Abstract
PURPOSE:
To conduct a systematic review to identify studies that assessed the association between CYP2C19 polymorphisms and clinical outcomes in Peripheral artery disease (PAD) patients who took clopidogrel.
METHODS:
We systematically searched Ovid EMBASE, PubMed, and Web of Science from November 1997 (inception) to September 2020. We included observational studies evaluating how CYP2C19 polymorphism is associated with clopidogrel’s effectiveness and safety among patients with PAD. We extracted relevant information details from eligible studies (e.g., study type, patient population, study outcomes). We used the Risk of Bias in Non-randomised Studies-of Interventions (ROBINS-I) Tool to assess the risk of bias for included observational studies.
RESULTS:
The outcomes of interest were the effectiveness and safety of clopidogrel. The effectiveness outcomes included clinical ineffectiveness (e.g., restenosis). The safety outcomes included bleeding and death related to the use of clopidogrel. We identified four observational studies with a sample size ranging from 50 to 278. Outcomes and comparison groups of the studies varied. Three studies (75%) had an overall low risk of bias. All included studies demonstrated that carrying CYP2C19 loss of function (LOF) alleles was significantly associated with reduced clinical effectiveness and safety of clopidogrel.
CONCLUSIONS:
Our systematic review showed an association between CYP2C19 LOF alleles and reduced functions of clopidogrel. The use of CYP2C19 testing in PAD patients prescribed clopidogrel may help improve the clinical outcomes. However, based on the limited evidence, there is a need for randomized clinical trials in PAD patients to test both the effectiveness and safety outcomes of clopidogrel.
Keywords: Clopidogrel, CYP2C19, peripheral artery disease, effectiveness and safety, restenosis, occlusion
INTRODUCTION
Peripheral artery disease (PAD) affects up to 20% of individuals worldwide.[1] PAD commonly impacts the lower extremities by narrowing vessels that carry blood from the heart to the distal extremities, caused by atherosclerosis, a buildup of fatty plaque in the arteries.[2] PAD can be found in any vessel, but it is more common in lower extremities than upper extremities.[3] Approximately 6.5 million people aged ≥40 years suffered from this disease in the United States (US).[3] Individuals with PAD have a reduced life expectancy by up to 10 years as compared to the general population.
The current American Heart Association / American College of Cardiology (AHA/ACC) guidelines recommend antiplatelet therapy consisting of aspirin (range 75–325mg per day) or clopidogrel alone (75 mg per day) for risk reduction of myocardial infarction, stroke, and vascular death in symptomatic PAD patients.[4] The AHA/ACC guidelines also suggest antiplatelet therapy as a reasonable treatment option to reduce the risk of myocardial infarction, stroke, or vascular death for asymptomatic patients with PAD (i.e., ankle-brachial index ≤ 0.90).[4] Although alternative agents including prasugrel and ticagrelor are available for treating PAD,[5] clopidogrel remains the most commonly used antiplatelet, with over 20 million prescriptions per year.[6]
Clopidogrel is a thienopyridine prodrug that requires hepatic biotransformation to form an active metabolite. Only 15% of the clopidogrel prodrug will be transformed to an active agent. The conversion of clopidogrel to its active metabolite requires two sequential oxidative steps involving several cytochrome P450 (CYP) enzymes, primarily CYP2C19. Therefore, the effectiveness of clopidogrel can be influenced among individuals who are poor and intermediate metabolizers of CYP2C19. In March 2010, the US Food and Drug Administration (FDA) issued a boxed warning that CYP2C19 polymorphism may diminish the clopidogrel’s effectiveness in patients with cardiovascular diseases (CVD).[7] There has been an increasing interest in providing pharmacogenetic testing on CYP2C19 to tailor and personalize clopidogrel therapy in order to improve patient clinical outcomes.[8] The need for pharmacogenomic testing was a central component of the new clopidogrel boxed warning.[9]
Despite the interests, there is a lack of clear recommendations on CYP2C19 genetic testing for clopidogrel use. Existing major guidelines list common CYP2C19 alleles and their clinical relevance, or provide recommendations when pharmacogenomics results are available but none of them explicitly recommend specific CYP2C19 variant alleles testing.[10–12] Most importantly, there is a lack of pharmacogenomic recommendations for PAD population because the associations between CYP2C19 polymorphism and clinical outcomes among clopidogrel users with PAD are not well studied. The objective of this study, therefore, was to systematically review current evidence and evaluate the association between CYP2C19 polymorphisms and effectiveness and safety outcomes in PAD patients who used clopidogrel.
METHODS
Data Sources and Search strategy
This systematic review complied with the internationally accepted gold standard guidelines for systematic reviews as stated in the Cochrane Handbook for Systematic Reviews of Interventions and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.[13, 14] The study protocol was registered with PROSPERO (i.e., International Prospective Register of Systematic Reviews) website (Registration ID: CRD42020203278). We systematically searched databases including Ovid EMBASE, PubMed, and Web of Science from their inception to September 2020. The search strategy combined database-specific controlled vocabulary truncated and phrase-searched keywords in titles and abstracts as available: (“clopidogrel” OR “plavix”) AND “peripheral vascular disease” AND “Cytochrome P-450 CYP2C19” AND (“pharmacogenetics” OR “loss of function mutation”) AND (“randomized controlled trial” OR “Observational Study”) (Appendix 1).
The intervention or exposure of this study is CYP2C19 genetic variants. The outcomes of interest were the effectiveness and safety of clopidogrel. Effectiveness outcomes included clinical ineffectiveness such as clinical nonresponses, amputation events, restenosis or occlusion, ischemic events, and target limb reintervention. Safety outcomes included bleeding and death related to the use of clopidogrel.
Eligibility criteria
In this systematic review, we restricted our search to randomized clinical trials and prospective or retrospective observational human studies written in English. We included studies focusing on patients with PAD using clopidogrel with any available scientific, generic, or brand name. Eligible studies should assess the effect or association of CYP2C19 polymorphisms with PAD outcomes, and the outcome of the study can be bleeding, clinical ineffectiveness, or death. We excluded case reports, letters to the editor, reviews, commentaries, editorials, as well as animal or in vitro studies. We also excluded abstracts from conferences with insufficient information about the research are available.
Data extraction and synthesis
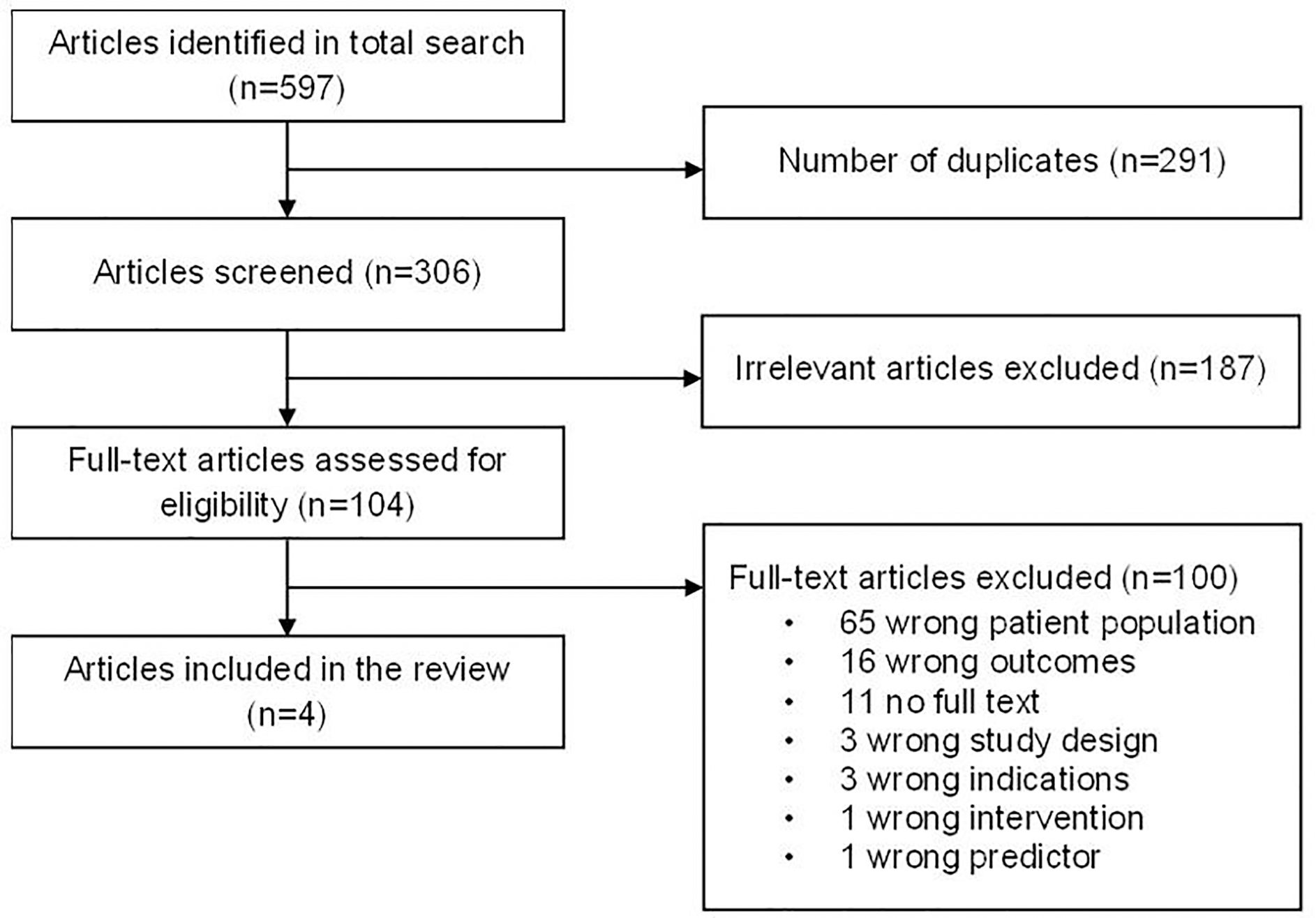
After a comprehensive literature search and removal of duplicates, four reviewers (SH, SY, RY, and SL) double screened the articles’ titles and abstracts and independently screened for inclusion and exclusion eligibility based on the full text. We used Covidence to assign articles to reviewers and manage the progress of the systematic review.[15] We extracted the study information and details using a standardized data collection sheet (Appendix 2). We summarized key study information from each article, including author names, study year, country/region, study type, patient population (e.g., sample size, conditions, treatments, clinical sites), measuring period, interventions, study outcomes, statistical methods, risk factors, and risk estimates (e.g., point estimate of hazard ratio [HR] or odds ratios [OR], 95% confidence intervals [95%CI], and p-values). We calculated the OR and HR based on the results reported in each included study. Next, four reviewers (SH, SY, RY, and SL) independently assessed the risk of bias of each article using the Risk of Bias in Non-randomized Studies - of Interventions (ROBINS-I) tool and the fifth reviewer (KN) aggregated the results and assigned the overall score.[16] ROBINS-I tool is designed specifically to assess the risk of bias in studies of interventions that did not use randomization to allocate units. Appendix 3 lists bias evaluation from our reviewers using 7 categories from the ROBINS-I tool. Any discrepancies on the relevance between reviewers were consulted with the fifth reviewer (KN). Figure 1 reveals the PRISMA flowchart detailing the selection process.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flowchart of the Systematic Review.
Risk of bias assessment
We used the ROBINS-I tool to assess the risk of bias for included observational studies.[16] We compared the Newcastle-Ottawa Scale and ROBINS-I and determined the ROBINS-I tool was most appropriate because it has the strength to evaluate the risk of bias in non-randomized studies.[16, 17] The ROBINS-I tool is designed based on the Cochrane Risk of Bias (Cochrane RoB) tool, which is the most frequently used bias assessment tool for randomized control trials (RCT) in systematic reviews.[18] It assesses each result for a specific outcome across seven bias domains: bias due to confounding, bias in selection of participants into the study, bias in classification of interventions, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of the outcome, bias in selection of the reported results.[19] Each domain has a comprehensive list of well documented questions to help reviewers determine the effects of potential confounders. ROBINS-I tool also has separate questions for cohort and case-control studies.
RESULTS
Study Selection and Characteristics
A total of 597 articles were identified by the electronic database search. After removing duplicates, irrelevant articles were screened by abstract review. We retrieved all 104 (17.4%) full-text articles that were neither duplicates nor irrelevant studies. Of these, four (3.8%) met the inclusion criteria for this systematic review. Reasons for exclusion are shown in Figure 1. The top three reasons for exclusion were: wrong patient population (n=65), wrong outcomes (n=16), and no full text (n=11).
The characteristics of the final four included studies are presented in Table 1. In addition, Figure 2 provides forest plots of outcomes measured. All studies were non-randomized, including two prospective cohort studies,[20, 21] and two retrospective cohort studies.[22, 23] Among the four studies, three evaluated the association between CYP2C19 polymorphisms and clinical ineffectiveness,[20–22] and one focused on the antiplatelet responsiveness to clopidogrel treatment and clinical outcomes.[23] None of the studies reported bleeding as an outcome. The included studies were conducted in China,[20] Greece,[21] Spain,[22] and Taiwan.[23]
Table 1:
Summary of Included Studies
| Author, publication year, country | Study type | Patient population | Sample size | Outcome(s) | Statistical method | Comparison groups | Crude event proportion (# Events by group) | Risk estimate (95% CI), p-value |
|---|---|---|---|---|---|---|---|---|
| Diaz-Villamarin, 2016, Spain | Retrospective cohort | PAD pts following PTA treated with clopidogrel 75 mg/day at least 3 months | 72 | restenosis or occlusion | Multivariable Logistic regression | CYP2C19*2 vs. non CYP2C19*2 (Ref) | 14/25 vs. 11/25 | aOR: 4.37 (1.07–17.77), p=0.039 |
| ABCB1 TT vs. ABCB1 CT+CC (Ref) | 18/25 vs. 7/25 | aOR: 2.98 (0.75–11.86), p=0.12 |
||||||
| Combined LOF vs. non LOF (Ref) | 17/25 vs. 8/25 | aOR: 4.66 (1.37–15.84), p=0.014 |
||||||
| Guo, 2014, China | Prospective cohort | Patients with arteriosclerosis obliterans in the superficial femoral artery receiving dual antiplatelet therapy with clopidogrel 75 mg/day at least 5 days before EVT | 50 | ISR or occlusion | Multivariable Cox regression | CYP2C19 with LOF alleles vs. without LOF alleles (Ref) | 15/26 vs. 4/24 | aHR: 2.69 (1.37–5.29), p=0.004 |
| Pastromas, 2013, Greece | Prospective cohort | Patients with IC or CLI receiving dual antiplatelet therapy with clopidogrel 75 mg/day after PTA or stenting | 113 | TLR | Multivariable Cox regression | Non-Responders vs. Responders (Ref)* | 52/73 vs. 21/66 | aHR: 1.86 (1.11–3.22), p=0.01 |
| Lee, 2019, Taiwan | Retrospective cohort | Patients with CLI taking clopidogrel after EVT | 278 | Amputation | Multivariable Cox regression | IM vs. PM vs. CYP2C19 EM (Ref) | 24/79 vs. 20/46 vs. 28/153 | aHR: 2.65 (2.1–2.9), p=0.009 |
| Death | 18/79 vs. 14/46 vs. 25/153 | aHR: 1.39 (1.07–1.74), p=0.037 |
Abbreviations: CI: confidence interval, CLI: critical limb ischemia, EM: extensive metabolizer, EVT: endovascular therapy, aHR: adjusted hazard ratio, IC: intermittent claudication, IM: intermediate metabolizer, ISR: in-stent restenosis, LOF: loss of function, aOR: adjusted odds ratio, PAD: peripheral artery disease, PM: poor metabolizer, PTA: percutaneous transluminal angioplasty, Ref: reference group, TLR: target limb reintervention
Converted
Figure 2. Forest Plots of Included Studies.
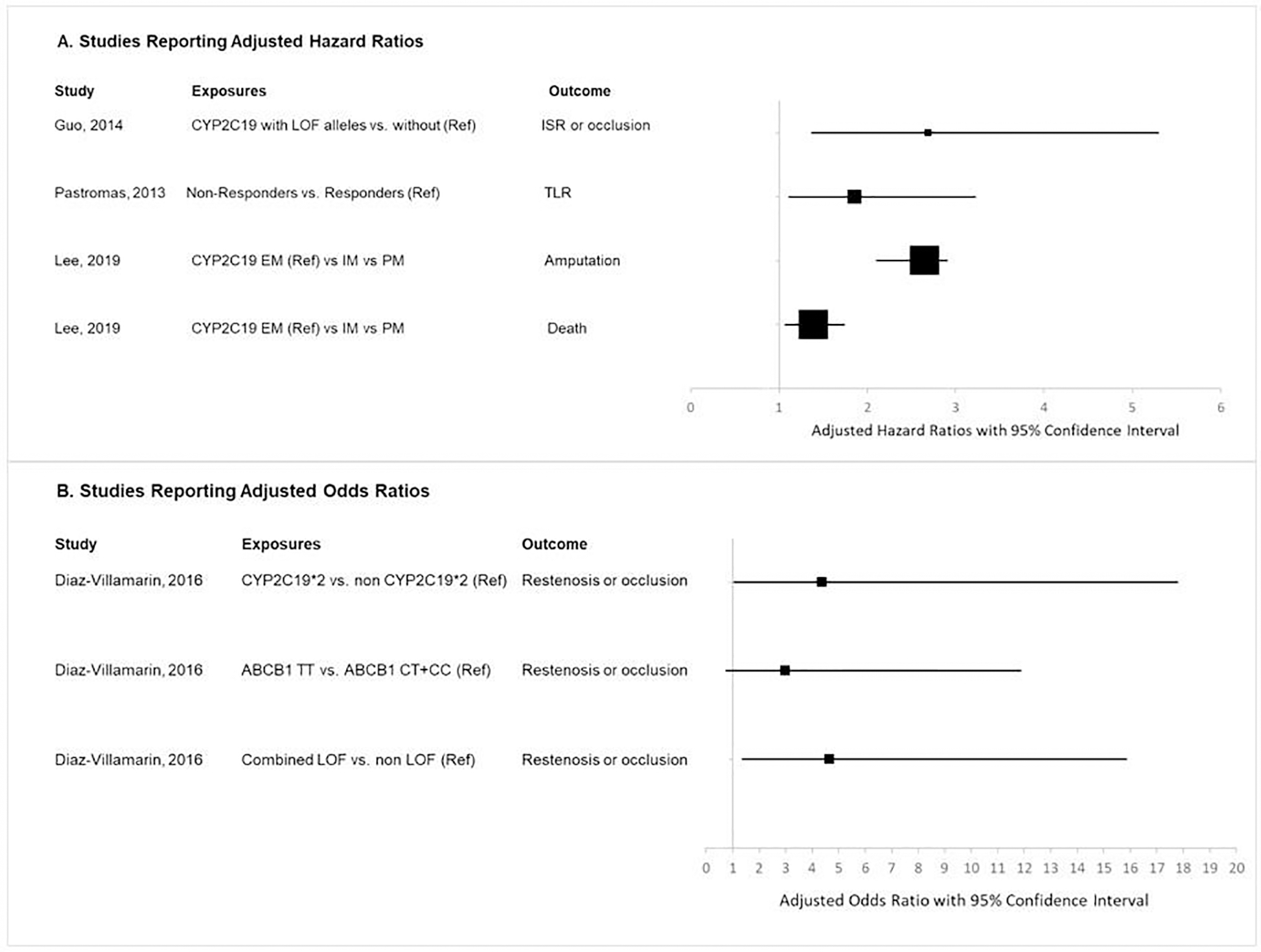
Abbreviations: ISR: in-stent restenosis, Ref: reference group, TLR: target limb reintervention
Study Summary
Diaz-Villamarin et al. studied the single and combined effect of three genotypes (ABCB1 3435C>T, CYP2C19*2, and CYP2C19*3) on clopidogrel response in 72 patients with peripheral artery disease following percutaneous transluminal angioplasty (PTA) receiving clopidogrel 75 mg for at least three months. Baseline characteristics (e.g. cardiovascular history, smokers, number of treated regions) were balanced between different genotype groups except for low-molecular-weight heparin (LMWH) and β-blockers. The study found that carriers of CYP2C19 loss of function (LOF) alleles and/or ABCB1 TT were at increased risk of restenosis or occlusion of previously treated lesions (adjusted OR: 7.04; 95%CI: 1.80–27.46; p=0.005) (Table 1 and Figure 2). The study concluded that the CYP2C19*2 and ABCB1 TT genotypes were independent determinants of atherothrombotic ischemic events in PAD patients following PTA and treated with clopidogrel.
Guo et al. assessed the association between CYP2C19 genotype and the development of ischemic events (i.e., in-stent restenosis or occlusion) among patients with arteriosclerosis obliterans in the superficial femoral artery receiving DAPT with clopidogrel 75 mg at least five days before endovascular therapy (EVT). Patients without LOF alleles and patients with one or more LOF alleles were compared in the study. Baseline clinical characteristics regarding presentation, lesion severity, and stent use were similar in the two groups. Of the 50 study subjects, 26 (52%) patients had one or more LOF alleles. The study found that LOF allele carriers had an increased risk of ischemic events compared with non-carriers. The percentage of patients with ischemic events was 20.8% in those without LOF alleles, 59.0% in carriers of one LOF allele, and 100% in carriers of two LOF alleles. In addition, the authors measured platelet function using thromboelastography (TEG) platelet mapping and classified the study subjects into high platelet reactivity (HPR) group (ADP- induced inhibition ≤30%) and normal on-treatment platelet reactivity (NPR) group (ADP-induced inhibition >30%). Among the 50 study participants, the authors identified 11 (22.0%) patients in the HPR group. Patients without LOF alleles had a greater platelet inhibition in response to clopidogrel compared to patients with LOF alleles. Furthermore, ischemic event rates at 1-year follow-up were significantly higher in patients with HPR than patients with NPR (log-rank test p=0.012).
Pastromas et al. examined the association between target limb reintervention (TLR) and platelet responsiveness among patients with intermittent claudication (IC) or critical limb ischemia (CLI) receiving daily treatment of 75 mg clopidogrel after peripheral percutaneous infrainguinal angioplasty (PTA) or stenting. The authors assessed platelet responsiveness using the VerifyNow P2Y12 assay after at least three months of clopidogrel therapy. They defined patients with residual platelet reactivity units (PRUs) ≥ 235 as non-responders (54.0%) and others as responders (46.0%). In this study, confounding by indication was minimized by excluding patients with any hypercoagulation disorders. Of the 113 study subjects, 58.4% were patients with CLI. Non-responders were more likely to have baseline diabetes (68.9% vs. 42.3%, p=0.007), chronic renal failure (21.3% vs. 7.7%, p=0.03), and CLI (70.5% vs. 44.2%, p=0.007). The study found that TLR-free survival rates at 7-year follow-up were 20.7% in responders vs. 1.9% in non-responders, with a significant difference between groups (log-rank test p=0.001). Resistance to clopidogrel was identified as an independent predictor for poor TLR-free survival in a multivariable Cox proportional hazards regression model. As a secondary outcome, amputation-free survival rates at 7-year follow-up was not significantly associated with platelet responsiveness (98.3% in responders vs. 96.7% in non-responders, log-rank test p=0.56)
Lee et al. investigated the association between clinical outcomes indicating ineffectiveness of clopidogrel treatment and CYP2C19 genotype among CLI patients with Rutherford classifications V and VI taking clopidogrel monotherapy after EVT. In this study, patients treated with other antiplatelet agents or anticoagulants at the time of EVT were excluded to avoid confounding bias. The study subjects were classified into three groups by the number of CYP2C19 LOF alleles: 1) extensive metabolizer (EM; no LOF), 2) intermediate metabolizer (IM; one LOF), and 3) poor metabolizer (PM; two LOFs). Among the total of 278 CLI patients, 55.0%, 28.4%, and 16.6% were identified as EM, IM, and PM, respectively. There were no significant differences in baseline demographic and clinical characteristics across groups. The study demonstrated that CYP2C19 genotypes were significantly associated with amputation and all-cause mortality in patients with CLI taking clopidogrel after EVT. Amputation-free survival rates at 1-year follow-up were 82.1% in EM, 66.1% in IM, and 56.6% in PM (log-rank test p=0.0006), and survival rates at 1-year follow-up were 83.7% in EM, 72.2% in IM, and 71.3% in PM (log-rank test p=0.01). The authors identified hemodialysis and the number of LOF alleles as independent predictors for both amputation and all-cause mortality. In this study, platelet aggregation was also measured using the VerifyNow P2Y12 assay. They demonstrated that the EM group had the greatest platelet inhibition after clopidogrel initiation with a mean PRU of 174.6, whereas the PM group had the lowest platelet inhibition with a mean PRU of 245.7.
Bias assessment
The overall quality of the four included studies was high according to the ROBINS-I assessment tool. The bias assessment results are summarized in Table 2.
Table 2.
Risk of Bias in Non-randomised Studies-of Interventions (ROBINS-I) Bias Assessment Results
| Author, year | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from intended interventions | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | ROBINS-I overall |
|---|---|---|---|---|---|---|---|---|
| Diaz-Villamarin, 2016 | Moderate | Low | Low | Low | Low | Low | Low | Low |
| Guo, 2014 | Low | Low | Low | Low | Moderate | Low | Moderate | Moderate |
| Pastromas, 2013 | Low | Low | Low | Low | Low | Low | Low | Low |
| Lee, 2019 | Moderate | Low | Low | Low | Low | Low | Low | Low |
Diaz-Villamarin et al. received a moderate risk in the domain of bias due to confounding and low risk in all other six domains. The overall risk of bias for this article was low. The authors adjusted clinical variables in the multivariable logistic regression and reported adjusted OR. However, the small sample size from one clinical site could have potential selection bias. In addition, among the 72 patients in the study, the length of clopidogrel treatment varied, with 19 (26.4%) patients treated for six months or less. Current guidelines recommend 12 months of clopidogrel treatment after percutaneous transluminal angioplasty.[24] The duration of clopidogrel treatment may impact the effectiveness; therefore, it should be adjusted for in the regression model.
Guo et al. received a moderate risk in two domains (bias due to missing data and bias in selection of the reported result) and a low risk in all other five domains. The overall risk of bias for this article was moderate. The main concern regarding this study was the high loss to follow-up rate (32.4%), resulting in a reduced power. The authors excluded the 24 patients with missing values in the data analysis which may introduce selection bias.
Pastromas et al. received a low risk in all seven domains. Therefore, the overall risk of bias for this article was low. This study investigated a relatively small number of patients using a single-center observational design, which may lead to inherent bias.
Lee et al. received a moderate risk in the domain of bias due to confounding and a low risk in all other six domains. The overall risk of bias for this article was low. The study included a large sample size from a single clinical site which limits the external validity. Misclassifications could occur due to the controversial methods and timing of platelet function testing.
DISCUSSION
This study is a systematic review that focuses on pharmacogenomics for PAD. Specifically, we evaluated the association between CYP2C19 LOF alleles and adverse events or ineffectiveness in individuals with PAD. Many prior systematic reviews have assessed the effect of CYP2C19 function on clopidogrel effectiveness in CVD. [25, 26] However, this study highlights the importance of CYP2C19 function in PAD, a condition that has garnered significantly less attention. As a result of our rigorous review process, we were able to identify four relevant observational cohort studies. Our analysis revealed a consistent effect of CYP2C19 on clopidogrel effectiveness in PAD patients, suggesting a need to assess recommendations regarding the use of CYP2C19 pharmacogenomic testing in PAD patients prior to prescribing clopidogrel.
All four studies found a higher risk of reduced clinical effectiveness in patients with a CYP2C19 LOF allele. Patients who have LOF alleles for CYP2C19 (*2, *3), especially those with both LOF alleles (also known as poor metabolizers) are at higher risk of low concentrations of the active metabolite of clopidogrel and clinical ineffectiveness.[27] These results suggest the need for CYP2C19 genetic results to evaluate the risk of ineffective clopidogrel use in PAD patients.
In addition to clinical ineffectiveness, bleeding risk is a serious side effects of clopidogrel use. This side effect had been frequently reported in the literature. A recent study by Nguyen et al. for patients with CVD identified two definite risk factors for major bleeding and four risk factors for any bleeding in clopidogrel use.[28] This study also identified CYP2C19 LOF as potential risk factor, but aggregated results showed that CYP2C19 LOF carrier was not a risk factor for either major bleeding or any bleeding (OR = 1.14, 95% CI 0.73–1.80 and OR= 0.65, 95% CI 0.33–1.30, respectively.) In PAD population, all four studies included in this review only focused on the clinical ineffectiveness from LOF alleles. None of these studies evaluated increased function allele (*17), especially in patients with two increased function alleles (*17/*17.) While *17 allele is associated with higher concentration of active metabolites [29, 30], there are fewer studies on its affect to bleeding risk. Li’s evaluation of 782 CAD patients from 4 studies showed that the risk of bleeding was 23% increase when compared CYP2C19*17 variants with wild type (OR = 1.25, 95% CI 1.07–1.47.). [31–34] Currently, either FDA label or guidelines have any recommendation regarding bleeding risks with CYP2C19*17 alleles [7, 35], especially in PAD patients. Overall, additional clinical studies are needed to evaluate the bleeding risk of clopidogrel in PAD population and CYP2C19*17 risk alleles.
This systematic review supports the finding that CYP2C19 polymorphisms may affect clinical outcomes in subjects with PAD. Pharmacogenomic testing would allow healthcare providers and others to employ genotype-guided approaches to make clinical decisions. CYP2C19 testing has been implemented across many healthcare settings worldwide for clopidogrel use in CAD patients [36–39], the current data included in this review suggests an opportunity to expand the utilization of CYP2C19 testing results to the PAD population. [40, 41] Genetic testing has the potential to add clinical value. [42–44] In addition, Klarin et al. study provided genetic evidence that therapies targeting specific risk factors could mitigate the rising incidence of PAD.[45] Although the costs of genetic testing vary by laboratory and health system, many health insurance plans currently cover CYP2C19 testing.[46] There are still ethical, and discrimination concerns regarding genetic testing; however, with the development of laws and medical guidelines, patients and health care providers are more and more perceptive to genetic testing given its high accuracy and fast speed to receive the test results.[47]
Preemptive genetic testing for CYP2C19 may help clinicians decide on clopidogrel therapy, alternative antiplatelet agents, or DAPT. In CAD patients, alternatives to replace clopidogrel include prasugrel and ticagrelor.[48] However, these two alternatives (prasugrel and ticagrelor) are not recommended for PAD treatment according to the AHA/ACC guideline.[49] Current recommend treatment include either aspirin alone or clopidogrel alone for PAD patients with symptomatic. Only in patients with both PAD and CAD, ticagrelor can be used concomitant with aspirin to reduce major adverse cardiac events. Alternatives to replace clopidogrel, therefore, are limited in patients with PAD compared to those with CAD. Electronic clinical decision support tools should be developed to assist clinicians with medication choice in patients with CYP2C19 polymorphism. Such tools can help educate clinicians on risks and benefits of clopidogrel use and provide appropriate alternatives to optimize treatment decisions.
Despite the findings, this study had several limitations. We were not able to pool all data due to differences in outcomes, measurement, and stratified cohorts. For instance, Lee et al. stratified the study cohort by metabolism phenotypes (IM, PM, and NM) for clopidogrel treatments, while the other three studies conducted genotype analysis (e.g., CYP2C19 with vs. without LOF alleles). In addition, the study outcomes varied in the four included articles. The primary outcome of Diaz-Villamarin et al. and Guo et al.’s study was restenosis or occlusion of the treated lesions; the study of Pastromas et al. focused on target limb reintervention free survival time; and Lee et al. measured the risks of amputation and death. Finally, patients included in each study were treated with clopidogrel and followed for different lengths of time which may also affect the study outcomes. Given those challenges and the small sample size we obtained, it was extremely hard to calculate a pooled OR with interpretable clinical meanings. Therefore, we analyzed each study separately.
CONCLUSION
Based on our findings, there is a need for additional RCTs in PAD patients to test the effect of pharmacogenomic testing on effectiveness and safety outcomes. Current evidence demonstrates an association of carrying CYP2C19 LOF alleles with reduced clinical effectiveness and safety of clopidogrel in patients with PAD. We recommend that investigators and clinical practitioners expand the use of CYP2C19 testing beyond CAD patients to include individuals with PAD to improve the effectiveness of clopidogrel therapy in this population.
Supplementary Material
FUNDING
This project was made possible, in part, by support from the Indiana Clinical and Translational Sciences Institute (funded in part by Award Number UL1TR002529 from the National Institutes of Health, National Center for Advancing Translational Sciences) Clinical and Translational Sciences Award, and the Lilly Endowment, Inc. Physician Scientist Initiative. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors, and do not necessarily reflect the views of the funding agencies.
Footnotes
STATEMENTS AND DECLARATIONS
FINANCIAL INTERESTS: The authors declare they have no financial interests.
NON-FINANCIAL INTERESTS: The authors declare they have no non-financial interests.
DATA AVAILABILITY:
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
REFERENCES
- 1.Bauersachs R, et al. , Burden of Coronary Artery Disease and Peripheral Artery Disease: A Literature Review. Cardiovasc Ther, 2019. 2019: p. 8295054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feringa HHH, et al. , A Prognostic Risk Index for Long-term Mortality in Patients With Peripheral Arterial Disease. Archives of Internal Medicine, 2007. 167(22): p. 2482–2489. [DOI] [PubMed] [Google Scholar]
- 3.CDC. Peripheral Arterial Disease (PAD). Septmber 2, 2021]; Available from: https://www.cdc.gov/heartdisease/PAD.htm.
- 4.Gerhard-Herman MD, et al. , 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation, 2017. 135(12): p. e686–e725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whayne TF, A review of the role of anticoagulation in the treatment of peripheral arterial disease. Int J Angiol, 2012. 21(4): p. 187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kane SP Clopidogrel. September 12, 2021. [cited 2022 Feburary 28]; Version 21.2:[Available from: https://clincalc.com/DrugStats/Drugs/Clopidogrel. [Google Scholar]
- 7.FDA. FDA Drug Safety Communication: Reduced effectiveness of Plavix (clopidogrel) in patients who are poor metabolizers of the drug. 2017; Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-review-finds-long-term-treatment-blood-thinning-medicine-plavix.
- 8.Scott SA, et al. , Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther, 2013. 94(3): p. 317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmes DR, et al. , ACCF/AHA Clopidogrel Clinical Alert: Approaches to the FDA Boxed Warning. Circulation, 2010. 122(5): p. 537–557. [DOI] [PubMed] [Google Scholar]
- 10.Pratt VM, et al. , Recommendations for Clinical CYP2C19 Genotyping Allele Selection: A Report of the Association for Molecular Pathology. J Mol Diagn, 2018. 20(3): p. 269–276. [DOI] [PubMed] [Google Scholar]
- 11.Electronic Code of Federal Regulations Part 493—Laboratory Requirements. Available from: https://www.ecfr.gov/current/title-42/chapter-IV/subchapter-G/part-493.
- 12.Clinical Pharmacogenetics Implementation Consortium Guidelines. Available from: https://cpicpgx.org/guidelines/.
- 13.Hutton B, et al. , The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med, 2015. 162(11): p. 777–84. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). 2021; Available from: www.training.cochrane.org/handbook.
- 15.Veritas Health Innovation. Covidence systematic review software. Available from: www.covidence.org.
- 16.Sterne JA, et al. , ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ, 2016. 355: p. i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lo CK-L, Mertz D, and Loeb M, Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Medical Research Methodology, 2014. 14(1): p. 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farrah K, et al. , Risk of bias tools in systematic reviews of health interventions: an analysis of PROSPERO-registered protocols. Systematic Reviews, 2019. 8(1): p. 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sterne JAC, Higgins JPT, Elbers RG, Reeves BC and the development group for ROBINSI. Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I): detailed guidance. October 20, 2016. [cited 2021 September 3]; Available from: http://www.riskofbias.info.
- 20.Guo B, et al. , Patients carrying CYP2C19 loss of function alleles have a reduced response to clopidogrel therapy and a greater risk of in-stent restenosis after endovascular treatment of lower extremity peripheral arterial disease. J Vasc Surg, 2014. 60(4): p. 993–1001. [DOI] [PubMed] [Google Scholar]
- 21.Pastromas G, et al. , Clopidogrel responsiveness in patients undergoing peripheral angioplasty. Cardiovasc Intervent Radiol, 2013. 36(6): p. 1493–1499. [DOI] [PubMed] [Google Scholar]
- 22.Díaz-Villamarín X, et al. , Genetic polymorphisms influence on the response to clopidogrel in peripheral artery disease patients following percutaneous transluminal angioplasty. Pharmacogenomics, 2016. 17(12): p. 1327–38. [DOI] [PubMed] [Google Scholar]
- 23.Lee J, et al. , CYP2C19 Polymorphism is Associated With Amputation Rates in Patients Taking Clopidogrel After Endovascular Intervention for Critical Limb Ischaemia. Eur J Vasc Endovasc Surg, 2019. 58(3): p. 373–382. [DOI] [PubMed] [Google Scholar]
- 24.Stone GW and Aronow HD, Long-term Care After Percutaneous Coronary Intervention: Focus on the Role of Antiplatelet Therapy. Mayo Clinic Proceedings, 2006. 81(5): p. 641–652. [DOI] [PubMed] [Google Scholar]
- 25.Holmes MV, et al. , CYP2C19 genotype, clopidogrel metabolism, platelet function, and cardiovascular events: a systematic review and meta-analysis. Jama, 2011. 306(24): p. 2704–14. [DOI] [PubMed] [Google Scholar]
- 26.Alakbarzade V, et al. , High on-clopidogrel platelet reactivity in ischaemic stroke or transient ischaemic attack: Systematic review and meta-analysis. J Stroke Cerebrovasc Dis, 2020. 29(7): p. 104877. [DOI] [PubMed] [Google Scholar]
- 27.Ford NF, The Metabolism of Clopidogrel: CYP2C19 Is a Minor Pathway. J Clin Pharmacol, 2016. 56(12): p. 1474–1483. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen KA, et al. , Risk Factors for Bleeding and Clinical Ineffectiveness Associated With Clopidogrel Therapy: A Comprehensive Meta-Analysis. Clinical and Translational Science, 2021. 14(2): p. 645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price MJ, et al. , Influence of genetic polymorphisms on the effect of high- and standard-dose clopidogrel after percutaneous coronary intervention: the GIFT (Genotype Information and Functional Testing) study. J Am Coll Cardiol, 2012. 59(22): p. 1928–37. [DOI] [PubMed] [Google Scholar]
- 30.Tiroch KA, et al. , Protective effect of the CYP2C19 *17 polymorphism with increased activation of clopidogrel on cardiovascular events. Am Heart J, 2010. 160(3): p. 506–12. [DOI] [PubMed] [Google Scholar]
- 31.Sibbing D, et al. , Cytochrome 2C19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement. Circulation, 2010. 121(4): p. 512–8. [DOI] [PubMed] [Google Scholar]
- 32.Paré G, et al. , Effects of CYP2C19 genotype on outcomes of clopidogrel treatment. N Engl J Med, 2010. 363(18): p. 1704–14. [DOI] [PubMed] [Google Scholar]
- 33.Wallentin L, et al. , Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet, 2010. 376(9749): p. 1320–8. [DOI] [PubMed] [Google Scholar]
- 34.Campo G, et al. , Prospective evaluation of on-clopidogrel platelet reactivity over time in patients treated with percutaneous coronary intervention relationship with gene polymorphisms and clinical outcome. J Am Coll Cardiol, 2011. 57(25): p. 2474–83. [DOI] [PubMed] [Google Scholar]
- 35.CPIC. CPIC guidelines. 2021; Available from: https://cpicpgx.org/guidelines/.
- 36.Cavallari LH, et al. , Clinical implementation of rapid CYP2C19 genotyping to guide antiplatelet therapy after percutaneous coronary intervention. Journal of Translational Medicine, 2018. 16(1): p. 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russmann S, et al. , Implementation and management outcomes of pharmacogenetic CYP2C19 testing for clopidogrel therapy in clinical practice. European Journal of Clinical Pharmacology, 2021. 77(5): p. 709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bergmeijer TO, et al. , Feasibility and implementation of CYP2C19 genotyping in patients using antiplatelet therapy. Pharmacogenomics, 2018. 19(7): p. 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson SG, et al. , Feasibility of clinical pharmacist-led CYP2C19 genotyping for patients receiving non-emergent cardiac catheterization in an integrated health system. Pharm Pract (Granada), 2017. 15(2): p. 946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erlinge D, et al. , Clopidogrel metaboliser status based on point-of-care CYP2C19 genetic testing in patients with coronary artery disease. Thromb Haemost, 2014. 111(5): p. 943–50. [DOI] [PubMed] [Google Scholar]
- 41.Arya V, et al. , Association of CYP2C19, CYP3A5 and GPIIb/IIIa gene polymorphisms with Aspirin and Clopidogrel Resistance in a cohort of Indian patients with Coronary Artery Disease. Int J Lab Hematol, 2015. 37(6): p. 809–18. [DOI] [PubMed] [Google Scholar]
- 42.Dean L, Clopidogrel Therapy and CYP2C19 Genotype, in Medical Genetics Summaries, Pratt VM, et al. , Editors. 2012, National Center for Biotechnology Information (US): Bethesda (MD). [Google Scholar]
- 43.Pereira NL, et al. , Effect of Genotype-Guided Oral P2Y12 Inhibitor Selection vs Conventional Clopidogrel Therapy on Ischemic Outcomes After Percutaneous Coronary Intervention: The TAILOR-PCI Randomized Clinical Trial. Jama, 2020. 324(8): p. 761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Claassens DMF, et al. , Clopidogrel in noncarriers of CYP2C19 loss-of-function alleles versus ticagrelor in elderly patients with acute coronary syndrome: A pre-specified sub analysis from the POPular Genetics and POPular Age trials CYP2C19 alleles in elderly patients. Int J Cardiol, 2021. 334: p. 10–17. [DOI] [PubMed] [Google Scholar]
- 45.Klarin D, et al. , Genome-wide association study of peripheral artery disease in the Million Veteran Program. Nat Med, 2019. 25(8): p. 1274–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Capasso JE, The cost of genetic testing for ocular disease: who pays? Curr Opin Ophthalmol, 2014. 25(5): p. 394–9. [DOI] [PubMed] [Google Scholar]
- 47.Bélisle-Pipon JC, et al. , Genetic testing, insurance discrimination and medical research: what the United States can learn from peer countries. Nat Med, 2019. 25(8): p. 1198–1204. [DOI] [PubMed] [Google Scholar]
- 48.Lawton JS, et al. , 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation, 2022. 145(3): p. e18–e114. [DOI] [PubMed] [Google Scholar]
- 49.Firnhaber JM and Powell CS, Lower Extremity Peripheral Artery Disease: Diagnosis and Treatment. Am Fam Physician, 2019. 99(6): p. 362–369. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.


