Abstract
Background
Cognitive assessment in acute stroke is relevant for identifying patients at risk of persistent post-stroke cognitive impairment (PSCI). Despite preliminary evidence on MoCA accuracy, there is no consensus on its optimal score in the acute stroke setting to predict PSCI.
Aims
(1) To explore whether the application of different normative datasets to MoCA scores obtained in the acute stroke setting results in variable frequency of patients defined as cognitively impaired; (2) to assess whether the normality cut-offs provided by three normative datasets predict PSCI at 6–9 months; (3) to calculate alternative MoCA cut-offs able to predict PSCI.
Methods
Consecutive stroke patients were reassessed at 6–9 months with extensive neuropsychological and functional batteries for PSCI determination.
Results
Out of 207 enrolled patients, 118 (57%) were followed-up (mean 7.4 ± 1.7 months), and 77 of them (65%) received a PSCI diagnosis. The application of the normality thresholds provided by the 3 normative datasets yielded to variable (from 28.5% to 41%) rates of patients having an impaired MoCA performance, and to an inadequate accuracy in predicting PSCI, maximizing specificity instead of sensitivity. In ROC analyses, a MoCA score of 22.82, adjusted according to the most recent normative dataset, achieved a good diagnostic accuracy in predicting PSCI.
Conclusions
The classification of acute stroke patients as normal/impaired based on MoCA thresholds proposed by general population normative datasets underestimated patients at risk of persistent PSCI. We calculated a new adjusted MoCA score predictive of PSCI in acute stroke patients to be further tested in larger studies.
Keywords: Acute stroke, Neuropsychology, Montreal Cognitive Assessment, Post-stroke cognitive impairment, Normality cut-off
Introduction
Post-stroke cognitive impairment (PSCI) encompasses all forms and degrees of cognitive disorders whose onset is temporally related with a stroke [1, 2]. The cognitive profile of PSCI is heterogeneous and may include deficits in cortical functions (e.g., aphasia, neglect, apraxia, agnosia) as well as a dysexecutive syndrome caused by the dysfunction of integrated brain networks [3–6]. In the acute phase, approximately 75% of stroke patients experience cognitive deficits [7–9]. In the chronic phase, cognitive impairment persists in approximately 50% of patients and is associated with poor functional and survival outcomes [2, 9, 10].
The identification of patients at risk of persistent PSCI in the acute phase might help clinicians to early plan treatment options as well as serial cognitive assessments. Because cognitive assessment in acute stroke must fulfill a feasibility criterion imposed by the setting and patients’ conditions, a multidomain, quick and easy to use, screening tool represents the gold standard. Diagnostic accuracy of such a tool would have to reach a sensitivity ≥ 80% and a specificity ≥ 60% and should be evaluated with respect to a long-term PSCI diagnosis based on a comprehensive neuropsychological test battery [8, 11].
During the last decade, several studies have tested Montreal Cognitive Assessment (MoCA) in acute stroke patients, and some encouraging evidence on its accuracy and predictive validity have made it one of the best candidates in this setting [12–15]. However, one unsolved issue in the use of MoCA in acute stroke patients concerns the definition of cut-offs able to predict patients at risk of persistent PSCI. In this peculiar setting, MoCA scores predictive of PSCI were variable across studies evaluating acute stroke patients, ranging from 19 to 22, and were based on raw scores [15, 16]. The latter represent a relevant issue because MoCA normative studies have clearly showed the wide impact of demographic and cultural factors on its performance [17].
At present, three general population normative datasets are available for the Italian version of the MoCA. Normative values were firstly published by Conti and colleagues in 2004 (225 subjects, age range 60–80), then by Santangelo and colleagues in 2005 (415 subjects, age range 21–95), and recently by Aiello and colleagues in 2021 (579 subjects, age range 21–96) [18–20]. The use of demographically and culturally appropriate correction norms for the evaluation of MoCA performances in acute stroke might represent an added value on the way of defining predictive scores in this setting.
The aims of this study were: (1) to explore whether the application of different normative datasets to the MoCA scores obtained in the acute stroke setting results in variable frequency of patients defined as cognitively impaired; (2) to assess whether the normality cut-offs provided by each of the three normative datasets predict PSCI at 6–9 months; (3) to calculate alternative cut-offs of MoCA performances obtained in the acute phase able to predict PSCI.
Methods
The present study is based on data collected in two previous studies carried out at the stroke units of the Luigi Sacco University Hospital (Milan) and of the Careggi University Hospital (Florence), Italy [21, 22].
Inclusion criteria were diagnosis of stroke (ischemic or hemorrhagic) or transient ischemic attack and age > 18 years. No exclusion criterion was applied. Informed consent was obtained by patients or caregivers.
During stroke unit hospitalization, patients were evaluated at bedside by means of the MoCA, and the National Institute of Health Stroke Scale (NIHSS) was used to estimate index stroke severity [23]. MoCA performance was evaluated using the corrections norms reported in the three normative datasets [18–20]. Demographically adjusted scores have been calculated based on the following regression equations extracted by normative studies:
All normative studies applied an equivalent score (ES) methodology that is a non-parametric (percentiles based) norming method that allows to convert age and education adjusted scores into an ordinal 5-point scale [24]. Definitions of ES classification and cut-offs of the normative studies were as follows: ES = 0, impaired performance (a demographically adjusted score below the outer confidence limit for the 5th centile of the normal population), corresponded to a cut-off of 18.58 in Aiello, 17.36 in Conti, and 15.50 in Santangelo normative datasets; ES = 1, borderline performance; ES = 2, 3 and 4, normal performance [18–20].
Between 6 and 9 months after the acute event, each patient was contacted to undergo an extensive neuropsychological and functional evaluation. Neuropsychological tests used at the follow-up examination in the two centers are shown in Table 1. Functional status was measured by means of the modified Rankin Scale (mRS) [35], ADL and IADL scales [36, 37].
Table 1.
Neuropsychological tests used at the follow-up examination
| Cognitive domain | Luigi Sacco University Hospital | Careggi University Hospital |
|---|---|---|
| Memory | Rey Auditory-Verbal Learning Test [25] | |
| Rey figure test—delayed recall [26] | ||
| Attention and executive functions | Visual search test [27] | |
| Symbol digit modalities test [28] | ||
| Trail making test (TMT, part A and B) [29] | ||
| Color word Stroop test [30] | ||
| Language | Phonemic and semantic verbal fluency tasks [31, 32] | |
| Phrase construction test [25] | ||
| Neuropsychological examination for aphasia [33] | ||
| Visuospatial functions | Rey figure test—copy [26] | |
| Apple test [34] | ||
The study outcome was PSCI diagnosis, including both Mild Cognitive Impairment (MCI) and dementia. Cognitive impairment was diagnosed based on the presence of an impaired performance in at least one test (a demographically adjusted score below the 5th centile of the normal population). The differential diagnosis between MCI and dementia was based on the presence of a functional dependence in ADL or IADL scales. Specifically, dementia was diagnosed only if the functional impairment was not a motor/sensory sequelae of the cerebrovascular event, nor a consequence of other diseases or physical limitations.
Statistical analysis
Descriptive statistics were used to describe the study cohort in terms of baseline demographic and clinical characteristics. To verify for a selection bias, bivariate statistical analyses (independent samples t-tests, Chi-square tests) were used to compare baseline characteristics between patients with or without follow-up.
For the statistical analyses purposes, MoCA performance was analyzed either as demographically adjusted scores (continuous), ES distribution (categorical), or impaired (ES = 0) vs. normal (ES = 1–4) (dichotomic).
Bivariate statistical analyses (independent samples t-tests, ANOVAs) were used to compare MoCA demographically adjusted scores between patients with or without PSCI at follow-up, as well as across cognitively normal, MCI and demented patients. Logistic regression models were further applied to evaluate if baseline MoCA adjusted scores were associated with the risk of PSCI at follow-up. Chi-square tests and accuracy indexes (sensitivity, specificity, positive and negative predictive values) were calculated for the normality thresholds of each normative study.
Receiver operator characteristic (ROC) analysis was used to examine the ability of the demographically adjusted MoCA scores to distinguish between patients with or without PSCI at follow-up. For each normative study, area under the curve, sensitivity, specificity, positive and negative predictive values were calculated to identify an optimal predictive score.
All data analyses were performed using SPSS 27 and a significance threshold set at p < 0.05.
Results
Two hundred and seven patients (mean age 76 ± 9.6 years, mean education 9.1 ± 4.5 years, females 34%) were recruited during their hospitalization in stroke unit (n = 82 Careggi University Hospital, n = 125 Luigi Sacco University Hospital).
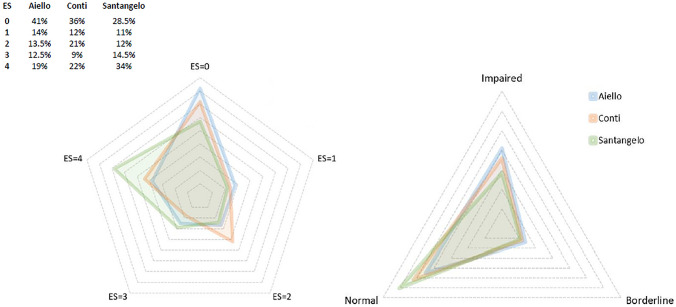
Applying the different normality thresholds of each of the 3 normative datasets, rates of patients having an impaired baseline MoCA performance ranged from 28.5% using Santangelo’s, to 36% using Conti’s, and 41% using Aiello’s normative datasets (Fig. 1).
Fig. 1.
Radar charts showing baseline Montreal Cognitive Assessment (MoCA) performances evaluated by means of Conti, Santangelo, and Aiello normative datasets. Left panel: equivalent scores (ES) distributions. Right panel: MoCA performances categorized as impaired (ES = 0), borderline (ES = 1), or normal (ES ≥ 2)
Out of the 207 enrolled patients, 118 (57%) were evaluated at follow-up (mean follow-up time 7.4 ± 1.7 months). As reported in Table 2, compared to patients with a follow-up evaluation, drop-outs were significantly older and had a worse MoCA performance at baseline.
Table 2.
Demographic and clinical characteristics of the baseline cohort, and comparisons between patients who completed the study and drop-outs
| Baseline cohort n = 207 |
Follow-up cohort n = 118 |
Drop-outs n = 89 |
p | |
|---|---|---|---|---|
| Age (years) | 76 ± 9.6 | 74.7 ± 8.9 | 77.7 ± 10.3 | 0.031 |
| Sex (female) | 70 (34%) | 38 (32%) | 32 (36%) | 0.572 |
| Education (years) | 9.1 ± 4.5 | 9.1 ± 4.6 | 9.1 ± 4.4 | 0.966 |
| Type of cerebrovascular event | ||||
| TIA | 13 (6%) | 7 (6%) | 6 (7%) | |
| Ischemic stroke | 174 (84%) | 99 (84%) | 75 (84%) | 0.938 |
| Hemorrhagic stroke | 20 (10%) | 12 (10%) | 8 (9%) | |
| Stroke severity (NIHSS score) | 2.1 ± 3.1 | 1.9 ± 2.9 | 2.4 ± 3.3 | 0.345 |
| MoCA (raw total score) | 17.1 ± 6.9 | 18.1 ± 6.8 | 15.8 ± 7.1 | 0.021 |
| MoCA impaired performance | ||||
| Aiello | 85 (41%) | 38 (32%) | 47 (53%) | 0.002 |
| Conti | 75 (36%) | 33 (28%) | 42 (47%) | 0.004 |
| Santagelo | 59 (28.5) | 28 (24%) | 31 (35%) | 0.080 |
Bold indicates statistically significant differences between the groups
MoCA Montreal Cognitive Assessment; NIHSS National Institute of Health Stroke Scale; TIA transient ischemic attack
Out of the 118 followed-up patients, 77 (65%) received a PSCI diagnosis (55 MCI and 22 dementia). No statistically significant differences were found in rates of patients diagnosed as PSCI between Careggi and Luigi Sacco University Hospitals (69% vs. 61%, respectively, p = 0.348).
Applying all the three normative studies, baseline MoCA adjusted scores were significantly different between patients with or without PSCI, and across the three categories of cognitively normal, MCI and demented patients (Table 3). For all the three normative datasets, the logistic regression models showed that the loss of one point on baseline MoCA increased the risk of PSCI of approximately 30%, specifically: Conti OR = 1.29 (95%CI 1.15–1.45), Santangelo OR = 1.37 (95%CI 1.19–1.58), Aiello OR = 1.39 (95%CI 1.21–1.59).
Table 3.
Baseline Montreal Cognitive Assessment (MoCA) adjusted scores (mean ± SD): comparison across patients with or without post-stroke cognitive impairment (PSCI) at follow-up
| No PSCI n = 41 |
PSCI n = 77 |
p* | MCI n = 55 |
Dementia n = 22 |
p# | |
|---|---|---|---|---|---|---|
| Aiello | 23.9 ± 3.2 | 17.9 ± 5.5 | 0.001 | 19.2 ± 5 | 14.8 ± 5.3 | 0.001 |
| Conti | 22.9 ± 3.3 | 17.5 ± 5.7 | 0.001 | 18.5 ± 5.1 | 14.9 ± 6.4 | 0.001 |
| Santangelo | 23.9 ± 3.2 | 17.9 ± 5.6 | 0.001 | 19.3 ± 5.1 | 14.6 ± 5.4 | 0.001 |
MCI mild cognitive impairment
*Comparisons between patients with or without PSCI: independent samples t tests
#Comparisons across no PSCI, MCI and demented patients: ANOVAs
However, rates of patients that had a normal MoCA performance in the acute phase according to normative datasets but received at follow-up a PSCI diagnosis ranged from 51% using Aiello’s, to 54% using Conti’s and 55% using Santangelo’s dataset. Accuracy indexes (Table 4) showed that the application of the normality thresholds maximized specificity (ranging from 95% to 98%) in respect of sensitivity (ranging from 35% to 47%); none of the normative studies achieved an adequate level of diagnostic accuracy (i.e., sensitivity ≥ 80%, specificity ≥ 60%).
Table 4.
Accuracy of the baseline Montreal Cognitive Assessment (MoCA) performance evaluated according to normality thresholds proposed by normative datasets on the post-stroke cognitive impairment (PSCI) diagnosis at follow-up
| Baseline MoCA performance | No PSCI n = 41 |
PSCI n = 77 |
p | SE | SP | PPV | NPV | |
|---|---|---|---|---|---|---|---|---|
| Aiello | Impaired | 2 (5%) | 36 (47%) | 0.001 | 47% | 95% | 95% | 49% |
| Normal | 39 (95%) | 41 (53%) | ||||||
| Conti | Impaired | 2 (5%) | 31 (40%) | 0.001 | 40% | 95% | 94% | 46% |
| Normal | 39 (95%) | 46 (60%) | ||||||
| Santangelo | Impaired | 1 (2%) | 27 (35%) | 0.001 | 35% | 98% | 96% | 44% |
| Normal | 40 (98%) | 50 (65%) |
Impaired baseline MoCA performance corresponded to an equivalent score = 0
Normal baseline MoCA performance corresponded to an equivalent score ≥ 1
SE sensitivity; SP specificity; PPV positive predictive value; NPV negative predictive value
To identify a demographically adjusted MoCA score able to detect patients at risk of PSCI, ROC analyses were conducted separately for the 3 normative studies. All areas under the curves (Conti = 0.800, Santangelo = 0.828, Aiello = 0.833) were statistically significant at p < 0.001 (Fig. 2). As shown in Table 5, an Aiello-adjusted MoCA score of 22.82 achieved a good diagnostic accuracy, i.e., sensitivity 81%, specificity 71%, positive predictive value 84%, negative predictive value 66%. The only other potential predictive score that achieved an adequate diagnostic accuracy (sensitivity 82%, specificity 61%, positive predictive value 80%, negative predictive value 64%) was a Santangelo-adjusted MoCA score of 23.45.
Fig. 2.
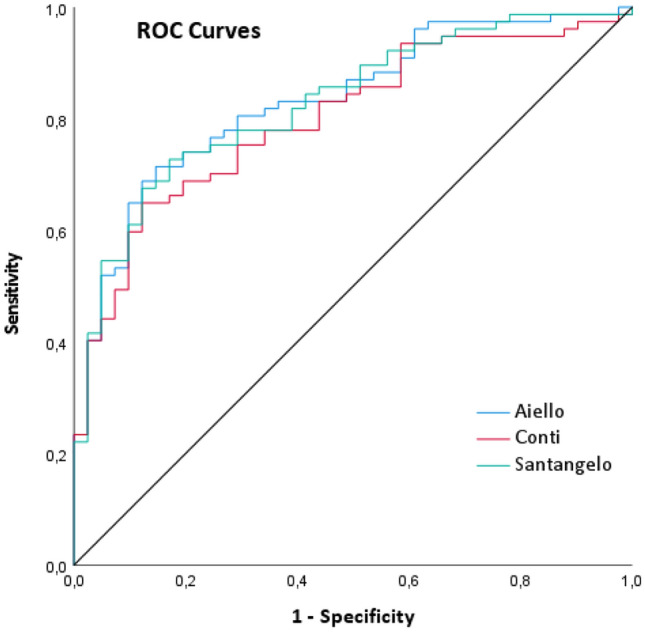
ROC curves showing sensibility and specificity of Montreal Cognitive Assessment (MoCA) scores demographically adjusted according to Conti, Santangelo and Aiello normative datasets (independent variables) for the identification of post stroke cognitive impairment at follow-up (dependent variable)
Table 5.
Sensitivity and specificity of Montreal Cognitive Assessment (MoCA) scores demographically adjusted according to normative datasets for the identification of Post Stroke Cognitive Impairment (PSCI) at follow-up
| Aiello | Conti | Santangelo | ||||||
|---|---|---|---|---|---|---|---|---|
| Cut-off | Sensitivity | Specificity | Cut-off | Sensitivity | Specificity | Cut-off | Sensitivity | Specificity |
| 21.16 | 70 | 85 | 20.73 | 70 | 76 | 21.41 | 70 | 83 |
| 21.20 | 71 | 85 | 20.86 | 70 | 73 | 21.53 | 71 | 83 |
| 21.26 | 71 | 83 | 20.92 | 70 | 71 | 21.64 | 73 | 83 |
| 21.38 | 71 | 81 | 20.95 | 71 | 71 | 21.69 | 73 | 81 |
| 21.54 | 73 | 81 | 20.99 | 73 | 71 | 21.75 | 74 | 81 |
| 21.74 | 74 | 81 | 21.13 | 74 | 71 | 21.86 | 74 | 78 |
| 21.99 | 74 | 78 | 21.28 | 75 | 71 | 22.06 | 74 | 76 |
| 22.17 | 74 | 76 | 21.33 | 75 | 68 | 22.34 | 75 | 76 |
| 22.28 | 75 | 76 | 21.42 | 75 | 66 | 22.50 | 75 | 73 |
| 22.40 | 77 | 76 | 21.55 | 77 | 66 | 22.63 | 75 | 71 |
| 22.48 | 77 | 73 | 21.64 | 78 | 66 | 22.75 | 77 | 71 |
| 22.54 | 78 | 73 | 21.73 | 78 | 63 | 22.79 | 78 | 71 |
| 22.58 | 78 | 71 | 21.97 | 78 | 61 | 22.86 | 78 | 68 |
| 22.68 | 79 | 71 | 22.24 | 78 | 59 | 22.91 | 78 | 66 |
| 22.82 | 81 | 71 | 22.35 | 78 | 56 | 23.14 | 78 | 63 |
| 22.92 | 81 | 68 | 22.41 | 79 | 56 | 23.37 | 78 | 61 |
| 22.97 | 81 | 66 | 22.50 | 81 | 56 | 23.40 | 79 | 61 |
| 23.05 | 82 | 66 | 22.59 | 82 | 56 | 23.44 | 81 | 61 |
| 23.10 | 82 | 63 | 22.64 | 83 | 56 | 23.45 | 82 | 61 |
| 23.18 | 83 | 63 | 22.90 | 83 | 54 | 23.50 | 82 | 59 |
| 23.30 | 83 | 61 | 23.16 | 83 | 51 | 23.56 | 83 | 59 |
| 23.40 | 83 | 59 | 23.19 | 84 | 51 | 23.58 | 84 | 59 |
| 23.45 | 83 | 56 | 23.23 | 84 | 49 | 23.61 | 84 | 56 |
| 23.64 | 83 | 54 | 23.66 | 86 | 56 | |||
| 23.81 | 83 | 51 | 23.86 | 86 | 54 | |||
| 23.83 | 84 | 51 | 24.07 | 86 | 51 | |||
| 23.90 | 86 | 51 | 24.19 | 86 | 49 | |||
| 23.98 | 87 | 51 | ||||||
| 24.06 | 87 | 49 | ||||||
Bold indicates the optimal cut-offs
Discussion
The present study represents an effort to examine and compare the use of different normative datasets, and thus of different general population normality thresholds, in the evaluation of MoCA performances in acute stroke and to evaluate the accuracy of these thresholds in detecting patients at risk of mid-term PSCI. Furthermore, we identified an optimal demographically adjusted MoCA score predictive of mid-term PSCI to be used in the acute stroke setting.
Our results show that the categorization of acute stroke patients into normal/impaired based on thresholds proposed for MoCA by normative datasets is not recommended. The different thresholds produced a variability of approximately 13% in rates of patients classified as having cognitive deficits in the acute phase. Even more relevant, the general population normality thresholds underestimated patients at risk of a persistent cognitive decline, and about half of patients could miss the opportunity to benefit from cognitive rehabilitation or other therapeutic strategies. However, demographically adjusted MoCA scores were able to weigh the risk of PSCI, and this allowed us to identify a new predictive score. A good level of diagnostic accuracy was reached applying the correction norms by Aiello and colleagues, and a demographically adjusted MoCA predictive score of 22.8 points appears recommendable in the acute stroke setting. The higher accuracy of the Aiello’s dataset in predicting PSCI is likely due to methodological and cultural factors. From the methodological point of view, the Aiello’s dataset is the most recent and is based on the largest sample, and this could have probably increased its representativeness of the population. On the other side, the relevance of region-specific norms is an emerging issue in reliability of normative datasets, and the Aiello’s datasets, which was collected in Northern Italy, could be more consistent also from the cultural point of view with our sample from Central-Northern Italy.
Despite the availability of several studies that evaluated the use of MoCA in the acute phase of stroke [38–46], data on its predictivity on mid- and long-term PSCI diagnosis reached by means of a comprehensive neuropsychological battery are quite limited [22, 47, 48]. Furthermore, previous studies estimated MoCA predictive scores in acute stroke based on raw scores and did not calculate correction norms based on demographics. Since performance on neuropsychological tests is known to vary as a function of several demographic variables, and available normative datasets confirmed these effects also for MoCA, normality thresholds based on raw scores could be inaccurate [49]. Moreover, also the heterogeneity among MoCA normality cut-offs found in previous studies in acute stroke patients could be partly due to the neglected cultural and demographic differences among populations. In line with this, a recent study on optimal MoCA cut-off scores for people with probable Alzheimer’s disease confirmed the discrepancy in cut-off points existing between Italian and other international validation studies and debated the influence of specific population characteristics [50]. Finally, compared with previous evidence, we found a higher MoCA normality threshold, and this discrepancy is likely due to the fact that demographic corrections overall increased MoCA scores in an elderly cohort.
Our sample also included a small group of TIA patients. When the analyses were repeated excluding this subgroup of patients, the main results (i.e., variability in rates of patients having an impaired MoCA performance, inadequate accuracy of the normative thresholds, and superiority of the Aiello’s dataset in predicting PSCI) were confirmed (data nor shown). Considering that the distinction between TIA and stroke is often not easy because many TIA patients develop indeed acute brain lesions, and also because this distinction is somehow questionable, we decided to keep this small number of TIA patients in our sample [51].
Limitations of our study need to be highlighted. First, neuropsychological tests used at the follow-up evaluation in the two centers were not completely superimposable. Despite the two neuropsychological batteries assessed all core cognitive domains and were partially overlapping, the Luigi Sacco University Hospital protocol was more comprehensive and strengthened the evaluation of specific cortical deficits, such as aphasia and neglect. However, it should be noted that, despite these differences, the rate of PSCI was similar between the two centers. A second limitation is the high number of drop-outs that resulted in a limited sample size. From the statistical point of view, these limitations have reduced the statistical power, and our results need to be taken with some caution and further tested in larger studies. On the other side, this could also represent a selection bias that might have underestimated the PSCI rate considering that patients lost to follow-up presented worst baseline cognitive efficiency. From the clinical point of view, patients with severe cognitive impairment in the acute phase are, in most cases, already addressed to rehabilitation or long-term care pathways, while mild stroke patients are frequently discharged at home, thus increasing the risk that persistent cognitive deficits are underdiagnosed and, ultimately, undertreated. This is why tools able to screen for these patients in the acute phase may be useful.
Further research efforts are needed for the determination of the optimal MoCA predictive score in the acute setting. National longitudinal studies based on large samples of acute stroke patients should be conducted in order to provide corrections norms and predictive scores that could be appropriate from both cultural and clinical points of view.
Acknowledgements
We wish to thank the nonprofit organization Associazione per la Ricerca sulle Demenze (ARD) ONLUS, Department of Neurology, Luigi Sacco Hospital, Milan, Italy for supporting the Stroke and Dementia Lab of the Luigi Sacco Department of Biomedical and Clinical Sciences, University of Milan.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by ES and LP. The first draft of the manuscript was written by ES and LP, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Open access funding provided by Università degli Studi di Firenze within the CRUI-CARE Agreement. No funding was received for conducting this study.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Financial interests
The authors declare they have no financial interests.
Ethics approval
The study was approved by the local ethics committee of the Careggi University Hospital and was performed in accordance with the 1964 Declaration of Helsinki and its later amendments.
Statement of human and animal rights
The study procedures were in accordance with the ethical standards of the responsible committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000 and 2008.
Consent to participate
Informed consent was obtained by patients or caregivers.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/14/2022
Missing Open Access funding information has been added in the Funding Note.
References
- 1.O’Brien JT, Erkinjuntti T, Reisberg B, et al. Vascular cognitive impairment. Lancet Neurol. 2003;2:89–98. doi: 10.1016/s1474-4422(03)00305-3. [DOI] [PubMed] [Google Scholar]
- 2.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American heart association/American stroke association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferro JM. Hyperacute cognitive stroke syndromes. J Neurol. 2001;248:841–849. doi: 10.1007/s004150170067. [DOI] [PubMed] [Google Scholar]
- 4.Sachdev PS, Brodaty H, Valenzuela MJ, et al. The neuropsychological profile of vascular cognitive impairment in stroke and TIA patients. Neurology. 2004;62:912–919. doi: 10.1212/01.wnl.0000115108.65264.4b. [DOI] [PubMed] [Google Scholar]
- 5.Hurford R, Charidimou A, Fox Z, et al. Domain-specific trends in cognitive impairment after acute ischaemic stroke. J Neurol. 2013;260:237–241. doi: 10.1007/s00415-012-6625-0. [DOI] [PubMed] [Google Scholar]
- 6.Yatawara C, Ng KP, Chander R, et al. Associations between lesions and domain-specific cognitive decline in poststroke dementia. Neurology. 2018;91:e45–e54. doi: 10.1212/WNL.0000000000005734. [DOI] [PubMed] [Google Scholar]
- 7.Leśniak M, Bak T, Czepiel W, et al. Frequency and prognostic value of cognitive disorders in stroke patients. Dement Geriatr Cogn Disord. 2008;26:356–63. doi: 10.1159/000162262. [DOI] [PubMed] [Google Scholar]
- 8.Stolwyk RJ, O'Neill MH, McKay AJ, et al. Are cognitive screening tools sensitive and specific enough for use after stroke? A systematic literature review. Stroke. 2014;45:3129–3134. doi: 10.1161/STROKEAHA.114.004232. [DOI] [PubMed] [Google Scholar]
- 9.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 10.Barbay M, Diouf M, Roussel M, et al. Systematic review and meta-analysis of prevalence in post-stroke neurocognitive disorders in hospital-based studies. Dement Geriatr Cogn Disord. 2018;46:322–334. doi: 10.1159/000492920. [DOI] [PubMed] [Google Scholar]
- 11.Quinn TJ, Elliott E, Langhorne P. Cognitive and mood assessment tools for use in stroke. Stroke. 2018;49:483–490. doi: 10.1161/STROKEAHA.117.016994. [DOI] [PubMed] [Google Scholar]
- 12.Hachinski V, Iadecola C, Petersen R, et al. National institute of neurological disorders and stroke-Canadian stroke network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 13.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 14.Van Heugten CM, Walton L, Hentschel U. Can we forget the mini-mental state examination? A systematic review of the validity of cognitive screening instruments within one month after stroke. Clin Rehabil. 2015;29:694–704. doi: 10.1177/0269215514553012. [DOI] [PubMed] [Google Scholar]
- 15.Chiti G, Pantoni L. Use of Montreal Cognitive Assessment in patients with stroke. Stroke. 2014;45:3135–3140. doi: 10.1161/STROKEAHA.114.004590. [DOI] [PubMed] [Google Scholar]
- 16.Shi D, Chen X, Li Z. Diagnostic test accuracy of the Montreal Cognitive Assessment in the detection of post-stroke cognitive impairment under different stages and cutoffs: a systematic review and meta-analysis. Neurol Sci. 2018;39:705–716. doi: 10.1007/s10072-018-3254-0. [DOI] [PubMed] [Google Scholar]
- 17.O'Driscoll C, Shaikh M. Cross-cultural applicability of the Montreal Cognitive Assessment (MoCA): a systematic review. J Alzheimers Dis. 2017;58:789–801. doi: 10.3233/JAD-161042. [DOI] [PubMed] [Google Scholar]
- 18.Conti S, Bonazzi S, Laiacona M, et al. Montreal Cognitive Assessment (MoCA)-Italian version: regression based norms and equivalent scores. Neurol Sci. 2015;36:209–214. doi: 10.1007/s10072-014-1921-3. [DOI] [PubMed] [Google Scholar]
- 19.Santangelo G, Siciliano M, Pedone R, et al. Normative data for the Montreal Cognitive Assessment in an Italian population sample. Neurol Sci. 2015;36:585–591. doi: 10.1007/s10072-014-1995-y. [DOI] [PubMed] [Google Scholar]
- 20.Aiello EN, Gramegna C, Esposito A, et al. The Montreal Cognitive Assessment (MoCA): updated norms and psychometric insights into adaptive testing from healthy individuals in Northern Italy. Aging Clin Exp Res. 2021 doi: 10.1007/s40520-021-01943-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cova I, Mele F, Zerini F, et al. The Clock Drawing Test as a predictor of cognitive decline in non-demented stroke patients. J Neurol. 2021 doi: 10.1007/s00415-021-10637-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salvadori E, Pasi M, Poggesi A, et al. Predictive value of MoCA in the acute phase of stroke on the diagnosis of mid-term cognitive impairment. J Neurol. 2013;260:2220–2227. doi: 10.1007/s00415-013-6962-7. [DOI] [PubMed] [Google Scholar]
- 23.Brott T, Marler JR, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.STR.20.7.864. [DOI] [PubMed] [Google Scholar]
- 24.Capitani E, Laiacona M. Composite neuropsychological batteries and demographic correction: Standardization based on equivalent scores, with a review of published data. The Italian Group for the Neuropsychological Study of Ageing. J Clin Exp Neuropsychol. 1997;19:795–809. doi: 10.1080/01688639708403761. [DOI] [PubMed] [Google Scholar]
- 25.Carlesimo GA, Caltagirone C, Gainotti G. The Mental Deterioration Battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. The Group for the Standardization of the Mental Deterioration Battery. Eur Neurol. 1996;36:378–384. doi: 10.1159/000117297. [DOI] [PubMed] [Google Scholar]
- 26.Caffarra P, Vezzadini G, Dieci F, et al. Rey-Osterrieth complex figure: normative values in an Italian population sample. Neurol Sci. 2002;22:443–447. doi: 10.1007/s100720200003. [DOI] [PubMed] [Google Scholar]
- 27.Della Sala S, Laiacona M, Spinnler H, et al. A cancellation test: its reliability in assessing attentional deficits in Alzheimer's disease. Psychol Med. 1992;22:885–901. doi: 10.1017/S0033291700038460. [DOI] [PubMed] [Google Scholar]
- 28.Nocentini U, Giordano A, Di Vincenzo S, et al. The symbol digit modalities test-oral version: Italian normative data. Funct Neurol. 2006;21:93–96. [PubMed] [Google Scholar]
- 29.Giovagnoli AR, Del Pesce M, Mascheroni S, et al. Trail making test: normative values from 287 normal adult controls. Ital J Neurol Sci. 1996;17:305–309. doi: 10.1007/BF01997792. [DOI] [PubMed] [Google Scholar]
- 30.Caffarra P, Vezzadini G, Dieci F, et al. A short version of the Stroop test: normative data in an Italian population sample. Nuova Riv Neurol. 2002;12:111–115. [Google Scholar]
- 31.Novelli G, Papagno C, Capitani E. Tre test clinici di ricerca e produzione lessicale. Taratura su soggetti normali. Arch Psicol Neurol Psichiatr. 1986;47:477–506. [Google Scholar]
- 32.Costa A, Bagoj E, Monaco M, et al. Standardization and normative data obtained in the Italian population for a new verbal fluency instrument, the phonemic/semantic alternate fluency test. Neurol Sci. 2014;35:365–372. doi: 10.1007/s10072-013-1520-8. [DOI] [PubMed] [Google Scholar]
- 33.Capasso R, Miceli G. Esame neuropsicologico per l'Afasia. E.N.P.A: Springer-Verlag Mailand; 2001. [Google Scholar]
- 34.Mancuso M, Rosadoni S, Capitani D, et al. Italian standardization of the Apples Cancellation Test. Neurol Sci. 2015;36:1233–1240. doi: 10.1007/s10072-015-2088-2. [DOI] [PubMed] [Google Scholar]
- 35.van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.STR.19.5.604. [DOI] [PubMed] [Google Scholar]
- 36.Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 37.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. doi: 10.1093/geront/9.3_Part_1.179. [DOI] [PubMed] [Google Scholar]
- 38.Pasi M, Salvadori E, Poggesi A, et al. Factors predicting the Montreal cognitive assessment (MoCA) applicability and performances in a stroke unit. J Neurol. 2013;260:1518–1526. doi: 10.1007/s00415-012-6819-5. [DOI] [PubMed] [Google Scholar]
- 39.Popović IM, Serić V, Demarin V. Mild cognitive impairment in symptomatic and asymptomatic cerebrovascular disease. J Neurol Sci. 2007;257:185–193. doi: 10.1016/j.jns.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 40.Dong Y, Sharma VK, Chan BP, et al. The Montreal Cognitive Assessment (MoCA) is superior to the Mini-Mental State Examination (MMSE) for the detection of vascular cognitive impairment after acute stroke. J Neurol Sci. 2010;299:15–18. doi: 10.1016/j.jns.2010.08.051. [DOI] [PubMed] [Google Scholar]
- 41.Toglia J, Fitzgerald KA, O'Dell MW, Mastrogiovanni AR, Lin CD. The Mini-Mental State Examination and Montreal Cognitive Assessment in persons with mild subacute stroke: relationship to functional outcome. Arch Phys Med Rehabil. 2011;92:792–798. doi: 10.1016/j.apmr.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 42.Blackburn DJ, Bafadhel L, Randall M, et al. Cognitive screening in the acute stroke setting. Age Ageing. 2013;42:113–116. doi: 10.1093/ageing/afs116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan E, Khan S, Oliver R, et al. Underestimation of cognitive impairments by the Montreal Cognitive Assessment (MoCA) in an acute stroke unit population. J Neurol Sci. 2014;343:176–179. doi: 10.1016/j.jns.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Horstmann S, Rizos T, Rauch G, et al. Feasibility of the Montreal Cognitive Assessment in acute stroke patients. Eur J Neurol. 2014;21:1387–1393. doi: 10.1111/ene.12505. [DOI] [PubMed] [Google Scholar]
- 45.Godefroy O, Fickl A, Roussel M, et al. Is the Montreal Cognitive Assessment superior to the Mini-Mental State Examination to detect poststroke cognitive impairment? A study with neuropsychological evaluation. Stroke. 2011;42:1712–1716. doi: 10.1161/STROKEAHA.110.606277. [DOI] [PubMed] [Google Scholar]
- 46.Zuo L, Dong Y, Zhu R, et al. Screening for cognitive impairment with the Montreal Cognitive Assessment in Chinese patients with acute mild stroke and transient ischaemic attack: a validation study. BMJ Open. 2016;6:e011310. doi: 10.1136/bmjopen-2016-011310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dong Y, Venketasubramanian N, Chan BP, et al. Brief screening tests during acute admission in patients with mild stroke are predictive of vascular cognitive impairment 3–6 months after stroke. J Neurol Neurosurg Psychiatry. 2012;83:580–585. doi: 10.1136/jnnp-2011-302070. [DOI] [PubMed] [Google Scholar]
- 48.Dong Y, Xu J, Chan BP, et al. The Montreal Cognitive Assessment is superior to National Institute of Neurological Disease and Stroke-Canadian Stroke Network 5-minute protocol in predicting vascular cognitive impairment at 1 year. BMC Neurol. 2016;16:46. doi: 10.1186/s12883-016-0570-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zimmerman ME. Normative data. In: Kreutzer JS, DeLuca J, Caplan B, editors. Encyclopedia of clinical neuropsychology. New York, NY: Springer; 2011. [Google Scholar]
- 50.Bosco A, Spano G, Caffò AO, et al. Italians do it worse. Montreal Cognitive Assessment (MoCA) optimal cut-off scores for people with probable Alzheimer's disease and with probable cognitive impairment. Aging Clin Exp Res. 2017;29:1113–1120. doi: 10.1007/s40520-017-0727-6. [DOI] [PubMed] [Google Scholar]
- 51.Easton JD, Johnston SC. Time to retire the concept of transient ischemic attack. JAMA. 2022;327:813–814. doi: 10.1001/jama.2022.0300. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.