Abstract
Background:
Extracellular cold-inducible RNA-binding protein (eCIRP) aggravates acute kidney injury (AKI) after renal ischemia/reperfusion (RIR). Although eCIRP activates triggering receptor expressed on myeloid cells (TREM-1), how this receptor and its antagonism with a novel peptide, M3, affect AKI is poorly understood. We therefore hypothesize that inhibiting the eCIRP/TREM-1 pathway with M3 attenuates AKI.
Methods:
Wild-type (WT) and TREM-1−/− mice were subjected to bilateral 30-minute renal hilum clamping followed by reperfusion or sham. After 4 hours, WT mice received M3 (10 mg/kg BW) or normal saline intraperitoneally. After 24 h, renal tissue and serum was collected for analysis. Additionally, WT mice were subjected to bilateral renal ischemia for 34 minutes and treated with M3 at 10 mg/kg BW or vehicle at the time of reperfusion. Survival was monitored for 10 days.
Results:
After RIR, TREM-1 mRNA expression increased by 9-fold in WT mice compared to sham. WT mice also demonstrated significant increases in serum BUN, creatinine, and IL-6 and renal tissue levels of IL-6 and NGAL after RIR compared to sham. TREM-1−/− mice demonstrated significant reductions in serum BUN, creatinine, and IL-6 compared to WT after RIR. Levels of renal IL-6 and NGAL were also significantly decreased in the kidneys of TREM-1−/− mice. Furthermore, treatment with M3 in WT mice significantly decreased serum and renal levels of IL-6 after RIR. M3 treatment demonstrated significant reductions in renal mRNA and protein levels of NGAL, serum BUN and creatinine, and histologic structural damage as well as apoptosis. Treatment with M3 also increased the survival rate from 35% to 65% in AKI mice.
Conclusions:
TREM-1 mediates the deleterious effects of eCIRP in AKI after RIR. The novel eCIRP/TREM-1 pathway antagonist, M3, attenuates AKI and has the potential to be developed as a therapeutic agent for AKI.
Keywords: Acute Kidney Injury, Renal Ischemia/Reperfusion Injury, Inflammation, TREM-1, eCIRP, DAMP
Two Sentence Summary
Following renal ischemia/reperfusion injury, the severity of acute kidney injury is attenuated by genetic deletion of TREM-1 and by M3, an inhibitory peptide of the eCIRP/TREM-1 interaction. The importance of these findings is that TREM-1 is a novel target of inhibition in the development of treatments for acute kidney injury.
INTRODUCTION
Acute kidney injury (AKI) is a common complication among hospitalized patients with acute illness, estimated at one in five adults and one in three children globally 1-3. AKI is an increasingly common comorbidity in the United States, with the incidence of AKI hospitalization increasing by 230% from 2000 to 2014 in nondiabetic patients 4. This disorder is defined as a sudden decrease in kidney function that develops over a period of hours to days4,5. This decrease in function ranges from transient changes in serum biochemical markers, blood urea nitrogen (BUN) and creatinine, to kidney failure requiring dialysis 1,5 The risk for mortality increases in proportion to the severity of the AKI, with the highest risk in patients requiring renal replacement therapy 1,3 Additionally, AKI can lead to prolonged hospitalizations, more clinical investigations and unplanned ICU stays, all of which increase the risk of hospital-acquired infections and other complications, as well as the hospital costs 1. AKI secondary to renal ischemia/reperfusion (RIR) injury is caused by a generalized lack of oxygen and nutrient delivery to the kidney secondary to the decreased or absent blood flow. This ischemia causes tubular epithelial damage and leads to the release of damage-associated molecular patterns (DAMPs), which activate innate immune cells such as neutrophils and macrophages and furthers cellular injury through the release of cytokines and reactive oxygen species 2,6,7. Additionally the subsequent re-oxygenation to the ischemic kidney with reperfusion generates reactive oxygen species that initiate a cascade of deleterious cellular responses leading to further inflammation, apoptosis, and AKI 8.
Cold-inducible RNA-binding protein (CIRP) is a 172-amino acid nuclear protein belonging to the family of cold shock proteins that regulates protein expression 9. We have discovered that, in hypoxic states such as ischemia/reperfusion, hemorrhagic shock, and sepsis, extracellular CIRP (eCIRP) is released and acts as new inflammatory mediator or damage associated molecular pattern (DAMP) that critically aggravates tissue injury and worsens the survival 10-12. During hemorrhagic shock and sepsis, inflammation triggers the translocation of CIRP from the nucleus to the cytosol and its release to the extracellular space, where it induces an inflammatory response in numerous cells such as macrophages, neutrophils, lymphocytes, and dendritic cells 11. We have also shown that eCIRP is increased in the kidneys and circulation following RIR, leading to an increased inflammatory response and renal cell damage. We have shown that genetic deficiency of CIRP and blockade of eCIRP decrease AKI after RIR by attenuating inflammation 2,13. However, the specific mechanism of how eCIRP contributes to the inflammation driving AKI remains elusive.
We have recently identified TREM-1 as a key receptor for eCIRP during sepsis 12. TREM-1 is a plasma membrane protein typically expressed by monocytes and neutrophils 12,14. Once activated, TREM-1 triggers a signal transduction cascade that includes the phosphorylation of intracellular signaling molecules DAP12 and SYK and leads to the release of inflammatory cytokines that cause excessive inflammation and proximal tissue injury 12. Although initially discovered to have a role in infectious diseases, there is increasing evidence that TREM-1 activation also plays a role in sterile inflammatory conditions such as ischemia/reperfusion-induced injury, atherosclerosis, colitis, fibrosis, and cancer 15. We have invented M3, a novel 7-amino acid (aa) peptide from the sequence of human eCIRP, and M3 inhibits the interaction between eCIRP with TREM-1 12,16. The M3 peptide has been effective in the treatment of intestinal ischemia-reperfusion injury by decreasing inflammation, reducing associated lung injury, and increasing survival 16. Both eCIRP and TREM-1 are upregulated in AKI 2,15,17-19, however their interaction in the context of RIR has not been adequately evaluated. In this study, we have demonstrated that AKI severity can be attenuated by inhibition of the eCIRP/TREM-1 interaction with M3 following RIR.
MATERIALS AND METHODS
Experimental animals
C57BL/6 male mice were purchased from Charles River Laboratories. The TREM-1−/− mice were generated and obtained by the trans-NIH Knockout Mouse Project (KOMP) and obtained from the KOMP Repository, University of California, Davis, and lines maintained by in-house breeding. All experiments used age-matched mice at 8–12 weeks old. All experiments involving live animals were previously evaluated and approved by the Institutional Animal Care and Use Committee (IACUC) at the Feinstein Institutes for Medical Research.
Animal model of RIR
Renal IR was performed as previously described 2,13. Mice were anesthetized with inhaled isoflurane on a surgical plane and secured in supine position. Briefly, the abdomen was shaved and disinfected with 70% ethanol. Delayed analgesia was provided with a subcutaneous injection of buprenorphine. A midline incision was utilized, and bowel was displaced to expose the right kidney. A clamp was placed on the renal hilum. The process was repeated for the left kidney and the bowel returned to an open abdominal cavity, which was then covered in a saline soaked gauze pad. Mice remained anesthetized and were monitored on a heating pad at 38°C during the ischemia time. For 24-hour experiments, ischemia was induced for 30 minutes. To create a more severe model of ischemia for the 10-day survival experiment, ischemia was induced for 34 minutes (LD60). After the allotted ischemia time, clamps were carefully removed, and the midline incision was closed in layers.
Treatment with M3
At the end of the RIR procedure, mice were randomly allocated to vehicle (normal saline) or treatment (M3, RGFFRGG; GenScript) groups. In 24-hour experiments, treatment mice received 10 mg/kg BW M3, injected i.p. at 4 hours following reperfusion. In the 10-day survival experiment, treatment mice received 10 mg/kg BW M3, instilled i.p. at the time of reperfusion, prior to abdominal closure. Vehicle groups received an equivalent volume of vehicle (normal saline) at the respective time points. Following closure, all mice received a 1-mL subcutaneous injection of normal saline for fluid resuscitation. In the 24-hour experiments, mice were anesthetized with isoflurane the following day, and blood and renal tissue was collected for analysis. The right kidney was preserved in 10% formalin for histopathologic analysis, and the left kidney was frozen in liquid nitrogen and stored at −80°C for quantitative PCR and Western blot analysis. For the 10-day survival experiment, mice were evaluated for humane criteria for euthanasia and survival status twice daily for the first 3 days, and then daily for the remaining week.
Determination of BUN, creatinine, and IL-6 levels
Serum levels of BUN and creatinine were determined using specific colorimetric enzymatic assays according to manufacturer instructions (Pointe Scientific). Serum was analyzed for IL-6 using a specific ELISA kit according to manufacturer instructions (BD Biosciences).
Isolation of the mRNA and real-time quantitative reverse transcription PCR
Kidneys were harvested at 24 hours following reperfusion and stored at −80°C. Approximately 100 mg of tissue powder was lysed using sonication and provided lysis buffer from Illustra RNAspin Mini RNA Isolation kit (GE Healthcare) according to manufacturer instructions. Total tissue RNA was extracted using the same kit. RNA was subsequently reversed-transcribed into cDNA using MLV reverse transcriptase (Applied Bio- systems, Thermo Fisher Scientific). The PCR reaction was performed in a final volume of 24 μL containing 4 βg cDNA, 0.08 μmol of forward and reverse primers, 10 μL SYBR Green PCR Master Mix (Applied Biosystems), and 11 μL nuclease-free water. cDNA was amplified using a Step One Plus real-time PCR machine (Applied Biosystems, Thermo Fisher Scientific). Mouse β-actin was used for normalization and relative expression of mRNA was calculated using the ΔΔCT method. Results were reported as fold change in comparison with the sham mice. Primer sequences are: β-actin: Forward: CGTGAAAAGATGACCCAGATCA, Reverse: TGGTACGACCAGAGGCATACAG, TREM-1: Forward: CTACAACCCGATCCCTACCC, Reverse: AAACCAGGCTCTTGCTGAGA, IL-6: Forward: CCGGAGAGGAGACTTCACAG, Reverse: CAGAATTGCCATTGCACAAC, NGAL: Forward: CTCAGAACTTGATCCCTGCC, Reverse: TCCTTGAGGCCCAGAGACTT
Western blot
Protein was extracted from approximately 100 mg of lysed renal tissue powder using in-house prepared RIPA lysis buffer and complete Protease Inhibitor Cocktail (Sigma Aldrich). Protein concentrations were determined by Bradford protein assay reagent (Bio-Rad). Equal amounts of protein were electrophoresed using polyacrylamide gels and transferred to nitrocellulose membranes (Invitrogen, Thermo Fisher Scientific). The blots were incubated with β-actin (monoclonal mouse anti-mouse IgG, catalog A5441; Sigma Aldrich) and NGAL (polyclonal rabbit anti-mouse IgG, catalog PA5-79590; Invitrogen, Thermo Fisher Scientific) primary antibodies, followed by immunofluorescent-labeled secondary antibodies (anti–mouse IgG, catalog 926-68070; anti-rabbit IgG, catalog 926-32211; Li-Cor Biosciences). Protein was detected by Odyssey CLx Imaging system (Li-Cor Biosciences) and relative expression measured using Image Studio 5.2 (Li-Cor Biosciences).
Renal histopathology
Renal tissue was preserved in formalin and then embedded in paraffin. Tissue was sectioned into 5-μm slices and stained using hematoxylin and eosin (H&E). Level of renal injury was assessed in a blinded fashion using light microscopy. Injury was assessed in the following categories: tubular cell injury, tubular cell detachment, loss of brush border, tubular simplification, and cast formation 2. Scores ranged from 0 (0% injury), 1 (<10%), 2 (10–25%), 3 (25–50%), 4 (50– 75%), 5 (>75%), for a maximal score of 25. Scores were averaged for each sample over 10 randomly selected fields 2.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
The number of apoptotic cells were assessed by in situ labeling of DNA fragmentation using the TUNEL assay. Renal tissue embedded in paraffin was sectioned into 5-μm slices and adhered to slides. Tissue was then deparaffinated and rehydrated using xylene, EtOH, and water. Cells were permeabilized using a proteinase K solution. An In Situ Cell Death Detection kit (Roche Diagnostics) was used to label apoptotic cells, and cells were counterstained with 4′,6-diamidino-2′-phenylindole dihydrochloride (DAPI). Positive and negative controls were prepared and utilized per manufacturer instructions.
RESULTS
TREM-1 is upregulated by RIR and promotes AKI
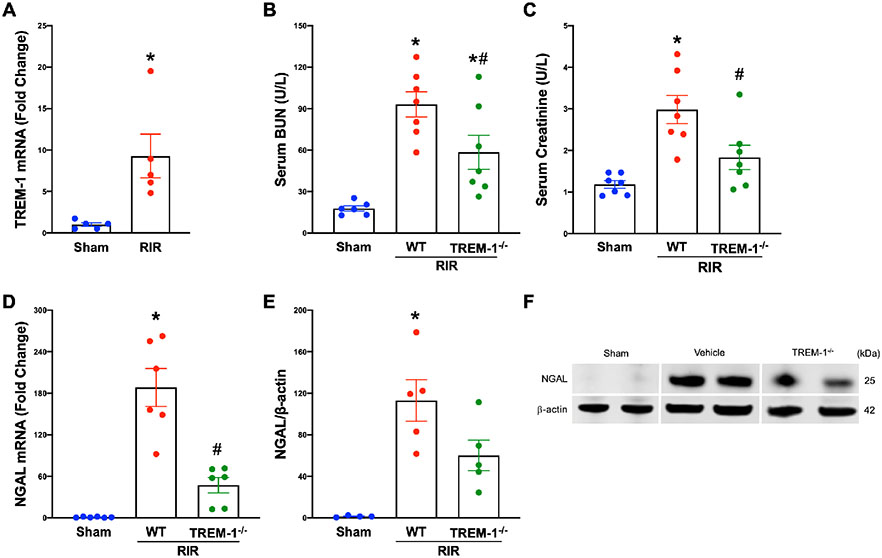
To determine whether TREM-1 expression is upregulated during AKI, we subjected WT mice to RIR injury. Kidney tissue was collected 24 hours after the insult, and gene expression of TREM-1 was determined to be 9-fold higher in RIR mice compared to sham (Fig. 1A). We then evaluated if the deficiency of TREM-1 attenuated the severity of AKI. We subjected TREM-1−/− and WT mice to the same RIR injury and demonstrated that absence of TREM-1 significantly reduced serum BUN and creatinine compared to WT mice (Fig. 1B-C). NGAL is a biomarker for AKI that is upregulated in renal tissue following injury 20. Following RIR, we demonstrated a marked increase of NGAL gene and protein expression in WT mice compared to sham. In TREM-1−/− mice, there was a 74.9% reduction in NGAL mRNA and a 46.8% reduction in NGAL protein expression in the kidneys compared with WT mice (Fig. 1D-F). These data indicate that TREM-1 activation plays a critical role in promoting AKI after RIR.
Fig. 1: TREM-1 is upregulated in RIR and promotes AKI.
Adult C57BL/6 and TREM-1−/− mice were randomly assigned to sham or RIR operation. At 24 hours after reperfusion, renal mRNA levels of (A) TREM-1 were measured by real-time PCR (RT-PCR), n=5 mice per group, *P<0.05, student’s t-test. (B) Serum BUN and (C) creatinine were determined using specific colorimetric enzymatic assays, n=6-7 mice per group. (D) Renal mRNA levels of NGAL were measured by RT-PCR, n=6 mice per group. (E) Extracted total protein from renal tissue measured by western blotting. NGAL expression was normalized to β-actin expression, mean values of sham mice were standardized as one for comparison, n=4-5 mice per group. (F) Representative image of western blot for NGAL and β-actin. All groups were compared by 1-way ANOVA and Tukey’s method. *P<0.05 vs. sham, #P<0.05 vs. wild-type (WT). Data are expressed as mean ± SEM.
TREM-1 deficiency ameliorates systemic and renal inflammation after RIR.
To further verify the role of TREM-1 in AKI, we evaluated if deficiency of TREM-1 decreased levels of pro-inflammatory marker, IL-6. IL-6 is released locally following tissue injury and stimulates acute phase inflammatory response 6,21. Following RIR, TREM-T−/− mice had a 55.6% reduction in renal IL-6 mRNA compared to WT mice (Fig. 2A). Serum IL-6 was increased 6.7-fold in WT mice who had ischemia/reperfusion injury compared to sham mice. This was markedly reduced in TREM-1−/− mice by 61.2% compared to WT mice who underwent RIR (Fig. 2B). This data show that deficiency of TREM-1 attenuates the inflammatory response after RIR.
Fig. 2: TREM-1 regulates systemic and renal inflammation after RIR.
Adult C57BL/6 and TREM-1−/− mice were randomly assigned to sham or RIR operation. At 24 hours after reperfusion, (A) Renal mRNA level of IL-6 was measured by RT-PCR, n=7 mice per group. (B) Serum level of IL-6 was measured by ELISA, n=9-10 mice per group. All groups were compared by 1-way ANOVA and Tukey’s method. *P<0.05 vs. sham, #P<0.05 vs. WT. Data are expressed as mean ± SEM.
Renal histological damage and apoptosis are decreased in TREM-1−/− mice.
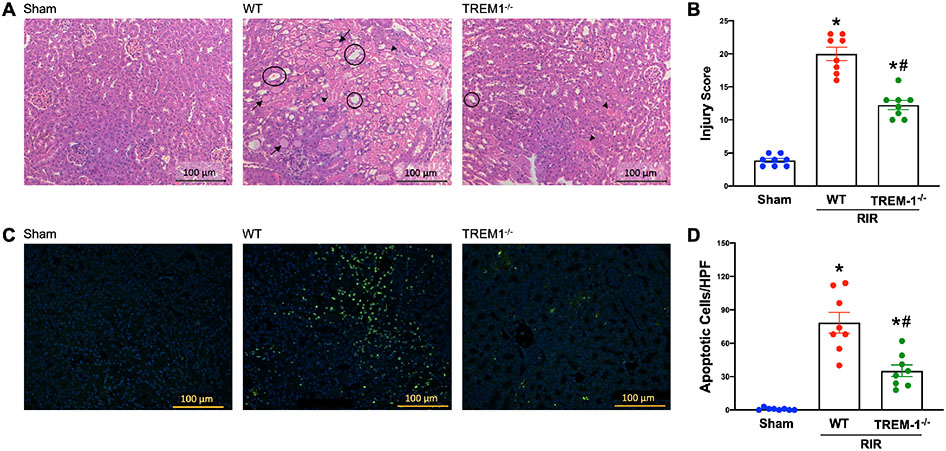
Following RIR, we examined the integrity of renal structure in WT and TREM-1−/− mice using histological evaluation. Using H&E staining, we demonstrated normal architecture in WT sham mice. Kidneys of WT and TREM-1−/− mice after RIR demonstrated varying degrees of renal histologic injury (Fig. 3A). Severity of histologic injury was scored based on tubular cell injury, tubular cell detachment, loss of brush border, tubular simplification, and cast formation. Out of a possible 25 points, WT mice averaged a score of 20, while the TREM-1−/− mice averaged a significantly lower score of 12.25 (Fig. 3B). Additionally, we examined cellular apoptosis in renal tissue using TUNEL staining. WT mice demonstrated prominent areas of TUNEL-positive staining following RIR, which was decreased in TREM-1−/− mice (Fig. 3C). Number of apoptotic cells per field was counted and averaged. After RIR, TREM-1−/− mice had a significant reduction of 55% in the number of apoptotic cells compared to WT mice (Fig. 3D). These data demonstrate that deficiency of TREM-1 attenuates renal architectural damage and cellular apoptosis after ischemia/reperfusion injury.
Fig. 3: TREM-1 regulates renal injury and cellular apoptosis after RIR.
Adult C57BL/6 and TREM-1−/− mice were randomly assigned to sham or RIR operation. At 24 hours after reperfusion, renal histology was evaluated. (A) Representative images of H&E-stained renal tissue at 200x magnification (bar, 100 μm). Areas of tubular cell injury/necrosis are marked with a short arrow, cast formation with a long arrow, and tubular simplification are circled. (B) Renal injury score calculated as described in methods. (C) Representative images of TUNEL staining (green fluorescence) for apoptotic cells and nuclear counterstaining (blue fluorescence) at 200x magnification (bar, 100 μm). (D) Number of apoptotic cells in each group. n=8 images per group. All groups were compared by 1-way ANOVA and Tukey’s method. *P<0.05 vs. sham, #P<0.05 vs. WT. Data are expressed as mean ± SEM.
M3 protects mice from AKI.
We next evaluated the efficacy of M3 to provide renal protection from AKI following RIR. WT mice underwent the same model of ischemia/reperfusion, and groups randomly assigned to treatment received a dose of M3 4 hours after reperfusion. We demonstrated that M3 treatment significantly reduced serum BUN (40.2%) and creatinine (44.8%) compared to vehicle treated mice (Fig. 4A-B). We also demonstrated a significant decrease in NGAL mRNA and protein expression in renal tissue in M3 treated mice compared to vehicle (Fig. 4C-E). These data suggest that using M3 as a treatment in RIR injury can attenuate the severity of the resulting kidney injury.
Fig. 4: M3 improves renal functioning following RIR.
Adult C57BL/6 mice were randomly assigned to sham operation, or RIR with administration of vehicle or treatment. Treatment mice received a 10 mg/kg BW dose of M3 i.p. and vehicle mice received a comparable volume of NS 4 hours following reperfusion. At 24 hours after reperfusion, (A) serum BUN and (B) creatinine were determined using specific colorimetric enzymatic assays, n=6-7 mice per group. (C) Renal mRNA levels of NGAL were measured by RT-PCR, n=6 mice per group. (D) Extracted total protein from renal tissue measured by western blotting. NGAL expression was normalized to β-actin expression, mean values of sham mice were standardized as one for comparison, n=4 mice per group. (E) Representative image of western blot for NGAL and β-actin. All groups were compared by 1-way ANOVA and Tukey’s method. *P<0.05 vs. sham, #P<0.05 vs. vehicle. Data are expressed as mean ± SEM.
M3 abrogates local and systemic inflammation associated with AKI.
Using IL-6 as a marker of injury induced inflammation, renal tissue mRNA was decreased 75.4% in M3 treated mice compared to WT (Fig. 5A). Additionally, serum IL-6 levels were decreased by 73.3% in M3 treated mice compared to WT (Fig. 5B). These data suggest that treatment with M3 can attenuate the renal and systemic inflammatory response after RIR injury.
Fig. 5: M3 attenuates systemic and renal inflammation after RIR.
Adult C57BL/6 mice were randomly assigned to sham operation, or RIR with administration of vehicle or treatment. Treatment mice received a 10 mg/kg BW dose of M3 i.p. and vehicle mice received a comparable volume of NS 4 hours following reperfusion. At 24 hours after reperfusion, (A) renal mRNA levels of IL-6 were measured by RT-PCR. (B) Serum level of IL-6 was measured by ELISA. n=6-8 mice per group. All groups were compared by 1-way ANOVA and Tukey’s method. *P<0.05 vs. sham, #P<0.05 vs. vehicle. Data are expressed as mean ± SEM.
M3 protects mice from renal histological damage and apoptosis following injury.
Following RIR, we performed histological examination of renal tissue using H&E staining to evaluate the renal architecture. Once again, we demonstrated normal architecture in WT sham mice. Renal injury was noted in both vehicle and M3 treated mice, with vehicle mice appearing to have more severe damage (Fig. 6A). Severity of histologic injury was scored using the same system as the TREM-1−/− mice. Out of a possible 25 points, sham mice averaged a score of 4 and mice that underwent RIR and received vehicle averaged a score of 20. Mice treated with M3 following RIR averaged a score of 11.5 (Fig. 6B). Additionally, we examined cellular apoptosis in renal tissue using TUNEL staining. Sham mice had virtually no areas of apoptosis, while M3 treated mice demonstrated a reduction in TUNEL-positive staining compared to vehicle mice. (Fig. 6C). The number of apoptotic cells per field was counted and averaged. After RIR, M3 treated mice had a significant reduction of 73.8% in the number of apoptotic cells compared to vehicle treated mice (Fig. 6D).
Fig. 6: M3 attenuates renal injury and cellular apoptosis after RIR.
Adult C57BL/6 mice were randomly assigned to sham operation, or RIR with administration of vehicle or treatment. Treatment mice received a 10 mg/kg BW dose of M3 i.p. and vehicle mice received a comparable volume of NS 4 hours following reperfusion. At 24 hours after reperfusion, renal histology was evaluated. (A) Representative images of H&E-stained renal tissue at200x magnification (bar, 100 μm). Areas of tubular cell injury/necrosis are marked with a short arrow, cast formation with a long arrow, and tubular simplification are circled. (B) Renal injury score calculated as described in methods. (C) Representative images of TUNEL staining for apoptotic cells (green fluorescence) and nuclear counterstaining (blue fluorescence) at 200x magnification (bar, 100 μm). (D) Number of apoptotic cells in each group. n=8 images per group. All groups were compared by 1-way ANOVA and Tukey’s method. *P<0.05 vs. sham, #P<0.05 vs. vehicle. Data are expressed as mean ± SEM.
M3 improves survival following severe AKI.
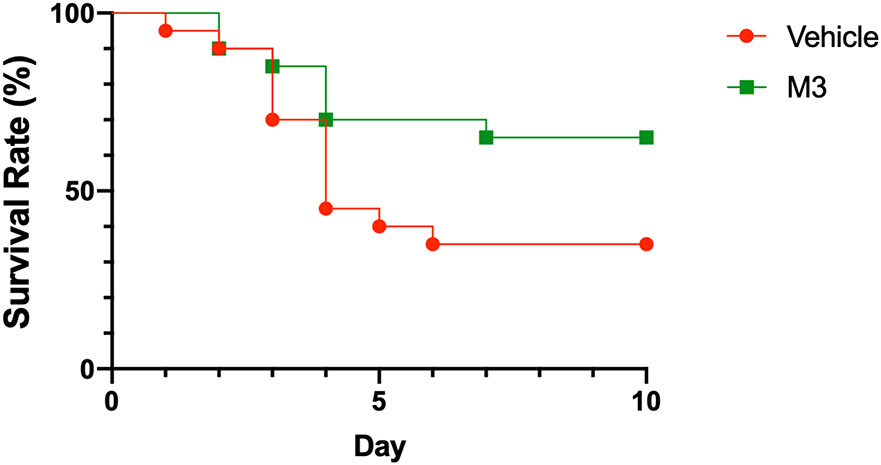
In order to determine if there was a long-term benefit of M3 treatment following a more severe model of RIR, we treated mice with either M3 or normal saline i.p. at the time of reperfusion. Mice were followed for 10 days for survival, and vehicle treated mice were noted to have a survival rate of 35%. Mice treated with M3 were noted to have an improved survival rate to 65%, demonstrating a marked survival benefit after treatment (Fig. 7). This finding suggests that inhibition of TREM-1 activation by eCIRP may have a beneficial effect on the survival in patients with AKI.
Fig. 7: M3 prolongs survival following RIR.
Adult C57BL/6 mice underwent bilateral RIR and were randomly assigned vehicle or treatment with M3 at the time of reperfusion. Treatment mice received a 10mg/kg BW i.p. and vehicle mice received a comparable volume of NS. Mice were monitored for 10 days for survival. (A) Kaplan-Meier survival curve generated from treatment (M3) and vehicle (NS) mice after increased-severity RIR is shown. n=20 mice per group. Data is compared using log-rank test, P=0.06 vs. vehicle
DISCUSSION
Extracellular CIRP is a DAMP originally discovered to be released in response to hemorrhagic shock and sepsis 2,9. In addition to serum eCIRP being elevated in patients admitted to the ICU with hemorrhagic shock 9, high levels of eCIRP in cardiac bypass patients was associated with an increased risk for developing AKI 22. In mice, deficiency of CIRP has been shown to attenuate the severity of renal injury following RIR 2, however the mechanism of this effect remains unclear. Sepsis induced eCIRP release has been demonstrated to bind to and activate TREM-1, which via intracellular signaling molecules DAP12 and SYK, induces the release of proinflammatory cytokines and activates neutrophils and macrophages 12,23. This pathway increases localized and systemic inflammation, and has been demonstrated to promote acute lung injury 12. In this study, we identified that the TREM-1 pathway also plays an integral role in the development of AKI by showing that mice deficient in the TREM-1 receptor have attenuated renal damage and inflammation following RIR. We further demonstrate that inhibiting the binding of eCIRP to TREM-1 using M3, a small peptide developed to inhibit this interaction 12,16, also decreases the severity of AKI following RIR.
Current data demonstrates that TREM-1 is upregulated in the kidneys and soluble TREM-1 is increased in the serum of patients with AKI 15,17,24. Additionally, levels of soluble TREM-1 in urine have been evaluated in septic patients as a biomarker of sepsis-associated AKI 25-27. Mice that underwent RIR to induce AKI have been found to have significant increases in serum and renal tissue protein levels of TREM-1 17,24, as well as increased transcript expression seen on histology 17. Although it remains unclear whether the increase in TREM-1 expression is from TREM-1+ cell migration to the kidney or from an increase in local surface expression, some data suggests renal TREM-1 mRNA-expressing cells are most likely infiltrating granulocytes 17. To verify that TREM-1 is upregulated during AKI induced by RIR, we assessed TREM-1 expression in the kidneys of mice subjected to RIR using RT-PCR. We found that mice with RIR-associated AKI had a significant increase in TREM-1 gene expression. It is possible that this upregulation in TREM-1 expression is due to eCIRP release following RIR, leading to activation and recruitment of TREM-1 expressing macrophages 2,12. This macrophage activation would lead to the release of inflammatory cytokines, furthering renal damage. Interestingly, however, the effect of TREM-1 in the development of renal injury has been contested. In agreement with our observations, Lo T. et al. reported that deficiency of TREM-1 alone ameliorated renal inflammation, injury, and fibrosis development following unilateral ureteral obstruction 18. Additionally, TREM-1−/− mice are protected in models of hepatocellular carcinoma, atherosclerosis, and myocardial ischemia/reperfusion, predominately via decreased recruitment of inflammatory cells 15,18,28-30. On the other hand, mice deficient in both TREM-1 and TREM-3 genes had no improvement in renal damage and inflammation in the acute phase following ischemia reperfusion injury, and conversely increased mortality over 10 days, compared to WT mice 24. In a milder model of RIR, TREM-1/3 double knockout mice had increased tubular damage and elevated renal injury markers at 10-days 24. The difference in phenotype between TREM-1/3 double KO and TREM-1−/− mice is an area for further investigation. However, it is possible that knockdown of both TREM-1/3 may also affect the expression of the proximal TREM-2 gene, given its proximity to both genes. When present, TREM-2 attenuates macrophage activation and the subsequent inflammatory response, so iatrogenic deletion of this gene could lead to increased inflammation and renal damage 17,18,31. Our findings provide further evidence that TREM-1−/− mice display protection from inflammation and injury, using a RIR model.
In this work, we also demonstrate that inhibiting TREM-1 with a 7-aa peptide, M3, is able to decrease the severity of renal injury and inflammation following RIR. Peptidoglycan recognition protein 1 (PGLYRP1) was one of the first ligands discovered to activate TREM-1 32. M3 was subsequently developed using an area of aa sequence homology between PGLYRPI and CIRP and was demonstrated to dramatically inhibit CIRP’s binding to TREM-1 12,16,32. Treatment with M3 has further been shown to protect against inflammation and damage following sterile and polymicrobial sepsis, and gut IR 12,16. Interestingly, Tammaro et al. have found that inhibiting TREM-1 using a different peptide, LP17, did not ameliorate RIR-induced injury 17. LP17 is a 17-aa peptide that was developed based on the TREM-1 sequence at a time when TREM-1’s natural ligand had not yet been identified 33,34. Although the mechanism by which LP17 inhibits TREM-1 is unknown, it has been shown to be protective in sepsis, mesenteric ischemia/reperfusion, and sterile inflammation in mice 12,34,35. However, since it is unknown if LP17 directly inhibits the binding of TREM-1’s natural ligands, the eCIRP ligand-specific targeted inhibition of TREM-1 with M3 could explain its superior efficacy in protecting against AKI. Additionally, Tammaro et al. only looked at renal mRNA expression of inflammatory cytokines and did not assess markers of renal damage like BUN, creatinine, and NGAL, or assess histological integrity 17. Using M3 as a treatment following RIR, we were able to demonstrate significant decreases in markers of inflammation, renal damage, apoptosis, and improve mortality.
In this study, we only used male mice based on evidence that male and female sex steroids modulate functions in immune responses differently, in both normal conditions and in diverse diseases 36. Female sex hormones can be protective against the deleterious effects of inflammation and sepsis compared to males 36,37. In response to endotoxin LPS, male mice produce significantly more inflammatory cytokines compared to females 38. Based on this knowledge, the decision was made to use only male mice at this stage to generate consistent findings and ensure adequate inflammatory response to RIR-induced injury. Considering our previous work and preliminary data demonstrated no difference between WT and TREM-1 sham mice, we decided to use only WT sham for comparison to decrease the amount of mice used 12. We decided to use 30 minutes of ischemia time in order to induce renal injury based on previous work 2, however we recognize that results may vary based on different ischemia times. Additionally, all data points were assessed at 24 hours following reperfusion and could vary based on different follow up times. For our survival study, despite previous studies demonstrating mortality at 30 minutes or less 13,24, we did not have mortality in vehicle treated mice with less than 34 minutes of ischemia time. This could be due to a variety of environmental factors, such as clip strength, heating pad temperature, and housing conditions pre and post operatively. Interestingly, we performed time optimization studies for M3 administration and found a slight benefit in treating 4 hours post reperfusion compared to immediately after reperfusion in reducing renal injury, although not significantly different. This effect was reversed in prolonging survival, and mice treated with M3 immediately after reperfusion had prolonged survival. One possibility is that using a larger sample size would demonstrate marked attenuated renal damage with treatment at the time of reperfusion, and that treating as fast as possible after an ischemic incidence has both a morbidity and mortality benefit. All treatment mice received a dose of 10 mg/kg BW, a dose previously established as effective and non-toxic 12,16. In vitro, M3 has been established to be nontoxic to macrophages at a dose 10 times higher than that used for all other in vitro work 12. Further studies on the half-life, absorption, and safety of M3 would be required prior to use in larger animal or human research and to elucidate differences in administration time and outcomes.
Conclusions
We have demonstrated that genetic depletion of TREM-1 exhibits protective effects against the development of AKI following RIR. We additionally show that that treatment with M3, a small peptide that inhibits the binding of CIRP with TREM-1, was able to successfully attenuate renal damage and inflammation following RIR. Treatment with M3 also prolonged survival in mice with a more severe ischemic insult. Future work should focus on the mechanism by which M3 protects against AKI, what cell types are affected by this treatment, and use of this peptide in other models of AKI and CKD. M3 shows promise as a potential treatment to improve AKI in patients.
Acknowledgements
We thank Dr. Monowar Aziz, Mr. HaoTing Yen, and Dr. Fangming Zhang of the Center for Immunology and Inflammation, Feinstein Institutes for Medical Research for their critical discussion and technical assistance.
Funding/Financial Support
This study was supported by the National Institutes of Health (NIH) grants R35GM118337 and R01HL076179
Footnotes
Conflict of Interest/Disclosure Statement
All authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol. Apr 2014;10(4):193–207. doi: 10.1038/nrneph.2013.282 [DOI] [PubMed] [Google Scholar]
- 2.Cen C, Yang WL, Yen HT, Nicastro JM, Coppa GF, Wang P. Deficiency of cold-inducible ribonucleic acid-binding protein reduces renal injury after ischemia-reperfusion. Surgery. August 2016;160(2):473–83. doi: 10.1016/j.surg.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. Jun 2009;53(6):961–73. doi: 10.1053/j.ajkd.2008.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavkov ME, Harding JL, Burrows NR. Trends in Hospitalizations for Acute Kidney Injury - United States, 2000-2014. MMWR Morb Mortal Wkly Rep. Mar 16 2018;67(10):289–293. doi: 10.15585/mmwr.mm6710a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makris K, Spanou L. Acute Kidney Injury: Definition, Pathophysiology and Clinical Phenotypes. Clin Biochem Rev. May 2016;37(2):85–98. [PMC free article] [PubMed] [Google Scholar]
- 6.Thurman JM. Triggers of inflammation after renal ischemia/reperfusion. Clin Immunol. Apr 2007;123(1):7–13. doi: 10.1016/j.clim.2006.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. Nov 2011;121(11):4210–21. doi: 10.1172/JCI45161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malek M, Nematbakhsh M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J Renal Inj Prev. 2015;4(2):20–7. doi: 10.12861/jrip.2015.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiang X, Yang WL, Wu R, et al. Cold-inducible RNA-binding protein (CIRP) triggers inflammatory responses in hemorrhagic shock and sepsis. Nat Med. Nov 2013;19(11):1489–95. doi: 10.1038/nm.3368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang WL, Sharma A, Wang Z, Li Z, Fan J, Wang P. Cold-inducible RNA-binding protein causes endothelial dysfunction via activation of Nlrp3 inflammasome. Sci Rep. May 24 2016;6:26571. doi: 10.1038/srep26571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aziz M, Brenner M, Wang P. Extracellular CIRP (eCIRP) and inflammation. J Leukoc Biol. Jul 2019; 106(1):133–146. doi: 10.1002/JLB.3MIR1118-443R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denning NL, Aziz M, Murao A, et al. Extracellular CIRP as an endogenous TREM-1 ligand to fuel inflammation in sepsis. JCI Insight. Mar 2020;5(5)doi: 10.1172/jci.insight.134172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGinn J, Zhang F, Aziz M, et al. The protective effect of a short peptide derived from cold-inducible RNA-binding protein in renal ischemia-reperfusion injury. Shock. Mar 2018;49(3):269–276. doi: 10.1097/SHK.0000000000000988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arts RJ, Joosten LA, van der Meer JW, Netea MG. TREM-1: intracellular signaling pathways and interaction with pattern recognition receptors. J Leukoc Biol. Feb 2013;93(2):209–15. doi: 10.1189/jlb.0312145 [DOI] [PubMed] [Google Scholar]
- 15.Tammaro A, Derive M, Gibot S, Leemans JC, Florquin S, Dessing MC. TREM-1 and its potential ligands in non-infectious diseases: from biology to clinical perspectives. Pharmacol Ther. Sep 2017;177:81–95. doi: 10.1016/j.pharmthera.2017.02.043 [DOI] [PubMed] [Google Scholar]
- 16.Denning NL, Aziz M, Ochani M, Prince JM, Wang P. Inhibition of a triggering receptor expressed on myeloid cells-1 (TREM-1) with an extracellular cold-inducible RNA-binding protein (eCIRP)-derived peptide protects mice from intestinal ischemia-reperfusion injury. Surgery. Sep 2020;168(3):478–485. doi: 10.1016/j.surg.2020.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tammaro A, Kers J, Emal D, et al. Effect of TREM-1 blockade and single nucleotide variants in experimental renal injury and kidney transplantation. Sci Rep. December 08 2016;6:38275. doi: 10.1038/srep38275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lo TH, Tseng KY, Tsao WS, et al. TREM-1 regulates macrophage polarization in ureteral obstruction. Kidney Int. Dec 2014;86(6):1174–86. doi: 10.1038/ki.2014.205 [DOI] [PubMed] [Google Scholar]
- 19.Campanholle G, Mittelsteadt K, Nakagawa S, et al. TLR-2/TLR-4 TREM-1 signaling pathway is dispensable in inflammatory myeloid cells during sterile kidney injury. PLoS One. 2013;8(7):e68640. doi: 10.1371/journal.pone.0068640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soni SS, Cruz D, Bobek I, et al. NGAL: a biomarker of acute kidney injury and other systemic conditions. Int Urol Nephrol. Mar 2010;42(1):141–50. doi: 10.1007/s11255-009-9608-z [DOI] [PubMed] [Google Scholar]
- 21.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. Sep 04 2014;6(10):a016295. doi: 10.1101/cshperspect.a016295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu W, Yan Y, Han D, et al. CIRP Secretion during Cardiopulmonary Bypass Is Associated with Increased Risk of Postoperative Acute Kidney Injury. Thorac Cardiovasc Surg. Jul 07 2021;doi: 10.1055/s-0041-1730450 [DOI] [PubMed] [Google Scholar]
- 23.Colonna M, Facchetti F. TREM-1 (triggering receptor expressed on myeloid cells): a new player in acute inflammatory responses. J Infect Dis. Jun 15 2003;187 Suppl 2:S397–401. doi: 10.1086/374754 [DOI] [PubMed] [Google Scholar]
- 24.Tammaro A, Scantlebery AML, Rampanelli E, et al. TREM1/3 Deficiency Impairs Tissue Repair After Acute Kidney Injury and Mitochondrial Metabolic Flexibility in Tubular Epithelial Cells. Front Immunol. 2019;10:1469. doi: 10.3389/fimmu.2019.01469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Derive M, Gibot S. Urine sTREM-1 assessment in diagnosing sepsis and sepsis-related acute kidney injury. Crit Care. 2011;15(6):1013. doi: 10.1186/cc10533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan ZK, Fang F, Liu CJ, Li J, Chen YF, Xu F. [Value of urine soluble triggering receptor expressed on myeloid cells-1 in the early diagnosis of sepsis associated acute kidney injury]. Zhonghua Er Ke Za Zhi. May 02 2018;56(5):342–346. doi: 10.3760/cma.j.issn.0578-1310.2018.05.007 [DOI] [PubMed] [Google Scholar]
- 27.Su LX, Feng L, Zhang J, et al. Diagnostic value of urine sTREM-1 for sepsis and relevant acute kidney injuries: a prospective study. Crit Care. 2011;15(5):R250. doi: 10.1186/cc10508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu J, Li J, Salcedo R, Mivechi NF, Trinchieri G, Horuzsko A. The proinflammatory myeloid cell receptor TREM-1 controls Kupffer cell activation and development of hepatocellular carcinoma. Cancer Res. Aug 15 2012;72(16):3977–86. doi: 10.1158/0008-5472.CAN-12-0938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joffre J, Potteaux S, Zeboudj L, et al. Genetic and Pharmacological Inhibition of TREM-1 Limits the Development of Experimental Atherosclerosis. J Am Coll Cardiol. Dec 27 2016;68(25):2776–2793. doi: 10.1016/j.jacc.2016.10.015 [DOI] [PubMed] [Google Scholar]
- 30.Boufenzer A, Lemarié J, Simon T, et al. TREM-1 Mediates Inflammatory Injury and Cardiac Remodeling Following Myocardial Infarction. Circ Res. May 22 2015;116(11):1772–82. doi: 10.1161/CIRCRESAHA.116.305628 [DOI] [PubMed] [Google Scholar]
- 31.Turnbull IR, Gilfillan S, Cella M, et al. Cutting edge: TREM-2 attenuates macrophage activation. J Immunol. Sep 15 2006;177(6):3520–4. doi: 10.4049/jimmunol.177.6.3520 [DOI] [PubMed] [Google Scholar]
- 32.Read CB, Kuijper JL, Hjorth SA, et al. Cutting Edge: identification of neutrophil PGLYRP1 as a ligand for TREM-1. J Immunol. Feb 15 2015;194(4):1417–21. doi: 10.4049/jimmunol.1402303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bleharski JR, Kiessler V, Buonsanti C, et al. A role for triggering receptor expressed on myeloid cells-1 in host defense during the early-induced and adaptive phases of the immune response. J Immunol. Apr 01 2003;170(7):3812–8. doi: 10.4049/jimmunol.170.7.3812 [DOI] [PubMed] [Google Scholar]
- 34.Gibot S, Kolopp-Sarda MN, Bene MC, et al. A soluble form of the triggering receptor expressed on myeloid cells-1 modulates the inflammatory response in murine sepsis. J Exp Med. Dec 6 2004;200(11):1419–26. doi: 10.1084/jem.20040708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gibot S, Massin F, Alauzet C, et al. Effects of the TREM-1 pathway modulation during mesenteric ischemia-reperfusion in rats. Crit Care Med. Feb 2008;36(2):504–10. doi: 10.1097/01.CCM.0B013E318161FAF3 [DOI] [PubMed] [Google Scholar]
- 36.Angele MK, Pratschke S, Hubbard WJ, Chaudry IH. Gender differences in sepsis: cardiovascular and immunological aspects. Virulence. Jan 01 2014;5(1):12–9. doi: 10.4161/viru.26982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zellweger R, Wichmann MW, Ayala A, Stein S, DeMaso CM, Chaudry IH. Females in proestrus state maintain splenic immune functions and tolerate sepsis better than males. Crit Care Med. Jan 1997;25(1):106–10. doi: 10.1097/00003246-199701000-00021 [DOI] [PubMed] [Google Scholar]
- 38.Kuo SM. Gender Difference in Bacteria Endotoxin-Induced Inflammatory and Anorexic Responses. PLoS One. 2016;11(9):e0162971. doi: 10.1371/journal.pone.0162971 [DOI] [PMC free article] [PubMed] [Google Scholar]