Abstract
Among 865 adults with early syphilis considered for a multicenter treatment trial, 234 (27%) were excluded prior to enrollment due to bacterial STI coinfection. Coinfection with Neisseria gonorrhoeae (29%), Chlamydia trachomatis (22%), or both (23%) were common. Study findings highlight the need for comprehensive bacterial STI screening in syphilis patients.
Keywords: chlamydia, gonorrhea, STI coinfection, STI screening, syphilis, Treponema pallidum
Short Summary:
Among 865 US adults with early syphilis, more than 1 in 4 had bacterial STI coinfection. This study supports routine mucosal screening for chlamydia and gonorrhea in people with syphilis.
Introduction
Bacterial sexually transmitted infection (STI) rates in the United States have risen over the past decade and 130,000 cases of syphilis, 1.8 million cases of chlamydia and 616,000 cases of gonorrhea were reported to the US Centers for Disease Control and Prevention (CDC) in 2019.1 Persons at risk of STI acquisition are also at risk of coinfection with more than one STI. For example, co-infection with Chlamydia trachomatis (CT) was detected in 45% of young women with Neisseria gonorrhoeae (NG) infection seen in family planning, prenatal and STI clinics.2,3 Surprisingly, few studies document the prevalence of chlamydia and gonorrhea coinfection among persons diagnosed with syphilis.
While serological syphilis screening is performed with a blood test, detection of mucosal STIs, such as chlamydia and gonorrhea, requires specimen collection from different urogenital and extragenital (rectal and pharyngeal) sites. CDC recommends routine bacterial STI screening at extragenital sites in people living with HIV, men who have sex with men (MSM) and consideration of screening in women based on risk.4 As multisite STI screening among sexually active adults is infrequent, asymptomatic infections can be missed, and co-infection rates in persons with syphilis are not well documented.5
In this note, we describe the proportion of adults with CT and/or NG coinfection among persons with early syphilis (defined as primary, secondary, or early latent infection).
Methods
Adults living with and without HIV were enrolled from 10 US outpatient clinical sites in a phase IV, open-label, randomized controlled trial comparing the efficacy of single dose vs 3 weekly doses of 2.4 million units of intramuscular benzathine penicillin G for early syphilis treatment (NCT03637660 at clinicaltrials.gov). As part of the eligibility pre-screening process at urban academic facilities and public health clinics located across the US, study staff pre-screened potential participants with incident syphilis infection. Staff recorded in a de-identified manner the reason for pre-screen failure for persons found to be ineligible based on pre-specified study eligibility criteria. No further data were collected on these individuals. In this report, we focused on people pre-screened between September 12th, 2018 and August 27th, 2021. Persons requiring treatment for a concurrent infection with antimicrobials active against Treponema pallidum were ineligible to participate. Therefore, individuals who underwent routine STI screening and were prescribed antimicrobials for chlamydia (azithromycin), gonorrhea (ceftriaxone), and/or non-gonococcal urethritis (doxycycline) therapies were ineligible. Chlamydia and gonorrhea results on tests performed prior to prescreening, when known, were collected by study staff and included in the weekly screening and eligibility report. Individual-level data including gender, age, sexual partner information, HIV status, coinfection site, and symptoms were not collected during the pre-screening process.
Basic descriptive statistics are presented. The parent study is approved by the University of Alabama at Birmingham IRB. Informed consent was obtained for enrolled participants but not pre-screened persons since only limited, non-identifiable information was collected during the pre-screening process.
Results
Among those prescreened, 865 adults were classified as having early syphilis and were potentially eligible for study participation. Exclusion criteria were present in 630 of these persons, and 235 were ultimately enrolled in the trial (See Figure 1). Among the 865 patients diagnosed with early syphilis, 234 (27%) had known co-infection with a bacterial STI. Bacterial STI coinfection requiring treatment with antibiotics active against T. pallidum was the most common reason for exclusion documented in 37% (234/630) of persons with early syphilis excluded from participation. Other common reasons for exclusion included receipt of antibiotics in the past 30 days (n=216; 34%) and known or reported allergy or hypersensitivity to penicillin or other ß-lactam antibiotics (n=99; 16%). Among 234 adults with known STI coinfection, STI type was documented for 179 (76%); 37% of these (67/179) were co-infected with N. gonorrhoeae, 28% (51/179) had chlamydial coinfection and 30% (53/179) had triple infection with NG, CT, and syphilis. Eight potential study participants with syphilis (4%) had non-gonococcal urethritis (6/179) or lymphogranuloma venereum (2/179). Figure 2 shows the proportion of each STI in addition to the unspecified STI category. Information about anatomic site of coinfection and symptoms were not recorded.
Figure 1: Flow Diagram.
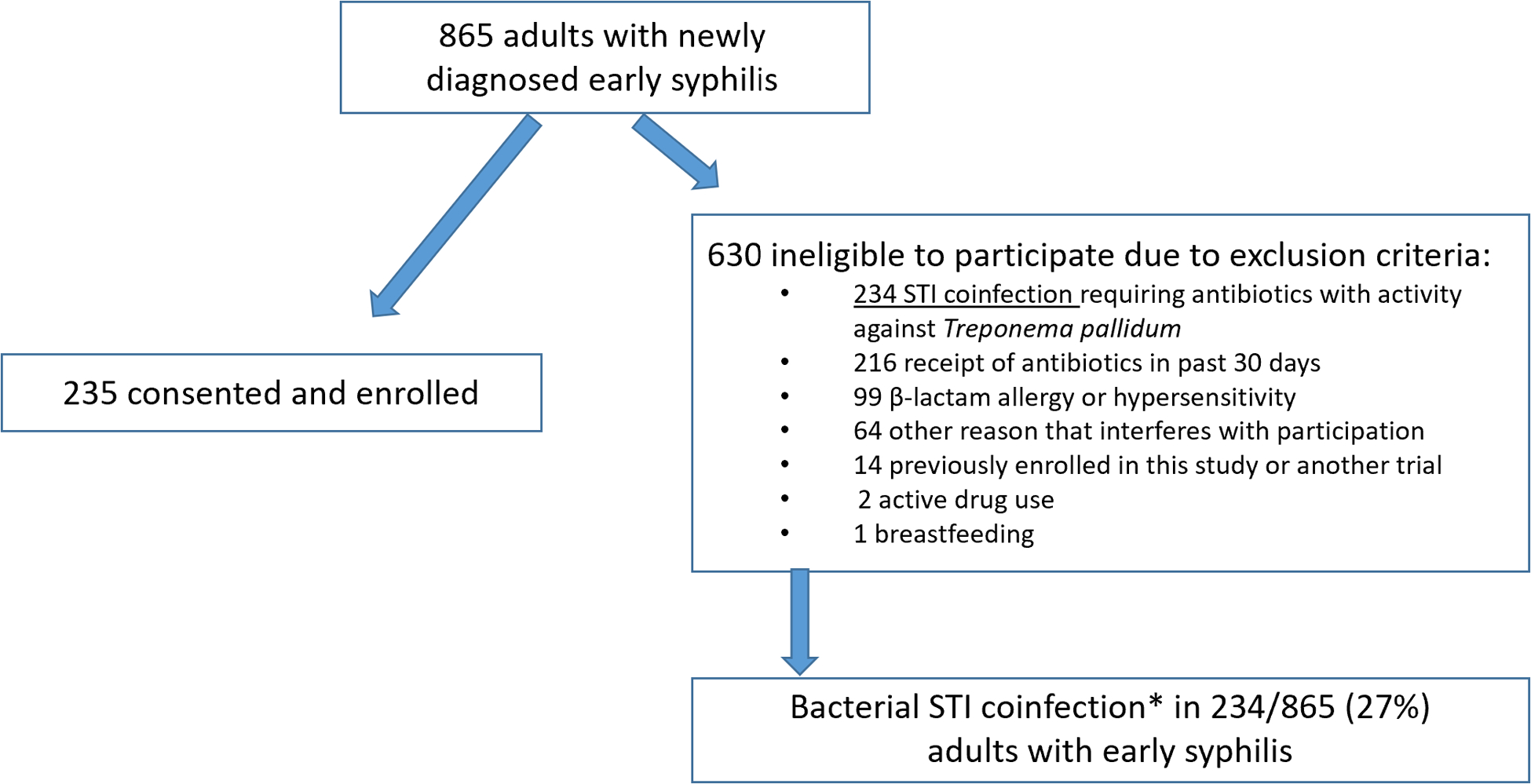
*STI coinfection type specified in Figure 2
Figure 2: Bacterial STI Coinfection Type among Adults with Early Syphilis * (n=234).
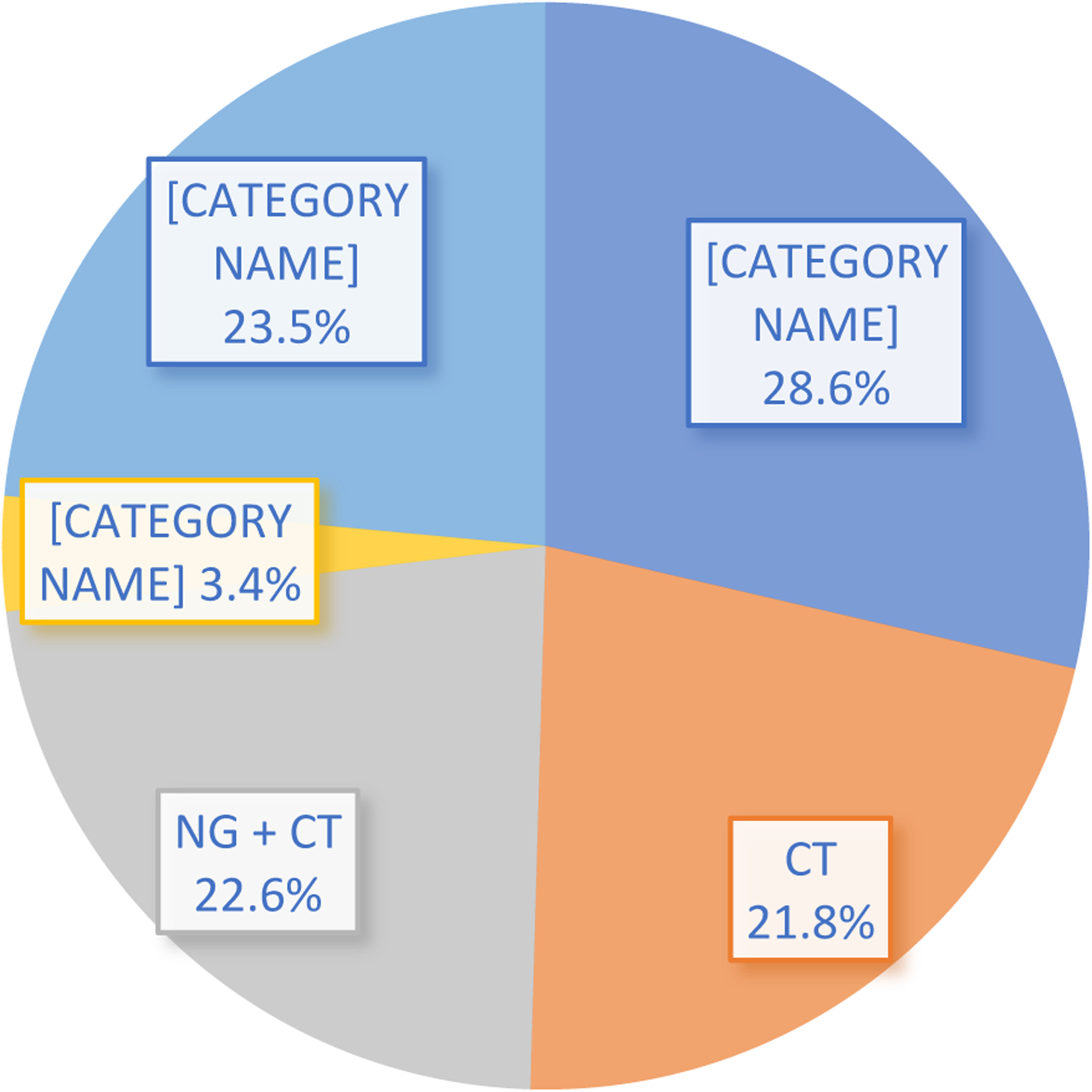
*These adults were found to be ineligible to participate in a syphilis treatment trial due to STI during the pre-screening process. The unspecified category was used between 09/12/2018 – 02/25/2019 and then STI type was specified between 02/26/19 – 08/27/21.
CT = Chlamydia trachomatis; NG = Neisseria gonorrhoeae; Other = non-gonococcal urethritis (NGU) (6), lymphogranuloma venereum (LGV) (2).
Discussion
In this study, more than one in four adults with recently diagnosed early syphilis were known to have bacterial STI coinfection (NG/CT) during the pre-screening process for enrollment in a syphilis treatment trial. While syphilis cases in the US increased by 74%, gonorrhea cases by 56%, and chlamydia cases by 19% between 2015 and 2019, CDC does not routinely collect data on coinfection rates.1 Despite longstanding routine NG/CT screening recommendations from the US Preventive Services Task Force (USPSTF) for sexually active adolescents and adults at risk of STI acquisition and/or adverse outcomes, (women under age 25, people living with HIV, MSM), data on multisite STI screening rates are limited. The annual NG/CT screening rate among people receiving HIV care was only 24% in the CDC Medical Monitoring Project and 42% among MSM (16% at extragenital sites) according to an internet survey study.5,6 Since most NG/CT among women and MSM is asymptomatic and rectal infection is frequently missed, routine screening and access to care are critical for identification and treatment of infection.7–9
Little is published about NG/CT coinfection rates in people with syphilis in the US, although high coinfection rates could be expected since NG/CT rates are higher than syphilis rates and risk factors for acquisition are similar. Among 548 European adults with newly diagnosed syphilis screened for genital and extragenital infection, 16% had NG/CT coinfection and 86% of coinfections were asymptomatic.10 Coinfection prevalence was 20% in MSM compared to 4% in heterosexuals (p<0.001). Given the well-known epidemiologic synergy between STI infection and HIV acquisition and transmission, undiagnosed STI has added significance in the setting of efforts to end the HIV epidemic – part of which relies on upscaling HIV prevention activities.11–13 In a recent study based on state-wide surveillance data in Tennessee, syphilis and a composite measure of STI coinfection (NG, CT and/or syphilis) were the strongest predictors of incident HIV infection.14 The strength of the association was similar among heterosexual women and men (aHR 2.8, 95% CI 1.9–4.2) and MSM (adjusted HR 2.3, 95% CI 1.4–3.8). Similarly, among women age 13–59 in Louisiana with a history of STI, the rate of HIV was higher in women with documented NG/CT/syphilis coinfection compared to women without coinfection (73 cases per 100,000 vs 47 cases per 100,000 person years).15 A recent CDC model based on published data assumed a relative risk (RR) of HIV acquisition of 2.0 in the presence of rectal NG/CT and RR 1.5 for urethral NG/CT. In this study, an estimated 10% of incident HIV among MSM was attributable to the presence of NG or CT infection.16
Serologic screening for syphilis in outpatient care settings (prenatal clinics, urgent care or emergency room visits, HIV care and prevention clinics) is often incorporated into standing orders for periodic routine phlebotomy. In contrast, testing for other common and reportable bacterial STIs (NG/CT) requires swab or urine collection and some clinics default to urine STI testing only for simplicity. Screening rates for mucosal NG/CT (particularly at extragenital sites) tend to lag behind syphilis screening rates in MSM and people living with HIV despite the fact that rectal STI screening is high yield.17–19 Screening based on exposure site is limited if patients are reluctant to disclose information about sexual practice or if providers do not ask about exposure sites as part of a complete sexual history.
The main limitations of our study are that we did not have STI test results on all patients prescreened for the study and testing for NG and CT was not required for participation. We also did not include NG/CT coinfections in enrolled participants who were diagnosed after randomization or who received antibiotics with no activity against T. pallidum. Findings on enrolled participants in this ongoing study will be published in future reports. As a result of these exclusions, current study findings could underrepresent the STI co-infection rate. We also suspect that coinfection estimates are conservative since some people excluded for recent antibiotic exposure may have been treated for STI. We did not capture information about the indication for recent antibiotics during the prescreening process. Other limitations include the lack of individual-level data about participant characteristics and the site and symptomatology of NG/CT infections. This also may have contributed to an underestimate of STI coinfection rates.
Our study has strengths, including representation from ten US clinical sites, and timeliness given the recent resurgence in STI rates noted during the COVID-19 pandemic.20–22 Concomitant STIs in persons with syphilis represent an important public health priority. The study also has implications for the conduct of STI clinical treatment trials, which often exclude persons with co-infections identified during screening. It may be useful to design trials that are permissive to coinfection. Further studies should confirm STI co-infection rates in persons with syphilis, stratify data by demographic and clinical settings, and compare treatment and health outcomes in those with and without bacterial STI co-infection.
In conclusion, NG/CT coinfection was common among US adults with early syphilis. In addition to HIV screening, all individuals diagnosed with early syphilis should also be screened for NG/CT. Clinics should develop protocols that facilitate routine NG/CT screening in this group.
Funding Statement:
This work was funded by the National Institute of Allergy and Infectious Diseases (Sexually Transmitted Infections Clinical Trials Group) Contract HHSN272201300012I. The views and opinions presented do not reflect those of the funding agency. JDO reports research funding from NIH/NICHD 1R01HD101545.
Footnotes
COI Statement: Authors declare no conflict of interest.
References
- 1.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2019. 2021.
- 2.Dicker LW, Mosure DJ, Berman SM, Levine WC. Gonorrhea prevalence and coinfection with chlamydia in women in the United States, 2000. Sex Transm Dis. 2003;30(5):472–6. [DOI] [PubMed] [Google Scholar]
- 3.Geisler WM, James AB. Chlamydial and gonococcal infections in women seeking pregnancy testing at family-planning clinics. Am J Obstet Gynecol. 2008;198(5):502.e1–4. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. STI Treatment Guidelines, 2021. 2021.
- 5.de Voux A, Bernstein KT, Kirkcaldy RD, et al. Self-Reported Extragenital Chlamydia and Gonorrhea Testing in the Past 12 Months Among Men Who Have Sex with Men in the United States-American Men’s Internet Survey, 2017. Sex Transm Dis. 2019;46(9):563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flagg EW, Weinstock HS, Frazier EL, et al. Bacterial Sexually Transmitted Infections Among HIV-Infected Patients in the United States: Estimates From the Medical Monitoring Project. Sex Transm Dis. 2015; 42(4): 171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patton ME, Kidd S, Llata E, et al. Extragenital gonorrhea and chlamydia testing and infection among men who have sex with men--STD Surveillance Network, United States, 2010–2012. Clin Infect Dis. 2014;58(11):1564–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Footman A, Dionne-Odom J, Aaron KJ, et al. Performance of 4 Molecular Assays for Detection of Chlamydia and Gonorrhea in a Sample of Human Immunodeficiency Virus-Positive Men Who Have Sex With Men. Sex Transm Dis. 2020;47(3):158–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abara WE, Llata EL, Schumacher C, et al. Extragenital Gonorrhea and Chlamydia Positivity and the Potential for Missed Extragenital Gonorrhea With Concurrent Urethral Chlamydia Among Men Who Have Sex With Men Attending Sexually Transmitted Disease Clinics-Sexually Transmitted Disease Surveillance Network, 2015–2019. Sex Transm Dis. 2020;47(6):361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rob F, Jůzlová K, Kružicová Z, et al. Prevalence of Chlamydia trachomatis and Neisseria gonorrhoeae co-infections among patients with newly diagnosed syphilis: a single-centre, cross-sectional study. Cent Eur J Public Health. 2019;27(4):285–291. [DOI] [PubMed] [Google Scholar]
- 11.Traeger MW, Cornelisse VJ, Asselin J, et al. Association of HIV Preexposure Prophylaxis With Incidence of Sexually Transmitted Infections Among Individuals at High Risk of HIV Infection. Jama. Apr 9 2019;321(14):1380–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen MS, Council OD, Chen JS. Sexually transmitted infections and HIV in the era of antiretroviral treatment and prevention: the biologic basis for epidemiologic synergy. J Int AIDS Soc. 2019;22 (Suppl 6):e25355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skinner JM, Distefano J, Warrington J, et al. Trends in Reported Syphilis and Gonorrhea among HIV-Infected People in Arizona: Implications for Prevention and Control. Public Health Rep. 2014;129(Suppl 1):85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grome HN, Rebeiro PF, Brantley M, et al. Risk of HIV diagnosis following bacterial sexually transmitted infections in Tennessee, 2013–2017. Sex Transm Dis. 2021, 48(11):873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newman DR, Rahman MM, Brantley A, Peterman TA. Rates of New Human Immunodeficiency Virus (HIV) Diagnoses After Reported Sexually Transmitted Infection in Women in Louisiana, 2000–2015: Implications for HIV Prevention. Clin Infect Dis. 2020;70(6):1115–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones J, Weiss K, Mermin J, et al. Proportion of Incident Human Immunodeficiency Virus Cases Among Men Who Have Sex With Men Attributable to Gonorrhea and Chlamydia: A Modeling Analysis. Sex Transm Dis. 2019;46(6):357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dionne-Odom J, Westfall AO, Van Der Pol B, et al. Sexually Transmitted Infection Prevalence in Women with HIV: Is There a Role for Targeted Screening? Sex Transm Dis. 2018, 45(11):762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farfour E, Dimi S, Chassany O, et al. Trends in asymptomatic STI among HIV-positive MSM and lessons for systematic screening. PLoS One. 2021;16(6):e0250557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watson RJ, Collibee C, Maksut JL, et al. High levels of undiagnosed rectal STIs suggest that screening remains inadequate among Black gay, bisexual and other men who have sex with men. Sex Transm Infect. 2021. Mar 31:sextrans-2020–054563. doi: 10.1136/sextrans-2020-054563. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanford KA, Almirol E, Schneider J, Hazra A. Rising Syphilis Rates During the COVID-19 Pandemic. Sex Transm Dis. 2021;48(6):e81–e83. [DOI] [PubMed] [Google Scholar]
- 21.Tao J, Napoleon SC, Maynard MA, et al. Impact of the COVID-19 Pandemic on Sexually Transmitted Infection Clinic Visits. Sex Transm Dis. 2021;48(1):e5–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pagaoa M, Grey J, Torrone E, et al. Trends in Nationally Notifiable Sexually Transmitted Disease Case Reports During the US COVID-19 Pandemic, January to December 2020. Sex Transm Dis. 2021;48(10):798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]


