Abstract
Background:
GNAO1 is an emerging disorder characterized with hypotonia, developmental delay, epilepsy, and movement disorder, which can be potentially life threatening during acute exacerbation. In the USA, deep brain stimulation (DBS) has been licensed for treating children with chronic, treatment-resistant primary dystonia, who are 7 years old or older.
Case Description:
A 4-year-old girl diagnosed to have GNAO1-related dyskinesia and severe global developmental delay. She had severe dyskinesia precipitated by intercurrent infection, requiring prolonged intensive care for heavy sedation and related complications. Her dyskinesia improved dramatically after DBS implantation. Technical difficulties and precautions of DBS in preschool children were discussed.
Conclusion:
DBS should be considered early in the treatment of drug-resistant movement disorders in young children with GNAO1, especially after dyskinetic crisis, as they tend to recur. Presurgical counseling to parents and close monitoring of complications is also important in the process.
Keywords: Deep brain stimulation, Dyskinesia, GNAO1, Preschool child

INTRODUCTION
GNAO1 is an emerging disorder characterized with developmental delay, early onset epilepsy, and movement disorder.[4,12] Life-threatening dystonic or hyperkinetic crisis is common.[8] There are increasing reports in using deep brain stimulation (DBS) to ameliorate the associated movement disorders.[13]
The Food and Drug Administration in the USA has approved DBS for the treatment of dystonia in 2003 for children older than 7 years with humanitarian device exemption.[10] However, National Health Service of England do not put forward an age limit for DBS in children.[5] A meta-analysis of DBS in more than 300 children with dystonia from various causes showed that two-thirds of them had clinically significant (>20%) improvement in Burke–Fahn–Marsden Dystonia Rating Scale (BFMDRS), but few received DBS at age younger than 7 years old.[6] Here, we reported our experience of using DBS in a young child with severe GNAO1-related dyskinesia.
CASE ILLUSTRATION
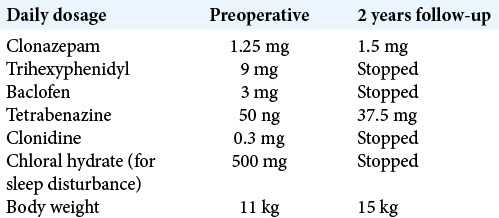
A 4-year-old girl has background history of global developmental delay and dyskinesia, caused by de novo pathogenic mutation in GNAO1 (c.709G>A, p.Glu237Lys). She could not sit independently, and she did not have any verbal expression. She was admitted with worsening of hyperkinetic movement with near continuous choreoathetoid movement involving the whole body, triggered by enterovirus infection. It was further complicated by pneumonia and rhabdomyolysis. She stayed in intensive care unit (ICU) for 7 weeks and required heavy sedation/anesthesia to control her movement, with a combination of midazolam, fentanyl, and propofol. Oral medications were also maximized [Table 1].
Table 1:
Drug regime.
She still had significant choreoathetoid movement after discharge from ICU, which severely affected her daily activities such as posturing and feeding. Parents eventually agreed for DBS as rescue therapy. She underwent preoperative magnetic resonant imaging (MRI) under general anesthesia (GA). The target of DBS was bilateral posterolateroventral portion of the globus pallidus internus (GPi). She was kept sedated and intubated after MRI. The next day, frame-based stereotactic surgery for DBS was performed under GA. Leksell ® Vantage™ Head Frame was fixed to the head, and computed tomography (CT) of brain was performed together with the frame. Afterward, the preoperative MRI with targeting plan was fused to the CT images. Directional leads (Vercise™ Cartesia™ Model DB-2202-45) were implanted on both sides and a rechargeable pulse generator (Vercise Gevia™ Model DB-1200) was implanted over the left subclavian subcutaneous pocket.
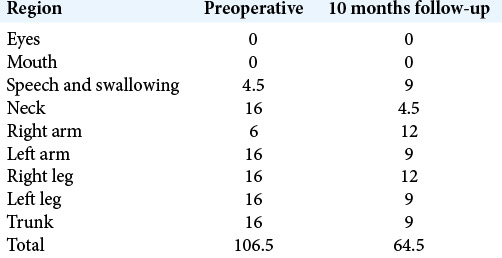
On postoperative day 1, sedation was stopped and the pulse generator was switched on. The initial setting of stimulation was monopolar configuration contact 1 and 9 which were the distalmost (ventral) pair of contact, with 3 mA, pulse width 60 µs, and frequency of 130 Hz. There was significant decrease in the hyperkinetic movement in early postoperative days. The patient was discharged home on postoperative day 8. Her choreoathetoid movement continued to improve with further DBS programming. There was no further exacerbation of dyskinesia at 24 months follow-up after DBS, even with intercurrent infection. The BFMDRS was 106.5/120 before DBS and 64.5/120 by 10 months follow-up [Table 2]. The latest DBS parameters remained the same monopolar configuration 1–9, while the amplitude was increased to 3.9 mA and 4.0 mA, respectively. The frequency and pulse width remained 130 Hz and 60 µs, respectively. We were able to step down her oral drugs [Table 1]. She was also making some progress in neurodevelopment that she could now sit independently and reach out for objects.
Table 2:
Scores of Burke–Fahn–Marsden Dystonia Rating Scale.
DISCUSSION
GNAO1 – Guanosine nucleotide-binding protein G (o) subunit α is a gene that is associated with neurodevelopmental problems, including early-onset epilepsy, mild-to-severe developmental delay, and various movement disorders, from hyperkinesia to lack of spontaneous movement.[13,16] The mutation is our patient has been reported in at least three patients[7,18] and its functional effect is still unknown.[17] The genotype-phenotype correlation is still unclear. The movement disorders may include variable combination of dystonia, choreoathetosis, and dyskinetic movement. Tetrabenazine appears most effective in certain patients, but response to medical treatment is often suboptimal.[17] Kelly et al., in 2019, have proposed that identification of genetic variant in GNAO1 gene with movement disorder should prompt earlier evaluation and counseling for DBS for the treatment of movement disorder because of frequent occurrence of potentially life-threatening dystonic/dyskinetic crisis.[12]
Up to date, there were 17 published DBS implantations in patients with GNAO1-associated movement disorders and all reported “positive” results, usually with dramatic improvement of the hyperkinetic movement and decrease in the recurrence of status dystonicus.[3,4,8,9,13,14,18-20] These reports could certainly be limited by reporting bias.
However, the response of DBS in these cases was dramatic as shown in video recordings with on and off stimulation.[13] Many patients improved within days and weeks with DBS stimulation.[12] Recently, there were also reports of long-term response of DBS in GNAO1-associated movement disorders with persistent benefit up to 10 years.[2,13] Ten out of 17 reported cases received the implantation in ICU with dystonic/hyperkinetic crisis, which reflected the severity of the movement disorder and potentially “life-saving effect” of DBS in these patients.[8,13,20]
All DBS implantations with GNAO1 mutations were targeted at GPi.[2,3,6,19] The optimal stimulation setting is still unknown. A range of settings has been reported: with amplitude, pulse width, and frequency ranging from 1 to 4 V, 50 to 450 µs, and 130 to 210 Hz, respectively.[2,19]
Recharging devices have been used in the series reported by Kaminska et al., in 2017. It reduces frequency of battery replacement. Yet, the DBS team needs continuous support to carers for using harness or belt for recharging. Loss of recharger and difficulty in maintaining connection between recharger and battery were common.[11]
Radiological studies from patients with DBS suggested a projected increase in distance between entry point and target of 5–10 mm from brain growth from 4 to 18 years of age, with most occurring before 7 years of age.[1] Younger age of implantation would necessitate need for future revision of leads as the child grows. Among 17 reported DBS implantations with GNAO1 mutations, five of them were younger than 7 years of age when they received surgery. Our patient is the second youngest patient with GNAO1 mutation who received DBS implantation.[14] Long-term clinical follow-up plus minor rescan for the DBS lead location is needed for our patient. Implantations in young children may be associated with more technical challenges, especially if they are malnourished and/or have severe dyskinetic movement. Complications with infections, skin erosions, and/or cerebrospinal fluid leakage may be increased. Different teams have proposed various remedial strategies, such as implanting stimulators in abdomen in malnourished children[2] or performing staged operations of electrode and stimulator placement.[15]
In the large pediatric cohort of DBS for dystonia reported by Kaminska et al., 26 out of 126 patients were 7 years or younger when they received the implantation. The youngest one was only 3.3 years of age. Surgical site infections were the most significant adverse events and occurred in 10.3%. They concluded that medical and surgical experience of the DBS team might also be an important factor for treatment success. Safe and early DBS implantation might mitigate functional skeletal deformities and adverse effects from drugs. It might also optimize developmental potential by activity-dependent plasticity.[11]
Families should be counseled on the risks of deterioration of hyperkinetic movement or even “dystonic storm” on sudden cessation of DBS, for example, system switch off unexpectedly, battery failure, or electrode malfunction. They should also be reminded about possible electrode contact point migration as the child grows and hence need for revision. Although movement disorder may improve, DBS may not help the neurodevelopment dramatically. Parents need to be realistic in developmental outcome of the patients.
CONCLUSION
DBS can be an effective treatment for severe hyperkinetic movement disorder in patients with pathogenic GNAO1 mutation. Age alone should not be a limitation in consideration of DBS. The risk and potential benefit of the procedure should be thoroughly discussed with the families.
Acknowledgment
The authors thanked Ms. Rosanna Wong, our occupational therapist, in the functional assessment of the patient.
Footnotes
How to cite this article: Fung EL, Mo C, Fung ST, Chan AY, Lau K, Chan EK, et al. Deep brain stimulation in a young child with GNAO1 mutation – Feasible and helpful. Surg Neurol Int 2022;13:285.
Contributor Information
Eva Lai-wah Fung, Email: eva_fung@cuhk.edu.hk.
Chung-yin Mo, Email: alexcymo@gmail.com.
Sharon Tsui-Hang Fung, Email: fungth@ha.org.hk.
Anne Yin-yan Chan, Email: yychan@gmail.com.
Ka-yee Lau, Email: yeekaclaire@surgery.cuhk.edu.hk.
Emily Kit-ying Chan, Email: emilychan@surgery.cuhk.edu.hk.
David Yuen-chung Chan, Email: davidchan@surgery.cuhk.edu.hk.
Xian-lun Zhu, Email: zhuxl@surgery.cuhk.edu.hk.
Danny Tat-ming Chan, Email: tmdanny@surgery.cuhk.edu.hk.
Wai-sang Poon, Email: wpoon@surgery.cuhk.edu.hk.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Air EL, Ostrem JL, Sanger TD, Starr PA. Deep brain stimulation in children: Experience and technical pearls. J Neurosurg Pediatr. 2011;8:566–74. doi: 10.3171/2011.8.PEDS11153. [DOI] [PubMed] [Google Scholar]
- 2.Benato A, Carecchio M, Burlina A, Paoloni F, Sartori S, Nosadini M, et al. Long-term effect of subthalamic and pallidal deep brain stimulation for status dystonicus in children with methylmalonic acidemia and GNAO1 mutation. J Neural Transm (Vienna) 2019;126:739–57. doi: 10.1007/s00702-019-02010-2. [DOI] [PubMed] [Google Scholar]
- 3.Danhofer P, Zech M, Bálintová Z, Baláž M, Jech R, Ošlejšková H. Brittle biballism-dystonia in a pediatric patient with GNAO1 mutation managed using pallidal deep brain stimulation. Mov Disord Clin Pract. 2021;8:153–5. doi: 10.1002/mdc3.13118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danti FR, Galosi S, Romani M, Montomoli M, Carss KJ, Raymond FL, et al. GNAO1 encephalopathy: Broadening the phenotype and evaluating treatment and outcome. Neurol Genet. 2017;3:e143. doi: 10.1212/NXG.0000000000000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deep Brain stimulation-NHS England. Available from: https://www.england.nhs.uk/wp-content/uploads/2013/04/d03-p-b.pdf [Last accessed on 2022 Jan 31]
- 6.Elkaim LM, Alotaibi NM, Sigal A, Alotaibi HM, Lipsman N, Kalia SK, et al. Deep brain stimulation for pediatric dystonia: A meta-analysis with individual participant data. Dev Med Child Neurol. 2019;61:49–56. doi: 10.1111/dmcn.14063. [DOI] [PubMed] [Google Scholar]
- 7.Feng H, Khalil S, Neubig RR, Sidiropoulos C. A mechanistic review on GNAO1-associated movement disorder. Neurobiol Dis. 2018;116:131–41. doi: 10.1016/j.nbd.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Honey CM, Malhotra AK, Tarailo-Graovac M, van Karnebeek CD, Horvath G, Sulistyanto A. GNAO1 mutation-induced pediatric dystonic storm rescue with pallidal deep brain stimulation. J Child Neurol. 2018;33:413–6. doi: 10.1177/0883073818756134. [DOI] [PubMed] [Google Scholar]
- 9.Hull M, Parnes M. Management of life threatening dyskinesias in GNAO1 related movement disorders: Two new cases and review of the literature. Mov Disord. 2019;34(Suppl 2) Available from: https://www.mdsabstracts.org/abstract/management-of-life-threatening-dyskinesias-in-gnao1-related-movement-disorder-two-new-cases-and-review-of-the-literature [Last accessed on 2021 Jul 07] [Google Scholar]
- 10.Humanitarian Device Exemption FDA. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf2/H020007b.pdf [Last accessed on 2022 Jan 31]
- 11.Kaminska M, Perides S, Lumsden DE, Nakou V, Selway R, Ashkan K, et al. Complications of deep brain stimulation (DBS) for dystonia in children the challenges and 10 year experience in a large paediatric cohort. Eur J Paediatr Neurol. 2017;21:168–75. doi: 10.1016/j.ejpn.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 12.Kelly M, Park M, Mihalek I, Rochtus A, Gramm M, Pérez-Palma E, et al. Spectrum of neurodevelopmental disease associated with the GNAO1 guanosine triphosphate-binding region. Epilepsia. 2019;60:406–18. doi: 10.1111/epi.14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koy A, Cirak S, Gonzalez Becker K, Roujeau T, Milesi C, Baleine J, et al. Deep brain stimulation is elective in pediatric patients with GNAO1 associated severe hyperkinesia. J Neurol Sci. 2018;391:31–9. doi: 10.1016/j.jns.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 14.Kulkarni N, Tang S, Bhardwaj R, Bernes S, Grebe TA. Progressive movement disorder in brothers carrying a GNAO1 mutation responsive to deep brain stimulation. J Child Neurol. 2016;31:211–4. doi: 10.1177/0883073815587945. [DOI] [PubMed] [Google Scholar]
- 15.Lumsden DE, Ashmore J, Charles-Edwards G, Selway R, Lin JP, Ashkan K. Observation and modelling of deep brain stimulation electrode depth in the pallidal target of the developing brain. World Neurosurg. 2015;83:438–46. doi: 10.1016/j.wneu.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Okumura A, Maruyama K, Shibata M, Kurahashi H, Ishii A, Numoto S, et al. A patient with a GNAO1 mutation with decreased spontaneous movements, hypotonia, and dystonic features. Brain Dev. 2018;40:926–30. doi: 10.1016/j.braindev.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Schirinzi T, Garone G, Travaglini L, Vasco G, Galosi S, Rios L, et al. Phenomenology and clinical course of movement disorder in GNAO1 variants: Results from an analytical review. Parkinsonism Relat Disord. 2019;61:19–25. doi: 10.1016/j.parkreldis.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Waak M, Mohammad SS, Coman D, Sinclair K, Copeland L, Silburn P, et al. GNAO1-related movement disorder with life-threatening exacerbations: Movement phenomenology and response to DBS. J Neurol Neurosurg Psychiatry. 2018;89:221–2. doi: 10.1136/jnnp-2017-315653. [DOI] [PubMed] [Google Scholar]
- 19.Yamashita Y, Ogawa T, Ogaki K, Kamo H, Sukigara T, Kitahara E, et al. Neuroimaging evaluation and successful treatment by using directional deep brain stimulation and levodopa in a patient with GNAO1-associated movement disorder: A case report. J Neurol Sci. 2020;411:116710. doi: 10.1016/j.jns.2020.116710. [DOI] [PubMed] [Google Scholar]
- 20.Yilmaz S, Turhan T, Ceylaner S, Gökben S, Tekgul H, Serdaroglu G. Excellent response to deep brain stimulation in a young girl with GNAO1-related progressive choreoathetosis. Childs Nerv Syst. 2016;32:1567–8. doi: 10.1007/s00381-016-3139-6. [DOI] [PubMed] [Google Scholar]


