Abstract
MicroRNAs [miRNAs], small non-coding RNAs, have recently been described as crucial contributors to intestinal homeostasis. They can interact with the gut microbiota in a reciprocal manner and deeply affect host health status, leading to several disorders when unbalanced. Inflammatory bowel disease [IBD] is a chronic inflammation of the gastrointestinal tract that co-occurs with alterations of the gut microbiota, and whose aetiology remains largely unclear. On one hand, host miRNA could be playing a relevant role in IBD pathophysiology by shaping the gut microbiota. The gut microbiome, on the other hand, may regulate the expression of host miRNAs, resulting in intestinal epithelial dysfunction, altered autophagy, and immune hyperactivation. Interestingly, it has been hypothesised that their reciprocal impact may be used for therapeutic goals. This review describes the latest research and suggests mechanisms through which miRNA and intestinal microbiota, as joint actors, may participate specifically in IBD pathophysiology. Furthermore, we discuss the diagnostic power and therapeutic potential resulting from their bidirectional communication after faecal transplantation, probiotics intake, or anti-miRNAs or miRNA mimics administration. The current literature is summarised in the present work in a comprehensive manner, hoping to provide a better understanding of the miRNA-microbiota cross-talk and to facilitate their application in IBD.
Keywords: MicroRNA, microbiota, inflammatory bowel disease
Graphical Abstract
1. Introduction
Inflammatory bowel disease [IBD] is the term that groups chronic inflammatory relapsing disorders of the gastrointestinal tract, primarily Crohn’s disease [CD] and ulcerative colitis [UC]. CD is characterised by a deep inflammation that can affect any segment of the gastrointestinal tract, whereas UC is a non-transmural inflammation limited to the colon. The most common symptoms presented in IBD are abdominal pain and diarrhoea, causing prominent disability.1 Patients with IBD have a higher risk of developing colorectal cancer than the general population2 which calls for surveillance colonoscopies that aim to reduce morbidity and mortality due to colorectal cancer.3 Moreover, the incidence and prevalence of IBD have been rising rapidly worldwide from 1990 to 2017, posing a substantial social and economic burden on governments and health systems in the coming years.4
Although the pathogenesis of IBD remains largely unknown, studies have shown an association between immunological abnormalities, genetic susceptibility, and environmental factors.5 Among those, the gut microbiota is considered an important factor in IBD pathogenesis. IBD patients exhibit an exacerbated host immune response characterised by a loss of tolerance towards the intestinal microbiota,6 potentially leading to chronic inflammation. Animal studies have demonstrated that the presence of intestinal microbiota is indispensable for driving colitis, the extent and severity of which are highly dependent on the microbial composition.7 The microbiome can be influenced by both external factors such as diet,8 and by host factors such as epithelium-associated factors including, notably, the mucus barrier, epithelial tight junctions, and microRNAs [miRNA].9
The latest research has explored miRNAs in IBD, offering new insights into its pathogenesis. The commonly known miRNAs are single-stranded, non-coding RNAs of 18 to 24 nucleotides length, able to mediate gene silencing and regulate up to 60% of the human transcriptome.10 They exert biological functions by complementary pairing within the 3′-UTR of mRNAs, resulting in the inhibition of their translocation and degradation which leads to a downregulation of the targeted genes’ expression.11 Importantly, miRNAs are not perfectly complementary to their target mRNA, a feature that makes them able to regulate simultaneously multiple transcripts.12 It is estimated that to maintain cellular homeostasis, 66% of all miRNA regulate critical cellular pathways, providing a certain degree of functional redundancy.13 Generally, miRNA circulate in the human peripheral blood in a stable form, but they can also be present in other body fluids such as urine, saliva, cerebrospinal fluid, and faeces.14
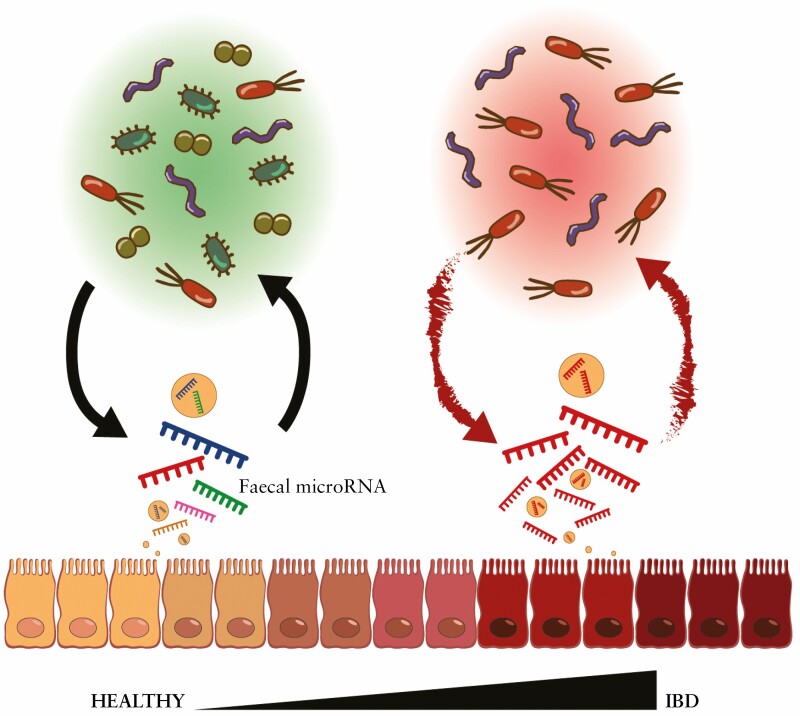
Recent studies have suggested bidirectional interactions between host cells and gut microbiota via miRNA which participate in shaping the gut microbiota after being secreted from intestinal epithelial cells, and which accumulate in faeces.15,16 On the other hand, host miRNA expression can be influenced by the microbiota through microbe-derived metabolites that might potentially influence the host physiology.17 Moreover, the dysregulation of miRNA functions has been recently associated with IBD pathogenesis.18
Several studies list in detail the known miRNA,19 or microbiota alterations in IBD,15,20,21 but the factors that mediate their combined interaction in impacting on IBD are poorly understood. The present work highlights the most relevant insights and interplays between miRNA and gut microbiota, as joint actors, exclusively during IBD. Here, we focus exclusively on miRNAs that are related to microbiota and vice versa. Further and uniquely, we aim to provide a fresh perspective classifying the mechanisms through which their complex cross-talk may lead to pathogenesis, as well as discuss the diagnostic power and therapeutic potential resulting from their bidirectional communication.
2. Main Characteristics of IBD Intestinal Microbiota
The human gut harbours more than 100 trillion different microorganisms that are in close relation with the host cells, establishing and maintaining beneficial interactions. It is estimated that the ‘normal gut microbiota’ is formed by 500 to 1000 bacterial species, mostly anaerobic, which include four major phyla. Firmicutes [49–76%] and Bacteroidetes [16–23%] are the dominant phyla,22 followed by the Proteobacteria and Actinobacteria phyla to a much lesser extent, which reside alongside an undetermined number of viruses, fungi, and other microbes.23 During the past century, revolutionary research studying the microbiome has proven its importance and has shown several associations with human diseases. The gut microbiota competes for nutrients and ecological niches with pathogens, inhibiting and excluding their colonisation through various mechanisms.24 Interestingly, the faecal microbial composition is associated with the incidence and frequency of abdominal pain even in the general population.25 A detrimental change in the bacterial composition, also called dysbiosis, has been extensively linked within IBD, but whether dysbiosis is the cause or the consequence of the inflammation remains unclear. Some studies in mice and the use of antibiotics in humans are suggesting that a disturbed microbiota is a prerequisite for IBD pathogenesis.26–28
The rare pieces of evidence demonstrating that a specific pathogen could induce, by itself, the disease as well as the need for immunodeficient animal models in order to develop colitis, indicate the importance of genetic susceptibility in IBD pathogenesis alongside other factors. Bacterial toxins, for instance, are considered virulent factors that can alter the host cells to facilitate bacterial colonisation.29 The presence of many bacterial organisms such as Mycobacterium avium paratuberculosis, Campylobacter, Helicobacter, Salmonella enterica serovar typhimurium,30 and adherent-invasive Escherichia coli [AIEC]31 have also been implicated in the exacerbation or development of IBD.
Overall, IBD patients present a compromised and less diverse microbial ecosystem characterised by a reduction of bacteria proven to have anti-inflammatory properties, and an increase of bacteria with pro-inflammatory properties ushered in with signs of oxidative stress and enhanced secretion of type II toxins.23 Strong shreds of evidence support that IBD patients have increased amounts of Proteobacteria [specially AIEC] and reduced Firmicutes diversity due to the decrease of Clostridium groups [mostly Faecalibacterium prausnitzii].22 Less consistently but still significant, distinct bacterial composition has been identified in UC compared with CD.32 The presence of Clostridium lachnospiraceae seems to be disease-specific to CD, whereas Clostridium clostridiaceae is specific to UC.32 Differences have also been found during active and inactive phases. Novel results from Metwaly et al. linked the presence of bacteria with sulphur metabolism to human disease activity.33 They identified members of Desulfovibrio, Enterococcus, Streptococcus, and Escherichia being more present during CD inflammation, whereas Burkholderiales, Desulfomicrobiaceae, Sutterella, and Butyrivibrio were more abundant in non-inflamed cases.33
3. The Relevance of miRNA in IBD Pathogenesis
It was only in 2008 that Wu et al. demonstrated for the very first time a differential miRNA colonic expression pattern between active UC and controls.34 Since then, miRNAs have been studied in several different types of biological samples. Due to the relatively easy access to and the high stability of miRNA in blood, many research groups have based their studies on systemic miRNAs and have tried to identify circulating biomarkers that reflect the local intestinal changes. Iborra et al. studied miRNA expression patterns in serum and colonic mucosa of IBD patients during all the stages of the disease. Although they studied around 700 miRNAs and detected significantly different expressions when comparing patients with healthy subjects, or active with inactive IBD phases, no serum miRNA corresponded with tissue miRNA.35 These results suggest that, in all likelihood, considerable intestinal damage alongside an exacerbated local expression of miRNA is required to detect the same elevated and correlated miRNA levels in both blood and tissue. Nevertheless, and as reviewed extensively by James and colleagues,20 several studies have demonstrated circulating miRNAs as useful disease and diagnostic biomarkers. For example, we identified serum miRNA signatures that could predict disease risk, inflammation type, and therapy response.36 The measure of miRNAs in faecal samples is a non-invasive alternative demonstrated to be better linked to the intestinal mucosa expression levels and shown to correlate with disease activity and surrogate inflammatory markers, such as calprotectin.37
As previously mentioned, the research field of miRNAs in IBD has grown rapidly after their discovery. Several studies have found significantly different profiles of hundreds of miRNAs20,38 between IBD and healthy individuals,39 paediatric and adult patients,40 inflamed and non-inflamed mucosa, and in relation to disease activity.15,35,37,40 To date, there are enough collected data to say that miRNAs are important regulatory factors that play a relevant role in the pathogenesis of IBD. One of the most studied miRNAs in IBD is miR-21. This miRNA is overexpressed systemically in the plasma39 as well as locally in colonic tissues.41 More specifically, miR-21 is overexpressed in the lamina propria and in subsets of macrophages and T cells of IBD patients compared with healthy controls.41 MiR-21 is also associated with UC, but not CD, as well as with disease activity42 and severity.40 Moreover, its levels are significantly increased in intestinal lesions of adults compared with paediatric UC patients.40 Thus, miR-21 is strongly recommended as a biomarker to distinguish between health and IBD, UC and CD, active and remission phases.41 Likewise, murine miR-21 is overexpressed during induced colitis and its absence in miR-21-knockout mice reduces the recruitment of inflammatory leukocytes and the production of pro-inflammatory cytokines such as tumour necrosis factor [TNF], thereby protecting against inflammation and tissue injury and improving the survival rate of dextran sulphate sodium [DSS]-induced colitis mice.43
Similarly, miR-31 levels also have been found to be increased in inflamed mucosa of IBD patients and mice with colitis compared with controls.44,45 Shi et al. demonstrated how miR-31 directly targets the expression of IL-25, a crucial counter-regulator cytokine of Th1/Th17 inflammatory responses.44 They also showed an inverse correlation between the anti-inflammatory cytokine IL-25 and miR-31 in the colon of both CD patients and mouse models.44 Moreover, the treatment with anti-miR-31 promoted IL-25 expression and improved significantly the induced colitis.44 Although these results suggested miR-31 as a pro-inflammatory molecule, Tian et al. recently showed that miR-31-knockout mice develop a more severe inflammatory response when inducing colitis. They demonstrated that miR-31, expressed predominantly in colonic epithelial cells, inhibits the expression of IL-17R and IL-17RA and GP130 inflammatory cytokine receptors and downregulates the pro-inflammatory nuclear factor-kB [NF-kB] and STAT3 signalling pathways.45 Despite the conflicting results, evidence indicates that miR-31 is a key regulatory factor of the Th17 response implicated in IBD.
Additionally, a recent meta-analysis used a novel bipartite approach to find miRNA associated with IBD.38 Among the most relevant was the Let-7 family. This is a multifaceted family of miRNA highly conserved across diverse animal species and related with cell proliferation, cell cycle, metabolism, and cell migration.38 In more detail, the expression of multiple members of this family appears to be altered during inflammation. Regulated by NF-kB46 and in direct relation with IL-6 expression, the Let-7 family is part of positive feedback loops that induce enhanced inflammatory responses and cell transformation.47 More specifically, Let-7f is increased in colonic tissue of patients with active UC compared with healthy colonic tissue,34 whereas Let-7e is increased by more than 5-fold in the colonic mucosa of quiescent UC patients compared with both active UC and controls.48 Moreover, in silico studies demonstrated the direct effect of the Let-7 family on IBD pathogenesis by targeting the gene LOH12CR1, associated with UC susceptibility.48
4. Mechanisms by Which miRNA-microbiota Interactions May Lead to IBD
Although the fascinating research on miRNA has grown exponentially over recent years, suggesting these tiny molecules as major players in the host-microbiota interaction, the exact mechanisms through which miRNAs are involved in IBD or dysbiosis are still unsolved. Thus, it is reasonable to consider miRNAs as signalling cascade components rather than the main drivers of pathogenesis. An example of this was shown for miR-187, which was identified as an IL-10 dependent miRNA produced by monocytes after lipopolysaccharide [LPS] Toll-like receptor-4 activation. Thereafter, the expression of miR-187 reduced the production of pro-inflammatory cytokines, such as TNF and IL-6.49 This finding demonstrates the presence of miRNAs as physiological regulators, in this case of the anti-inflammatory response driven by IL-10.
Besides the complexity of their communication, current evidence indicates an interaction between miRNA and microbiota. To begin with, the study of the miRNA profile along the gastrointestinal tract has shown a differential expression that is influenced by the presence of bacteria and/or inflammation.50,51 Moreover, a rising number of articles are investigating miRNA as part of the immune response to bacteria. As further discussed, host miRNA seems able to shape the intestinal microbiota,16 and the gut microbiome can conversely regulate the expression of miRNA,52 and thus improve or worsen the host health status, maybe even causing disease when dramatically unbalanced [Table 1]. One of the earliest pieces of evidence of this was declared by Dalmasso et al. in 2011.53 They studied the consequences of colonising germ-free mice with the microbiota from pathogen-free mice. Such colonisation prompted the differential expression of miRNA and host genes in both the ileum and the colon. Among those, the Abcc3 gene was identified as upregulated and a potential target of miR-665.53 Furthermore, we recently published evidence of the impact of the gut microbiota on faecal miRNA such as let-7, miR-148, miR-21, and miR-196, which levels are correlated with the microbiota composition and with its inflammatory potential.52 Similarly, Tomkovich et al. described the association between bacterial taxon abundance and faecal miRNAs, some of which were predicted to target host genes whereas others targeted bacterial genes, validating a complex bacteria-miRNA-host interaction.54 In line with our findings, Johnston et al. showed that the expression of miR-21 promotes intestinal inflammation following the alteration of the intestinal microbiota composition. They also proved that the lack of miR-21 protects from colitis, in part, due to the reduction of Bacteroidetes and an increase of protective Firmicutes and Clostridia.55
Table 1.
Summary of intestinal miRNA-microbiota interactions that may be associated with inflammatory bowel disease [IBD] pathophysiology. The arrows indicate the targeted component meaning bidirectional communication [⟷], miRNA targets microbiota [⟶] or vice versa [⟵]
| miRNA | Bacteria | Interaction Effect | Ref. | |
|---|---|---|---|---|
| miR-515-5p | ⟶ | Fusobacterium nucleatum | Promotion of bacterial growth. | 16 |
| miR-1226-5p | ⟶ | Escherichia Coli | Promotion of bacterial growth. | 16 |
| miR-194-5p and Let-7c-5p | ⟷ | Enterobacteriaceae | Positive correlation and modulation of dysbiosis-related inflammation. | 52 |
| miR-148-3p and miR-27-3p | ⟷ | Proteobacteria | Strong positive correlation and association with IBD pathogenesis. | 52 |
| miR-21 | ⟶ | Bacteroidetes: Barnesiella and Prevotella. Firmicutes and Clostridia: Coprococcus, Lactonifactor, Oscilibacter, Clostridium sensu stricto, Anaerosporobacter, Catonella. | Increases abundance of IBD related Bacteroidetes phylum.Reduces abundance of protective Firmicutes and Clostridia phylum. | 55 |
| Exogenous miR-7267-3p | ⟷ | Lactobacillus rhamnosus | Increases the expression of indole-3-carboxaldehyde, ligand for hydrocarbon receptor, and induces production of protective IL-22. | 63 |
| miR-155 and miR-233 | ⟵ | Lactobacillus fermentum and Lactobacillus salivarius | Reduce overexpression of miRNA, reducing inflammation and preserving intestinal barrier function. | 82 |
| miR-150 and miR-143 | ⟵ | Lactobacillus fermentum | Restore expression and preservation of intestinal barrier function. | 82 |
| miR-29a | ⟵ | Salmonella | Upregulation of miRNA, which targets and downregulates caveolin 2, increasing bacteria uptake. | 86 |
| miR-29b-2-5p | ⟵ | Shigella | Upregulation of miRNA enhances filopedia formation, promotes bacteria invasion and intracellular replication. | 87 |
| miR-29 | ⟵ | Adherent-invasive Escherichia Coli | Upregulation of miRNA limits the pro-inflammatory IL-21 release after interaction with NOD2, which is altered in IBD patients. | 88 |
| miR-30c, miR-93, miR-106b, and miR-130a | ⟵ | Adherent-invasive Escherichia Coli | Upregulation of miRNA reduces autophagy-related genes and clearance of bacteria. | 93,94 |
| miR-128 | ⟵ | Salmonella | Upregulation of miRNA downregulates epithelial cell-secreted macrophage colony-stimulating factor, reducing the recruitment of macrophages. | 107 |
| miR-155 | ⟵ | Salmonella | Upregulation of miRNA induces macrophages death and promotion of pro-inflammatory cytokines. | 108,109 |
| miR-16, miR-145, miR-146b, miR-155, and Let-7a1, miR-146a, miR-155, miR-125a-3p/5p, and miR-149, miR-29 | ⟵ | Listeria monocytogenes | Downregulation of miRNAs in different cell types alters the immune response. | 119-122 |
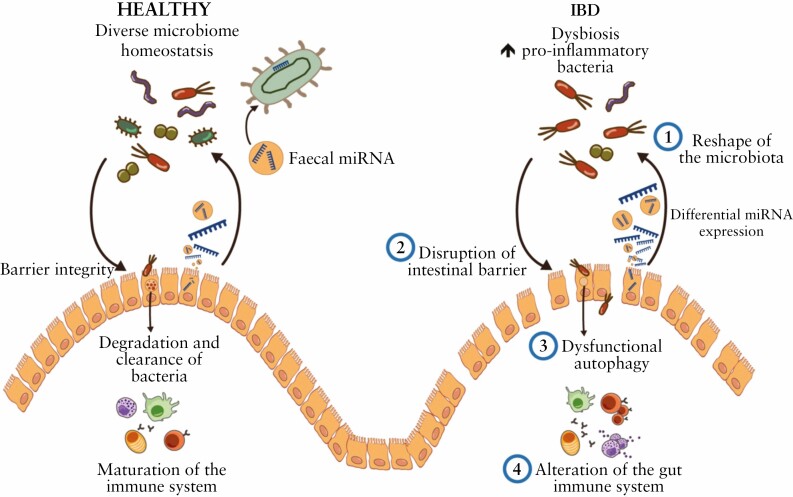
Altogether this compiled information suggests that both dysregulation and insufficiency of miRNAs could lead, among other effects, to microbiota changes, intestinal epithelial dysfunction, and immune hyperactivation50 [Figure 1]. In the following section, we review the possible mechanisms by which miRNA-microbiota interactions may directly or indirectly influence IBD initiation and progression.
Figure 1.
Overview of the interactions between microRNA [miRNA] and microbiota that may participate in inflammatory bowel disease [IBD] pathophysiology.
4.1.Reshaping the microbiota by miRNA
Although several studies have shown a link between host genes and gut microbiota, the influence of host miRNA on the microbiome remains poorly understood. Remarkable experiments performed by Liu et al. shed light on the matter and confirmed the crucial role of host miRNA in maintaining intestinal homeostasis and shaping gut microbiota.16 They identified intestinal epithelial cells and Hopx + cells as the main source of faecal miRNA, which accumulates in the faeces within extracellular vesicles. Some miRNAs, such as miR-101, miR-325, miR-515-5p, miR-623, miR-876-5p, miR-1224-5p, miR-1226-5p, and miR-1253, were identified to enter bacteria and directly regulate bacterial gene transcripts, subsequently promoting bacterial growth and motility, shaping the composition and distribution of the gut microbiota.16
An indirect way by which miRNA can reshape the microbiota is through the modification of antimicrobial peptides [AMP] expression. AMP, mainly secreted by Paneth cells and enterocytes, are a long-known part of the innate immunity and key regulatory factors of the commensal microbiota. AMP are cationic and amphipathic peptides that generally exert an antimicrobial effect by interacting with the bacterial membrane, generating leakage or disruption that ends in bacterial death.56 IBD patients present reduced gastrointestinal AMP production as a consequence of inflammation,57 a fact that potentially prompts gut dysbiosis. Defensins are the most abundant AMP in the intestinal mucosa; in physiological conditions, microbial infection triggers their production via the NF-kB signalling pathway as part of the host defence response.58 To date, only a small number of studies have demonstrated the role of miRNA regulating AMP. In vitro research has shown miR-124 and miR-924 to negatively regulate the expression of α-defensin 5.59 Mice that overexpress α-defensin 5 present a shift in the dominant bacterial species with a decrease of filamentous bacteria, which is associated with downregulation of Th17 cells in the intestinal tissue.60 Furthermore, miR-124 seems to induce inflammation through the inhibition of the aryl hydrocarbon receptor. Its suppression, on the other hand, drives the alleviation of induced colitis in animal models. In humans, miR-124 is upregulated in intestinal tissue of active CD and is considered to promote IBD pathogenesis.61
Another indirect way through which the host can considerably affect the gut microbial composition is through the diet. Indeed, recent studies have demonstrated direct links between faecal miRNA profiles, diet, and gut microbial composition.62,63 Teng and colleagues showed that miRNAs found in edible plants, enclosed in derived exosome-like nanoparticles, can be taken up preferentially by bacteria issuing changes in the microbiome composition, growth, and localisation.62 Exogenous miRNAs from plants may also be taken up by the host and accumulate in serum and tissue.64 Exogenous miRNAs could then promote the expression of anti-inflammatory cytokines and activate downstream molecular pathways that regulate antimicrobial immunity and tissue repair, mediating the cross-talk between the host immune system and gut microbiota.62 Nevertheless, this research is at an early stage and debates are rising regarding the evidence of cross-kingdom gene regulation or even the detection of exogenous miRNA after passing through the digestive tract.65 An example of this controversy was seen with the exogenous plant miR-168a detected in mammalian serum and tissue and assumed by Zhang et al. to be acquired through food intake.64 This group reported that miR-168a can regulate the expression of host genes, decreasing the serum amounts of low-density lipoprotein [LDL] in mice.64 Not long after, Dickinson et al. questioned those results and reported that the reduction of circulating LDL levels was a result of the nutritional imbalance between the groups, rather than a gene regulation by exogenous miR-168a, which they were unable to detect in the serum.66 Furthermore, a computational analysis study of public databases revealed that this number of observed plant miRNAs is significantly uncommon among animals, and its detection may be artefactual due to sequencing methodology67 or cross-contamination.68 A recent study did detect exogenous miRNAs but in low abundance, a fact which, according to the authors, suggests a non-functional role of these molecules.63 It should be noted that part of the current controversy might be the consequence of the capacity of exogenous miRNA to resist food processing, since the detectability and stability of miRNA might rely on the foodstuff nature and processing level.69
At present, the direct or indirect role of miRNA regulating the microbiota remains uncertain and further research on their association is required.
4.2.Disruption of the epithelial barrier function
The integrity of the intestinal barrier is fundamental for the maintenance of gut homeostasis. It allows the permeability of ions and nutrients, restricts the entry of dangerous insults, and facilitates antigen presentation and immunotolerance between the intestinal microbes and the immune system. Epithelial tight junction proteins seal the paracellular spaces between enterocytes and prevent the passage of molecules.70 Although a compromised mucosal permeability alone may not be sufficient to cause IBD, the increased intestinal permeability and barrier dysfunction that IBD patients present have been largely associated with the promotion of the mucosal inflammation.71 Previous research has shown that miRNAs are important regulators of the epithelial barrier function by controlling tight junctions’ expression and epithelial cells’ apoptosis, proliferation, and differentiation.72,73
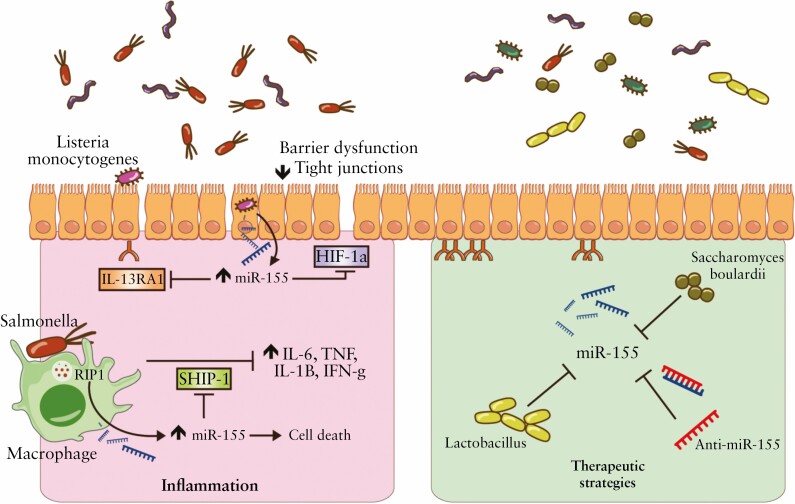
Studies working with intestinal epithelial cell miRNA-deficient [Dicer1ΔIEC] mice have determined an altered intestinal epithelium in this model which is associated with a reduced expression of the tight junctions zonula occludens 1, claudin 1, and occludin,16 which are widely known to be downregulated in IBD patients74 and regulated by symbiotic bacteria.75 Although knowledge regarding the influence of miRNA on the epithelial intestinal barrier is still lacking, specific microbiota-related miRNAs have recently been identified as important regulators. For instance, miR-375 regulates the proliferation of intestinal epithelial stem cells and miR-375 is notably underexpressed in active UC34 and suppressed in intestinal epithelial stem cells by the presence of microbiota.76 In contrast, the overexpression of miR-21 in UC patients is related to intestinal epithelial barrier impairment through targeting Rho GTPase77 and ADP ribosylation factor 4 [ARF4] GTPase.78 MiR-21 induces the degradation of RhoB mRNA and upregulation of ARF4. In this regard, miR-21 can decrease the transepithelial electrical resistance and increase the paracellular permeability,77 which may be related to occludin, claudin 1, and claudin 4 decrease.78,79 Another miRNA related to the breach of the integrity of tight junctions is miR-223.80. Shown to be overexpressed in the serum and faeces of CD and UC patients37 and DSS-induced colitis mice,81 miR-223 promotes IBD progression by downregulating the expression of the tight junction protein claudin 8.82 In this case, the sources of miR-223 were identified in mast cell-derived exosomes.82 Mast cells are innate immune cells that play an important role in the intestinal barrier function and were proven to be increased in number and activation level in IBD.83In vivo experiments have shown miR-223 as an important mediator in the cross-talk with the IL-23 pathway by targeting claudin 8. Inhibition of miR-223 can significantly restore claudin 8 levels and recover treated mice from induced colitis.80 Interestingly, the administration of the probiotics Lactobacillus fermentum and Lactobacillus salivarius, isolated from human breast milk, was shown to exert a beneficial effect in DSS-induced colitis by significantly reducing the overexpression of miR-223, as well as miR-155, preserving the intestinal barrier function81 [Figure 2].
Figure 2.
Overview of miR-155 mode of action in intestinal epithelium and potential role as therapeutic.
Pathogenic bacteria are known to own a vast range of strategies to invade, facilitate survival, and infect the host cells. These strategies include the alteration of miRNA expression. Salmonella typhimurium is a well-known Gram-negative facultative anaerobic pathogenic bacterium related to numerous gastric disorders. Salmonella pathogenicity significantly depends on its ability to adhere, invade, and survive intracellularly. This bacterium employs a complex protein system that injects bacterial toxins into enterocytes, triggering mechanisms of micropinocytosis, regulated by different sets of Rho GTPase, rearranging the actin cytoskeleton and enabling the bacterial internalisation.84 Hoeke et al. demonstrated how this process is partially controlled by miRNA, more specifically by miR-29a.85 The infection with Salmonella leads to upregulation of miR-29a and downregulation of the focal adhesion protein caveolin 2, which leads to the increase of bacterial uptake.85 Similarly, Sunkavalli et al. found that miR-29b-2-5p enhances the formation of filopodia, increasing the capture of the Gram-negative pathogenic enterobacterium Shigella, promoting its invasion and intracellular replication.86 Finally, the upregulation of miR-29 appears to be associated with the regulation of claudin 187 and with intestinal epithelial apoptosis in IBD patients and DSS mice.88
4.3.Dysfunctional autophagy
Autophagy is a cellular process through which intracellular content is degraded inside lysosomes for the recycling of molecules. This is a key process responsible for the capture, degradation, and clearance of bacteria while initiating a controlled immune reaction.89 IL-25 is one of the cytokines secreted by enterocytes during the autophagy process.90 It exerts a regulatory function inhibiting Th1/Th17 inflammatory responses and inducing Th2-related cytokines such as IL-4 and IL-13.90 These cytokines are involved in the induction of antimicrobial peptides and thus the maintenance of gut homeostasis. The alteration of autophagy-related genes is related to the exacerbation of colitis and the increase of bacterial invasion of the colonic mucosa in animal models.90 Recently, dysfunctional autophagy has not only been associated with IBD89 but also with dysbiosis.91 An example of this can be found in mice with silenced autophagy-related gene 7 [Atg7], which exhibited increased bacterial burden with microbiota alterations due to a reduction of mucin and antimicrobial peptides production.90 In the case of the local knockout expression of Atg5, the disruption of the gut autophagy dramatically altered the intestinal microbiota composition and increased the pro-inflammatory bacteria, just as observed in IBD.91
Although the specific influence of miRNA on autophagy during IBD is still largely unexplored, a few studies based on the AIEC infection have revealed novel insights. Both in vitro and in vivo experiments have proven that the abnormal colonisation of AIEC activates NF-kB in intestinal epithelial cells and upregulates miR-30c, miR-93, miR-106b, and miR-130a.92,93 Intestinal epithelial cells infected with pathogenic, but not commensal, AIEC are induced to release exosomes loaded with miR30c and miR-130a.93,94 These miRNAs can lead to the inhibition of Atg5 and Atg16L1 expression and so impair the autophagy-mediated AIEC elimination, leading to the increase of intracellular AIEC and the exacerbation of the inflammatory response.92,93 The suppression with anti-miR-30c and anti-miR-130a restored functional autophagy, increased clearance effectiveness, and decreased inflammation in vivo.92 Supporting these experiments, the analysis of the intestinal mucosa of active CD patients revealed increased levels of miR-106b,93 miR-30c, and miR-130a,92 and reduced levels of Atg5 and Atg16L,75 compared with controls.
Last, autophagy can be influenced by the status of the endoplasmic reticulum [ER]. This organelle is in charge of the synthesis and secretion of proteins. Unfolded or misfolded proteins undergo ubiquitination and, subsequently, degradation.95 In case of ER stress, imbalance and overproduction of incorrectly built proteins trigger the backup cytoprotective pathway known as unfolded protein response [UPR] to restore physiological cell functions.95 The possible involvement of ER stress in IBD has received mounting attention in recent years,95,96 showing that, although autophagy and UPR have compensatory roles in the intestinal epithelium, the deficiency or alteration of either one or the other is linked to an increased risk of IBD.96 New insights demonstrated that miR-665, expression of which is regulated by the presence of microbiota,53 is upregulated in the intestinal mucosa of mice with active colitis by repressing the UPR pathway, which promotes apoptosis and autophagy sensitivity that seem to exacerbate the intestinal inflammation.97 Other miRNAs, such as miR-150 and miR-346, have been speculated to be involved in IBD. They appear to regulate fibroblast autophagy98 and protect from inflammation and cell death,99 respectively. However, their exact mechanism and connection with the microbiota remain to be studied.
4.4.Alteration of the gut immune homeostasis due to bacteria
IBD is characterised by an excessive infiltration and hyperactivation of the immune system which lead to chronic inflammation and tissue damage. CD immune response is traditionally described to be based on Th17 cells, with an increase of IL-17, IL-23, and IL-32, whereas UC response leans towards a Th2 cells profile, with IL-5, IL-13, IL-15, and IL-33 increased.100 The relevance of the dysregulation of the immune system in the pathogenesis of IBD has been widely discussed101 and, more recently, associated with miRNA. A series of studies have also shown that the gut microbiota is in tight symbiosis with the host and has a great impact on the development of the mucosal immune system. In physiological conditions, pathogenic metabolites recognised by the innate immune system trigger stimulatory immune responses; whereas commensal microbes induce repression of immune responses with a tolerogenic outcome.
With the creation of germ-free mice, researchers were able to reveal the importance of the microbiome-immune system cross-talk. The absence of microbes, hence the lack of immune challenges, is associated with profound abnormalities in the maturation of the lymphoid tissue, the number of cells, the expression of cytokines, and the intestinal architecture.102 Experiments performed in germ-free and antibiotic-treated mice showed that the presence of bacteria is essential for the development of colitis.103–105 Compared with wild-type mice, the faeces of animals that lack microbiota present differently expressed miRNA profiles.16 On the other hand, the absence of miRNAs generates uncontrolled gut microbiota and exacerbated colitis in Dicer1ΔIEC mice which can be ameliorated after faecal replacement from wild-type mice, due to the restoration of intestinal microbes.16
Furthermore, the effect of pathogens on the immune system via miRNA is being studied by altering the microbiota ecosystem with the introduction of single pathogenic bacteria. For instance, the presence of the traditional Salmonella upregulates the intestinal expression of miR-128 and reduces the recruitment of macrophages throughout the downregulation of macrophage colony-stimulating factor.106Salmonella’s presence can also upregulate miR-155 and induce macrophage death.107 Considered one of the most well-investigated miRNAs, miR-155 is considered a complex pro-inflammatory miRNA able to promote the secretion of IL-6, IL-8, TNF, IL-1B, and IFN-γ, through the direct repression of SHIP-1108 and SOCS1109 [Figure 2]. MiR-155 can also repress IL-13 receptors, characteristic of UC,110 and increase a Th17 pro-inflammatory response, characteristic of CD.111 Recent reports suggested the contribution of miR-155 in DSS-induced colitis via the downregulation of the expression of hypoxia-inducible factor-1α, tight junctions, and consequently an increase of the intestinal barrier dysfunction.112 The absence of miR-155 in knockout mice grants resistance towards intestinal inflammation caused by DSS treatment, with a reduction of mucosal inflammatory cytokines and decrease of CD4+T cells and Th1/Th17 response.113 Furthermore, miR-155 is increased in both adult109 and paediatric114 inflamed colonic mucosal samples, and under the presence of LPS,109 proven to be a key regulatory factor in inflammatory responses concerning bacteria and thus in IBD [Figure 2]. Similarly to miR-155, and as discussed earlier, miR-31 is considered a key regulatory factor of Th17 response44 and is associated with the development of Crohn’s phenotypes.115 Like several other miRNAs, studies reporting a direct connection between miR-31 and microbiota are lacking. Nonetheless, Ghorpade et al. demonstrated that its expression is induced in macrophages during Mycobacterium bovis Bacillus Calmette-Guérin infections after the activation of Toll-like receptor 2 and subsequent expression of TNF.116
It has been postulated that IBD patients may be at risk for more frequent and serious opportunistic pathogens due to, at least partially, miRNA alterations. Listeria monocytogenes is an intracellular Gram-positive bacillus that can turn invasive when the immune system is compromised. A recent study reported cases of listeriosis during treatment with anti-TNF biological agents in IBD patients.117 A differential expression of 90 miRNAs [39 upregulated and 51 downregulated] was detected upon Listeria monocytogenes infection,118 which showed to be cell type-dependent. Infected intestinal epithelial cells had a dysregulated expression of miR-16, miR-145, miR-146b, miR-155, and Let-7a1119; infected macrophages had altered expression of miR-146a, miR-155, miR-125a-3p/5p, and miR-149; and natural killers and T cells, both CD8+ and CD4+, presented a largely reduced expression of miR-29.120 Interestingly, among the modified miRNAs triggered by Listeria monocytogenes infection, the intestinal expression of miR-143, miR-148a, miR-200b, miR-200c, and miR-378 was demonstrated to be dependent on the presence of the microbiota.121
The already mentioned miR-29, upregulation of which is related to disruption of the intestinal barrier function,85,86,88 seems to also be involved in the development, general physiology, and more importantly immunomodulation of the gut. Brian et al. found that the expression of miR-29 is regulated by the intracellular sensor NOD2. When NOD2 recognises intracellular bacteria in human dendritic cells, miR-29 is upregulated. Thereafter, miR-29 targets and downregulates the IBD susceptibility gene IL-12p40 and the pro-inflammatory cytokine IL-23.87 Unlike healthy controls, IBD patients present NOD2 variants that lead to an insufficient miR-29 expression and possibly to the abnormal elevation of IL-23 in response to AIEC, thereby exacerbating inflammatory responses.87 Interestingly, NOD2 expression can be downregulated by another miRNA, miR-320, expression of which is induced by IL-33. MiR-320 promotes the repair of the intestinal epithelium and the resolution of inflammation.122 Although blood levels of miR-320 are correlated with disease activity123 and increased in patients with IBD compared with healthy controls, its regulatory function has been shown to be altered as a consequence of inflammation.124
5. Therapeutic and Diagnostic Potential of miRNA and the Gut Microbiota in IBD
The current main therapeutic goal for IBD patients is to obtain remission and mucosal healing, and thereby lower surgery rates. Classical therapies include corticosteroids, thiopurines, and aminosalicylates, which are most useful for the treatment of mild to moderate UC.125 The newest therapies use biologic agents to target key elements of the inflammatory process. To date, the most successful biologic agents are those targeting TNF. However, anti-TNF drugs have distinctive pharmacodynamic profiles that result in heterogeneous therapeutic efficacies at a high economic cost.126 New biomarkers and therapeutic agents that help personalise the treatment and predict the therapeutic responses are required. Remarkably, the microbiota itself can be used to both monitor inflammation and predict therapeutic outcomes.52,127
5.1.Modulation of the microbiota in IBD as a therapeutic option
Inflammation is associated with reduction of intestinal microbial diversity but, if the treatment with anti-TNF agents is successful, a normalisation of the microbiota composition occurs.128 Specifically in children with IBD, the mentioned dysbiosis has been linked to an increase of butyrate producers and Gram-positive bacteria such as Clostridiums.127 The presence of Clostridium sphenoids and Haemophilus species seems to be associated with the levels of calprotectin, and together could be used as a predictor of clinical response to anti-TNF therapy.127
Because of the high relevance of the gut microbiota in IBD, efforts are being made to develop strategies that modulate and improve the properties of the indigenous microbiome. One of the most common strategies includes the use of probiotics, ‘live strains of strictly selected microorganisms which, when administered in adequate amounts, confer a health benefit on the host’.129 Probiotics are widely evaluated in IBD patients for their capacity to induce and maintain remission. Promising beneficial effects were obtained with the administration of single species, such as Escherichia coli Nissle 1917130 or Saccharomyces boulardii.131 More in detail and in the scenario of UC, a meta-analysis described the treatment with Escherichia coli Nissle 1917 as effective as the treatment with aminosalicylates, preventing disease relapse.130 Experiments performed in an in vitro study suggested that its beneficial effect may be related to the induction of miR-146a,132 known to modulate Toll-like receptor signalling and to be necessary for a good response against LPS.133 In the case of the probiotic Saccharomyces boulardii, there is not enough evidence to prove its beneficial effect in IBD patients.134 Nonetheless, a study reported that the administration of Saccharomyces boulardii to DSS-treated mice had multiple beneficial anti-inflammatory effects and directly affected the overexpression of miR-155 and miR-223 in induced-colitis mice131 [Figure 2]. Despite some positive results, the benefits of probiotics seem to be limited and effects do not persist after the cessation of their consumption.135 Probiotics efficacy may depend on several factors such as the diet,136 the initial composition of the indigenous microbiota,135 the dose,137 treatment length,137 bacterial strain,137 and manufacturing process.138 Consequently, the literature still bears discrepancies and a lack of coherence regarding their efficacy in IBD. In addition, a recent report questioned probiotics adverse effects and demonstrated that their use delays and persistently disrupts indigenous microbiome reconstitution after antibiotic treatment. Unlike probiotics, autologous faecal microbiota transplantation [FMT] induced a rapid and efficient reconstitution of the microbiota to a near-complete recovery.139
FMT is a more novel and controversial method that refers to the procedure of transplanting faecal bacterial content from a healthy donor to a patient, with a therapeutic goal. Thereby, FMT can increase the gut microbiota diversity, restore the impaired intestinal permeability, and improve the immune system, mitigating the colonic inflammation.140 Recent findings revealed new evidence that local and circulating levels of miRNAs are modified consequent to FMT, normalising their levels and acting as cytoprotective molecules by targeting IL-12β, IL-18, FGF21, and TNFRSF9.141 This study demonstrated miR-23a, miR-150, miR-26b, and miR-28 as the main miRNAs involved in the downregulation of pro-inflammatory protein expression and protection of epithelial cells.141
Although FMT efficacy is robustly proven for recurrent Clostridioides difficile infections,142 its application in IBD is still under discussion. The insufficient data regarding the safety and tolerability of this approach for many years have increased concerns regarding its use. A recent retrospective study showed that the most common adverse effects in UC patients who received multiple FMT were mild symptoms.143 Hence, it was concluded FMT was a safe and well-tolerated procedure. Aside from its safety, different meta-analyses have highlighted the inconsistency of FMT effectiveness in inducing clinical remission,144 especially in CD.145 More promising outcomes are found in UC patients, where FMT allows maintainenance of clinical and endoscopic remission.146 The greater success of FMT engraftment has been described in those patients who shared more bacterial strains with the donor,147 suggesting that the current standardised approach of ‘one-stool-fits-all’ may not be the most clinically suitable.148
Altogether these data indicate that host, as well as environmental, factors can influence the microbiota composition shift following FMT and determine the compatibility of the donor and recipient microbiomes.148 However, exceptionally little is known about the molecular mechanism underlying the effects of microbiota changes, the epitranscriptomic changes associated with FMT and probiotics, and which optimal initial status quo is required to ensure successful outcomes. Thus, further mapping of miRNA and microbiota profiles before and after treatment are required.
5.2.The potential therapeutic value of miRNA in IBD
As already mentioned, some of the unsuccessful therapeutic outcomes obtained with the currently available treatments can be mitigated due to the incorporation of miRNA expression as predictors of response.149 Given the need for new predictive tools, we recently suggested the use of miRNA to evaluate the host-microbiota healthiness before FMT, to ensure more satisfactory results.52 Similarly, we also demonstrated that the use of a 9 miRNA signature from serum can be used as a therapeutic response to anti-TNF treatment.36 In humans, the use of algorithms based on the levels of miRNAs can discriminate responders from non-responders to steroids, infliximab, and cyclosporine with 93%, 84%, and 80% accuracy, respectively.150
More than a diagnostic or prognostic tool, miRNAs are very interesting for their potential therapeutic application [Table 2]. In theory, miRNAs can be used as antagonists, suppressing overexpression; or as mimics, enhancing reduced miRNA and restoring aberrant functions.151 To date and in the context of IBD, these approaches have only been investigated in induced-colitis animal models, with some promising results. The most studied strategy is based on the inhibition of exacerbated miRNAs that use antisense oligonucleotides. Although further validation is required, the development of new chemically modified antisense oligonucleotides is starting to prove their worth in silencing aberrant miRNA with high affinity and low toxicity.152,153 The use of anti-miR-155, for example, was shown to regulate Th17/Treg cell balance through Jarid2/Wnt/B-catenin proteins, and to decrease the activation of Akt leading to an overall diminished inflammatory response and amelioration of DSS-induced colitis.108,154 The most promising example of an anti-miRNA based agent is miravirsen, the first miRNA molecule that entered into clinical trials. Miravirsen [SPC3649] is an anti miR-122 that has shown long-term safety and efficacy in chronic hepatitis C patients.155 This drug is currently under phase II clinical trials.156
Table 2.
Summary of the current anti-miRNAs that are being tested due to their possible therapeutic effects in the context of intestinal inflammation and infection
| Anti-miRNA | Model tested | Effect | Ref. |
|---|---|---|---|
| Anti-miR-31 | TNBS-induced colitis | Restores colonic IL-25 expression and blocks Th1/Th17 responses | 44 |
| Anti-miR-30c + anti-miR-130a | In vitro | Restores functional autophagy resulting in more effective clearance of intracellular bacteria | 92 |
| Anti-miR-155 | DSS-induced colitis IL-10 knockout In vitro |
Alleviation of colitis due to the restoration of SHIP-1 Downregulation of pro-inflammatory cytokines Regulation of Th17/Treg cell balance |
105,108,155 |
| Anti-miR-128 |
In vitro
Mouse infection model |
Increases macrophage recruitment and suppresses Salmonella infection | 106 |
| Anti-miR-665 | DSS-induced colitis | Inhibition of apoptosis and restoration of phagocytosis functions, impairing induced colitis | 97 |
| Anti-miR-106b + anti-miR-93 | In vitro | Restoration of autophagy process | 93 |
| Anti-miR-223 | TNBS-induced colitis | Restoration of Claudin 8 levels, improvement of barrier function and alleviation of colitis | 80 |
TNBS, 2,4,6-Trinitrobenzenesulphonic acid; DSS, dextran sulphate sodium.
The replacement of deficient miRNA seems to be even more challenging than the creation of anti-miRNA. First, to succeed, synthetic miRNA mimics have to be incorporated in the RNA-induced silencing complex to perform its lost biological function.151 Moreover, the carrier should target specifically interested cells in enough amount and potency to perform its function before the clearance. Most commonly viral vectors, which are highly associated with toxicity and immunogenicity, are used to deliver the synthetic miRNA via intravenous or colonic administration.151 Unconventionally, Tian et al. recently showed in a very sophisticated manner that the administration by enema of microspheres encapsulating the miRNA mimic of interest is a very effective delivery method. They were able to successfully deliver miR-31, reducing intestinal inflammation, increasing body weight, and promoting intestinal epithelial cell regeneration in mice by regulating Wnt and Hippo signalling.45 Similarly, we used nanoparticles derived from edible ginger, rich in lipids, and developed a natural delivery mechanism of miRNAs that attenuated acute colitis and prevented chronic colitis and colitis-associated cancer in mice.157 Although no mimic therapy is currently available for IBD, several miRNA-mimic based treatments are being developed in the oncology field. MRX34, for instance, is a liposomal encapsulated molecule that mimics miR-34a.158 This miRNA, the function of which is lost or attenuated in a vast number of malignancies, can downregulate the expression of more than 30 oncogenes. MRX34 is currently under phase I clinical trial for different types of cancers.156,158
6. Concluding Remarks
Intestinal miRNAs are secreted in their majority from intestinal epithelial cells to the lumen, accumulating in the faeces and interacting with the microbiota. Increasing evidence suggests miRNA to be key regulatory factors in the maintenance of gastrointestinal homeostasis through bidirectional cross-talk with the microbes. Although miRNA-microbiota communication is complex and not fully elucidated yet, studies have demonstrated that both host and extrinsic miRNA can shape the gut ecosystem, modulating its composition and distribution along the gastrointestinal tract. Concomitantly, the presence of pathogenic microbes or an imbalanced microbiome can disrupt the intestinal epithelial barrier, prompt dysfunctional autophagy, and impair the intestinal immune system via the modification of host miRNA. Thus, an imbalance between these two compartments can alter health status and affect the host’s inflammation at different levels, impacting on IBD pathophysiology.
Moreover, the study of miRNA signature is a powerful tool that can facilitate the diagnosis and the prediction of treatment outcomes, increasing the chances of personalised medicine and successful therapies. Altogether, this indicates that the targeted intervention of the microbiome and/or miRNA, with either mimics or anti-miRNA, may be potential therapy for IBD and other disorders that co-occur with dysbiosis. Despite the tremendous advances, the field faces many challenges due to the functional redundancy of miRNA and off-target effects that can result in unwanted silencing and toxicity, justifying the need for elaborate potent delivery systems.
The present review compiled the most novel findings that describe the association of miRNA-microbiota in IBD, sorted the most relevant mechanisms that may lead to it, and discussed their therapeutic potential, providing new and comprehensive insights into the topic.
Acknowledgements
We thank Dr Melissa C. Kordahi for carefully proofreading our manuscript.
Contributor Information
Maite Casado-Bedmar, INSERM, U1149, Center for Research on Inflammation, Université de Paris, Paris, France.
Emilie Viennois, INSERM, U1149, Center for Research on Inflammation, Université de Paris, Paris, France.
Funding
This work was supported by ANR-11-IDEX-0005-02 Laboratory of Excellence INFLAMEX. EV is recipient of an Individual Fellowship Marie Sklodowska-Curie grant from the European Research Executive Agency.
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
MC-B: scientific literature research, wrote the manuscript, creation of the figures and tables. EV: original idea, first manuscript drafting, reviewed scientific literature. Both authors read and approved the final version.
References
- 1. Sairenji T, Collins KL, Evans DV. An update on inflammatory bowel disease. Prim Care 2017;44:673–92. [DOI] [PubMed] [Google Scholar]
- 2. Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 2001;48:526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Annese V, Daperno M, Rutter MD, et al. ; European Crohn’s and Colitis Organisation. European evidence-based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis 2013;7:982–1018. [DOI] [PubMed] [Google Scholar]
- 4. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2020;5:17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol 2010;28:573–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pirzer U, Schönhaar A, Fleischer B, Hermann E, Meyer zum Büschenfelde KH. Reactivity of infiltrating T lymphocytes with microbial antigens in Crohn’s disease. Lancet 1991;338:1238–9. [DOI] [PubMed] [Google Scholar]
- 7. Keubler LM, Buettner M, Häger C, Bleich A. A multihit model: colitis lessons from the interleukin-10-deficient mouse. Inflamm Bowel Dis 2015;21:1967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu GD, Chen J, Hoffmann C, et al. . Linking long-term dietary patterns with gut microbial enterotypes. Science 2011;334:105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chang CS, Kao CY. Current understanding of the gut microbiota shaping mechanisms. J Biomed Sci 2019;26:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 2009;19:92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gebert LFR, MacRae IJ. Regulation of microRNA function in animals. Nat Rev Mol Cell Biol. 2019;20:21–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu W, Wang X. Prediction of functional microRNA targets by integrative modeling of microRNA binding and target expression data. Genome Biol 2019;20:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fischer S, Handrick R, Aschrafi A, Otte K. Unveiling the principle of microRNA-mediated redundancy in cellular pathway regulation. RNA Biol 2015;12:238–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weber JA, Baxter DH, Zhang S, et al. . The microRNA spectrum in 12 body fluids. Clin Chem 2010;56:1733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ji Y, Li X, Zhu Y, Li N, Zhang N, Niu M. Faecal microRNA as a biomarker of the activity and prognosis of inflammatory bowel diseases. Biochem Biophys Res Commun 2018;503:2443–50. [DOI] [PubMed] [Google Scholar]
- 16. Liu S, da Cunha AP, Rezende RM, et al. . The host shapes the gut microbiota via fecal microRNA. Cell Host Microbe 2016;19:32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hu S, Liu L, Chang EB, Wang JY, Raufman JP. Butyrate inhibits pro-proliferative miR-92a by diminishing c-Myc-induced miR-17-92a cluster transcription in human colon cancer cells. Mol Cancer 2015;14:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park JH, Peyrin-Biroulet L, Eisenhut M, Shin JI. IBD immunopathogenesis: A comprehensive review of inflammatory molecules. Autoimmun Rev 2017;16:416–26. [DOI] [PubMed] [Google Scholar]
- 19. Kalla R, Ventham NT, Kennedy NA, et al. . MicroRNAs: new players in IBD. Gut 2015;64:504–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. James JP, Riis LB, Malham M, Høgdall E, Langholz E, Nielsen BS. MicroRNA Biomarkers in IBD-Differential Diagnosis and Prediction of Colitis-Associated Cancer. Int J Mol Sci 2020. doi: 10.3390/ijms21217893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qin J, Li R, Raes J, et al. ; MetaHIT Consortium. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010;464:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matsuoka K, Kanai T. The gut microbiota and inflammatory bowel disease. Semin Immunopathol 2015;37:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Costea PI, Hildebrand F, Arumugam M, et al. . Enterotypes in the landscape of gut microbial community composition. Nat Microbiol 2018;3:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sommer F, Bäckhed F. The gut microbiota—masters of host development and physiology. Nat Rev Microbiol 2013;11:227–38. [DOI] [PubMed] [Google Scholar]
- 25. Hadizadeh F, Bonfiglio F, Belheouane M, et al. . Faecal microbiota composition associates with abdominal pain in the general population. Gut 2018;67:778–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gevers D, Kugathasan S, Denson LA, et al. . The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014;15:382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007;448:427–34. [DOI] [PubMed] [Google Scholar]
- 28. Nell S, Suerbaum S, Josenhans C. The impact of the microbiota on the pathogenesis of IBD: lessons from mouse infection models. Nat Rev Microbiol 2010;8:564–77. [DOI] [PubMed] [Google Scholar]
- 29. do Vale A, Cabanes D, Sousa S. Bacterial toxins as pathogen weapons against phagocytes. Front Microbiol 2016;7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schultz BM, Paduro CA, Salazar GA, et al. . A potential role of salmonella infection in the onset of inflammatory bowel diseases. Front Immunol 2017;8:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Darfeuille-Michaud A, Boudeau J, Bulois P, et al. . High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology 2004;127:412–21. [DOI] [PubMed] [Google Scholar]
- 32. Sankarasubramanian J, Ahmad R, Avuthu N, Singh AB, Guda C. Gut microbiota and metabolic specificity in ulcerative colitis and Crohn’s disease. Front Med [Lausanne] 2020;7:606298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Metwaly A, Dunkel A, Waldschmitt N, et al. . Integrated microbiota and metabolite profiles link Crohn’s disease to sulfur metabolism. Nat Commun 2020;11:4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu F, Zikusoka M, Trindade A, et al. . MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology 2008;135:1624–35.e24. [DOI] [PubMed] [Google Scholar]
- 35. Iborra M, Bernuzzi F, Correale C, et al. . Identification of serum and tissue micro-RNA expression profiles in different stages of inflammatory bowel disease. Clin Exp Immunol 2013;173:250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Viennois E, Zhao Y, Han MK, et al. . Serum miRNA signature diagnoses and discriminates murine colitis subtypes and predicts ulcerative colitis in humans. Sci Rep 2017;7:2520.,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schönauen K, Le N, von Arnim U, Schulz C, Malfertheiner P, Link A. Circulating and fecal microRNAs as biomarkers for inflammatory bowel diseases. Inflamm Bowel Dis 2018;24:1547–57. [DOI] [PubMed] [Google Scholar]
- 38. Altaf-Ul-Amin M, Karim MB., Hu P, Ono N, Kanaya S. Discovery of inflammatory bowel disease-associated miRNAs using a novel bipartite clustering approach. BMC Med Genet 2020;13:10.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ahmed Hassan E, El-Din Abd El-Rehim AS, Mohammed Kholef EF, Abd-Elgwad Elsewify W. Potential role of plasma miR-21 and miR-92a in distinguishing between irritable bowel syndrome, ulcerative colitis, and colorectal cancer. Gastroenterol Hepatol Bed Bench 2020;13:147–54. [PMC free article] [PubMed] [Google Scholar]
- 40. Malham M, James J, Jakobsen C, et al. . The mucosal microRNA profile relates to age and severity of disease in patients with ulcerative colitis. Inflamm Bowel Dis 2021;27:S11. [Google Scholar]
- 41. Thorlacius-Ussing G, Schnack Nielsen B, Andersen V, Holmstrøm K, Pedersen AE. Expression and localization of miR-21 and miR-126 in mucosal tissue from patients with inflammatory bowel disease. Inflamm Bowel Dis 2017;23:739–52. [DOI] [PubMed] [Google Scholar]
- 42. Yan H, Zhang X, Xu Y. Aberrant expression of miR-21 in patients with inflammatory bowel disease: A protocol for systematic review and meta analysis. Medicine 2020;99:e19693.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shi C, Liang Y, Yang J, et al. . MicroRNA-21 knockout improve the survival rate in DSS induced fatal colitis through protecting against inflammation and tissue injury. PLoS One 2013;8:e66814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shi T, Xie Y, Fu Y, et al. . The signaling axis of microRNA-31/interleukin-25 regulates Th1/Th17-mediated inflammation response in colitis. Mucosal Immunol 2017;10:983–95. [DOI] [PubMed] [Google Scholar]
- 45. Tian Y, Xu J, Li Y, et al. . MicroRNA-31 reduces inflammatory signaling and promotes regeneration in colon epithelium, and delivery of mimics in microspheres reduces colitis in mice. Gastroenterology 2019;156:2281–96.e6. [DOI] [PubMed] [Google Scholar]
- 46. Wang DJ, Legesse-Miller A, Johnson EL, Coller HA. Regulation of the let-7a-3 promoter by NF-κB. PLoS One 2012;7:e31240.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell 2009;139:693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Coskun M, Bjerrum JT, Seidelin JB, Troelsen JT, Olsen J, Nielsen OH. miR-20b, miR-98, miR-125b-1*, and let-7e* as new potential diagnostic biomarkers in ulcerative colitis. World J Gastroenterol 2013;19:4289–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rossato M, Curtale G, Tamassia N, et al. . IL-10–induced microRNA-187 negatively regulates TNF-α, IL-6, and IL-12p40 production in TLR4-stimulated monocytes. ProcNatl Acad Sci 2012;109:E3101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wu LY, Ma XP, Shi Y, et al. . Alterations in microRNA expression profiles in inflamed and noninflamed ascending colon mucosae of patients with active Crohn’s disease. J Gastroenterol Hepatol 2017;32:1706–15. [DOI] [PubMed] [Google Scholar]
- 51. Guo Z, Wu R, Gong J, et al. . Altered microRNA expression in inflamed and non-inflamed terminal ileal mucosa of adult patients with active Crohn’s disease. J Gastroenterol Hepatol 2015;30:109–16. [DOI] [PubMed] [Google Scholar]
- 52. Viennois E, Chassaing B, Tahsin A, et al. . Host-derived fecal microRNAs can indicate gut microbiota healthiness and ability to induce inflammation. Theranostics 2019;9:4542–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dalmasso G, Nguyen HT, Yan Y, et al. . Microbiota modulate host gene expression via microRNAs. PLoS One 2011;6:e19293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tomkovich S, Gharaibeh RZ, Dejea CM, et al. . Human colon mucosal biofilms and murine host communicate via altered mRNA and microRNA expression during cancer. mSystems 2020;5:e00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Johnston DGW, Williams MA, Thaiss CA, et al. . Loss of microRNA-21 influences the gut microbiota, causing reduced susceptibility in a murine model of colitis. J Crohns Colitis 2018;12:835–48. [DOI] [PubMed] [Google Scholar]
- 56. Huan Y, Kong Q, Mou H, Yi H. Antimicrobial peptides: classification, design, application and research progress in multiple fields. Front Microbiol 2020;11:582779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Arijs I, De Hertogh G, Lemaire K, et al. . Mucosal gene expression of antimicrobial peptides in inflammatory bowel disease before and after first infliximab treatment. PLoS One 2009;4:e7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Contreras G, Shirdel I, Braun MS, Wink M. Defensins: Transcriptional regulation and function beyond antimicrobial activity. Dev Comp Immunol 2020;104:103556. [DOI] [PubMed] [Google Scholar]
- 59. Miles DR, Shen J, Chuang AY, et al. . Alpha-defensin 5 expression is regulated by microRNAs in the Caco-2 intestinal epithelial cell line. J Inflamm Bowel Dis Disord 2016;1:105. [PMC free article] [PubMed] [Google Scholar]
- 60. Salzman NH, Hung K, Haribhai D, et al. . Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol 2010;11:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhao Y, Ma T, Chen W, et al. . MicroRNA-124 promotes intestinal inflammation by targeting aryl hydrocarbon receptor in Crohn’s disease. J Crohns Colitis 2016;10:703–12. [DOI] [PubMed] [Google Scholar]
- 62. Teng Y, Ren Y, Sayed M, et al. . Plant-derived exosomal microRNAs shape the gut microbiota. Cell Host Microbe 2018;24:637–52.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tarallo S, Ferrero G, De Filippis F, et al. . Stool microRNA profiles reflect different dietary and gut microbiome patterns in healthy individuals. Gut 2021. doi: 10.1136/gutjnl-2021-325168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang L, Hou D, Chen X, et al. . Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of cross-kingdom regulation by microRNA. Cell Res 2012;22:107–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Micó V, Martín R, Lasunción MA, Ordovás JM, Daimiel L. Unsuccessful detection of plant microRNAs in beer, extra virgin olive oil and human plasma after an acute ingestion of extra virgin olive oil. Plant Foods Hum Nutr 2016;71:102–8. [DOI] [PubMed] [Google Scholar]
- 66. Dickinson B, Zhang Y, Petrick JS, Heck G, Ivashuta S, Marshall WS. Lack of detectable oral bioavailability of plant microRNAs after feeding in mice. Nat Biotechnol 2013;31:965–7. [DOI] [PubMed] [Google Scholar]
- 67. Zhang Y, Wiggins BE, Lawrence C, et al. . Analysis of plant-derived miRNAs in animal small RNA datasets. BMC Genomics 2012;13:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tosar JP, Rovira C, Naya H, Cayota A. Mining of public sequencing databases supports a non-dietary origin for putative foreign miRNAs: underestimated effects of contamination in NGS. RNA 2014;20:754–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang M, Viennois E, Xu C, Merlin D. Plant derived edible nanoparticles as a new therapeutic approach against diseases. Tissue Barriers 2016;4:e1134415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chelakkot C, Ghim J, Ryu SH. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp Mol Med 2018;50:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Michielan A, D’Incà R. Intestinal permeability in inflammatory bowel disease: pathogenesis, clinical evaluation, and therapy of leaky gut. Mediators Inflamm 2015;2015:628157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dalmasso G, Nguyen HT, Yan Y, et al. . MicroRNAs determine human intestinal epithelial cell fate. Differentiation 2010;80:147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nguyen HT, Dalmasso G, Yan Y, et al. . MicroRNA-7 modulates CD98 expression during intestinal epithelial cell differentiation. J Biol Chem 2010;285:1479–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kucharzik T, Walsh SV, Chen J, Parkos CA, Nusrat A. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am J Pathol 2001;159:2001–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Braniste V, Al-Asmakh M, Kowal C, et al. . The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med 2014;6:263ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Peck BC, Mah AT, Pitman WA, Ding S, Lund PK, Sethupathy P. Functional transcriptomics in diverse intestinal epithelial cell types reveals robust microRNA sensitivity in intestinal stem cells to microbial status. J Biol Chem 2017;292:2586–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yang Y, Ma Y, Shi C, et al. . Overexpression of miR-21 in patients with ulcerative colitis impairs intestinal epithelial barrier function through targeting the Rho GTPase RhoB. Biochem Biophys Res Commun 2013;434:746–52. [DOI] [PubMed] [Google Scholar]
- 78. Nakata K, Sugi Y, Narabayashi H, et al. . Commensal microbiota-induced microRNA modulates intestinal epithelial permeability through the small GTPase ARF4. J Biol Chem 2017;292:15426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhang L, Zhang F, He DK, Fan XM, Shen J. MicroRNA-21 is upregulated during intestinal barrier dysfunction induced by ischemia reperfusion. Kaohsiung J Med Sci 2018;34:556–63. [DOI] [PubMed] [Google Scholar]
- 80. Wang H, Chao K, Ng SC, et al. . Pro-inflammatory miR-223 mediates the cross-talk between the IL23 pathway and the intestinal barrier in inflammatory bowel disease. Genome Biol 2016;17:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rodríguez-Nogales A, Algieri F, Garrido-Mesa J, et al. . Differential intestinal anti-inflammatory effects of Lactobacillus fermentum and Lactobacillus salivarius in DSS mouse colitis: impact on microRNAs expression and microbiota composition. Mol Nutr Food Res 2017. doi: 10.1002/mnfr.201700144. [DOI] [PubMed] [Google Scholar]
- 82. Li M, Zhang S, Qiu Y, et al. . Mast cells-derived MiR-223 destroys intestinal barrier function by inhibition of CLDN8 expression in intestinal epithelial cells. Biol Res 2020;53:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Casado-Bedmar M, Heil SDS, Myrelid P, Söderholm JD, Keita ÅV. Upregulation of intestinal mucosal mast cells expressing VPAC1 in close proximity to vasoactive intestinal polypeptide in inflammatory bowel disease and murine colitis. Neurogastroenterol Motil 2019;31:e13503. [DOI] [PubMed] [Google Scholar]
- 84. Friebel A, Ilchmann H, Aepfelbacher M, Ehrbar K, Machleidt W, Hardt WD. SopE and SopE2 from Salmonella typhimurium activate different sets of RhoGTPases of the host cell. J Biol Chem 2001;276:34035–40. [DOI] [PubMed] [Google Scholar]
- 85. Hoeke L, Sharbati J, Pawar K, Keller A, Einspanier R, Sharbati S. Intestinal Salmonella typhimurium infection leads to miR-29a induced caveolin 2 regulation. PLoS One 2013;8:e67300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sunkavalli U, Aguilar C, Silva RJ, et al. . Analysis of host microRNA function uncovers a role for miR-29b-2-5p in Shigella capture by filopodia. PLoS Pathog 2017;13:e1006327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Brain O, Owens BM, Pichulik T, et al. . The intracellular sensor NOD2 induces microRNA-29 expression in human dendritic cells to limit IL-23 release. Immunity 2013;39:521–36. [DOI] [PubMed] [Google Scholar]
- 88. Lv B, Liu Z, Wang S, et al. . MiR-29a promotes intestinal epithelial apoptosis in ulcerative colitis by down-regulating Mcl-1. Int J Clin Exp Pathol 2014;7:8542–52. [PMC free article] [PubMed] [Google Scholar]
- 89. Shao BZ, Yao Y, Zhai JS, Zhu JH, Li JP, Wu K. The role of autophagy in inflammatory bowel disease. Front Physiol 2021;12:621132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Tsuboi K, Nishitani M, Takakura A, Imai Y, Komatsu M, Kawashima H. Autophagy protects against colitis by the maintenance of normal gut microflora and secretion of mucus. J Biol Chem 2015;290:20511–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yang L, Liu C, Zhao W, et al. . Impaired autophagy in intestinal epithelial cells alters gut microbiota and host immune responses. Appl Environ Microbiol 2018. doi: 10.1128/aem.00880-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Nguyen HT, Dalmasso G, Müller S, Carrière J, Seibold F, Darfeuille-Michaud A. Crohn’s disease-associated adherent invasive Escherichia coli modulate levels of microRNAs in intestinal epithelial cells to reduce autophagy. Gastroenterology 2014;146:508–19. [DOI] [PubMed] [Google Scholar]
- 93. Lu C, Chen J, Xu HG, et al. . MIR106B and MIR93 prevent removal of bacteria from epithelial cells by disrupting ATG16L1-mediated autophagy. Gastroenterology 2014;146:188–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Larabi A, Dalmasso G, Delmas J, Barnich N, Nguyen HTT. Exosomes transfer miRNAs from cell-to-cell to inhibit autophagy during infection with Crohn’s disease-associated adherent-invasive E. coli. Gut Microbes 2020;11:1677–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Li C. Unfolded protein response and Crohn’s diseases: a molecular mechanism of wound healing in the gut. Gastrointestinal Disorders 2021;3:31–43. [Google Scholar]
- 96. Hosomi S, Kaser A, Blumberg RS. Role of endoplasmic reticulum stress and autophagy as interlinking pathways in the pathogenesis of inflammatory bowel disease. Curr Opin Gastroenterol 2015;31:81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Li M, Zhang S, Qiu Y, et al. . Upregulation of miR-665 promotes apoptosis and colitis in inflammatory bowel disease by repressing the endoplasmic reticulum stress components XBP1 and ORMDL3. Cell Death Dis 2017;8:e2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Heindryckx F, Binet F, Ponticos M, et al. . Endoplasmic reticulum stress enhances fibrosis through IRE1α-mediated degradation of miR-150 and XBP-1 splicing. EMBO Mol Med 2016;8:729–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Guo J, Yang Z, Yang X, Li T, Liu M, Tang H. miR-346 functions as a pro-survival factor under ER stress by activating mitophagy. Cancer Lett 2018;413:69–81. [DOI] [PubMed] [Google Scholar]
- 100. Nemeth ZH, Bogdanovski DA, Barratt-Stopper P, Paglinco SR, Antonioli L, Rolandelli RH. Crohn’s disease and ulcerative colitis show unique cytokine profiles. Cureus 2017;9:e1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Guan Q. A Comprehensive review and update on the pathogenesis of inflammatory bowel disease. J Immunol Res 2019;2019:7247238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res 2020;30:492–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hernández-Chirlaque C, Aranda CJ, Ocón B, et al. . Germ-free and antibiotic-treated mice are highly susceptible to epithelial injury in DSS colitis. J Crohns Colitis 2016;10:1324–1335. [DOI] [PubMed] [Google Scholar]
- 104. Sellon RK, Tonkonogy S, Schultz M, et al. . Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun 1998;66:5224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Li Y, Tian Y, Zhu W, et al. . IL-10/microRNA-155/SHIP-1 signaling pathway is crucial for commensal bacteria induced spontaneous colitis. Biochem Pharmacol 2016;116:100–6. [DOI] [PubMed] [Google Scholar]
- 106. Zhang T, Yu J, Zhang Y, et al. . Salmonella enterica serovar enteritidis modulates intestinal epithelial miR-128 levels to decrease macrophage recruitment via macrophage colony-stimulating factor. J Infect Dis 2014;209:2000–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ro YT, Jo GH, Jung SA, Lee EH, Shin J, Lee JH. Salmonella‑induced miR‑155 enhances necroptotic death in macrophage cells via targeting RIP1/3. Mol Med Rep 2018;18:5133–40. [DOI] [PubMed] [Google Scholar]
- 108. Lu ZJ, Wu JJ, Jiang WL, et al. . MicroRNA-155 promotes the pathogenesis of experimental colitis by repressing SHIP-1 expression. World J Gastroenterol 2017;23:976–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Pathak S, Grillo AR, Scarpa M, et al. . MiR-155 modulates the inflammatory phenotype of intestinal myofibroblasts by targeting SOCS1 in ulcerative colitis. Exp Mol Med 2015;47:e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gwiggner M, Martinez-Nunez RT, Whiteoak SR, et al. . MicroRNA-31 and microRNA-155 are overexpressed in ulcerative colitis and regulate IL-13 signaling by targeting interleukin 13 receptor α-1. Genes [Basel] 2018. doi: 10.3390/genes9020085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hou J, Hu X, Chen B, et al. . miR-155 targets Est-1 and induces ulcerative colitis via the IL-23/17/6-mediated Th17 pathway. Pathol Res Pract 2017;213:1289–95. [DOI] [PubMed] [Google Scholar]
- 112. Liu Y, Zhu F, Li H, et al. . MiR-155 contributes to intestinal barrier dysfunction in DSS-induced mice colitis via targeting HIF-1α/TFF-3 axis. Aging [Albany NY] 2020;12:14966–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Singh UP, Murphy AE, Enos RT, et al. . miR-155 deficiency protects mice from experimental colitis by reducing T helper type 1/type 17 responses. Immunology 2014;143:478–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Béres NJ, Szabó D, Kocsis D, et al. . Role of altered expression of miR-146a, miR-155, and miR-122 in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis 2016;22:327–35. [DOI] [PubMed] [Google Scholar]
- 115. Keith BP, Barrow JB, Toyonaga T, et al. . Colonic epithelial miR-31 associates with the development of Crohn’s phenotypes. JCI Insight 2018. doi: 10.1172/jci.insight.122788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ghorpade DS, Holla S, Kaveri SV, Bayry J, Patil SA, Balaji KN. Sonic hedgehog-dependent induction of microRNA 31 and microRNA 150 regulates Mycobacterium bovis BCG-driven toll-like receptor 2 signaling. Mol Cell Biol 2013;33:543–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Horigome R, Sato H, Honma T, Terai S. Septicemic listeriosis during adalimumab- and golimumab-based treatment for ulcerative colitis: case presentation and literature review. Clin J Gastroenterol 2020;13:22–5. [DOI] [PubMed] [Google Scholar]
- 118. Mannala GK, Izar B, Rupp O, et al. . Listeria monocytogenes induces a virulence-dependent microRNA signature that regulates the immune response in Galleria mellonella. Front Microbiol 2017;8:2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Izar B, Mannala GK, Mraheil MA, Chakraborty T, Hain T. microRNA response to Listeria monocytogenes infection in epithelial cells. Int J Mol Sci 2012;13:1173–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ma F, Xu S, Liu X, et al. . The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-γ. Nat Immunol 2011;12:861–9. [DOI] [PubMed] [Google Scholar]
- 121. Archambaud C, Sismeiro O, Toedling J, et al. . The intestinal microbiota interferes with the microRNA response upon oral Listeria infection. mBio 2013;4:e00707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lopetuso LR, De Salvo C, Pastorelli L, et al. . IL-33 promotes recovery from acute colitis by inducing miR-320 to stimulate epithelial restitution and repair. Proc Natl Acad Sci U S A 2018;115:E9362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Cordes F, Demmig C, Bokemeyer A, et al. . MicroRNA-320a monitors intestinal disease activity in patients with inflammatory bowel disease. Clin Transl Gastroenterol 2020;11:e00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Pierdomenico M, Cesi V, Cucchiara S, et al. . NOD2 is regulated by Mir-320 in physiological conditions but this control is altered in inflamed tissues of patients with inflammatory bowel disease. Inflamm Bowel Dis 2016;22:315–26. [DOI] [PubMed] [Google Scholar]
- 125. Williams C, Panaccione R, Ghosh S, Rioux K. Optimizing clinical use of mesalazine [5-aminosalicylic acid] in inflammatory bowel disease. Therap Adv Gastroenterol 2011;4:237–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Atreya R, Neurath MF, Siegmund B. Personalizing treatment in IBD: Hype or reality in 2020? Can we predict response to anti-TNF? Front Med [Lausanne] 2020;7:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kolho KL, Korpela K, Jaakkola T, et al. . Fecal microbiota in pediatric inflammatory bowel disease and its relation to inflammation. Am J Gastroenterol 2015;110:921–30. [DOI] [PubMed] [Google Scholar]
- 128. Kowalska-Duplaga K, Kapusta P, Gosiewski T, et al. . Changes in the intestinal microbiota are seen following treatment with infliximab in children with Crohn’s disease. J Clin Med 2020. doi: 10.3390/jcm9030687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Hill C, Guarner F, Reid G, et al. . Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 2014;11:506–14. [DOI] [PubMed] [Google Scholar]
- 130. Losurdo G, Iannone A, Contaldo A, Ierardi E, Di Leo A, Principi M. Escherichia coli Nissle 1917 in ulcerative colitis treatment: systematic review and meta-analysis. J Gastrointestin Liver Dis 2015;24:499–505. [DOI] [PubMed] [Google Scholar]
- 131. Rodríguez-Nogales A, Algieri F, Garrido-Mesa J, et al. . Intestinal anti-inflammatory effect of the probiotic Saccharomyces boulardii in DSS-induced colitis in mice: Impact on microRNAs expression and gut microbiota composition. J Nutr Biochem 2018;61:129–39. [DOI] [PubMed] [Google Scholar]
- 132. Sabharwal H, Cichon C, Ölschläger TA, Sonnenborn U, Schmidt MA. Interleukin-8, CXCL1, and microRNA miR-146a responses to probiotic Escherichia coli Nissle 1917 and enteropathogenic E. coli in human intestinal epithelial T84 and monocytic THP-1 cells after apical or basolateral infection. Infect Immun 2016;84:2482–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Boldin MP, Taganov KD, Rao DS, et al. . miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J Exp Med 2011;208:1189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Sivananthan K, Petersen AM. Review of Saccharomyces boulardii as a treatment option in IBD. Immunopharmacol Immunotoxicol 2018;40:465–75. [DOI] [PubMed] [Google Scholar]
- 135. Tannock GW, Munro K, Harmsen HJ, Welling GW, Smart J, Gopal PK. Analysis of the fecal microflora of human subjects consuming a probiotic product containing Lactobacillus rhamnosus DR20. Appl Environ Microbiol 2000;66:2578–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Son J, Jang LG, Kim BY, Lee S, Park H. The effect of athletes’ probiotic intake may depend on protein and dietary fiber intake. Nutrients 2020. doi: 10.3390/nu12102947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Ritchie ML, Romanuk TN. A meta-analysis of probiotic efficacy for gastrointestinal diseases. PLoS One 2012;7:e34938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Trinchieri V, Laghi L, Vitali B, et al. . Efficacy and safety of a multistrain probiotic formulation depends from manufacturing. Front Immunol 2017;8:1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Suez J, Zmora N, Zilberman-Schapira G, et al. . Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell 2018;174:1406–23.e16. [DOI] [PubMed] [Google Scholar]
- 140. Fong W, Li Q, Yu J. Gut microbiota modulation: a novel strategy for prevention and treatment of colorectal cancer. Oncogene 2020;39:4925–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Monaghan TM, Seekatz AM, Markham NO, et al. . Fecal microbiota transplantation for recurrent Clostridioides difficile infection associates with functional alterations in circulating microRNAs. Gastroenterology 2021;161:255–270.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Baunwall SMD, Lee MM, Eriksen MK, et al. . Faecal microbiota transplantation for recurrent Clostridioides difficile infection: An updated systematic review and meta-analysis. EClinicalMedicine 2020;29–30:100642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Sood A, Singh A, Mahajan R, et al. . Acceptability, tolerability, and safety of fecal microbiota transplantation in patients with active ulcerative colitis [AT&S Study]. J Gastroenterol Hepatol 2020;35:418–24. [DOI] [PubMed] [Google Scholar]
- 144. Fang H, Fu L, Wang J. Protocol for fecal microbiota transplantation in inflammatory bowel disease: a systematic review and meta-Analysis. Biomed Res Int 2018;2018:8941340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Xiang L, Ding X, Li Q, et al. . Efficacy of faecal microbiota transplantation in Crohn’s disease: a new target treatment? Microb Biotechnol 2020;13:760–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Sood A, Mahajan R, Singh A, et al. . Role of faecal microbiota transplantation for maintenance of remission in patients with ulcerative colitis: a pilot study. J Crohns Colitis 2019;13:1311–7. [DOI] [PubMed] [Google Scholar]
- 147. Li SS, Zhu A, Benes V, et al. . Durable coexistence of donor and recipient strains after fecal microbiota transplantation. Science 2016;352:586–9. [DOI] [PubMed] [Google Scholar]
- 148. Danne C, Rolhion N, Sokol H. Recipient factors in faecal microbiota transplantation: one stool does not fit all. Nat Rev Gastroenterol Hepatol 2021;18:503–13. [DOI] [PubMed] [Google Scholar]
- 149. Kalla R, Adams AT, Ventham NT, et al. . Whole blood profiling of T-cell derived miRNA allows the development of prognostic models in inflammatory bowel disease. J Crohns Colitis 2020;14:1724–1733. [DOI] [PubMed] [Google Scholar]
- 150. Morilla I, Uzzan M, Laharie D, et al. . Colonic MicroRNA profiles, identified by a deep learning algorithm, that predict responses to therapy of patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol 2019;17:905–13. [DOI] [PubMed] [Google Scholar]
- 151. Wang C, Chen J. microRNAs as therapeutic targets in intestinal diseases. ExRNA 2019;1:23. [Google Scholar]
- 152. Lennox KA, Owczarzy R, Thomas DM, Walder JA, Behlke MA. Improved performance of anti-miRNA oligonucleotides using a novel non-nucleotide modifier. Mol Ther Nucleic Acids 2013;2:e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Lima JF, Cerqueira L, Figueiredo C, Oliveira C, Azevedo NF. Anti-miRNA oligonucleotides: A comprehensive guide for design. RNA Biol 2018;15:338–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Zhu F, Li H, Liu Y, et al. . miR-155 antagomir protect against DSS-induced colitis in mice through regulating Th17/Treg cell balance by Jarid2/Wnt/β-catenin. Biomed Pharmacother 2020;126:109909. [DOI] [PubMed] [Google Scholar]
- 155. van der Ree MH, van der Meer AJ, van Nuenen AC, et al. . Miravirsen dosing in chronic hepatitis C patients results in decreased microRNA-122 levels without affecting other microRNAs in plasma. Aliment Pharmacol Ther 2016;43:102–13. [DOI] [PubMed] [Google Scholar]
- 156. Chakraborty C, Sharma AR, Sharma G, Lee SS. Therapeutic advances of miRNAs: A preclinical and clinical update. J Adv Res 2021;28:127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Zhang M, Viennois E, Prasad M, et al. . Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 2016;101:321–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Hong DS, Kang YK, Borad M, et al. . Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br J Cancer 2020;122:1630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]