Abstract
Background:
The relation between a novel measure of total skeletal muscle mass (assessed by D3-creatine dilution, D3Cr) and incident fracture is unknown.
Methods:
In 1,363 men (mean age, 84.2 yrs), we determined D3Cr muscle mass; FRAX 10-year probability of hip and major osteoporotic (hip, humerus, vertebral, forearm) fracture; and femoral neck BMD (by DXA). Incident fractures were centrally adjudicated by review of radiology reports over 4.6 years. Correlations adjusted for weight and height were calculated between femoral neck BMD and D3Cr muscle mass. Across quartiles of D3Cr muscle mass/weight, proportional hazards models calculated hazard ratios for any (N=180); non-spine (N=153); major osteoporotic fracture (N=85); and hip fracture (N=40) after adjustment for age, femoral neck BMD, recurrent fall history, and FRAX probability. Models were then adjusted to evaluate the mediating influence of physical performance (walking speed, chair stands, and grip strength).
Results:
D3Cr muscle mass was weakly correlated with femoral BMD (r=0.10, p<.001). Compared to men in the highest quartile, those in the lowest quartile of D3Cr muscle mass/weight had an increased risk of any clinical fracture (HR: 1.8, 95%CI: 1.1 – 2.8); non-spine fracture (HR: 1.8, 95%CI: 1.1 – 3.0), major osteoporotic fracture (HR: 2.3, 95%CI: 1.2 – 4.6) and hip fracture (HR: 5.9, 95% CI: 1.6– 21.1). Results were attentuated after adjustment for physical performance, but associations remained borderline signficant for hip and major osteoporotic fractures (p≥0.05–0.10).
Conclusions:
Low D3Cr muscle mass/weight is associated with a markedly high risk of hip and potentially other fractures in older men; this association is partially mediated by physical performance.
Introduction
Low muscle mass likely predisposes individuals to fracture for several reasons. First, bone remodels in response to muscle force. Thus, lower muscle strength (presumably due to lower muscle mass) should result in weaker bones and therefore more fractures.1 Second, lower muscle mass also increases fall risk, whereby smaller, weaker muscles are associated with worse muscle function and physical performance, which should in turn result in more falls and fractures. There is compelling evidence that poor muscle function (quantified as strength and power) and impaired physical performance (gait speed, chair stands) are strongly related to risk of hip, vertebral and other fractures.2–6 Third, through bone-muscle crosstalk, ‘myokines’ may also influence bone directly.7,8
The evidence for the direct association between muscle mass and fracture are less clear. A commonly used approximation of muscle mass is appendicular lean body mass (ALM, kg) derived from dual x-ray absorptiometry (DXA). While DXA is widely used to assess bone mass and density for the diagnosis of osteoporosis,9 DXA also measures soft tissue lean mass. Lean mass from DXA is not a direct measure of muscle mass. Despite this, DXA lean mass has been considered a “reference standard” for muscle mass by some,10 a designation that is not without controversy.11,12 DXA total body lean mass includes soft tissue from organs like kidney and liver; fibrotic and other lean tissue; and water. Operationally, lean mass from DXA is usually analyzed as lean mass in the arms and legs, standardized to body height (m2), ALM/ht2.13,14 While ALM/ht2 is a commonly used approximation of muscle, it is not consistently or independently related to fractures, particularly after consideration of bone mineral density.5,15,16 In some cases, after accounting for BMD, those with low ALM have a decreased risk of fracture.17,18 We posit that the lack of an independent association between DXA low lean mass and fracture risk is due to limitations in the use of DXA as a surrogate measure of muscle mass. Therefore, it is possible that a more direct measure of muscle mass may be related to fracture risk even when DXA lean mass is not. D3Cr dilution assessment of muscle mass (D3Cr muscle mass) relies on several aspects of creatine biology to estimate muscle mass19 that does not rely on the same assumptions of compartment models (such as DXA), and may therefore represent a more accurate assessment of muscle mass.
We recently demonstrated that low muscle mass by D3Cr dilution is strongly related to weakness, poor physical performance, increased likelihood of prevalent disability and incident mobility limitations, incident disability, risk of serious injurious falls and mortality in older men, while DXA lean mass is not consistently associated with these outcomes.19,20 We have also demonstrated that D3Cr muscle mass (not adjusted to weight) is only moderately correlated with DXA ALM (r=0.68, p<.001) and DXA ALM/ht2 (r=0.55, p<.001).19 We hypothesize that the accurate assessment of muscle mass provided by the D3Cr dilution method will reveal a strong association of muscle mass with fracture risk. We tested this hypothesis in the prospective Osteoporotic Fractures in Men (MrOS) study, a cohort of community-dwelling older men. Since much of the association of muscle mass may act through strength and performance, we also aimed to test whether any association observed between D3Cr muscle mass and fracture was mediated by these factors.
Methods
MrOS Cohort
In 2000–2002 the MrOS multi-center cohort study of aging and osteoporosis recruited 5,994 ambulatory community-dwelling men aged ≥65 years without bilateral hip replacements.21,22 All men provided written informed consent, and the study was approved by the Institutional Review Board at each center. In 2014–2016, 2,786 survivors were contacted to participate in “Visit 4” (Year 14) clinic visit. Of these, 362 refused participation, 583 completed questionnaires only, and 1,841 completed questionnaires and at least part of the clinic visit (Figure 1). MrOS data is available here: https://mrosonline.ucsf.edu/
Figure 1.
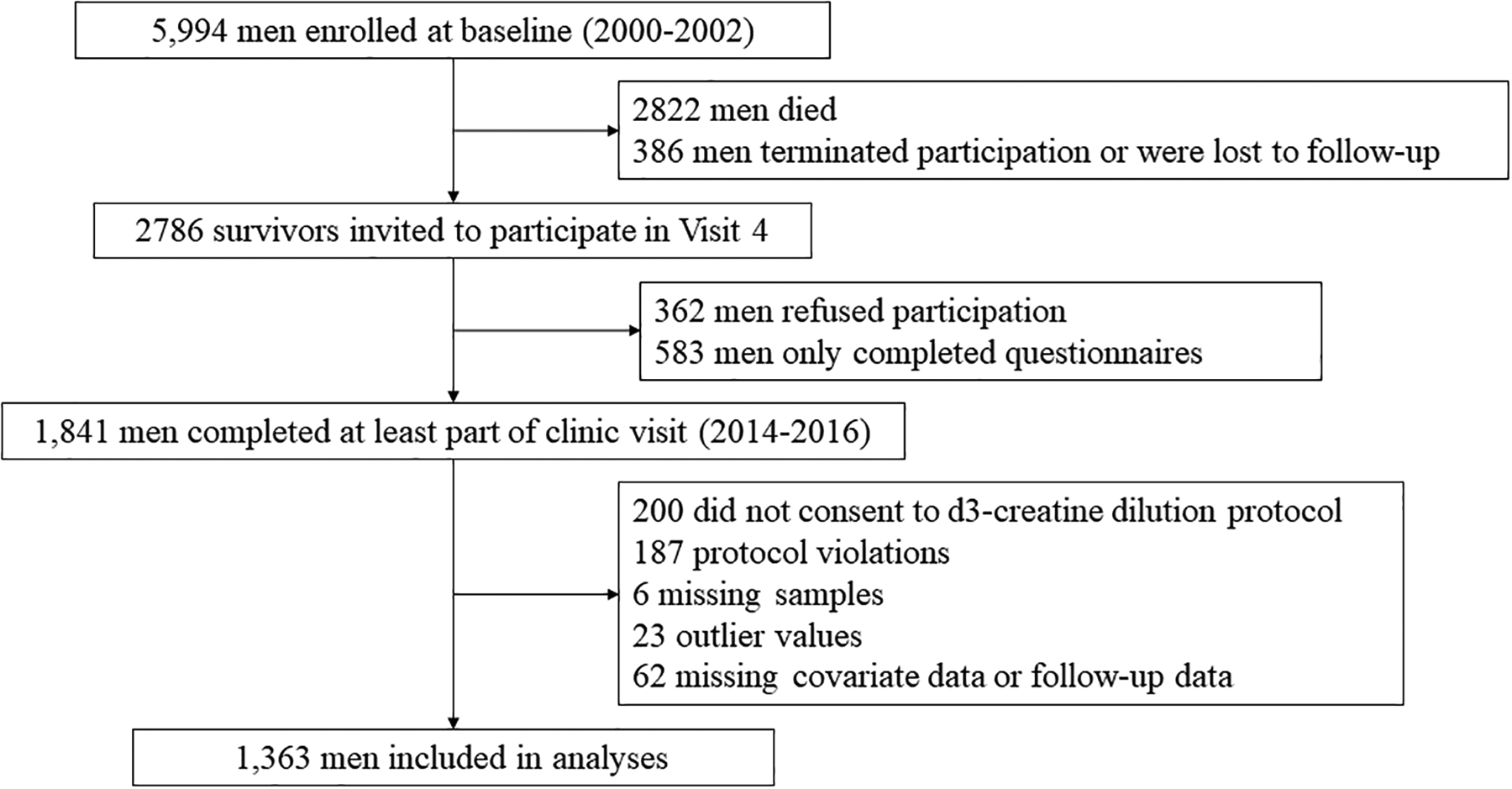
Analysis flow chart
D3Cr dilution method to estimate muscle mass
To complete the D3Cr dilution method, a participant takes a “dose” (30-mg) labeled creatine (D3-creatine), and the provides a fasting, morning urine sample 72–144 hours (3–6 days) later as previously described.19 In the urine, high performance liquid chromatography (HPLC) and tandem mass spectrometry (MS/MS) are then used to measure d3-creatinine, unlabeled creatinine, and creatine. These measures are then included in an algorithm to determine total body creatine pool size and skeletal muscle mass.23 The D3Cr dilution method is not dependent on creatinine clearance or renal function, s the enrichment of creatinine is measured (i.e., the ratio of D3-creatinine to unlabeled creatinine). No special dietary control (other than the need for a fasting morning spot urine sample) is required. To account for the relation between body weight and muscle mass we analyzed D3Cr muscle mass divided by weight as the primary independent variable.
Other clinical measurements
DXA scans performed Hologic 4500 scanners (Waltham, MA, USA) were used to determine femoral neck and total hip BMD, and appendicular lean mass (ALM) standardized to height squared (ALM/ht2) as previously described.24 Grip strength (kg) was measured twice on each hand with Jamar handheld dynamometers; the maximum value obtained across all tests was analyzed. Walking speed at usual pace was measured over a 6-m course using the average of two trials (m/s).3 Ability and time to rise from a chair five times without the use of the arms was recorded. This was analyzed as chair stands per 10 seconds with those unable to complete coded as 0 chair stands/10 seconds. Self-report of parental history of hip fracture; alcohol use, rheumatoid arthritis, history of fracture; and history of falls as recorded. Recurrent fall history was considered 2 or more falls in the year prior to the visit (compared to 0–1 falls). Height (measured with wall mounted stadiometers) and weight (measured by digital or balance beam scales) were used to calculate body mass index [weight (kg) divided by height2 (m2)]. Participants were asked to bring in all prescription and non-prescription medications; medication was matched to its ingredient(s) based on the Iowa Drug Information Service (IDIS) Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA).25 Using data from the Year 14 visit, the 10-year probability of hip fracture, or major osteoporotic fracture (hip, humerus, vertebral, forearm), with BMD were calculated with U.S. FRAX models.5,26
Fractures
Every four months (March, July and November), MrOS men completed questionnaires about fractures that had occurred in the preceding 4 months. These events were then centrally adjudicated by physician review of radiology reports, and radiographs if needed. The study radiologist confirmed clinically identified vertebral fractures by reviewing lateral spine radiographs or the image used to make the diagnosis in the community clinical practice setting (e.g., MRI) as available. We analyzed the following fracture outcomes: any clinical fracture; any non-spine fracture, major osteoporotic fracture (hip, humerus, clinical vertebral, forearm), and hip fracture. Fractures following the Year 14 visit date through August 2020 were analyzed.
Study sample
As previously described,19 we invited all 1,841 men with a Year 14 clinic visit to complete the D3Cr dilution protocol (without any inclusion/exclusion criteria) and 1425 had valid measures of D3Cr muscle mass/weight (Figure 1). Of these, 62 were missing fracture follow-up or covariate data. Thus, the main analysis sample included 1,363 men. In the models further adjusted for strength and physical performance, 50 men were missing grip strength or walking speed; therefore, the N for these mediation models is 1313 men.
Statistical approach
We have previously reported characteristics of the cohort by quartiles of D3Cr muscle mass/wgt.19 We compared characteristics of participants by occurrence of incident hip fracture using t-tests, Kruskall-Wallis tests and chi-square tests as appropriate. Proportional hazards regression was used to estimate the hazard ratio (HR) and 95% CI for fracture. To test the proportional hazards assumption, we tested time dependent covariates by creating interactions of each of the predictors with a function of survival time, and added them to the model. Since these interactions were not significant, the assumption is not violated. D3Cr muscle mass/weight was analyzed as a continuous variable with the hazards ratio expressed per SD increment, and also by quartiles. Test for trend across quartiles was performed by using a four level indicator variable with values of 1–4 representing each quartile. All models were adjusted for age, femoral neck BMD, FRAX 10-year probability of fracture (hip fracture for hip fracture models; major osteoporotic fracture for all other models), and history of recurrent falls. To determine whether the association between D3Cr muscle mass/weight was mediated by physical performance and strength, we subsequently adjusted all models for walking speed, chair stands, and grip strength.
We considered the competing risk for mortality in sensitivity analyses using the Fine-Grey extension of the Cox model. These results yielded virtually identical results to the standard proportional models (data not shown) so only the standard proportional hazard results are presented.
Results
Mean age of the 1363 men included in these analyses was 84.2 years. At the Year 14 visit, men who went on to suffer an incident hip fracture (N=40) were older, taller, had lower D3Cr muscle mass/wgt, walked slower, had a slower rate of chair stands (p<.0.05 for all, Table 1). Compared to those without an incident hip fracture, those with a hip fracture were somewhat more likely to report use of glucocorticoids; rheumatoid arthritis; previous fracture; or recurrent falls (p<.0.05 for all). In addition, men with an incident hip fracture had lower BMD at the total hip and femoral neck, and a higher FRAX probability than men without an incident fracture (P<0.01 for all). There were no significant differences in weight, BMI, grip strength, parental history of fracture, or alcohol use (p>0.05 for all). D3Cr muscle mass was weakly corrleated with femoral neck BMD (r=0.10, p<.001) and total hip BMD (r=0.14, p<.001) after adjustment for height and weight (Figure 2).
Table 1.
Characteristics of MrOS participants by incident hip fracture status after the Year 14 clinic visit
| Characteristic | No incident hip fracture (N=1326) | Incident hip fracture (N=40) | p-value |
|---|---|---|---|
| Age (years) | 84.1 ± 4.0 | 86.0 ± 4.6 | 0.004 |
| Weight (kg) | 79.6 ± 12.5 | 78.4 ± 11.2 | 0.532 |
| Height (m) | 172.2 ± 6.8 | 174.4 ± 5.7 | 0.048 |
| BMI (kg/m2) | 26.8 ± 3.7 | 25.8 ± 3.4 | 0.075 |
| D3Cr muscle mass/weight | 0.31 ± 0.05 | 0.28 ± 0.04 | 0.004 |
| ALM/ht2 (kg/m2) | 7.55 ± 0.87 | 7.20 ± 0.65 | 0.013 |
| Walk speed (m/s) | 1.13 ± 0.25 | 0.99 ± 0.23 | 0.002 |
| Chair stands per 10 seconds (stands/10 sec) | 3.77 ± 1.75 | 2.43 ± 1.95 | <0.001 |
| Grip strength (kg) | 35.7 ± 7.8 | 35.2 ± 8.5 | 0.683 |
| Parental history of fracture (Yes/no/unknown) | 0.915 | ||
| Yes | 362 (27.4) | 11 (27.5) | |
| No | 492 (37.2) | 16 (40) | |
| Unknown | 469 (35.5) | 13 (32.5) | |
| Previous fracture | 796 (60.2) | 33 (82.5) | 0.004 |
| Glucocorticoid use | 33 (2.5) | 3 (7.5) | 0.052 |
| Rheumatoid arthritis | 80 (6.1) | 7 (17.5) | 0.004 |
| Alcohol use | 0.628 | ||
| 0–2 drinks/week | 792 (60.1) | 23 (57.5) | |
| >2–13 drinks/week | 456 (34.6) | 16 (40) | |
| >13 drinks/week | 69 (5.2) | 1 (2.5) | |
| 2 or more falls in past year | 222 (16.8) | 12 (30) | 0.029 |
| Femoral neck BMD | 0.760 ± 0.134 | 0.633 ± 0.088 | <0.001 |
| Total hip BMD | 0.937 ± 0.147 | 0.808 ± 0.110 | <0.001 |
| 10-year probability of hip fracture (with BMD) | 4.4 ± 4.89 | 8.3 ± 6.3 | <0.001 |
| 10-year probability of hip fracture (without BMD) | 8.1 ± 6.1 | 12.0 ± 8.7 | <0.001 |
| 10-year probability of MOF* fracture (with BMD) | 9.3 ± 5.8 | 14.7 ± 6.8 | <0.001 |
| 10-year probability of MOF* fracture (without BMD) | 13.7 ± 6.7 | 18.2 ± 8.8 | <0.001 |
MOF: major osteoporotic fracture: hip, humerus, vertebral, forearm.
Figure 2.
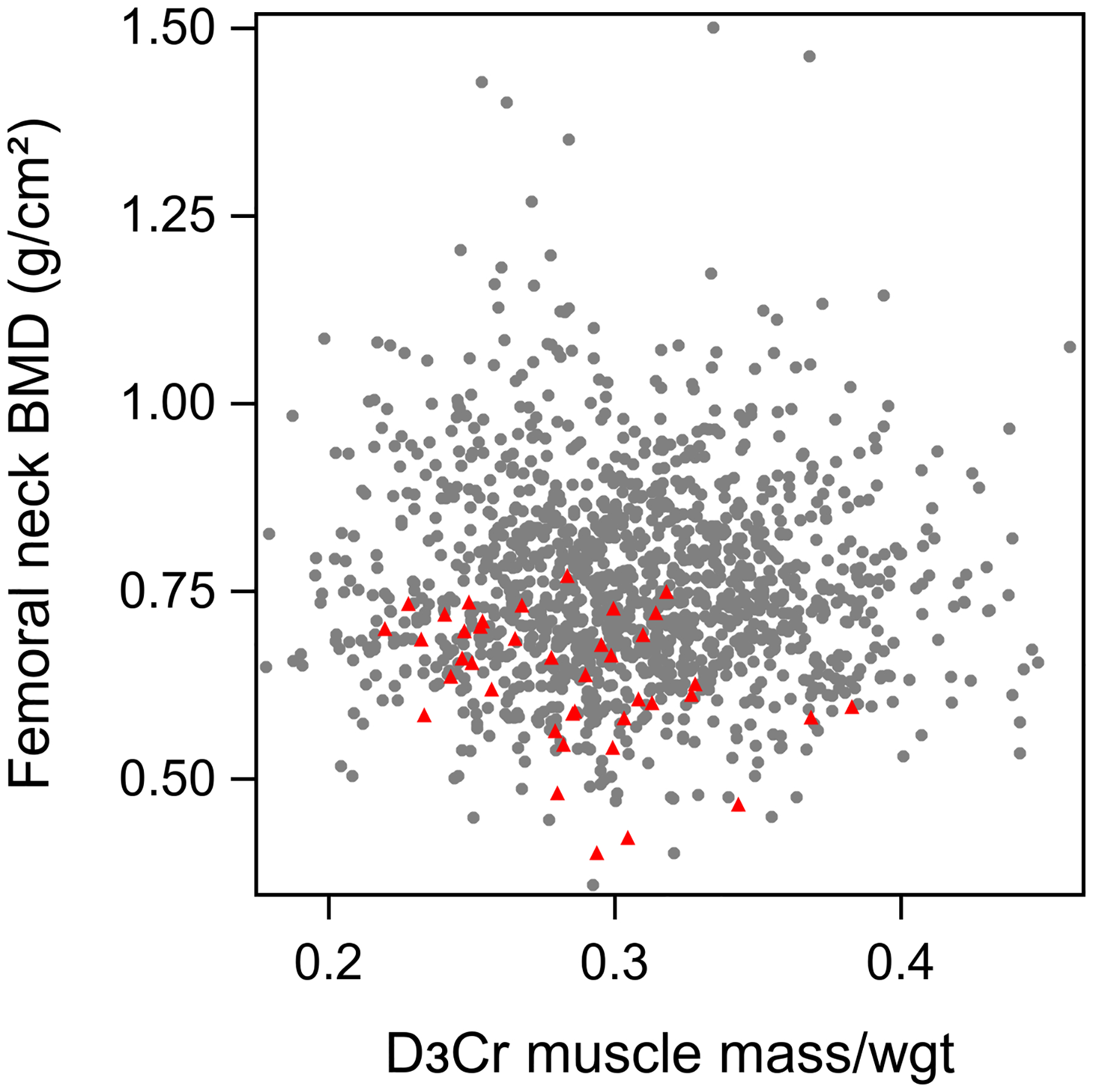
Scatterplot of D3Cr muscle mass/wgt vs femoral neck BMD.
Red triangle indicates participants with incident hip fracture; grey dots represent those without incident hip fracture
*partial correlation for D3Cr muscle mass/wgt with femoral neck BMD (r=0.10, p<.001) and with total hip BMD (r=0.14, p<.001), adjusted for height and weight
Over 4.6 years of follow-up, 180 men (13.2%) had at least one clinical fracture, including 153 (11.2%) men with a non-spine fracture, 85 men (6.2%) with a major osteoporotic fracture and 40 men (2.9%) with a hip fracture. The incidence of fracture was highest in the lower quartiles of D3Cr muscle mass/wgt, and the lowest fracture rates were seen in the highest quartile of D3Cr muscle mass/wgt (Figure 3).
Figure 3.
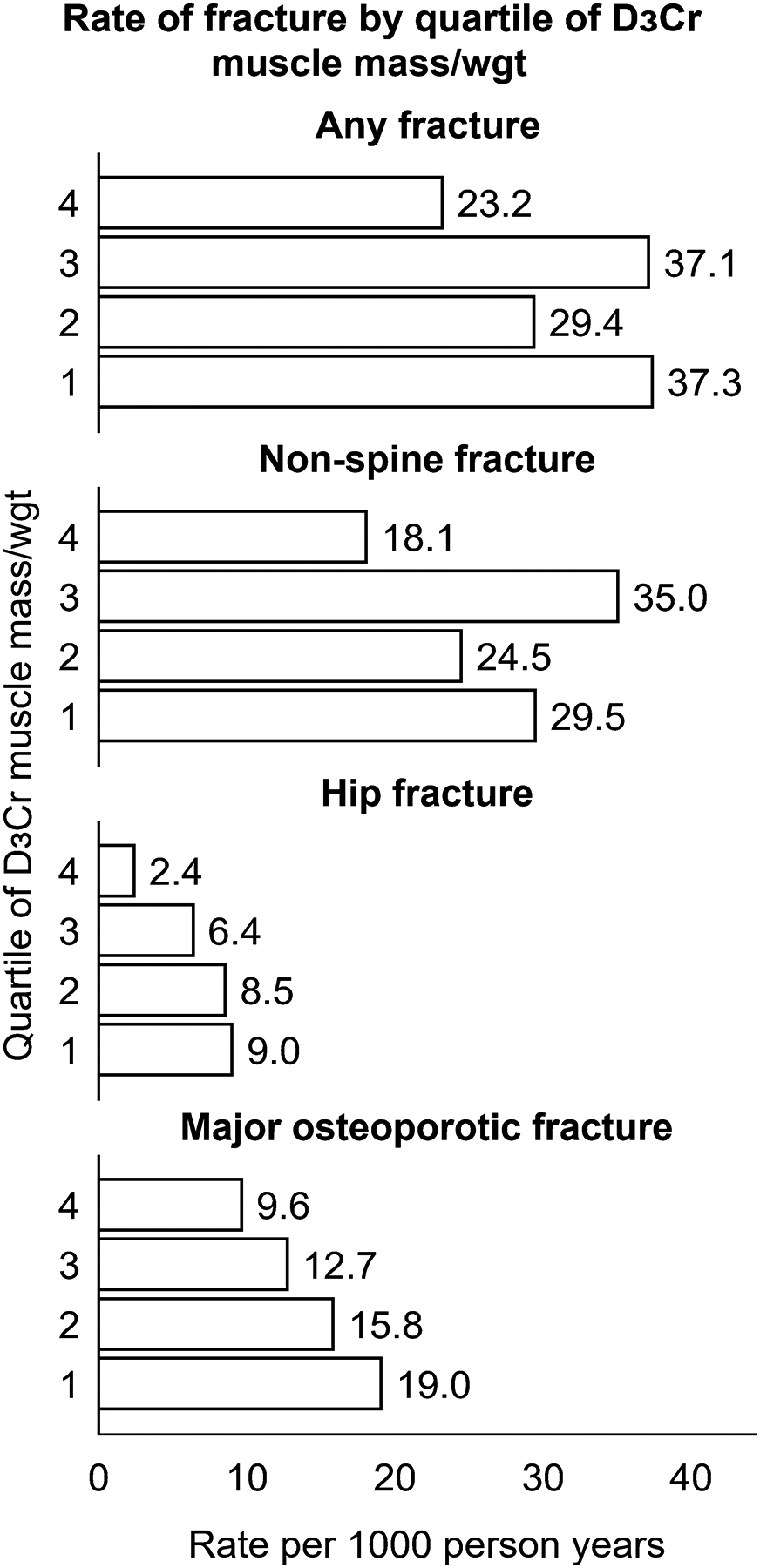
Age-adjusted incidence of fracture per 1000 person years, by quartile of D3Cr muscle mass/wgt
D3Cr muscle mass/wgt quartiles were defined as follows: quartile 1 (lowest): <0.273 (median 0.25); quartile 2: ≥0.273 – <0.303 (median: 0.289); quartile 3: ≥0.303 – <0.339 (median: 0.319), quartile 4 (highest): ≥0.339 (median: 0.363).
In multivariate models adjusted for age, femoral neck BMD, FRAX fracture probability and recurrent fall history, each SD decrement in D3Cr muscle mass/wgt was associated with a 1.8-fold higher risk of hip fracture (HR per SD: 1.75, 95%CI: 1.22 to 2.50, mean follow up 4.6 years); a 1.4-fold higher risk of major osteoporotic fracture (HR per SD: 1.37, 95%CI: 1.08 to 1.74, mean follow up 4.5 years); a 1.2-fold higher risk of non-spine fracture (HR per SD: 1.21, 95%CI: 1.02 to 1.43, mean follow up 4.4 years); and a 1.2 fold higher risk of any clinical fracture (HR per SD: 1.22, 95%CI: 1.04 to 1.43, mean follow up 4.3 years). Further, men in the lowest quartile of D3Cr muscle mass/wgt had a 5-fold higher risk of hip fracture and a 2-fold higher risk of major osteoporotic fracture than men in the highest quartile of D3Cr muscle mass/wgt (Figure 4) When we further accounted for the potentially mediating effects of muscle function (grip strength) and physical performance (chair stands, walking speed), the associations between D3Cr muscle mass/wgt and fracture were attenuated but remained borderline statistically significant for hip and major osteoporotic fracture (p≥0.05–0.10). The point estimates for the HR for the associations of D3Cr muscle mass/wgt with non-spine and clinical fracture remained above 1.0 (HR: 1.6 for Q1 vs Q4) but were not statistically significant (Supplemental Figure 1).
Figure 4.
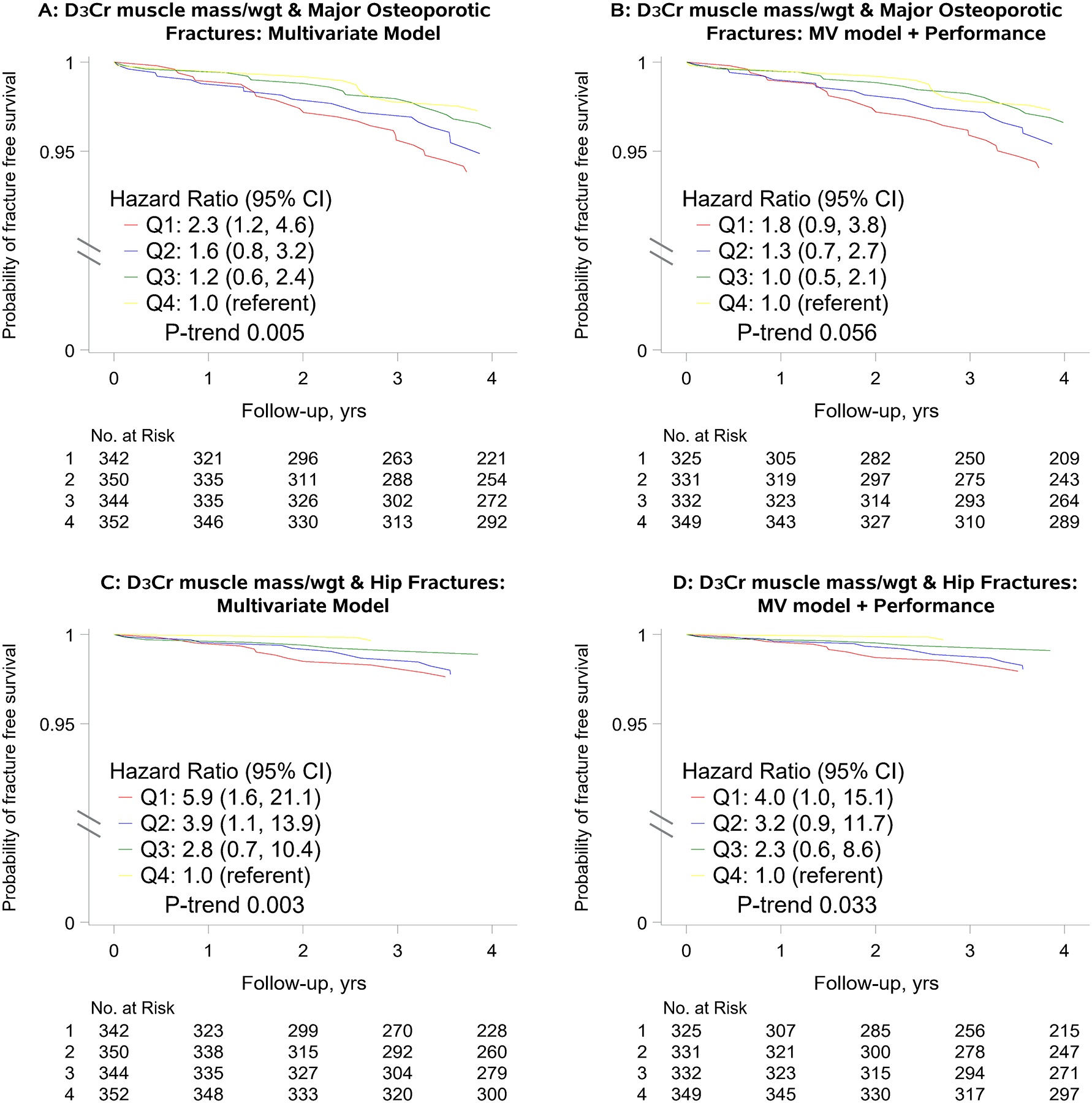
Multivariate* adjusted hazard ratio (HR) for major osteoporotic and hip fracture, by quartile of D3Cr muscle mass/wgt
*Panels A and C: Adjusted for age, history of falls, femoral neck BMD, and FRAX 10-year risk of hip fracture or major osteoporotic fracture
*Panels B and D: Adjusted for age, history of falls, femoral neck BMD, FRAX 10-year risk of hip fracture or major osteoporotic fracture, grip strength, walking speed and chair stand rate.
*Hazard ratios per SD decrement in D3Cr muscle mass/wgt in models adjusted for age, history of falls, femoral neck BMD and FRAX 10-year risk of hip fracture or major osteoporotic fracture were 1.75 (95%CI: 1.22, 2.50) for hip fracture and 1.37 (95%CI: 1.08, 1.74) for major osteoporotic fracture.
*Hazard ratios per SD decrement in D3Cr muscle mass/wgt in models adjusted for age, history of falls, femoral neck BMD and FRAX 10-year risk of hip fracture or major osteoporotic fracture, grip strength, walking speed and chair stand rate were 1.44 (95%CI: 0.98, 2.10) for hip fracture and 1.23 (95%CI: 0.94, 1.59) for major osteoporotic fracture.
D3Cr muscle mass/wgt quartiles were defined as follows: quartile 1 (lowest): <0.273 (median 0.25); quartile 2: ≥0.273 – <0.303 (median: 0.289); quartile 3: ≥0.303 – <0.339 (median: 0.319), quartile 4 (highest): ≥0.339 (median: 0.363).
Discussion
Our findings suggest that low D3Cr muscle mass/wgt is a risk factor for incident fractures, particularly hip fractures, in older men even after accounting for potential confounders that may influence both muscle mass and fracture risk. This association is partially mediated by physical performance, particularly for any fracture and non-spine fracture outcomes. We posit that the relationship between low muscle mass and fractures has not been previously observed because other studies have used inaccurate approximations of muscle mass, namely lean mass assessed by DXA.
Physical performance partially mediated the effects of D3Cr muscle mass/wgt on fractures, as the effect estimates for D3Cr muscle mass/wgt were attenuated but remained significant (for p-for-trend and Q1 vs Q4 for hip fractures, p<.05) or borderline significant (for HR per SD decrement for hip fractures, 0.05≤p<.1) after these factors were included in the models. The most simplistic explanation for these findings is that low D3Cr muscle mass results in poor physical performance, and then poor physical performance impacts fracture risk; this paradigm is supported by our previous work.20,27 However, this hypothesis is difficult to test currently as we only have longitudinal data on D3Cr muscle mass and physical performance in a small subset of ~40 MrOS men.28 Although there appears to be a relationship between muscle mass and declines in strength and performance and muscle mass, full characterization of these changes has not been completed. Low D3Cr muscle mass/wgt is associated with increased risk of serious injurious falls in MrOS, but not significantly associated with any incident fall or recurrent falls.19 Adjusting the present results for fall history did not fully explain the associations observed. Thus, our results suggest that the association between low muscle mass and fracture risk may due to additional factors aside from just poor function and fall risk. Muscle is known to release myokines and these may directly act on bone which then influence fracture risk;7,8 other pathways (such as inflammation or senescence) impact both muscle and bone.29–32 Future research will investigate which of these intermediaries most likely explain the D3Cr muscle mass – fracture relationship, as such measurements are not available in all MrOS participants at the same time point when D3Cr muscle mass was assessed.
Our implementation of the D3-creatine dilution protocol in MrOS as well as previous data33 show that this approach is an accurate and precise measurement of muscle mass. The finding that D3Cr muscle mass is associated with fractures even after adjustment for potential confounders including FRAX risk suggest that D3Cr muscle mass may be useful to further identify those at risk of fracture beyond traditional risk factors. In contrast, a number of reports have noted that adjustment for BMD attenuates the association between low ALM and increased fracture risk; in some cases, adjustment for BMD even reverses the association such that after account for BMD, those with low ALM have a decreased risk of fracture.17,18 In our study, however, there were relatively few fractures which precludes our ability to conduct meaningful reclassification and discrimination analyses for the D3Cr muscle mass data. More research in larger cohorts with a greater number of fractures is needed to make this determination.
This study has several strengths, including its large, well characterized population, prospective design, and centrally adjudicated fracture outcomes. However, a number of limitations should be noted. First, MrOS is all men with a very high proportion of white participants. Whether or not these results generalize to women or all race/ethnic groups is unclear. A small study in women (N=74) suggested that D3Cr muscle mass was strongly related to self-reported function, and that this association was stronger than the relationship between the observed significant association of DXA lean mass and function in women.34 Men have a much lower fracture risk than women, and men and women have very different body sizes and body composition. Thus, it is reasonable to suspect that there may be sex differences in the relation between muscle mass and fracture. It does appear that sex differences may exist for the relationship between obesity and fracture, with obesity associated with a higher risk of fracture in men but a reduce risk of fracture in women.35 Thus, it is not clear that D3Cr muscle mass and fracture associations would be similar in women as they are in men. Second, despite the large size of the study, hip fractures were still relatively rare which resulted in wide confidence intervals for the effect estimates and limited our ability to adjust for additional potential confounders and ability to detect even moderate associations. Thus, these results should be replicated in other studies with more fracture events.
Another limitation of our project is that we did not adjust for physical activity, which may have led to unresolved confounding. However, this concern is tempered by previous findings in MrOS that suggested that physical activity was not causally related to hip fracture risk, making unresolved confounding by failure to adjust for physical activity unlikely. Further, as this is an observational study, we cannot rule out unmeasured confounding as an explanation for our findings. However, we adjusted for potentially confounding factors, and it is unlikely that unmeasured factors would fully explain the compelling associations observed. While we considered grip strength, we did not include gold standard measures of strength such as isokinetic dynamometry of the lower extremities as a potentially mediating factor. In addition, the D3Cr dilution method does not allow for regional assessment of muscle mass (that is, quantification of the amount of muscle only in the legs, or the amount of muscle only in the arms) so it is not possible with these data to disentangle which particular muscle groups (if any) are driving the association between D3Cr muscle mass and fractures.
Assessment of muscle mass by D3Cr dilution is in not yet available in clinical settings. Potential barriers to its use in clinical settings (or research studies) could include the coordination required for the timing of the dose and urine collection. However, we note several advantages of the D3Cr dilution approach: this method does not require participant interaction with expensive machinery, as the assays are completed at a single central lab, allowing the use of this method in a variety of settings (including the home). Further, the D3Cr dilution method does not require trained technologists to interact with participants (unlike DXA, CT or MR), and does not expose participants to radiation (unlike DXA or CT).
Our findings, if replicated in additional studies in diverse populations with additional fracture outcomes, may impact the continuing evolution of operational definitions of sarcopenia, for example the Sarcopenia Definitions and Outcomes Consortium (SDOC) or the revised European Working Group for Sarcopenia in Older Persons (EWGSOP2) definitions.36–38 If low D3Cr muscle mass is consistently found to be a risk factor for fracture, then it may be considered for inclusion in such operational definitions, at least in research settings. Whether or not D3Cr muscle mass would be included as part of a composite definition of sarcopenia in addition to the grip strength and walking speed components that are already part of the SDOC and EWGSOP2 definitions, or if D3Cr muscle mass could replace these components, would depend on future work, including analyses of risk prediction, discrimination, and reclassification.
In summary, low D3Cr muscle mass/weight is associated with a markedly high risk of hip and potentially other fractures in older men; this association is partially mediated by physical performance. Further research should further evaluate whether D3Cr muscle mass is a useful clinical tool to identify those at risk of fracture, particularly in more diverse populations.
Supplementary Material
Acknowledgement
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: R01 AG066671 and UL1 TR000128. Funding for the D3Cr muscle mass measure was provided by NIAMS (grant number R01 AR065268). GlaxoSmithKline provided in-kind support by providing the d3-creatine dose and analysis of urine samples.
Conflict of interest statement:
Dr. Cawthon has grants to her institution from Nestle and Abbott outside of the work of this manuscript. Dr. Evans is listed as coinventor on the granted patents for the D3-Cr dilution method. However, he does not derive any income or royalties or own the intellectual property for the method. No other authors have conflicts to declare.
Data availability
The MrOS data are available at https://mrosonline.ucsf.edu.
References
- 1.Frost HM, Ferretti JL, Jee WS. Perspectives: some roles of mechanical usage, muscle strength, and the mechanostat in skeletal physiology, disease, and research. Calcified tissue international. 1998;62(1):1–7. [DOI] [PubMed] [Google Scholar]
- 2.Cawthon PM, Blackwell TL, Marshall LM, et al. Physical performance and radiographic and clinical vertebral fractures in older men. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2014;29(9):2101–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cawthon PM, Fullman RL, Marshall L, et al. Physical performance and risk of hip fractures in older men. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2008;23(7):1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cauley JA, Harrison SL, Cawthon PM, et al. Objective measures of physical activity, fractures and falls: the osteoporotic fractures in men study. Journal of the American Geriatrics Society. 2013;61(7):1080–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harvey NC, Oden A, Orwoll E, et al. Measures of Physical Performance and Muscle Strength as Predictors of Fracture Risk Independent of FRAX, Falls, and aBMD: A Meta-Analysis of the Osteoporotic Fractures in Men (MrOS) Study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2018;33(12):2150–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. The New England journal of medicine. 1995;332(12):767–773. [DOI] [PubMed] [Google Scholar]
- 7.Hamrick MW. A role for myokines in muscle-bone interactions. Exerc Sport Sci Rev. 2011;39(1):43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaji H. Effects of myokines on bone. BoneKEy reports. 2016;5:826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cummings SR, Bates D, Black DM. Clinical use of bone densitometry - Scientific review. Jama-Journal of the American Medical Association. 2002;288(15):1889–1897. [DOI] [PubMed] [Google Scholar]
- 10.Buckinx F, Landi F, Cesari M, et al. Pitfalls in the measurement of muscle mass: a need for a reference standard. Journal of cachexia, sarcopenia and muscle. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark BC, Tavoian D, Goodpaster BH, Cawthon PM, Hansen RD, Manini TM. Comment on: “Pitfalls in the measurement of muscle mass: a need for a reference standard” by Buckinx et al et al. Journal of cachexia, sarcopenia and muscle. 2018;9(7):1269–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scafoglieri A, Clarys JP. Dual energy X-ray absorptiometry: gold standard for muscle mass? Journal of cachexia, sarcopenia and muscle. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. American journal of epidemiology. 1998;147(8):755–763. [DOI] [PubMed] [Google Scholar]
- 14.Newman AB, Kupelian V, Visser M, et al. Sarcopenia: alternative definitions and associations with lower extremity function. Journal of the American Geriatrics Society. 2003;51(11):1602–1609. [DOI] [PubMed] [Google Scholar]
- 15.Schott AM, Cormier C, Hans D, et al. How hip and whole-body bone mineral density predict hip fracture in elderly women: the EPIDOS Prospective Study. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 1998;8(3):247–254. [DOI] [PubMed] [Google Scholar]
- 16.Zaslavsky O, Li W, Going S, Datta M, Snetselaar L, Zelber-Sagi S. Association between body composition and hip fractures in older women with physical frailty. Geriatrics & gerontology international. 2017;17(6):898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harvey NC, Kanis JA, Liu E, Johansson H, Lorentzon M, McCloskey E. Appendicular lean mass and fracture risk assessment: implications for FRAX(R) and sarcopenia. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLean RR, Kiel DP, Berry SD, et al. Lower Lean Mass Measured by Dual-Energy X-ray Absorptiometry (DXA) is Not Associated with Increased Risk of Hip Fracture in Women: The Framingham Osteoporosis Study. Calcified tissue international. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cawthon PM, Orwoll ES, Peters KE, et al. Strong Relation Between Muscle Mass Determined by D3-creatine Dilution, Physical Performance, and Incidence of Falls and Mobility Limitations in a Prospective Cohort of Older Men. The journals of gerontology Series A, Biological sciences and medical sciences. 2019;74(6):844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cawthon PM, Blackwell T, Cummings SR, et al. Muscle mass assessed by D3-Creatine dilution method and incident self-reported disability and mortality in a prospective observational study of community dwelling older men. The journals of gerontology Series A, Biological sciences and medical sciences. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemporary clinical trials. 2005;26(5):557–568. [DOI] [PubMed] [Google Scholar]
- 22.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemporary clinical trials. 2005;26(5):569–585. [DOI] [PubMed] [Google Scholar]
- 23.Shankaran M, Czerwieniec G, Fessler C, et al. Dilution of oral D3 -Creatine to measure creatine pool size and estimate skeletal muscle mass: development of a correction algorithm. Journal of cachexia, sarcopenia and muscle. 2018;9(3):540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee CG, Boyko EJ, Nielson CM, et al. Mortality risk in older men associated with changes in weight, lean mass, and fat mass. Journal of the American Geriatrics Society. 2011;59(2):233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10(4):405–411. [DOI] [PubMed] [Google Scholar]
- 26.Kanis JA, Harvey NC, Cooper C, Johansson H, Oden A, McCloskey EV. A systematic review of intervention thresholds based on FRAX : A report prepared for the National Osteoporosis Guideline Group and the International Osteoporosis Foundation. Archives of osteoporosis. 2016;11(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cawthon PM, Fullman RL, Marshall L, et al. Physical performance and risk of hip fractures in older men. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2008;23(7):1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duchowny KA, Peters KE, Cummings SR, et al. Association of change in muscle mass assessed by D3 -creatine dilution with changes in grip strength and walking speed. Journal of cachexia, sarcopenia and muscle. 2020;11(1):55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bano G, Trevisan C, Carraro S, et al. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas. 2017;96:10–15. [DOI] [PubMed] [Google Scholar]
- 30.Barbour KE, Lui LY, Ensrud KE, et al. Inflammatory markers and risk of hip fracture in older white women: the study of osteoporotic fractures. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2014;29(9):2057–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cauley JA, Barbour KE, Harrison SL, et al. Inflammatory Markers and the Risk of Hip and Vertebral Fractures in Men: the Osteoporotic Fractures in Men (MrOS). Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2016;31(12):2129–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stojanovic D, Buzkova P, Mukamal KJ, et al. Soluble Inflammatory Markers and Risk of Incident Fractures in Older Adults: The Cardiovascular Health Study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark RV, Walker AC, O’Connor-Semmes RL, et al. Total body skeletal muscle mass: estimation by creatine (methyl-d3) dilution in humans. Journal of applied physiology (Bethesda, Md : 1985). 2014;116(12):1605–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu K, Wactawski-Wende J, Ochs-Balcom HM, et al. The Association of Muscle Mass Measured by D3-Creatine Dilution Method with Dual Energy X-ray Absorptiometry and Physical Function in Postmenopausal Women. The journals of gerontology Series A, Biological sciences and medical sciences. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nielson CM, Srikanth P, Orwoll ES. Obesity and fracture in men and women: an epidemiologic perspective. J Bone Miner Res. 2012;27(1):1–10. [DOI] [PubMed] [Google Scholar]
- 36.Bhasin S, Travison TG, Manini TM, et al. Sarcopenia Definition: The Position Statements of the Sarcopenia Definition and Outcomes Consortium. Journal of the American Geriatrics Society. 2020;68(7):1410–1418. [DOI] [PubMed] [Google Scholar]
- 37.Cawthon PM, Manini T, Patel SM, et al. Putative Cut-Points in Sarcopenia Components and Incident Adverse Health Outcomes: An SDOC Analysis. Journal of the American Geriatrics Society. 2020;68(7):1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age and ageing. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The MrOS data are available at https://mrosonline.ucsf.edu.


