Abstract
Pathogenic variants in ACTA2, encoding smooth muscle α-actin, predispose to thoracic aortic aneurysms and dissections. ACTA2 variants altering arginine 179 predispose to a more severe, multisystemic disease termed smooth muscle dysfunction syndrome (SMDS; OMIM 613834). Vascular complications of SMDS include patent ductus arteriosus (PDA) or aortopulmonary window, early-onset thoracic aortic disease (TAD), moyamoya-like cerebrovascular disease, and primary pulmonary hypertension. Patients also have dysfunction of other smooth muscle-dependent systems, including congenital mydriasis, hypotonic bladder, and gut hypoperistalsis. Here, we describe five patients with novel heterozygous ACTA2 missense variants, p.Arg179Gly, p.Met46Arg, p.Thr204Ile, p.Arg39Cys, and p.Ile66Asn, who have clinical complications that align or overlap with SMDS. Patients with the ACTA2 p.Arg179Gly and p.Thr204Ile variants display classic features of SMDS. The patient with the ACTA2 p.Met46Arg variant exhibits exclusively vascular complications of SMDS, including early-onset TAD, PDA, and moyamoya-like cerebrovascular disease. The patient with the ACTA2 p.Ile66Asn variant has an unusual vascular complication: a large fusiform internal carotid artery aneurysm. The patient with the ACTA2 p.Arg39Cys variant has pulmonary, gastrointestinal, and genitourinary but no vascular SMDS manifestations. Identifying pathogenic ACTA2 variants associated with features of SMDS is critical for aggressive surveillance and management of vascular and non-vascular complications and delineating the molecular pathogenesis of SMDS.
Introduction
Pathogenic variants in ACTA2, which encodes the smooth muscle (SM)-specific α-actin monomer (SM α-actin), are the most frequent cause of non-syndromic heritable thoracic aortic aneurysms and dissections (Guo et al., 2007; Guo et al., 2009; Morisaki et al., 2009). SM α-actin polymerizes to form thin filaments that interact with smooth muscle myosin-containing thick filaments to form the contractile unit in smooth muscle cells (SMCs) (Milewicz et al., 2008). ACTA2 missense mutations disrupt SM α-actin polymerization and interaction with myosin, highlighting the importance of altered SMC contractile function in the pathogenesis of thoracic aortic disease (Lu et al., 2015; Lu et al., 2016; Milewicz et al., 2008; Milewicz et al., 2017). Specific ACTA2 mutations also predispose to other vascular diseases, either early onset ischemic strokes due to moyamoya-like occlusion of the distal internal carotid artery (ICA) or early onset coronary artery disease (Guo et al., 2009; Milewicz et al., 2010; Regalado et al., 2015; Regalado et al., 2018). At the severe end of the disease spectrum, de novo variants disrupting arginine 179 cause a syndrome termed Smooth Muscle Dysfunction Syndrome (SMDS), which is characterized by early onset vascular complications along with disruption of other organs dependent on SMCs for their function (Milewicz et al., 2010). This syndrome is characterized by patent ductus arteriosus (PDA) or aortopulmonary window (APW), early-onset and highly penetrant thoracic aortic disease, moyamoya-like cerebrovascular disease, and congenital mydriasis, along with less penetrant pulmonary hypertension, chronic lung disease, hypoperistalsis, malrotation of the gut, prune belly syndrome, hypotonic bladder, megacystis, and hydronephrosis (Milewicz et al., 2010; Regalado et al., 2018; Richer et al., 2012). Cerebrovascular disease in these patients has features of moyamoya disease (MMD), specifically stenosis or occlusion of the terminal portion of the ICA that may extend into the middle and anterior cerebral arteries, without formation of collateral arteries typically observed in MMD patients (Munot et al., 2012). SMDS patients also have fusiform dilation of the proximal internal carotid arteries, abnormally straight intracranial arteries, and periventricular hyperintensities suggestive of small vessel disease (Georgescu et al., 2015; Lauer et al., 2021; Milewicz et al., 2010; Munot et al., 2012; Regalado et al., 2018). The most common ACTA2 variant associated with SMDS is p.Arg179His, but p.Arg179Cys, p.Arg179Leu, and p.Arg179Ser variants have also been identified in SMDS patients (Regalado et al., 2018). We report here five patients, all of European descent, with novel ACTA2 pathogenic variants who have features of SMDS.
Materials and Methods
The study was approved by the institutional review board of the University of Texas Health Science Center at Houston (UTHealth). Participants were recruited for the Montalcino Consortium patient registry at UTHealth. De-identified medical records, including imaging studies and interpretations, surgical reports, pathology reports, and physicians’ notes, were reviewed after obtaining informed consent by proband and/or family members. The ACTA2 variants were identified through clinical genetic testing.
Results
Patient 1 was a female infant with a de novo missense ACTA2 p.Arg179Gly variant (c.535C>G; likely pathogenic) identified via whole exome sequencing shortly after birth. The patient was diagnosed antenatally with bladder dilation and bilateral ventriculomegaly in the brain by ultrasound at 20 weeks gestation. Brain fetal magnetic resonance imaging (MRI) at 27 weeks gestation confirmed bilateral ventriculomegaly. She was found to have mild hydronephrosis on the left kidney at 35 weeks gestation and bilateral moderate hydronephrosis by 37 weeks gestation. The patient was delivered at 37 weeks gestation via Cesarean section due to breech presentation. Congenital mydriasis was noted at birth. She displayed regular respiratory effort and required continuous positive airway pressure at 7.0 cm H2O and FiO2 of 35%. Abdominal distension was noted at birth, and 100 ml of urine was drained from her bladder. The patient was also diagnosed with megacystis-microcolon-intestinal hypoperistalsis syndrome, previously associated with variants altering ACTA2 p.Arg179, and underwent surgery to correct gut malrotation (Richer et al., 2012). Postnatal brain MRI showed periventricular white matter loss. Cardiac assessment revealed a massively aneurysmal PDA with large volume bidirectional shunting, causing branch pulmonary artery dilatation, bronchial compression, and collapse of the left lower lobe medial basal segment. She developed progressive systemic steal through the PDA with increasing left to right shunting, leading to respiratory difficulties and systemic hypoperfusion. She went on to be diagnosed with bilateral interstitial pulmonary edema with scattered subpleural atelectasis in the right upper lobe. She also had innominate artery syndrome, with tracheal stenosis due to compression of the trachea by the brachiocephalic artery. PDA repair was not pursued due to the complexity of the procedure with the prospect of a prolonged recovery period. Patient received palliative care and died at 27 days old.
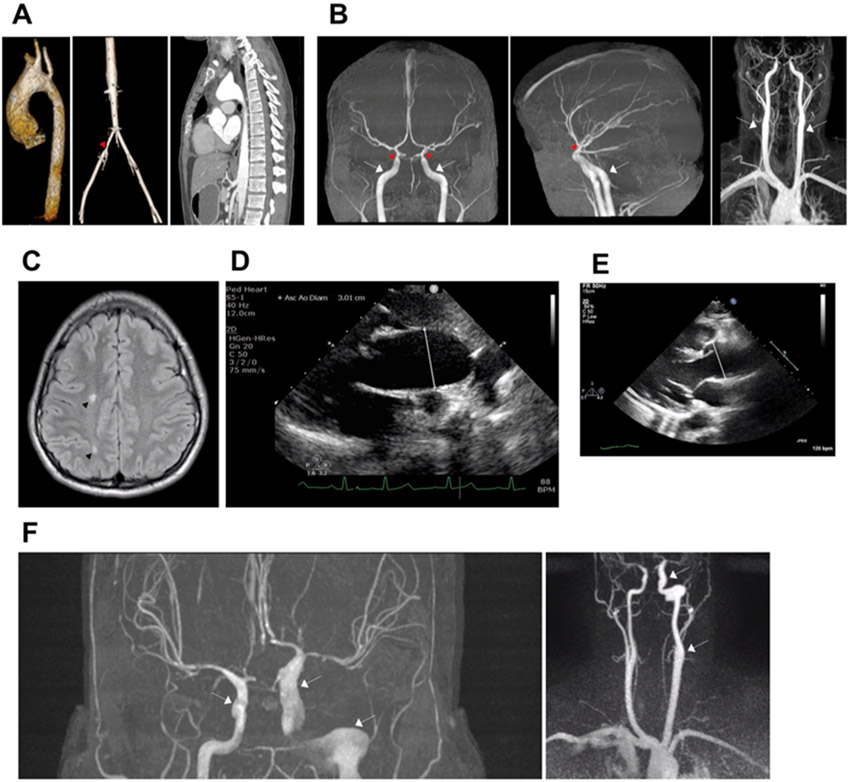
Patient 2 is a 23-year-old male who had a patent foramen ovale and PDA repaired at birth. At 19 years of age, the patient was found to have abdominal and femoral bruit on routine examination. Echocardiography revealed a dilated aortic root and ascending aorta with maximum diameter at the aortic root sinuses of Valsalva of 40 mm (Z-score +3.70) (Fig. 1E). Three-dimensional magnetic resonance angiography (MRA) confirmed mildly dilated aortic root measuring 40 x 40 x 41 mm in systole and a mildly dilated distal ascending aorta just proximal to origin of great vessels from arch measuring 39 x 36 mm (Fig. 1A, left). Valve sparing surgical repair of the aortic root and ascending aorta was performed at the age of 20 years, and postoperative computed tomography (CT) angiography a year later identified fusiform dilation of the proximal and mid aortic arch with the largest diameter of 47 mm (Fig. 1A, right). MRA showed continued mild fusiform aneurysm of the proximal abdominal aorta and at the origin of the celiac trunk (25 x 24 mm). He also has diffuse narrowing of the left common iliac artery (4.7 mm) (Fig. 1A, middle). Just proximal to bifurcation into external and internal iliacs, a short segment (8 mm) of more pronounced stenosis was noted, with a minimum vessel diameter of 4.1 mm compared to 8.6 mm on the contralateral side (Fig. 1A, middle). There was also post-stenotic dilation of the proximal left internal and external iliac arteries (Fig. 1A, middle). The patient’s brain MRA revealed robust appearance of the petrous and cavernous portions of the ICAs, then abrupt tapering and straightening of the clinoid and supraclinoid portions of the ICAs bilaterally without occlusion (Fig. 1B). Extracranial segments of ICA demonstrate bilateral symmetric fusiform dilation and straightened appearance (Fig. 1B). There was a straightened appearance to the intracerebral arteries, as well as diffuse and symmetric narrowing of the bilateral MCAs, ACAs, PCAs and their major branches (Fig. 1B, left, middle). The patient had onset of TIAs and psychosis, and MRI of the brain showed stable stenotic lesions with scattered focal areas of T2 or FLAIR hyperintense signal abnormalities suspicious for chronic ischemia in the bilateral cerebral hemispheres (Fig. 1C). MRA of the head and neck four years after diagnosis showed no significant changes. A de novo missense ACTA2 p.Met46Arg variant (c.137T>G; likely pathogenic) was identified, as previously described, and the patient is on currently taking atenolol (Zhang et al., 2019).
Figure 1.
A. 3D MR angiogram from Patient 2 (p.Met46Arg) shows dilation of the aortic root and ascending aorta (pre-surgery) (left image) and stenosis of the left common iliac artery (red arrowhead, middle image). CT angiogram (post-surgical repair) shows fusiform dilation of the proximal and mid aortic arch with the largest diameter of 47 mm (post-surgery) (right image). B. MR angiogram images from Patient 2 (p.Met46Arg) show dilation of proximal ICAs (white arrows), stenosis of distal ICAs (red arrowheads), and straightening of intracranial arteries bilaterally. C. MRI of the brain of Patient 2 (p.Met46Arg) shows white matter signal change/hyperintensity on axial T2-weighted images (black arrowheads). D. Transthoracic echocardiography from Patient 3 (p.Thr204Ile) shows dilated aortic root (28.3 mm) and ascending aorta (30.1 mm) with point of measurement indicated by the white line. E. Transthoracic echocardiography from Patient 2 (p.Met46Arg) shows dilated aortic root (40 mm, white line). F. MRA from Patient 5 (p.Ile66Asn) shows fusiform aneurysm of the left intracranial ICA at its entrance into the skull to its terminous measuring 10 mm in the cavernous segment with maximal diameter measuring 13 mm in the petrous segment and tapering to 6 mm and mild dilation of the distal right cavernous carotid to its supraclinoid segment measuring 6 mm (white arrows). MR; magnetic resonance. CT; computed tomography. ICA; internal carotid artery.
Patient 3, a 6 year old female with a de novo ACTA2 p.Thr204Ile variant (c.611C>T; likely pathogenic), had a prenatal history of choroid plexus cyst, single umbilical artery, and megacystis at 20 week anatomy ultrasound. At birth, she had a hemodynamically significant PDA that was repaired, an atrial septal defect, and significant dilation of the aortic root (26 mm, Z-score +3.63) and ascending aorta (25 mm, Z-score +4.28) (Fig. 1D) with mild aortic insufficiency. She also exhibited bladder dysfunction at the time of birth requiring catheterizations until 20 months of age. She was diagnosed with pulmonary hypertension, and at the age of 6 years, with asthma. The patient was started on atenolol due to relatively rapid growth of aortic root and ascending aorta, which was later switched to losartan due to worsening of her asthma. She has no developmental delay or stroke-like symptoms and has not had imaging of her brain.
Patient 4 is a 5 year old female who inherited a familial ACTA2, p.Arg39Cys variant (c. 115C>T; likely pathogenic, Fig. 2). The patient was born at 37 weeks gestation and was hospitalized for 4 days due to feeding issues, difficulty breathing, and jaundice; echocardiogram was normal. MRI of the brain and MRA of the head, neck, chest, abdomen, and pelvis showed no abnormalities. She had obstructive sleep apnea secondary to severe tonsillar hyperplasia and underwent adenoidectomy at 2 years of age. She continued to experience mild obstructive sleep hypopnea and mild fatigue and was placed on bilevel positive airway pressure at 4 years old, with some relief. Chest x-ray at 2 years of age identified streaky perihilar changes and bilateral peribronchial cuffing, suggestive of chronic aspiration or viral/reactive airway disease. She was diagnosed with persistent asthma and recurrent laryngotracheobronchitis responsive to bronchodilators and dexamethasone. Chest x-ray imaging, a clinical history of asthma, and use of positive pressure in this patient are suggestive of the chronic lung disease observed in SMDS patients (Regalado et al., 2018).
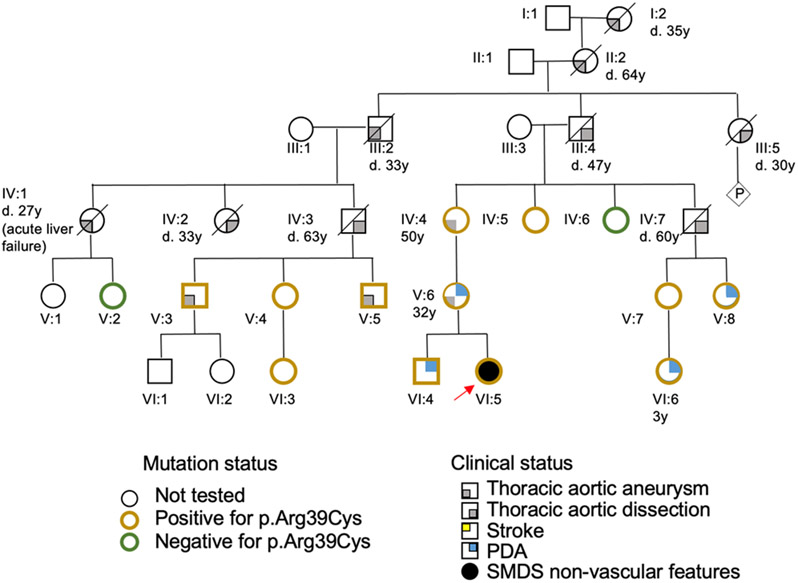
Figure 2.
Pedigree for Patient 4 (ACTA2, p.Arg39Cys) shows inheritance of predominantly thoracic aortic disease segregating with the variant in this family. Males are designated as squares, females as circles, and presence of clinical presentation and genetic test results is indicated according to the legend. Age of death (d. age) or diagnosis (age) is indicated underneath the individual when available. The proband (Patient 4) is indicated with a red arrow (individual VI:5). PDA; patent ductus arteriosus.
Patient 4 also has severe oropharyngeal phase dysphagia with recurrent aspiration pneumonia, gastrointestinal dysmotility, and chronic constipation. On fluoroscopy, no evidence of malrotation or gastric outlet obstruction was found, but significant stool burden in rectum was identified. A gastrostomy tube was placed at 2 years of age and at 5 years of age, she was advanced to oral thickened fluids but still receives 80% of her nutrition through the gastrostomy tube. Finally, Patient 4 has urinary retention, requiring catheterization to void the bladder.
The ACTA2 p.Arg39Cys variant, inherited from her mother, is the cause of heritable thoracic aortic disease associated with variably penetrant PDA in the mother’s family (Fig. 2). The patient’s mother has a history of atrial septal defect and a surgically repaired PDA, and the patient’s brother has a PDA (Fig. 2). The maternal grandmother is heterozygous for the ACTA2 pathogenic variant and was diagnosed with aortic enlargement identified in her 30s; she underwent thoracic aneurysm repair at age of 50 years (Fig. 2). The father of the maternal grandmother passed away at age 47 years due to an acute aortic dissection (Fig. 2). A cousin’s daughter with the ACTA2 variant had her PDA surgically corrected. No family members have lung, urinary, or gastrointestinal problems similar to the proband nor MMD-like cerebrovascular disease.
Patient 5 is a 27-year-old woman who presented with a type A aortic dissection at the age of 21, three months after giving birth to her first child. Her ascending aorta diameter was 59 mm at the time of dissection, and a bicuspid aortic valve was identified. She underwent emergent valve sparing aortic root and ascending aortic repair. There is no family history of thoracic aortic disease in the proband’s large family, and genetic testing identified an ACTA2 p.Ile66Asn variant (c.197T>A; variant of unknown significance). MRA four years later revealed fusiform aneurysm of the left intracranial ICA at its entrance into the skull to its terminus measuring 10 mm in the cavernous segment and tapering to 6 mm along with mild dilation of the distal right cavernous carotid measuring 6 mm to its supraclinoid segment (Fig. 1F). The maximum caliber of the left petrous segment is 13 mm (Fig. 1F). She also has hypoplastic cerebral arteries, suggesting diffuse stenosis, and non-specific periventricular white matter changes without classic findings of embolic stroke. Previously, relative narrowing of the terminal ICA to the petrous ICA was shown to correlate with acute ischemic stroke in ACTA2 p.Arg179 patients (Lauer et al., 2021). Patient 5 has a relative terminal ICA stenosis score >0.6, suggesting increased risk for acute ischemic stroke. She is currently taking metoprolol, losartan, and aspirin.
Discussion
We report here five patients with complications typically seen in SMDS that have missense variants not previously associated with SMDS. Patient 1 has classic features of SMDS and a de novo variant leading to an amino acid substitution, p.Arg179Gly, not previously identified in SMDS patients (Fig. 3, Table S1). In patients with SMDS, ACTA2 p.Arg179 is most commonly altered to histidine but can also be changed to cysteine, leucine and serine, and all these missense variants are associated with similar age of onset and features of SMDS (Fig. 3, Table S1). Patient 1 was the first patient identified with an ACTA2 p.Arg179Gly variant and presented with typical features of SMDS but had severe complications leading to early death including cardiovascular, cerebrovascular, respiratory, gastrointestinal, and genitourinary issues. Thus, SMDS-causing variants alter Arg179 to 5 different amino acids, specifically histidine, cysteine, serine, leucine, and glycine. An additional amino acid substitution to proline is possible but has not been observed in SMDS patients.
Figure 3.
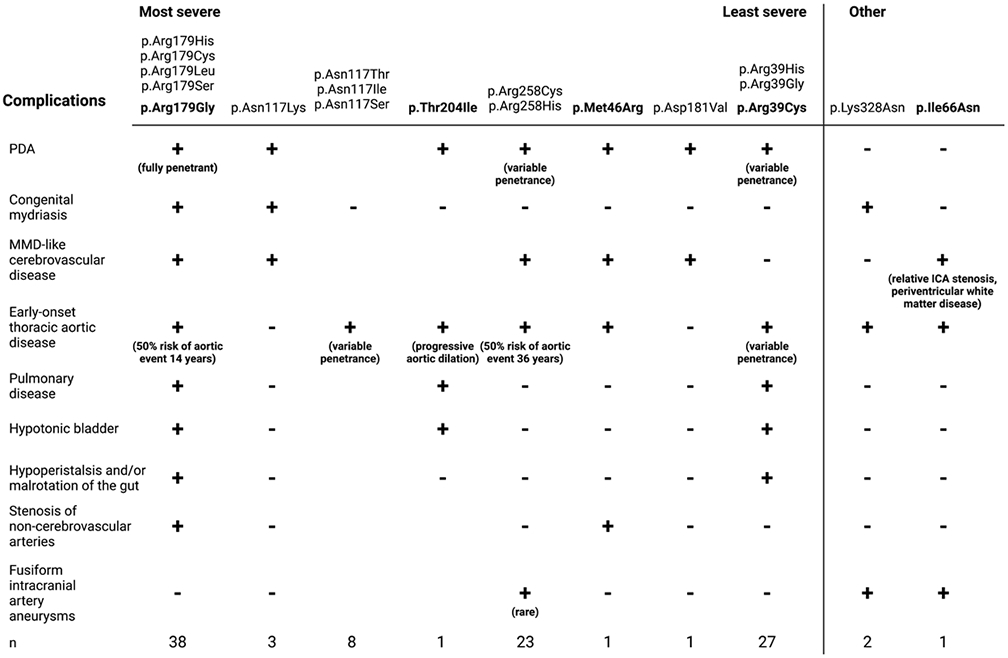
ACTA2 mutations associated with the increasingly severe spectrum of complications observed in SMDS patients. *Aortic events defined as aortic aneurysm repair or presentation with dissection. SMDS; smooth muscle dysfunction syndrome. ICA; internal carotid artery. Created with BioRender.com.
The moyamoya-like cerebrovascular disease can be observed in other recurrent inherited pathogenic variants in ACTA2 disrupting arginine 258 (p.Arg258Cys, p.Arg258His) (Fig. 3, Table S1) (Diness et al., 2020; Guo et al., 2009). These patients have variable penetrance of PDA, along with later onset thoracic aortic disease, moyamoya-like stenosis of the distal ICAs, white matter hyperintensities, and intracranial artery straightening seen in SMDS patients but do not have hypotonic bladders or gut complications (Fig. 3, Table S1) (Diness et al., 2020; Guo et al., 2009). A family with the ACTA2 p.Asn117Lys variant had MMD-like cerebrovascular disease, PDA, poorly reactive pupils, and retinal artery tortuosity without evidence of thoracic aortic disease. Other families with variants affecting p.Asn117 had adult onset thoracic aortic disease but were not reported to have cerebrovascular disease (Fig. 3, Table S1) (Ke et al., 2016; Mc Glacken-Byrne et al., 2020). Additionally, a patient with a de novo ACTA2 p.Asp181Val mutation was reported to have MMD-like disease, periventricular white matter hyperintensities, hypoplasia of the corpus callosum, and a prior history of PDA without evidence of aortic disease or ocular dysfunction (Fig. 3, Table S1) (Pinto, 2020). Patient 2 has a phenotype similar to these patients, including a PDA, early onset thoracic aortic disease and moyamoya-like cerebrovascular disease, intracranial artery straightening, and white matter hyperintensities, and he has a novel, de novo ACTA2 p.Met46Arg variant (Fig. 3, Table S1). This variant does not meet the American College of Medical Genetics criteria to be a pathogenic variant but given that the variant is de novo and the phenotype of the proband overlaps with SMDS, it is likely to be a pathogenic variant. He also has stenosis of the left common iliac artery. SMDS patients have been found to have steno-occlusive lesions in other muscular arteries, including the pulmonary, hepatic (unpublished data), and vasa vasorum arteries (Milewicz et al., 2010).
Patient 3 had a PDA, early onset aortic disease, genitourinary, and pulmonary features of SMDS due to a de novo ACTA2 missense variant not previously associated with SMDS, p.Thr204Ile, but the status of her cerebrovascular disease is unknown (Fig. 3, Table S1). Patient 4 has an ACTA2 pathogenic variant, p.Arg39Cys, established to predispose to thoracic aortic disease and causing heritable thoracic aortic disease associated with PDA in her family (Fig. 3, Table S1) (Hoffjan et al., 2011; Regalado et al., 2015). The proband had early-onset lung, bowel, and bladder complications typical for SMDS without vascular manifestations. We hypothesize that Patient 4 has an additional, unidentified rare variant in another gene that disrupts primarily the SMCs in the gut and bladder, and therefore augments the effect of the inherited ACTA2 variant and leads to her SMDS complications. Loss of function variants in MYLK and MYH11 have been associated with megacystis-microcolon-intestinal hypoperistalsis syndrome, supporting that genes causing thoracic aortic disease can also disrupt the gut and bladder (Gauthier et al., 2015; Halim et al., 2017; Wang et al., 2019).
Finally, Patient 5 had early onset thoracic aortic disease, along with a distinct phenotype of cerebrovascular disease: a large fusiform aneurysm of her left ICA (Fig. 3, Table S1). Cerebral aneurysms are unusual in patients with ACTA2 pathogenic variants but have been associated with the ACTA2 p.Lys328Asn variant in addition to early-onset thoracic aortic aneurysms and dissections (<50 years of age) and distal ICA stenosis (Fig. 3, Table S1) (Amans et al., 2013; Shalhub et al., 2020; Ware et al., 2014). The patients with the ACTA2 p.Lys328Asn variants also presented with congenital mydriasis but did not have lung, gastrointestinal or bladder complications (Ware et al., 2014).
The patients reported here raise a critical question of how to classify ACTA2 variants with variable features of SMDS (Fig. 3). The major causes of death in SMDS patients are due to aortic disease, MMD-like cerebrovascular disease, and pulmonary disease. Therefore, it is essential to correctly designate patients with ACTA2 variants associated with these features of SMDS. Based on the current recommendations, a dyadic approach to delineation of genetic conditions is recommended (Biesecker et al., 2021). Thus, both patients with fully penetrant SMDS and patients with variants previously reported to cause SMDS should be designated ACTA2-related SMDS. We recommend that patients with some features but not all the complications of SMDS (e.g, p.Arg258 variants) be designated ACTA2-related SMDS-like.
Patients with disease-causing ACTA2 variants can present with a range of complications associated with SMDS, and it is essential for physicians to recognize these complications in order to ensure patients receive proper surveillance and treatment. It is also critical to identify complications of SMDS since these clinical features indicate more severe clinical prognosis. Congenital mydriasis, PDA, and gut and bladder complications, which when present in infancy in all patients with ACTA2 p.Arg179 variants, appear to be the reliable predictors of a diagnosis of SMDS (Fig. 3). The presence of a PDA and/or congenital mydriasis in the absence of other features of SMDS suggests higher risk for early-onset thoracic aortic disease and MMD-like cerebrovascular disease, as illustrated by Patient 2 with ACTA2 p.Met46Arg variant, Patient 3 with the ACTA2 p.Thr204Ile variant, and the patients with ACTA2 p.Arg258, p.Asn117, p.Lys328Asn, and p.Asp181Val variants (Fig. 3). These patients should receive vascular disease surveillance and an annual ophthalmologic exam for congenital mydriasis, similar to the current recommendations for SMDS patients (Regalado et al., 2018). Based on this study and previous studies assessing aortic disease progression in SMDS patients, it is critical to consider concomitant repair of a normal arch when the root and ascending aorta are repaired in patients with SMDS-like features, as illustrated by Patient 2 in this report and as previously recommended for SMDS patients (Regalado et al., 2018). ICA aneurysms are rare in ACTA2 patients, and not all patients with ACTA2 variants require cerebrovascular imaging, but these cases are associated with early-onset thoracic aortic disease. Importantly, individuals who have de novo novel variants, PDA, congenital mydriasis, and/or onset of thoracic aortic surgery or dissection before 30 years of age should have cerebrovascular imaging.
Supplementary Material
Acknowledgments:
We would like to thank the patients and their families for participating in this study, along with Josipa Paska and Ludmilla Temerty for their continued support of the Montalcino Aortic Consortium.
Sources of funding:
This work was supported by the National Heart, Lung and Blood Institute (NIH R01HL109942 to D.M.M) and (RO1 HL146583 to D.M.M), America Heart Association Merit Award (D.M.M), Genetic Aortic Disorders Association Canada, Marylin and Frederick R. Lummis, MD, Fellowship in the Biomedical Sciences (A.K.), NIH TL1TR003169 (A.K.), NIH UL1TR003167 (A.K.), NIH T32GM120011 (K.K.), and American Heart Association Grant 20CDA35310689 (C.S.K). This content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Disclosures: None.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Amans MR, Stout C, Fox C, Narvid J, Hetts SW, Cooke DL, Higashida RT, Dowd CF, McSwain H, & Halbach VV (2013, Nov 29). Cerebral arteriopathy associated with Arg179His ACTA2 mutation. BMJ Case Rep, 2013. 10.1136/bcr-2013-010997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesecker LG, Adam MP, Alkuraya FS, Amemiya AR, Bamshad MJ, Beck AE, Bennett JT, Bird LM, Carey JC, Chung B, Clark RD, Cox TC, Curry C, Dinulos MBP, Dobyns WB, Giampietro PF, Girisha KM, Glass IA, Graham JM Jr., Gripp KW, Haldeman-Englert CR, Hall BD, Innes AM, Kalish JM, Keppler-Noreuil KM, Kosaki K, Kozel BA, Mirzaa GM, Mulvihill JJ, Nowaczyk MJM, Pagon RA, Retterer K, Rope AF, Sanchez-Lara PA, Seaver LH, Shieh JT, Slavotinek AM, Sobering AK, Stevens CA, Stevenson DA, Tan TY, Tan WH, Tsai AC, Weaver DD, Williams MS, Zackai E, & Zarate YA (2021, Jan 7). A dyadic approach to the delineation of diagnostic entities in clinical genomics. Am J Hum Genet, 108(1), 8–15. 10.1016/j.ajhg.2020.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diness BR, Palmquist RN, Norling R, Hove H, Bundgaard H, Hertz JM, Kondziella D, Krieger D, Dunø M, & Grønborg S (2020, 2020/August/15/). Expanding the cerebrovascular phenotype of the p.R258H variant in ACTA2 related hereditary thoracic aortic disease (HTAD). Journal of the Neurological Sciences, 415, 116897. https://doi.org/ 10.1016/j.jns.2020.116897 [DOI] [PubMed] [Google Scholar]
- Gauthier J, Ouled Amar Bencheikh B, Hamdan FF, Harrison SM, Baker LA, Couture F, Thiffault I, Ouazzani R, Samuels ME, Mitchell GA, Rouleau GA, Michaud JL, & Soucy J-F (2015, 2015/September/01). A homozygous loss-of-function variant in MYH11 in a case with megacystis-microcolon-intestinal hypoperistalsis syndrome. European Journal of Human Genetics, 23(9), 1266–1268. 10.1038/ejhg.2014.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu MM, Pinho Mda C, Richardson TE, Torrealba J, Buja LM, Milewicz DM, Raisanen JM, & Burns DK (2015, Dec 4). The defining pathology of the new clinical and histopathologic entity ACTA2-related cerebrovascular disease. Acta Neuropathol Commun, 3, 81. 10.1186/s40478-015-0262-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo DC, Pannu H, Tran-Fadulu V, Papke CL, Yu RK, Avidan N, Bourgeois S, Estrera AL, Safi HJ, Sparks E, Amor D, Ades L, McConnell V, Willoughby CE, Abuelo D, Willing M, Lewis RA, Kim DH, Scherer S, Tung PP, Ahn C, Buja LM, Raman CS, Shete SS, & Milewicz DM (2007, Dec). Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet, 39(12), 1488–1493. 10.1038/ng.2007.6 [DOI] [PubMed] [Google Scholar]
- Guo DC, Papke CL, Tran-Fadulu V, Regalado ES, Avidan N, Johnson RJ, Kim DH, Pannu H, Willing MC, Sparks E, Pyeritz RE, Singh MN, Dalman RL, Grotta JC, Marian AJ, Boerwinkle EA, Frazier LQ, LeMaire SA, Coselli JS, Estrera AL, Safi HJ, Veeraraghavan S, Muzny DM, Wheeler DA, Willerson JT, Yu RK, Shete SS, Scherer SE, Raman CS, Buja LM, & Milewicz DM (2009, May). Mutations in smooth muscle alpha-actin (ACTA2) cause coronary artery disease, stroke, and Moyamoya disease, along with thoracic aortic disease. Am J Hum Genet, 84(5), 617–627. 10.1016/j.ajhg.2009.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halim D, Brosens E, Muller F, Wangler MF, Beaudet AL, Lupski JR, Akdemir ZHC, Doukas M, Stoop HJ, de Graaf BM, Brouwer RWW, van Ijcken WFJ, Oury J-F, Rosenblatt J, Burns AJ, Tibboel D, Hofstra RMW, & Alves MM (2017). Loss-of-Function Variants in MYLK Cause Recessive Megacystis Microcolon Intestinal Hypoperistalsis Syndrome. The American Journal of Human Genetics, 101(1), 123–129. 10.1016/j.ajhg.2017.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffjan S, Waldmüller S, Blankenfeldt W, Kötting J, Gehle P, Binner P, Epplen JT, & Scheffold T (2011, May). Three novel mutations in the ACTA2 gene in German patients with thoracic aortic aneurysms and dissections. Eur J Hum Genet, 19(5), 520–524. 10.1038/ejhg.2010.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke T, Han M, Zhao M, Wang QK, Zhang H, Zhao Y, Ruan X, Li H, Xu C, & Sun T (2016, 2016/July/18). Alpha-actin-2 mutations in Chinese patients with a non-syndromatic thoracic aortic aneurysm. BMC Medical Genetics, 17(1), 45. 10.1186/s12881-016-0310-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer A, Speroni SL, Patel JB, Regalado E, Choi M, Smith E, Kalpathy-Kramer J, Caruso P, Milewicz DM, & Musolino PL (2021). Cerebrovascular Disease Progression in Patients With ACTA2 Arg179 Pathogenic Variants. Neurology, 96(4), e538–e552. 10.1212/wnl.0000000000011210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Fagnant PM, Bookwalter CS, Joel P, & Trybus KM (2015). Vascular disease-causing mutation R258C in ACTA2 disrupts actin dynamics and interaction with myosin. Proceedings of the National Academy of Sciences, 112(31), E4168–E4177. 10.1073/pnas.1507587112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Fagnant PM, Krementsova EB, & Trybus KM (2016, Oct 7). Severe Molecular Defects Exhibited by the R179H Mutation in Human Vascular Smooth Muscle α-Actin. J Biol Chem, 291(41), 21729–21739. 10.1074/jbc.M116.744011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Glacken-Byrne AB, Prentice D, Roshandel D, Brown MR, Tuch P, Yau KSY, Sivadorai P, Davis MR, Laing NG, & Chen FK (2020). High-resolution iris and retinal imaging in multisystemic smooth muscle dysfunction syndrome due to a novel Asn117Lys substitution in ACTA2: a case report. BMC ophthalmology, 20(1), 68–68. 10.1186/s12886-020-01344-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milewicz DM, Guo DC, Tran-Fadulu V, Lafont AL, Papke CL, Inamoto S, Kwartler CS, & Pannu H (2008). Genetic basis of thoracic aortic aneurysms and dissections: focus on smooth muscle cell contractile dysfunction. Annu Rev Genomics Hum Genet, 9, 283–302. 10.1146/annurev.genom.8.080706.092303 [DOI] [PubMed] [Google Scholar]
- Milewicz DM, Ostergaard JR, Ala-Kokko LM, Khan N, Grange DK, Mendoza-Londono R, Bradley TJ, Olney AH, Ades L, Maher JF, Guo D, Buja LM, Kim D, Hyland JC, & Regalado ES (2010, Oct). De novo ACTA2 mutation causes a novel syndrome of multisystemic smooth muscle dysfunction. Am J Med Genet A, 152A(10), 2437–2443. 10.1002/ajmg.a.33657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milewicz DM, Trybus KM, Guo DC, Sweeney HL, Regalado E, Kamm K, & Stull JT (2017, Jan). Altered Smooth Muscle Cell Force Generation as a Driver of Thoracic Aortic Aneurysms and Dissections. Arterioscler Thromb Vasc Biol, 37(1), 26–34. 10.1161/atvbaha.116.303229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisaki H, Akutsu K, Ogino H, Kondo N, Yamanaka I, Tsutsumi Y, Yoshimuta T, Okajima T, Matsuda H, Minatoya K, Sasaki H, Tanaka H, Ishibashi-Ueda H, & Morisaki T (2009). Mutation of ACTA2 gene as an important cause of familial and nonfamilial non-syndromatic thoracic aortic aneurysm and/or dissection (TAAD). Human Mutation, 30(10), 1406–1411. https://doi.org/ 10.1002/humu.21081 [DOI] [PubMed] [Google Scholar]
- Munot P, Saunders DE, Milewicz DM, Regalado ES, Ostergaard JR, Braun KP, Kerr T, Lichtenbelt KD, Philip S, Rittey C, Jacques TS, Cox TC, & Ganesan V (2012, Aug). A novel distinctive cerebrovascular phenotype is associated with heterozygous Arg179 ACTA2 mutations. Brain, 135(Pt 8), 2506–2514. 10.1093/brain/aws172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto M (2020). A Novel ACTA2 Gene Disease-Causing Variant Presenting with a Complex Brain Phenotype. Sinapse, 20(4), 181–183. 10.46531/sinapse/CC/200032/2020 [DOI] [Google Scholar]
- Regalado ES, Guo D.-c., Prakash S, Bensend TA, Flynn K, Estrera A, Safi H, Liang D, Hyland J, Child A, Arno G, Boileau C, Jondeau G, Braverman A, Moran R, Morisaki T, Morisaki H, Pyeritz R, Coselli J, LeMaire S, & Milewicz DM (2015). Aortic Disease Presentation and Outcome Associated With ACTA2 Mutations. Circulation: Cardiovascular Genetics, 8(3), 457–464. 10.1161/CIRCGENETICS.114.000943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regalado ES, Mellor-Crummey L, De Backer J, Braverman AC, Ades L, Benedict S, Bradley TJ, Brickner ME, Chatfield KC, Child A, Feist C, Holmes KW, Iannucci G, Lorenz B, Mark P, Morisaki T, Morisaki H, Morris SA, Mitchell AL, Ostergaard JR, Richer J, Sallee D, Shalhub S, Tekin M, Estrera A, Musolino P, Yetman A, Pyeritz R, & Milewicz DM (2018, Oct). Clinical history and management recommendations of the smooth muscle dysfunction syndrome due to ACTA2 arginine 179 alterations. Genet Med, 20(10), 1206–1215. 10.1038/gim.2017.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richer J, Milewicz DM, Gow R, de Nanassy J, Maharajh G, Miller E, Oppenheimer L, Weiler G, & O'Connor M (2012). R179H mutation in ACTA2 expanding the phenotype to include prune-belly sequence and skin manifestations. American Journal of Medical Genetics Part A, 158A(3), 664–668. https://doi.org/ 10.1002/ajmg.a.35206 [DOI] [PubMed] [Google Scholar]
- Shalhub S, Roman MJ, Eagle KA, LeMaire SA, Zhang Q, Evangelista A, & Milewicz DM (2020). Type B Aortic Dissection in Young Individuals With Confirmed and Presumed Heritable Thoracic Aortic Disease. The Annals of Thoracic Surgery, 109(2), 534–540. 10.1016/j.athoracsur.2019.07.004 [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhang J, Wang H, Feng Q, Luo F, & Xie J (2019, Nov). Compound heterozygous variants in MYH11 underlie autosomal recessive megacystis-microcolon-intestinal hypoperistalsis syndrome in a Chinese family. J Hum Genet, 64(11), 1067–1073. 10.1038/s10038-019-0651-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware SM, Shikany A, Landis BJ, James JF, & Hinton RB (2014). Twins With Progressive Thoracic Aortic Aneurysm, Recurrent Dissection and ACTA2 Mutation. Pediatrics, 134(4), e1218–e1223. 10.1542/peds.2013-2503 [DOI] [PubMed] [Google Scholar]
- Zhang A, Jo A, Grajewski K, & Kim J (2019). Characteristic Cerebrovascular Findings Associated with ACTA2 Gene Mutations. Canadian Journal of Neurological Sciences / Journal Canadien des Sciences Neurologiques, 46(3), 342–343. 10.1017/cjn.2019.20 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.