Abstract
Background
Reducing hospital admissions among people dying with dementia is a policy priority.
Aim
To explore associations between primary care contacts, continuity of primary care, identification of palliative care needs, and unplanned hospital admissions among people dying with dementia.
Design and setting
This was a retrospective cohort study using the Clinical Practice Research Datalink linked with hospital records and Office for National Statistics data. Adults (>18 years) who died between 2009 and 2018 with a diagnosis of dementia were included in the study.
Method
The association between GP contacts, Herfindahl–Hirschman Index continuity of care score, palliative care needs identification before the last 90 days of life, and multiple unplanned hospital admissions in the last 90 days was evaluated using random-effects Poisson regression.
Results
In total, 33 714 decedents with dementia were identified: 64.1% (n = 21 623) female, mean age 86.6 years (SD 8.1), mean comorbidities 2.2 (SD 1.6). Of these, 1894 (5.6%) had multiple hospital admissions in the last 90 days of life (increase from 4.9%, 95% confidence interval [CI] = 4.2 to 5.6 in 2009 to 7.1%, 95% CI = 5.7 to 8.4 in 2018). Participants with more GP contacts had higher risk of multiple hospital admissions (incidence risk ratio [IRR] 1.08, 95% CI = 1.05 to 1.11). Higher continuity of care scores (IRR 0.79, 95% CI = 0.68 to 0.92) and identification of palliative care needs (IRR 0.66, 95% CI = 0.56 to 0.78) were associated with lower frequency of these admissions.
Conclusion
Multiple hospital admissions among people dying with dementia are increasing. Higher continuity of care and identification of palliative care needs are associated with a lower risk of multiple hospital admissions in this population, and might help prevent these admissions at the end of life.
Keywords: dementia, end of life, family practice, palliative care, primary health care, hospitalisation
INTRODUCTION
Dementia is one of the leading causes of death in high-income nations,1 and the number of people dying with dementia requiring symptom management is projected to increase.2,3 It is essential, for patients and the system, to better understand how to provide high-quality end-of-life care for this population.
People with dementia experience a rapid increase in symptoms,4,5 emergency department visits,6,7 and hospital admissions in their last year of life.8,9 Transitions to hospital among people dying with dementia have been associated with markers of poor-quality end-of-life care,10 and poor health outcomes such as delirium, falls, and cognitive and functional decline.11–13 Multiple hospital admissions in the last 90 days of life has been suggested as an indicator of poor end-of-life care in people with dementia.14,15
Primary care services, including GPs, nurses, or other healthcare services provided in the community, are likely to contribute to reducing unnecessary transitions at the end of life by providing timely access to patient-centred care.16 Community-based palliative care services have been associated with fewer hospital admissions in people with dementia in Australia17 and the US.18 However, people with dementia experience several barriers to access palliative care services.19 Being the first point of contact, GPs play an important role in providing end-of-life care, and contacts with GPs20 and home health care21 have been associated with lower risk of end-of-life admissions to hospital among older adults and people with dementia. It is not known how this relationship is affected by the frequency and length of contacts,21 the level of continuity of care experienced,22,23 or whether palliative care needs are being identified by the GP.24
The aim of this study was 1) to describe primary care service use among individuals with dementia in the last year of life, and 2) explore associations between contacts, continuity of care with GPs, palliative care needs identification, and unplanned hospital admissions in people dying with dementia in their last 90 days of life.
METHOD
Design and data sources
This is a nationwide population-based retrospective cohort study in England using the Clinical Practice Research Datalink (CPRD), linked with hospital records and mortality data from the Office for National Statistics. CPRD contains anonymised medical records from over 19 million people enrolled in 952 general practices across the UK.25 People currently registered in CPRD primary care practices represent approximately 4.6% of the UK population.26
How this fits in
| People with dementia are at high risk of multiple hospital admissions at the end of life and preventing these admissions is a policy priority. This study found that people with dementia who had better continuity of care with GPs were less likely to have multiple hospital admissions in the last 90 days of life, in particular if they lived at home and had multiple comorbidities. People living in care homes and with an identification of palliative care needs in their primary care records were less likely to experience these admissions. |
Population
People included in the study were adults (≥18 years) who died between 1 January 2009 and 31 December 2018, had a dementia diagnosis recorded in primary care or hospital records, and a 12-month before death registration period in a GP practice with continuous high-quality data based on CPRD quality checks.25 Dementia diagnosis was identified from primary care records (using Read codes, standard clinical codes used in UK GP practices to record diagnosis and procedures)27 and hospital records (using International Statistical Classification of Diseases and Related Health Problems 10 [ICD-10] codes), based on previous studies28 (see Supplementary Box S1 and Table S1).
Outcome
The primary outcome was multiple unplanned admissions to hospital in the last 90 days of life (0 if no, 1 if yes), based on Gozalo et al as either more than two unplanned admissions for any reason or more than one unplanned admission for respiratory infection, urinary tract infection, dehydration, or sepsis10 (see Supplementary Box S2).
Explanatory variables
To describe primary care service use, the number of participant’s consultations with a GP in the last 12 months of life were determined. Face-to-face and telephone consultations were included regardless of where the consultation took place (practice, home, or out-of-hours).
An exposure period was defined between months 12 and 4 before death (day 365 until day 91 before death). To account for the fact that participants in hospital cannot visit their GP, a rate of consultations with GPs by month was calculated by dividing the total number of consultations with GPs by the number of days participants were in the community (excluding days in hospital) during the exposure period (Box 1).
Box 1.
Formulas for calculating rate GP contacts and continuity of care score
| ni = the number of contacts the patient had with a GP during the exposure period (months 12 and 4 before death)N = the total number of GP contacts during exposure period. |
The Consultation and Staff files provided by CPRD include an anonymised code identifying the physician who recorded each consultation, which was used to calculate the Herfindahl–Hirschman Index continuity of care score (Box 1).29 This continuity of care score measures the extent to which consultations during a certain period of time are with the same physician, and has a range from 0 to 1 (1 means all contacts the patient has in that period were with the same GP). Only contacts with GPs during the exposure period were considered.
Identification of palliative care needs was derived from the Palliative Care Register, an electronic register introduced in 2004 in England that aims to identify people in the GP practice who might benefit from a palliative care approach.30 When patients are identified as having palliative care needs, their GP adds a code in the patients’ clinical records that is then captured by the Palliative Care Register. In this study, people with a relevant code at any point before the last 90 days of life were identified in order to recognise people who had been identified by their GPs as having palliative care needs (see Supplementary Table S2).27,31–33
Covariables
Factors associated with multiple hospital admissions in the last 90 days of life were examined based on previous research and theoretical models.34,35 Age at death was calculated using the year of death and year of birth. Sex and GP practice region were extracted from the CPRD. The 2011 England and Wales rural–urban classification of the GP practice where participants were enrolled and the 2015 English Index of Multiple Deprivation quintiles at lower super output areas level from the latest available postcode of residence for participants were used.
The underlying cause of death, place, and date of death were identified from the Office for National Statistics. The underlying cause of death was grouped into ICD-10 block codes (see Supplementary Table S3). The number of comorbidities (excluding dementia) were calculated using the count of chronic diseases from the Quality and Outcomes Framework Read codes rules (see Supplementary Table S2).27,36 Read codes were used to identify whether participants had a record of living in a care home (nursing or residential care home) based on previous publications (see Supplementary Table S4).37
Analysis
Changes in the annual proportion of participants with multiple hospital admissions in the last 90 days of life between 2009 and 2018 were explored using a scatter plot, with the proportion of multiple hospital admissions by year of death adjusted by age and sex. The mean (95% confidence interval) number of contacts with GPs by month before death was explored.
A multilevel Poisson regression with robust error variance and a random intercept for the region and participant’s GP practice was used to estimate the association between the rate of GP contacts per month, continuity of care score, identification of palliative care needs during the exposure period, and multiple hospital admissions in the last 90 days of life. As the Herfindahl–Hirschman continuity of care score can only be calculated with at least two contacts, participants with fewer than two contacts with GPs were excluded (2920/33 714, 8.7%). Missing values for covariables were small (<1%) and therefore excluded.
A subgroup analysis was performed to explore the influence of sociodemographic and illness-related factors on the association between the rate of GP contacts per month, continuity of care, identification of palliative care needs, and the outcome.
Three sensitivity analyses were conducted:
as the continuity of care score has been shown to be less stable when participants have <4 contacts, an analysis excluding those participants was performed;
to explore the effect of excluding participants with <2 contacts with GPs, the same general multivariate model was performed excluding continuity of care; and
an analysis was performed including people with at least 1 day of enrolment during the last year of life (n = 57 659). As a notable proportion of people with <365 days of enrolment had <2 contacts with GPs, the continuity of care score was excluded in this analysis.
All analysis were performed using Stata® version 16.1.
RESULTS
Characteristics of the study sample
This study identified 57 659 people with dementia who died between 2009 and 2018, and who were registered in a GP practice during the last year of life. After excluding 23 945 people without a complete year of registration before death, 33 714 participants were included in the analysis (Supplementary Figure S1).
Demographic characteristics for people with and without a complete year of registration are described in Supplementary Table S5. The cohort had an average age at death of 86.6 years (SD 8.1), 64.1% (n = 21 623) were female, 21.5% (n = 7260) lived in the least deprived quintile, and 56.0% (n = 18 896) of the cohort had a code for living in a care home. The most common underlying cause of death was dementia (36.8%, n = 12 404) followed by cerebrovascular disease (10.7%, n = 3615), and cancer (8.7%, n = 2926) (Table 1).
Table 1.
Characteristics of participants by multiple hospital admissions in the last 90 days
| Total | Multiple unplanned hospital admissions last 90 days | ||
|---|---|---|---|
|
| |||
| No | Yes | ||
| Total, n | 33 714 | 31 820 (94.4) | 1894 (5.6) |
|
| |||
| Age, mean (SD) | 86.56 (8.07) | 86.69 (8.01) | 84.67 (8.79) |
|
| |||
| Sex, n (%) | |||
| Male | 12 091 (35.9) | 11 171 (35.1) | 920 (48.6) |
| Female | 21 623 (64.1) | 20 649 (64.9) | 974 (51.4) |
|
| |||
| IMD quintiles, n (%) | |||
| (Least deprived) 1 | 7260 (21.5) | 6906 (21.7) | 354 (18.7) |
| 2 | 7451 (22.1) | 7062 (22.2) | 389 (20.5) |
| 3 | 7837 (23.2) | 7459 (23.4) | 378 (20.0) |
| 4 | 5895 (17.5) | 5549 (17.4) | 346 (18.3) |
| 5 | 5258 (15.6) | 4831 (15.2) | 427 (22.5) |
| Missing | 13 | 13 | 0 |
|
| |||
| Lived in care home, n (%) | |||
| No | 14 818 (44.0) | 13 816 (43.4) | 1002 (52.9) |
| Yes | 18 896 (56.0) | 18 004 (56.6) | 892 (47.1) |
|
| |||
| Rurality, n (%) | |||
| Urban | 28 921 (85.8) | 27 192 (85.5) | 1729 (91.3) |
| Rural | 4793 (14.2) | 4628 (14.5) | 165 (8.7) |
|
| |||
| Region, n (%) | |||
| North East | 703 (2.1) | 666 (2.1) | 37 (2.0) |
| North West | 5947 (17.6) | 5510 (17.3) | 437 (23.1) |
| Yorkshire & The Humber | 1047 (3.1) | 981 (3.1) | 66 (3.5) |
| East Midlands | 364 (1.1) | 342 (1.1) | 22 (1.2) |
| West Midlands | 4028 (11.9) | 3785 (11.9) | 243 (12.8) |
| East of England | 2755 (8.2) | 2618 (8.2) | 137 (7.2) |
| South West | 4855 (14.4) | 4678 (14.7) | 177 (9.3) |
| South Central | 5311 (15.8) | 5121 (16.1) | 190 (10.0) |
| London | 3386 (10.0) | 3107 (9.8) | 279 (14.7) |
| South East Coast | 5318 (15.8) | 5012 (15.8) | 306 (16.2) |
|
| |||
| Cause of death, n (%) | |||
| Dementia | 12 404 (36.8) | 11 917 (37.5) | 487 (25.7) |
| Cancer | 2926 (8.7) | 2731 (8.6) | 195 (10.3) |
| Cerebrovascular disease | 3615 (10.7) | 3429 (10.8) | 186 (9.8) |
| Ischaemic heart disease | 2510 (7.4) | 2363 (7.4) | 147 (7.8) |
| Influenza and pneumonia | 1800 (5.3) | 1657 (5.2) | 143 (7.6) |
| Chronic pulmonary disease | 1148 (3.4) | 1003 (3.2) | 145 (7.7) |
| Chronic heart disease | 1160 (3.4) | 1087 (3.4) | 73 (3.9) |
| Parkinson’s disease | 831 (2.5) | 801 (2.5) | 30 (1.6) |
| Senility | 750 (2.2) | 737 (2.3) | 13 (0.7) |
| Other | 6565 (19.5) | 6090 (19.1) | 475 (25.1) |
| Missing | 5 | 5 | 0 |
|
| |||
| Number of QoF comorbidities, mean (SD) | 2.23 (1.60) | 2.20 (1.59) | 2.72 (1.69) |
|
| |||
| Place of death, n (%) | |||
| No home | 30 376 (90.1) | 28 577 (89.8) | 1799 (95.0) |
| Home | 3338 (9.9) | 3243 (10.2) | 95 (5.0) |
IMD = Index of Multiple Deprivation. QoF = Quality and Outcomes Framework. SD = standard deviation.
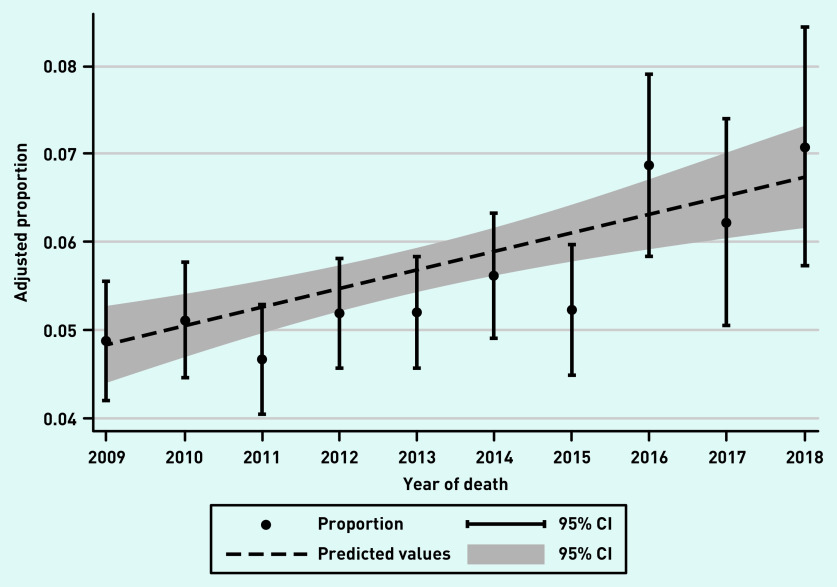
Of 33 714 participants, 1894 (5.6%) had multiple hospital admissions in the last 90 days of life. This proportion increased from 4.9% (95% CI = 4.2 to 5.6) in 2009 to 7.1% (95% CI = 5.7 to 8.4) in 2018 (Figure 1). The mean continuity of care score in the cohort was 0.41 (SD 0.30). Participants with multiple hospital admissions in the last 90 days had lower continuity of care scores than those without these admissions (Table 2). There were 3169 (9.4%) participants who were identified as having palliative care needs by a GP before their last 90 days of life, and they were less likely to have multiple hospital admissions in the last 90 days (Table 2).
Figure 1.
Age-and sex-adjusted proportion of decedents who experienced multiple hospital admissions in the last 90 days of life by year of death. CI = confidence interval.
Table 2.
Association between GP contacts, continuity of care score, identification of palliative care needs, and multiple hospital admissions
| Multiple hospital admissions in the last 90 days | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| No (n = 31 820) | Yes (n = 1894) | IRRa | 95% CI | P-value | |||||
|
| |||||||||
| Events, n | Days in community, n | IR × 30 days | Events, n | Days in community, n | IR × 30 days | ||||
| GP contact rate (12–4 months before death) | 313 501 | 8 395 512 | 1.12 | 21 259 | 488 090 | 1.31 | 1.08 | (1.05 to 1.11) | <0.001 |
|
| |||||||||
| Mean | SD | IMD | Mean | SD | |||||
|
| |||||||||
| Continuity of care score (12–4 months before death) | 0.41 | 0.30 | 0.38 | 0.28 | 0.79 | (0.68 to 0.92) | 0.003 | ||
|
| |||||||||
| Freq | % | Freq | % | ||||||
|
| |||||||||
| Palliative care QoF any time before last 90 days | |||||||||
| No | 28 782 | 90.5 | 1763 | 93.1 | |||||
| Yes | 3038 | 9.5 | 131 | 6.9 | 0.66 | (0.56 to 0.78) | 0.001 | ||
Multilevel Poisson model with a random intercept for region and GP practice, adjusted by age, number of QoF comorbidities, sex, IMD, rurality, living in a care home, cause of death, and year of death. The model includes only participants with at least two contacts with the GP during the exposure period. The full model is available in Supplementary Table S7. CI = confidence interval. IR = incidence rate. IRR = incidence risk ratio. IMD = Index of Multiple Deprivation. QoF = Quality and Outcomes Framework. SD = standard deviation.
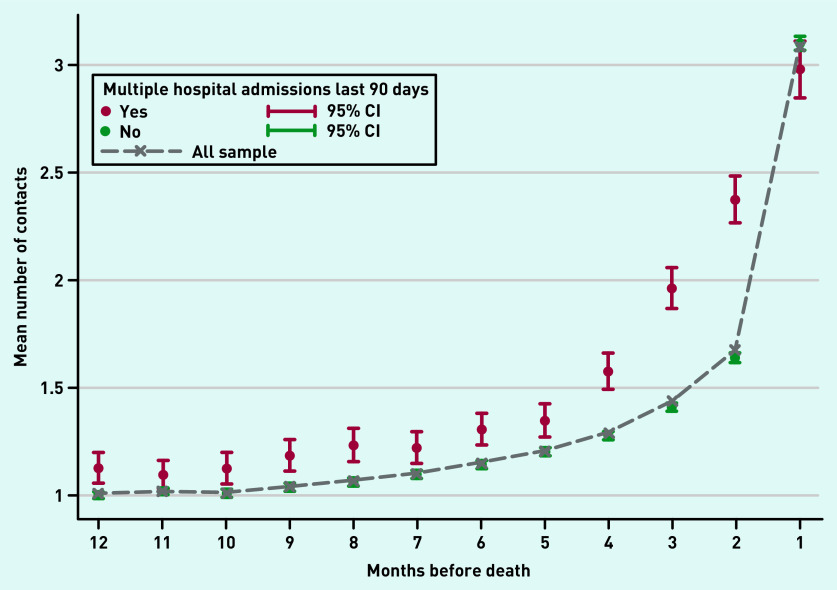
Participants had on average 16.1 (SD 11.6) contacts with GPs in their last year of life, which increased closer to death, particularly in the last month of life (mean 3.1, SD 2.9). Participants with multiple hospital admissions had a higher mean number of contacts with GPs throughout the whole last year of life, except for the last month before death (Figure 2).
Figure 2.
GP contacts for participants with and without multiple hospital admissions by month before death. Mean number of contacts with GPs in the last 12 months of life for participants with dementia with and without multiple hospital admissions in the last 90 days by month before death. CI = confidence interval.
Multilevel adjusted model
In the adjusted model, participants with a higher rate of contacts with GPs per month were more likely to have multiple hospital admissions in the last 90 days of life. Participants with greater continuity of care scores and identification of palliative care needs were less likely to have multiple hospital admissions (Table 2).
The subgroup analysis showed the positive association between the number of contacts with GPs and multiple hospital admissions in the last 90 days was significant for all groups except for participants <75 years of age, and for those whose underlying cause of death was dementia or cancer (Figure 3 and Supplementary Table S6). Better continuity of care with GPs was associated with a lower risk of multiple hospital admissions mainly for participants >95 years, with more comorbidities, living in urban areas, and not living in care homes. Identification of palliative care needs was associated with a lower risk of multiple admissions in older participants (>85 years old), those with no comorbidities, living in urban areas and in care homes, and for those whose underlying cause of death was dementia (Figure 3 and Supplementary Table S6).
Figure 3.
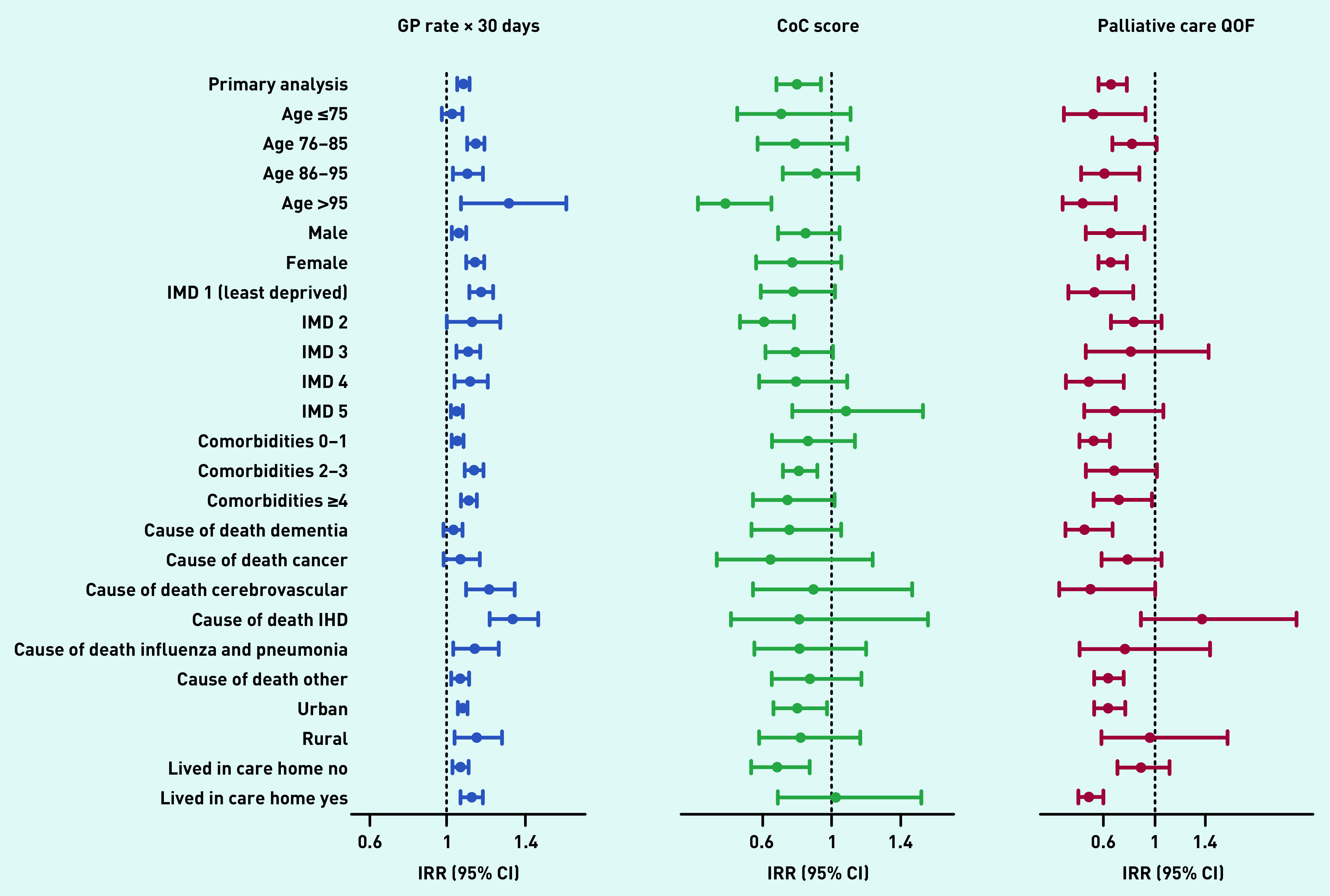
Subgroup analysis. The figure shows results from the subgroup analyses exploring the influence of sociodemographic and illness-related factors in the association between the rate of GP contacts per month, continuity of care score, identification of palliative care needs before the last 90 days of life, and multiple hospital admissions in the last 90 days of life (primary analysis). The IRR represents the risk of multiple hospital admissions for the monthly rate of GP contacts, level of continuity of care, and identification of palliative care needs before the last 90 days of life. All models are adjusted for age in years, number of comorbidities, IMD quintile, underlying cause of death, rurality, sex, living in care homes, and year of death, excluding the variable used for the subgroup analysis, and include a random intercept for region and GP practice. CI = confidence interval. CoC = continuity of care. IHD = ischaemic heart disease. IMD = Index of Multiple Deprivation. IRR = incidence risk ratio. QoF = Quality and Outcomes Framework.
All sensitivity analysis performed showed similar results (see Supplementary Table S7).
DISCUSSION
Summary
In this large population-based cohort of people who died with dementia in England, results show that more contacts with GPs was positively associated with multiple hospital admissions in the last 90 days of life, whereas continuity of care with GPs and identification of palliative care needs were negatively associated with these hospital admissions. Continuity of care was particularly relevant for participants >95 years of age, those with more comorbidities, living in urban areas, and not living in care homes. Identification of palliative care needs was particularly relevant in participants without comorbidities, and those living in urban areas and care homes.
Strengths and limitations
This study uses a large nationwide population-based cohort linked with hospital and death certificates records. Participants with dementia were identified from primary and hospital care records, reducing the risk of missing people with incomplete records.
This study has some limitations. Restricting the sample to participants with a full year of enrolment in a GP practice is likely to exclude people who changed their GP practice because of deterioration or severe cognitive impairment.38,39 However, the sensitivity analysis including people with <365 days of enrolment showed similar results. Information on the appropriateness of admissions to hospital, quality of care, or reasons for GP visits, which are likely to influence the risk of end-of-life admissions, was not available. Palliative Care Quality and Outcomes Framework codes were used to identify people who have been recognised as having palliative care needs in primary care. However, these codes do not identify all people whose death is anticipated by the GP.33
The measure of continuity of care used in this study does not capture the nature of the relationship between physicians and patients or the quality of care received.40,41 Although other measures of continuity of care exist, the Herfindahl–Hirschman Index continuity of care score has been widely used in the literature and does not rely on the need to identify a usual provider.29,41–43
Comparison with existing literature
Studies investigating the association between contacts with GPs and hospital use at the end of life show conflicting results. Contacts with GPs have been positively associated with end-of-life hospital admissions among people with cancer in Canada22 and older adults in Australia,44 and negatively associated with end-of-life hospital admissions in the US in patients with congestive heart failure and chronic obstructive pulmonary disease.20 In Chen et al,21 people with dementia receiving home health care in Taiwan had a higher risk of multiple hospital admissions in the last 90 days. However, this effect varied depending on the frequency and duration of home health care. Differences in results could also be explained by differences in healthcare systems45 and type of conditions. People with frequent hospital admissions are likely to have more GP contacts because of higher healthcare needs. However, it is possible that more GP contacts might reflect poor coordination and integration between healthcare services. More research is needed to understand how interdisciplinary work between GPs and other community care services might have an impact on admissions to hospital in this population.46,47
Two studies (both on cancer) have explored the relationship between continuity of care and hospital admissions for people approaching the end of life in Canada22 and England48 with similar findings to the current study. Continuity of care can increase trust between patients and doctors, increase adherence to long-term treatments, improve the quality of management, and reduce over-aggressive treatment, in particular among people with multimorbidity and those living in the community.49–52 Continuity of care was low in the sample of this study (mean continuity of care score 0.4). This is similar to findings from a study in older adults in the UK.53 Indices that measure concentration of care, such as the continuity of care score used in this study, are highly influenced by the number of contacts and type of professional considered (GP versus specialists), which could explain differences across studies.
Implications for research and practice
Despite the evidence for the potential benefit of continuity of care,54 the proportion of patients who were able to see their preferred GP has declined by 9% between 2012 and 2017 in England.55 Strategies such as assigning a key worker,34 assigning patients to small multidisciplinary teams within a practice, enhancing the role of receptionists to support continuity, and prioritising continuity for patients who may benefit the most have been recommended to improve the level of continuity of care,56 and might help prevent unnecessary admissions to hospital in older people with dementia and multimorbidity living at home.
The finding of this study suggests that identifying people who might benefit from a palliative care approach could help to reduce unnecessary end-of-life transitions to hospital. These findings are consistent with results from a previous study in a London population.24 However, recognising when people with dementia are approaching the end of life is challenging, and GPs have reported barriers to doing so, such as lack of knowledge and training.57,58 Screening tools such as the Supportive and Palliative Care Indicators Tool or the Electronic Frailty Index developed in the UK for primary care settings,59,60 and the IPOS-Dem developed specially for people with dementia,61,62 might help GPs flag people with high risk of deteriorating and dying, assess patients’ needs, and identify those who might benefit from a palliative care approach.
Funding
Javiera Leniz is funded by a Royal Marsden Partners Pan London Research Fellowship Award and the Programa Formacion de Capital Humano Avanzado, Doctorado Becas Chile, 2018 (folio 72190265). Deokhee Yi is supported by Cicely Saunders International. Katherine E Sleeman is funded by a National Institute for Health Research (NIHR) Clinician Scientist Fellowship (CS-2015-15-005) and is the Laing Galazka Chair in Palliative Care at King’s College London, funded by an endowment from Cicely Saunders International and the Kirby Laing Foundation. Irene J Higginson is an NIHR Senior Investigator Emeritus and is supported by the NIHR Applied Research Collaboration South London (NIHR ARC South London) at King’s College Hospital NHS Foundation Trust. Irene J Higginson leads the Palliative and End of Life Care theme of the NIHR ARC South London, and co-leads the national theme in this. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health and Social Care, or the funding charities.
Ethical approval
The Clinical Practice Research Datalink (CPRD) has broad National Research Ethics Service Committee ethics approval for purely observational research. No further ethical approval was required for the analysis of the data as this study used anonymised data only.
Data
The data that support the findings of this study are available from the CPRD. Access to CPRD data is subject to protocol approval by an Independent Scientific Advisory Committee (ISAC). The protocol of this study was approved by the ISAC in April 2021 (Protocol 20_031).
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.World Health Organization The top 10 causes of death WHO’s global health estimates. 2020 https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (1 May 2022). [Google Scholar]
- 2.Etkind SN, Bone AE, Gomes B, et al. How many people will need palliative care in 2040? Past trends, future projections and implications for services. BMC Med. 2017;15(1):102. doi: 10.1186/s12916-017-0860-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sleeman KE, de Brito M, Etkind S, et al. The escalating global burden of serious health-related suffering: projections to 2060 by world regions, age groups, and health conditions. Lancet Glob Health. 2019;7(7):e883–e892. doi: 10.1016/S2214-109X(19)30172-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaudhry SI, Murphy TE, Gahbauer E, et al. Restricting symptoms in the last year of life: a prospective cohort study. JAMA Intern Med. 2013;173(16):1534–1540. doi: 10.1001/jamainternmed.2013.8732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sampson EL, Candy B, Davis S, et al. Living and dying with advanced dementia: a prospective cohort study of symptoms, service use and care at the end of life. Palliat Med. 2018;32(3):668–681. doi: 10.1177/0269216317726443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunt LJ, Ritchie CS, Cataldo JK, et al. Pain and emergency department use in the last month of life among older adults with dementia. J Pain Symptom Manage. 2018;56(6):871–877.e7. doi: 10.1016/j.jpainsymman.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sleeman KE, Perera G, Stewart R, Higginson IJ. Predictors of emergency department attendance by people with dementia in their last year of life: retrospective cohort study using linked clinical and administrative data. Alzheimers Dement. 2018;14(1):20–27. doi: 10.1016/j.jalz.2017.06.2267. [DOI] [PubMed] [Google Scholar]
- 8.Leniz J, Higginson IJ, Stewart R, Sleeman KE. Understanding which people with dementia are at risk of inappropriate care and avoidable transitions to hospital near the end-of-life: a retrospective cohort study. Age Ageing. 2019;48(5):672–679. doi: 10.1093/ageing/afz052. [DOI] [PubMed] [Google Scholar]
- 9.Aaltonen M, Raitanen J, Forma L, et al. Burdensome transitions at the end of life among long-term care residents with dementia. J Am Med Dir Assoc. 2014;15(9):643–648. doi: 10.1016/j.jamda.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Gozalo P, Teno JM, Mitchell SL, et al. End-of-life transitions among nursing home residents with cognitive issues. N Engl J Med. 2011;365(13):1212–1221. doi: 10.1056/NEJMsa1100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehmann J, Michalowsky B, Kaczynski A, et al. The impact of hospitalization on readmission, institutionalization, and mortality of people with dementia: a systematic review and meta-analysis. J Alzheimers Dis. 2018;64(3):735–749. doi: 10.3233/JAD-171128. [DOI] [PubMed] [Google Scholar]
- 12.Sprung J, Knopman DS, Petersen RC, et al. Association of hospitalization with long-term cognitive trajectories in older adults. J Am Geriatr Soc. 2020;69:660–668. doi: 10.1111/jgs.16909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Travers C, Byrne GJ, Pachana NA, et al. Prospective observational study of dementia in older patients admitted to acute hospitals. Australas J Ageing. 2014;33(1):55–58. doi: 10.1111/ajag.12021. [DOI] [PubMed] [Google Scholar]
- 14.De Schreye R, Smets T, Deliens L, et al. Appropriateness of end-of-life care in people dying with dementia: applying quality indicators on linked administrative databases. J Am Med Dir Assoc. 2020;21(8):1093–1101.e1. doi: 10.1016/j.jamda.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 15.Dequanter S, Buyl R, Fobelets M. Quality indicators for community dementia care: a systematic review. Eur J Public Health. 2020;30(5):879–885. doi: 10.1093/eurpub/ckaa096. [DOI] [PubMed] [Google Scholar]
- 16.Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83(3):457–502. doi: 10.1111/j.1468-0009.2005.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenwax L, Spilsbury K, Arendts G, et al. Community-based palliative care is associated with reduced emergency department use by people with dementia in their last year of life: a retrospective cohort study. Palliat Med. 2015;29(8):727–736. doi: 10.1177/0269216315576309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brian Cassel J, Kerr KM, McClish DK, et al. Effect of a home-based palliative care program on healthcare use and costs. J Am Geriatr Soc. 2016;64(11):2288–2295. doi: 10.1111/jgs.14354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dening KH, Greenish W, Jones L, et al. Barriers to providing end-of-life care for people with dementia: a whole-system qualitative study. BMJ Support Palliat Care. 2012;2(2):103–107. doi: 10.1136/bmjspcare-2011-000178. [DOI] [PubMed] [Google Scholar]
- 20.Kronman AC, Ash AS, Freund KM, et al. Can primary care visits reduce hospital utilization among Medicare beneficiaries at the end of life? J Gen Intern Med. 2008;23(9):1330–1335. doi: 10.1007/s11606-008-0638-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen PJ, Ho CH, Liao JY, et al. The association between home healthcare and burdensome transitions at the end-of-life in people with dementia: a 12-year nationwide population-based cohort study. Int J Environ Res Public Health. 2020;17(24):9255. doi: 10.3390/ijerph17249255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almaawiy U, Pond GR, Sussman J, et al. Are family physician visits and continuity of care associated with acute care use at end-of-life? A population-based cohort study of homecare cancer patients. Palliat Med. 2014;28(2):176–183. doi: 10.1177/0269216313493125. [DOI] [PubMed] [Google Scholar]
- 23.Godard-Sebillotte C, Strumpf E, Sourial N, et al. Primary care continuity and potentially avoidable hospitalization in persons with dementia. J Am Geriatr Soc. 2021;69(5):1208–1220. doi: 10.1111/jgs.17049. [DOI] [PubMed] [Google Scholar]
- 24.Leniz J, Higginson IJ, Yi D, et al. Identification of palliative care needs among people with dementia and its association with acute hospital care and community service use at the end-of-life: a retrospective cohort study using linked primary, community, and secondary care data. Palliat Med. 2021;35(9):1691–1700. doi: 10.1177/02692163211019897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: Clinical Practice Research Datalink (CPRD) Int J Epidemiol. 2015;44(3):827–836. doi: 10.1093/ije/dyv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CPRD Release notes: CPRD GOLD March 2021. 2021 https://www.cprd.com/DOIs?page=3 (accessed 1 May 2022). [Google Scholar]
- 27.NHS Digital Business rules for Quality and Outcomes Framework (QOF) 2017/2018. 2017. https://digital.nhs.uk/data-and-information/data-collections-and-data-sets/data-collections/quality-and-outcomes-framework-qof (accessed 1 May 2022).
- 28.Jaakkimainen RL, Bronskill SE, Tierney MC, et al. Identification of physician-diagnosed Alzheimer’s disease and related dementias in population-based administrative data: a validation study using family physicians’ electronic medical records. J Alzheimers Dis. 2016;54(1):337–349. doi: 10.3233/JAD-160105. [DOI] [PubMed] [Google Scholar]
- 29.Nyweide DJ, Anthony DL, Bynum JP, et al. Continuity of care and the risk of preventable hospitalization in older adults. JAMA Intern Med. 2013;173(20):1879–1885. doi: 10.1001/jamainternmed.2013.10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansford P, Meehan H. Gold standards framework: improving community care. End Life Care. 2007;1(3):56–61. https://www.goldstandardsframework.org.uk/cd-content/uploads/files/Library%2C%20Tools%20%26%20resources/Gold%20Standards%20Framework%20-%20improving%20community%20care.pdf (accessed 1 May 2022). [Google Scholar]
- 31.Gadoud A, Kane E, Macleod U, et al. Palliative care among heart failure patients in primary care: a comparison to cancer patients using English family practice data. PLoS One. 2014;9(11):e113188. doi: 10.1371/journal.pone.0113188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gadoud A, Kane E, Oliver SE, et al. Palliative care for non-cancer conditions in primary care: a time trend analysis in the UK 2009–2014. BMJ Support Palliat Care. 2020 doi: 10.1136/bmjspcare-2019-001833. [DOI] [PubMed] [Google Scholar]
- 33.Stow D, Matthews FE, Hanratty B. Timing of GP end-of-life recognition in people aged ≥75 years: retrospective cohort study using data from primary healthcare records in England. Br J Gen Pract. 2020. DOI: . [DOI] [PMC free article] [PubMed]
- 34.Bone AE, Evans CJ, Henson LA, et al. Patterns of emergency department attendance among older people in the last three months of life and factors associated with frequent attendance: a mortality follow-back survey. Age Ageing. 2019;48(5):680–687. doi: 10.1093/ageing/afz043. [DOI] [PubMed] [Google Scholar]
- 35.Gomes B, Higginson IJ. Factors influencing death at home in terminally ill patients with cancer: systematic review. BMJ. 2006;332(7540):515–521. doi: 10.1136/bmj.38740.614954.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brilleman SL, Salisbury C. Comparing measures of multimorbidity to predict outcomes in primary care: a cross sectional study. Fam Pract. 2013;30(2):172–178. doi: 10.1093/fampra/cms060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jain A, van Hoek AJ, Walker JL, et al. Identifying social factors amongst older individuals in linked electronic health records: an assessment in a population based study. PLoS One. 2017;12(11):e0189038. doi: 10.1371/journal.pone.0189038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perrels AJ, Fleming J, Zhao J, et al. Place of death and end-of-life transitions experienced by very old people with differing cognitive status: retrospective analysis of a prospective population-based cohort aged 85 and over. Palliat Med. 2014;28(3):220–233. doi: 10.1177/0269216313510341. [DOI] [PubMed] [Google Scholar]
- 39.Welberry HJ, Jorm LR, Barbieri S, et al. The impact of dementia on aged care service transitions in the last five years of life. Age Ageing. 2020;50(4):1159–1165. doi: 10.1093/ageing/afaa254. [DOI] [PubMed] [Google Scholar]
- 40.Bentler SE, Morgan RO, Virnig BA, Wolinsky FD. Do claims-based continuity of care measures reflect the patient perspective? Med Care Res Rev. 2014;71(2):156–173. doi: 10.1177/1077558713505909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nyweide DJ. Concordance between continuity of care reported by patients and measured from administrative data. Med Care Res Rev. 2014;71(2):138–155. doi: 10.1177/1077558713505685. [DOI] [PubMed] [Google Scholar]
- 42.Sidaway-Lee K, Gray DP, Evans P. A method for measuring continuity of care in day-to-day general practice: a quantitative analysis of appointment data. Br J Gen Pract. 2019. DOI: [DOI] [PMC free article] [PubMed]
- 43.Jee SH, Cabana MD. Indices for continuity of care: a systematic review of the literature. Med Care Res Rev. 2006;63(2):158–188. doi: 10.1177/1077558705285294. [DOI] [PubMed] [Google Scholar]
- 44.Tran B, Falster MO, Girosi F, Jorm L. Relationship between use of general practice and healthcare costs at the end of life: a data linkage study in New South Wales, Australia. BMJ Open. 2016;6(1):e009410. doi: 10.1136/bmjopen-2015-009410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Loenen T, van den Berg MJ, Westert GP, Faber MJ. Organizational aspects of primary care related to avoidable hospitalization: a systematic review. Fam Pract. 2014;31(5):502–516. doi: 10.1093/fampra/cmu053. [DOI] [PubMed] [Google Scholar]
- 46.Offen J. The role of UK district nurses in providing care for adult patients with a terminal diagnosis: a meta-ethnography. Int J Palliat Nurs. 2015;21(3):134–141. doi: 10.12968/ijpn.2015.21.3.134. [DOI] [PubMed] [Google Scholar]
- 47.Walshe C, Luker KA. District nurses’ role in palliative care provision: a realist review. Int J Nurs Stud. 2010;47(9):1167–1183. doi: 10.1016/j.ijnurstu.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 48.Barker I, Steventon A, Deeny SR. Association between continuity of care in general practice and hospital admissions for ambulatory care sensitive conditions: cross sectional study of routinely collected, person level data. BMJ. 2017;356:j84. doi: 10.1136/bmj.j84. [DOI] [PubMed] [Google Scholar]
- 49.Cabana MD, Jee SH. Does continuity of care improve patient outcomes? J Fam Pract. 2004;53(12):974–980. [PubMed] [Google Scholar]
- 50.Gruneir A, Bronskill SE, Maxwell CJ, et al. The association between multimorbidity and hospitalization is modified by individual demographics and physician continuity of care: a retrospective cohort study. BMC Health Serv Res. 2016;16:154. doi: 10.1186/s12913-016-1415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kao YH, Lin WT, Chen WH, et al. Continuity of outpatient care and avoidable hospitalization: a systematic review. Am J Manag Care. 2019;25(4):e126–e134. [PubMed] [Google Scholar]
- 52.Freeman GK, Hughes J. Continuity of care and the patient experience. 2010. https://www.kingsfund.org.uk/sites/default/files/field/field_document/continuity-care-patient-experience-gp-inquiry-research-paper-mar11.pdf (accessed 1 May 2022).
- 53.Tammes P, Purdy S, Salisbury C, et al. Continuity of primary care and emergency hospital admissions among older patients in England. Ann Fam Med. 2017;15(6):515–522. doi: 10.1370/afm.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santomassino M, Costantini GD, McDermott M, et al. A systematic review on the effectiveness of continuity of care and its role in patient satisfaction and decreased hospital readmissions in the adult patient receiving home care services. JBI Libr Syst Rev. 2012;10(21):1214–1259. doi: 10.11124/01938924-201210210-00001. [DOI] [PubMed] [Google Scholar]
- 55.Tammes P, Morris RW, Murphy M, Salisbury C. Is continuity of primary care declining in England? Practice-level longitudinal study from 2012 to 2017. Br J Gen Pract. 2020. DOI: . [DOI] [PMC free article] [PubMed]
- 56.Palmer W, Hemmings N, Rosen R, et al. Improving access and continuity in general practice Practical and policy lessons. 2018 https://www.nuffieldtrust.org.uk/research/improving-access-and-continuity-in-general-practice (accessed 1 May 2022). [Google Scholar]
- 57.Harrison N, Cavers D, Campbell C, Murray SA. Are UK primary care teams formally identifying patients for palliative care before they die? Br J Gen Pract. 2012. DOI: . [DOI] [PMC free article] [PubMed]
- 58.Pocock LV, Wye L, French LRM, Purdy S. Barriers to GPs identifying patients at the end-of-life and discussions about their care: a qualitative study. Fam Pract. 2019;36(5):639–643. doi: 10.1093/fampra/cmy135. [DOI] [PubMed] [Google Scholar]
- 59.ElMokhallalati Y, Bradley SH, Chapman E, et al. Identification of patients with potential palliative care needs: a systematic review of screening tools in primary care. Palliat Med. 2020;34(8):989–1005. doi: 10.1177/0269216320929552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Highet G, Crawford D, Murray SA, Boyd K. Development and evaluation of the Supportive and Palliative Care Indicators Tool (SPICT): a mixed-methods study. BMJ Support Palliat Care. 2014;4(3):285–290. doi: 10.1136/bmjspcare-2013-000488. [DOI] [PubMed] [Google Scholar]
- 61.Ellis-Smith C, Higginson IJ, Daveson BA, et al. How can a measure improve assessment and management of symptoms and concerns for people with dementia in care homes? A mixed-methods feasibility and process evaluation of IPOS-Dem. PLoS One. 2018;13(7):e0200240. doi: 10.1371/journal.pone.0200240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spichiger F, Koppitz AL, De Wolf-Linder S, et al. Improving caring quality for people with dementia in nursing homes using IPOS-Dem: a stepped-wedge cluster randomized controlled trial protocol. J Adv Nurs. 2021;77(10):4234–4245. doi: 10.1111/jan.14953. [DOI] [PMC free article] [PubMed] [Google Scholar]