Abstract
Aims
Although in persistent atrial fibrillation (AF) a complex AF substrate characterized by a high incidence of conduction block has been reported, relatively little is known about AF complexity in paroxysmal AF (pAF). Also, the relative contribution of various aspects of structural alterations to conduction disturbances is not clear. In particular, the contribution of endomysial fibrosis to conduction disturbances during progression of AF has not been studied yet.
Methods and results
During cardiac surgery, epicardial high-density mapping was performed in patients with acutely induced (aAF, n = 11), pAF (n = 12), and longstanding persistent AF (persAF, n = 9) on the right atrial (RA) wall, the posterior left atrial wall (pLA) and the LA appendage (LAA). In RA appendages, overall and endomysial (myocyte-to-myocyte distances) fibrosis and connexin 43 (Cx43) distribution were quantified. Unipolar AF electrogram analysis showed a more complex pattern with a larger number of narrower waves, more breakthroughs and a higher fractionation index (FI) in persAF compared with aAF and pAF, with no differences between aAF and pAF. The FI was consistently higher at the pLA compared with the RA. Structurally, Cx43 lateralization increased with AF progression (aAF = 7.5 ± 8.9%, pAF = 24.7 ± 11.1%, persAF = 35.1 ± 11.4%, P < 0.001). Endomysial but not overall fibrosis correlated with AF complexity (r = 0.57, P = 0.001; r = 0.23, P = 0.20; respectively).
Conclusions
Atrial fibrillation complexity is highly variable in patients with pAF, but not significantly higher than in patients with acutely induced AF, while in patients with persistent AF complexity is higher. Among the structural alterations studied, endomysial fibrosis, but not overall fibrosis, is the strongest determinant of AF complexity.
Keywords: Atrial fibrillation, Fibrosis, Endomysial fibrosis, AF complexity, AF substrate, Epicardial mapping
What’s new?
Atrial fibrillation complexity is highly variable in patients with paroxysmal AF.
Atrial fibrillation complexity is comparable in acute and paroxysmal AF, but higher in persistent AF.
Endomysial, but not overall fibrosis, is the strongest structural determinant of AF complexity.
Introduction
Although various pathophysiological processes have been identified in the onset and perpetuation of persistent atrial fibrillation (AF), the exact underlying mechanism remains to be determined. While progressive electrical and structural atrial remodelling is induced by AF itself structural alterations can also occur because of underlying structural heart diseases. They play an important role in the persistence of AF and lead to an increased complexity of conduction patterns during AF. However, the relative contribution of structural alterations during the progression of AF is still unknown. An example of such an individual substrate determinant is fibrosis.1,2 Both histological and delayed enhancement magnetic resonance imaging studies suggest that progression of AF goes hand in hand with an increase in atrial fibrosis.3
The type of fibrosis and its localization within the atrial wall have an important impact on the resulting arrhythmogenic effect on atrial conduction. For example, animal studies suggest that fibrous tissue within individual myocytes (endomysial fibrosis), rather than fibrous tissue surrounding bundles of myocytes, is responsible for the increased complexity of fibrillatory conduction.4 Nevertheless, a comprehensive study of the correlation between direct contact mapping and underlying tissue alterations is still lacking.
Furthermore, recent epicardial mapping has shown that sinus rhythm (SR) patients with structural heart disease already harbour a substrate capable of maintaining AF.5 Indeed, several studies have also highlighted an abnormal substrate in pAF but epicardial mapping data in patients with paroxysmal AF are still scarce. Thus, the question remains whether the increase in AF complexity occurs already in patients with paroxysmal AF or only after AF has become persistent.
To address these questions, we evaluated the electrophysiological substrate in patients with acutely induced AF, with paroxysmal and with persistent AF using direct contact AF wave mapping algorithms.6 In addition, mapping data from the right atrium were compared with the histological features of the right atrial (RA) appendage in order to study the relative contribution of various structural features on the complexity of AF.
Methods
Patient population
Patients without a history of AF (n = 11, aAF), with pAF (n = 12) and longstanding persistent AF (persAF, n = 9) were included. In the aAF-group, preoperative AF was excluded using the medical history and rhythm monitoring (7 days, Vitaphone™). The Institutional Review board approved the study and informed consent was obtained from each patient (Dutch Trial register NTR1301).
Epicardial mapping and atrial fibrillation electrogram analysis
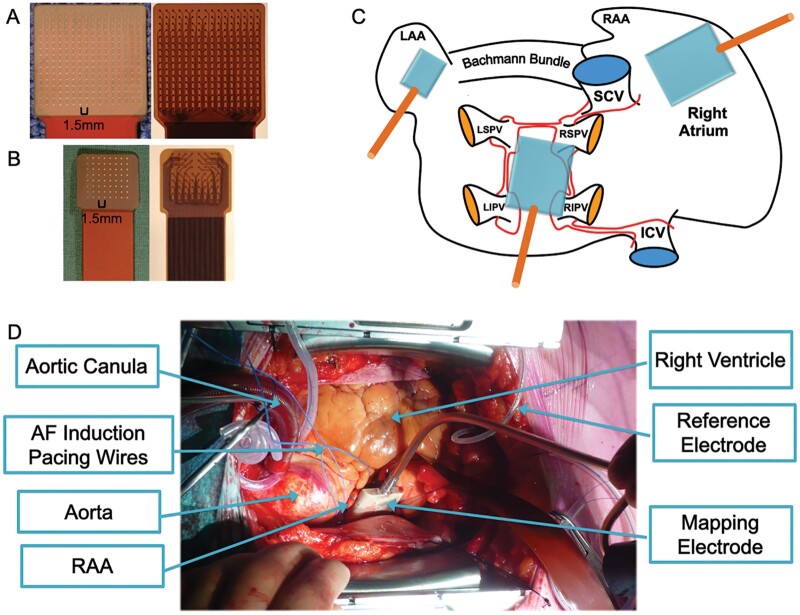
Epicardial high-density mapping of AF was performed using two custom-made single-use grid electrodes (inter-electrode distance 1.5 mm): a 256-channel electrode (22.5x22.5 mm) for mapping of the RA-free wall and posterior left atrium (pLA) and a 64-channel electrode (10.5x10.5 mm) for the left atrial appendage (LAA, Figure 1). Right atrial and pLA mapping was performed off-pump while LAA mapping was performed on-pump for hemodynamic reasons. Atrial filling pressure was preserved. If necessary, AF was induced via incremental pacing (3.3 Hz to 16.7 Hz). Recording of fibrillation electrograms (10 s; sampling rate 1 kHz; filtering bandwidth 0.5-500Hz) was started 30 s after AF induction and data were stored for off-line analysis. Unipolar AF electrogram analysis and wave mapping was performed using a fully automated analysis has been described in-depth elsewhere and we refer for detailed explanation to Zeemering et al.6 Briefly, electrogram preprocessing included filtering with a third order zero-phase Chebyshev 0.5 Hz high-pass filter to remove any baseline drift and removal of ventricular far-field deflections by ventricular R-wave detection followed by single-beat QRST-template cancellation. Intrinsic deflections—representing local activations underneath the electrode—were identified based on a recently validated probabilistic algorithm.6 Non-intrinsic deflections were considered as far field effects caused by fibrillation waves propagating in the close vicinity of the electrode. If they were larger than 25% of the median intrinsic deflection amplitude they were counted as fractionated activity. As an additional substrate parameter, the fractionation index (FI) was defined as the ratio of non-intrinsic to intrinsic deflections.
Figure 1.
Examples of the epicardial 256-channel (A) and 64-channel (B) mapping electrode. (C) Diagram of the atria with locations of electrode application. Larger electrode cartoons represent the 256 electrode, smaller electrode cartoon represents the 64 electrode (D). Illustrative view on the mapping procedure during cardiac surgery.
Atrial fibrillation wave construction consisted of three phases: (1) partial wave creation by identifying wave borders based on conduction block, defined by a local conduction velocity (CV)<20cm/s; (2) determining the distribution of CV and conduction deviation (tortuosity) within one wave; (3) allocation of unassigned deflections to adjacent waves based on a maximum local CV and conduction direction probability.6
These fibrillation waves were used to analyse the conduction characteristics during AF. The following electrophysiological wave parameters were determined: CV, AF cycle length, dominant frequency, number of waves and epicardial breakthroughs, wave width and FI. Atrial fibrillation complexity was defined as the number of fibrillation waves/AFCL, a parameter that correlates very well with the fractionation index.
Histological and immunohistochemical analysis
During surgery, RAA were harvested and stored in formaldehyde 0.4%. All analysis was undertaken in batches with the investigator blinded to the patient group.
Histology
After paraffin embedding and slicing, tissue sections were fixed at 56°C overnight, deparaffinized, and rehydrated. Two types of staining were used:
After Sirius red staining the overall myocardial connective tissue content was determined. Epicardial and perivascular fibrosis were blanked out and total myocardial fibrosis was colour-identified and quantified as the ratio of fibrosis to total tissue. The amount of fibrosis was averaged per patient (5–8 sections, 20× magnification).
On haematoxylin and eosin staining, cardiomyocyte diameter and intermyocyte distance (endomysial fibrosis) were determined. For the cell diameter, the shortest diameter was used in transversely cut cells to exclude errors related to oblique cross-sections through cells. All measurements were averaged per patient (12 ± 5 measurements/section, 5 sections/patient, 40x magnification). All measurements were performed with manually ImageJ (National Institutes of Health, USA) with the investigator blinded to the patient group.
Connexin 43 analysis
Immunostaining for connexin 43 (Cx43) in formalin fixed RAA was performed as described elsewhere.7 Briefly, expression of Cx43 at the lateral and polar membrane of myocytes was analysed in 2–3 sections per sample, and 5–10 cells per section, so that each data point is representing 100–250 cells. The Cx43-ratio ([lateral Cx43/polar Cx43] *100) was determined.
Statistical analysis
All data are expressed as mean ±SD if continuous, median ±interquartile ranges if ordinal and percentages if dichotomous. Significant difference in means was calculated using a Student’s t-test or one-way analysis of variance with Fisher’s least significant difference post hoc test. Significant difference in proportions was calculated with a χ2-test. Correlation between different parameters was calculated with bivariate (overall) and partial correlation (corrected for differences related to patient groups). P-values < 0.05 were considered statistically significant.
Results
Patient population
Patients with persAF and pAF were older than aAF patients (Table 1). Diabetes was present in 22% of patients. Overall, the difference in CHA2DS2-VASc score was not different (P = 0.09), but it was higher in persAF compared with pAF (P = 0.03). Also the type of surgery was not different between groups (P = 0.40). The indication for surgery in the lone AF patients was arrhythmia surgery for AF. Right atrial volume and LA diameter and volume were higher in persAF compared with aAF and pAF patients. When compared with pAF, patients with persAF were more frequently prescribed with digoxin, but less frequently with amiodarone or sotalol. Patients in persAF used more frequently diuretics preoperatively compared with patients in the aAF and pAF group. Patients with persAF were longer in AF than patients with pAF.
Table 1.
Patient characteristics
| Acute AF (n = 11) | Paroxysmal AF (n = 12) | Persistent AF (n = 9) | P-value | |
|---|---|---|---|---|
| Age (years) | 61.6 ± 9.3 | 68.6 ± 4.0* | 69.1 ± 5.8* | 0.03 |
| Male sex (%) | 72.7 | 75.0 | 55.6 | 0.63 |
| Clinical characteristics | ||||
| Hypertension (%) | 63.6 | 66.7 | 77.8 | 0.80 |
| Diabetes (%) | 18.2 | 8.3 | 44.4 | 0.14 |
| Peripheral arterial disease (%) | 27.3 | 16.7 | 22.2 | 0.84 |
| Myocardial infarction (%) | 36.4 | 25.0 | 11.1 | 0.46 |
| GFR < 60 mL/min (%) | 0 | 25.0 | 22.2 | 0.22 |
| BMI (kg/m2) | 26.9 ± 2.5 | 27.5 ± 5.1 | 26.9 ± 3.1 | 0.35 |
| NYHA class | 2 (2–3) | 2 (1–2.75) | 2 (2–3) | 0.63 |
| CHA2DS2-Vasc | 3 (3–4) | 3 (2–3.75) | 3 (3–6)§ | 0.09 |
| Surgery | 0.40 | |||
| CABG+MVS | 2 | – | – | |
| CABG | 7 | 8 | 4 | |
| MVS | 2 | 1 | 2 | |
| AVS | – | 2 | 2 | |
| Lone AF | – | 1 | 1 | |
| Echocardiography | ||||
| LA diameter (mm) | 43.0 ± 7.1 | 43.1 ± 7.9 | 53.1 ± 6.1*,§ | 0.005 |
| LA volume (cm3) | 87.4 ± 35.0 | 79.2 ± 26.1 | 151.0 ± 55.3*,§ | <0.001 |
| RA volume (cm3) | 47.9 ± 17.0 | 50.9 ± 25.7 | 102.8 ± 32.3*,§ | <0.001 |
| LVEF (%) | 55.5 ± 14.1 | 64.0 ± 8.7 | 61.4 ± 6.4 | 0.18 |
| Drugs | ||||
| Betablocker (%) | 72.7 | 83.3 | 77.8 | 0.84 |
| Digoxin (%) | – | 0 | 33.3§ | 0.01 |
| Amiodarone/sotalol (%) | – | 58.3 | 11.1§ | 0.001 |
| CCB (%) | 18.2 | 33.3 | 33.3 | 0.69 |
| AF duration (years) | – | 7.0 ± 6.9 | 9.6 ± 6.6§ | 0.004 |
AF, atrial fibrillation; AVS, aortic valve surgery; BMI, body mass index; CABG, coronary artery bypass grafting; CCB, Calcium channel blocker; GFR, glomerular filtration rate; LA(A), left atrial (appendage); LVEDD, left ventricular end diastolic diameter; MVS, mitral valve surgery; NYHA, New York Heart Association; RA, right atrial.
P < 0.05 vs. acute AF.
P < 0.05 vs. paroxysmal AF.
Epicardial mapping
Basic characteristics of atrial fibrillation
Overall, AF cycle length (AFCL) was shorter in persAF compared with pAF (169 ± 13 vs. 186 ± 36 ms, P < 0.05, see Supplementary material online, Table S1). Within each group, AFCL and dominant frequency were not different between locations. Per location, there were no significant differences in AFCL between the three groups. Dominant frequency was higher in persAF compared with aAF and pAF only in the LAA (6.2 ± 0.5 vs. 5.2 ± 1.1 Hz, P < 0.05, see Supplementary material online, Table S1). Conduction velocity was lower in persAF compared with aAF only in the RA (0.64 ± 0.04 vs. 0.75 ± 0.12 m/s, P < 0.05, see Supplementary material online, Table S1).
Parameters of atrial fibrillation conduction pattern
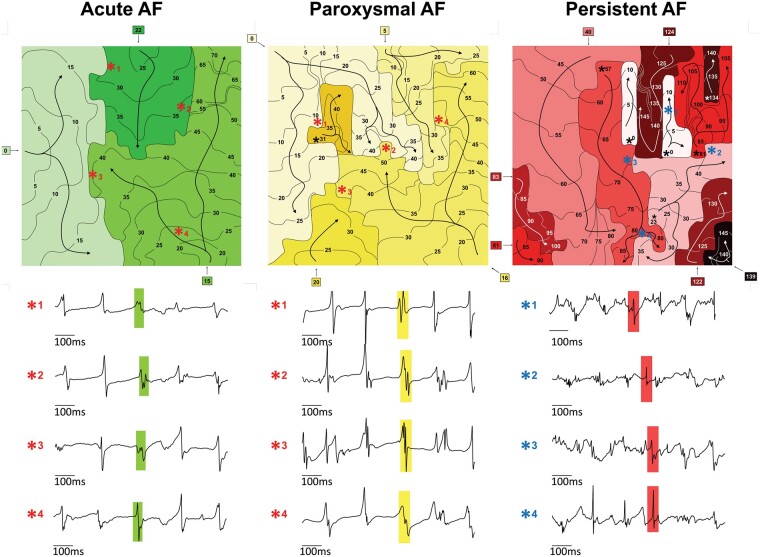
In Figure 2, representative isochronal maps in the pLA are depicted. The number of waves and breakthroughs was markedly higher in persAF compared with aAF and pAF. Moreover, the representative electrograms, corresponding to different locations on the map (asterisks), are clearly more fractionated in persAF than in aAF and pAF, supporting the presence of a more complex AF substrate.
Figure 2.
Representative isochrone maps of 1 AF beat in the posterior left atrium and the corresponding electrograms of patients with aAF, pAF, and persAF. Separate waves are coloured different shades of green for aAF, yellow for pAF and red for persAF. Electrograms are preceded by numbered asterisks that corresponds to the asterisks on the map. Four-way arrow symbols represent breakthrough conduction. AF, atrial fibrillation; aAF, acutely induced AF; pAF, paroxysmal AF; persAF, longstanding persistent AF.
The number of fibrillation waves per AF cycle (waves/cycle) and breakthroughs per cycle (BT/cycle) were significantly higher in persAF compared with aAF and pAF at the RA and pLA, but not at the LAA (Table 2). There were no differences in both parameters between pAF and aAF at any location. Furthermore, there were more waves/cycle and BT/cycle at the pLA than at the RA in aAF and persAF patients. Correspondingly, significantly smaller waves were present in persAF when compared with aAF or pAF for all 3 mapping sites. Fibrillatory waves were also smaller in pLA compared with RA for all groups. As the LAA was mapped with a smaller electrode, we did not directly compare LAA mapping data with other mapping sites, only between patient groups.
Table 2.
Parameters of the AF conduction pattern
| Acute AF (n = 11) | Paroxysmal AF (n = 12) | Persistent AF (n = 9) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| RA | pLA | LAA | RA | pLA | LAA | RA | pLA | LAA | |
| Waves/cycle | 5.7 ± 2.2 | 9.6 ± 3.6‡ | 2.8 ± 1.4 | 6.5 ± 2.7 | 8.5 ± 3.8 | 3.2 ± 0.7 | 11.2 ± 3.9*,§ | 17.9 ± 3.9*,§,‡ | 3.7 ± 1.1 |
| BT/cycle | 1.9 ± 0.9 | 4.2 ± 2.3‡ | 0.6 ± 0.6 | 2.4 ± 1.8 | 3.4 ± 2.3 | 0.7 ± 0.4 | 4.8 ± 2.3*,§ | 9.0 ± 2.6*,§,‡ | 0.9 ± 0.5 |
| Wave size (mm2) | 120.1 ± 75.9 | 65.6 ± 27.4‡ | 61.1 ± 30.5 | 98.8 ± 43.1 | 66.1 ± 23.8‡ | 46.4 ± 12.7 | 53.7 ± 20.0*,§ | 28.9 ± 8.4*,§,‡ | 34.0 ± 13.1*,§ |
| FI | 1.0 ± 0.4 | 2.5 ± 0.7‡ | 1.6 ± 1.0# | 1.3 ± 0.5 | 2.3 ± 1.1‡ | 2.9 ± 1.7*,‡ | 1.9 ± 0.8*,§ | 4.5 ± 1.1*,§,‡ | 4.0 ± 1.5*,‡ |
AF, atrial fibrillation; BT, breakthrough; FI, fractionation index; RA, right atrium; pLA, posterior left atrium; LAA, left atrial appendage.
P < 0.05 vs. acute AF.
P < 0.05 vs. paroxysmal AF.
P < 0.05 vs. RA.
P < 0.05 vs. PLA (only for FI).
To evaluate the difference in AF complexity within the groups of aAF, pAF and persAF, the variability in AF complexity is illustrated in a boxplot (Supplementary material online, Figure S1). In both atria, the variability in AF complexity appears to be higher in the pAF group than in the aAF and persAF group (coefficients of variation at the RA: 56.1%, 63.5% and 30.3% for aAF, pAF and persAF, respectively; coefficients of variation at the pLA: 36.7%, 58.4% and 21.0% for aAF, pAF and persAF, respectively).
Electrogram fractionation
The fractionation index (FI) was higher in the persAF group compared with the aAF group for all three locations and compared with pAF at the RA and pLA (Table 2). Only at the LAA, the FI was higher in the pAF group compared with the aAF group. When comparing between locations, the FI was consistently higher at the pLA when compared with the RA. At the LAA, the FI was higher than at the RA in the pAF and the persAF group, but not in the aAF group. In the aAF group, the FI was lower at the LAA compared with the PLA.
The correlation between the FI and AF complexity (expressed as waves/cycle) was very high in the RA and pLA (r = 0.90, P < 0.001; r = 0.84, P < 0.001, respectively, Supplementary material online, Table S2) and lower but still significant in the LAA (r = 0.40, P = 0.04). Even after correction for the effect of the individual group characteristics, the correlation remained significant in both the RA and the pLA (partial correlation, RA: r = 0.86, p < 0.01, pLA: r = 0.76, p < 0.01, LAA: 0.29, p = 0.14). The unipolar FI is therefore a strong surrogate parameter for the quantification of AF complexity.
Histological and immunohistochemical alterations
Myocyte dimensions
Myocyte diameters were significantly larger in persAF compared with aAF. Myocyte diameter and length did not show a significant correlation with AF complexity (see Supplementary material online, Tables S2 and S3).
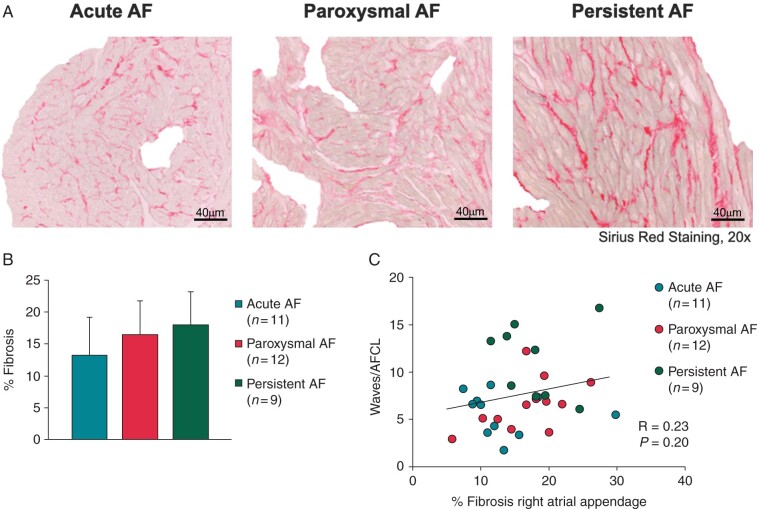
Total amount of fibrosis
Representative examples of Sirius red staining for patients with aAF, pAF and persAF are depicted in Figure 3A. There is a trend towards more fibrosis in the persAF compared with the aAF group (Figure 3B), however this difference was not significant (aAF = 13.4 ± 6.3%, pAF = 16.6 ± 5.5%, persAF = 18.0 ± 5.2%, P = 0.17). Moreover, no significant correlation was found between RA AF complexity (expressed as waves/cycle) and the degree of fibrosis (r = 0.23, P = 0.20, Figure 3C). Also after correction for group differences, no correlation was present (r = 0.06, P = 0.76, Supplementary material online, Table S1).
Figure 3.
(A) Representative pictures of Sirius red staining after exclusion of pericardial and perivascular fibrosis for aAF, pAF, and persAF. (B) Total amount of fibrosis (%) in the right atrial appendage. (C) Correlation between total amount of fibrosis (%) and AF complexity. AF, atrial fibrillation; aAF, acutely induced AF; pAF, paroxysmal AF; persAF, longstanding persistent AF.
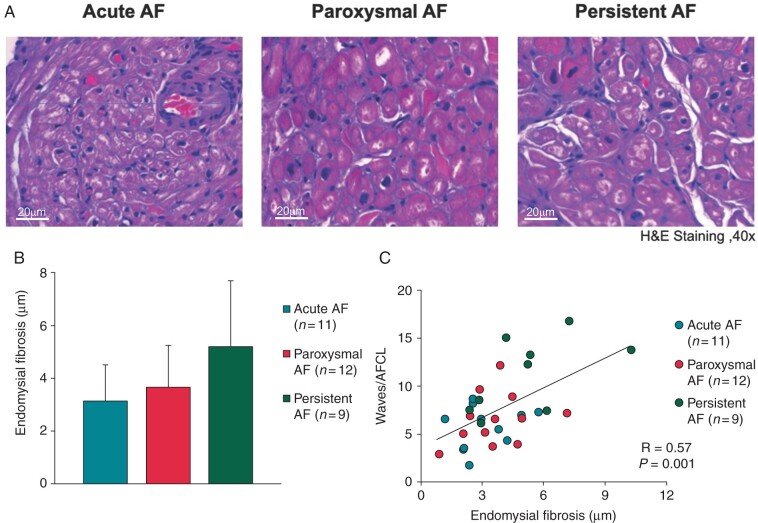
Endomysial fibrosis
Examples of endomysial fibrosis (fibrosis within bundles between myocytes) for all groups are shown in Figure 4A. The difference in endomysial fibrosis among groups almost reached statistical significance (aAF = 3.1 ± 1.4 μm, pAF = 3.7 ± 1.6 μm, persAF = 5.2 ± 2.5 μm, P = 0.054, Figure 4B). Importantly, a good correlation between RA AF complexity and the degree of endomysial fibrosis was present (r = 0.57, P = 0.001, Figure 4C). This correlation was still present after correction for group differences (r = 0.44, P = 0.01, Supplementary material online, Table S1).
Figure 4.
(A) Representative pictures of haematoxylin and eosin (H&E) staining with endomysial fibrosis (i.e. myocyte-to-myocyte distance) for aAF, pAF, and persAF. (B) Endomysial fibrosis in the right atrial appendage. (C) Correlation between endomysial fibrosis and AF complexity. AF, atrial fibrillation; aAF, acutely induced AF; pAF, paroxysmal AF; persAF, longstanding persistent AF.
Cx43 expression and distribution
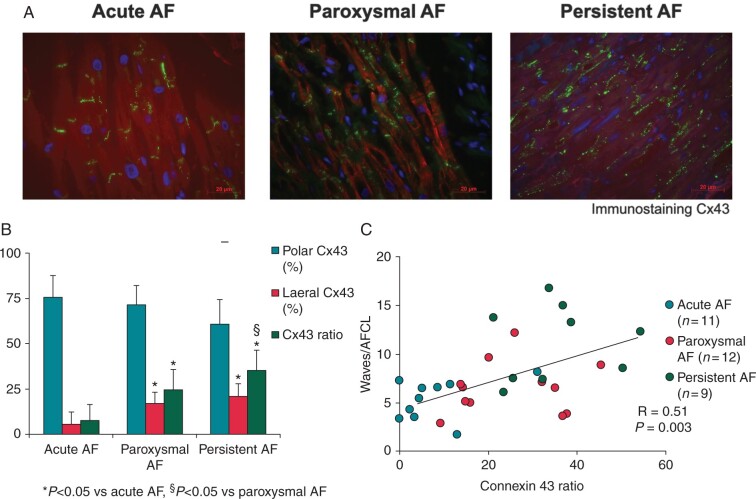
In Figure 5A, lateralization of Cx43 is shown in 3 representative examples of Cx43 immunostaining. A decreasing trend in polar Cx43 was present between the groups (aAF = 75.4 ± 12.1%, pAF = 71.3 ± 10.9%, persAF = 60.7 ± 13.7%, P = 0.058; Figure 5B). Also, both lateral Cx43 (aAF = 5.7 ± 6.5%, pAF = 16.9 ± 6.3%, persAF = 20.8 ± 7.1%, P < 0.001) and the Cx43-ratio (aAF = 7.5 ± 8.9, pAF = 24.7 ± 11.1, persAF = 35.1 ± 11.4, P < 0.001) were higher in pAF and persAF than in aAF (Figure 5B). The correlation between the Cx43 distribution and AF complexity (correlation r = 0.51, P = 0.003, Figure 5C) lost significance after correction for group differences (r = 0.14, P = 0.47, Supplementary material online, Table S1).
Figure 5.
(A) Representative pictures of immunostaining for Cx43 for aAF, pAF, and persAF. In aAF, Cx43 is mainly localized at the longitudinal junction between myocytes. In persAF, distribution of Cx43 is more lateralized than in aAF. (B) Amount of polar Cx43, lateral Cx43, and Cx43 ratio in right atrial appendages. (C) Correlation between the Cx43 ratio and AF complexity. AF, atrial fibrillation; aAF, acutely induced AF; Cx43, connexin 43; pAF, paroxysmal AF; persAF, longstanding persistent AF.
Discussion
Main findings
This study provides a direct comparison of structural abnormalities and AF conduction patterns between patients without a history of AF, patients with paroxysmal AF and patients with persistent AF.
The main findings of this study are as follows: (1) AF complexity was only increased in patients with persistent, but not in patients with paroxysmal AF. (2) Of all structural alterations we have investigated (gap junction remodelling, total fibrosis, myocyte size, endomysial fibrosis), only endomysial fibrosis and not overall myocardial fibrosis, was independently from group effects associated with AF complexity.
Paroxysmal atrial fibrillation in the process of atrial fibrillation progression
Since the description of focal pulmonary veins triggers initiating AF paroxysms, a huge number of patients have been treated with ablation procedures aimed at electrical isolation of the pulmonary veins. Several electrophysiological changes have been described in human pAF compared with control: prolonged signal-averaged p-wave durations,8 increased spatial dispersion of refractoriness,9 globally shortened refractoriness with increased spatial heterogeneity,10 and higher anisotropy indices.11 To date, however, detailed high-density epicardial mapping studies addressing the substrate in human pAF are very scarce. The conduction pattern in persAF is much more complex and is characterized by multiple narrow fibrillation waves, a high incidence of epicardial breakthroughs and highly fractionated electrograms.1 Allessie et al.12 demonstrated that longstanding persistent human AF is characterized by longitudinal electrical dissociation and a high incidence of epicardial breakthroughs. In a goat model of lone AF, we reported that AF persistence goes hand-in-hand with an increase in endo-epicardial dissociation of electrical activity, in three-dimensional conduction (breakthrough) and with rearrangement of atrial bundle anatomy and AF conduction.13,14 We and others could confirm endo-epicardial dissociation of electrical activity in human mapping studies.15,16 In high-density maps of human persistent AF, Lee et al.17 described activation patterns with multiple wavefronts and disorganized electrical activity, with only sporadic focal activity or rotating wavefronts.
In this study, we report comparable AF complexity in terms of numbers of fibrillation waves, epicardial breakthroughs and electrogram fractionation between patients without a history of AF and patients with pAF, supporting the importance of triggers in the occurrence of pAF. In persAF, these measures for AF complexity were much higher compared with aAF and pAF, and also higher in the pLA than in the RA. These data are consistent with other high-density human mapping studies.12,14
Atrial fibrillation complexity is higher in the pLA than at other mapping sites which might be explainable by the complex underlying anatomy.18 The RA-free wall consists of trabeculae known as pectinate muscles that run perpendicular to the terminal crest in a parallel fashion towards the vestibular portion and the RA appendage. These pectinate muscles are interspersed with very thin areas of the epicardial layer. This relative uniform organization in muscle fibres stands in contrast with the posterior left atrium. Although the posterior left atrium consists of a smooth atrial wall, it is not uniform in thickness or fibre direction, but has a complex architecture of overlapping myofibres of different orientation.18 It is therefore to be expected that endomysial fibrosis at this location will lead to more chaotic and complex conduction patters during AF.19
Loss of side-to-side cell coupling due to structural remodelling results in insulated muscle fibres that can promote conduction block and (micro-)reentry.18,20
Endomysial fibrosis as main determinant of conduction disturbances
Several structural alterations have been identified as possible determinants of conduction disturbances during AF. Both in animal and human studies, cellular hypertrophy has been associated with progression of AF.1,4,21 In our study, atrial myocyte diameter was larger in persAF patients than in aAF patients. This is in line with another study analysing appendages removed during Cox-maze surgery.21 Although myocyte hypertrophy occurs with persistence of AF, it was not associated with an increase in AF complexity and as such seems to play at most a bystander role in conduction disturbances.
Atrial fibrosis is often thought to play the most important role in the structural remodelling process of AF, however the relation between fibrosis and AF in experimental and human studies is not consistent.1 For example, the canine heart failure model is known to produce a high degree of (replacement) fibrosis, yet the AF conduction pattern in this model is relatively simple.22 In patients, Platonov et al.23 found more fibrosis in AF patients than control at different atrial locations, but found no clear correlation with AF duration. Anné et al.24 showed interstitial fibrosis to be associated with the underlying valvular disease rather than with the presence of AF. However, in this context it is important to differentiate reparative from reactive processes in the development of fibrosis.2 Reparative or replacement fibrosis develops following myocyte death for example caused by myocardial infarction, while the formation reactive or interstitial fibrosis is caused when stressors such as stretch or inflammation activate profibrotic signalling pathways in the absence of myocyte death. In cardiac tissue, interstitial fibrosis usually leads to collagen septa thickening both between muscle bundles (perimysial fibrosis) and myocytes (endomysial fibrosis), and therefore has a much larger effect on atrial conduction compared with replacement fibrosis.1,2 This is reflected in our findings. We neither found any differences in total amount of fibrosis between aAF, pAF, and persAF nor any correlation between total amount of fibrosis and AF complexity (independent of classifying patients in pAF or persAF). Instead, endomysial fibrosis correlated well with AF complexity. Importantly, this correlation was still present after correction for differences between the patient groups. Therefore, endomysial fibrosis rather than total amount of fibrosis plays an important role in the structural remodelling underlying conduction disturbance in fibrillating atria.
Finally, altered connexin expression might play a role in micro-reentry, however their exact contribution remains to be determined.1 Cx43 is usually localized at the end-to-end connections between myocytes, but in AF, translocation of Cx43 to the lateral membranes of the myocytes has been reported.7 Also in our study, a higher Cx43 ratio (lateral/polar) was found in persAF compared with aAF and pAF and also a higher Cx43 ratio in pAF compared with aAF. Furthermore, lateralization of Cx43 correlated overall with AF complexity and this change in connexin expression pattern may reduce conduction velocity and could contribute to enhanced incidence of conduction block.7
Limitations
Since this study is performed in patients undergoing cardiac surgery, not in ‘lone-AF’ patients, the results might be influenced by the underlying pathology. However, the study was not powered to show differences in the type of surgery between groups. Recording on the LAAs was performed on cardiopulmonary bypass but with preserved filling pressures. Initiation of cardiopulmonary bypass might alter cardiac pressures and therefore also AF conduction. Structural analysis was performed on RAA biopsies only. As such, no direct comparison of right and left atrial anatomical atrial wall structure could be performed and differences in AF complexity between RA and pLA could not be directly related to differences in atrial histology. Atrial histology at the RAA might be different from the pLA. However, fibrosis, total or endomysial, is a bi-atrial disease and AF conduction patterns in the RA also showed high AF complexity. Moreover, LAA biopsies might not be representative for the pLA, as electrical activation at the LAA is often very regular and far less fractionated than at the pLA due to underlying structural differences. The size of the mapping electrode did not allow us to simultaneously map the electrical activation of both atria during AF. However, the electrode did allow to map the entire pLA between the pulmonary veins, sequential mapping at different locations at the RA did not reveal differences in conduction pattern and no consecutive waves propagating in the same direction or linking was observed. Therefore, the constructed wave maps do represent the actual AF conduction pattern. Finally, our results are based on the analysis of unipolar atrial electrograms recorded at the epicardium only, with the potential bias of misrepresenting 3-D AF conduction patterns throughout the atrial wall. However, previous reports of our group and others13,16,25,26 studied simultaneous endo-epicardial mapping and showed no difference in AF complexity or fibrillatory conduction maps between the endocardial and epicardial layer.
Conclusions
In this in-depth electrophysiological and structural analysis of human AF, we were able to show that the complexity of the AF substrate in patients with pAF can be highly variable. High AF complexity appears to be present in patients with persistent AF only, but not in patients with paroxysmal AF. Structural abnormalities in lateralization of Cx43 and endomysial but not total amount of fibrosis may play an important role in the development of a substrate for AF.
Supplementary material
Supplementary material is available at Europace online.
Funding
This work was supported by the Netherlands Heart Foundation (CVON2014-09, RACE V Reappraisal of Atrial Fibrillation: Interaction between hyperCoagulability, Electrical remodelling, and Vascular Destabilisation in the Progression of AF), the European Union (ITN Network Personalize AF: Personalized Therapies for Atrial Fibrillation: a translational network, grant number 860974; CATCH ME: Characterizing Atrial fibrillation by Translating its Causes into Health Modifiers in the Elderly, grant number 633196; MAESTRIA: Machine Learning Artificial Intelligence Early Detection Stroke Atrial Fibrillation, grant number 965286). European Network for Translational Research in AF (FP7 collaborative project EUTRAF, No.261057), Netherlands Heart Foundation (CVON2014-09, RACE V), European Union (CATCH ME, No.633196; ITN Network AFib TrainNet, No.675351; ERACoSysMED H2020 ERA-NET Cofund project), and EU ITN Network RADOX, PITN-GA-2012–316738.
Conflict of interest: B.M. is a consultant for Medtronic and Atricure. M.L.M. is a consultant for Atricure. U.S. received consultancy fees or honoraria from Università della Svizzera Italiana (USI, Switzerland), Roche Diagnostics (Switzerland), EP Solutions Inc. (Switzerland), Johnson & Johnson Medical Limited, (United Kingdom), Bayer Healthcare (Germany). U.S. is co-founder and shareholder of YourRhythmics BV, a spin-off company of the University Maastricht. All remaining authors have declared no conflicts of interest.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
Contributor Information
Bart Maesen, Department of Cardio-Thoracic Surgery, Maastricht University Medical Center, Maastricht, The Netherlands; Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, The Netherlands.
Sander Verheule, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, The Netherlands; Department of Physiology, Maastricht University, Universiteitssingel 50, PO Box 616, 6200MD Maastricht, The Netherlands.
Stef Zeemering, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, The Netherlands; Department of Physiology, Maastricht University, Universiteitssingel 50, PO Box 616, 6200MD Maastricht, The Netherlands.
Mark La Meir, Department of Cardiac Surgery, UZ Brussels, Brussels, Belgium.
Jan Nijs, Department of Cardiac Surgery, UZ Brussels, Brussels, Belgium.
Stijn Lumeij, Department of Physiology, Maastricht University, Universiteitssingel 50, PO Box 616, 6200MD Maastricht, The Netherlands.
Dennis H Lau, Department of Physiology, Maastricht University, Universiteitssingel 50, PO Box 616, 6200MD Maastricht, The Netherlands.
Mathieu Granier, Department of Physiology, Maastricht University, Universiteitssingel 50, PO Box 616, 6200MD Maastricht, The Netherlands.
Harry Jgm Crijns, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, The Netherlands; Department of Cardiology, Maastricht University Medical Center, Maastricht, The Netherlands.
Jos G Maessen, Department of Cardio-Thoracic Surgery, Maastricht University Medical Center, Maastricht, The Netherlands; Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, The Netherlands.
Stefan Dhein, Department of Cardiac Surgery, Clinic for Cardiac Surgery, Heart Centre Leipzig, Leipzig, Germany.
Ulrich Schotten, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, The Netherlands; Department of Physiology, Maastricht University, Universiteitssingel 50, PO Box 616, 6200MD Maastricht, The Netherlands.
References
- 1. Schotten U, Verheule S, Kirchhof P, Goette A.. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev 2011;91:265–325. [DOI] [PubMed] [Google Scholar]
- 2. Winters J, von Braunmuhl ME, Zeemering S, Gilbers M, Brink TT, Scaf Bet al. JavaCyte, a novel open-source tool for automated quantification of key hallmarks of cardiac structural remodeling. Sci Rep 2020;10:20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pontecorboli G, Figueras I, Ventura RM, Carlosena A, Benito E, Prat-Gonzales S, Padeletti Let al. Use of delayed-enhancement magnetic resonance imaging for fibrosis detection in the atria: a review. Europace 2017;19:180–9. [DOI] [PubMed] [Google Scholar]
- 4. Verheule S, Tuyls E, Gharaviri A, Hulsmans S, van Hunnik A, Kuiper Met al. Loss of continuity in the thin epicardial layer because of endomysial fibrosis increases the complexity of atrial fibrillatory conduction. Circ Arrhythm Electrophysiol 2013;6:202–11. [DOI] [PubMed] [Google Scholar]
- 5. Kanagaratnam P, Kojodjojo P, Peters NS.. electrophysiological abnormalities occur prior to the development of clinical episodes of atrial fibrillation: observations from human epicardial mapping. Pacing Clin Electro 2008;31:443–53. [DOI] [PubMed] [Google Scholar]
- 6. Zeemering S, Maesen B, Nijs J, Lau DH, Granier M, Verheule S.. Automated quantification of atrial fibrillation complexity by probabilistic electrogram analysis and fibrillation wave reconstruction. Conf Proc IEEE Eng Med Biol Soc 2012;2012:6357–60. [DOI] [PubMed] [Google Scholar]
- 7. Polontchouk L, Haefliger JA, Ebelt B, Schaefer T, Stuhlmann D, Mehlhorn Uet al. Effects of chronic atrial fibrillation on gap junction distribution in human and rat atria. J Am Coll Cardiol 2001;38:883–91. [DOI] [PubMed] [Google Scholar]
- 8. Fukunami M, Yamada T, Ohmori M, Kumagai K, Umemoto K, Sakai Aet al. Detection of patients at risk for paroxysmal atrial fibrillation during sinus rhythm by P wave-triggered signal-averaged electrocardiogram. Circulation 1991;83:162–9. [DOI] [PubMed] [Google Scholar]
- 9. Ramanna H, Hauer RN, Wittkampf FH, de Bakker JM, Wever EF, Elvan Aet al. Identification of the substrate of atrial vulnerability in patients with idiopathic atrial fibrillation. Circulation 2000;101:995–1001. [DOI] [PubMed] [Google Scholar]
- 10. Kojodjojo P, Peters NS, Davies DW, Kanagaratnam P.. Characterization of the electroanatomical substrate in human atrial fibrillation: the relationship between changes in atrial volume, refractoriness, wavefront propagation velocities, and AF burden. J Cardiovasc Electrophysiol 2007;18:269–75. [DOI] [PubMed] [Google Scholar]
- 11. Wong CX, Stiles MK, John B, Brooks AG, Lau DH, Dimitri Het al. Direction-dependent conduction in lone atrial fibrillation. Heart Rhythm 2010;7:1192–9. [DOI] [PubMed] [Google Scholar]
- 12. Allessie MA, de Groot NM, Houben RP, Schotten U, Boersma E, Smeets JLet al. Electropathological substrate of long-standing persistent atrial fibrillation in patients with structural heart disease: longitudinal dissociation. Circ Arrhythm Electrophysiol 2010;3:606–15. [DOI] [PubMed] [Google Scholar]
- 13. Eckstein J, Maesen B, Linz D, Zeemering S, van Hunnik A, Verheule Set al. Time course and mechanisms of endo-epicardial electrical dissociation during atrial fibrillation in the goat. Cardiovasc Res 2011;89:816–24. [DOI] [PubMed] [Google Scholar]
- 14. Maesen B, Zeemering S, Afonso C, Eckstein J, Burton RA, van Hunnik Aet al. Rearrangement of atrial bundle architecture and consequent changes in anisotropy of conduction constitute the 3-dimensional substrate for atrial fibrillation. Circ Arrhythm Electrophysiol 2013;6:967–75. [DOI] [PubMed] [Google Scholar]
- 15. Gharaviri A, Bidar E, Potse M, Zeemering S, Verheule S, Pezzuto Set al. Epicardial fibrosis explains increased endo-epicardial dissociation and epicardial breakthroughs in human atrial fibrillation. Front Physiol 2020;11:68. 10.3389/fphys.2020.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Groot N, van der Does L, Yaksh A, Lanters E, Teuwen C, Knops Pet al. Direct proof of endo-epicardial asynchrony of the atrial wall during atrial fibrillation in humans. Circulation Arrhythmia and Electrophysiology 2016;9:e003648. 10.1161/circep.115.003648. [DOI] [PubMed] [Google Scholar]
- 17. Lee G, Kumar S, Teh A, Madry A, Spence S, Larobina Met al. Epicardial wave mapping in human long-lasting persistent atrial fibrillation: transient rotational circuits, complex wavefronts, and disorganized activity. Eur Heart J 2014;35:86–97. [DOI] [PubMed] [Google Scholar]
- 18. Ho SY, Sánchez-Quintana D.. The importance of atrial structure and fibers. Clin Anat 2009;22:52–63. [DOI] [PubMed] [Google Scholar]
- 19. Hocini M, Ho SY, Kawara T, Linnenbank Andre C, Potse M, Shah Det al. Electrical conduction in canine pulmonary veins: electrophysiological and anatomic correlation. Circulation 2002;105:2442–8. [DOI] [PubMed] [Google Scholar]
- 20. Spach MS, Boineau JP.. Microfibrosis produces electrical load variations due to loss of side-to-side cell connections; a major mechanism of structural heart disease arrhythmias. Pacing Clin Electrophysiol 1997;20:397–413. [DOI] [PubMed] [Google Scholar]
- 21. Castonguay MC, Wang Y, Gerhart JL, Miller DV, Stulak JM, Edwards WDet al. Surgical pathology of atrial appendages removed during the Cox-Maze procedure: a review of 86 cases (2004 to 2005) with implications for prognosis. Am J Surg Pathol 2013;37:890–7. [DOI] [PubMed] [Google Scholar]
- 22. Li D, Fareh S, Leung TK, Nattel S.. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation 1999;100:87–95. [DOI] [PubMed] [Google Scholar]
- 23. Platonov PG, Mitrofanova LB, Orshanskaya V, Ho SY.. Structural abnormalities in atrial walls are associated with presence and persistency of atrial fibrillation but not with age. J Am Coll Cardiol 2011;58:2225–32. [DOI] [PubMed] [Google Scholar]
- 24. Anné W, Willems R, Roskams T, Sergeant P, Herijgers P, Holemans Pet al. Matrix metalloproteinases and atrial remodeling in patients with mitral valve disease and atrial fibrillation. Cardiovasc Res 2005;67:655–66. [DOI] [PubMed] [Google Scholar]
- 25. Eckstein J, Schotten U.. Rotors and breakthroughs as three-dimensional perpetuators of atrial fibrillation. Cardiovasc Res 2012;94:8–9. [DOI] [PubMed] [Google Scholar]
- 26. Eckstein J, Zeemering S, Linz D, Maesen B, Verheule S, van Hunnik Aet al. Transmural conduction is the predominant mechanism of breakthrough during atrial fibrillation: evidence from simultaneous endo-epicardial high-density activation mapping. Circ Arrhythm Electrophysiol 2013;6:334–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.