rare. Our patients responded well to potent topical corticosteroids (cream clobetasol propionate 0.05% for 2–3 months daily and later tapering to twice weekly ± calcineurin inhibitors on other days). We propose guidelines for long-term management including counseling in cases of LS. The major counseling in these patients includes general care practices to be stressed upon such as frequent emollient application, avoidance of scratching, harsh detergent for washing, sexual trauma, or prolonged contact with irritants such as urine and feces to avoid Koebnerization.[15] Lubricating jellies can be advised to females for decreasing sexual trauma, and to ease intercourse, males can be advised circumcision. All patients should be advised long-term follow-up keeping in view the malignant potential of disease, albeit low. We recommend that systemic agents such as etretinate, ciclosporin, and methotrexate to be kept as reserve drugs for their higher incidences of side effects and longer duration of treatment.
Conclusion
Therefore, from this study, we conclude that LS is an important disorder presenting to the dermatological outpatient department with considerable morbidity in the form of disfigured genitalia, excoriations, erosions, bleeding, and development of SCC along with increased VQLI. Early diagnosis with proper counseling of the patient and his/her partner regarding various aspects of disease is essential for a wholesome approach. The main limitation of our study was small sample size. Hence, more such studies with larger sample size are advocated for establishing a proper correlation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kaufman RH, DiPaola GR, Friedrich EG, Jr, Hewitt J, Woodruff JD. New nomenclature for vulvar disease. J Cutan Pathol. 1976;3:159–61. doi: 10.1111/j.1600-0560.1976.tb01105.x. [DOI] [PubMed] [Google Scholar]
- 2.Neill SM, Lewis FM, Tatnall FM, Cox NH British Association of Dermatologists. British Association of dermatologists’ guidelines for the management of lichen sclerosus 2010. Br J Dermatol. 2010;163:672–82. doi: 10.1111/j.1365-2133.2010.09997.x. [DOI] [PubMed] [Google Scholar]
- 3.Powell JJ, Wojnarowska F. Lichen sclerosus. The Lancet. 1999;353:1777–83. doi: 10.1016/s0140-6736(98)08228-2. [DOI] [PubMed] [Google Scholar]
- 4.Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393–416. doi: 10.1016/0190-9622(95)90060-8. [DOI] [PubMed] [Google Scholar]
- 5.Wallace HJ. Lichen sclerosus et atrophicus. Trans St Johns Hosp Dermatol Soc. 1971;57:9–30. [PubMed] [Google Scholar]
- 6.Mallon E, Hawkins D, Dinneen M, Francics N, Fearfield L, Newson R, et al. Circumcision and genital dermatoses. Arch Dermatol. 2000;136:350–4. doi: 10.1001/archderm.136.3.350. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein AT, Marinoff SC, Cristopher K, Srodon M. Prevalence of vulvar lichen sclerosus in a general gynecology practices. J Reprod Med. 2005;50:477. [PubMed] [Google Scholar]
- 8.Cooper SM, Gao XH, Powell JJ, Wojnarowska F. Does treatment of vulvar lichen sclerosus influence its prognosis? Arch Dermatol. 2004;140:702–6. doi: 10.1001/archderm.140.6.702. [DOI] [PubMed] [Google Scholar]
- 9.Saunderson RB, Harris V, Yeh R, Mallitt KA, Fischer G. Vulvar Quality of Life Index (VQLI)-A simple tool to measure quality of life in patients with vulvar disease. Australas J Dermatol. 2020;61:152–7. doi: 10.1111/ajd.13235. [DOI] [PubMed] [Google Scholar]
- 10.Felmingham C, Chan L, Doyle LW, Veysey E. The Vulval Disease Quality of Life Index in women with vulval lichen sclerosus correlates with clinician and symptom scores. Australas J Dermatol. 2020;61:110–8. doi: 10.1111/ajd.13197. [DOI] [PubMed] [Google Scholar]
- 11.Thomas RH, Ridley CM, McGibbon DH, Black MM. Anogenital lichen sclerosus in women. J R Soc Med. 1996;89:694–8. doi: 10.1177/014107689608901210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlson JA, Ambros R, Malfetano J, Ross J, Grabowski R, Lamb P, et al. Vulvar lichen sclerosus and squamous cell carcinoma: A cohort, case control, and investigational study with historical perspective; implications for chronic inflammation and sclerosis in the development of neoplasia. Hum Pathol. 1998;29:932–48. doi: 10.1016/s0046-8177(98)90198-8. [DOI] [PubMed] [Google Scholar]
- 13.Carli P, Cattaneo A, De Magnis A, Biggeri A, Taddei G, Giannotti B. Squamous cell carcinoma arising in vulval lichen sclerosus: A longitudinal cohort study. Eur J Cancer Prev. 1995;4:491–5. doi: 10.1097/00008469-199512000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Guo H, Peng X, Jin C, Wang L, Chen F, Sa Y. Lichen sclerosus accompanied by urethral squamous cell carcinoma: A retrospective study from a urethral referral center. Am J Mens Health. 2018;12:1692–9. doi: 10.1177/1557988318782095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis FM, Tatnall FM, Velangi SS, Bunker CB, Kumar A, Brackenbury F, et al. British association of dermatologists guidelines for the management of lichen sclerosus, 2018. Br J Dermatol. 2018;178:839–53. doi: 10.1111/bjd.16241. [DOI] [PubMed] [Google Scholar]


