Abstract
Aims
Complexity of the ventricular tachycardia (VT) substrate and the size and thickness of infarction area border zones differ based on location of myocardial infarctions (MIs). These differences may translate into heterogeneity in the effectiveness of treatments. This study aims to examine the influence of infarct location on the effectiveness of VT ablation in comparison with escalated pharmacological therapy in patients with prior MI and antiarrhythmic drug (AAD)-refractory VT.
Methods and results
VANISH trial participants were categorized based on the presence or absence of an inferior MI scar. Inverse probability of treatment weighted Cox models were calculated for each subgroup. Of 259 randomized patients (median age 69.8 years, 7.0% women), 135 had an inferior MI and 124 had a non-inferior MI. Among patients with an inferior MI, no statistically significant difference in the composite primary outcome of all-cause mortality, appropriate implantable cardioverter-defibrillator (ICD) shock, and VT storm was detected between treatment arms [adjusted hazard ratio (aHR) 0.80, 95% confidence interval (CI) 0.51–1.20]. In contrast, patients with non-inferior MIs had a statistically significant reduction in the incidence of the primary outcome with ablation (aHR 0.48, 95% CI 0.27–0.86). In a sensitivity analysis of anterior MI patients (n = 83), a trend towards a reduction in the primary outcome with ablation was detected (aHR 0.50, 95% CI 0.23–1.09).
Conclusion
The effectiveness of VT ablation versus escalated AADs varies based on the location of the MI. Patients with MI scars located only in non-inferior regions of the ventricles derive greater benefit from VT ablation in comparison to escalation of AADs in reducing VT-related events.
Keywords: Ventricular tachycardia, Catheter ablation, Antiarrhythmic drugs, Myocardial infarction
What’s new?
Patients with prior myocardial infarction (MI) and drug-refractory ventricular tachycardia (VT) have different characteristics based upon the infarction location.
Patients with non-inferior wall infarctions were more likely to be female, have heart failure, and renal dysfunction, while those with inferior infarctions were more likely to have diabetes and hypertension.
Patients presenting with a prior inferior MI and drug-refractory vVT were more likely to have presented with VT despite sotalol, while those with prior non-inferior MI were more likely to have presented with VT despite amiodarone.
Patients with non-inferior infarction had greater benefit from catheter ablation in comparison to escalated antiarrhythmic drug (AAD) therapy in terms of reductions in the composite outcome of death, appropriate implantable cardioverter-defibrillator (ICD) shock, and VT storm. In contrast, the difference in VT event-free survival between treatment arms was not statistically significant for patients with an inferior infarction.
Introduction
In recent years, catheter ablation for ventricular tachycardia (VT) has emerged as a safe and efficacious treatment option for VT patients with a previous myocardial infarction (MI), in which antiarrhythmic drugs (AADs) have been ineffective to prevent VT recurrences.1–4 Compared to implantable cardioverter-defibrillator (ICD) therapy alone, catheter ablation decreased appropriate ICD shocks by 39–65% in post-MI patients.5–7 In the Ventricular tachycardia AblatioN versus escalated antiarrhythmic drug therapy in ISchemic Heart disease (VANISH) trial, catheter ablation improved VT event-free survival by 28% compared to escalated AAD therapy.8 As a result, current clinical guidelines recommend catheter ablation as second-line therapy for post-MI patients with AAD-refractory VT.1–4
Despite significant improvements in VT event-free survival with catheter ablation among the high-risk post-MI population, 59.1% of ablation patients of the VANISH trial had a VT recurrence or died.8 The substantial residual risk of events among ablation patients raises the possibility that a proportion of patients may receive differential benefit from ablation. A potential source of heterogeneity may be variability in the ventricular scar due to the location of the MI. Complexity of the VT substrate, technical aspects of catheter manipulation and tip contact, and the size and thickness of infarction area border zones differ based on location of MI.9–12 These differences may translate into a VT substrate that is more susceptible to VT recurrences or less responsive to treatment with catheter ablation. Small observational studies suggest that VT from inferior MIs have higher risk of early recurrence despite smaller infarct areas.10,12–14 However, the differential effectiveness of VT treatments based on the location of MI has not been definitively established.
As the utilization of VT ablation among post-MI patients has increased almost three-fold over the last decade,15 identification of patients who derive the most benefit from ablation is warranted. The MI scar harbours the substrate for VT and is the target of ablation lesions such that the location of the MI, specifically inferior wall MI, may be a plausible effect modifier for the association between catheter ablation and VT event-free survival. Thus, the objective of this sub-study of the VANISH randomized trial was to compare the effectiveness of VT ablation among those with and without inferior MI in reducing the composite endpoint of all-cause mortality, VT storm, or appropriate ICD therapy when compared with escalated AAD therapy in VT patients with a prior MI.
Methods
Clinical trial
The VANISH trial was a multicentre (22 centres) randomized controlled trial which compared the incidence of VT event-free survival (composite of all-cause mortality, appropriate ICD shock, and VT storm) among patients with a prior MI randomized to ablation or escalated AAD therapy.8 Patients were enrolled from July 2009 to November 2014. Enrolled patients had a prior MI, an ICD implant, and a monomorphic sustained VT (<250 b.p.m.) event during treatment with amiodarone or another Class I or III antiarrhythmic drug (AAD) during a 6-month period prior to enrolment. Patients were randomized 1:1 to ablation or escalated therapy with a block randomization design. Randomization was stratified by centre and AAD (amiodarone or other AAD) during the qualifying VT event.
Patients randomized to escalated therapy were treated with amiodarone alone or amiodarone and mexiletine, dependent on AAD and dose during qualifying VT event. Patients who experienced the qualifying VT while treated with a low dose of amiodarone (<300 mg per day) were prescribed a loading dose of 400 mg twice daily for 2 weeks, followed by 400 mg once daily for 1 week, and 300 mg once daily for the remainder of the study follow-up. Patients who had the qualifying VT during treatment with a high dose of amiodarone (≥300 mg per day) continued amiodarone treatment with the addition of mexiletine (200 mg three times daily). Patients whose qualifying VT event was during treatment with another AAD (i.e. not amiodarone) were prescribed a loading dose of amiodarone at 400 mg twice daily for 2 weeks, followed by 400 mg daily for 4 weeks, and 200 mg daily for the remainder of the study.
Patients randomized to catheter ablation underwent a standardized endocardial ablation procedure to target all inducible VTs. Ablations were performed within 14 days after randomization.
Detailed descriptions of the trial design, eligibility criteria, ablation procedure, and protocol have been previously published.8 The VANISH trial was approved by institutional review boards at all 22 participating centres.
Subgroups by location of infarction
VANISH trial participants were categorized based on the presence or absence of MI scar in the inferior wall of the left ventricle (LV). Location of MI scar was identified from medical history (clinical MI localization). Patients were allocated to (i) inferior and (ii) non-inferior MI subgroups based on the presence or absence of an inferior MI, respectively. As some patients had multiple infarct locations, patients in the inferior MI subgroup often had additional infarcts in non-inferior regions of the LV. However, the non-inferior MI subgroup had no patients with an inferior infarct.
A sensitivity analysis was conducted on a subgroup of patients with anterior MIs. The anterior MI subgroup included patients with isolated anterior infarctions, as well as additional infarctions in inferior and other non-inferior regions of the LV.
Inverse probability of treatment weighting
In the VANISH trial, randomization was not stratified based on the MI location. As such, the balance of patient characteristics from randomization was not maintained,16–20 and patient characteristics were unbalanced between treatment arms (ablation vs. escalated drug therapy) within each MI location subgroup. To balance baseline characteristics between treatment arms, inverse probability of treatment weights (IPTW) were calculated within each MI location subgroup as the inverse propensity for being randomized to ablation (fitted multivariable logistic regression). Predefined clinically important patient characteristics were incorporated into the propensity score model, which included age, sex, comorbidities, heart rate, type of ICD, number of ICD shocksand antitachycardia pacing (ATP) within the 3 months prior to randomization, medications, and the location of additional infarctions. Location of additional infarctions was included in the propensity score to isolate the effect of the specific infarct location investigated (i.e. inferior, non-inferior, anterior) on the hazards of the outcomes. Continuous variables, such as age, were included as linear variables in propensity score models (lowest deviance compared to continuous variables modelled flexibly).21,22 Weights were stabilized and the average treatment effect in the treated was estimated.23–26
Outcomes
The primary outcome investigated was a composite of all-cause mortality, appropriate ICD shock after 30 days of treatment, and VT storm (≥3 documented VTs within 24 h) after 30 days of treatment. Secondary outcomes were the components of the primary outcome. All VT events were adjudicated by two blinded members of the independent adjudication committee. Further details regarding outcomes in the VANISH trial have previously been described elsewhere.8
Statistical analyses
Due to the small sample sizes and use of propensity score methods, standardized mean differences (SMDs) were calculated to assess patient characteristics between treatment arms within each MI location subgroup (before and after IPTW), as well as to compare characteristics between patients with infarcts in different regions of the LV. Standardized mean differences <10% denoted that the distribution of patient characteristics was balanced between groups.
All analyses were performed within the intention-to-treat (ITT) framework and associations between catheter ablation and outcomes were evaluated with time-to-event analyses. Individual event time was defined as the time from index date to the date of the first event of interest or till November 2015 for patients with no events during the study period (right censored). Crude cumulative incidence rates were calculated as the number of events per 100 person-years. Cox proportional hazards models were weighted (IPTW) and adjusted for covariates that were not balanced after IPTW (doubly robust). For non-fatal secondary outcomes, the competing risk of all-cause mortality was accounted for using the Lunn–McNeil (cause-specific) extension of the Cox model. Adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) were calculated separately for each MI location subgroup and for each outcome investigated. All 95% CIs were calculated with 1000 bootstrap resampling. In addition, weighted and adjusted Kaplan–Meier curves were created for the primary composite outcome and all-cause mortality. Cumulative incidence function (CIF) curves accounting for the competing risk of all-cause mortality were created for the component outcomes of appropriate ICD shock and VT storm.
Additional sensitivity analyses included (i) a subgroup analysis of patients with an inferior MI only and (ii) stratified analyses by AAD at baseline (amiodarone or sotalol) within inferior and non-inferior MI subgroups. Patients in the inferior MI only subgroup had no additional infarctions in other locations of the LV.
For the analyses, Stata statistical software (Version 16, StataCorp, College Station, TX, USA) and R version 3.6.0 (RStudio, Inc., Boston, MA, USA) were used.
Results
Patient characteristics
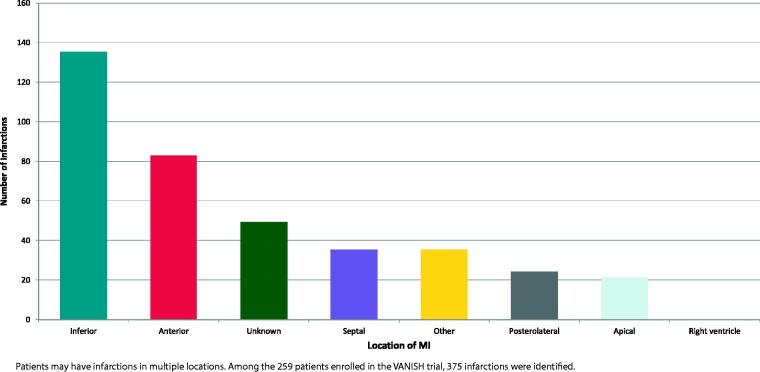
Of 259 patients enrolled in the VANISH trial (median age 69.8 [interquartile range (IQR) 63.0–74.2] years, 7.0% women), 135 patients had an inferior MI and 124 patients a non-inferior MI. Patients often had infarctions in multiple locations in the LV, with a total of 375 MI locations identified (Figure 1). Inferior infarctions were the most common (36%) (Figure 1).
Figure 1.
Locations of myocardial infarctions. MI, myocardial infarction.
Compared to patients with inferior MIs, patients with non-inferior MIs were more likely to be women (5.2% vs. 8.9%) and have congestive heart failure (55.9% vs. 73.7%) and renal insufficiency (16.3% vs. 21.8%) (Table 1; inferior vs. non-inferior, respectively; SMDs >10% for all). However, inferior MI patients had a higher prevalence of comorbid disease, such as diabetes (32.6% vs. 26.6%), hypertension (73.3% vs. 65.3%), and VF arrests (17.9% vs. 11.4%) within 6 months prior to enrolment (Table 1; inferior vs. non-inferior, respectively; SMDs > 10% for all).
Table 1.
Comparison of patient characteristics by infarct location (crude)
| Patient characteristics | Inferior infarct (N = 135), N (%) | Non-inferior infarct (N = 124), N (%) | Sensitivity: anterior infarcts (N = 83), N (%) |
|---|---|---|---|
| Age (years)a | 68.9 ± 7.5 | 68.3 ± 8.8 | 67.2 ± 9.1 |
| Women | 7 (5.2) | 11 (8.9)b | 7 (8.4)b |
| Time since MI (years)a | 15.6 ± 9.4 | 15.7 ± 9.7 | 15.1 ± 7.9 |
| Coronary artery disease | 126 (93.3) | 106 (85.5)b | 74 (89.2)b |
| Prior PCI | 59 (43.7) | 53 (42.7) | 34 (41.0) |
| Prior CABG | 59 (43.7) | 59 (47.6) | 33 (39.8) |
| Diabetes | 44 (32.6) | 33 (26.6)b | 16 (19.3)b |
| Hypertension | 99 (73.3) | 81 (65.3)b | 55 (66.3)b |
| Renal insufficiency | 22 (16.3) | 27 (21.8)b | 18 (21.7)b |
| Atrial fibrillation/flutter | 51 (37.8) | 48 (38.7) | 30 (36.1) |
| Hypercholesterolaemia | 124 (91.9) | 96 (77.4)b | 67 (80.7)b |
| Stroke/TIA | 15 (11.1) | 22 (17.7)b | 14 (16.9)b |
| VF arrest | 20 (17.9) | 13 (11.4)b | 7 (9.2)b |
| SVT | 11 (8.2) | 12 (9.7) | 8 (9.6) |
| Torsades de pointes | 1 (0.7) | 0 (0.0)b | 0 (0.0)b |
| Heart rate (b.p.m.)a | 62.9 ± 9.7 | 63.3 ± 11.5 | 63.9 ± 11.2 |
| CHF | 62 (55.9) | 84 (73.7)b | 51 (70.8)b |
| NYHA class | |||
| I | 35 (25.9) | 27 (21.8) | 17 (20.5)b |
| II | 70 (51.9) | 66 (53.2) | 45 (54.2) |
| III | 30 (22.2) | 31 (25.0) | 21 (25.3) |
| Ejection fraction (%)a | 37.5 ± 8.8 | 33.0 ± 11.7b | 29.7 ± 11.3b |
| Location of infarctions | |||
| Inferior | 135 (100.0) | 0 (0.0)b | 23 (27.7) |
| Anterior | 23 (17.0) | 60 (48.4)b | 83 (100.0)b |
| Septal | 15 (11.1) | 20 (16.1)b | 26 (31.3)b |
| Posterolateral | 21 (15.6) | 3 (2.4)b | 3 (3.6)b |
| Apical | 17 (12.6) | 4 (3.2)b | 9 (10.8) |
| Right ventricle | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Unknown | 0 (0.0) | 49 (39.5)b | 0 (0.0) |
| Other | 13 (9.6) | 15 (12.1) | 8 (9.6) |
| ICD characteristics | |||
| Indication for ICD | |||
| Primary prevention | 34 (25.2) | 60 (48.4)b | 39 (47.0)b |
| Secondary prevention | 101 (74.8) | 64 (51.6)b | 44 (53.0)b |
| ICD | |||
| Single chamber | 49 (36.3) | 38 (30.7)b | 32 (38.6) |
| Dual chamber | 64 (47.4) | 57 (46.0) | 30 (36.1)b |
| CRT-D | 22 (16.3) | 29 (23.4)b | 21 (25.3)b |
| Number of shocks (3 months prior)a | 1.4 ± 1.7 | 1.6 ± 3.2 | 1.6 ± 2.4 |
| Number of ATP (3 months prior)a | 20.0 ± 63.4 | 15.6 ± 30.1 | 14.5 ± 27.0b |
| VT below detection (3 months prior)a | 0.3 ± 0.5 | 0.4 ± 0.5b | 0.4 ± 0.5b |
| Medications at randomization | |||
| AADsc | |||
| Amiodarone | 80 (59.3) | 89 (72.4)b | 63 (75.9)b |
| <300 mg/day | 68 (86.1) | 82 (81.1)b | 57 (89.1) |
| ≥300 mg/day | 11 (13.9) | 8 (8.9)b | 7 (10.9) |
| Sotalol | 55 (40.7) | 34 (27.6)b | 20 (24.1)b |
| Procainamide | 0 (0.0) | 1 (0.8) | 0 (0.0) |
| Beta blockers | 91 (67.4) | 104 (83.9)b | 67 (80.7)b |
| ACE inhibitor | 89 (65.9) | 79 (63.7) | 51 (61.5) |
| ARB | 31 (23.0) | 28 (22.6) | 27 (32.5)b |
| Diuretics | 85 (63.0) | 94 (75.8)b | 59 (71.1)b |
| Digoxin | 24 (17.8) | 28 (22.6)b | 21 (25.3)b |
| Clopidogrel | 29 (23.6) | 23 (21.7) | 23 (30.7) |
| Aspirin | 100 (80.7) | 81 (79.3) | 58 (77.3) |
| Calcium channel blocker | 23 (17.0) | 10 (8.1)b | 10 (12.1)b |
| Warfarin | 39 (31.5) | 50 (46.7)b | 33 (43.4)b |
| Lab measurementsa | |||
| Creatinine | 105.3 ± 32.0 | 117.1 ± 38.1b | 113.4 ± 34.8b |
| Sodium (mmol/L) | 138.7 ± 3.3 | 138.1 ± 3.1b | 138.3 ± 3.2b |
| Potassium (mmol/L) | 4.3 ± 0.4 | 4.3 ± 0.4 | 4.3 ± 0.4 |
| NT-proBNP (pg/ml) | 934.5 ± 1101.6 | 1034.7 ± 1054.1 | 1326.9 ± 1325.6b |
AADs, antiarrhythmic drugs; ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blocker; ATP, antitachycardia pacing; CABG, coronary artery bypass graft; CHF, congestive heart failure; CRT-D, cardiac resynchronization therapy defibrillator; ICD, implantable cardioverter-defibrillator; MI, myocardial infarction; NT-proBNP, N-terminal-proB-type natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; SMD, standardized mean differences; SVT, supraventricular tachycardia; TIA, transient ischemic attack; VF, ventricular fibrillation; VT, ventricular tachycardia.
Presented as a mean (±standard deviation).
SMD > 10% are considered unbalanced. SMDs compared noni-inferior and anterior infarct patients to inferior infarct patients.
AADs taken within 4 weeks prior to randomization were included.
At baseline, a statistically significantly higher proportion of patients with non-inferior MIs were prescribed amiodarone (59.3% vs. 72.4%); while inferior MI patients were more likely to be prescribed sotalol (40.7% vs. 27.6%) (Table 1; inferior vs. non-inferior, respectively; SMDs > 10% for all).
Within each MI location subgroup, patient characteristics were not balanced between treatment arms before IPTW (Supplementary material online, Table S1). However, after IPTW, most patient characteristics were balanced between treatment arms (SMD < 10%; Table 2 and Figure 2A–B).
Table 2.
Inverse probability of treatment weighted patient characteristics by infarct location
| Patient characteristics | Inferior infarct | Non-inferior infarct | Sensitivity: anterior infarct | |||
|---|---|---|---|---|---|---|
| Catheter ablation (N = 63) | Escalated therapy (N = 72) | Catheter ablation (N = 69) | Escalated therapy (N = 55) | Catheter ablation (N = 46) | Escalated therapy (N = 37) | |
| Age (years)b | 68.4 ± 7.9 | 67.8 ± 8.0 | 66.1 ± 6.8 | 67.2 ± 7.3 | 64.6 ± 9.3 | 65.3 ± 9.1 |
| Women | 8% | 7% | 6% | 4% | 0.3% | 0.0% |
| Hypertension | 70% | 69% | 64% | 65% | 67% | 69% |
| Diabetes mellitus | 32% | 38%a | 24% | 22% | 15% | 14% |
| Coronary artery disease | 97% | 97% | 70% | 74% | 97% | 95% |
| Renal insufficiency | 20% | 18% | 17% | 19% | 12% | 12% |
| Atrial fibrillation/flutter | 38% | 42% | 42% | 52%a | 48% | 56%a |
| Prior stroke/TIA | 10% | 12% | 8% | 7% | 8% | 7% |
| SVT | 8% | 9% | 16% | 0%a | 15% | 13% |
| Torsades de pointes | 0% | 1% | 0% | 0% | 0% | 0% |
| Heart rate (b.p.m.)b | 63.5 ± 9.6 | 63.4 ± 10.1 | 63.2 ± 11.5 | 61.3 ± 11.4 | 63.4 ± 10.5 | 65.1 ± 10.9 |
| NYHA class | ||||||
| I | 28% | 24% | 16% | 16% | 28% | 30% |
| II | 55% | 58% | 53% | 51% | 14% | 16% |
| III | 17% | 18% | 31% | 33% | 58% | 54% |
| Location of infarctions | ||||||
| Inferior | 100% | 100% | 0% | 0% | 15% | 12% |
| Anterior | 14% | 18% | 46% | 48% | 100% | 100% |
| Septal | 13% | 13% | 18% | 17% | 10% | 7% |
| Posterolateral | 19% | 17% | 2% | 1% | 4% | 0%a |
| Apical | 17% | 18% | 6% | 5% | 12% | 12% |
| RV | 0% | 0% | 0% | 0% | 0% | 0% |
| Unknown | 0% | 0% | 0% | 0% | 0% | 0% |
| Other | 10% | 10% | 9% | 11% | 9% | 8% |
| ICD characteristics | ||||||
| ICD | ||||||
| Single chamber | 42% | 43% | 22% | 25% | 21% | 19% |
| Dual chamber | 44% | 44% | 48% | 44% | 42% | 40% |
| CRT-D | 14% | 13% | 30% | 31% | 37% | 41% |
| Mean number of shocks (3 months prior)b | 1.4 ± 1.2 | 1.6 ± 1.3 | 1.24 ± 3.3 | 0.71 ± 3.5a | 1.3 ± 2.9 | 1.2 ± 2.4 |
| Mean number ATP (3 months prior)b | 12.0 ± 29.4 | 13.3 ± 26.9 | 8.6 ± 19.5 | 9.0 ± 17.7 | 19.2 ± 26.9 | 13.5 ± 28.0a |
| Medications | ||||||
| AADsc | ||||||
| Amiodarone | 55% | 51% | 100% | 98% | 89% | 86% |
| <300 mg/day | 49% | 48% | 12% | 10% | 6% | 9% |
| ≥300 mg/day | 6% | 3% | 88% | 88% | 83% | 77% |
| Sotalol | 45% | 49% | 0% | 2% | 11% | 14% |
| Beta blockers | 63% | 62% | 96% | 96% | 97% | 97% |
| Digoxin | 22% | 26% | 18% | 15% | 24% | 23% |
AADs, antiarrhythmic drugs; ATP, antitachycardia pacing; CRT-D, cardiac resynchronization therapy defibrillator; ICD, implantable cardioverter-defibrillator; NYHA, New York Heart Association; RV, right ventricle; SVT, supraventricular tachycardia; TIA, transient ischemic attack
SMD >10% are considered unbalanced. SMDs compared ablation patients to escalated therapy patients.
Presented as a mean (±standard deviation).
AADs taken within 4 weeks prior to randomization were included.
Figure 2.
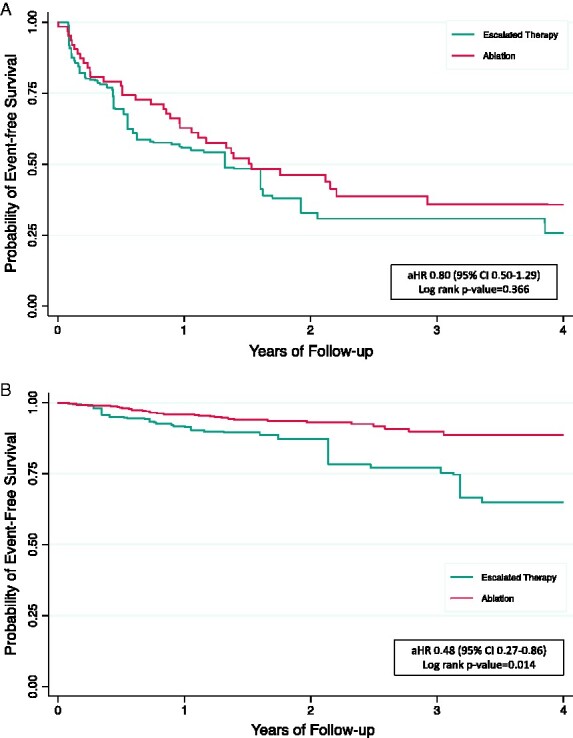
Kaplan–Meier curves for primary composite outcome. (A) Patients with inferior infarctions (N = 135); (B) patients with non-inferior infarctions (N = 124). aHR, adjusted hazard ratio; CI, confidence interval; IPTW, inverse probability of treatment weighting; SVT, supraventricular tachycardia. aKaplan–Meier curves were IPTW and adjusted for the presence of diabetes mellitus. bKaplan–Meier curves were IPTW and adjusted for the presence of SVT and the number of shocks within the 3 months prior to randomization.
Catheter ablation procedure by location of myocardial infarction
Among the 174 patients who underwent ablation, patients with an inferior MI were more likely to have inducible VT (95% vs. 86.8%) and activation mapping performed (45% vs. 33.8%), and had a higher number of clinical VTs identified pre-ablation [means: 1.5 (SD 1.0) vs. 1.3 (SD 0.7)] compared with patients with non-inferior MIs (Table 3; inferior vs. non-inferior, respectively; SMDs > 10% for all). During the ablation procedure, non-inferior MI patients had a statistically significantly longer duration of radiofrequency (RF) time, more RF applications, and greater number of VTs interrupted with RF (Table 3; SMDs > 10% for all). Of the 79.5% of patients who underwent post-ablation induction testing, non-inducible arrhythmias were more prevalent among non-inferior MI patients (41.7% vs. 56.9%) and statistically more clinical VTs were still inducible in inferior MI patients (6.3% vs. 1.7%) (Table 3; inferior vs. non-inferior, respectively; SMDs > 10% for all).
Table 3.
Comparison of ablation procedure by infarct location (crude)
| Procedure variable | Inferior infarct (N = 61), N (%) | Non-inferior infarct (N = 68), N (%) | Sensitivity: anterior infarct (N = 46), N (%) |
|---|---|---|---|
| General anaesthesia used | 13 (21.3) | 22 (32.4)a | 8 (17.4)a |
| Mapping method | |||
| Activation mapping | 27 (45.0) | 23 (33.8)a | 18 (39.1)a |
| Entrainment mapping | 19 (31.7) | 20 (29.4) | 20 (43.5)a |
| Substrate mapping | 54 (90.0) | 62 (91.2) | 40 (87.0) |
| Pace mapping | 42 (70.0) | 50 (73.5) | 34 (73.9) |
| Irrigated catheter used | 60 (100.0) | 68 (100.0) | 46 (100.0) |
| LV mapped | 57 (95.0) | 63 (92.7)a | 40 (87.0)a |
| RV mapped | 15 (25.0) | 12 (17.7)a | 9 (19.6)a |
| Intracardiac echo used | 13 (21.7) | 19 (27.9) | 10 (21.7) |
| Mean (SD) fluoroscopy time (min) | 31.3 ± 18.9 | 33.4 ± 23.4 | 31.8 ± 22.9 |
| Total radiofrequency (RF) time (min) | 36.3 ± 21.8 | 41.3 ± 21.1a | 46.7 ± 23.1a |
| Mean (SD) RF applications | 37.0 ± 25.0 | 39.6 ± 26.2 | 45.5 ± 31.2a |
| Inducible VT at baseline | 57 (95.0) | 59 (86.8)a | 40 (87.0)a |
| Distinct monomorphic VTs induced | |||
| 0 | 1 (1.7) | 4 (6.5)a | 3 (7.0)a |
| 1 | 14 (23.7) | 13 (21.0) | 3 (7.0)a |
| 2 | 16 (27.1) | 10 (16.1)a | 8 (18.6)a |
| ≥3 | 28 (47.5) | 41 (56.4)a | 29 (67.4)a |
| Induction testing performed post-ablation | 48 (80.0) | 56 (83.6)a | 36 (78.3) |
| VT inducible in post-ablation induction testing | |||
| Non-inducible arrhythmia | 20 (41.7) | 33 (56.9)a | 19 (51.4)a |
| Clinical VT still inducible | 3 (6.3) | 1 (1.7)a | 0 (0.0)a |
| Non-clinical VT still inducible with ≥300 ms | 6 (12.5) | 8 (13.8) | 5 (13.5) |
| Non-clinical VT still inducible with <300 ms | 19 (39.6) | 16 (27.6)a | 13 (35.1) |
| Acute complications | 3 (4.9) | 6 (8.8)a | 3 (6.7)a |
LV, left ventricle; RF, radiofrequency; RV, right ventricle; SD, standard deviation; VT, ventricular tachycardia.
SMD >10% are considered unbalanced. SMDs compared noni-inferior and anterior infarct patients to inferior infarct patients.
The per protocol ablation population was used because 129 of 132 patients randomized to ablation underwent the procedure.
Compared to inferior MI patients who underwent ablation, a greater proportion of non-inferior MI patients had acute procedural complications (4.9% of inferior MI patients vs. 8.8% of non-inferior MI patients, P < 0.05). Two non-inferior MI patients had heart failure decompensation and one patient had a major bleeding event, which accounted for the difference between acute complications in the inferior and non-inferior MI groups. All other complications were equally distributed between MI location groups. Acute complications are listed in Supplementary material online.
After the index ablation, a greater proportion of inferior MI patients (26.2%) had repeat ablations compared to non-inferior MI patients (14.7%; SMD = 0.26).
Primary composite outcome
The median follow-up was 25.5 (IQR 16.4–41.9) months and 22.9 (IQR 13.7–38.8) months for inferior and non-inferior MI patients, respectively. Among patients with an inferior MI (N = 135), 38 (60.3%) ablation patients and 47 (65.3%) escalated therapy patients experienced the primary composite endpoint (Table 4). After IPTW and adjustment, the trend to improved outcomes with ablation was not statistically significant between patients randomized to ablation or escalated therapy (aHR 0.80, 95% CI 0.51–1.20; Table 4; log-rank P-value = 0.37, Figure 3).
Table 4.
Effectiveness outcomes by infarct location
| Inferior infarct (N = 135) | Non-inferior infarct (N = 124) | Sensitivity: anterior infarct (N = 83) | ||||
|---|---|---|---|---|---|---|
| Ablation (N = 63) | Escalated therapy (N = 72) | Ablation (N = 69) | Escalated therapy (N = 55) | Ablation (N = 46) | Escalated therapy (N = 37) | |
| Primary composite outcome: all-cause mortality, appropriate ICD shock (>30 days post), VT storm (>30 days post) | ||||||
| Crude events, N (%) | 38 (60.3%) | 47 (65.3%) | 40 (58.0%) | 40 (72.7%) | 29 (63.0%) | 27 (72.9%) |
| Crude incidence per 100 person-years (95% CI) | 37.8 (27.5–51.9) | 49.9 (37.5–66.4) | 35.9 (26.3–48.9) | 54.6 (40.1–74.5) | 40.6 (28.2–58.5) | 57.5 (39.3–83.6) |
| aHR (95% CI)a | 0.80 (0.50–1.29) | 0.48 (0.27–0.86) | 0.50 (0.23–1.09) | |||
| All-cause mortality | ||||||
| Crude events, N (%) | 17 (27.0%) | 16 (22.2%) | 19 (27.5%) | 19 (34.6%) | 12 (26.1%) | 13 (35.1%) |
| Crude incidence per 100 person-years (95% CI) | 10.2 (6.3–16.4) | 9.3 (5.7–15.2) | 11.9 (7.6–18.7) | 15.5 (9.9–24.3) | 10.6 (6.0–18.6) | 17.8 (10.3–30.6) |
| aHR (95% CI)a | 0.99 (0.46–2.16) | 0.58 (0.26–1.32) | 0.84 (0.24–3.01) | |||
| Appropriate ICD shock (>30 days post) | ||||||
| Crude events, N (%) | 25 (39.7%) | 29 (40.3%) | 25 (26.2%) | 25 (45.5%) | 19 (41.3%) | 16 (43.2%) |
| Crude incidence per 100 person-years (95% CI) | 22.1 (14.9–32.7) | 24.5 (17.0–35.2) | 20.0 (13.5–29.5) | 30.7 (20.8–45.5) | 23.0 (14.7–16.2) | 30.7 (18.8–59.1) |
| aHR (95% CI)a | 0.99 (0.53–1.84) | 0.55 (0.28–1.09) | 0.75 (0.31–1.86) | |||
| VT storm (>30 days post) | ||||||
| Crude events, N (%) | 19 (35.9%) | 21 (29.2%) | 15 (21.7%) | 19 (34.6%) | 12 (26.1%) | 7 (18.9%) |
| Crude incidence per 100 person-years (95% CI) | 15.1 (9.6–23.6) | 16.6 (10.8–25.5) | 11.7 (7.0–19.3) | 20.6 (20.8–45.5) | 15.3 (8.7–16.9) | 10.7 (5.1–22.4) |
| aHR (95% CI)a | 1.30 (0.56–2.86) | 0.62 (0.27–1.43) | 2.02 (0.59–6.93) | |||
aHR, adjusted hazard ratio; CI, confidence interval; ICD, implantable cardioverter-defibrillator; VT, ventricular tachycardia.
HRs were inverse probability of treatment weighted. The variables included in the propensity score for the weights included age, sex, comorbidities, heart rate, type of ICD, number of ICD shocks, and ATP within the 3 months prior to randomization, medications, and the location of additional infarctions.
Figure 3.
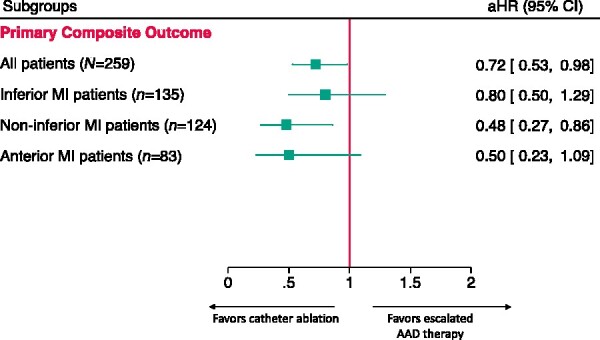
Forest plot comparing the effectiveness of treatment by MI localization to overall VANISH trial for the primary composite outcome. MI, myocardial infarction.
In contrast, 40 (58.0%) ablation patients and 40 (72.7%) escalated therapy patients with non-inferior MIs (N = 124) experienced the primary outcome. For patients with only non-inferior MIs, catheter ablation was associated with a statistically significant reduction in the hazard of the primary outcome (aHR 0.48, 95% CI 0.27–0.86) compared with patients randomized to escalated therapy (Table 4). Consistent with these results, comparison of the IPTW Kaplan–Meier curves illustrates the sustained reduction in the incidence of the primary composite endpoint in ablation patients, compared to escalated therapy patients, over the follow-up period (log-rank P-value = 0.014; Figure 3).
Secondary outcomes
The results for the secondary outcomes are presented in Table 4 and Supplementary material online. There was no statistically significant difference detected between ablation and escalated therapy for all component (secondary) outcomes within each MI location subgroup (Table 4 and Supplementary material online). However, there was a trend towards a protective effect of ablation against appropriate ICD shocks in non-inferior MI patients (aHR 0.55, 95% CI 0.28–1.09) (Table 4).
Sensitivity analysis: anterior myocardial infarction subgroup
A total of 83 (32.0%) patients had an anterior MI, among whom 23 (27.7%) patients also had infarction in the inferior region of the LV. Patient characteristics and the catheter ablation procedure were similar to those of the non-inferior MI subgroup (Tables 1 and 3).
In the anterior MI subgroup, 29 (63.0%) ablation patients had the primary outcome over the study period compared to 27 (72.9%) escalated therapy patients. After IPTW and adjustment, there was a trend towards a reduction in the primary outcome with ablation, although statistical significance was not reached (aHR 0.50, 95% CI 0.23–1.09) (Table 4; log-rank value = 0.08, Figure 3).
Sensitivity analyses: inferior myocardial infarction only and baseline antiarrhythmic drug
After exclusion of inferior MI patients with additional infarcts in other areas of the LV (n = 75), the hazards of the primary outcome (aHR 0.88, 95% CI 0.43–1.81) was similar to the overall inferior MI group (aHR 0.80, 95% CI 0.51–1.20) (Supplementary material online).
Stratification of inferior and non-inferior MI patients by baseline AAD produced similar results to the overall study; however, no statistically significant difference was detected among non-inferior patients on sotalol at baseline (n = 34; Supplementary material online).
Discussion
In this sub-study of the VANISH trial, we demonstrated that (i) comorbidities, AADs prescribed, and the ablation procedure differed by the location of MI; (ii) there was a statistically significant improvement in the incidence of the primary composite outcome for non-inferior MI patients who underwent catheter ablation compared to escalated AAD therapy which was not observed for those with inferior MI; and (iii) there was a trend towards a reduction in VT-related events with ablation in anterior MI patients. To our knowledge, this is the largest study to assess heterogeneity in the effectiveness of ablation by location of MI and the first study to evaluate it using a randomized controlled trial with a standardized ablation approach.
Comparison to overall VANISH trial
The VANISH randomized trial established catheter ablation as a viable and efficacious treatment option for the challenging post-MI population with AAD-refractory VT.1–3,27 The overall trial included all patients with infarcts in any area of the LV myocardium (N = 259) and found that catheter ablation reduced the incidence of the primary outcome by 28% compared with escalated therapy (HR 0.72, 95% CI 0.53–0.98). In this VANISH subgroup analysis, we found that the relative protective effect of catheter ablation is greater in patients with non-inferior MIs (n = 124); with a 52% reduction in the primary outcome compared to patients randomized to escalated therapy (aHR 0.48, 95% CI 0.27–0.86) (Figure 3). In contrast, there was not a significant difference in the incidence of the primary outcome between treatment arms among inferior MI patients [N = 135; aHR 0.80 (95% CI 0.50–1.29)]. As shown in Figure 3, the comparison of these effect estimates suggests that the results of the overall VANISH trial may have been driven in part by the enhanced protective effect of ablation compared to escalated therapy among non-inferior MI patients. Although the subgroup analyses were not powered to detect a statistically significant difference, the sample size and subgroup event rates were similar between inferior and non-inferior MI patients; further emphasizing the strong association in non-inferior MI patients.
A trend towards a reduction in the primary outcome was detected in anterior MI patients; however, statistical significance was not achieved. The point estimate of an aHR of 0.50 suggests the results may be similar to the non-inferior MI subgroup which included anterior MI patients, but may not have reached statistical significance due to the smaller sample size (n = 83) and that 27.7% of patients with an anterior MI also had an inferior MI.
Observational studies on myocardial infarction localization and catheter ablation outcomes
A few small observational studies have compared catheter ablation outcomes based on the location of the LV scar. In this study, inferior MI patients had a higher left ventricular ejection fraction (LVEF) and were less likely to be prescribed amiodarone than anterior MI patients.10,12–14 Further, RF duration and number of RF applications were fewer among inferior MI patients.10,14 In Wasmer et al. (N = 152), no statistically significant difference in mortality (aHR 1.10, 95% CI 0.55–2.23) or ICD shocks (46% of anterior MI patients vs. 34% of inferior MI patients, P = 0.22) was detected between patients with an anterior and inferior MI over a mean follow-up of 3 (SD 2) years.14 Analogous results were reported by Antz et al. (N = 69) and Yoshiga et al. (N = 70). However, all of these studies were underpowered and significant residual confounding was present due to minimum regression adjustment, if any.10,12,14
Increased arrhythmogenicity and decreased response to ablation in inferior myocardial infarction patients
Several studies have found that VT originating from inferior MIs are more arrhythmogenic despite lesser impairment of LVEF, as compared to non-inferior MIs.10,12,13,28 In studies by Pascale et al. (N = 252) and Sosa et al. (N = 14), the correlation between arrhythmogenicity and inferior MIs was stronger among patients with VT due to more remote MIs (mean 12.4 ± 9.4 years),13,29 which is similar to the VANISH trial patients whose MIs occurred a mean of 15 years prior to enrolment. The underlying mechanism for increased arrhythmogenicity of inferior MI scars is unknown. Hypotheses for the heightened propensity for electrical instability range from increased right ventricular involvement (30–50%), damage to greater density of receptors with vagal afferents clustered in the inferior wall, and anatomic factors such as the proximity to the mitral annulus or close relationship with the papillary muscles.11–14,30–34 Comparisons of MIs in inferior and non-inferior regions of the LV indicate that the VT substrate in inferior regions is more complex with shorter activation times (VT cycle lengths), lower incidences of complete superficial re-entry and aneurysms, and the VT substrates are smaller with thicker border zones.9,10,12,13,35 Thus, the heightened complexity of the VT substrate due to anatomical and functional remodelling in the inferior region may hinder effective lesion delivery with an ablation catheter.
Further, in the VANISH trial, the index ablation was endocardial only; however, it is possible that epicardial substrates may be more prevalent among patients with an inferior MI.12,36 Pathologic case series have shown that scars due to inferior MIs are usually transmural with a primarily endocardial scar which may become more epicardial towards the apex.9,11,12,34,37,38 Although epicardial mapping and ablation is infrequently needed for VT due to ischaemic cardiomyopathy, several reports consistently showed that while 0% of patients with anterior MIs needed epicardial ablation, epicardial ablation was warranted in 15–39% of patients with an inferior MI.11,12,29,37–39 Thus, as demonstrated in the present study, an endocardial-only ablation may be insufficient to improve VT event-free survival among inferior MI patients when compared to escalated AAD therapy. It is possible that a combined endocardial and epicardial VT ablation approach may yield superior outcomes in inferior MI patients. Therefore, future studies are warranted.
Limitations
Although this is the largest study evaluating the effect of modification by the location of MI on the association between VT ablation and VT event-free survival, several important limitations remain. First, the present study was a post hoc analysis of the VANISH trial and was not a pre-specified sub-study. Although clinical information regarding MI location was available, details about the infarctions (e.g. infarct size, anatomy) were not collected. Such explanatory variables may shed further light on the findings of this study. Further, the location of MI was captured from patient medical records (clinical MI localization). Scar location identified by voltage mapping or magnetic resonance imaging could enhance precision. Also, the transaortic approach was preferred in VANISH trial, but the approach used to access the left ventricle was not collected in the trial. Use of propensity score methods balanced patient characteristics between treatment arms in each subgroup; however, potential residual confounding may still be present. Also, the VANISH trial was not powered to detect a difference in the incidence of the primary or secondary outcomes within subgroups. Nevertheless, statistical significance was detected among non-inferior MI patients and a trend towards a reduction was detected among anterior MI patients despite the smaller sample size and number of events. Finally, selective populations are enrolled in randomized trials, thus the generalizability of results is limited to the target population of the VANISH trial.
Conclusion
The effectiveness of VT ablation vs. escalated pharmacological therapy varies based on the location of the MI. Patients with MI scars located only in non-inferior regions of the ventricles derived greater benefit from VT ablation in reducing VT-related events. These results suggest that MI localization may be considered when deciding on the choice of therapy. Further clinical and mechanistic studies are required to assess the impact of VT treatment strategies based on MI location in optimizing outcomes.
Supplementary material
Supplementary material is available at Europace online.
Funding
The VANISH trial was funded by the Canadian Institutes of Health Research (CIHR, Funding Reference Number 102695), with additional financial support from St. Jude Medical and Biosense Webster.
Conflict of interest: J.S.: research funding from Biosense Webster and Abbott, and speaker/consulting honoraria from Medtronic, Abbott, and Biosense Webster. I.N.: honorarium from Biosense Webster, Servier, BMS-Pfizer, and Bayer. V.E.: FRQS clinical research scholar award and honoraria from Medtronic Inc, Abbott, and Biosense Webster. R.P.: research funding from Abbott, Medtronic, and Novartis. P.K.: André Chagnon chair in electrophysiology and congenital heart disease. M.S.: nothing to disclose.
Data availability
The data underlying this article will be shared upon reasonable request to the corresponding author.
Supplementary Material
Acknowledgements
The authors thank Karen Giddens for her contributions towards the conduct of the VANISH trial and present sub-study.
Contributor Information
Michelle Samuel, Department of Medicine, Montreal Heart Institute, Université de Montréal, Montreal, Quebec, Canada.
Lena Rivard, Department of Medicine, Montreal Heart Institute, Université de Montréal, Montreal, Quebec, Canada.
Isabelle Nault, Department of Medicine, Quebec Heart and Lung Institute, Quebec City, Quebec, Canada.
Lorne Gula, Department of Medicine, Western University, London, Ontario, Canada.
Vidal Essebag, Department of Medicine, McGill University Health Centre, Montreal, Quebec, Canada.
Ratika Parkash, Department of Medicine, Queen Elizabeth II Health Sciences Centre, Dalhousie University, Room 2501B Halifax Infirmary, 1796 Summer St, Halifax, Nova Scotia B3H 3A7, Canada.
Laurence D Sterns, Department of Medicine, Royal Jubilee Hospital, Victoria, British Columbia, Canada.
Paul Khairy, Department of Medicine, Montreal Heart Institute, Université de Montréal, Montreal, Quebec, Canada.
John L Sapp, Department of Medicine, Queen Elizabeth II Health Sciences Centre, Dalhousie University, Room 2501B Halifax Infirmary, 1796 Summer St, Halifax, Nova Scotia B3H 3A7, Canada.
References
- 1. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm Jet al. 2015. ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Europace 2015;17:1601–87. [DOI] [PubMed] [Google Scholar]
- 2. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis ABet al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm 2018;15:e73–189. [DOI] [PubMed] [Google Scholar]
- 3. Deyell MW, AbdelWahab A, Angaran P, Essebag V, Glover B, Gula LJet al. Members of the Secondary Panel . 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society position statement on the management of ventricular tachycardia and fibrillation in patients with structural heart disease. Can J Cardiol 2020;36:822–36. [DOI] [PubMed] [Google Scholar]
- 4. Cronin EM, Bogun FM, Maury P, Peichl P, Chen M, Namboodiri Net al. 2019. HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Europace 2019;21:1143–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kuck KH, Schaumann A, Eckardt L, Willems S, Ventura R, Delacrétaz Eet al. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): a multicentre randomised controlled trial. Lancet 2010;375:31–40. [DOI] [PubMed] [Google Scholar]
- 6. Kuck KH, Tilz RR, Deneke T, Hoffmann BA, Ventura R, Hansen PSet al. Impact of substrate modification by catheter ablation on implantable cardioverter-defibrillator interventions in patients with unstable ventricular arrhythmias and coronary artery disease: results from the multicenter randomized controlled SMS (Substrate Modification Study). Circ Arrhythm Electrophysiol 2017;10:e004422. [DOI] [PubMed] [Google Scholar]
- 7. Reddy VY, Reynolds MR, Neuzil P, Richardson AW, Taborsky M, Jongnarangsin Ket al. Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl J Med 2007;357:2657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sapp JL, Wells GA, Parkash R, Stevenson WG, Blier L, Sarrazin JFet al. Ventricular tachycardia ablation versus escalation of antiarrhythmic drugs. N Engl J Med 2016;375:111–21. [DOI] [PubMed] [Google Scholar]
- 9. Lacroix D, Warembourg H, Klug D, Decoene C, Kacet S.. Intraoperative computerized mapping of ventricular tachycardia: differences between anterior and inferior myocardial infarctions. J Cardiovasc Electrophysiol 1999;10:781–90. [DOI] [PubMed] [Google Scholar]
- 10. Antz M, Berodt K, Bansch D, Ernst S, Chun KJ, Satomi Ket al. Catheter-ablation of ventricular tachycardia in patients with coronary artery disease: influence of the endocardial substrate size on clinical outcome. Clin Res Cardiol 2008;97:110–7. [DOI] [PubMed] [Google Scholar]
- 11. Cesario DA, Vaseghi M, Boyle NG, Fishbein MC, Valderrabano M, Narasimhan Cet al. Value of high-density endocardial and epicardial mapping for catheter ablation of hemodynamically unstable ventricular tachycardia. Heart Rhythm 2006;3:1–10. [DOI] [PubMed] [Google Scholar]
- 12. Yoshiga Y, Mathew S, Wissner E, Tilz R, Fuernkranz A, Metzner Aet al. Correlation between substrate location and ablation strategy in patients with ventricular tachycardia late after myocardial infarction. Heart Rhythm 2012;9:1192–9. [DOI] [PubMed] [Google Scholar]
- 13. Pascale P, Schlaepfer J, Oddo M, Schaller MD, Vogt P, Fromer M.. Ventricular arrhythmia in coronary artery disease: limits of a risk stratification strategy based on the ejection fraction alone and impact of infarct localization. Europace 2009;11:1639–46. [DOI] [PubMed] [Google Scholar]
- 14. Wasmer K, Reinecke H, Heitmann M, Dechering DG, Reinke F, Lange PSet al. Clinical, procedural and long-term outcome of ischemic VT ablation in patients with previous anterior versus inferior myocardial infarction. Clin Res Cardiol 2020;109:1282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Palaniswamy C, Kolte D, Harikrishnan P, Khera S, Aronow WS, Mujib Met al. Catheter ablation of postinfarction ventricular tachycardia: ten-year trends in utilization, in-hospital complications, and in-hospital mortality in the United States. Heart Rhythm 2014;11:2056–63. [DOI] [PubMed] [Google Scholar]
- 16. Pocock SJ, Hughes MD, Lee RJ.. Statistical problems in the reporting of clinical trials. A survey of three medical journals. N Engl J Med 1987;317:426–32. [DOI] [PubMed] [Google Scholar]
- 17. Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM.. Statistics in medicine–reporting of subgroup analyses in clinical trials. N Engl J Med 2007;357:2189–94. [DOI] [PubMed] [Google Scholar]
- 18. Yusuf S, Wittes J, Probstfield J, Tyroler HA.. Analysis and interpretation of treatment effects in subgroups of patients in randomized clinical trials. JAMA 1991;266:93–8. [PubMed] [Google Scholar]
- 19. Parker AB, Naylor CD.. Subgroups, treatment effects, and baseline risks: some lessons from major cardiovascular trials. Am Heart J 2000;139:952–61. [DOI] [PubMed] [Google Scholar]
- 20. Assmann SF, Pocock SJ, Enos LE, Kasten LE.. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet 2000;355:1064–9. [DOI] [PubMed] [Google Scholar]
- 21. Royston P, Sauerbrei W.. Multivariable Model-Building: A Pragmatic Approach to Regression Analysis Based on Fractional Polynomials for Modelling Continuous Variables. England, UK: John Wiley & Sons; 2008. [Google Scholar]
- 22. Kyle RP, Moodie EE, Klein MB, Abrahamowicz M.. Evaluating flexible modeling of continuous covariates in inverse-weighted estimators. Am J Epidemiol 2019;188:1181–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lunceford JK, Davidian M.. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med 2004;23:2937–60. [DOI] [PubMed] [Google Scholar]
- 24. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rosenbaum PR, Rubin DB.. The central role of the propensity score in observational studies for causal effects. J Biometrika 1983;70:41–55. [Google Scholar]
- 26. Imbens GW. Nonparametric estimation of average treatment effects under exogeneity: a review. J Rev Econ Stat 2004;86:4–29. [Google Scholar]
- 27. Callans DJ, Reynolds M, Zimetbaum PJ.. Electrophysiology's identity crisis: what our clinical trials do and do not say about us. Arrhythm Electrophysiol Rev 2020;9:15–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mentias A, Raza MQ, Barakat AF, Hill E, Youssef D, Krishnaswamy Aet al. Outcomes of ischaemic mitral regurgitation in anterior versus inferior ST elevation myocardial infarction. Open Heart 2016;3:e000493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sosa E, Scanavacca M, d'Avila A, Oliveira F, Ramires JA.. Nonsurgical transthoracic epicardial catheter ablation to treat recurrent ventricular tachycardia occurring late after myocardial infarction. J Am Coll Cardiol 2000;35:1442–9. [DOI] [PubMed] [Google Scholar]
- 30. Bogun F, Desjardins B, Crawford T, Good E, Jongnarangsin K, Oral Het al. Post-infarction ventricular arrhythmias originating in papillary muscles. J Am Coll Cardiol 2008;51:1794–802. [DOI] [PubMed] [Google Scholar]
- 31. Schmidt A, Azevedo CF, Cheng A, Gupta SN, Bluemke DA, Foo TKet al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation 2007;115:2006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mehta RH, Starr AZ, Lopes RD, Hochman JS, Widimsky P, Pieper KSet al. ; APEX AMI Investigators . Incidence of and outcomes associated with ventricular tachycardia or fibrillation in patients undergoing primary percutaneous coronary intervention. JAMA 2009;301:1779–89. [DOI] [PubMed] [Google Scholar]
- 33. Thames MD, Klopfenstein HS, Abboud FM, Mark AL, Walker JL.. Preferential distribution of inhibitory cardiac receptors with vagal afferents to the inferoposterior wall of the left ventricle activated during coronary occlusion in the dog. Circ Res 1978;43:512–9. [DOI] [PubMed] [Google Scholar]
- 34. Brugada J, Berruezo A, Cuesta A, Osca J, Chueca E, Fosch Xet al. Nonsurgical transthoracic epicardial radiofrequency ablation: an alternative in incessant ventricular tachycardia. J Am Coll Cardiol 2003;41:2036–43. [DOI] [PubMed] [Google Scholar]
- 35. Woie L, Eftestol T, Engan K, Kvaloy JT, Nilsen DW, Orn S.. The heart rate of ventricular tachycardia following an old myocardial infarction is inversely related to the size of scarring. Europace 2011;13:864–8. [DOI] [PubMed] [Google Scholar]
- 36. Marchlinski FE, Callans DJ, Gottlieb CD, Zado E.. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation 2000;101:1288–96. [DOI] [PubMed] [Google Scholar]
- 37. Nakahara S, Tung R, Ramirez RJ, Gima J, Wiener I, Mahajan Aet al. Distribution of late potentials within infarct scars assessed by ultra high-density mapping. Heart Rhythm 2010;7:1817–24. [DOI] [PubMed] [Google Scholar]
- 38. Ashikaga H, Sasano T, Dong J, Zviman MM, Evers R, Hopenfeld Bet al. Magnetic resonance-based anatomical analysis of scar-related ventricular tachycardia: implications for catheter ablation. Circ Res 2007;101:939–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sacher F, Roberts-Thomson K, Maury P, Tedrow U, Nault I, Steven Det al. Epicardial ventricular tachycardia ablation a multicenter safety study. J Am Coll Cardiol 2010;55:2366–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.