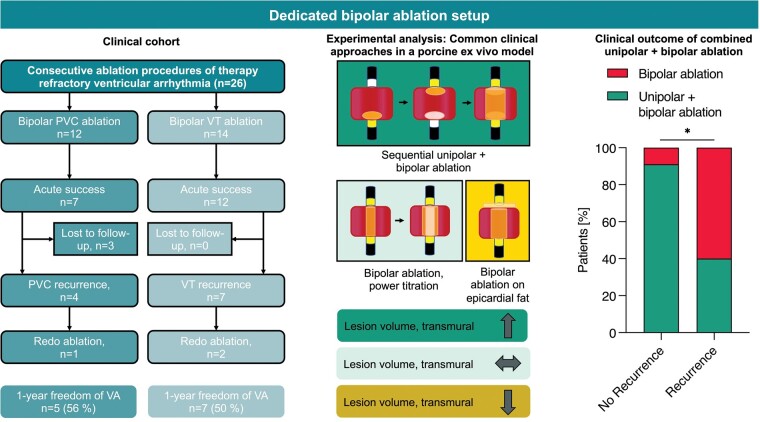
Abstract
Aims
Bipolar radiofrequency ablation (B-RFA) has been reported as a bail-out strategy for the treatment of therapy refractory ventricular arrhythmias (VA). Currently, existing setups have not been standardized for B-RFA, while the impact of conventional B-RFA approaches on lesion formation remains unclear.
Methods and results
(i) In a multicentre observational study, patients undergoing B-RFA for previously therapy-refractory VA using a dedicated B-RFA setup were retrospectively analysed. (ii) Additionally, in an ex vivo model lesion formation during B-RFA was evaluated using porcine hearts. In a total of 26 procedures (24 patients), acute success was achieved in all 14 ventricular tachycardia (VT) procedures and 7/12 procedures with premature ventricular contractions (PVC), with major complications occurring in 1 procedure (atrioventricular block). During a median follow-up of 211 days in 21 patients, 6/11 patients (VT) and 5/10 patients (PVC) remained arrhythmia-free. Lesion formation in the ex vivo model during energy titration from 30 to 50 W led to similar lesion volumes compared with initial high-power 50 W B-RFA. Lesion size significantly increased when combining sequential unipolar and B-RFA (1429 mm3 vs. titration 501 mm3 vs. B-RFA 50 W 423 mm3, P < 0.001), an approach used in overall 58% of procedures and more frequently applied in procedures without VA recurrence (92% vs. 36%, P = 0.009). Adipose tissue severely limited lesion formation during B-RFA.
Conclusion
Using a dedicated device for B-RFA for therapy-refractory VA appears feasible and safe. While some patients need repeat ablation, success rates were encouraging. Sequential unipolar and B-RFA may be favourable for lesion formation.
Keywords: Bipolar ablation, Ventricular tachycardia, Ventricular arrhythmias, Premature ventricular contractions, Radiofrequency generator
Graphical Abstract
Graphical Abstract.
What’s new?
This case series reports the experience of the first dedicated approach using a radiofrequency generator for bipolar ablation.
In patients with otherwise therapy refractory ventricular arrhythmias, bipolar ablation was associated with a freedom from arrhythmia in 52% of patients in a follow-up of up to 2 years.
The combination of unipolar ablation followed by bipolar radiofrequency ablation led to enhanced lesion formation in a porcine ex vivo model and the clinical cohort, whereas epicardial adipose tissue significantly reduces bipolar ablation efficacy ex vivo.
Introduction
Catheter ablation is increasingly used to treat ventricular arrhythmias (VA) refractory to medical therapy. However, arrhythmias of deep intramural origin may not be accessible through unipolar ablation, as often encountered at the interventricular septum or the left ventricular summit.1,2 Bipolar radiofrequency ablation (B-RFA) is described as a bail-out method in such a scenario.3 However, clinical setups for B-RFA often employ custom solutions with cable connectors using a single generator.4 The 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of VA emphasizes the concern over present custom-made approaches particularly due to the lack of temperature monitoring for both catheter tips.5 In 2017, the first dedicated device for B-RFA received its CE mark (HAT500, Osypka AG, Rheinfelden, Germany). This device allows for a standardized dedicated approach with two ablation catheters and separate irrigation pumps. Clinical approaches often employ prior unipolar ablation before B-RFA or titration of power from lower to higher levels. The rationale behind these strategies is a supposed reduction in risk for adverse events, whereas optimal B-RFA approaches remain unclear. Data supporting specific approaches are limited especially when joined by additional challenges such as the presence of significant epicardial adipose tissue (EA).2
This study reports (i) the first multicentre experience of B-RFA using a dedicated single ablation generator in patients presenting with VA refractory to conventional therapy and (ii) assessment of common clinically used B-RFA approaches in an ex vivo model.
Methods
Clinical cohort
Consecutive patients that underwent B-RFA for VA were retrospectively studied at participating tertiary care centres from July 2017 until July 2020. Inclusion criteria were defined as therapy refractory VA with a history of previous unsuccessful antiarrhythmic medication and/or unipolar RF ablation. Moreover, patients with suspected deep intramural VA origin were included based on previous 12-channel ECG and pre-procedural cardiac imaging including transthoracic/transesophageal echocardiography, cardiac computed tomography or cardiac magnetic resonance imaging. Patients with idiopathic VA or known structural heart disease were included. Exclusion criteria were current critical illness, severe acute liver or kidney dysfunction, acute hyperthyroidism, age <18 years and patients with insufficient procedural data. Baseline patient characteristics and history were evaluated via a standardized questionnaire sent to the treating electrophysiologist at the respective centres [University Medical Center Hamburg-Eppendorf, University Hospital Düsseldorf, EVK Düsseldorf, Karlsruhe Hospital, Heart Center Bad-Neustadt, German Heart Center Munich (all Germany) and Grochowski Hospital Warsaw, Poland]. Data collection and analysis were performed with approval of the institutional ethics committee. All patients gave written informed consent.
Electrophysiological study setup
The procedures were carried out in accordance to existing consensus statements as well as previously published protocols.6 Patients underwent the procedure in a fasting state under conscious sedation using propofol or midazolam and fentanyl. General anaesthesia was applied, if deemed necessary by the respective centres. Access to the ventricles was gained anterograde via the femoral vein or transseptal access and retrograde via the femoral artery.
In short, the diagnostic setup included catheters placed in the coronary sinus and the right ventricular apex. Substrate mapping was conducted during sinus rhythm in the right (RV) and/or left ventricle (LV) using the Smarttouch Thermocool single-tip catheter, the 20-pole Pentaray catheter (Biosense Webster, Diamond Bar, CA, USA) or the 64-pole Orion catheter (Boston Scientific, Marlborough, MA, USA). Maps were created with the Carto® 3 (Biosense Webster) or Rhythmia™ mapping system (Boston Scientific) and left at the treating physicians discretion. For VT induction, programmed ventricular stimulation was conducted with two different baseline cycle lengths and up to three extra stimuli with a minimal coupling interval of 180 ms. Additional incremental atrial and ventricular pacing was performed following programmed stimulation. The induced VT was regarded as the clinical VT when cycle length and morphology conformed with previous recording.6 Activation mapping was performed whenever VT was haemodynamically tolerated, with additional entrainment or pace mapping conducted at the operator’s discretion. In case of non-inducibility, additional intravenous orciprenaline or isoproterenol administration was performed.
Procedural workflow and bipolar ablation
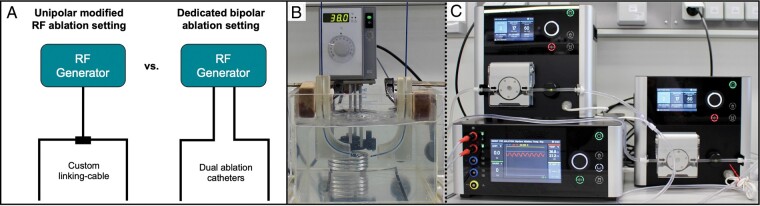
A schematic ablation generator setup is shown in Figure 1A. The HAT500 single ablation generator (OSYPKA AG, Rheinfelden, Germany) enables usage of two ablation catheters with individual irrigation pumps. This allows for separate monitoring of power levels, temperature and either separate unipolar or bipolar impedance for both catheters individually. Furthermore, it is possible to switch between unipolar and B-RFA without changing cable connections. The general unipolar ablation setting has been described before.7 For B-RFA, two open-irrigated 3.5 or 4 mm tip ablation catheters were used, with one catheter being placed at the endocardial site in the RV/LV and the other at the opposing epicardial or septal site or within the coronary sinus/great cardiac vein. In either system, one ablation catheter is declared as a diagnostic catheter and disappears during energy application. Additional coronary angiography and intracardiac echocardiography were performed before energy application in selected cases if the suspected critical VA site was in close proximity to the coronary vessels or for better visualization of catheter positioning.
Figure 1.
Clinical and ex vivo bipolar ablation setup. (A) Schematic overview on custom-made solutions and presented approach using a dedicated radiofrequency (RF) generator setup for bipolar ablation. (B) Experimental setup of custom catheter guiding blocks in a temperature-controlled saline bath with two irrigated 3.5 mm ablation catheters on either side. (C) Catheters are connected to a single ablation generator with two separate irrigation pumps.
Ablation points were chosen based on the earliest activation site and/or best pace map. Unipolar RF energy current was first applied to the suspected critical isthmus site followed by B-RFA with a maximum power of 50 W. Immediate B-RFA without prior unipolar ablation was conducted in selected cases with ≥2 prior ablation procedures and/or at sites of high myocardial tissue thickness such as the aorto-mitral continuity or left/right ventricular outflow tract.
Follow-up
Acute procedural success was defined as non-inducibility of the clinical VT for VT ablation and complete cessation or PVC burden reduction >90% after 30 min waiting time and subsequent isoproterenol/orciprenaline provocation testing during PVC ablation. Antiarrhythmic treatment was continued at the operator’s discretion. Patients were asked to visit the outpatient clinic every 3–6 months after the initial catheter ablation at respective centre site. A 12-lead ECG or 24-h Holter ECG monitoring was performed at every visit and whenever patients complained about symptoms. Device interrogation for VA detection was additionally conducted whenever applicable. Long-term recurrence of VT was defined as sustained VT or appropriate ICD therapy and PVC recurrence as a PVC burden reduction ≤90% during 24-h Holter ECG monitoring after a blanking period >3 months.
Ex vivo model setup
An experimental setup overview is displayed in Figure 1B and C. For evaluation of lesion formation during different clinical approaches, ablation was conducted in freshly explanted swine hearts using the HAT500 generator and two irrigated 3.5 mm ablation catheters (Cerablade cool, Osypka AG, Rheinfelden, Germany). Left ventricular and septal myocardium was dissected in regions with a myocardial thickness ≥15 mm. Extracted tissue was positioned in a temperature-controlled saline bath at 37°C with active and ground ablation catheter positioned orthogonally to each side of the myocardium applying a constant force of ∼10 g for 60 s with an irrigation rate of 30 mL/min. Each investigated ablation approach was conducted for 60 s, with 30 s resting period separating sequences within each group: (i) 30 W B-RFA followed by 50 W B-RFA (titration group); (ii) 30 W sequential unipolar ablation (SUA) followed by 50 W B-RFA (SUA+B-RFA group); (iii) 50 W B-RFA in areas of significant epicardial adipose (EA) tissue (EA group); (iv) sole B-RFA using 30/40/50 W (B-RFA group); (v) sole 30 W SUA (SUA group). Baseline generator impedance and maximum generator impedance drop were assessed. Only lesions without occurrence of steam pops were considered for analysis. The following lesion parameters were macroscopically analysed using a digital caliper for estimation of the intramural lesion volume8: maximal lesion depth (A), maximal intramural diameter (B), depth at maximum diameter (C), and lesion surface diameter (D).
In non-transmural B-RFA lesions, the maximal lesion depth was determined based on the deepest lesion from either active or returning ablation catheter, whereas in transmural lesions it was calculated by halving the measured total myocardial thickness. Furthermore, a combined lesion depth of both endocardial and epicardial lesions as well as the ratio of the combined lesion depth to the myocardial tissue thickness was calculated accordingly. Transmurality was defined as a macroscopically visible continuous lesion between both catheters reviewed by two independent investigators (F.-A.A. and K.S.). The transmurality rate was calculated as the percentage of transmural lesions within all set lesions of the respective approach.
Statistical analysis
All continuous variables were tested for normal distribution using the Shapiro–Wilk test. Parametric data are expressed as mean ± standard deviation, whereas results of non-parametric data are provided as medians with inter-quartile ranges. If applicable, minimum and maximum values are also indicated. Analysis was performed using Graphpad Prism 9® (Graphpad Inc., La Jolla, CA, USA) and Microsoft Excel (Microsoft Corporation, Redmond, WA, USA). For calculation of differences of continuous data, either Student’s t-test or ordinary one-way ANOVA tests were conducted for parametric data. For non-parametric data, either the Mann–Whitney U test or Kruskal–Wallis test were applied. Differences of categorical variables were determined using a χ2 test. A P-value <0.05 was considered statistically significant.
Results
Patients and baseline characteristics
Baseline characteristics are shown in Table 1. A total of 26 procedures in 24 patients were investigated (VT n = 13/PVC n = 11). Underlying structural heart diseases included ischaemic cardiomyopathy (n = 4), dilatative cardiomyopathy (n = 6), previous myocarditis (n = 3) and cardiac sarcoidosis (n = 1), whereas the majority of patients presented with idiopathic VA (n = 12). Recurrent VT (14/26) or a relevant PVC burden (12/26) was targeted. Patients without structural heart disease presented more often for PVC ablation and less often for VT ablation compared with patients with structural heart disease (PVC P = 0.02; VT P = 0.008). A median of 1 previous ablation (range 0–5) of various approaches had been performed before inclusion, with n = 2 patients presenting for initial VA ablation (all PVC).
Table 1.
Baseline characteristics
| Parameter | All patients | Idiopathic | SHD | Idiopathic vs. SHD, P-value |
|---|---|---|---|---|
| n = 24 | n = 12 | n = 12 | ||
| Age (years) | 57 ± 15 | 55 ± 17 | 61 ± 13 | 0.29 |
| Female sex, n (%) | 5 (21) | 3 (25) | 2 (17) | >0.99 |
| Body mass index (kg/m2) | 28 ± 4.9 | 26 ± 3 | 29 ± 6 | 0.31 |
| CHA2DS2VASc score, n (inter-quartile range) | 3 (0–3) | 1 (0–3) | 3 (3–4) | 0.01 |
| HAS-BLED score, n (inter-quartile range) | 1 (0–2) | 0 (0–1) | 1 (0–2) | 0.12 |
| LVEF, % (total range) | 47 ± 15 (23–74) | 61 ± 8 (47–74) | 36 ± 9 (23–50) | <0.001 |
| ICD present, n (%) | 12 (50) | 0 0 | 12 | <0.001 |
| Antiarrhythmic medication, n (%) | ||||
| Ajmalin | 1 (4) | 0 0 | 1 (8) | >0.99 |
| Flecainid | 1 (4) | 1 (8) | 0 0 | >0.99 |
| Propafenone | 0 0 | 0 0 | 0 0 | >0.99 |
| Amiodaron | 7 (29) | 1 (8) | 6 (50) | 0.07 |
| Sotalol | 0 0 | 0 0 | 0 0 | >0.99 |
| Mexilitine | 2 (8) | 0 0 | 2 (17) | 0.48 |
| Quinidine | 1 (4) | 0 0 | 1 (8) | >0.99 |
| Underlying condition, n (%) | ||||
| Post-myocarditis | 3 (13) | 0 0 | 3 (25) | 0.22 |
| Dilated cardiomyopathy | 6 (25) | 0 0 | 6 (50) | 0.01 |
| Ischaemic cardiomyopathy | 2 (8) | 0 0 | 2 (17) | 0.48 |
| Idiopathic | 12 (50) | 12 (50) | 0 0 | <0.001 |
| Sarcoidosis | 1 (4) | 0 0 | 1 (8) | >0.99 |
| Procedure indication, n (%) | ||||
| Recurrent PVC | 12/26 (46) | 9/12 (75) | 3/14 (21) | 0.02 |
| Recurrent VT | 12/26 (38) | 2/12 (17) | 10/14 (71) | 0.008 |
| VT storm | 2/26 (8) | 1/12 (8) | 1/14 (7) | >0.99 |
| Previous VA Ablation, n (%) | 22/26 (85) | 9/12 (75) | 13/14 (93) | 0.31 |
| No. of previous ablation, median (inter-quartile range) | 2 (1–2) | 1 (0–2) | 2 (1–3) | 0.01 |
Variables are expressed as absolute values, mean ± standard deviation, or median with inter-quartile ranges.
ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; PVC, premature ventricular contraction; SHD, structural heart disease; VA, ventricular arrhythmia; VT, ventricular tachycardia.
Procedural data
Procedural data are listed in Table 2. High-density mapping was employed in 10 out of 26 procedures. In 92% of the cases, the clinical arrhythmia was inducible or occurred spontaneously. In one procedure, prophylactic mechanical circulatory support via Impella™ (Abiomed Europe, Aachen, Germany) was established to prevent cardiogenic shock during the procedure. Coronary angiography was performed before energy application in 12 procedures (Table 3). Unipolar ablation was carried out in 15 out of 26 procedures before switching to B-RFA. Maximum applied energy ranged between 20 and 50 W with an average of 37.5 ± 8.9 W.
Table 2.
Procedural data
| Electroanatomic mapping system, n (%) | |
| Carto | 23 (88) |
| Rhythmia | 3 (12) |
| Pre- or periprocedural imaging, n (%) | |
| Intracardiac echocardiography | 4/26 (15) |
| Magnetic resonance imaging | 4/26 (15) |
| Computed tomography | 1/26 (4) |
| Clinical VT inducible, n (%) | 24 (92) |
| Number of inducible VT, n (inter-quartile range) | 1 (1–1) |
| Clinical VT cycle length (ms) | 385 (343–428) |
| Haemodynamic support, n (%) | |
| General anaesthesia | 1 (4) |
| Impella | 1 (4) |
| Procedure duration (min) | 196.5 ± 55.8 |
| Fluoroscopy time (min) | 19.5 ± 9.4 |
| Fluoroscopy dose (cGy·cm2) | 3847 ± 5613 |
| Coronary angiography before energy application, n (%) | 12 (46) |
| Unipolar ablation in same procedure, n (%) | 15 (58) |
| Mean maximum applied energy during B-RFA (W) (total range) | 37.5 ± 8.9 (24–50) |
| Acute success, n (%) | |
| PVC, suppression | 7/12 (58) |
| Clinical VT, non-inducibility | 14/14 (100) |
| Overall VT, non-inducibility | 12/14 (86) |
| Periprocedural complications, n (%) | |
| Overall | 3 (12) |
| Groin complications | 2 (8) |
| Atrioventricular block with pre-existing ICD | 1 (4) |
| Follow-up (n = 23 patients) | |
| Median follow-up duration, days (inter-quartile range) | 139 (75–245) |
| Freedom of arrhythmia, n (%) | |
| PVC | 5/9 (56) |
| VT | 7/14 (50) |
| Re-do ablation, n (%) | |
| PVC | 1/9 (11) |
| VT | 2/14 (14) |
Variables expressed as n (%) or n ± standard deviation.
B-RFA, bipolar radiofrequency ablation; ICD, implantable cardioverter-defibrillator; PVC, premature ventricular contraction; VA, ventricular arrhythmia; VT, ventricular tachycardia.
Table 3.
Detailed procedure characteristics
| Case no. | Cardiomyopathy | Procedure | Maximum energy (W) | Unipolar ablation in same procedure | Coronary angiography conducted | Position catheter 1 | Size (mm) | Position catheter 2 | Size (mm) | Target region |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Post myocarditis | VT | 45 | No | Yes | Anteroseptal left | 3.5 | Epicardial | 4 | IVS |
| 2 | Post myocarditis | VT | 50 | No | No | Septal LV | 3.5 | Septal RV | 4 | IVS |
| 3 | Post myocarditis | VT | 42 | No | No | Septal LV | 3.5 | Septal RV | 3.5 | IVS |
| 4 | Post myocarditis | VT | 45 | No | Yes | Septal LV | 3.5 | Epicardial LV | 3.5 | Septal LV |
| 5 | DCM | VT | 35 | Yes | No | Septal LV | 3.5 | Septal RV | 4 | IVS |
| 6 | DCM | VT | 40 | Yes | No | RVOT | 3.5 | CS | 4 | RVOT |
| 7 | DCM | VT | 50 | Yes | No | Septal LV | 3.5 | Septal RV | 3.5 | IVS |
| 8 | DCM | VT | 50 | Yes | No | Septal LV | 3.5 | Septal RV | 3.5 | IVS |
| 9 | DCM | PVC | 36 | Yes | No | LV antero-basal | 3.5 | CS | 3.5 | Anterobasal LV |
| 10 | DCM | PVC | 36 | Yes | Yes | LVOT | 3.5 | RVOT | 3.5 | LCC/RVOT |
| 11 | DCM | PVC | 29 | Yes | Yes | RVOT | 3.5 | CS | 4 | RVOT |
| 12 | ICM | VT | 45 | Yes | No | Septal LV | 3.5 | Septal RV | 4 | IVS |
| 13 | ICM | VT | 30 | No | Yes | Posterior wall LV epicardial | 3.5 | LV posterior wall | 3.5 | LV posterior wall |
| 14 | Idiopathic | VT | 41 | Yes | No | Septal LV | 4 | Septal RV | 3.5 | IVS |
| 15 | Idiopathic | PVC | 30 | No | No | RVOT | 3.5 | CS | 3.5 | RVOT |
| 16 | Idiopathic | VT | 24 | No | No | LVOT | 3.5 | CS | 3.5 | LVOT |
| 17 | Idiopathic | PVC | 50 | Yes | Yes | RVOT | 3.5 | CS | 3.5 | RVOT/CS |
| 18 | Idiopathic | PVC | 50 | Yes | Yes | AMC | 3.5 | CS | 3.5 | AMC |
| 19 | Idiopathic | VT | 40 | Yes | Yes | AMC | 3.5 | CS | 3.5 | AMC |
| 20 | Idiopathic | PVC | 25 | No | No | LVOT | 3.5 | CS/GCV | 3.5 | LVOT/CS |
| 21 | Idiopathic | PVC | 30 | Yes | Yes | LVOT | 3.5 | Pulmonary cusp | 3.5 | LVOT |
| 22 | Idiopathic | PVC | 20 | Yes | Yes | RVOT/LVOT | 3.5 | CS/GCV | 3.5 | RVOT |
| 23 | Idiopathic | PVC | 35 | Yes | Yes | LVOT | 3.5 | CS | 3.5 | LVOT |
| 24 | Idiopathic | PVC | 32 | No | Yes | RVOT | 3.5 | CS | 3.5 | RVOT |
| 25 | Idiopathic | PVC | 30 | No | No | Septal LV | 3.5 | CS/GCV | 3.5 | Septal LV |
| 26 | Sarcoidosis | VT | 35 | Yes | No | Epicardial left | 3.5 | Septal left | 3.5 | Septal LV |
B-RFA, bipolar radiofrequency ablation; CS, coronary sinus; DCM, dilatative cardiomyopathy; GCV, great cardiac vein; ICM, ischaemic cardiomyopathy; IVS, interventricular septum; LCC, left coronary cusp; LV, left ventricle; LVOT, left ventricular outflow tract; MVA, mitral valve annulus; RF, radiofrequency energy; RVOT, right ventricular outflow tract.
Target location and mid-term outcome
The placement of both active and grounding catheter as well as detailed procedure characteristics are presented in Table 3. Main target sites were located at the right/left ventricular outflow tract (11/26) with n = 2 involving either the left coronary or pulmonary cusp, interventricular septum (8/26), left ventricular anterior/septal/posterior wall (n = 5) and aorto-mitral continuity (2/26). Clinical PVC morphologies included left bundle branch block morphology in 10/12 cases (83%), while in two procedures of the same patient a right bundle branch block morphology was present with subsequent B-RFA between the right ventricular outflow tract and the left coronary cusp/coronary sinus. A median of 1 VT per procedure with a median cycle length of 385 ms was induced, with the clinical VT morphologies being left bundle branch block in 8/14 procedures (57%) and right bundle branch block in the remaining 6/14 cases (43%). The VA origin in the n = 2 patients with VT storm were the aorto-mitral continuity (LBBB morphology) and the interventricular septum (RBBB morphology).
Acute success with non-inducibility of the clinical VT was achieved in all 14 VT cases. In 7/12 patients undergoing ablation of PVCs, PVCs were successfully suppressed. Minor groin complications without need for surgical intervention were observed in two procedures. In one patient with an existing implantable cardioverter-defibrillator, a permanent atrioventricular block III° occurred after B-RFA at the high interventricular septum.
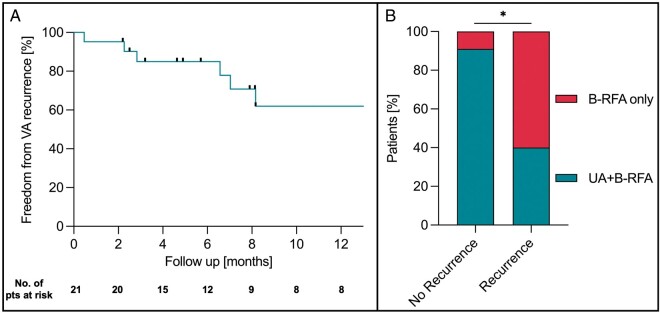
A median follow-up of 211 days (inter-quartile range: 116–453 days) was obtained in 21 patients (Figure 2), with three patients lost to follow-up (n = 2 VT/n = 1 PVC). Freedom from any VT was found in 6/11 patients undergoing VT ablation. One patient presented with recurrence of electrical storm and underwent additional B-RFA VT ablation 50 days after the initial B-RFA procedure.
Figure 2.
Mid-term follow-up after bipolar ablation. (A) Freedom of ventricular arrhythmia (VA) was observed in 59% of patients within 12 months after initial B-RFA. (B) Lower VA recurrence rates were observed in patients with unipolar ablation followed by bipolar ablation (UA+B-RFA) compared with B-RFA alone. B-RFA, bipolar radiofrequency ablation; UA, unipolar ablation; VA, ventricular arrhythmia.
Successful PVC ablation was observed in 5/10 patients. One patient received repeat B-RFA PVC ablation 69 days after the initial procedure with success during 66 days follow-up. In the remaining four patients, burden reduction varied between 55% and 75%, with one patient showing no PVC burden decrease. No difference in VA recurrence was observed between patients with or without structural heart disease (57% vs. 33%, P = 0.40).
Ex vivo ablation
A total of 88 ablation lesions (LV free wall n = 67, interventricular septum n = 21) were analysed. A detailed overview about lesion characteristics is given in Table 4. The mean myocardial tissue thickness was 19 ± 2 mm (range 15–23 mm). Audible and macroscopic steam pops were registered in 13 lesions (range 0–27%) without significant differences between groups (P = 0.29). The maximum bipolar impedance drop was not associated with steam pop occurrence [no steam pop: median—31 (inter-quartile range 24–49) vs. steam pop—38 (30–59) Ω, P = 0.24] and did not differ between all groups (P = 0.08).
Table 4.
Lesion characteristics during ex vivo ablation
| B-RFA | B-RFA | B-RFA | SUA | EA | Titration | SUA 30 W + B-RFA 50 W | P-value | |
|---|---|---|---|---|---|---|---|---|
| 30 W | 40 W | 50 W | 30 W | 50 W | (B-RFA 30 + 50 W) | All groups | ||
| Number of lesions, n | 15 | 9 | 15 | 11 | 15 | 12 | 11 | |
| Myocardial tissue thickness (mm) | 19.4 ± 2.2 | 18.8 ± 1.8 | 19.0 ± 1.8 | 18.0 ± 2.1 | 18.7 ± 1.7 | 18.2 ± 1.9 | 18.4 ± 2.1 | 0.59 |
| Transmural lesions without steam pop, n (%) | 0 0 | 0 0 | 1 (7) | 1 (9) | 1 (7) | 5 (42) | 8 (73)*,† | <0.001 |
| Steam pops, n (%) | 0 0 | 0 0 | 2 (13) | 2 (18) | 4 (27) | 2 (17) | 3 (27) | 0.29 |
| Lesion volume (mm3) | 231* | 224 | 307 | 463 | 95* | 421 | 1386*,† | <0.001 |
| (97–382) | (163–452) | (261–561) | (303–1405) | (41–313) | (259–683) | (1303–1647) | ||
| Lesion surface diameter endocardial (mm) | 3.9 | 4.0 | 4.5 | 5.5 | 4.2† | 4.3† | 7.1*,† | 0.001 |
| (2.5–5.9) | (3.2–5.3) | (3.8–5.2) | (4.5–6.4) | (0–4.7) | (3.2–4.9) | (6.1–8.6) | ||
| Lesion surface diameter epicardial (mm) | 1.4*,† | 3.7 | 3.8 | 3.9 | 2.1 | 3.4 | 8.3*,† | <0.001 |
| (0–3.2) | (2.8–5.4) | (3.1–4.6) | (3.2–6.7) | (0–5.4) | (2.8–4.4) | (7.8–10.0) | ||
| Maximum lesion depth (mm) | 4.8 | 4.8 | 5.9 | 8.1 | 4.1 | 8.1 | 9.3*,† | <0.001 |
| (3.0–6.9) | (4.0–6.0) | (4.0–8.9) | (5.2–8.7) | (3.8–5.9) | (5.2–8.7) | (8.9–10.5) | ||
| Combined endo- + epicardial lesion | 7.5*,† | 11.0 | 11.7 | 11.4 | 8.7* | 13.5 | 18.7*,† | <0.001 |
| depth (mm) | (5.7–9.5) | (8.9–12.4) | (10.4–14.2) | (10.4–14.0) | (3.8–11.8) | (11.1–16.9) | (17.6–21.0) | |
| Combined lesion depth/tissue thickness | 37*,† | 58 | 63 | 65 | 48*,† | 68 | 100*,† | <0.001 |
| ratio (%) | (28–56) | (47–68) | (55–76) | (53–77) | (20–61) | (61–100) | (100–100) | |
| Maximum intramural lesion diameter (mm) | 9.3 ± 3.7 | 9.5 ± 1.5 | 10.1 ± 1.4 | 12.1 ± 2.5 | 8.3 ± 3.7† | 10.5 ± 2.2 | 12.0 ± 0.9* | 0.018 |
| Depth at maximum diameter (mm) | 2.3 | 2.3 | 2.2 | 2.4 | 2.5 | 2.5 | 3.8* | 0.023 |
| (1.1–2.6) | (2.0–2.8) | (1.8–2.6) | (2.1–2.8) | (2.2–3.3) | (2.0–3.7) | (2.5–4.6) |
Values are reported as mean ± standard deviation or absolute numbers and percentages. In case of non-parametric data, results were provided as medians with inter-quartile ranges.
P < 0.05 vs. B-RFA 50 W.
P < 0.05 vs. SUA 30 W.
B-RFA, bipolar radiofrequency ablation; EA, epicardial adipose tissue; SUA, sequential unipolar ablation.
Influence of bipolar energy titration on lesion formation
Energy titration resulted in similar mean lesion volume compared with direct B-RFA using 50 W (P = 0.52) and a trend towards higher transmurality rates without reaching statistical significance (P = 0.052). Steam pop rates were similar between both groups, ranging between 0% and 27% (P > 0.99). Titration did not influence the endo-/epicardial lesion surface diameter (P = 0.44; P = 0.33), maximum lesion depth (P = 0.10), maximum intramural diameter (P = 0.61), or depth at the maximum intramural diameter (P = 0.10).
Combined sequential unipolar and bipolar ablation increases lesion volume and transmurality rates
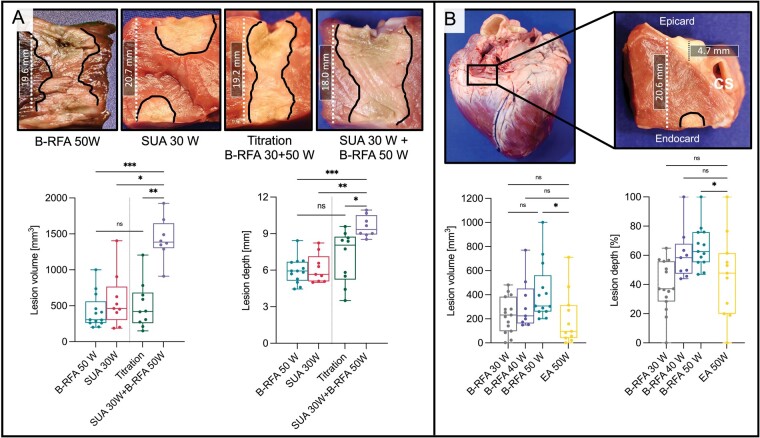
Combined unipolar and B-RFA was a common approach in our cohort used in 58% of cases. Figure 3A displays lesion characteristics of energy titration and combined SUA+B-RFA approaches in comparison to B-RFA 50 W and SUA 30 W controls. B-RFA with 50 W resulted in increased lesion volume but not depth in comparison to SUA 30 W (lesion volume: P = 0.05; lesion depth P = 0.31). Combined SUA+B-RFA resulted in an increased transmurality rate compared with the titration, B-RFA 50 W and SUA 30 W control group (P < 0.001). Furthermore, the lesion volume increased in comparison to all three groups (P < 0.001) and displayed the highest combined endo- and epicardial lesion depth (P < 0.001). The epicardial lesion surface diameter was also increased compared with all three groups (P < 0.001), whereas the endocardial lesion surface diameter appeared larger compared with titration as well as B-RFA 50 W group only. In the clinical cohort, this approach was more frequently observed in patients with arrhythmia freedom during follow-up compared with patients with VA recurrence irrespective of underlying myocardial substrate and applied maximum power (92% vs. 36%, P = 0.009).
Figure 3.
Lesion formation during common clinical bipolar ablation approaches. (A) Lesion volume and depth significantly increased after combination of sequential unipolar (SUA) and bipolar ablation (B-RFA), whereas energy titration did not significantly alter macroscopic lesion characteristics. (B) B-RFA with 50 W in regions of significant epicardial adipose tissue (EA) close to the coronary sinus (CS) significantly reduced lesion volume and depth compared with B-RFA at regions without EA. Commonly, no lesion was visible at the epicardial site (upper right image). B-RFA, bipolar radiofrequency ablation; EA, epicardial adipose tissue; SUA, sequential unipolar ablation.
Epicardial adipose tissue impacts lesion formation
In our clinical cohort, B-RFA was conducted in 81% of procedures at sites where high EA thickness can be expected (outflow tract, aorto-mitral continuity, left ventricular summit, epicardial LV). Figure 3B displays lesion characteristics during B-RFA in EA. Lesion transmurality was similar between EA and B-RFA 30–50 W control groups (range 0–7%, P = 0.55). The total lesion volume as well as the combined endo- and epicardial lesion depth were lower in the EA group compared with the B-RFA 50 W group (endocardial P = 0.03; epicardial P = 0.018). No epicardial lesion was detected in 36% of lesions in the EA group, significantly less compared with the B-RFA 50 W group (P = 0.03). The maximum intramural diameter, depth at the maximum diameter, endocardial and epicardial lesion surface diameter were similar between EA and B-RFA 30–50 W groups.
Discussion
To our knowledge, this study provides the first multicentre experience of patients with therapy-refractory ventricular arrhythmias undergoing B-RFA using a dedicated setup. The major findings are as follows: (i) B-RFA using a dedicated RF generator appears feasible and safe, with a high acute success rate and minor adverse events without need for surgical intervention or prolonged hospital stay. During a follow-up of up to 2 years, 52% of patients remained arrhythmia-free after one B-RFA procedure, a high success rate in view of the unsuccessful prior procedures in all patients. (ii) The combination of unipolar ablation followed by B-RFA might enhance lesion formation.
Challenges of bipolar radiofrequency ablation
The 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement addresses current challenges of B-RFA VA catheter ablation, which was not recommended routinely because of the lack of standardized settings.5 Previous studies using different custom built setups for B-RFA did not address the limitation of lacking temperature and/or impedance monitoring for both catheters.9,10 With power settings ranging between 25 and 50 W, reported complication rates varied overall.9,11,12 In our multicentre cohort, B-RFA using a dedicated setup was performed safely at an average maximum power of 40 W (range 20–50 W). One non-randomized study comparing unipolar to B-RFA using a custom setup in VA was recently suspended because of higher adverse events in the bipolar group (NCT02374476). The dedicated B-RFA setup appears to be safe in this report. Unlike other systems, it addresses the need for simultaneous monitoring of temperature, impedance and irrigation of both catheters as well as changing between unipolar and B-RFA without significant setup changes needed during the procedure.
An overview on published clinical B-RFA studies in patients with VA can be found in the supplementary material. Long-term outcome after B-RFA remains challenging: Two B-RFA multicentre series reported acutely reduced VA burden, but relevant VT recurrence rates in ∼50% and 67% of patients.9,12 This is in line with our observed acute and mid-term outcome. However, in mentioned studies only patients with structural heart disease were included for B-RFA, whereas our collective consisted of 50% of patients without detectable structural heart disease. As deep intramural foci are a relevant cause for unipolar ablation failure,13 they are common not only in patients with structural heart diseases: Approximately >10% of idiopathic septal VA and 20% of idiopathic LVOT VA have been described to be of intramural origin.14,15 In our cohort, 83% of patients without structural heart disease presented VA originating in the left/right ventricular outflow tract or aorto-mitral continuity. Therefore, B-RFA displays a valuable therapeutic option in patients with idiopathic VA.
Nevertheless, when considering the bail-out situations in which B-RFA is mostly employed often with several unsuccessful previous ablation attempts, we believe that this partial success is promising. Della Bella et al.12 recently published a case series using a dedicated 3-D software for B-RFA enabling visualization of both catheters during ablation. Although not described so far in detail, this might be additionally helpful in combination with presented dedicated B-RFA setup as a next step to improve B-RFA applications and procedural workflow.
Impact of commonly used clinical approaches during bipolar ablation
The effect of B-RFA on lesion depth and transmurality rates may be limited in myocardial tissue exceeding 15 mm thickness resulting in similar lesion volumes compared with unipolar ablation.16Ex vivo power titration during sole B-RFA resulted in similar lesion volume levels and steam pop rates compared with immediate higher power B-RFA, while being associated with a trend towards increased transmurality rates. Furthermore, combining unipolar with consecutive B-RFA was conducted in approximately two-thirds of presented cases, which significantly increased lesion volume and transmurality rates without increasing steam pop rates in our ex vivo model. Interestingly, patients without VA recurrence underwent combined unipolar and B-RFA significantly more frequently than patients with recurrence in our clinical cohort. Therefore, this may be a valuable B-RFA approach for therapy-refractory VA in the clinical setting. How a systematic application of these specific approaches may influence lesion formation in vivo needs further investigation.
Importance of epicardial adipose tissue assessment
Areas of thickest EA are commonly found along the basal perivascular segments, reaching >5 mm in 60% of wall segments during systole.17 Of interest, our data on ablation in areas with significant EA confirmed recent ex vivo study results18: EA has lower electrical conductivity compared with pericardial fluid, leading to RF current preferably flowing through surrounding fluid instead of EA and underlying myocardial tissue.2 Accordingly, epicardial sites did not show any significant lesion formation in one-third of lesions in our ex vivo model. Whether emerging catheter systems such as RF needle catheters, pulsed-field or high intensity ultrasound ablation may be able to overcome limited lesion formation at EA sites needs further investigation.19–21
Limitations
This study has several limitations. Being a retrospective multicentre study, ablation protocols and settings differed between centres with possible selection bias. As we report the so far largest clinical cohort with a dedicated B-RFA setup, the total number of procedures remains small with no matched control group which has to be considered during interpretation of presented safety data. Furthermore, an exact validation of the distance between active and return catheter and their respective position was not possible. Limitations of our ex vivo model include presence of ischaemic myocardium due to non-perfusion as well as altered cellular and inflammatory reaction to RF ablation compared with in vivo models. The used model did not allow to investigate lesion formation in areas of myocardial scar. Immediate translation of presented ex vivo data into our clinical cohort remains challenging, as there are no histological data displaying the relationship between lesions and ablation targets. Passive catheter cooling characteristics may also differ compared with epicardial catheter positioning/positioning in the coronary sinus.
Conclusion
Bipolar catheter ablation using a dedicated approach is feasible and safe for the treatment of VA. Using this standardized setting, acute efficacy is high with low complication rates. Overall follow-up is encouraging as more than half of the patients remain arrhythmia-free within 12 months. Combined SUA and B-RFA may enhance ablation efficacy.
Supplementary Material
Acknowledgements
We thank Osypka AG for providing technical assistance during the performance of the ex vivo ablation procedures.
Funding
European Union BigData@Heart (grant agreement EU IMI 116074), British Heart Foundation (FS/13/43/30324, PG/17/30/32961, and PG/20/22/35093), German Centre for Cardiovascular Research supported by the German Ministry of Education and Research (DZHK, via a grant to AFNET), and Leducq Foundation to P.K.; P.K. is listed as inventor on two patents held by University of Birmingham (Atrial Fibrillation Therapy WO 2015140571, Markers for Atrial Fibrillation WO 2016012783). P.K. receives research support for basic, translational, and clinical research projects from European Union, British Heart Foundation, Leducq Foundation, Medical Research Council (UK), and German Centre for Cardiovascular Research. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest: S.K., R.S., K.S., L.D., and R.P. declared no conflict of interest; F.-A.A.: travel grants from Boston Scientific and Bayer Pharmaceuticals; C.M.: speaker and consulting: Abbott, Boston Scientific; speaker: Biosense Webster; A.R.: travel grants and lecture fees from Biosense Webster, Medtronic, Ablacon, EPD/Phillips, Cardiofocus, Abott, and Boehringer Ingelheim; J.B. and P.K.: speaker and consulting fees from Biosense Webster.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Contributor Information
Shinwan Kany, Department of Cardiology, University Heart and Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistraße 52, 20251 Hamburg, Germany; DZHK (German Center for Cardiovascular Research), Partner Site Hamburg/Kiel/Lübeck, Berlin, Germany.
Fares Alexander Alken, DZHK (German Center for Cardiovascular Research), Partner Site Hamburg/Kiel/Lübeck, Berlin, Germany; Division of Cardiology/Angiology/Intensive Care, EVK Düsseldorf, Cardiac Neuro- and Electrophysiology Research Consortium (cNEP), Kirchfeldstr. 40, 40217 Düsseldorf, Germany.
Ruben Schleberger, Department of Cardiology, University Heart and Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistraße 52, 20251 Hamburg, Germany.
Jakub Baran, Division of Clinical Electrophysiology, Department of Cardiology, Centre of Postgraduate Medical Education, Grochowski Hospital, Warsaw, Poland.
Armin Luik, Department of Medicine IV, Hospital Karlsruhe GmbH, Karlsruhe, Germany.
Annika Haas, Department of Medicine IV, Hospital Karlsruhe GmbH, Karlsruhe, Germany.
Elena Ene, Division of Cardiology II, Röhn Hospital, Campus Bad Neustadt, Bad Neustadt/Saale, Germany.
Thomas Deneke, Division of Cardiology II, Röhn Hospital, Campus Bad Neustadt, Bad Neustadt/Saale, Germany.
L Dinshaw, Department of Cardiology, University Heart and Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistraße 52, 20251 Hamburg, Germany.
Andreas Rillig, Department of Cardiology, University Heart and Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistraße 52, 20251 Hamburg, Germany.
Andreas Metzner, Department of Cardiology, University Heart and Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistraße 52, 20251 Hamburg, Germany.
Bruno Reissmann, Department of Cardiology, University Heart and Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistraße 52, 20251 Hamburg, Germany.
Hisaki Makimoto, Division of Cardiology, University Hospital Düsseldorf, Düsseldorf, Germany.
Tilko Reents, Division of Cardiology, German Heart Center Munich, Munich, Germany.
Miruna Andrea Popa, Division of Cardiology, German Heart Center Munich, Munich, Germany.
Isabel Deisenhofer, Division of Cardiology, German Heart Center Munich, Munich, Germany.
Roman Piotrowski, Division of Clinical Electrophysiology, Department of Cardiology, Centre of Postgraduate Medical Education, Grochowski Hospital, Warsaw, Poland.
Piotr Kulakowski, Division of Clinical Electrophysiology, Department of Cardiology, Centre of Postgraduate Medical Education, Grochowski Hospital, Warsaw, Poland.
Paulus Kirchhof, Department of Cardiology, University Heart and Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistraße 52, 20251 Hamburg, Germany; DZHK (German Center for Cardiovascular Research), Partner Site Hamburg/Kiel/Lübeck, Berlin, Germany; Institute of Cardiovascular Sciences, University of Birmingham, Birmingham, UK.
Katharina Scherschel, DZHK (German Center for Cardiovascular Research), Partner Site Hamburg/Kiel/Lübeck, Berlin, Germany; Division of Cardiology/Angiology/Intensive Care, EVK Düsseldorf, Cardiac Neuro- and Electrophysiology Research Consortium (cNEP), Kirchfeldstr. 40, 40217 Düsseldorf, Germany; Institute for Neural and Sensory Physiology, Cardiac Neuro- and Electrophysiology Research Consortium (cNEP), Medical Faculty, Heinrich Heine University Düsseldorf, Düsseldorf, Germany.
Christian Meyer, DZHK (German Center for Cardiovascular Research), Partner Site Hamburg/Kiel/Lübeck, Berlin, Germany; Division of Cardiology/Angiology/Intensive Care, EVK Düsseldorf, Cardiac Neuro- and Electrophysiology Research Consortium (cNEP), Kirchfeldstr. 40, 40217 Düsseldorf, Germany; Institute for Neural and Sensory Physiology, Cardiac Neuro- and Electrophysiology Research Consortium (cNEP), Medical Faculty, Heinrich Heine University Düsseldorf, Düsseldorf, Germany.
References
- 1. Burnes JE, Taccardi B, Ershler PR, Rudy Y.. Noninvasive electrocardiogram imaging of substrate and intramural ventricular tachycardia in infarcted hearts. J Am Coll Cardiol 2001;38:2071–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. González-Suárez A, Trujillo M, Koruth J, d'Avila A, Berjano E.. Radiofrequency cardiac ablation with catheters placed on opposing sides of the ventricular wall: computer modelling comparing bipolar and unipolar modes. Int J Hyperthermia 2014;30:372–84. [DOI] [PubMed] [Google Scholar]
- 3. Sauer WH, Steckman DA, Zipse MM, Tzou WS, Aleong RG.. High-power bipolar ablation for incessant ventricular tachycardia utilizing a deep midmyocardial septal circuit. Heart Rhythm Case Rep 2015;1:397–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Teh AW, Reddy VY, Koruth JS, Miller MA, Choudry S, D'Avila Aet al. Bipolar radiofrequency catheter ablation for refractory ventricular outflow tract arrhythmias. J Cardiovasc Electrophysiol 2014;25:1093–9. [DOI] [PubMed] [Google Scholar]
- 5. Cronin EM, Bogun FM, Maury P, Peichl P, Chen M, Namboodiri Net al. ; ESC Scientific Document Group . 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Europace 2019;21:1143–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schleberger R, Jularic M, Salzbrunn T, Hacke C, Schwarzl JM, Hoffmann BAet al. Outcome of catheter ablation of non-reentrant ventricular arrhythmias in patients with and without structural heart disease. Eur J Med Res 2020;25:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nührich JM, Kaiser L, Akbulak RÖ, Schäffer BN, Eickholt C, Schwarzl Met al. Substrate characterization and catheter ablation in patients with scar-related ventricular tachycardia using ultra high-density 3-D mapping. J Cardiovasc Electrophysiol 2017;28:1058–67. [DOI] [PubMed] [Google Scholar]
- 8. Nguyen DT, Zheng L, Zipse MM, Borne RT, Tzou WS, Fleeman Bet al. Bipolar radiofrequency ablation creates different lesion characteristics compared to simultaneous unipolar ablation. J Cardiovasc Electrophysiol 2019;30:2960–7. [DOI] [PubMed] [Google Scholar]
- 9. Igarashi M, Nogami A, Fukamizu S, Sekiguchi Y, Nitta J, Sakamoto Net al. Acute and long-term results of bipolar radiofrequency catheter ablation of refractory ventricular arrhythmias of deep intramural origin. Heart Rhythm 2020;17:1500–7. [DOI] [PubMed] [Google Scholar]
- 10. Futyma P, Ciąpała K, Głuszczyk R, Sander J, Futyma M, Kułakowski P.. Bipolar ablation of refractory atrial and ventricular arrhythmias: importance of temperature values of intracardiac return electrodes. J Cardiovasc Electrophysiol 2019;30:1718–26. [DOI] [PubMed] [Google Scholar]
- 11. Futyma P, Głuszczyk R, Futyma M, Kułakowski P.. Right atrial position of a return electrode for bipolar ablation of the left posterosuperior process ventricular tachycardia. Pacing Clin Electrophysiol 2019;42:474–7. [DOI] [PubMed] [Google Scholar]
- 12. Della Bella P, Peretto G, Paglino G, Bisceglia C, Radinovic A, Sala Set al. Bipolar radiofrequency ablation for ventricular tachycardias originating from the interventricular septum: safety and efficacy in a pilot cohort study. Heart Rhythm 2020;17:2111–8. [DOI] [PubMed] [Google Scholar]
- 13. Tokuda M, Kojodjojo P, Tung S, Tedrow UB, Nof E, Inada Ket al. Acute failure of catheter ablation for ventricular tachycardia due to structural heart disease: causes and significance. JAHA 2013;2:e000072–e000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yamada T, Doppalapudi H, Maddox WR, McElderry HT, Plumb VJ, Kay GN.. Prevalence and electrocardiographic and electrophysiological characteristics of idiopathic ventricular arrhythmias originating from intramural foci in the left ventricular outflow tract. Circ: Arrhythmia Electrophysiol 2016;9:e004079. [DOI] [PubMed] [Google Scholar]
- 15. Yokokawa M, Good E, Chugh A, Pelosi F, Crawford T, Jongnarangsin Ket al. Intramural idiopathic ventricular arrhythmias originating in the intraventricular septum. Circ Arrhyth Electrophysiol 2012;5:258–63. [DOI] [PubMed] [Google Scholar]
- 16. Nguyen DT, Tzou WS, Brunnquell M, Zipse M, Schuller JL, Zheng Let al. Clinical and biophysical evaluation of variable bipolar configurations during radiofrequency ablation for treatment of ventricular arrhythmias. Heart Rhythm 2016;13:2161–71. [DOI] [PubMed] [Google Scholar]
- 17. Sourwine M, Jeudy J, Miller B, Vunnam R, Imanli H, Mesubi Oet al. Location, variations, and predictors of epicardial fat mapping using multidetector computed tomography to assist epicardial ventricular tachycardia ablation. Pacing Clin Electrophysiol 2017;40:1059–66. [DOI] [PubMed] [Google Scholar]
- 18. Zipse MM, Edward JA, Zheng L, Tzou WS, Borne RT, Sauer WHet al. Impact of epicardial adipose tissue and catheter ablation strategy on biophysical parameters and ablation lesion characteristics. J Cardiovasc Electrophysiol 2020;31:1114–24. [DOI] [PubMed] [Google Scholar]
- 19. Dickow J, Suzuki A, Henz BD, Madhavan M, Lehmann HI, Wang Set al. Characterization of lesions created by a heated, saline irrigated needle-tip catheter in the normal and infarcted canine heart. Circ Arrhythm Electrophysiol 2020;13:e009090. [DOI] [PubMed] [Google Scholar]
- 20. Koruth JS, Kuroki K, Iwasawa J, Viswanathan R, Brose R, Buck EDet al. Endocardial ventricular pulsed field ablation: a proof-of-concept preclinical evaluation. Europace 2020;22:434–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nazer B, Giraud D, Zhao Y, Hodovan J, Elman MR, Masri Aet al. High-intensity ultrasound catheter ablation achieves deep mid-myocardial lesions in vivo. Heart Rhythm 2021;18:623–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.