Abstract
Survival of bacteria in changing environments depends on their ability to adapt to abiotic stresses. Microorganisms used in food technology face acid stress during fermentation processes. Similarly, probiotic bacteria have to survive acid stress imposed within the stomach in order to reach the intestine and play a beneficial role. Propionibacteria are used both as cheese starters and as probiotics in human alimentation. Adaptation to low pH thus constitutes a limit to their efficacy. Acid stress adaptation in the probiotic SI41 strain of Propionibacterium freudenreichii was therefore investigated. The acid tolerance response (ATR) was evidenced in a chemically defined medium. Transient exposure to pH 5 afforded protection toward acid challenge at pH 2. Protein neosynthesis was shown to be required for optimal ATR, since chloramphenicol reduced the acquired acid tolerance. Important changes in genetic expression were observed with two-dimensional electrophoresis during adaptation. Among the up-regulated polypeptides, a biotin carboxyl carrier protein and enzymes involved in DNA synthesis and repair were identified during the early stress response, while the universal chaperonins GroEL and GroES corresponded to a later response. The beneficial effect of ATR was evident at both the physiological and morphological levels. This study constitutes a first step toward understanding the very efficient ATR described in P. freudenreichii.
Bacteria are periodically exposed to abiotic stresses in a variety of environments. In this context, survival involves sensing changes in environmental parameters such as temperature, pH, or the presence of toxic compounds and adapting quickly in order to exhibit a greater tolerance. Bacteria have evolved a set of inducible responses, including stress proteins, leading to tolerance, which implies the complex regulation of gene expression (41).
Acid stress is of particular importance for bacteria used in food technology. Indeed, a variety of food products are acidified during fermentation by lactic acid bacteria. Probiotic microorganisms, in particular, are usually provided in the form of fermented milk and suffer lactic acid stress. Consequently, probiotics, including Bifidobacterium and Lactobacillus strains, undergo severe mortality during the processing and storage of such products. For this reason, less acidified products such as cheeses were proposed as carriers of these bacteria (8). Probiotics are further challenged by extreme acid stress when reaching the stomach lumen where hydrochloric acid is present. It is thus clear that the ability to efficiently adapt to acid stress is a sine qua non condition for a probiotic microorganism in order to reach the intestine and exert the expected beneficial effects (10).
As for other stresses, a sublethal acidic environment can trigger an adaptive response in bacteria and offer protection toward a subsequent exposure to a lethal acidic pH, a mechanism known as acid tolerance response (ATR). ATR has been well documented for a number of gastrointestinal or food-borne pathogenic bacteria, such as Escherichia coli (36), Salmonella enterica serovar Typhimurium (7), Aeromonas hydrophila (18), Vibrio parahaemolyticus (45), Helicobacter pylori (27), Listeria monocytogenes (4), and Enterococcus faecalis (6), as well as the oral cariogenic organism Streptococcus mutans (12). Indeed adaptation and survival at low pH might be important factors in the pathogenicity of gastrointestinal bacteria and are of great concern in food safety and health. In contrast, less is known about the mechanisms of acid tolerance in beneficial microorganisms used in the dairy industry, except Lactococcus lactis (13) and Lactobacillus acidophilus (21).
Propionibacteria are gram-positive, nonmotile, anaerobic to aerotolerant bacteria producing propionic acid, acetic acid, and carbon dioxide as products of the fermentation of sugars and lactic acid. They are thus used for the industrial fermentative production of propionic acid (30). Dairy propionibacteria, such as Propionibacterium freudenreichii, are traditionally used as starter cultures in cheese technology. They are also considered probiotics due to their ability to inhibit the growth of undesirable flora (23), to stimulate the growth of bifidobacteria (3), and to beneficially modify the enzymatic activities within the gut (25). It is likely that the acid tolerance of dairy propionibacteria contributes to their potentiality as both starters and probiotics.
Acid susceptibility was shown to be highly dependent on the species and strain of propionibacteria studied (34). In a previous report, we described the ability of a P. freudenreichii strain isolated from Swiss-type cheese to develop ATR in a complex medium (17). Here, we investigated ATR mechanisms in a probiotic strain of the same species. Although a chemically defined medium is radically different from conditions encountered in situ, it allows physiological and molecular investigations under reproducible and controllable conditions. We thus developed such a medium for dairy propionibacteria and demonstrated that growth and ATR (this study) were similar to what was observed in rich medium (17). This allowed us to study changes in protein synthesis in order to elucidate the mechanism involved in ATR. Acid-induced polypeptides were determined, their relative rate of synthesis was monitored in a time-dependent manner, and five proteins were identified by N-terminal sequencing. In addition, changes in cell morphology were observed by scanning electron microscopy during acid adaptation and extreme acid challenge.
MATERIALS AND METHODS
Microorganism and growth conditions.
The SI41 strain of P. freudenreichii subsp. shermanii used in this study was kindly provided by Standa Industrie (Caen, France) and is part of the probiotic preparation Propiofidus. This strain was routinely cultured in yeast extract lactate (YEL) medium (24) and stored long-term at −80°C in the same medium complemented with 10% glycerol. However, for this study, we developed a chemically defined medium, MDP, to favor the incorporation of radiolabeled amino acids. MDP medium contained (per liter of distilled water) 12.8 g of sodium lactate, 0.6 g of KH2PO4, 0.4 g of potassium acetate, 50 mg of MgSO4O · 7H2O, 5 mg of MnSO4 · 4H2O, 2.5 mg of FeSO4 · 7H2O, 2.5 mg of CuSO4, 2.5 mg of NaCl, 0.25 mg of cobalt acetate, 15 μg of ZnSO4, 1 μg of H3BO3, 1 μg of Na2MoO4, 50 μg of thiamine, 100 μg of pyridoxal, 50 μg of calcium pantothenate, 50 μg of riboflavin, 100 μg of nicotinamide, 10 μg of p-aminobenzoic acid, 4 μg of biotin, 20 μg of folic acid, and 2 μg of cyanocobalamin. Amino acids were supplied at the following concentrations (per liter): 50 mg of l-Ala, 160 mg of l-Arg, 150 mg of l-Asn, 250 mg of l-Asp, 140 mg of l-Cys, 80 mg of Gly, 190 mg of l-Glu, 150 mg of l-Gln, 100 mg of l-His, 180 mg of l-Ile, 300 mg of l-Leu, 220 mg of l-Lys, 60 mg of dl-Met, 460 mg of l-Pro, 170 mg of l-Phe, 180 mg of l-Ser, 150 mg of l-Thr, 50 mg of l-Trp, 60 mg of l-Tyr, and 480 mg of dl-Val. Five milligrams each of the bases adenine, guanine, uracil, and xanthine were also added. The pH of the medium was adjusted to 7.0 with NaOH prior to filter sterilization (0.45-μm pore diameter; Millipore, Bedford, Mass.). Growth was carried out at 30°C without shaking and monitored spectrophotometrically at 650 nm as well as by CFU counting.
Acid adaptation and extreme acid challenge.
Log-phase cells were obtained as follows. A starter culture (10 ml in MDP medium) was diluted 1,000-fold in fresh MDP medium. During exponential growth, this preculture was again diluted 1,000-fold in 100 ml of fresh medium. When the culture reached a cell density of 5 × 108 cells per ml (optical density at 650 nm [OD650] = 0.5), bacteria were harvested by centrifugation (6,000 × g 30°C, 5 min). For acid adaptation, cells were recovered in an equal volume of MDP medium adjusted to pH 5.0 and incubated at 30°C for 60 min.
Adapted and nonadapted cells were harvested by centrifugation and recovered in an equal volume of MDP medium at pH 2.0. Viable-cell counts were determined after 30 min of acid challenge. Samples were diluted in peptone water (0.1% peptic digest of meat; Biokar Diagnostics, Beauvais, France), pH 7.0, containing 0.9% NaCl and poured into YEL medium containing 1.5% agar for maximal recovery. The number of CFU was determined after 6 days of anaerobic incubation at 30°C.
Chloramphenicol treatment.
The MIC of chloramphenicol was 4 μg/ml, and chloramphenicol was used at a concentration of 40 μg/ml where bacteriostasis occurred and where no lethal effect was observed in P. freudenreichii. It was added to the culture 60 min before adaptation and during adaptation at pH 5.0 and acid challenge at pH 2.0.
Radioactive labeling and whole-cell protein extraction.
Log-phase cells grown in MDP medium were labeled essentially as described by Flahaut et al. (6). Bacteria were harvested by centrifugation (6,000 × g, 30°C, 5 min) and recovered in an equal volume of MDP medium devoid of cysteine and methionine, at either pH 7.0 (control cells) or pH 5.0 (adapted cells). A 1-ml subsample of bacterial suspension was labeled with 500 μCi of [35S]methionine/cysteine protein labeling mix (ICN Pharmaceuticals, Orsay, France) for 30 min at 30°C. Incorporation was stopped by rapidly washing the bacteria in 1 ml of stop solution (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM methionine, 1 mM cysteine, 1 mg of chloramphenicol per ml, 0.4 mM phenylmethylsulfonyl fluoride [PMSF]). Washed cells were then recovered in protoplastization solution (25 mM Tris-HCl [pH 7.5], 0.5 M sucrose, 5 mg of lysozyme per ml, 1 mg of chloramphenicol per ml, 0.4 mM PMSF) and incubated for 5 min at 37°C prior to centrifugation (6,000 × g, 30°C, 5 min). The cell pellet was recovered in 200 μl of lysis solution consisting of 50 mM Tris-HCl [pH 7.5], 0.3% sodium dodecyl sulfate (SDS), 200 mM dithiothreitol (DTT), and 0.4 mM PMSF and immediately sonicated on ice with a Vibra Cell sonicator (Bioblock Scientific, Illkirch, France) equipped with a tapered microtip (three bursts of 1 min at 1-min intervals, output of 2.5). The lysate was brought to 95°C for 10 min to improve protein solubilization, and insoluble materials were removed by centrifugation (10 000 × g, 10 min, room temperature). Four volumes of ice-cold acetone was added, and proteins were precipitated for 30 min on ice prior to centrifugation (12 000 × g, 10 min, 4°C) and then air dried.
Two-dimensional electrophoresis.
Preliminary experiments performed with pH 3 to 10 gradients have shown that the majority of P. freudenreichii polypeptides were focused in the pH 4 to 7 range. For this reason, pH 4 to 7 Dry Strips (Amersham Pharmacia Biotech, Uppsala, Sweden) were used in this study. Air-dried protein pellets were solubilized in sample solution containing 7 M urea, 2 M thiourea, 25 mM DTT, 4% 3-[(3-cholamidopropyl)dimethylammonio]-1-propane-sulfonate (CHAPS), and 2% IPG-Buffer (Amersham Pharmacia Biotech). The surfactant CHAPS and the chaotropic agent thiourea were used throughout the isoeletric focusing to improve protein solubility and transfer to the second dimension (33). Equal amounts of radioactivity (106 dpm) were loaded onto the gel in the first dimension. Isoelectric focusing was carried out as described by Görg et al. (11) with Immobiline Dry Strips on a Multiphor II electrophoresis system (Amersham Pharmacia Biotech). The strips were equilibrated in the presence of DTT and then iodoacetamide before being placed on the top of the second-dimension gel. SDS-polyacrylamide gel electrophoresis (PAGE) (12.5% polyacrylamide) was performed according to the method of Laemmli (19) in slab (16 by 16 by 0.1 cm) gels (Protean II; Bio-Rad, Hercules, Calif.). For analysis of small proteins, the second dimension of some two-dimensional gels was performed by discontinuous SDS-PAGE in Tris-Tricine buffer according to the method of Schägger and von Jagow (38). This electrophoretic system consisted of a separating gel, a spacer gel, and a stacking gel at concentrations of 16, 10, and 4%, respectively. The radioactivity in the dried gels was detected with a Storm Phosphorimager (Amersham Pharmacia Biotech). Image analysis, gel matching, and quantification of the radioactivity in individual spots were performed with the Melanie II software (Bio-Rad). Molecular masses were calibrated by comigration of low-molecular-mass markers (94.0, 67.0, 43.0, 30.0, 20.1, and 14.4 kDa) and peptide markers (16,949, 14,404, 10,700, 8,159, 6,214, and 2,512 Da) with bacterial proteins. Similarly, isoelectric points were calibrated with the broad pI kit (isoelectric points 4.55, 5.20, 5.85, 6.55, and 6.85 were resolved on such gels). All markers were obtained from Amersham Pharmacia Biotech. Relative rates of synthesis were determined by calculating the ratio of radioactivity in a spot to radioactivity in the entire gel. Results are the means of determinations from at least three independent experiments, with a standard deviation of less than 15%.
N-terminal amino acid sequence determination.
Cells from a 200-ml culture in MDP medium were lysed and proteins were extracted with SDS as described above. Protein content in the extract was determined according to the Lowry method (22) by using bovine serum albumin as a standard. Aliquots of 200 mg of total proteins were acetone precipitated and separated by two-dimensional electrophoresis. The resulting two-dimensional gels were transferred to polyvinylidene difluoride (PVDF) membranes (Hyperbond; Beckman Instruments, Inc., Fullerton, Calif.) by immersed electroblotting in 10 mM 3-[cyclohexylamino]-1-propanesulfonic acid (CAPS) according to the method of Matsudaira (26). Proteins were visualized by staining with Coomassie blue R-250 by the method of Pryor et al. (32). Spots were cut from the membrane and applied to an automatic Beckman/Porton LF3000 protein sequencer (Beckman Instruments) as described by the manufacturer. Sequence homologies were searched with the FASTA program (31).
Electron microscopy.
P. freudenreichii cells were laid by gentle filtration onto 0.2-μm-pore-diameter membranes (Isopore membrane filters; Millipore). Membranes were fixed for 48 h with 2% (wt/vol) glutaraldehyde in 0.1 M sodium cacodylate buffer [pH 6.8] and rinsed in the same buffer. Samples were dehydrated with ethanol (10, 25, 50, 75, 95, and finally 100%), critical point dried by the CO2 method, and coated with gold. Cells were examined and photographed with a Philips XL 20 scanning electron microscope operating at 10 kV.
RESULTS
ATR of P. freudenreichii SI 41 in MDP medium.
Propionibacteria are polyauxotrophic bacteria routinely cultivated on complex medium supplemented with yeast extract, such as YEL medium. For a better understanding of stress adaptation, we developed the MDP defined medium and compared it with YEL medium. In this medium, the SI41 strain displayed growth characteristics slightly different from those observed in the complex one. The generation time was 6 h, and the final OD was 2, corresponding to a cell density of 5.108 CFU/ml, while the generation time was 5 h, and the final OD was 2.5, corresponding to 109 CFU/ml in YEL medium. Therefore, MDP medium fulfilled the nutrient requirements of P. freudenreichii and was used in these experiments.
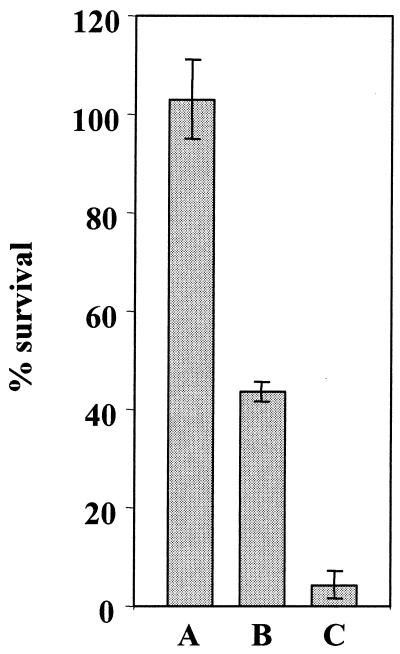
ATR was then explored for the probiotic SI41 strain grown in this medium. Cells were challenged during exponential growth in MDP medium. (The pH of such cultures was typically between 6.5 and 7.0.) They underwent severe mortality when shifted to pH 2.0 (Fig. 1). Indeed, only 4.3% of viable propionibacteria were detected after 30 min of this challenge. In contrast, when identical cultures were exposed to a sublethal pH of 5.0 for 60 min prior to challenge, they survived at pH 2.0 without significant loss of viability. Furthermore, the same protective effect of acid pretreatment was observed when SI41 was grown in rich YEL medium (data not shown). This is clearly evidence of an ATR for P. freudenreichii SI 41 grown in both media.
FIG. 1.
ATR in P. freudenreichii SI41 and effect of protein synthesis inhibition. Log-phase cells were harvested and submitted to acid adaptation at pH 5 with (B) or without (A) chloramphenicol treatment. Viability was then determined after 30 min of a subsequent extreme acid challenge at pH 2. As a control, nonadapted cells were subjected to the same challenge (C). Results are expressed as the percentage of viable cells at the end of the challenge. Error bars represent the standard deviation for triplicate experiments.
To determine the requirement for neosynthesis of specific protective proteins in P. freudenreichii SI 41 ATR, cells were treated with the protein synthesis inhibitor chloramphenicol before and during adaptation at pH 5.0. In this case, a notable mortality was observed during subsequent acid challenge at pH 2.0. Indeed, 43% of the adapted cells survived the extreme acid challenge, while 100% survived after adaptation in the absence of antibiotic. However, these cells displayed a reduced tolerance toward acid stress, and survival was still higher than that of nonadapted bacteria. Thus, inhibition of protein neosynthesis prevented ATR, at least partly, suggesting a role of acid-induced stress proteins in acid adaptation.
Morphological changes of P. freudenreichii upon exposure to acid stress.
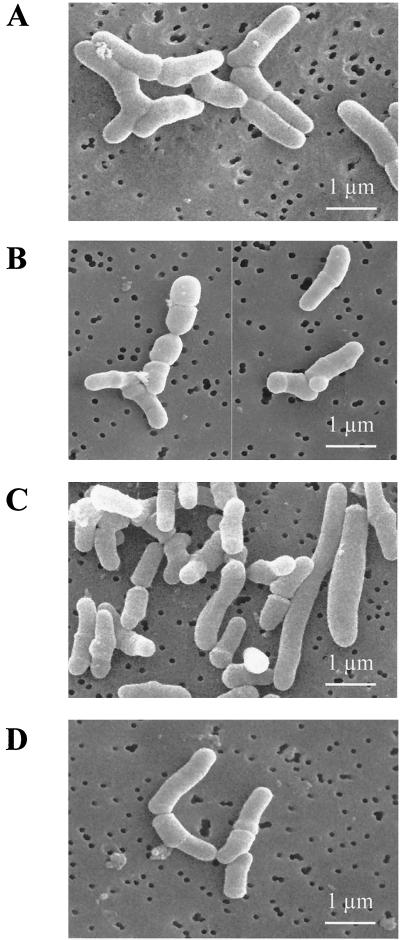
Scanning electron micrographs revealed the characteristic morphology of dairy propionibacteria for cells grown at pH 7.0: pleomorphic rods arranged in “Chinese characters” were observed, and the average length of individual cells (calculated from at least 100 measurements for each observation) was 1.22 μm (Fig. 2A). In contrast, cells incubated at pH 2.0 showed dramatic changes in morphology. The rod shape was lost, and bacteria showed a segmented aspect in which individual segments were shorter (0.48 μm on average). In addition, bleb-like structures on the surface of a significant number of bacterial cells were observed (Fig. 2B). Less morphological disturbances were observed upon exposure to pH 5.0. The rod shape was generally preserved, and deformations of the cell surface were almost absent (Fig. 2C). In addition, an elongation of certain cells was observed, and the average size of bacteria was then 1.57 μm. Furthermore, when challenged at pH 2.0, these bacteria adapted at pH 5.0 did not present modifications comparable to those undergone by nonadapted bacteria (Fig. 2D). This illustrates, at the morphological level, the protective effect of ATR.
FIG. 2.
Analysis of morphological changes of P. freudenreichii SI41 during acid stress. Nontreated cells (A), cells undergoing extreme acid challenge at pH 2 (B), and pH 5 acid-adapted cells (C) were analyzed by scanning electron microscopy. For comparison, cells subjected to acid adaptation (pH 5) and then to extreme acid challenge (pH 2) were also analyzed (D).
Changes in protein synthesis during ATR.
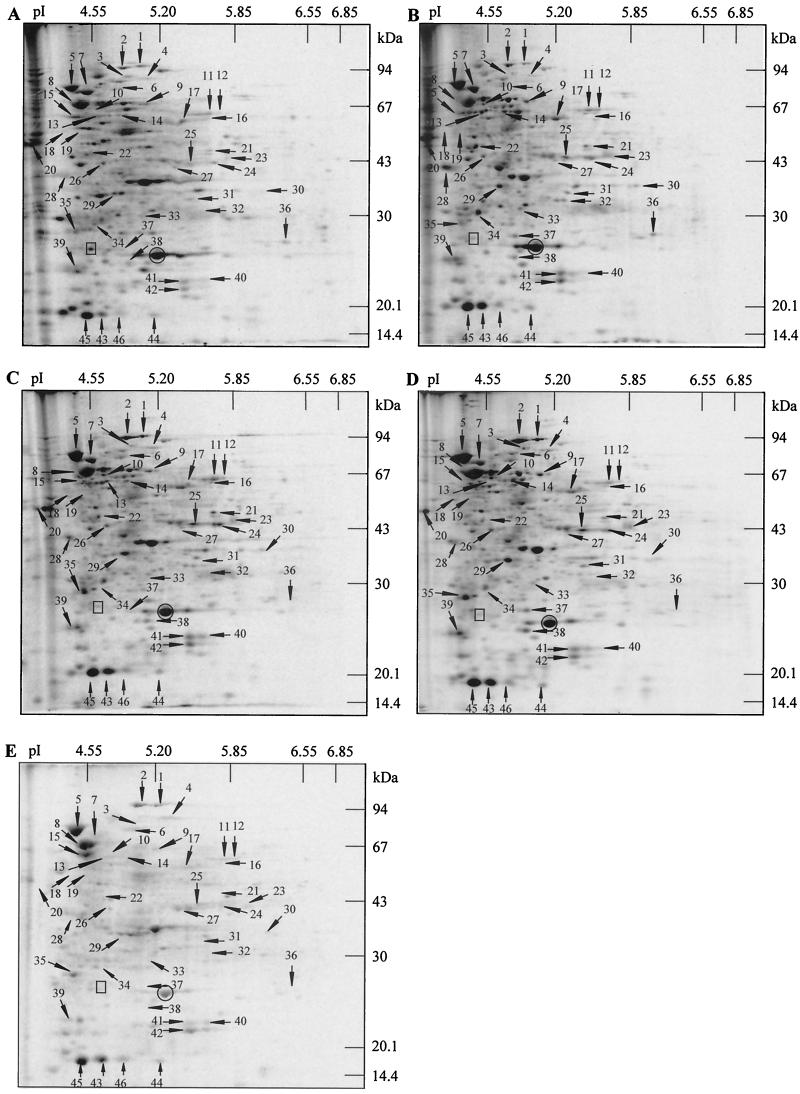
The requirements of ATR-specific polypeptides for maximal protection having been shown, we studied the rate of protein synthesis during acid adaptation. After pulse-labeling, we analyzed whole-cell protein extracts of the SI41 strain by two-dimensional electrophoresis. Electrophoregrams of control cells growing at pH 7.0 and cells undergoing acid adaptation were compared (Fig. 3). Pulse-labeling was performed during four successive 15-min periods during 60 min of acid adaptation.
FIG. 3.
Two-dimensional analysis of protein expression during acid adaptation in P. freudenreichii SI41. Protein synthesis was monitored during 60 min of pH 5 adaptation. Cells were pulse-labeled between 0 and 15 min (B), 15 and 30 min (C), 30 and 45 min (D), or 45 and 60 min (E) of adaptation. As a control, nontreated cells were labeled at pH 7 for 15 min (A). Whole-cell protein extracts were analyzed by two-dimensional electrophoresis followed by autoradiography. The arrows indicate polypeptides displaying an increased relative rate of synthesis during acid adaptation compared with that of nonadapted cells. Two polypeptides displaying, respectively, an unchanged (○) and a reduced (□) relative rate of synthesis are also shown.
On autoradiograms of control gels, the synthesis of 433 discrete cellular proteins was detected, in a molecular mass range of 14 to 130 kDa and a pI range of 4 to 7 (Fig. 3A). During the first 15 min of acid adaptation, 17 protein synthesis inductions were detected (out of the 46 induced polypeptides observed on these gels). This is the case with the acid stress proteins (ASPs) 7, 14, 34, and 45 (Fig. 3B). Among these early inductions, some were maintained throughout the experiment (see ASP 45 in Fig. 3 and ASP 47 in Fig. 4 and 5), while others (ASPs 14 and 22) returned to their initial level (Fig. 3C, 3D and 5). Certain stress proteins, such as ASP 8, were induced only at the end of the adaptation (Fig. 3D and 5), while others were only transiently overexpressed (ASPs 11, 35, and 39). The most important changes concerned the ASPs 1, 2, and 25, which showed maximal induction factors of 4.8, 3.9, and 3.8, respectively. The induction factors of the other acid-induced polypeptides were weaker: between 1.5 and 3.5. Finally, keeping the bacteria at pH 5.0 for up to 60 min led to a shutdown of synthesis of the majority of the polypeptides expressed in growing cells. Indeed, only 303 polypeptides were labeled between 45 and 60 min of treatment, and most of them were almost undetectable on the autoradiograms (Fig. 3E). In contrast, some ASPs were still expressed with a consequently high relative rate of synthesis (ASPs 5, 8, and 15).
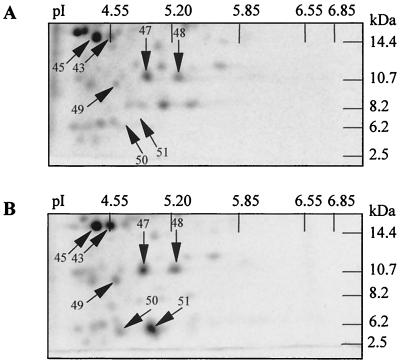
FIG. 4.
Two-dimensional analysis of expression of small polypeptides during acid adaptation in P. freudenreichii SI41. Protein extracts were prepared as described in the legend to Fig. 3, and two-dimensional electrophoresis was performed with discontinuous Tris-Tricine gels in the second dimension. The arrows indicate polypeptides displaying an increased rate of synthesis during acid adaptation (B) compared with that of nonadapted cells (A).
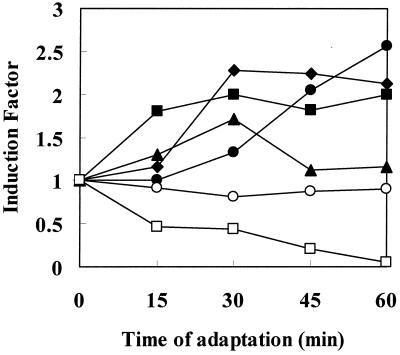
FIG. 5.
Variations in induction factors during acid adaptation. For each acid-induced polypeptide, the induction factor (defined as the ratio between relative rates of synthesis in adapted and control cells) was determined at 15, 30, 45, and 60 min of adaptation. Results are shown for ASPs 5 (♦), 8 (●), 11 (▴), and 47 (▪) as well as for the unchanged (○) and repressed (□) polypeptides indicated in Fig. 3.
Stress-induced polypeptides with molecular masses below 14 kDa have been described previously. We thus looked for acid-induced proteins by using discontinuous SDS-PAGE as a second dimension. As seen in Fig. 4, these gels were able to efficiently separate polypeptides in the 2.5- to 14.4-kDa range. Five additional ASPs were detected on these gels (ASPs 47 to 51). ASPs 50 and 51, in particular, are not detected on control gels and correspond to neosynthesized proteins in response to moderate acid stress.
The induction factors varied in a time-dependent manner, as shown in Fig. 5. This provides further evidence of the early, transient, late, or permanent nature of acid-induced proteins. As shown on the electrophoregrams, most of the cellular proteins were repressed, while some remained unchanged (two examples are illustrated in Fig. 3 and 5).
Analysis of ASPs.
From the observed ASPs, five belonged to the different classes of induction rate and were obviously detectable on Coomassie blue-stained gels. They were then subjected to N-terminal sequencing. Databases were screened and revealed homologies to known proteins. Results of five identifications are shown in Table 1.
TABLE 1.
Sequenced P. freudenreichii acid shock proteins
|
P. freudenreichii ASP characteristic
|
Most closely matching protein characteristic
|
||||||
|---|---|---|---|---|---|---|---|
| ASP no. | N-terminal sequence | pIa | Molecular mass (kDa)a | Protein | Microorganism | % Identity | Accession no. in databaseb |
| 8 | AKEIEFDEE | 4.5 | 67 | GroEL | B. subtilis | 78 | owl/D1092 |
| 49 | ATDIKXLEDRVL | 4.6 | 11 | GroES | M. bovis | 60 | sw/P15020 |
| 5 | QIPEPISLXXDS | 4.4 | 79 | RecR | B. subtilis | 67 | owl/P24277 |
| 11 | NERDSNER | 5.4 | 64 | RepB | L. lactis | 88 | owl/U90222 |
| 47 | MKLKVTV | 4.9 | 12 | BCCP | P. freudenreichii | 100 | owl/P02904 |
Determined from two-dimensional gels calibrated with Amersham Pharmacia Biotech molecular mass and isoelectric point calibration kits and Bio-Rad Melanie II software.
owl, OWL nonredundant library; sw, SwissProt library (searched by using the FASTA program).
ASP 8 was shown to be homologous to the protein chaperone GroEL from Bacillus subtilis (42), but also displayed homologies to 60-kDa chaperonins from various bacterial species. Similarly, ASP 49 showed homologies with the GroES chaperonin from Mycobacterium bovis (46) and with a series of 10-kDa chaperonins of both bacterial and eucaryotic origins. Moreover, ASPs 8 and 49 displayed molecular masses (67 and 11 kDa, respectively) and isolelectric points (4.5 and 4.6, respectively) in agreement with those reported for the GroE system of B. subtilis (39) and E. coli (43).
ASP5 revealed homologies to the RecR protein involved in DNA repair and genetic recombination in B. subtilis (1), and ASP 11 was homologous to an L. lactis plasmid-encoded replication protein, RepB (40).
ASP 47 showed 100% identity with biotin carboxyl carrier protein (BCCP), a biotin containing a 1.3S carboxyl carrier subunit contained in P. freudenreichii transcarboxylase (28).
DISCUSSION
In P. freudenreichii, we demonstrated a very efficient ATR, in both rich and chemically defined media. The ATR affords protection toward a pH as low as 2.0 without mortality, in contrast to nonadapted cells. To better understand the mechanisms underlying this phenomenon, in this work, we evaluated changes in morphology and protein synthesis during development of ATR.
P. freudenreichii cells subjected to extreme acid environments were shown to undergo dramatic changes in morphology, while viability decreased. In contrast, cell integrity was preserved in adapted bacteria, while viability was not affected. This indicates that bacteria preexposed to a sublethal environment can adapt and retain a normal shape during the challenge. Stress-induced morphological changes in nonsporulating bacteria were previously described. Shrinkage of E. coli cells, resulting from the induction of the bolA morphogene belonging to the rpoS regulon, is triggered by entry into stationary phase and a variety of stresses, including low pH (37). Similarly, modifications of the cell morphology in the gram-positive organism E. faecalis are caused by stress-induced modifications of the transcription of many genes (9).
ATR has been reported to be linked to a concomitant modification of protein synthesis. However, inhibition of ASP synthesis did not abolish ATR in all the studies. Protein neo-synthesis inhibition by chloramphenicol reversed ATR in L. monocytogenes (4), in A. hydrophila (18), and in the enterobacteria S. enterica serovar Typhimurium and E. coli (7, 36). In contrast, it showed no effect on acid adaptation in L. lactis subsp. lactis (13) and in E. faecalis (5). In L. acidophilus, blocking of protein synthesis reduced ATR during adaptation at pH 5, but had no effect on ATR developed at pH 4.2 (21). In P. freudenreichii, we showed that chloramphenicol reduced ATR, suggesting an important role of ASPs. However, this reduction was not complete, suggesting that both an inducible preexisting system and a mechanism dependent on de novo protein synthesis coexist in this Propionibacterium and that they together afford maximal protection against extreme acid stress, as suggested for L. acidophilus (21).
Analysis of ASPs revealed that general stress response proteins, indicative of damage to macromolecules, were induced during ATR in P. freudenreichii. Indeed, ASPs 8 and 49 showed homologies with the highly conserved chaperonins GroEL and GroES. These heat shock proteins are also induced by acid stress in E. coli (16), L. lactis (13), and Lactobacillus delbrueckii subsp. bulgaricus (20). The similar levels and kinetics of induction for these chaperonins are consistent with the previous report that they belong to the same operon, under the control of a regulatory agent, CIRCE (controlling inverted repeat for chaperone expression), in B. subtilis (14). ASPs 5 and 11 showed homologies with proteins involved in DNA synthesis or repair (RecR and RepB). Such proteins, which belong to the SOS regulon, have been shown to be induced by mutagenic agents, starvation, and entry into stationary phase (44). Indeed, most stresses that cause growth arrest may be responsible for oxidative stress, at least in E. coli (29).
ASP 47 was unambiguously identified as P. freudenreichii BCCP (28). This 12-kDa polypeptide is part of a multimeric (30 subunits) transcarboxylase responsible for decarboxylation of methylmalonyl-coenzyme A and carboxylation of pyruvate, a metabolic reaction specific to propionibacteria (15). It is noteworthy that this carboxyl carrier protein is labile under acidic conditions (28). Glutamate, lysine, and arginine decarboxylases are key actors of the pH homeostasis in enterobacteria (2). Because glutamate is by far the most abundant intracellular free amino acid in P. freudenreichii (35) and BCCP is a very abundant polypeptide, such a crucial role of a biotin-containing carboxyl carrier protein in the P. freudenreichii adaptative response to acidic environments must be investigated.
In conclusion, a chemically defined medium was developed and the two-dimensional electrophoresis method was adapted to monitor protein synthesis in P. freudenreichii. This allowed a kinetic study of both general and specific stress proteins during acid adaptation in this bacterium to be carried out. The present work constitutes a first insight into the mechanisms leading to efficient acid tolerance in dairy propionibacteria. Moreover, it will allow a proteomic study of other stresses, which should lead to the characterization of stress regulons in P. freudenreichii.
ACKNOWLEDGMENTS
We thank A. Rouault for expert technical assistance, M. Gautier for interest in this work, V. Rioux for help with protein blotting, and Y. Auffray and J. C. Giard for critical reading of the manuscript.
REFERENCES
- 1.Alonso J C, Shirahige K, Ogasawara N. Molecular cloning, genetic characterization, and DNA sequence analysis of the recM region of Bacillus subtilis. Nucleic Acids Res. 1990;18:6771–6777. doi: 10.1093/nar/18.23.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bearson S, Bearson B, Foster J W. Acid stress responses in enterobacteria. FEMS Microbiol Lett. 1997;147:173–180. doi: 10.1111/j.1574-6968.1997.tb10238.x. [DOI] [PubMed] [Google Scholar]
- 3.Bougle D, Roland N, Lebeurrier F, Arhan P. Effect of propionibacteria supplementation on fecal bifidobacteria and segmental colonic transit time in healthy human subjects. Scand J Gastroenterol. 1999;34:144–148. doi: 10.1080/00365529950172998. [DOI] [PubMed] [Google Scholar]
- 4.Davis J M, Coote P J, O'Byrne C P. Acid tolerance in Listeria monocytogenes: the adaptive acid tolerance response (ATR) and growth-phase-dependent acid resistance. Microbiology. 1996;142:2975–2982. doi: 10.1099/13500872-142-10-2975. [DOI] [PubMed] [Google Scholar]
- 5.Flahaut S. Incidences physiologiques et biochimiques de différents stress chez Enterococcus faecalis ATCC 19433. Ph.D. thesis. Caen, France: University of Caen; 1996. [Google Scholar]
- 6.Flahaut S, Hartke A, Giard J C, Benachour A, Boutibonnes P, Auffray Y. Relationship between stress response toward bile salts, acid and heat treatment in Enterococcus faecalis. FEMS Microbiol Lett. 1996;138:49–54. doi: 10.1111/j.1574-6968.1996.tb08133.x. [DOI] [PubMed] [Google Scholar]
- 7.Foster J W. The acid tolerance response of Salmonella typhimurium involves transient synthesis of key acid shock proteins. J Bacteriol. 1993;175:1981–1987. doi: 10.1128/jb.175.7.1981-1987.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gardiner G, Ross R P, Collins J K, Fitzgerald G, Stanton C. Development of a probiotic cheddar cheese containing human-derived Lactobacillus paracasei strains. Appl Environ Microbiol. 1998;64:2192–2199. doi: 10.1128/aem.64.6.2192-2199.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giard J C, Rince A, Capiaux H, Auffray Y, Hartke A. Inactivation of the stress- and starvation-inducible gls24 operon has a pleiotrophic effect on cell morphology, stress sensitivity, and gene expression in Enterococcus faecalis. J Bacteriol. 2000;182:4512–4520. doi: 10.1128/jb.182.16.4512-4520.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldin B R, Gorbach S L. Probiotics for humans. In: Fuller R, editor. Probiotics, the scientific basis. London, United Kingdom: Chapman & Hall; 1992. pp. 355–376. [Google Scholar]
- 11.Görg A, Postel W, Gunther S. The current state of two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis. 1988;9:531–546. doi: 10.1002/elps.1150090913. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton I R, Buckley N D. Adaptation by Streptococcus mutans to acid tolerance. Oral Microbiol Immunol. 1991;6:65–71. doi: 10.1111/j.1399-302x.1991.tb00453.x. [DOI] [PubMed] [Google Scholar]
- 13.Hartke A, Bouché S, Giard J C, Benachour A, Boutibonnes P, Auffray Y. The lactic acid stress response of Lactococcus lactis subsp. lactis. Curr Microbiol. 1996;33:194–199. doi: 10.1007/s002849900099. [DOI] [PubMed] [Google Scholar]
- 14.Hecker M, Schumann W, Volker U. Heat-shock and general stress response in Bacillus subtilis. Mol Microbiol. 1996;19:417–428. doi: 10.1046/j.1365-2958.1996.396932.x. [DOI] [PubMed] [Google Scholar]
- 15.Hettinga D H, Reinbold G W. The propionic acid bacteria—a review. II Metabolism J Milk Food Technol. 1972;35:358–372. [Google Scholar]
- 16.Heyde M, Portalier R. Acid shock proteins of Escherichia coli. FEMS Microbiol Lett. 1990;57:19–26. doi: 10.1016/0378-1097(90)90406-g. [DOI] [PubMed] [Google Scholar]
- 17.Jan G, Rouault A, Maubois J L. Acid stress susceptibility and acid adaptation of Propionibacterium freudenreichii subsp. shermanii. Lait. 2000;80:325–336. [Google Scholar]
- 18.Karem K L, Foster J W, Bej A K. Adaptive acid tolerance response (ATR) in Aeromonas hydrophila. Microbiology. 1994;140:1731–1736. doi: 10.1099/13500872-140-7-1731. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Lim E M, Ehrlich S D, Maguin E. Identification of stress-inducible proteins in Lactobacillus delbrueckii subsp. bulgaricus. Electrophoresis. 2000;21:2557–2561. doi: 10.1002/1522-2683(20000701)21:12<2557::AID-ELPS2557>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 21.Lorca G L, Raya R R, Taranto M P, De Valdez G F. Adaptative acid tolerance response in Lactobacillus acidophilus. Biotechnol Lett. 1998;20:239–241. [Google Scholar]
- 22.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 23.Lyon W J, Sethi J K, Glatz B A. Inhibition of psychrotrophic organisms by propionicin PLG-1, a bacteriocin produced by Propionibacterium thoenii. J Dairy Sci. 1993;76:1506–1513. doi: 10.3168/jds.S0022-0302(93)77482-2. [DOI] [PubMed] [Google Scholar]
- 24.Malik A C, Reinbold G W, Vedamuthu E R. An evaluation of the taxonomy of Propionibacterium. Can J Microbiol. 1968;14:1185–1191. doi: 10.1139/m68-199. [DOI] [PubMed] [Google Scholar]
- 25.Mantere-Alhonene S. Propionibacteria used as probiotics—a review. Lait. 1995;75:447–452. [Google Scholar]
- 26.Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- 27.Mooney C, Munster D J, Bagshaw P F, Allardyce R A. Helicobacter pylori acid resistance. Lancet. 1990;335:1232. doi: 10.1016/0140-6736(90)92764-9. [DOI] [PubMed] [Google Scholar]
- 28.Murtif V L, Bahler C R, Samols D. Cloning and expression of the 1.3S biotin-containing subunit of transcarboxylase. Proc Natl Acad Sci USA. 1985;82:5617–5621. doi: 10.1073/pnas.82.17.5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nystrom T. Starvation, cessation of growth and bacterial aging. Curr Opin Microbiol. 1999;2:214–219. doi: 10.1016/S1369-5274(99)80037-X. [DOI] [PubMed] [Google Scholar]
- 30.Ozadali F, Glatz B A, Glatz C E. Fed-batch fermentation with and without on-line extraction for propionic and acetic acid production by Propionibacterium acidipropionici. Appl Microbiol Biotechnol. 1996;44:710–716. doi: 10.1007/BF00178607. [DOI] [PubMed] [Google Scholar]
- 31.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pryor J L, Xu W, Hamilton D W. Immunodetection after complete destaining of Coomassie blue-stained proteins on Immobilon-PVDF. Anal Biochem. 1992;202:100–104. doi: 10.1016/0003-2697(92)90213-q. [DOI] [PubMed] [Google Scholar]
- 33.Rabilloud T. Use of thiourea to increase the solubility of membrane proteins in two-dimensional electrophoresis. Electrophoresis. 1998;19:758–760. doi: 10.1002/elps.1150190526. [DOI] [PubMed] [Google Scholar]
- 34.Rehberger J L, Glatz B A. Response of cultures of Propionibacterium to acid and low pH: tolerance and inhibition. J Food Prot. 1998;61:211–216. doi: 10.4315/0362-028x-61.2.211. [DOI] [PubMed] [Google Scholar]
- 35.Rolin D B, Girard F, de Certaines J D, Boyaval P. 13C-NMR study of lactate metabolism in Propionibacterium freudenreichii subsp. shermanii. Appl Microbiol Biotechnol. 1995;44:210–217. [Google Scholar]
- 36.Rowbury R J, Goodson M. PhoE porin of Escherichia coli and phosphate reversal of acid damage and killing and of acid induction of the CadA gene product. J Appl Bacteriol. 1993;74:652–661. doi: 10.1111/j.1365-2672.1993.tb05199.x. [DOI] [PubMed] [Google Scholar]
- 37.Santos J M, Freire P, Vicente M, Arraiano C M. The stationary-phase morphogene bolA from Escherichia coli is induced by stress during early stages of growth. Mol Microbiol. 1999;32:789–798. doi: 10.1046/j.1365-2958.1999.01397.x. [DOI] [PubMed] [Google Scholar]
- 38.Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt A, Schiesswohl M, Völker U, Hecker M, Schumann W. Cloning, sequencing, mapping, and transcriptional analysis of the groESL operon from Bacillus subtilis. J Bacteriol. 1992;174:3993–3999. doi: 10.1128/jb.174.12.3993-3999.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schouler C, Clier F, Lerayer A L, Ehrlich S D, Chopin M-C. A type IC restriction-modification system in Lactococcus lactis. J Bacteriol. 1998;180:407–411. doi: 10.1128/jb.180.2.407-411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Segal G, Ron E Z. Regulation of heat-shock response in bacteria. Ann N Y Acad Sci. 1998;851:147–151. doi: 10.1111/j.1749-6632.1998.tb08988.x. [DOI] [PubMed] [Google Scholar]
- 42.Tozawa Y, Yoshikawa H, Kawamura F, Itaya M, Takahashi H. Isolation and characterization of the groES and groEL genes of Bacillus subtilis Marburg. Biosci Biotechnol Biochem. 1992;56:1995–2002. doi: 10.1271/bbb.56.1995. [DOI] [PubMed] [Google Scholar]
- 43.VanBogelen R A, Sankar P, Clark R L, Bogan J A, Neidhardt F C. The gene-protein database of Escherichia coli: edition 5. Electrophoresis. 1992;13:1014–1054. doi: 10.1002/elps.11501301203. [DOI] [PubMed] [Google Scholar]
- 44.Villarroya M, Perez-Roger I, Macian F, Armengod M E. Stationary phase induction of dnaN and recF, two genes of Escherichia coli involved in DNA replication and repair. EMBO J. 1998;17:1829–1837. doi: 10.1093/emboj/17.6.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong H-C, Peng P-Y, Han J-M, Chang C-Y, Lan S-L. Effect of mild acid treatment on the survival, enteropathogenicity, and protein production in Vibrio parahaemolyticus. Infect Immun. 1998;66:3066–3071. doi: 10.1128/iai.66.7.3066-3071.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamaguchi R, Matsuo K, Yamazaki A, Nagai S, Terasaka K, Yamada T. Immunogenic protein MPB57 from Mycobacterium bovis BCG: molecular cloning, nucleotide sequence and expression. FEBS Lett. 1988;240:115–117. doi: 10.1016/0014-5793(88)80350-8. [DOI] [PubMed] [Google Scholar]