Abstract
The COVID-19 pandemic severely impacted long-term care facilities resulting in the death of approximately 8% of residents nationwide as of March 2021. As COVID-19 case rates declined and state and county restrictions were lifted in spring 2021, facility managers, local and state health agencies were challenged with defining their own policies moving forward to appropriately mitigate disease transmission. The continued emergence of variants of concern and variable vaccine uptake across facilities highlighted the need for a readily available tool that can be employed at the facility-level to determine best practices for mitigation and ensure resident and staff safety. To assist leadership in determining the impact of various infection surveillance and response strategies, we developed an agent-based model and an online dashboard interface that simulates COVID-19 infection within congregate care settings under various mitigation measures. This dashboard quantifies the continued risk for COVID-19 infections within a facility given a designated testing schedule and vaccine requirements. Key findings were that choice of COVID-19 diagnostic (ex. nasal swab qRT-PCR vs rapid antigen) and testing cadence has less impact on attack rate and staff workdays missed than does vaccination rates among staff and residents. Specifically, low vaccine uptake among staff at long-term care facilities puts staff and residents at risk of ongoing COVID-19 outbreaks. Here we present our model and dashboard as an exemplar of a tool for state public health officials and facility directors to gain insights from an infectious disease model that can directly inform policy decisions in the midst of a pandemic.
Keywords: Agent-based model, Nonpharmaceutical interventions, Vaccine uptake, COVID-19
Abbreviations: ABM, agent-based model; CDC, Centers for Disease Control; CMS, Centers for Medicare and Medicaid Services; LTCF, long term care facility
1. Introduction
Nursing homes, skilled nursing facilities and assisted living facilities, collectively known as long-term care facilities (LTCFs), provide care for some of the most vulnerable populations in society. Shared sleeping quarters, bathrooms, dining facilities, and common spaces; and the need for daily contact between staff and residents create an opportunistic environment for the spread of respiratory pathogens. In particular, the morbidity and mortality within LTCFs throughout the COVID-19 pandemic was estimated at nearly 8% as of March 2021, demonstrating the extreme vulnerability of both LTCF residents and staff to a transmissible viral illness (Curiskis et al., 2021). Approximately 40% of COVID-related deaths in the United States were among residents of LTCFs as of March 2021, and yet clinical research in LTCFs during COVID-19 has been limited (Kim et al., 2020; Quinn et al., 2021). Moreover, LTCF staff represent a disproportionately high percentage of SARS-CoV-2 infections as compared to non-healthcare community members regardless of whether LTCF staff have direct patient contact. In one report, whole-genome sequencing suggested that SARS-CoV-2 infection among LTCF staff had more likely come from staff-staff transmission than community import events (Gallichotte et al., 2020).
In recognition of the difficulty mitigating or preventing SARS-CoV-2 within LTCFs, the Centers for Disease Control (CDC) recommended that LTCFs have first priority for vaccine access and developed The Pharmacy Partnership for Long Term Care Program to distribute the first available vaccines to LTCF residents and staff. However, early vaccine acceptance among LTCF staff was quite variable. Before vaccination requirements started rolling out, one study reported that among the 11,460 LTCFs with at least one vaccination clinic conducted via the CDC Pharmacy Partnership for Long Term Care Program, only 37.5% of staff members received the vaccine as compared to 77.8% of residents (Gharpure et al., 2021). These data were and are concerning because unvaccinated staff can sustain SARS CoV-2 infection within LTCFs, making infection control extremely difficult. Furthermore, even with higher vaccination rates, new variants (e.g., delta and omicron variant) pose a risk because they may be more resistant to vaccine-induced immunity (Cavanaugh, 2021; White et al., 2021). In a study of infections in LTCFs in Catalonia, Spain during spring 2021, researchers found that once more than 70% of the facility population was vaccinated, approximately 75% of COVID-19 deaths and infections in the facility were prevented (Salazar et al., 2021). Vaccination rates are of critical importance even for LTCF residents that were previously infected with COVID-19 as a substantial proportion have been found to have nondetectable antibodies six months post infection (Moore et al., 2021).
Centers for Medicare and Medicaid Services (CMS), and state and local public health recommendations for SARS-CoV-2 surveillance testing in LTCFs to identify and isolate presymptomatic/asymptomatic SARS-CoV-2 positive individuals evolved over time. A number of learnings have emerged throughout the pandemic that highlight limitations with a “one size fits all” approach to surveillance and outbreak response in LTCFs. Mitigation and prevention approaches rarely considered the simultaneous influences of test type, predicted sensitivity/specificity, testing frequency, testing goal (surveillance versus diagnostic), test result latency period, and vaccine acceptance rates. Given the rapidly changing climate surrounding COVID-19 prevalence, testing availability, vaccination acceptance, and community prevalence, we sought to create a model to better understand potential outcomes within LTCFs using inputs related to these real-life and fluid variables as they change throughout time.
Agent-based models (ABMs) are a powerful tool for understanding complex dynamic processes, such as infectious disease transmission (Bonabeau, 2002). During the COVID-19 pandemic, these models have been used to assess the impact of nonpharmaceutical interventions on infections within schools, a small town, and France (Naimark et al., 2021; Truszkowska et al., 2021; Hoertel et al., 2020). Researchers further demonstrated that delaying the second dose of mRNA vaccines for those under 65 led to more positive outcomes under certain conditions with an ABM (Romero-Brufau et al., 2021). Within LTCFs specifically, ABMs were used to study the impact of testing and immunity-based staffing interventions, and early in the pandemic were used to simulate the spatiotemporal transmission process of COVID-19 under varied virus infectiousness levels and within-facility mobility restrictions (Holmdahl et al., 2021; Cuevas, 2020). An advantage of ABMs over compartmental models based on differential equations is that they can be programmed to capture important micro-level behavior unique to a specific setting.
The agent-based framework has several advantages for modeling LTCFs. First, LTCFs are focused settings with relatively low populations. Second, the ABM permits simple tracking of individuals' health and behavior over time. This point is important in the LTCF setting, where staff are transient members of the population as they move in and out of the population according to work schedules. Accounting for this behavior is key as infectious individuals only pose a risk to the facility when present. Moreover, ABMs track the state of individual agents in the model. If a member of the night staff is infected, that specific agent can be isolated until they no longer pose an infection risk to other agents in the facility. These features make the ABM a suitable framework for a decision support tool used to analyze the impact of different testing protocols, vaccination rates, and visitation policies.
State and county COVID-19 regulations began to relax heavily in May 2021. This left LTCF managers and local health officials to define their own COVID-19 policies. Some facilities required their staff to be vaccinated, leaning on existing requirements for the influenza vaccine to argue this was a natural policy (Paulin, 2021). Other facilities decided not to require vaccination and instead relied on surveillance testing to continue to monitor their facility for infection in unvaccinated individuals. While both strategies have advantages and disadvantages, it was unclear how much continued risk a facility is at given a specific level of staff vaccine uptake, testing regime, and community prevalence.
Due to slight nuances across facilities that can greatly affect transmission patterns, we created an interactive online dashboard that accepts facility-specific parameters and forecasts infection rates and worker days missed under a set of transmission characteristics and testing protocols (dashboard is accessible here: https://ltcf-covid.shinyapps.io/ltcf_covid_dashboard/) (Dilliott, 2021). This dashboard allowed facility administrators to evaluate the relative impact of various strategies moving forward. In what follows, we explore the trade-off between a high cadence testing regime at a moderately vaccinated facility to a no testing regime at a highly vaccinated facility to demonstrate how the ABM and dashboard could be utilized by practioners.
2. Methods
Consider the scenario where a facility manager is tasked with deciding on policies for summer 2021. The facility has 90 residents, 125 day staff, and 45 night staff. Assume residents and staff are vaccinated at rates mirroring the national averages, 78%, and 38%, respectively at this time, and the 7-day case rate average for the community is approximately 84 people per 100,000/week. The manager is concerned about potential future outbreaks due to their impact on resident and staff physical health, the number of worker days missed due to quarantined staff, and the emotional toll of a return to a lock-down state. Thus, the manager considers a facility-wide vaccination requirement for staff and asks: How many fewer worker days would be missed? How many fewer individuals would be infected with COVID-19? Could infection levels instead be limited by regular testing of unvaccinated staff and residents?
To answer these concrete questions, we programed an agent-based model that simulates the key daily behaviors and events that impact disease transmission in a facility. Like the traditional infectious disease compartmental models, all agents (staff and residents) are labeled as either susceptible, exposed, asymptomatic infected, symptomatic infected, or immune (also referred to as recovered). Each agent transitions between these states based on events in the simulation. For example, all unvaccinated individuals start out as a ‘susceptible’, but after they come into contact with an infectious agent, there is a chance that they contract the virus and become ‘exposed’ for a short period of time before being labeled as ‘infected’ and able to spread COVID-19 to others. Finally, after the active infection period has subsided, the agent moves to the ‘immune’ state. Recognizing vaccine efficacy is not 100%, most vaccinated individuals are given the ‘immune’ label from the start with a small number remaining ‘susceptible’.
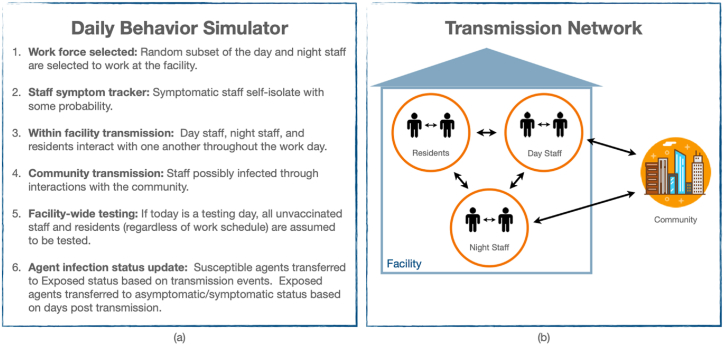
The progression of events in a day is described in Fig. 1(a). A random fifth of the day staff and fifth of the night staff are selected to show up to work. Each infected staff member has a chance of showing symptoms, self-isolating, and staying home from work. All other staff attend work and have interactions with each other and the residents. While interactions are assumed to be random each day, a contact matrix dictates the relative number of contacts between different subgroups. For example, day staff and night staff are assumed to have limited contact that occurs just during shift changes. Finally, we model staff infection via community transmission based on the local 7-day case rate. Community case rates were shown to be the strongest predictor of COVID-19 cases and outbreaks at LTCFs (Gorges & Konetzka, 2020). Fig. 1(b) depicts the transmission network for the ABM. We assume a vaccine efficacy of 95% in an “all-or-nothing” framework, such that 5% of those vaccinated remain susceptible to infection (Bubar et al., 2021).
Fig. 1.
(a) Description of events throughout a day during the simulation. (b) Avenues of transmission within and into the facility.
The primary means for COVID-19 monitoring and outbreak response during the latter half of 2020 and today is facility-wide testing. There are two primary options for surveillance testing: rapid antigen and qRT-PCR tests. The advantage of qRT-PCR tests is that they are extremely accurate, with a sensitivity and specificity greater than 95%; however, the time between the test and receiving the result is often 36–72 h depending on the lab processing it. Conversely, antigen test results can be acquired the same day but are limited by lower sensitivity. In this study, we assume an qRT-PCR test with 95% sensitivity and 99.5% specificity, and a rapid antigen test with 64.2% sensitivity on symptomatic positive individuals, 35.8% sensitivity on asymptomatic individuals and 99.5% specificity. If vaccine uptake among staff and residents is limited or moderate, a facility director may consider regular facility-wide testing of unvaccinated individuals to combat the possibility and extent of an outbreak. Upon a positive test result, we use a 14-day quarantine for staff. A complete description of the ABM is provided in Appendix A.
3. Results
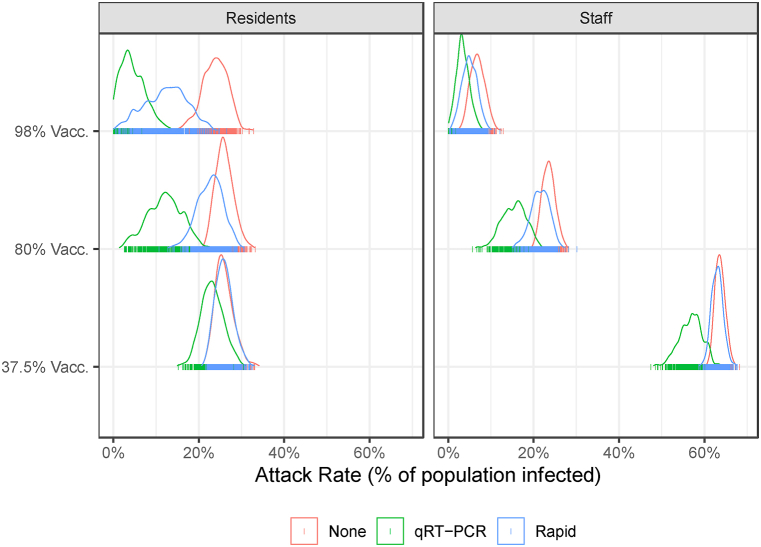
We simulate the LTCF-ABM to illustrate two continued risks to LTCFs: 1) low staff vaccination rates, and 2) relaxed screening protocols. LTCF staff are a conduit for risk to LTCF residence because they interact with the outside community when not at work. Our model suggests that outbreaks are likely to occur when 78% of residents and 37.5% of staff are vaccinated even with a weekly testing protocol in place. Fig. 2 shows the distribution of the total number of infections across three vaccination scenarios (37.5%, 80%, and 90%) and three testing scenarios (None, qRT-PCR, and Rapid) for 500 simulations. The variation in results across simulations is due to the stochastic transmission functions in the model. The results indicate that while a testing protocol makes a difference, staff vaccination rates are the primary determinant of the total number of infections in both staff and residents. In staff, increasing vaccination from 37.5% to 98% reduces the attack rate by 89% (from 63% to 7%) without a testing protocol. A weekly qRT-PCR-based testing protocol can provide further protection reducing the staff attack rate to a mean of 3% in the 98% staff vaccination case. The combination of high staff vaccination (98%) and weekly qRT-PCR-based testing protocol reduces the attack rate in residents by 78% (from 23% to 5%). In the low staff vaccination scenario, the qRT-PCR-based testing protocol reduces the attack rate in residents by only 11% (from 26% to 23%). The reduced sensitivity of the rapid antigen test has a much smaller effect, especially with relatively low staff vaccination rates.
Fig. 2.
The attack rate in residents (left panel) and staff (right panel) populations over 60-day simulation by vaccination and testing scenario. The distribution represents the variation in results due to stochastic transmission during 500 simulations. Summary statistics are reported in Table A.3.
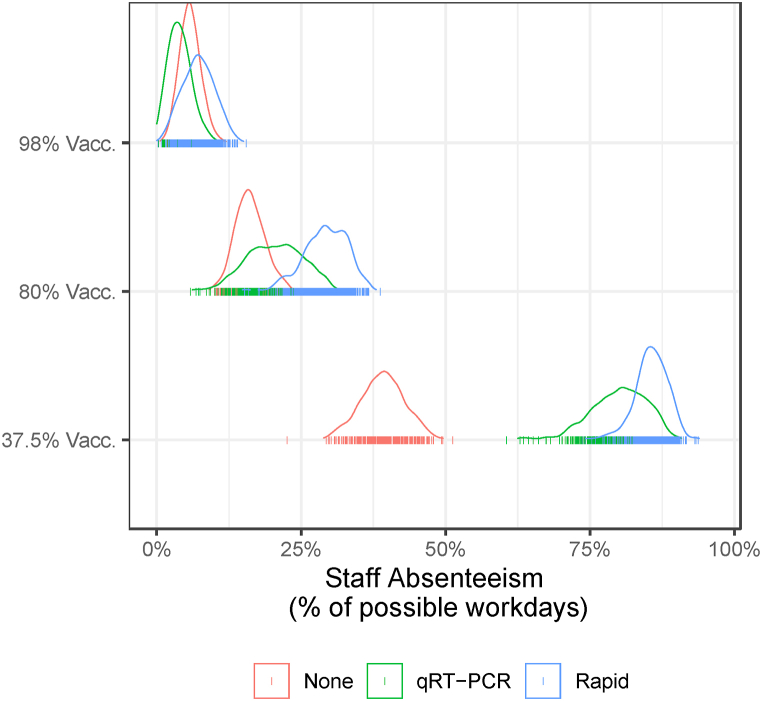
While testing protocols provide protection against transmission, they also lead to staff absenteeism as individuals that test positive are required to isolate. We assume that symptomatic staff will report their illness and self-isolate with some probability. Fig. 3 plots the distribution of staff absenteeism measured by the number of days staff are assigned to work but are unable to because of a positive test or self-isolation. Again, higher staff vaccination rates are the most effective mechanism for reducing staff absenteeism because vaccination prevents infection. When comparing the qRT-PCR to the rapid antigen testing protocols, we find that absenteeism is higher under the rapid antigen protocol due to the shorter delay in receiving the test result (immediate versus two days). In the low vaccination scenario (37.5% staff vaccination rate), the absenteeism rate is 86% under the rapid antigen testing protocol versus 80% under the qRT-PCR testing protocol. The difference between the testing protocols becomes smaller as staff vaccination rates increase.
Fig. 3.
Staff absenteeism by vaccination and testing scenario over 60-day simulation. Absenteeism is defined as workdays missed because of a positive test result or symptomatic self-reporting. The distributions are based on 500 model simulations. Summary statistics are reported in Table A.4.
4. Discussion
Our model and dashboard highlighted the potential for COVID-19 outbreaks in long-term care facilities even when staff and residents are partially vaccinated and screened regularly in summer 2021. This result was not just theoretical. 627 cases of COVID-19 in staff and residents at 75 skilled nursing facilities were documented from December 28, 2020 to March 31, 2021 (Teran, 2021). Of those 627, 22 occurred in fully vaccinated individuals more than two weeks after the second dose, and 145 occurred in partially vaccinated individuals.
While our model and analysis are tailored to LTCFs in the United States, our model could be adapted to other countries in which vulnerable populations live in facilities with staff that provide care. Our ABM model of LTFCs could also be coupled with forecasting models that predict community prevalence (Dawoud, 2020) via the probability that staff become infected while off duty.
The results of the LTCF-ABM should be considered along with model limitations. We strived to use realistic parameters when estimates were available from the literature. However, some parameters were not well studied at this time. We assumed a contact structure between staff and residents that is static over the simulation. This may not be true if staff limit their contact with each other and residents in the event of an outbreak. Researchers have also documented increased risk to facilities whose staff work at other facilities (Chen et al., 2021). This additional risk was not explicitly modeled here but could be captured by assuming a higher community prevalence quantifying the risk of outside infection to staff.
The testing protocol is determined at the beginning of the simulation and does not change. Facilities that detect a positive case may engage in investigation and more rigorous testing procedures to mitigate the further spread of the virus. However, one important result from our analysis is that when community transmission is present, there is a continual risk that a staff member reintroduces the virus.
5. Conclusions
Despite increasing vaccination and declining cases in spring 2021, the future of the pandemic was very much uncertain. There existed potential for outbreaks in unvaccinated health care worker populations and vaccinated populations with the arrival of new variants such as delta and omicron, which were more infectious or more resistant to vaccines (Wingerter, 2021). Our decision support tool was created to help LTCFs and regional public health departments develop strategies to mitigate the risk of outbreaks and contain them as they emerge.
While test availability, decaying immunity, and COVID-19 treatments changed over the latter half of 2021, the ABM and dashboard developed here gradually lost is applicability. However, minor updates of the model parameters and structure could make the tool reflective of the current pandemic landscape. Collaboration between researchers and policy makers is essential for modeling tools, such as ABMs, to inform decision making. Our work here acts as an example of how a rich infectious disease model can be programed and integrated into a user-friendly dashboard to serve public health administrators. Importantly, this tool could also be adapted relatively easily to model the particulars of any existing or future respiratory pathogen.
Ethics approval and consent to participate
Not applicable.
Availability of data and materials
The dashboard is available at https://ltcf-covid.shinyapps.io/ltcf_covid_dashboard/. The code for the simulations and model underlying the dashboard are available at https://www.doi.org/10.5281/zenodo.4984845.
Funding
This work was supported by Colorado State University's Center for Healthy Aging, the Office of the Vice President for Research, the College of Health and Human Sciences, the College of Natural Sciences, the College of Veterinary Medicine and Biomedical Sciences, and the Walter Scott Jr College of Engineering. It was also supported by Colorado Department of Public Health and the Environment, COVID-19 Epidemic Modeling Services (RFAA 202000011320).
Consent for publication
Not applicable.
CRediT authorship contribution statement
Bailey K. Fosdick: Conceptualization, Methodology, Writing – original draft, Visualization. Jude Bayham: Conceptualization, Methodology, Software, Writing – original draft, Visualization, Funding acquisition. Jake Dilliott: Methodology, Software. Gregory D. Ebel: Conceptualization, Methodology, Writing – original draft. Nicole Ehrhart: Conceptualization, Methodology, Writing – original draft, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the Vivage Living Communities and Staff, families and residents of LTCFs. We also thank Laura Nolt for her comments and edits.
Handling editor. Dr Y. Shao
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Appendix A. Overview, Design concepts and Details Protocol for LTCF Agent-Based Model
-
1.
Purpose
This model's purpose is to study the spread of SARS-CoV-2 in a long-term care facility environment over a short time window, e.g. 3–6 months, under various vaccination levels of staff and residents and differing testing protocols. It is designed to allow facility managers to understand the trade-offs between adopting, for example, a vaccination mandate versus rapid testing staff weekly versus requiring qRT-PCR tests biweekly. The primary outcomes considered are the total number of infected individuals in the facility and the number of staff workday missed as a result of isolation. Complete code for the dashboard and model are available (Dilliott, 2021).
-
2.
Entities, State Variables, and Scales
The model includes three types of entities: day staff, night staff, and residents. Each entity is an individual and is characterized by their vaccination status (vaccinated or unvaccinated), their current infection state (Susceptible, Exposed, Asymptomatic infected, symptomatic Infected or Recovered) and whether they are in quarantine from a positive test. An all-or-nothing vaccine model is assumed such that 5% of vaccinated individuals are still susceptible to infection, while the remaining 95% are fully protected after being vaccinated (Bubar et al., 2021). Vaccine status of each entity is held constant. The simulation proceeds 1 day at a time, where entity infection and quarantine status is updated.
-
3.
Process overview, scheduling
At the start of each day, a random subset of day staff and night staff are selected to work at the LTCF. Staff and residents have contact with one another based on the contact matrix. Susceptible individuals that have contact with infected individuals stochastically transition to the Exposed state based on their number of infectious symptomatic and asymptomatic contacts in the facility. Staff are also susceptible to becoming exposed from the community based on the community prevalence, where it is assumed they contact 5 community individuals per day. Individuals in the Exposed state stochastically transition to an infected state, either Asymptomatic infected or symptomatic Infected, according to an exponential distribution. In addition, Asymptomatic infected and symptomatic Infected individuals stochastically transition to Recovered according to an exponential distribution. If testing is scheduled for the given day, all non-quarantined staff and residents are tested. If the test result delay is zero, such as for a rapid test, then those that test positive, whether a staff member or resident, are immediately placed in quarantine where they have no contact with others. Otherwise, positive testers are placed into quarantine the after the test delay has elapsed. Although positive residents would continue to contact the staff at the LTCF, it is assumed that the PPE protocols for staff are such that there is no probability of transmission. Note there is nonzero probability of a false positive or false negative test based on the sensitivity and specificity of the test used. Symptomatic Infected individuals possibly self-quarantine. Finally, Asymptomatic infected and symptomatic Infected individuals that complete their quarantine are transitioned to the Recovered state, and Susceptible individuals that completed their quarantine (due to a false positive test) remain Susceptible.
-
4.
Design concepts
Basic principles – This model adapts a basic SEIR agent-based model to a LTCF environment by incorporating variability in day and night staff schedules, as well as the relative number of contacts between day staff, night staff and residents.
Emergence – The emergence of new infections results from onsite contacts among the scheduled staff and residents at the LTCF, as well as from staff contracting COVID-19 from the community.
Sensing – Individuals do not have any knowledge of others’ infection status.
Interaction – Agents present at work interact according to a contact matrix based on agent type (i.e., day staff, night staff, resident). Table A.2 defines the contact matrix used in simulations.
Stochasticity – A random subset of non-quarantined staff show up to work at the LTCF each day. While interactions between agents present at the facility are dictated by the contact matrix, viral transmission is random and occurs when an agent interacts with an infectious (symptomatic or asymptomatic) agent and follows a Poisson distribution. The number of days in the Exposed state is random and follows an exponential distribution. The number of days in the symptomatic Infected and Asymptomatic infected states are also random and exponentially distributed. Symptomatic infected individuals independently randomly decide whether to self-quarantine each day according to a fixed probability. The accuracy of the test results are random according to the sensitivity and specificity of the test used by the facility.
Observation – The total number of staff and resident infections is monitored, as well as the number of staff workdays missed due to quarantining.
-
5.
Initialization
The composition of the facility in terms of number of day staff, night staff and residents must be specified and is fixed throughout the simulation. A fixed fraction of staff and residents are specified to be fully vaccinated at the start of the simulation and a fixed fraction of staff and residents are assumed to be initially infected. No individuals are in quarantine at the start of the simulation.
-
6.
Input data
This model does not include any input of external data.
-
7.
Submodels
All model parameters are listed in Tables A.1 and A.2 below.
Table A.1.
Input parameters and values used in simulations.
| Input parameter | Simulation values | Source |
|---|---|---|
| Facility |
||
| Number of day staff | 125 | Example facility |
| Number of night staff | 45 | Example facility |
| Number of residents | 90 | Example facility |
| Number of day staff scheduled each day | 25 | Example facility |
| Number of night staff scheduled each day | 9 | Example facility |
| Starting conditions |
||
| Symptomatic residents (%) | 0% | – |
| Asymptomatic staff and residents (%) | 2% | – |
| Staff vaccinated or previously infected in last 6 months (%) | 37.5%, 80%, 98% | Gharpure et al. (2021) |
| Residents vaccinated or previously infected in last 6 months (%) | 78% | Gharpure et al. (2021) |
| Testing |
||
| Test result delay (days) | 2 (qRT-PCR), 0 (Rapid) |
– |
| Testing cadence (days) | 7 | – |
| Test sensitivity symptomatic (%) | 95% (qRT-PCR), 64.2% (Rapid) |
Hanson et al. (2020) (qRT-PCR), Prince-Guerra et al. (2021) (Rapid) |
| Test sensitivity asymptomatic (%) | 95% (qRT-PCR), 35.8% (Rapid) | Prince-Guerra et al. (2021) (Rapid) |
| Test specificity (%) | 99.5% |
Hanson et al. (2020) (qRT-PCR), Hanson et al. (2021) (Rapid) |
| Epidemiology (Advanced) |
||
| R0∗ | 1.4 | – |
| Community 14 day case rate/100K | 168 | NYT Covid Tracker |
| Probability of symptomatic infection | 0.4286 | Buchwald et al. (2021) |
| Mean latent period (days in Exposed category) | 5 | Sanche et al. (2020) |
| Mean time in infected state (either asymptomatic or symptomatic) before transition to recovered | 8 | Bi et al. (2020) |
| Quarantine period (days) | 14 | LTCF Recommendation |
| Probability symptomatic infected individual self-quarantines each day | 0.28+ | – |
| Relative secondary attack rate for asymptomatic individuals compared to symptomatic individuals | .667 | Buchwald et al. (2021) |
| Simulation (Advanced) |
||
| Start date | 2021-05-01 | – |
| Duration (days) | 60 | – |
| Number of runs | 500 | – |
∗ Given the heavy usage of personal protective equipment in LTCFs, R0 values estimated for other settings, such as schools or the community, are unlikely to hold in LTCFs. The R0 value was selected by the authors as something reasonable to explore the impacts of other transmission factors.
+ Probability was specified such that cumulative probability a symptomatic infected individual self-quarantines over the course of 7 days is 0.9, where the probability of self-quarantining each day is assumed independent.
Table A.2.
Daily contact matrix governing interactions between entities in different subgroups.
| Day staff | Night staff | Residents | |
|---|---|---|---|
| Day staff | 7 | 1 | 7 |
| Night staff | 1 | 3 | 2 |
| Residents | 7 | 2 | 1 |
Probability staff member infected from community
The probability a random individual in the community is infectious is estimated to be 14/100,000 times the 14-day case rate, which roughly assumes an individual is infectious for a full 14-day interval. Although this estimate may be high based on literature on the infectiousness period of COVID-19, it is known that case rates are censored due to lack of testing and thus, the hope is this provides a reasonable estimate of a staff member's risk of obtaining COVID-19 through community spread. Each staff member is assumed to come into contact with five individuals each day so this probability they are infected from the community is 1 – (1–14/100,000∗(14-daycase rate))^5.
Translating R0 to transmission parameter
The user inputs an value, which we use to calibrate the parameter . Beta represents the probability that a contact between an infectious agent and a susceptible agent results in transmission. Define as
where is the probability that an agent is infectious and is the maximum eigen value of the contact matrix, (Diekmann et al., 1990). We rearrange this equation solving for as a function of ,
Timing of transmission
In a typical compartmental epidemiological model (e.g., SEIR), the generation interval is the mean duration in the exposed class (latent period) plus the mean duration in the infectious class (infectious period), which we assume are 5 and 8 days, respectively. These assumptions imply a generation interval of 13 days which is longer than the time between generations observed during the COVID-19 pandemic (Tang et al., 2021). However, this calculation of the generation interval is based on a model without testing and quarantine. In our model, agents are routinely testing and isolating if infected, which effectively reduces the mean generation interval since many infectious agents stop making contacts shortly after infection is detected. The effective time between generations depends on the testing cadence and compliance of the agents in LTCFs.
Appendix B. Additional Results
Table A.3.
Attack rate by vaccination and testing scenario over 500 simulations. Mean (10th percentile, 90th percentile).
| Vaccination Rate | None | qRT-PCR | Rapid | |
|---|---|---|---|---|
| Residents | 37.5%. | 26% (23%, 29%) | 23% (20%, 27%) | 26% (23%, 29%) |
| 80% | 26% (23%, 29%) | 12% (7%, 18%) | 23% (19%, 27%) | |
| 98% | 24% (20%, 28%) | 5% (1%, 9%) | 12% (5%, 19%) | |
| Staff | 37.5% | 64% (62%, 65%) | 57% (53%, 61%) | 63% (61%, 65%) |
| 80% | 24% (22%, 25%) | 15% (12%, 19%) | 22% (19%, 24%) | |
| 98% | 7% (5%, 9%) | 3% (2%, 5%) | 5% (3%, 7%) |
Table A.4.
Staff absenteeism by vaccination and testing scenario over 500 simulations. Mean (10th percentile, 90th percentile).
| Vaccination Rate | None | qRT-PCR | Rapid |
|---|---|---|---|
| 37.5% | 39% (34%, 44%) | 80% (74%, 86%) | 86% (82%, 89%) |
| 80% | 16% (13%, 20%) | 20% (14%, 27%) | 29% (24%, 34%) |
| 98% | 6% (4%, 8%) | 4% (1%, 7%) | 7% (4%, 11%) |
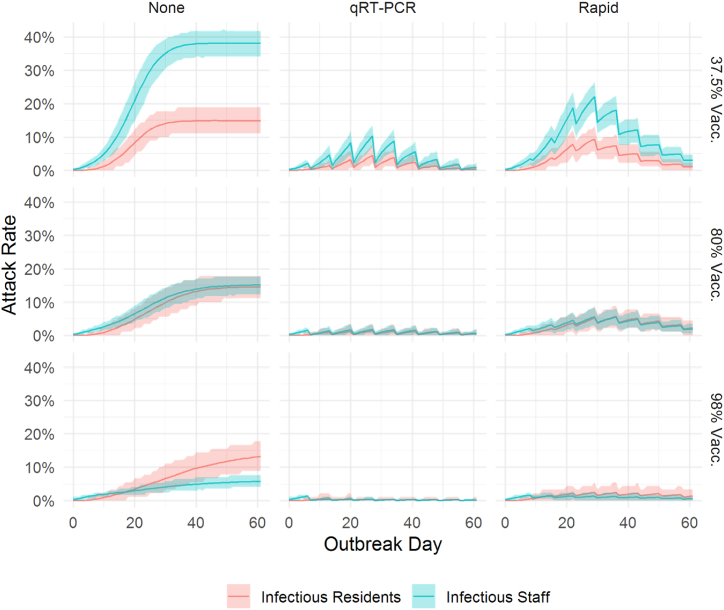
Fig. A.1.
Number of infectious residents (red) and staff (blue) at the facility by day over 60 days. The line represents the mean daily value and the shaded area represents the tenth (lower) and ninetieth (upper) percentile of the distribution based on 500 model simulations.
References
- Bi Q., Wu Y., Mei S., Ye C., Zou X., Zhang Z., et al. Epidemiology and Transmission of COVID-19 in Shenzhen China: Analysis of 391 cases and 1,286 of their close contacts. medRxiv. 2020 doi: 10.1101/2020.03.03.20028423. 2020, 03.03.20028423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonabeau E. Agent-based modeling: Methods and techniques for simulating human systems. Proceedings of the National Academy of Sciences. 2002;99:7280–7287. doi: 10.1073/pnas.082080899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubar K.M., et al. Model-informed COVID-19 vaccine prioritization strategies by age and serostatus. Science. 2021;371:916–921. doi: 10.1126/science.abe6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwald A.G., Bayham J., Adams J., Bortz D., Colborn K., Zarella O., et al. Estimating the impact of statewide policies to reduce spread of severe acute respiratory syndrome coronavirus 2 in real time, Colorado, USA. Emerging Infectious Diseases. 2021;27(9):2312–2322. doi: 10.3201/eid2709.204167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh A.M. COVID-19 outbreak associated with a SARS-CoV-2 R.1 lineage variant in a skilled nursing facility after vaccination Program — Kentucky, March 2021. MMWR Morb Mortal Wkly Rep. 2021;70 doi: 10.15585/mmwr.mm7017e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.K., Chevalier J.A., Long E.F. Nursing home staff networks and COVID-19. Proceedings of the National Academy of Sciences. 2021;118 doi: 10.1073/pnas.2015455118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas E. An agent-based model to evaluate the COVID-19 transmission risks in facilities. Computers in Biology and Medicine. 2020;121 doi: 10.1016/j.compbiomed.2020.103827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiskis A., Kelly C., Kissane E., Oehler K. 2021. Analysis & updates | what we know—and what we don't know—about the impact of the pandemic on our most vulnerable community. The COVID tracking project.https://covidtracking.com/analysis-updates/what-we-know-about-the-impact-of-the-pandemic-on-our-most-vulnerable-community [Google Scholar]
- Dawoud I. Modeling Palestinian COVID-19 cumulative confirmed cases: A comparative study. Infectious Disease Modelling. 2020;5:748–754. doi: 10.1016/j.idm.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekmann O., Heesterbeek J.A.P., Metz J.A. On the definition and the computation of the basic reproduction ratio R 0 in models for infectious diseases in heterogeneous populations. Journal of Mathematical Biology. 1990;28(4):365–382. doi: 10.1007/BF00178324. [DOI] [PubMed] [Google Scholar]
- Dilliott J. First Release. (Zenodo; 2021. jakedilliott/ltcf_covid_dashboard. [DOI] [Google Scholar]
- Gallichotte E.N., et al. Longitudinal surveillance for SARS-CoV-2 among staff in six Colorado long-term care facilities: Epidemiologic, virologic and sequence analysis. medRxiv. 2020;2020 doi: 10.1101/2020.06.08.20125989. 06.08.20125989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharpure R., et al. Early COVID-19 first-dose vaccination coverage among residents and staff members of skilled nursing facilities participating in the pharmacy partnership for long-term care Program — United States, December 2020–January 2021. MMWR Morb Mortal Wkly Rep. 2021;70:178–182. doi: 10.15585/mmwr.mm7005e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorges R.J., Konetzka R.T. Staffing levels and COVID-19 cases and outbreaks in U.S. Nursing homes. Journal of the American Geriatrics Society. 2020;68:2462–2466. doi: 10.1111/jgs.16787. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson K.E., Altayar O., Caliendo A.M., Arias C.A., Englund J.A., Hayden M.K., et al. Infectious Diseases Society of America, Version 1.0.0; 2021. Infectious diseases society of America guidelines on the diagnosis of COVID-19: Antigen testing.https://www.idsociety.org/practice-guideline/covid-19-guideline-antigen testing/ Available from: (See Figure s2b) [DOI] [PubMed] [Google Scholar]
- Hanson K.E., Caliendo A.M., Arias C.A., Hayden M.K., Englund J.A., Lee M.J., et al. Infectious Diseases Society of America, Version 2.0.0; 2020. Infectious diseases society of America guidelines on the diagnosis of COVID-19: Molecular diagnostic testing.https://www.idsociety.org/practice-guideline/covid-19-guideline-diagnostics/(SeeFiguress9aands9b) Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoertel N., Blachier M., Blanco C., et al. A stochastic agent-based model of the SARS-CoV-2 epidemic in France. Nature Medicine. 2020;26:1417–1421. doi: 10.1038/s41591-020-1001-6. [DOI] [PubMed] [Google Scholar]
- Holmdahl I., Kahn R., Hay J.A., Buckee C.O., Mina M.J. Estimation of transmission of COVID-19 in simulated nursing homes with frequent testing and immunity-based staffing. JAMA Network Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.J., Coffey K.C., Morgan D.J., Roghmann M.-C. Lessons learned - outbreaks of COVID-19 in nursing homes. American Journal of Infection Control. 2020;48:1279–1280. doi: 10.1016/j.ajic.2020.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J., Groves T., Pilkerton C.S., Ashcraft A.M., Shrader C.D. Geriatric antibody response to COVID-19. Journal of the American Geriatrics Societyn/a. 2021 doi: 10.1111/jgs.17210. 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naimark D., et al. Simulation-based estimation of SARS-CoV-2 infections associated with school closures and community-based nonpharmaceutical interventions in Ontario, Canada. JAMA Network Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulin E. Nursing homes are requiring staff COVID-19 vaccinations. 2021. https://www.aarp.org/caregiving/health/info-2021/nursing-homes-covid-vaccine-mandate.html AARP.
- Prince-Guerra J.L., et al. Evaluation of abbott BinaxNOW rapid antigen test for SARS-CoV-2 infection at two community-based testing sites - Pima county, Arizona, November 3-17, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:100–105. doi: 10.15585/mmwr.mm7003e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn C.C., Adams A.S., Magaziner J.S., Gurwitz J.H. Coronavirus disease 2019 and clinical research in U.S. nursing homes. Journal of the American Geriatrics Societyn/a. 2021 doi: 10.1111/jgs.17191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Brufau S., et al. Public health impact of delaying second dose of BNT162b2 or mRNA-1273 covid-19 vaccine: Simulation agent based modeling study. BMJ. 2021;373:n1087. doi: 10.1136/bmj.n1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar P.D., Link N., Lamarca K., Santillana M. High coverage COVID-19 mRNA vaccination rapidly controls SARS-CoV-2 transmission in Long-Term Care Facilities. Res Sq rs. 2021;3 doi: 10.21203/rs.3.rs-355257/v1. rs-355257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanche S., Lin Y.T., Xu C., Romero-Severson E., Hengartner N., Ke R. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2—volume 26, number 7—July 2020—emerging infectious diseases journal—CDC. Emerging Infectious Diseases. 2020;26:7. doi: 10.3201/eid2607.200282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Musa S.S., Zhao S., Mei S., He D. Using proper mean generation intervals in modeling of COVID-19. Frontiers in Public Health. 2021;9 doi: 10.3389/fpubh.2021.691262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teran R.A. Postvaccination SARS-CoV-2 infections among skilled nursing facility residents and staff members — chicago, Illinois, December 2020–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70 doi: 10.15585/mmwr.mm7017e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truszkowska A., et al. High-resolution agent-based modeling of COVID-19 spreading in a small town. Advanced Theory and Simulations. 2021;4 doi: 10.1002/adts.202000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E.M., et al. Incident SARS-CoV-2 infection among mRNA-vaccinated and unvaccinated nursing home residents. New England Journal of Medicine. 2021 doi: 10.1056/NEJMc2104849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingerter M. 2021. CDC investigating delta variant in Mesa County as it gains growing foothold in Colorado – the Denver Post. Denver Post. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dashboard is available at https://ltcf-covid.shinyapps.io/ltcf_covid_dashboard/. The code for the simulations and model underlying the dashboard are available at https://www.doi.org/10.5281/zenodo.4984845.