Abstract
A strong polychlorinated biphenyl (PCB) degrader, Rhodococcus sp. strain RHA1, has diverse biphenyl/PCB degradative genes and harbors huge linear plasmids, including pRHL1 (1,100 kb), pRHL2 (450 kb), and pRHL3 (330 kb). The diverse degradative genes are distributed mainly on the pRHL1 and pRHL2 plasmids. In this study, the structural and functional characteristics of pRHL2 were determined. We constructed a physical map of pRHL2, and the degradative enzyme genes, including bphB2, etbD2, etbC, bphDEF, bphC2, and bphC4, were localized in three regions. Conjugal transfer of pRHL2 between RHA1 mutant derivatives was observed at a frequency of 7.5 × 10−5 transconjugant per recipient. These results suggested that the linear plasmid is a possible determinant of propagation of the diverse degradative genes in rhodococci. The termini of pRHL2 were cloned and sequenced. The left and right termini of pRHL2 had 3-bp perfect terminal inverted repeats and were not as similar to each other (64% identity) as the known actinomycete linear replicons are. Southern hybridization analysis with pRHL2 terminal probes suggested that the right terminus of pRHL2 is similar to pRHL1 and pRHL3 termini. Retardation of both terminal fragments in the gel shift assay indicated that each terminus of pRHL2 is linked to a protein. We suggest that pRHL2 has invertron termini, as has been reported previously for Streptomyces linear replicons.
Linear DNA elements have been described in various gram-positive and gram-negative genera (9). Some of the linear elements, have inverted repeats at their ends and proteins bound to their 5′ ends. Such structural characteristics have been found in the linear elements of actinomycetes, bacteriophages, and viruses. This class of elements has been termed invertrons (18). A second class of bacterial linear plasmids having covalently closed ends at the termini of their DNAs has been found in the genus Borrelia (8). Linear plasmids were first described in Streptomyces sp. (7). Among the mycolic acid-containing actinomycetes, linear plasmids have been described in Rhodococcus opacus (11), Mycobacterium sp. (17), Rhodococcus fascians ( 1), and Rhodococcus erythropolis (2, 14).
Rhodococcus sp. strain RHA1 is a gram-positive polychlorinated biphenyl (PCB) degrader that efficiently transforms a wide range of PCB congeners (20, 21). Diverse degradative genes have been isolated from this strain, as shown in Table 1. These include three α-subunit genes (bphA1, ORF1, and ORF3), three β-subunit genes (bphA2, ORF2, and ORF4), two ferredoxin component genes (bphA3 and ORF5), and one ferredoxin reductase component gene (bphA4) of the ring-hydroxylating dioxygenases, two dihydrodiol dehydrogenase genes (bphB and bphB2), seven extradiol ring cleavage dioxygenase genes (bphC, bphC2, bphC3, bphC4, bphC5, bphC6, and etbC), three ring cleavage compound hydrolase genes (bphD, etbD1, and etbD2), one hydroxypentadienoate hydrolase gene (bphE), and one hydroxyoxovalerate aldolase gene (bphF). RHA1 contains three linear plasmids, pRHL1 (1,100 kb), pRHL2 (390 kb), and pRHL3 (280 kb), and the bphA1A2A3A4CB and bphDEF gene clusters have been found to be localized on pRHL1 and pRHL2, respectively (16). Some deletions in these plasmids have resulted in the loss of some degradative genes and in growth deficiencies on biphenyl (3).
TABLE 1.
Degradative genes in RHA1
| Gene Cluster | Localization | Gene | Function or probable function | Reference(s) |
|---|---|---|---|---|
| bphA1A2A3A4CB | pRHL1 | bphA1 | α Subunit of ring-hydroxylating dioxygenase | 15 |
| bphA2 | β Subunit of ring-hydroxylating dioxygenase | 15 | ||
| bphA3 | Ferredoxin component of ring-hydroxylating dioxygenase | 15 | ||
| bphA4 | Ferredoxin reductase component of ring-hydroxylating dioxygenase | 15 | ||
| bphB | Dihydrodiol dehydrogenase | 15 | ||
| bphC | Extradiol ring cleavage dioxygenase | 15 | ||
| ORF1·2 etbC bphDEF | pRHL2 | bphD | Ring cleavage compound hydrolase | 16 |
| bphE | Hydroxypentadienoate hydratase | 16 | ||
| bphF | Hydroxyoxovalerate aldolase | 16 | ||
| etbC | Extradiol ring cleavage dioxygenase | 6, 16 | ||
| ORF1 | α Subunit of ring-hydroxylating dioxygenasea | 24 | ||
| ORF2 | β Subunit of ring-hydroxylating dioxygenasea | 24 | ||
| ORF3·4·5 etbD2 | pRHL2 | etbD2 | Ring cleavage compound hydrolase | 24 |
| ORF3 | α Subunit of ring-hydroxylating dioxygenasea | 24 | ||
| ORF4 | β Subunit of ring-hydroxylating dioxygenasea | 24 | ||
| ORF5 | Ferredoxin component of ring-hydroxylating dioxygenasea | 24 | ||
| Unknown | pRHL2 | bphB2 | Dihydrodiol dehydrogenasea | Unpublished data |
| pRHL2 | bphC2 | Extradiol ring cleavage dioxygenase | Unpublished data | |
| Chromosome | bphC3 | Extradiol ring cleavage dioxygenase | Unpublished data | |
| pRHL2 | bphC4 | Extradiol ring cleavage dioxygenase | Unpublished data | |
| Chromosome | bphC5 | Extradiol ring cleavage dioxygenase | Unpublished data | |
| Chromosome | bphC6 | Extradiol ring cleavage dioxygenase | Unpublished data | |
| pRHL1 | etbD1 | Ring cleavage compound hydrolase | 5, 24 |
Putative gene products were deduced based on similarity to other genes whose functions are known.
In this study, to obtain insight into the involvement of the RHA1 linear plasmids in propagation and assembly of degradative genes, localization of the degradative genes on plasmids and transfer of linear plasmids were examined with a particular focus on pRHL2. We then characterized the termini of pRHL2 to address the structural features of this linear element.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The strains and plasmids used in this study are shown in Table 2. Rhodococcus strains were grown at 30°C in Luria-Bertani (LB) medium, diluted LB medium, or W minimal salt medium supplemented with 0.2% biphenyl or ethylbenzene vapor (15). RCA1 is a bphA1A2A3A4CB deletion mutant of RHA1. RHA1 mutant RDO5 lacks the pRHL2 plasmid and carries a kanamycin resistance gene in its chromosome. This strain was grown in the presence of 100 μg of kanamycin per ml.
TABLE 2.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| Rhodococcus sp. strains | ||
| RHA1 | Wild type; BP+; pRHL1, pRHL2, and pRHL3 carrier | 15 |
| RCA1 | Spontaneous mutant of RHA1; BP−; ΔbphA1A2A3A4CB; pRHL1-1, pRHL2, and pRHL3 carrierb | This study |
| RDO5 | Kmr gene insertion mutant of RHA1; BP− Kmr; pRHL1 and pRHL3 carrier | This study |
| Plasmids | ||
| pUC18 | Cloning vector; Apr | 25 |
| pUC118 and pUC119 | Cloning vectors; Apr | 23 |
| pBluescript II SK(+) | Cloning vector; Apr | 22 |
| pK4 | Rhodococcus-E. coli shuttle vector; Kmr | 4 |
| pK4HKcos | pK4 containing cos region | 24 |
| pC4A | pUC119 with a 2.6-kb fragment of RHA1 carrying bphC2 | Unpublished data |
| pC4B | pUC119 with a 2.8-kb fragment of RHA1 carrying bphC3 | Unpublished data |
| pC4C | pUC119 with a 2.5-kb fragment of RHA1 carrying bphC4 | Unpublished data |
| pC4F | pUC119 with a 2.8-kb fragment of RHA1 carrying bphC5 | Unpublished data |
| pTN8 | pUC119 with a 1.1-kb BglII fragment of RHA1 carrying bphC6 | Unpublished data |
| pHE1 | pUC119 with a 4.1-kb EcoRV fragment of RHA1 carrying ORF3·4·5 etbD2 | 24 |
| pKHD1 | pUC118 with a 3-kb EcoRI fragment of RHA1 carrying etbD1 | 24 |
| pHA101 | pUC119 with a 6-kb BamHI fragment of RHA1 carrying bphA1A2A3A4CB | 15 |
| pEB2 | pUC119 with a 1-kb BamHI fragment of RHA1 carrying bphDE | 16 |
| pUH5A2 | pUC119 with a 5.6-kb EcoRI fragment of RHA1 carrying bphB2 | Unpublished data |
| pTPS3 | pUC18 with a 1.9-kb fragment carrying the right end of pRHL2 | This study |
| pTPS4 | pUC18 with a 2-kb fragment carrying the left end of pRHL2 | This study |
BP+, growth on biphenyl; BP−, no growth on biphenyl; Apr, ampicillin resistance; Kmr, kanamycin resistance.
pRHL1-1 is a derivative of pRHL1 lacking bphA1A2A3A4CB.
Preparation and detection of linear plasmid DNA.
Rhodococcus strains were grown in 10 ml of diluted LB medium to an optical density at 600 nm of 0.8. Cells were harvested by centrifugation at 2,000 × g for 10 min, washed twice with 0.4 M sucrose, and recentrifuged. Then they were resuspended in 0.5 ml of TES (0.3 M sucrose, 0.25 M EDTA, 0.25 M Tris-HCl; pH 8) and mixed with 1.4% low-melting-point SeaPlaque agarose (FMC, Rockland, Maine) in TBE (0.49 M Tris, 0.49 M boric acid, 0.001 M EDTA; pH 8) (12). The resulting mixture was pipetted into plug molds (Bio-Rad Laboratories, Richmond, Calif.). After incubation at 4°C for 15 min, the agarose plugs were pushed out of the molds into 5 ml of a lysozyme solution (1 mg/ml) in TES. After incubation at 37°C for 2 h with swaying, the plugs were transferred to 5 ml of NDS (0.5 M EDTA, 0.01 M Tris [pH 9], 1% [wt/vol] lauroyl sarcosine) containing proteinase K (1 g/ml) and incubated at 50°C for 20 to 40 h. The proteinase K solution was predigested at 50°C for 1 h. For preparation of non-proteinase K-treated plasmid DNA, proteinase K was omitted. To digest DNA with restriction enzymes, an agarose plug containing DNA was soaked in 200 μl of restriction enzyme buffer for 30 min. The buffer was replaced with 200 μl of fresh buffer, and 9 to 36 U of restriction enzymes was added. The plugs were incubated at 37°C for at least 20 h. For pulsed-field gel electrophoresis (PFGE), the plugs were inserted into the wells of agarose gels consisting of 1% agarose in TBE. Electrophoresis was performed with TBE as the running buffer at 14°C by using a CHEF DRII PFGE system (Bio-Rad). The voltage, pulse time, and total running time were varied according to the size range of the fragments to be separated. Specific conditions are provided in the legends to the figures. Saccharomyces cerevisiae YNN295 chromosomes (Bio-Rad), a bacteriophage lambda ladder (Bio-Rad), and the Kb DNA ladder (Stratagene, La Jolla, Calif.) were used as size markers. Sodium dodecyl sulfate (SDS)-PFGE analysis was carried out by adding SDS (final concentration, 0.2%) to both the buffer and the agarose gel (13). SDS-PFGE was performed for 23 h in a 200-V electric field by using pulse times ranging from 60 to 90 s. Conventional agarose gel electrophoresis was carried out with 1.5% agarose in TAE buffer (0.04 M Tris, 0.04 M acetate, 0.001 M EDTA).
Southern hybridization analysis.
For gene assignment, DNA fragments separated by PFGE or conventional agarose gel electrophoresis were transferred to Hybond N nylon membrane filters (Amersham International plc, Buckinghamshire, United Kingdom). Southern hybridization was carried out by using the protocols provided by Amersham and a probe labeled with the digoxygenin (DIG) system (Boehringer Manheim Biochemicals, Indianapolis, Ind.). Probes were labeled as described in the DIG system manual. pRHL2 DNAs used for probes were recovered from PFGE gels by electroelution. A block of gel containing pRHL2 DNA was cut out and was enclosed in a dialysis bag filled with TBE. The dialysis bag was placed in TBE in the PFGE apparatus, and the DNA was eluted from the gel block by PFGE. PFGE was performed for 6 h by using the conditions described above for SDS-PFGE. The DNA was recovered from TBE in the dialysis bag by butyl alcohol concentration and ethyl alcohol precipitation. The DNA recovered was digested with a restriction enzyme and labeled with the DIG system.
Construction of subclones.
Subclones of pRHL2 were selected from an RHA1 total DNA cosmid library constructed by using pK4HKcos (24). Screening of pRHL2 subclones was performed by colony hybridization using restriction fragments of pRHL2 as the probes. Colony hybridization was carried out by using the protocols of Amersham. The degradative gene and terminal fragment probes were employed to obtain subclones containing the corresponding genes or fragments.
Mating experiment.
Mating was performed by using RCA1 as the donor and RDO5 as the recipient. Three milliliters of RDO5 cells grown in 10 ml of LB medium containing kanamycin and 3 ml of RCA1 cells grown in 10 ml of LB medium were mixed and transferred onto a nitrocellulose filter with a diameter of 25 mm and a pore size of 0.45 μm (Advantec, Tokyo, Japan) by filtration. The filter was placed on the surface of an LB medium plate and incubated at 30°C for 40 h. Then the cells were suspended in 1 ml of 0.9% NaCl, and 100-μl portions of appropriate dilutions were spread onto a W minimal salt medium agar plate containing 100 mg of kanamycin per liter. The plates were supplemented with biphenyl and incubated at 30°C for 2 days. The colonies formed on the plates were isolated as candidates for transconjugants and were subjected to plasmid DNA analysis by PFGE.
Cloning and sequencing of the terminal fragments.
pRHL2 DNA was separated by PFGE and recovered by electroelution. It was digested with PstI and was ligated with pUC18 linearized with PstI and SmaI. Escherichia coli JM109 was transformed by this ligation mixture, and transformants were selected on LB agar plates containing 50 mg of ampicillin per liter, 2 mM isopropyl-β-d-thiogalactopyranoside, and 0.04% 5-bromo-4-chloro-3-indolyl-β-d-galactoside. The terminal fragments of pRHL2 were subcloned in pUC18 and pUC19 and were sequenced by the dideoxy termination method (19) using an ALF Express DNA sequencer (Pharmacia). Sequence analysis was carried out by using the GeneWorks program (IntelliGenetics Inc., Mountain View, Calif.) and the FASTA program provided by DDBJ.
Nucleotide sequence accession number.
The nucleotide sequences of the pRHL2 left and right ends determined in this study have been deposited in the DDBJ, EMBL, and GenBank nucleotide sequence detabases under accession numbers AB048369 and AB048370, respectively.
RESULTS AND DISCUSSION
Localization of degradative genes on the linear plasmids in Rhodococcus sp. strain RHA1.
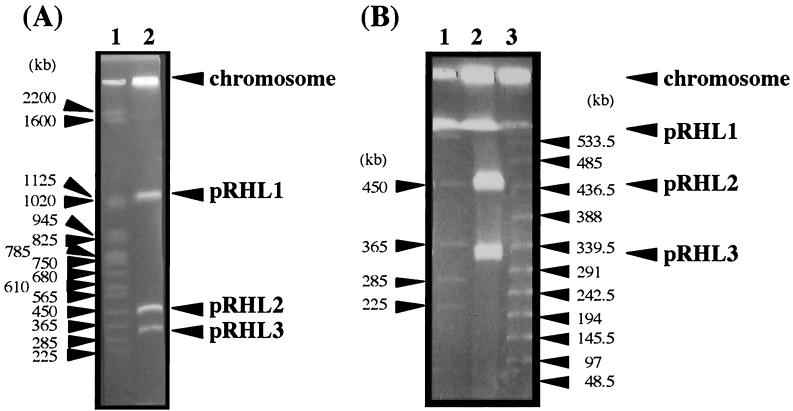
We have previously reported that Rhodococcus sp. strain RHA1 has three linear plasmids, pRHL1 (1,100 kb), pRHL2 (390 kb), and pRHL3 (280 kb) (16). In this study, the sizes of these linear plasmids were reevaluated by PFGE with improved conditions suitable for separating DNA fragments ranging from 200 to 500 kb long. The sizes of pRHL2 and pRHL3 were estimated to be 450 and 330 kb, respectively (Fig. 1). To localize the variety of degradative genes shown in Table 1, Southern hybridization analysis of the linear plasmids separated by PFGE was carried out using each gene probe. As a result, etbD1 was localized on pRHL1, which contained the bphA1A2A3A4CB genes, and bphC2, bphC4, and ORF3·4·5 etbD2 were localized on pRHL2, which included the etbC bphDEF genes (Table 1). It is assumed that the etb genes are involved in ethylbenzene degradation based on the profile of induction by ethylbenzene and the substrate specificity of their products (5, 6, 24). In contrast, bphC3, bphC5, and bphC6 hybridized at the position in the wells in which the chromosomal DNA should remain. These results indicated that degradative genes are widely distributed in the RHA1 genome, except for pRHL3.
FIG. 1.
PFGE of RHA1 linear plasmids performed with long (A) and short (B) pulse times. The positions of the chromosome and linear plasmids are indicated. (A) The pulse time was increased from 60 to 90 s during 23 h of electrophoresis. The voltage was adjusted to 6 V/cm. (B) The pulse time was increased from 20 to 30 s during 23 h of electrophoresis. The voltage was adjusted to 6 V/cm. Lane 1, S. cerevisiae chromosome marker, lane 2, total DNA of RHA1; lane 3, λ ladder marker.
It has been reported that the bphC genes of R. erythropolis TA421 and the isopropylbenzene degradation genes (ipb) of R. erythropolis BD2 are localized on the linear plasmids pTA421 (14) and pBD2 (2), respectively. These observations suggest that linear plasmids may play a role in propagation of the various degradative genes in rhodococci.
Physical map of pRHL2.
We constructed restriction maps of pRHL2 using physical methods and hybridization analysis. Because the G+C content of Rhodococcus DNA is high, restriction enzymes AflII, AseI, and HpaI with AT-rich recognition sequences were employed to generate a manageable number of fragments from pRHL2. All the DNA fragments generated by restriction enzyme digestion were separated by PFGE (data not shown). Five HpaI fragments smaller than 6 kb were separated by conventional agarose gel electrophoresis (data not shown). The estimated sizes of the restriction fragments are shown in Table 3. The total size of pRHL2 was estimated to be approximately 450 kb.
TABLE 3.
Fragment sizes of pRHL2 digested with restriction enzymes
| Restriction enzyme | Fragment | Fragment size (kb)a |
|---|---|---|
| AflII | F1 | 240 |
| F2 | 200 | |
| F3 | 11 | |
| AseI | A1 | 120 |
| A2 | 115 | |
| A3 | 100 | |
| A4 | 45 | |
| A5 | 35 | |
| A6 | 23 | |
| A7 | 18 | |
| HpaI | H1 | 108 |
| H2 | 80 | |
| H3 | 60 | |
| H4 | 55 | |
| H5 | 50 | |
| H6 | 35 | |
| H7 | 28 | |
| H8 | 8 | |
| H9 | 5.5 | |
| H10 | 5.2 | |
| H11 | 4.7 | |
| H12 | 4.5 | |
| H13 | 4 |
The total sizes of the AflII, AseI, and HpaI fragments are 451, 456, and 448 kb, respectively.
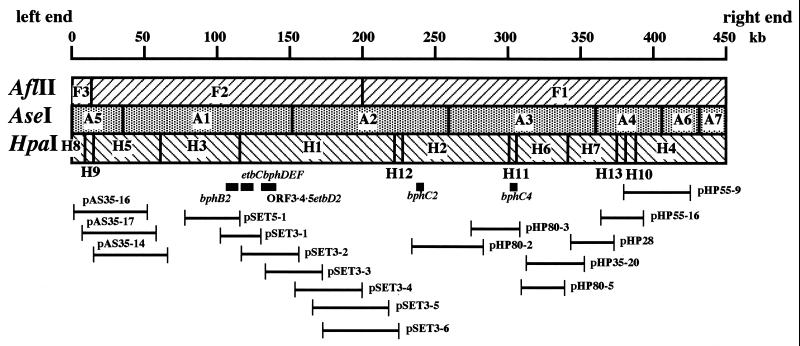
The orders of the restriction fragments were determined by Southern hybridization analysis. Each restriction fragment was extracted from the gel after PFGE and was used to prepare the fragment probes. Small HpaI fragments H9, H10, H11, H12, and H13 were independently cloned in the EcoRV site of pBluescript II SK(+), and the resulting plasmids were used for preparation of the fragment probes. If a fragment probe contains a restriction site between two adjacent fragments, it hybridizes to both of the adjacent fragments. Thus, the A5 and A2 probes, specified the connections between F3 and F2 and between F2 and F1, respectively, indicating that the order is F3-F2-F1 (Fig. 2). The H5, H1, H2, H7, and H4 probes determined the connections between A5 and A1, between A1 and A2, between A2 and A3, between A3 and A4, and between A4 and A6 or A7, respectively, indicating that the order is A5-A1-A2-A3-A4-A6/A7. If a fragment probe spans three joining fragments, the fragment in the middle hybridizes only to the probe. The A1, A2, A3, and A4 probes determined the partial orders H5-H3-H1, H1-H12-H2, H2-H11/H6-H7, and H7-H13/H10-H4, respectively. Conversely, the order of the AseI fragments specified the following order for the HpaI fragments: H8/H9-H5-H3-H1-H12-H2-H11/H6-H7-H13/H10/H4.
FIG. 2.
Physical map of pRHL2 and localization of degradative genes. The locations of degradative genes are indicated by bars below the map. The insert region of each pRHL2 subclone is also indicated below the map.
The orders of the fragments A6 and A7, H8 and H9, H11 and H6, and H13 and H10 were determined by restriction analysis of pRHL2 subclones. Restriction analysis of pHP55-9 specified the partial order A4-A6-A7, indicating that the complete order of the AseI fragments is A5-A1-A2-A3-A4-A6-A7. Restriction analyses of pAS35-16, pHP80-3, and pHP55-16 determined the partial orders H8-H9-H5, H2-H11-H6, and H7-H13-H10, respectively, indicating that the complete order of HpaI fragments is H8-H9-H5-H3-H1-H12-H2-H11-H6-H7-H13-H10-H4.
Localization of the degradative genes was determined by Southern hybridization analysis of pRHL2 restriction fragments using each degradative gene probe. The bphB2 probe hybridized to A1 and H3. The bphD and etbD2 probes hybridized to A1 and H1 (Fig. 2). On the other hand, the bphC2 probe hybridized to A2 and H2, and the bphC4 probe hybridized to A3 and H11. The detailed locations of the bphB2, bphD, etbD2, bphC2, and bphC4 genes were determined, as illustrated in Fig. 2, by Southern hybridization analysis of subclones pSET3-1, pSET3-2, pHP80-2, and pHP80-3. These results indicated that bphB2 and ORF3·4·5 etbD2 are localized proximal to etbC bphDEF, while bphC2 and bphC4 are separated from these genes.
Conjugal transfer of pRHL2.
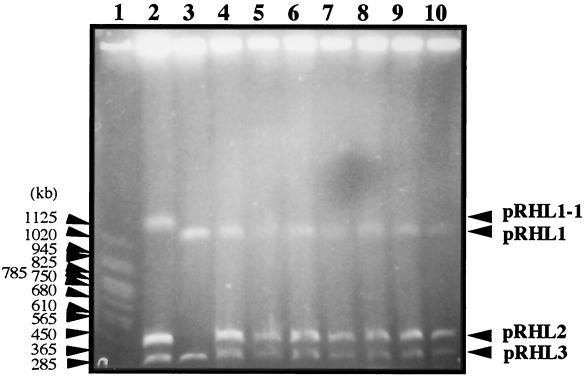
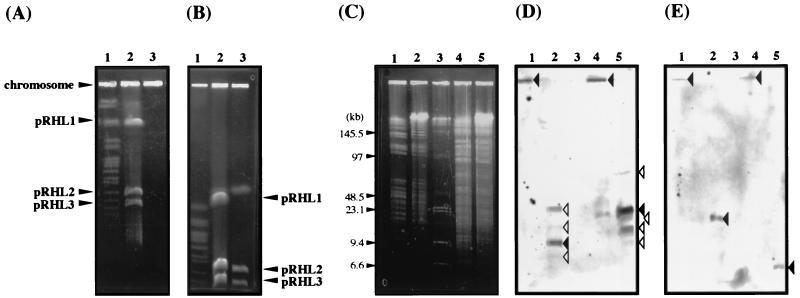
To investigate whether pRHL2 is self-transmissible, mating experiments were carried out using RHA1 mutant strains RCA1 and RDO5 as the donor and the recipient, respectively. RCA1 contained a derivative of pRHL1, pRHL1-1, which lacks the bphA1A2A3A4CB region. pRHL1-1 is 100 kb larger than pRHL1, suggesting that pRHL1-1 was generated by not only deletion of the bphACB region but also an unknown insertion. Strain RDO5 lacked all of pRHL2 and contained an insertion of the kanamycin resistance (Kmr) gene in its chromosome. RCA1 and RDO5 were not able to grow on biphenyl because of deficiencies in bphA1A2A3A4CB and bphD, respectively. Thus, only the derivative of RDO5 that received pRHL2 from RCA1 could grow on biphenyl in the minimum medium containing kanamycin. BP+ and Kmr colonies were obtained at a frequency of 7.5 × 10−5 colony per recipient. Seven independently isolated transconjugants were examined in the presence of pRHL2 and the Kmr gene by PFGE (Fig. 3) and Southern hybridization analysis (data not shown), respectively. All the transconjugants tested possessed both pRHL2 and the Kmr gene, indicating that pRHL2 was transmitted from RCA1 to RDO5.
FIG. 3.
Linear plasmids in transconjugants. Linear plasmids of a donor (RCA1), a recipient (RDO5), and transconjugants were separated by PFGE. The pulse time was increased from 60 to 90 s during 23 h of electrophoresis. The voltage was adjusted to 6 V/cm. Lane 1, S. cerevisiae chromosome marker; lane 2, RCA1; lane 3, RDO5; lanes 4 to 10, RDO5 transconjugants.
Interestingly, the isopropylbenzene dioxygenase genes (ipbA1A2A3A4) and the 3-isopropylcatechol 2,3-dioxygenase gene (ipbC) encoded on the 200-kb transmissible linear plasmid of R. erythropolis BD2 (2) exhibited extremely high identities (90 to 99%) with the corresponding bphA1A2A3A4 and bphC genes of RHA1, all of which are located on pRHL1 (15). Furthermore, the gene organization, including the spaces between coding regions, was well-conserved in BD2 and RHA1. These data suggest that the ipbA1A2A3A4C and RHA1 bphA1A2A3A4C genes diverged only recently. Kosono et al. have reported that the PCB degraders R. erythropolis TA421 and Rhodococcus globerulus P6 share seven bphC genes, and three of them are located on the linear plasmids of both strains (14). Although these linear plasmids are not similar in size, they are related on the basis of the degradation genes. Taken together, these findings suggest that linear plasmids play a key role in propagation among rhodococci of the genes for catabolism of aromatic compounds.
Terminal structure of pRHL2.
Blunt-ended terminal structures have been reported for linear plasmids in Rhodococcus (11) and actinomycetes (10). On the basis of the assumption that the ends of pRHL2 are blunt, we cloned the terminal fragments of pRHL2. pRHL2 DNA was isolated following separation by PFGE and was digested with PstI. The resulting fragments were ligated with pUC18 linearized with PstI and SmaI and used to transform E. coli JM109 cells. The transformants obtained harbored 4.5 and 4.6-kb plasmids that were designated pTPS3 and pTPS4, respectively. Southern hybridization analysis of pRHL2 fragments generated with AseI or HpaI was performed by using the inserts of pTPS3 and pTPS4 as probes. The insert of pTPS3 hybridized to A7 and H4, and the insert of pTPS4 hybridized to A5 and H8, indicating that pTPS3 and pTPS4 carry the right and left ends of pRHL2 shown in Fig. 2, respectively.
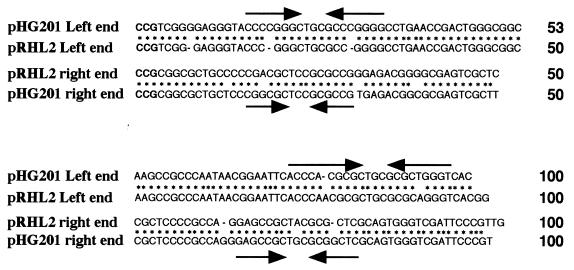
The nucleotide sequences of the inserts of pTPS3 (994 bp) and pTPS4 (1,975 bp) were determined. Alignment of the right and left terminal 100-bp sequences of pRHL2 is shown in Fig. 4. The right end 600-bp sequence of pRHL2 exhibited 84 and 90% identities with the right end sequences of linear plasmid pHG201 of R. opacus MR11 and linear plasmid pHG204 of R. opacus MR22, respectively (11). The pRHL2 left end sequence exhibited 96% identity with the 1,150-bp left end sequence of pHG201 (11). The 34-bp right and left terminal sequences of pHG201 exhibit 65% identity and are not as similar to each other as the known linear replicons in actinomyces are. This is also the case with pRHL2. The 100 terminal bases of the two ends exhibit 64% identity. The levels of identity of the terminal sequences of pRHL2 and pBD2 (accession no. U83846) of isopropylbenzene-degrading strain BD2 were less than 70% and were not significant in spite of the remarkable levels of identity of degradative genes of host strains RHA1 and BD2. However, the RHA1 counterparts of isopropylbenzene degradation genes in BD2 are bphA1A2A3A4C, as mentioned previously, and they are located on pRHL1. Although the size of pRHL1 (1,100 kb) is different from the size of pBD2 (ca. 210 kb), pRHL1 may have some profiles in common with pBD2. pRHL2 has 3-bp perfect terminal inverted repeats (Fig. 4). Except for these 3-bp repeats, the left and right termini of pRHL2 do not have apparent identity. Thus, the terminal inverted repeats of pRHL2 are very short compared with the linear replicons of Streptomyces. Kalkus et al. suggested that the two sets of inverted repeats that have the same central motif, GCTXCGC, are conserved in the terminal sequences of linear plasmids, including pHG201 (11). They hypothesized that these inverted repeats, including the 3-bp perfect terminal inverted repeats, might play a role in terminus-specific replication, which is thought to be primed by a terminal protein. Both termini of pRHL2 also have such sets of GCTXCGC-containing inverted repeats (Fig. 4).
FIG. 4.
Alignment of the terminal sequences of pRHL2 and pHG201. The dashes indicate gaps; the asterisks indicate identical nucleotide residues in pRHL2 and pHG201. The 3-bp perfect terminal inverted repeats are indicated by boldface type. The inverted repeats of pHG201 containing the control motif GCTXCGC which were identified by Kalkus et al. (11) are indicated by converging arrows.
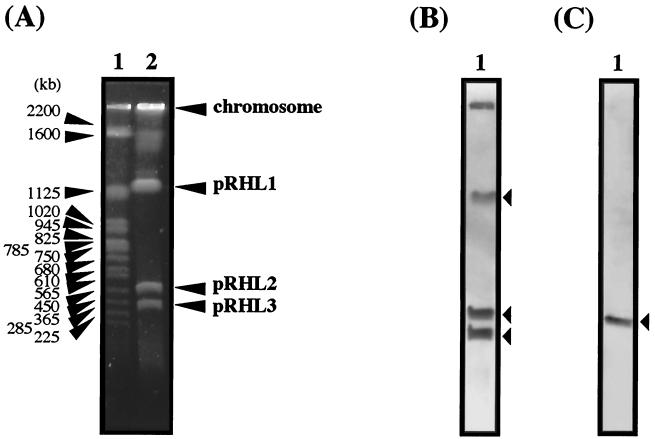
No aromatic degradation genes have been reported for pHG201. The size of pHG201 is distinct from the sizes of pRHL1 and pRHL2. Thus, pHG201 and pRHL2 are different. The remarkable identity of the termini of pRHL2 and pHG201 suggests that a terminal sequence is conserved in some linear plasmids in the genus Rhodococcus. To examine whether the terminal sequences are conserved in linear plasmids in RHA1, a Southern hybridization analysis of RHA1 linear plasmids separated by PFGE was performed by using the terminal sequences of pRHL2 as probes. The right end of pRHL2 hybridized to pRHL1, pRHL2, and pRHL3. In addition, it hybridized to the origin of electrophoresis, which is expected to retain chromosomal DNA. The left end hybridized only to pRHL2 (Fig. 5). Southern hybridization of the PstI digest of RHA1 total DNA gave four signals, including a 1.9-kb major signal with the right end probe and a unique 2.0-kb signal with the left end probe (data not shown). These results suggest that pRHL1 and pRHL3, (possibly their termini) have a sequence similar to the sequence of the right end of pRHL2. They also suggest that the RHA1 chromosome may have similarity with the right end of pRHL2.
FIG. 5.
Southern hybridization of linear plasmids of RHA1 with the terminus probes of pRHL2. (A) Results of PFGE. The sizes of marker fragments and the positions of the chromosome and linear plasmids are indicated on the left and right, respectively. The pulse time was increased from 60 to 90 s during 23 h of electrophoresis. The voltage was adjusted to 6 V/cm. Lane 1, S. cerevisiae chromosome marker; lane 2, linear plasmids of RHA1. (B and C) Results of hybridization performed with the right (B) and left (C) end probes. The 1.1-kb EcoRI and 0.45-kb EcoRI-SalI fragments of pRHL2 were used as the right and left end probes, respectively. The lanes contained linear plasmids of RHA1. The bands of linear plasmids are indicated by arrowheads.
Association of proteins with the plasmid termini.
Linear plasmids in actinomyces have been well-characterized. A general feature of linear replicons of actinomycetes is the presence of terminal proteins that covalently bind to the 5′ end of the plasmid DNA (18). Kinashi and Shimaji-Murayama (13) have reported that an intact protein-bound linear plasmid of Streptomyces can move during PFGE in the presence of SDS but not in the absence of SDS. To prepare intact protein-bound plasmids, proteinase K was omitted from the lysis treatment used for the cells during plasmid DNA preparation in a gel plug. The resultant DNA was analyzed by PFGE in the absence or presence of SDS. Plasmid DNAs that were not treated with proteinase K remained at the origin of electrophoresis (Fig. 6A). In the presence of 0.2% SDS (Fig. 6B), the plasmid DNAs moved as far as the proteinase K-treated plasmid DNAs. These results suggest that the proteins did bind to these linear plasmids. To determine whether proteins were bound to the termini of pRHL2, proteinase K-treated and non-proteinase K-treated total DNAs were digested with AseI or HpaI in gel plugs. The resultant DNA fragments were separated by PFGE and were subjected to Southern hybridization analysis by using the terminal fragments of pRHL2 as probes. When the cells were lysed without proteinase K, the terminal fragment probes hybridized to the DNAs in the wells (Fig. 6C to E). When proteinase K was employed in cell lysis, the probes hybridized to the positions appropriate for the sizes of the A5, A7, H4, and H8 fragments. The right terminus probe also produced minor bands in the presence of SDS that seemed to originate from the termini of pRHL1 and pRHL3. These results suggest that each terminus of pRHL2 is linked to a protein. Based on the presence of blunt-ended termini and perfect terminal inverted repeats and the association between proteins and termini, we concluded that pRHL2 has invertron termini, as has been reported for linear replicons in Streptomyces (18).
FIG. 6.
(A and B) Normal PFGE (A) and SDS-PFGE (B) of linear plasmid DNAs obtained with and without proteinase K treatment. The pulse time was increased from 60 to 90 s during 23 h of electrophoresis. The voltage was adjusted to 6 V/cm. Lane 1, S. cerevisiae chromosome marker; lane 2, proteinase K-treated plasmid DNA; lane 3, non-proteinase K-treated plasmid DNA. The positions of the chromosome and plasmids are indicated on the left and right. (C to E) Results of PFGE (C) and hybridization (D and E) of total restriction fragments obtained with and without proteinase K treatment. Hybridization was performed with the right (D) and left (E) end probes. The 1.1-kb EcoRI and 0.45-kb EcoRI-SalI fragments of pRHL2 were used as the right and left end probes, respectively. The pulse time was increased from 3 to 12 s during 21 h of electrophoresis. The voltage was adjusted to 5.1 V/cm. The sizes of marker fragments in panel C are indicated on the left. The bands of interest in panels D and E are indicated by solid arrowheads. Minor bands in panel D which seemed to be derived from the termini of pRHL1 and pRHL3 are indicated by open arrowheads. Lane 1, non-proteinase K-treated AseI digests; lane 2, proteinase K-treated AseI digests; lane 3, λ ladder plus HindIII-digested λ DNA markers; lane 4, non-proteinase K-treated HpaI digests; lane 5, proteinase K-treated HpaI digests.
In the present study, we constructed a physical map of pRHL2 and determined the structural features of its terminal ends, which suggested that pRHL2 has invertron termini. The self-transmissible feature of this plasmid strongly suggests that it is involved in propagation of diverse aromatic compound catabolic genes in the genus Rhodococcus.
ACKNOWLEDGMENTS
This study was supported in part by the Program for Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN) in Japan and a grant-in-aid for scientific research from the Ministry of Education (no. 12876018).
REFERENCES
- 1.Crespi M, Messens E, Caplan A B, van Montagu M, Desomer J. Fasciation induction by the phytopathogen Rhodococcus fascians depends upon a linear plasmid encoding a cytokine cyntase gene. EMBO J. 1992;11:795–804. doi: 10.1002/j.1460-2075.1992.tb05116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dabrock B, Keßeler M, Averhoff B, Gottschalk G. Identification and characterization of a transmissible linear plasmid from Rhodococcus erythropolis BD2 that encodes isopropylbenzene and trichloroethene catabolism. Appl Environ Microbiol. 1994;60:853–860. doi: 10.1128/aem.60.3.853-860.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukuda M, Shimizu S, Okita N, Seto M, Masai E. Structural alteration of linear plasmids encoding the genes for polychlorinated biphenyl degradation in Rhodococcus strain RHA1. Antonie Leeuwenhoek. 1998;74:169–173. doi: 10.1023/a:1001732718159. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto Y, Nishiyama M, Yu F, Watanabe I, Horinouchi S, Beppu T. Development of a host-vector system in a Rhodococcus strain and its use for expression of the cloned nitrile hydratase gene cluster. J Gen Microbiol. 1992;138:1003–1010. doi: 10.1099/00221287-138-5-1003. [DOI] [PubMed] [Google Scholar]
- 5.Hatta T, Shimada T, Yoshihara T, Yamada A, Masai E, Fukuda M, Kiyohara H. meta-Fission product hydrolases from a strong PCB degrader Rhodococcus sp. strain RHA1. J Ferment Bioeng. 1998;85:174–179. [Google Scholar]
- 6.Hauschild J E, Masai E, Sugiyama K, Hatta T, Kimbara K, Fukuda M, Yano K. Identification of an alternative 2,3-dihydroxybiphenyl 1,2-dioxygenase in Rhodococcus sp. strain RHA1 and cloning of the gene. Appl Environ Microbiol. 1996;62:2940–2946. doi: 10.1128/aem.62.8.2940-2946.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayakawa T, Tanaka T, Sakaguchi K, Otake N, Yonehara H. A linear plasmid-like DNA in Strepromyces sp. producing lankacidin group antibiotics. J Gen Appl Microbiol. 1979;25:255–260. [Google Scholar]
- 8.Hinnebush J, Bergstrom S, Barbour A G. Cloning and sequence analysis of linear plasmid telomeres of the bacterium Borrelia burgdorferi. Mol Microbiol. 1990;4:811–820. doi: 10.1111/j.1365-2958.1990.tb00651.x. [DOI] [PubMed] [Google Scholar]
- 9.Hinnebush J, Tilly K. Linear plasmids and chromosomes in bacteria. Mol Microbiol. 1993;10:917–922. doi: 10.1111/j.1365-2958.1993.tb00963.x. [DOI] [PubMed] [Google Scholar]
- 10.Hirochika H, Sakaguchi K. Analysis of linear plasmids isolated from Streptomyces: association of protein with the ends of the plasmid DNA. Plasmid. 1982;7:59–65. doi: 10.1016/0147-619x(82)90027-0. [DOI] [PubMed] [Google Scholar]
- 11.Kalkus J, Menne R, Reh M, Schlegel H G. The terminal structures of linear plasmids from Rhodococcus opacus. Microbiology. 1998;144:1271–1279. doi: 10.1099/00221287-144-5-1271. [DOI] [PubMed] [Google Scholar]
- 12.Kieser M H, Kieser T, Hopwood A D. A combined genetic and physical map of the Streptomyces coelicolor A3(2) chromosome. J Bacteriol. 1992;174:5496–5507. doi: 10.1128/jb.174.17.5496-5507.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinashi H, Shimaji-Murayama M. Physical characterization of SCP1, a giant linear plasmid from Streptomyces coelicolor. J Bacteriol. 1991;173:1523–1529. doi: 10.1128/jb.173.4.1523-1529.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kosono S, Maeda M, Fuji F, Arai H, Kudo T. Three of the seven bphC genes of Rhodococcus erythropolis TA421, isolated from a termite ecosystem, are located on an indigenous plasmid associated with biphenyl degradation. Appl Environ Microbiol. 1997;63:3282–3285. doi: 10.1128/aem.63.8.3282-3285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masai E, Yamada A, Healy J M, Hatta T, Kimbara K, Fukuda M, Yano K. Characterization of biphenyl catabolic genes of gram-positive polychlorinated biphenyl degrader Rhodococcus sp. strain RHA1. Appl Environ Microbiol. 1995;61:2079–2085. doi: 10.1128/aem.61.6.2079-2085.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masai E, Sugiyama K, Iwashita N, Shimizu S, Hauschild J E, Hatta T, Kimbara K, Yano K, Fukuda M. The bphDEF meta-cleavage pathway genes involved in biphenyl/polychlorinated biphenyl degradation are located on a linear plasmid and separated from the initial bphACB genes in Rhodococcus sp. strain RHA1. Gene. 1997;187:141–149. doi: 10.1016/s0378-1119(96)00748-2. [DOI] [PubMed] [Google Scholar]
- 17.Picardeau M, Vincent V. Characterization of large linear plasmids in mycobacteria. J Bacteriol. 1997;179:2753–2756. doi: 10.1128/jb.179.8.2753-2756.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakaguchi K. Invertrons, a class of structurally and functionally related genetic elements that includes linear DNA plasmids, transposable elements, and genomes of adeno-type viruses. Microbiol Rev. 1990;54:66–74. doi: 10.1128/mr.54.1.66-74.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seto M, Kimbara K, Shimura M, Hatta T, Fukuda M, Yano K. A novel transformation of polychlorinated biphenyls by Rhodococcus sp. strain RHA1. Appl Environ Microbiol. 1995;61:3353–3358. doi: 10.1128/aem.61.9.3353-3358.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seto M, Masai E, Ida M, Hatta T, Kimbara K, Fukuda M, Yano K. Multiple polychlorinated biphenyl transformation systems in the gram-positive bacterium Rhodococcus sp. strain RHA1. Appl Environ Microbiol. 1995;61:4510–4513. doi: 10.1128/aem.61.12.4510-4513.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Short J M, Fernandez J M, Sorge J A, Huse W. λ ZAP: a bacteriophage λ expression vector with in vivo excision properties. Nucleic Acids Res. 1988;16:7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vieira J, Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- 24.Yamada A, Kishi H, Sugiyama K, Hatta T, Nakamura K, Masai E, Fukuda M. Two nearly identical aromatic compound hydrolase genes in a strong polychlorinated biphenyl degrader, Rhodococcus sp. strain RHA1. Appl Environ Microbiol. 1998;64:2006–2012. doi: 10.1128/aem.64.6.2006-2012.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]