Abstract
Aims
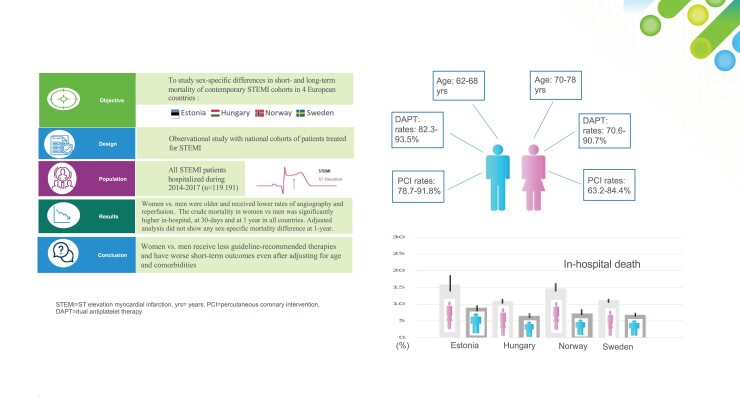
Data on how differences in risk factors, treatments, and outcomes differ between sexes in European countries are scarce. We aimed to study sex-related differences regarding baseline characteristics, in-hospital managements, and mortality of ST-elevation myocardial infarction (STEMI) patients in different European countries.
Methods and results
Patients over the age of 18 with STEMI who were treated in hospitals in 2014–17 and registered in one of the national myocardial infarction registers in Estonia (n = 5817), Hungary (n = 30 787), Norway (n = 33 054), and Sweden (n = 49 533) were included. Cardiovascular risk factors, hospital treatment, and recommendation of discharge medications were obtained from the infarction registries. The primary outcome was mortality, in-hospital, after 30 days and after 1 year. Logistic and cox regression models were used to study the associations of sex and outcomes in the respective countries. Women were older than men (70–78 and 62–68 years, respectively) and received coronary angiography, percutaneous coronary intervention, left ventricular ejection fraction assessment, and evidence-based drugs to a lesser extent than men, in all countries. The crude mortality in-hospital rates (10.9–15.9 and 6.5–8.9%, respectively) at 30 days (13.0–19.9 and 8.2–10.9%, respectively) and at 1 year (20.3–28.1 and 12.4–17.2%, respectively) after hospitalization were higher in women than in men. In all countries, the sex-specific differences in mortality were attenuated in the adjusted analysis for 1-year mortality.
Conclusion
Despite improved awareness of the sex-specific inequalities on managing patients with acute myocardial infarction in Europe, country-level data from this study show that women still receive less guideline-recommended management.
Keywords: Acute coronary syndrome, Acute myocardial infarction, Real-world data, Sex, Equality, Country comparisons
Graphical Abstract
Graphical Abstract.
Introduction
Cardiovascular disease (CVD) is the most common cause of death in Europe, accounting for 47% of deaths of women and 39% of deaths of men.1 Cardiovascular disease mortality has declined in many European countries due to improvements in both primary and secondary prevention during the last few decades.2 Despite decreased overall rates of cardiovascular mortality in women, the annual cardiovascular mortality rates are still higher in women than in men, and women still have higher rates of comorbidities, complications, and in-hospital mortality.3–8 Although sex-related differences in short-term outcomes in patients with acute myocardial infarction (AMI) are found to be worse in women than in men, the associations between sex and long-term outcomes often seem to attenuate or disappear after adjustments for age, comorbidities, and cardiovascular risk factors.9,10
However, some uncertainties remain with regard to sex-specific differences in mortality, due to non-contemporary studies, conflicting results, heterogenous study populations including patients with both non-ST-elevation myocardial infarction (NSTEMI) and ST-elevation myocardial infarction (STEMI) or lack of adjustments for several clinical confounders.10–13
Large-scale myocardial infarction (MI) registries provide important insights into how evidence-based therapies are applied in the real-world setting. In our previous work,14 we compared four different European ongoing national MI registries and observed that the presentation, management, and outcomes of STEMI patients differ between countries. Given the increased awareness and attention on sex-related topics in recent years, we specifically aimed to study sex-specific differences in short- and long-term mortalities of contemporary STEMI cohorts in four European countries (Estonia, Hungary, Norway, and Sweden). Our secondary objective was to study sex-related features of baseline characteristics and in-hospital following standard care of STEMI patients.
Methods
Study population
We included consecutive patients over 18 years of age who were hospitalized due to STEMI from 1 January 2014 to 31 December 2017 and registered in one of the four MI registries in Estonia (The Estonian Myocardial Infarction Registry, EMIR), Hungary (The Hungarian Myocardial Infarction Registry, HUMIR), Norway (The Norwegian Myocardial Infarction Registry, NORMI), and Sweden (The Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies, SWEDEHEART). If a patient was re-hospitalized with STEMI, only the first episode was counted as the index event and followed over time, to avoid multiple entries in the registries. The STEMI diagnosis was defined by the International Classification of Diseases codes provided by the treating physician and was based on the European Society of Cardiology (ESC) third universal definition of MI.15
The study complied with the Declaration of Helsinki and was approved by the local ethics committee of each country: Research Ethics Committee of the University of Tartu, Estonia (253/T-13); Hungarian Medical Research Council (34858-3/2019/EKU); the Regional Committee for Medical and Health Research Ethics North in Norway (REK 2016/170); and the regional ethics committee in Stockholm, Sweden (2012/60-31/2).
Data sources and acute myocardial infarction registries
The EMIR was founded in 2012 and is a national government–funded registry that reports data electronically via an internet-based standardized form for all hospitalized MI cases at 19 Estonian hospitals. Annually, about 2700 cases are enrolled, and data collection is mandatory by law. About 100 variables are entered in the registry, including demographic data, previous history of CVD and risk factors, clinical features at presentation, in-hospital medications and interventions, complications, discharge data, and autopsies. The data set and definitions conform to the Cardiology Audit and Registration Data Standards.16 The coverage of the registry is over 95%, and data validity is subject to routine error checking.17
The HUMIR was launched in 2010, and registry data collection is mandatory for all 81 Hungarian hospitals. The registry collects demographic and clinical data on all consecutive patients treated for MI regardless of age. Yearly, about 16 000 patients are enrolled, and data are reported via a national internet-based portal. About 178 structured categories, including pre-hospital data, prior medical history, in-hospital treatment, and coronary interventions, are covered. The case coverage is high (92% in 2017) and data are continuously checked and validated by specially trained personnel.
The NORMI is a government-funded registry established in 2013. The registration of all patients admitted to all Norwegian hospitals with MI are mandatory and does not require patient consent. Annually, the registry enrols about 11 000 patients with MI admitted to at all Norwegian hospitals. The registry contains information on sex, age, known risk factors, medical history and previous medication, symptoms and clinical presentation, treatment, in-hospital complications, and discharge medications. The registry has >90% case coverage with a high degree of completeness and accuracy.18
The SWEDEHEART was established in 1995 and is also funded by the government. About 18 000 patients with MI admitted to a coronary care unit or other specialized facility at all 72 Swedish hospitals are enrolled annually in the registry. More than 100 different clinical variables such as demography, previous medical history, symptom presentation, laboratory measurements, and data on in-hospital course including information on interventional therapies, discharge diagnosis, and medication at discharge are collected prospectively. Annual evaluation of entered data and medical records ensures a high level of data correctness of about 96%.19 SWEDEHEART covers about 87% of all AMI hospitalizations in Sweden.20
By using the respective countries’ unique personal identification number for the residents, further cross-matching with other national registries enabled merging and further analysis of vital status. A list of all variables, the variable definition, and the assessment of comparability (defined as a consensus agreement between the authors of this study) are described in detail in the Supplementary material online, Tables S1 and S2, and the ESC AMI quality indicators were kept in mind when reporting results.21
Statistical analysis
Data were analysed on a country level and compared as such. Categorical variables were expressed as percentages with 95% confidence intervals (CIs) and continuous variables as mean with standard deviations, or as medians with interquartile ranges. The χ2 and Wilcoxon rank-sum tests were used to test for significant differences between men and women for selected variables. In all analyses, a P-value of <0.05 was regarded as statistically significant. The primary objective of this study was to describe mortality in-hospital at 30 days and at 1 year. The secondary objective of this study was to describe the prevalence of patient characteristics and in-hospital management in women and men. Logistic regression was used to study the odds ratio (OR) for women vs. men for primary outcomes (in-hospital death, and mortality at 30 days). The model assumption of the proportional hazards was checked graphically, and Cox regression analysis was utilized for the 1-year mortality outcome. The adjusted logistic and cox regression models included data on previous history of PCI, AMI, stroke or heart failure, smoking, hypertension, diabetes, body mass index and age (as continuous variables), hyperlipidaemia, and peripheral artery disease (PAD). Treatments in-hospital or at discharge were considered to be dependent on the treating physician’s choice or on the institution the patient was treated at, rather than sex specific as such and were not adjusted for. Missing data were handled with multiple imputation using all covariates and outcomes as predictors. As a subgroup analysis, the primary outcomes were studied in patients <60 years of age.
All registries had a full 1-year follow-up for all patients, except for SWEDEHEART, where data on only vital status were available until 30 June 2018, which lead to the mortality analysis being restricted to the 2014–16 cohort. Analyses were performed using the Stata statistical software version 11 and 16 (StataCorp LLC, College Station, TX, USA) and SPSS version 26 (IBM Corp, Armonk, NY, USA).
Results
Baseline characteristics
In total, 64 025 STEMI patients were recorded during the study period 2014–17: 4584 in Estonia, 23 685 in Hungary, 12 083 in Norway, and 23 342 in Sweden. In Sweden and Norway, there were approximately twice as many men than women, while Estonia and Hungary had about one and a half times as many men than women. The median age was higher in women (range 70–78 years) than in men (range 62–68 year) in all four countries (Table 1). Key patient characteristics that differed between sexes were active smoking status, hypertension, diabetes, hyperlipidaemia, chronic kidney disease (CKD), and previous cardiovascular history (Table 1).
Table 1.
Baseline characteristics
| Estonia (n = 4584) | Hungary (n = 23 685) | Norway (n = 12 083) | Sweden (n = 23 342) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men (n = 2816) | Women (n = 1768) | P-value | Men (n = 14 580) | Women (n = 9105) | P-value | Men (n = 8613) | Women (n = 3470) | P-value | Men (n = 16 161) | Women (n = 7181) | P-value | |
| Demography | ||||||||||||
| Median age, years (IQR) | 65 (56–74) | 78 (69–84) | <0.001 | 62 (53–70) | 70 (60–79) | <0.001 | 63 (55–72) | 74 (63–84) | <0.001 | 68 (59–75) | 75 (66–83) | <0.001 |
| <60 years, % (95% CI) | 35.1 (33.3–36.9) | 9.3 (8.1–10.8) | <0.001 | 44.0 (43.2–44.8) | 24.3 (23.4–25.2) | <0.001 | 38.7 (37.6–39.7) | 18.2 (17.0–19.5) | <0.001 | 27.3 (26.7–28.0) | 13.6 (12.8–14.4) | <0.001 |
| 60–69 years, % (95% CI) | 31.8 (30.1–33.6) | 19.7 (17.9–21.7) | <0.001 | 30.6 (29.8–31.3) | 25.4 (24.5–26.3) | <0.001 | 29.6 (28.7–30.6) | 20.8 (19.5–22.2) | <0.001 | 29.5 (28.8–30.2) | 20.9 (20.0–21.8) | <0.001 |
| 70–79 years, % (95% CI) | 19.5 (18.1–21.0) | 29.2 (27.1–31.4) | <0.001 | 17.4 (16.8–18) | 26.6 (25.7–27.6) | <0.001 | 19.5 (18.7–20.4) | 24.2 (22.8–25.7) | <0.001 | 27.2 (26.5–27.9) | 28.4 (27.4–29.5) | <0.001 |
| >80 years, % (95% CI) | 13.6 (12.3–15.9) | 41.7 (39.4–44.1) | <0.001 | 8.1 (7.6–8.5) | 23.6 (22.7–24.5) | <0.001 | 12.2 (11.5–12.9) | 36.8 (35.2–38.4) | <0.001 | 15.9 (15.4–16.5) | 37.1 (36.0–38.2) | <0.001 |
| Risk factors | ||||||||||||
| BMI, median (IQR) | 27 (25–31) | 28 (24–32) | 0.097 | 28 (25–31) | 27 (24–30) | <0.001 | 27 (25–29) | 25 (23–29) | <0.001 | 27 (24–29) | 26 (23–29) | <0.001 |
| Previous smoker, % (95% CI) | 18.9 (17.5–20.4) | 5.7 (4.7–6.9) | <0.001 | 16.5 (15.7–17.2) | 9 (8.2–9.7) | <0.001 | 31.6 (30.6–32.6) | 19.3 (18.0–20.6) | <0.001 | 30.6 (30.0–31.2) | 23.2 (22.2–24.2) | <0.001 |
| Current smoker, % (95% CI) | 45.2 (43.4–47.1) | 17.4 (15.6–19.2) | <0.001 | 54.2 (53.3–55.2) | 39.6 (38.4–40.9) | <0.001 | 37.6 (36.6–38.6) | 34.7 (33.2–36.3) | 0.003 | 25.1 (24.6–25.7) | 24.6 (23.6–25.6) | 0.27 |
| Hypertension, % (95% CI) | 74.6 (73.2–76.5) | 84.8 (83.1–86.5) | <0.001 | 70.5 (69.7–71.2) | 80.6 (79.8–81.5) | <0.001 | 35.3 (34.3–36.3) | 46.7 (45.1–48.4) | <0.001 | 45.9 (45.1–46.6) | 57.3 (56.1–58.4) | <0.001 |
| Diabetes mellitus, % (95% CI) | 17.3 (15.9–18.7) | 26.0 (24.0–28.1) | <0.001 | 26.8 (26–27.5) | 31.6 (31.2–32.1) | <0.001 | 13.9 (13.2–14.7) | 16.5 (15.3–17.8) | <0.001 | 18.6 (18.0–19.2) | 19.4 (18.5–20.4) | 0.08 |
| Hyperlipidaemia, % (95% CI) | 68.8 (67.1–70.5) | 61.7 (59.4–64.0) | <0.001 | 29.4 (28.5–30.2) | 29.5 (28.5–30.6) | 0.82095 | 22.4 (21.5–23.3) | 21.9 (20.6–23.3) | 0.55 | 24.0 (23.3–24.6) | 21.5 (20.6–22.5) | <0.001 |
| CKD (eGFR < 60), % (95% CI) | NC | NC | NA | 20.3 (19.4–21.1) | 38.3 (37–39.6) | <0.001 | 16.9 (16.1–17.7) | 33.1 (31.6–34.7) | <0.001 | 20.3 (19.6–20.9) | 32.4 (31.3–33.5) | <0.001 |
| Previous CVD | ||||||||||||
| Myocardial infarction, % (95% CI) | 17.6 (16.2–19.1) | 13.5 (11.9–15.1) | <0.001 | 17.4 (16.8–18.1) | 14.7 (13.9–15.4) | <0.001 | 13.4 (12.7–14.2) | 11.0 (10.0–12.1) | <0.001 | 19.9 (19.2–20.5) | 16.5 (15.7–17.4) | <0.001 |
| Heart failure, % (95% CI) | 22.6 (21.1–24.2) | 36.8 (34.6–39.1) | <0.001 | 8.5 (8–8.9) | 10.9 (10.2–11.6) | <0.001 | 2.2 (1.9–2.6) | 4.8 (4.1–5.6) | <0.001 | 8.4 (8.0–8.9) | 10.8 (10.1–11.5) | <0.001 |
| Stroke, % (95% CI) | 8.7 (7.7–9.8) | 11.3 (9.9–12.9) | 0.004 | 7.3 (6.9–7.8) | 8.5 (7.9–9.1) | 0.00129 | 4.6 (4.2–5.0) | 7.6 (6.8–8.5) | <0.001 | 6.2 (5.8–6.6) | 8.1 (7.5–8.8) | <0.001 |
| Peripheral artery disease, % (95% CI) | 9.1 (8.0–10.2) | 7.9 (6.7–9.3) | 0.169 | 10.4 (9.9–10.9) | 10.6 (9.9–11.3) | 0.62904 | NC | NC | 3.8 (3.5–4.1) | 4.9 (4.4–5.4) | <0.001 | |
| Presentation | ||||||||||||
| Pre-hospital cardiac arrest, % (95% CI) | NC | NC | NC | 5.8 (5.4–6.2) | 4.7 (4.3–5.2) | <0.001 | 8.3 (7.7–8.9) | 5.6 (4.9–6.5) | <0.001 | 5.5 (5.1–5.8) | 3.4 (3.0–3.8) | 0.45 |
| Median heart rate, b.p.m. (IQR) | 77 (66–90) | 80 (67–93) | 0.999 | 80 (70–92) | 80 (70–93) | 0.108 | 77 (64–90) | 80 (65–93) | <0.001 | 76 (64–90) | 80 (66–94) | <0.001 |
| Mean systolic BP, mmHg (IQR) | 137 (118–154) | 140 (119–157) | 0.896 | 130 (116–150) | 130 (110–150) | <0.001 | 132 (116–150) | 131 (110–152) | 0.04 | 140 (121–160) | 140 (120–160) | <0.001 |
| Killip Classes II–IV, % (95% CI) | 24.4 (22.7–25.9) | 33.9 (31.7–36.1) | <0.001 | 13.0 (12.4–13.6) | 17.9 (17.1–18.7) | <0.001 | NC | NC | 9.5 (9.1–9.9) | 11.9 (11.2–12.7) | <0.001 | |
BMI, body mass index; b.p.m., beats per minute; CI, confidence interval; CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; IQR, interquartile range; NC, data not collected; SD, standard deviation.
In-hospital management
Angiography and PCI were performed to a lesser extent in women than in men in all countries, and Norway and Estonia had the lowest proportions of performed angiographies and PCI (Table 2). The lowest rate of fibrinolysis was observed in Sweden (Hungary does not use fibrinolysis), whereas Estonia and Norway reported the highest rates of thrombolysis, and coronary artery bypass grafting was performed to a higher extent in men than in women in all countries (Table 2). Female sex was associated with a 38–75% lower risk of receiving reperfusion therapy in the studied countries (Supplementary material online, Table S3).
Table 2.
In-hospital course describing management, complications, and medications at discharge
| Estonia (n = 4584) | Hungary (n = 23 685) | Norway (n = 12 083) | Sweden (n = 23 342) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men (n = 2816) | Women (n = 1768) | P-value | Men (n = 14 580) | Women (n = 9105) | P-value | Men (n = 8613) | Women (n = 3470) | P-value | Men (n = 16 161) | Women (n = 7181) | P-value | |
| In-hospital management % (95% CI) | ||||||||||||
| Coronary angiography | 86.2 (84.9–87.5) | 71.1 (69.0–73.3) | <0.001 | 85.8 (85.3–86.4) | 78.6 (77.8–79.5) | <0.001 | 90.5 (89.8–91.1) | 72.3 (70.8–73.8) | <0.001 | 95.2 (94.8–95.5) | 88.3 (87.5–89.0) | <0.001 |
| PCI | 78.7 (77.2–80.2) | 63.2 (60.9–65.5) | <0.001 | 84.3 (83.7–84.9) | 76.5 (75.6–77.3) | <0.001 | 84.4 (83.7–85.2) | 64.5 (62.9–66.1) | <0.001 | 91.8 (91.4–92.2) | 83.1 (82.2–84.0) | <0.001 |
| Fibrinolysis | 14.3 (13.1–15.7) | 9.2 (7.9–10.7) | <0.001 | —a | —a | 14.8 (14.1–15.6) | 11.5 (10.5–12.6) | <0.001 | 1.6 (1.4–1.8) | 1.1 (0.9–1.4) | <0.001 | |
| CABG | 4.3 (3.4–5.1) | 2.15 (1.5–2.9) | <0.001 | NA | NA | 2.7 (2.4–3.1) | 1.5 (1.1–1.9) | <0.001 | 2.3 (2.1–2.6) | 1.3 (1.0–1.6) | <0.001 | |
| LVEF assessment performed with echocardiography | 93.9 (93.0–94.8) | 88.2 (0.87–89.6) | <0.001 | 81.0 (80.3–81.6) | 77.4 (76.5–78.3) | <0.001 | 88.1 (87.4–88.8) | 81.3 (80.0–82.6) | <0.001 | 88.2 (87.6–88.7) | 83.3 (82.5–84.2) | <0.001 |
| LVEF ≥50% | 41.1 (39.3–43.0) | 46.3 (43.8–48.8) | 40.4 (39.6–41.2) | 38.1 (37.1–39.1) | <0.001 | NC | NC | 47.3 (46.5–48.1) | 48.1 (46.8–49.4) | |||
| LVEF 40 − 49% | 29.6 (27.8–31.4) | 28.2 (26.0–30.5) | 23.5 (22.8–24.2) | 22.6 (21.7–23.5) | 0.09775 | NC | NC | 27.5 (26.8–28.3) | 25.1 (24.0–26.2) | |||
| LVEF 30 − 39 | 21.4 (19.9–23.1) | 19.5 (17.6–21.6) | 12.5 (11.9–13) | 12.2 (11.5–12.9) | 0.48995 | NC | NC | 16.6 (16.0–17.3) | 17.8 (16.8–18.8) | |||
| LVEF <30% | 7.8 (6.8–8.9) | 6.0 (4.9–7.3) | 4.6 (4.3–5) | 4.5 (4.1–5.0) | 0.80878 | NC | NC | 7.6 (7.1–8.0) | 7.8 (7.2–8.6) | |||
| Complications % (95% CI) | ||||||||||||
| Reinfarction | 0.5 (0.2–0.8) | 0.5 (0.2–1.0) | 0.956 | NA | NA | 1.1 (0.9–1.3) | 1.1 (0.8–1.5) | 0.79 | 0.9 (0.8–1.1) | 0.8 (0.6–1.0) | <0.001 | |
| Cardiac arrest | 10.6 (9.4–11.7) | 13.6 (12.1–15.3) | 0.002 | 6.0 (5.7–6.4) | 7.7 (7.1–8.2) | <0.001 | 3.1 (2.8–3.5) | 2.8 (2.3–3.4) | 0.32 | 7.5 (7.1–7.9) | 4.4 (4.0–4.9) | 0.87 |
| Severe bleeding | 2.1 (1.6–2.7) | 1.9 (1.3–2.7) | 0.629 | 0.9 (0.8–1.1) | 1.7 (1.4–2) | <0.001 | 1.3 (1.1–1.6) | 1.7 (1.3–2.2) | 0.18 | 0.1 (0.0–0.1) | 0.3 (0.2–0.4) | 0.42 |
| Medications at discharge % (95% CI) | ||||||||||||
| Aspirin | 93.8 (92.9–94.8) | 88.8 (87.1–90.4) | <0.001 | 96.5 (96.2–96.8) | 94.3 (93.7–94.8) | <0.001 | 96.8 (96.4–97.2) | 94.4 (93.5–95.2) | <0.001 | 93.6 (93.2–93.9) | 89.4 (88.6–90.1) | <0.001 |
| DAPT | 82.3 (80.8–83.8) | 70.6 (68.4–73.1) | <0.001 | 93.5 (93–93.9) | 90.7 (90.1–91.4) | <0.001 | 92.6 (92.0–93.1) | 85.5 (84.2–86.8) | <0.001 | 85.4 (84.9–86.0) | 77.9 (76.9–78.9) | <0.001 |
| Oral anticoagulants | 9.6 (8.5–10.8) | 12.0 (10.4–13.7) | <0.001 | 5.9 (5.5–6.3) | 6.0 (5.5–6.5) | 0.83659 | 13.6 (12.9–14.4) | 15.2 (14.0–16.5) | 0.03 | 10.9 (10.4–11.4) | 12.0 (11.3–12.8) | 0.01 |
| Beta-blockers | 84.4 (82.5–85.4) | 84.3 (82.4–86.1) | 0.748 | 87.3 (86.7–87.8) | 86.6 (85.8–87.3) | 0.13311 | 81.0 (80.1–81.8) | 76.9 (75.3–78.4) | <0.001 | 89.5 (89.0–90.0) | 87.1 (86.3–87.9) | <0.001 |
| Statins | 89.2 (87.9–90.4) | 81.7 (79.7–83.6) | <0.001 | 92.7 (92.3–93.2) | 90.3 (89.7–91.0) | <0.001 | 93.6 (93.1–94.1) | 83.6 (82.3–84.9) | <0.001 | 94.3 (93.9–94.6) | 87.1 (86.3–87.9) | <0.001 |
| ACE-I/ARB | 78.4 (76.8–80.0) | 77.3 (75.1–79.4) | 0.413 | 85.2 (84.6–85.8) | 81.8 (80.9–82.6) | <0.001 | 64.3 (63.3–65.4) | 61.6 (59.9–63.4) | 0.01 | 86.1 (85.5–86.6) | 81.3 (80.4–82.2) | <0.001 |
ACE-I/ARB, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers; CABG, coronary artery bypass grafting; CI, confidence interval; DAPT, dual antiplatelet treatment; IQR, interquartile range; LVEF, left ventricular ejection fraction; NC, data not collected; PCI, percutaneous coronary intervention.
Thrombolysis is no longer used in Hungary.
Medications at discharge
Aspirin and dual antiplatelet treatment (DAPT) prescription at discharge was underutilized in women when compared with men in all countries (Table 2). Beta-blocker was prescribed to a lesser degree in women than in men in Norway and Sweden, and statins were prescribed to a lesser extent in women than in men in all countries (Table 2).
Primary outcomes
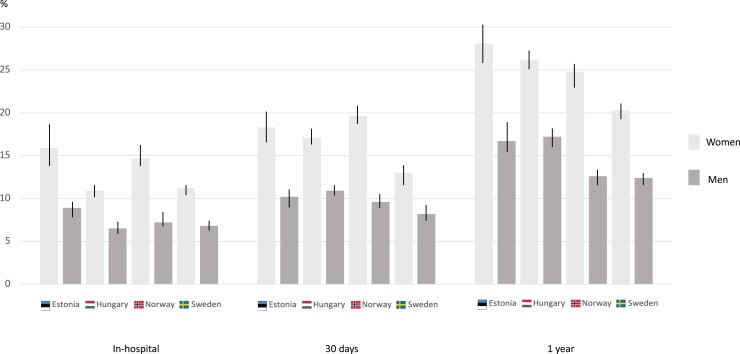
The in-hospital mortality rates ranged from 11.2 to 15.9% and from 6.8 to 8.9% in women and men, respectively (Figure 1, Table 3). The crude 30-day mortality and 1-year mortality rates were numerically higher in women (13.0–18.3 and 20.3–28.1%, respectively) than in men (8.2–10.9 and 12.4–17.2%, respectively) in all countries (Table 3). Female sex was significantly associated with a higher risk of death in-hospital, at 30 days and at 1 year in all countries (see Supplementary material online, Table S4). However, these differences were partly attenuated in the adjusted analysis, where women showed a significantly higher risk than men in terms of in-hospital [hazard ratio (HR) 1.12 (95% CI 1.01–1.25) and HR 1.17 (95% CI 1.02–1.35), for Hungary and Norway, respectively] and 30 days [HR 1.11 (95% CI 1.01–1.21) and HR 1.14 (95% CI 1.01–1.29), for Hungary and Norway, respectively] in Hungary and Norway (see Supplementary material online, Table S4). For 1-year mortality, the adjusted analysis did not show any significant difference between sexes (see Supplementary material online, Table S4).
Figure 1.
In-hospital, 30-day, and 1-year mortality rates stratified on sex in Estonia, Hungary, Norway, and Sweden.
Table 3.
In- and out-of-hospital mortality rates
| Estonia (n = 4584) | Hungary (n = 23 685) | Norway (n = 12 083) | Sweden (n = 23 342) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % (95% CI) | Men (n = 2816) | Women (n = 1768) | P-value | Men (n = 14 580) | Women (n = 9105) | P-value | Men (n = 8613) | Women (n = 3470) | P-value | Men (n = 16 161) | Women (n = 7181) | P-value |
| In-hospital | 8.9 (7.9–10.0) | 15.9 (14.2–17.7) | <0.001 | 6.5 (6.1–6.9) | 10.9 (10.3–11.6) | <0.001 | 7.2 (6.9–7.8) | 14.7 (13.6–15.9) | <0.001 | 6.8 (6.5–7.2) | 11.2 (10.5–12.0) | <0.001 |
| 30 days | 10.2 (9.1–11.4) | 18.3 (16.6–20.2) | <0.001 | 10.9 (10.4–11.4) | 17.1 (16.4–17.9) | <0.001 | 9.6 (9.0–10.3) | 19.6 (18.3–20.9) | <0.001 | 8.2 (7.8–8.6) | 13.0 (12.2–13.8) | <0.001 |
| 1 year | 16.7 (15.3–18.1) | 28.1 (26.0–30.2) | <0.001 | 17.2 (16.6 –17.8) | 26.2 (25.3–27.1) | <0.001 | 12.6 (11.8–13.2) | 24.8 (23.4–26.3) | <0.001 | 12.4 (11.8–13.0) | 20.3 (19.3–21.4) | <0.001 |
Subgroup of younger patients
In a subgroup analysis of STEMI patients under the age of 60, current smoking status rates were numerically higher in women and ranged from 56.0 to 72.8%, than in women in the full study population where smoking rates were 17.4 to 39.6% (Table 1, Supplementary material online, Table S5). In line with the full study population, diabetes, CKD, and hypertension were more common in women than in men (Table 1, Supplementary material online, Table S5). In contrast to the main population, sex-related differences in rates of reperfusion therapy with PCI or thrombolysis were less evident in younger patients (see Supplementary material online, Table S5). Female sex was associated with a lower risk of receiving reperfusion therapy than the male sex in Hungary and Norway [OR 0.84 (95% CI 0.72–0.97) and OR 0.44 (95% CI 0.35–0.59), respectively; see Supplementary material online, Table S6]. The in-hospital, 30-day, and 1-year mortality rates were numerically higher in women than in men all the countries, except for in-hospital mortality and 30-day mortality in Estonia (see Supplementary material online, Table S5). Following crude analyses, no significant sex differences were observed for mortality in any of the countries, except in Sweden, where the adjusted analyses for 1-year mortality were significantly higher in women (HR 1.44, 95% CI 1.02–2.03; see Supplementary material online, Table S7). In the adjusted analyses, no sex-specific differences were observed in the individual countries with regard to mortality, except for in-hospital mortality in Sweden [OR 1.58 (95% CI 1.01–2.48); see Supplementary material online, Table S7].
Discussion
This is the largest contemporary study of four ongoing European AMI registries that specifically examines sex-related differences in baseline characteristics, in-hospital management, discharge treatments, and outcomes in STEMI patients following routine care. We found that women were older and had heart failure, hypertension, and diabetes to a larger proportion than men. In addition, women underwent angiography, PCI, and LVEF assessment with echocardiography and received guideline-recommended therapies to a lesser extent than men in of all the studied countries. Even though women had higher crude short- and long-term all-cause mortality rates in all the studied countries, and the fact that many of these differences seemed to attenuate in the adjusted analyses in most of the countries, this study highlights important areas that can be addressed to reduce sex-specific disparities in access to care and outcomes for patients hospitalized with STEMI.
Sex-specific differences in the presentation, management, and prognosis of AMI are well-described, but not fully understood. Men and women with AMI differ in terms of risk factors, symptom presentation, coronary artery anatomy and function, comorbidities, treatment efficiency, and types of outcomes.3,9,22,23 Several studies have shown that women with AMI are older, have a higher extent of traditional risk factors such as diabetes mellitus, hypertension, and previous congestive heart failure, and may present with different types of symptoms, when compared with men,10,24–26 which is also in line with the results of this study. The underlying mechanisms of AMI differ in many ways in men and women. In addition to the classic plaque rupture and thrombus formation pathophysiology often described in men, several studies have identified other mechanisms of AMI (plaque erosion linked to inflammation, endothelial dysfunction and leukocyte activation, connective tissue disorders, coronary vasospasm, and spontaneous coronary artery dissection) that may play an important role in women.9,18,25,27 This may underline the discussion if sex-specific management approaches in MI are indicated.
Even though secondary prevention with antiplatelet medication is recommended in STEMI patients in all ESC countries, sex-specific differences were observed, which are also in line with previous findings.10 Lower rates of DAPT in women than in men could be explained by a higher proportion of conservative treatment, combined with poorer adherence of guideline recommendations of conservative treatment, as well as a higher degree of prescription of OAC in women than in men. Furthermore, several studies have shown that women have a higher risk of bleeding than men, even after adjusting for demography and comorbidities such as heart failure, diabetes, CKD, and hypertension.28,29 Therefore, the fear of a higher bleeding risk may partly explain the lower prescription rates of DAPT and aspirin in women than in men. However, even though women are at a higher risk of bleeding, extended monitoring of weight and renal function could still justify antiplatelet medication.30 Norway had the lowest proportions of prescribed angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers of countries, both for men and for women. Although this result stood out from the others, it could be explained by the low rates of heart failure, diabetes mellitus, and hypertension in the country.
The role of early invasive therapy is proved to be beneficial in both men and women with STEMI, when compared with conservative treatment, and the ESC guidelines recommend early invasive treatment for all STEMI patients regardless of sex.30,31 Despite this, several studies in patients with AMI have shown that angiography is performed less often in women, and women are less likely to receive reperfusion therapy with primary PCI or fibrinolytic therapy when compared with men.23,32 Similarly, these findings were also confirmed in our study that showed sex-related lower percentages of performed coronary angiography, PCI, and fibrinolysis for women in all the countries studied. Since women with STEMI often present with an older age, and more CV- and non-CV-related comorbidities, it is plausible to believe that frailty issues may have led to a more conservative treatment approach in the countries of this study. Since data on frailty are difficult to capture and missing in most registries, residual confounding may exist. However, even after adjusting for age, smoking, and clinically relevant comorbidities, there was still a significant association between female sex and undertreatment with reperfusion in all the studied countries. In contrast, among STEMI patients in the subgroup of patients <60 years, the likelihood of receiving reperfusion therapy was the same regardless of sex in two of the countries. Notably, in the younger population in Sweden and Norway, the rates of current smokers were higher in women than in men, which is an alarming finding that should be taken into account when designing antismoking campaigns. It is possible that women with MI to a larger extent than men are either misdiagnosed or underdiagnosed due to atypical symptom presentation, thereby also leading to undertreatment with reperfusion therapies. The lower proportions of women undergoing PCI in this study are, however, probably not related to underdiagnosis of STEMI in women, since this study included all patients with this diagnosis. It is possible that PCI utilization rates differ between countries due to different economic predispositions of the countries, but Estonia and Norway had the lowest proportions of PCI treatments for both men and women, even though Norway had the highest health expenditure of GDP rate. The low overall utilization rate of PCI in Norway may be explained by geographical and low-dense population issues.
The fact that women had significantly higher rates of crude short-term mortality in this study is also in line with previous studies.24,33 The reasons for the discrepancy in short-term prognosis for STEMI in women and men are unknown, but older age at STEMI presentation, comorbidities, and frailty are possible explanations. However, as outlined in this study, as well as in previous studies, sex-specific mortality differences remained after multivariable adjustments for clinical factors and treatments.12,24,34 It is possible that factors that are difficult to adjust for, such as different or late presentation and frailty-associated factors, may play an important role. Furthermore, CKD, menopause, and psychiatric conditions such as depression are additional risk factors that are linked to an increased risk of adverse prognosis, especially in young women, which can explain differences in mortality between sexes.9
In younger patients (<60 years of age), reperfusion therapy rates were distributed more equally between sexes, and the adjusted sex-specific associations with short- and long-term mortalities were not significant in any of the studied countries. Since women with STEMI are older than men, it may be plausible that older age rather than sex determines whether a STEMI patient receives conservative management or not. However, the age-adjusted analysis did not support this, as a lower probability to receive reperfusion therapy still existed for women when compared with men. It remains unclear though, if higher rates of reperfusion in the older population would lead to reduced differences in mortality between sexes.
Strengths and limitations
General limitations related to registry studies are also applicable to this study. Foremost, the observational nature of this study limits the conclusions that can be drawn to strictly generate hypothesis and with no causality. The lack of individually pooled patient-level data may limit inferences as to differences in patient characteristics, treatments, and outcomes of the studied patient cohorts between countries. Also, country comparisons are limited by the large regional variability in epidemiology, social, economic, cultural, and healthcare conditions, which may influence sex differences in the management and outcomes of acute coronary syndrome (ACS), which may have led to residual confounding. This study analysed and presented the results of four countries in a side-by-side manner since pooling of data from all countries was not possible. The impact of numerical differences between countries, both at patient and treatment and outcome levels, may, therefore, be a limit to the study. Time to PCI was not available in all countries, which is a weakness of this study, since this indicator may have influenced the outcomes. The strengths of this study are the large sample size of the populations and the high coverage rate, since almost all patients (>87%) with a registered STEMI during the study period in all the four countries were included, which leads to a high level of external validity. The baseline data were defined and recorded in a similar way making comparisons both feasible and appropriate, but some variables were not homogenous, which may lead to low internal validity that limits the comparisons between countries. This study may act as a quality measure for how countries follow guideline-recommended therapy in the real world with regard to sex-specific aspects. In addition, this study highlights the importance of continuous national AMI registries in detecting treatment and outcomes differences. Future studies should consider other outcomes (such as recurrent ACS) and include further countries that participate in real-world registries that record clinical variables in a similar and homogenous manner as well as creating a unified European heart registry with pooled data.
Conclusions
In conclusion, women were older, had more comorbidities, and underwent relevant assessments to a lesser degree than men in all countries included in this study. Despite improved awareness of the sex-specific inequalities on managing patients with AMI in Europe, country-level data from this study show that women still receive less guideline-recommended management. The observed sex-specific differences in mortality were small and may be due to conditions we were not able to adjust for. Even though the obvious healthcare inequity between the sex specifics may have several explanations, the overall evidence calls for an improvement in the clinical management of Sweden, Norway, Estonia, and Hungary towards a more sex-specific equal standard of care. Further detailed analyses of subsets of the populations and/or multivariate analyses based on individual patient data of randomized registry studies are needed.
Supplementary Material
Acknowledgements
The authors are thankful to all patients who participated in the registries and to all co-workers at the national MI registries, which enabled the conduct of analyses of this study. This work was supported by the Estonian Research Council [PRG435].
Conflict of interest: None declared.
Contributor Information
Tora Hellgren, Department of Clinical Sciences, Danderyd Hospital, Karolinska Institutet, Nobels väg 6, Sweden.
Mai Blöndal, Department of Cardiology, Heart Clinic, Tartu University Hospital, 8 L. Puusepa Street, Estonia; Department of Cardiology, University of Tartu, 8 L. Puusepa Street, 50406 Tartu, Estonia.
Jarle Jortveit, Department of Cardiology, Sorlandet Hospital, Arendal, Norway.
Tamas Ferenci, John von Neumann Faculty of Informatics, Obuda University, 1034 Budapest, Hungary; Department of Statistics, Corvinus University of Budapest, Keleti Karoly street 5–7, 1024 Budapest, Hungary.
Jonas Faxén, Department of Cardiology, Karolinska University Hospital, Eugeniavagen 23, 17165 Stockholm, Sweden; Department of Medicine, Huddinge, Karolinska Institutet, Halsovagen 13, 14157 Stockholm, Sweden.
Christian Lewinter, Department of Cardiology, Karolinska University Hospital, Eugeniavagen 23, 17165 Stockholm, Sweden.
Jaan Eha, Department of Cardiology, Heart Clinic, Tartu University Hospital, 8 L. Puusepa Street, Estonia; Department of Cardiology, University of Tartu, 8 L. Puusepa Street, 50406 Tartu, Estonia.
Piret Lõiveke, Department of Cardiology, University of Tartu, 8 L. Puusepa Street, 50406 Tartu, Estonia; Centre of Cardiology, North Estonia Medical Centre, 19 J. Sutiste Street, 13419 Tallinn, Estonia.
Toomas Marandi, Department of Cardiology, University of Tartu, 8 L. Puusepa Street, 50406 Tartu, Estonia; Centre of Cardiology, North Estonia Medical Centre, 19 J. Sutiste Street, 13419 Tallinn, Estonia.
Tiia Ainla, Department of Cardiology, University of Tartu, 8 L. Puusepa Street, 50406 Tartu, Estonia; Centre of Cardiology, North Estonia Medical Centre, 19 J. Sutiste Street, 13419 Tallinn, Estonia.
Aet Saar, Centre of Cardiology, North Estonia Medical Centre, 19 J. Sutiste Street, 13419 Tallinn, Estonia.
Gudrun Veldre, Department of Cardiology, University of Tartu, 8 L. Puusepa Street, 50406 Tartu, Estonia; Estonian Myocardial Infarction Registry, Tartu University Hospital, 8 L. Puusepa Street, 50406 Tartu, Estonia.
Péter Andréka, Hungarian Myocardial Infarction Registry, Gottsegen Hungarian Institute of Cardiology, Budapest, Hungary.
Sigrun Halvorsen, Department of Cardiology, Oslo University Hospital Ulleval, Oslo and University of Oslo, Kirkeveien 166, 0450 Oslo, Norway.
András Jánosi, Hungarian Myocardial Infarction Registry, Gottsegen Hungarian Institute of Cardiology, Budapest, Hungary.
Robert Edfors, Bayer AG, Cardiovascular Studies & Pipeline, Pharmaceuticals, Building S102, 13342 Berlin, Germany; Department of Clinical Sciences, Danderyd Hospital, Karolinska Institutet, Morbygardsvagen 5, 182 57 Stockholm, Sweden.
Lead author biography
 Robert Edfors is a cardiologist with a PhD in CV Epidemiology. He currently holds a position as clinical research physician at Bayer and is an affiliated post-doc researcher at the Department of Clinical Sciences, Danderyd Hospital, Karolinska Institutet in Stockholm, Sweden. His research interest encompasses cardiorenal topics and myocardial ischaemia. The authors of this paper are collaborating in research projects that involve comparisons of ongoing national myocardial infarction registries in Hungary, Estonia, Norway, and Sweden.
Robert Edfors is a cardiologist with a PhD in CV Epidemiology. He currently holds a position as clinical research physician at Bayer and is an affiliated post-doc researcher at the Department of Clinical Sciences, Danderyd Hospital, Karolinska Institutet in Stockholm, Sweden. His research interest encompasses cardiorenal topics and myocardial ischaemia. The authors of this paper are collaborating in research projects that involve comparisons of ongoing national myocardial infarction registries in Hungary, Estonia, Norway, and Sweden.
Supplementary material
Supplementary material is available at European Heart Journal Open online.
Data availability
The data underlying this article can be shared on reasonable request to the corresponding author.
References
- 1. Timmis A, Townsend N, Gale CP, Torbica A, Lettino M, Petersen SE, Mossialos EA, Maggioni AP, Kazakiewicz D, May HT, De Smedt D, Flather M, Zuhlke L, Beltrame JF, Huculeci R, Tavazzi L, Hindricks G, Bax J, Casadei B, Achenbach S, Wright L, Vardas P, European Society of Cardiology . European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur Heart J 2020;41:12–85. doi: 10.1093/eurheartj/ehz859 [DOI] [PubMed] [Google Scholar]
- 2. Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J 2016;37:3232–3245. doi: 10.1093/eurheartj/ehw334 [DOI] [PubMed] [Google Scholar]
- 3. Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN, Lindley KJ, Vaccarino V, Wang TY, Watson KE, Wenger NK. Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation 2016;133:916–947. doi: 10.1161/CIR.0000000000000351 [DOI] [PubMed] [Google Scholar]
- 4. Gupta A, Wang Y, Spertus JA, Geda M, Lorenze N, Nkonde-Price C, D'Onofrio G, Lichtman JH, Krumholz HM. Trends in acute myocardial infarction in young patients and differences by sex and race, 2001 to 2010. J Am Coll Cardiol 2014;64:337–345. doi: 10.1016/j.jacc.2014.04.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chandrasekhar J, Gill A, Mehran R. Acute myocardial infarction in young women: current perspectives. Int J Womens Health 2018;10:267–284. doi: 10.2147/IJWH.S107371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dreyer RP, Dharmarajan K, Kennedy KF, Jones PG, Vaccarino V, Murugiah K, Nuti SV, Smolderen KG, Buchanan DM, Spertus JA, Krumholz HM. Sex differences in 1-year all-cause rehospitalization in patients after acute myocardial infarction: a prospective observational study. Circulation 2017;135:521–531. doi: 10.1161/CIRCULATIONAHA.116.024993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu J, Elbadawi A, Elgendy IY, Megaly M, Ogunbayo GO, Krittanawong C, Tamis-Holland JE, Ballantyne CM, Khalid MU, Virani S, Gulati M, Albert M, Bozkurt B, Jneid H. Age-stratified sex disparities in care and outcomes in patients with ST-elevation myocardial infarction. Am J Med 2020;133:1293–1301.e1. doi: 10.1016/j.amjmed.2020.03.059 [DOI] [PubMed] [Google Scholar]
- 8. Kunadian V, Qiu W, Lagerqvist B, Johnston N, Sinclair H, Tan Y, Ludman P, James S, Sarno G. Gender differences in outcomes and predictors of all-cause mortality after percutaneous coronary intervention (data from United Kingdom and Sweden). Am J Cardiol 2017;119:210–216. doi: 10.1016/j.amjcard.2016.09.052 [DOI] [PubMed] [Google Scholar]
- 9. Pagidipati NJ, Peterson ED. Acute coronary syndromes in women and men. Nat Rev Cardiol 2016;13:471–480. doi: 10.1038/nrcardio.2016.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lawesson SS, Alfredsson J, Fredrikson M, Swahn E. A gender perspective on short- and long term mortality in ST-elevation myocardial infarction–a report from the SWEDEHEART register. Int J Cardiol 2013;168:1041–1047. doi: 10.1016/j.ijcard.2012.10.028 [DOI] [PubMed] [Google Scholar]
- 11. Heer T, Hochadel M, Schmidt K, Mehilli J, Zahn R, Kuck KH, Hamm C, Böhm M, Ertl G, Hoffmeister HM, Sack S, Senges J, Massberg S, Gitt AK, Zeymer U. Sex differences in percutaneous coronary intervention-insights from the coronary angiography and PCI registry of the German Society of Cardiology. J Am Heart Assoc 2017;6:e004972. doi: 10.1161/JAHA.116.004972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bavishi C, Bangalore S, Patel D, Chatterjee S, Trivedi V, Tamis-Holland JE. Short and long-term mortality in women and men undergoing primary angioplasty: a comprehensive meta-analysis. Int J Cardiol 2015;198:123–130. doi: 10.1016/j.ijcard.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 13. Berger JS, Brown DL. Gender-age interaction in early mortality following primary angioplasty for acute myocardial infarction. Am J Cardiol 2006;98:1140–1143. doi: 10.1016/j.amjcard.2006.06.012 [DOI] [PubMed] [Google Scholar]
- 14. Blondal M, Ainla T, Eha J, Loiveke P, Marandi T, Saar A, Veldre G, Edfors R, Lewinter C, Jernberg T, Jortveit J, Halvorsen S, Becker D, Csanádi Z, Ferenci T, Andréka P, Jánosi A. Comparison of management and outcomes of ST-segment elevation myocardial infarction patients in Estonia, Hungary, Norway and Sweden according to national ongoing registries. Eur Heart J Qual Care Clin Outcomes 2022;8:307–314. doi: 10.1093/ehjqcco/qcaa098 [DOI] [PubMed] [Google Scholar]
- 15. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Katus HA, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasché P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S, Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction . Third universal definition of myocardial infarction. Circulation 2012;126:2020–2035. doi: 10.1161/CIR.0b013e31826e1058 [DOI] [PubMed] [Google Scholar]
- 16. Flynn MR, Barrett C, Cosio FG, Gitt AK, Wallentin L, Kearney P, Lonergan M, Shelley E, Simoons ML. The Cardiology Audit and Registration Data Standards (CARDS), European data standards for clinical cardiology practice. Eur Heart J 2005;26:308–313. doi: 10.1093/eurheartj/ehi079 [DOI] [PubMed] [Google Scholar]
- 17. Internet source available at: http://www.infarkt.ee/et/aruanded. 2020.
- 18. Govatsmark RES, Janszky I, Slordahl SA, Ebbing M, Wiseth R, Grenne B, Vesterbekkmo E, Bønaa KH. Completeness and correctness of acute myocardial infarction diagnoses in a medical quality register and an administrative health register. Scand J Public Health 2020;48:5–13. doi: 10.1177/1403494818803256 [DOI] [PubMed] [Google Scholar]
- 19. Jernberg T, Attebring MF, Hambraeus K, Ivert T, James S, Jeppsson A, Lagerqvist B, Lindahl B, Stenestrand U, Wallentin L. The Swedish web-system for enhancement and development of evidence-based care in heart disease evaluated according to recommended therapies (SWEDEHEART). Heart 2010;96:1617–1621. doi: 10.1136/hrt.2010.198804 [DOI] [PubMed] [Google Scholar]
- 20. Swedeheart . Annual report. 2020. Internet source available at: https://www.ucr.uu.se/swedeheart/dokument-sh/arsrapporter-sh/arsrapport-2020/1-swedeheart-annual-report-2020-english-2.
- 21. Rossello X, Masso-van Roessel A, Perello-Bordoy A, Mas-Llado C, Ramis-Barcelo MF, Vives-Borras M, Pons J, Peral V. Assessment of the ESC quality indicators in patients with acute myocardial infarction: a systematic review. Eur Heart J Acute Cardiovasc Care 2021;10:878–889. doi: 10.1093/ehjacc/zuab042 [DOI] [PubMed] [Google Scholar]
- 22. Smilowitz NR, Gupta N, Guo Y, Zhong J, Weinberg CR, Reynolds HR, Bangalore S. Acute myocardial infarction during pregnancy and the puerperium in the United States. Mayo Clin Proc 2018;93:1404–1414. doi: 10.1016/j.mayocp.2018.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anand SS, Xie CC, Mehta S, Franzosi MG, Joyner C, Chrolavicius S, Fox KAA, Yusuf S. Differences in the management and prognosis of women and men who suffer from acute coronary syndromes. J Am Coll Cardiol 2005;46:1845–1851. doi: 10.1016/j.jacc.2005.05.091 [DOI] [PubMed] [Google Scholar]
- 24. Berger JS, Elliott L, Gallup D, Roe M, Granger CB, Armstrong PW, Simes RJ, White HD, Van de Werf F, Topol EJ, Hochman JS, Newby LK, Harrington RA, Califf RM, Becker RC, Douglas PS. Sex differences in mortality following acute coronary syndromes. JAMA 2009;302:874–882. doi: 10.1001/jama.2009.1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stein GY, Herscovici G, Korenfeld R, Matetzky S, Gottlieb S, Alon D, Gevrielov-Yusim Ne, Iakobishvili Z, Fuchs S, Guo Y. Type-II myocardial infarction–patient characteristics, management and outcomes. PLoS One 2014;9:e84285. doi: 10.1371/journal.pone.0084285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sielski J, Kazirod-Wolski K, Jurys K, Walek P, Siudak Z. The effect of periprocedural clinical factors related to the course of STEMI in men and women based on the National Registry of Invasive Cardiology Procedures (ORPKI) between 2014 and 2019. J Clin Med 2021;10:5716. doi: 10.3390/jcm10235716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bucholz EM, Butala NM, Rathore SS, Dreyer RP, Lansky AJ, Krumholz HM. Sex differences in long-term mortality after myocardial infarction: a systematic review. Circulation 2014;130:757–767. doi: 10.1161/CIRCULATIONAHA.114.009480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mehran R, Pocock SJ, Nikolsky E, Clayton T, Dangas GD, Kirtane AJ, Parise H, Fahy M, Manoukian SV, Feit F, Ohman ME, Witzenbichler B, Guagliumi G, Lansky AJ, Stone GW. A risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol 2010;55:2556–2566. doi: 10.1016/j.jacc.2009.09.076 [DOI] [PubMed] [Google Scholar]
- 29. Lansky AJ, Hochman JS, Ward PA, Mintz GS, Fabunmi R, Berger PB, New G, Grines CL, Pietras CG, Kern MJ, Ferrell M, Leon MB, Mehran R, White C, Mieres JH, Moses JW, Stone GW, Jacobs AK. Percutaneous coronary intervention and adjunctive pharmacotherapy in women: a statement for healthcare professionals from the American Heart Association. Circulation 2005;111:940–953. doi: 10.1161/01.CIR.0000155337.50423.C9 [DOI] [PubMed] [Google Scholar]
- 30. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Kardiol Pol 2018;76:229–313. doi: 10.5603/KP.2018.0041 [DOI] [PubMed] [Google Scholar]
- 31. Tamis-Holland JE, Palazzo A, Stebbins AL, Slater JN, Boland J, Ellis SG, Hochman JS. Benefits of direct angioplasty for women and men with acute myocardial infarction: results of the Global Use of Strategies to Open Occluded Arteries in Acute Coronary Syndromes Angioplasty (GUSTO II-B) Angioplasty Substudy. Am Heart J 2004;147:133–139. doi: 10.1016/j.ahj.2003.06.002 [DOI] [PubMed] [Google Scholar]
- 32. Lawesson SS, Alfredsson J, Fredrikson M, Swahn E. Time trends in STEMI–improved treatment and outcome but still a gender gap: a prospective observational cohort study from the SWEDEHEART register. BMJ Open 2012;2:e000726. doi: 10.1136/bmjopen-2011-000726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jneid H, Fonarow GC, Cannon CP, Hernandez AF, Palacios IF, Maree AO, Wells Q, Bozkurt B, LaBresh KA, Liang L, Hong Y, Newby LK, Fletcher G, Peterson E, Wexler L. Sex differences in medical care and early death after acute myocardial infarction. Circulation 2008;118:2803–2810. doi: 10.1161/CIRCULATIONAHA.108.789800 [DOI] [PubMed] [Google Scholar]
- 34. Canto JG, Goldberg RJ, Hand MM, Bonow RO, Sopko G, Pepine CJ, Long T. Symptom presentation of women with acute coronary syndromes: myth vs reality. Arch Intern Med 2007;167:2405–2413. doi: 10.1001/archinte.167.22.2405 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article can be shared on reasonable request to the corresponding author.