Abstract
Objective:
Despite lower plasma HIV RNA levels, women progress faster to AIDS than men. The reasons for these differences are not clear but might be a consequence of an elevated inflammatory response in women.
Methods:
We investigated sex differences in cytokine profiles by measuring the concentrations of 36 cytokine/chemokines by Luminex in blood of women and men (sex at birth) with chronic HIV infection under suppressive therapy. We initially performed a principal component analysis to see if participants clustered by sex, and then fit a PLS-DA model where we used cytokines to predict sex at birth. The significance of the difference in 9 cytokines with VIP>1 was tested using Wilcoxon test-rank. Further, potential confounding factors were tested by multivariate linear regression models.
Results:
Overall, we predicted sex at birth in the PLS-DA model with an error rate of approximately 13%. We identified 5 cytokines which were significantly higher in women compared to men, namely the pro-inflammatory chemokines CXCL1 (Gro-α), CCL5 (RANTES), CCL3 (MIP-1α), CCL4 (MIP-1β), as well as the T-cell homeostatic factor IL-7. The effect of sex remained significant after adjusting for CD4, age, ethnicity, and race for all cytokines, except for CCL3 and race.
Conclusions:
The observed sex-based differences in cytokines might contribute to higher immune activation in women compared to men despite suppressive therapy. Increased levels of IL-7 in women suggest that homeostatic proliferation may have a differential contribution to HIV reservoir maintenance in females and males. Our study emphasizes the importance of sex-specific studies of viral pathogenesis.
Keywords: cytokine, women, men, blood, HIV, chemokine
INTRODUCTION
Many clinical studies have reported sex-based differences in the outcome of infectious diseases (reviewed in [1]). In HIV infection, epidemiological studies have reported differences between men and women in the risk acquisition and its clinical course [2]. In many cases, sex inequities, underlying socioeconomic factors, and behavior account for the sex differences. However, distinct biological manifestations of sex-based differences have also been described.
Women with HIV have lower plasma viral loads [3–8], especially during the early phases of infection, and a 1.6-fold higher risk of developing AIDS when accounting for viral load levels in chronic infection [2]. Substantial differences in immune activation between men and women with HIV have been described [9–13], but the reasons for these differences are not fully elucidated. One important factor may be the cytokine response evoked during infection.
Cytokines are part of the language used by the innate and adaptive immune system to orchestrate an effective immune response to infectious pathogens. The crucial role of cytokines in sex-specific responses of the immune system to infectious pathogens has recently gained tremendous attention during the COVID-19 pandemic. Although infection rates are similar, male sex was shown to be a significant risk factor for more serious COVID-19 disease and death due to higher cytokine storm [14–16].
Here, we investigated the sex differences in plasma cytokine profiles in men and women with chronic HIV on suppressive ART. Towards this goal, we measured the concentrations of 36 cytokine/chemokines in blood plasma of men and women with chronic HIV on suppressive ART. ART is known to delay the progression of HIV-related disease and prolongs survival, but does not normalize cytokine levels [17].
We found that IL-7 and 4 inflammatory chemokines were higher in women on suppressive ART compared to men on suppressive ART. Our results are consistent with higher cellular activation observed in women. More studies are needed to better understand the underlying mechanisms that contribute to sex-based immune cell regulation in people with HIV [18]. Our study together with others (reviewed in [1, 18]) reveal the importance of sex/gender-specific studies, which too often remain a neglected area of biomedical research [11].
METHODS
Study Participants
Stored plasma samples from 50 women (sex assigned at birth) with HIV on suppressive ART enrolled at Weill Cornell Medicine and 52 men (sex assigned at birth) with HIV on suppressive ART enrolled in the California Collaborative Treatment Group (CCTG) 592 were used in this study to investigate the cytokine differences in men and women with HIV. The studies were conducted with written consent and were approved by the Human Research Protections Program at the University of California, Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, the University of Southern California, and Weill Cornell Medicine [19].
Multiplex Bead Array Assay for Cytokines/Chemokines Quantification
We measured the concentration of 36 cytokines/chemokines involved in different immunological functions in blood plasma of 102 participants (52 men and 50 women) using an in-house multiplexed bead-based assay. The NIH laboratory that performed Luminex measurements is part of the Microbicide Quality Assurance Program [20].
Antibody pairs and standards were purchased from R&D Systems (Minneapolis, MN), except for IL-4 and IL-9 (Biolegend (San Diego, CA)) or IL-21 (Thermo Fisher, MA). All reagents were tested to ensure there was no cross-reactivity or interference. Specific buffers were used to reduce non-specific binding and to account for the plasma matrix. In particular, heteroblock (Fisher Scientific, MA) was used to prevent aspecific interaction between antibodies. Standards and samples were diluted in ProCarta Universal Assay Buffer (Thermo Fisher, MA). Bead coupling was prepared according to manufacturer’s recommendations. Assay was performed as previously described [17]. Additional details of cytokine selection and measurement are provided in Supplemental Methods.
Statistical Analysis
Cytokines with >30% detectable values were included in subsequent analyses with undetectable values imputed with lower limit of detection / 2 values (n = 16). Cytokines with <30% detectable values were excluded from further analysis (n = 20) as shown in supplementary Table S1. Concentrations of cytokines were log-transformed and normalized for Principal Component Analysis (PCA) and Partial least squares discriminant analysis (PLS-DA) analyses. PCA was used as an unsupervised approach to investigate whether cytokine profiles were different between men compared to women. PLS-DA was used to identify which specific cytokines were different between the two groups. The classification performance of the PLS-DA model was assessed using 5-fold cross-validation repeated 100 times and the optimal number of components in our model was based on t-tests that test for a significant difference in the mean error rate between components (Figure S1). The statistical significance of the PLS-DA analysis was tested using the E-statistic for a two-sample difference in the multivariate normal distribution. Bootstrap replicates numbering 500,000 were used to generate p values for the E-statistic. Analyses were performed using R 4.1, the E-test was performed using the “energy” package, and the PLS-DA was performed using the “mixOmics” package.
PLS-DA projections allowed us to visualize the separation between men and women cytokine profiles and generated VIP (Variable Importance in Projection) scores for each cytokine. The VIP reflects the relative importance of each cytokine with each variate in the PLS-DA model. The statistical significance of the cytokines different between men and women with VIP>1 was further tested with the Wilcoxon rank-sum test. To account for the differences in baseline characteristics between our cohorts, we fit a multivariate linear regression model for each cytokine. We used log-transformed cytokine concentration as the outcome and sex as predictor. To adjust for differences in baseline characteristics, we individually included a term for each baseline characteristic that was significantly different.
RESULTS
Demographic and clinical information
Plasma samples from 52 men and 50 women were analyzed in this retrospective study to investigate the potential sex-based differences in blood plasma cytokines of people with HIV on suppressive ART (Table 1). Men and women were significantly different for age, race, ethnicity, and CD4 counts. The median age of men was 46 (range 30-68) and 53 (range, 22-65) in women; The race of men was white (75%), African American and Asian (21.2%) and Asian (3.8%). The race of women was white (16%), African American (74%), Asian (2%), Native American (2%) and other/multiracial (6%). Current CD4 and nadir CD4 counts were respectively 635 (range, 282-1149) and 215 (range, 0-667) in men compared to 721 (range, 300-1362) and 172 (range, 0-600) in women.
Table 1: Demographic and clinical information.
Median (range) or count (percentage) shown for continuous or categorical variables respectively. P-values were calculated using a two-sample t-test for continuous variables and a chi-square or Fisher’s exact test was used for categorical variables.
| Men (n = 52) | Women (n = 50) | Overall (n = 102) | p-value | |
|---|---|---|---|---|
| Demographic | ||||
| Age | 46 (30, 68) | 53 (22, 65) | 50.00 (22, 68) | < .001*** |
| Race | ||||
| White | 39 (75.0%) | 8 (16.0%) | 47 (46.1%) | <.001*** |
| African American | 11 (21.2%) | 37 (74.0%) | 48 (47.1%) | |
| Asian | 2 (3.8%) | 1 (2.0%) | 3 (2.9%) | |
| Native American | 0 (0.0%) | 1 (2.0%) | 1 (1.0%) | |
| Other | 0 (0.0%) | 3 (6.0%) | 3 (2.9%) | |
| Ethnicity | ||||
| Non-Hispanic | 32 (61.5%) | 43 (86.0%) | 75 (73.5%) | 0.010* |
| Hispanic | 20 (38.5%) | 7 (14.0%) | 27 (26.5%) | |
| CD4 count | 635 (282, 1149) | 721 (300, 1362) | 660 (282, 1362) | 0.046* |
| Nadir CD4 count | 215 (0, 667) | 172 (0, 600) | 195 (0, 667) | 0.128 |
| ARTClass | ||||
| Integrase Inhibitor | 3 (6.2%) | 20 (40.0%) | 23 (23.5%) | < .001*** |
| Multi Drug | 5 (10.4%) | 7 (14.0%) | 12 (12.2%) | |
| NNRTI | 18 (37.5%) | 12 (24.0%) | 30 (30.6%) | |
| PI | 22 (45.8%) | 11 (22.0%) | 33 (33.7%) |
Principal component analysis revealed differences in cytokine profiles between men and women
Large datasets are often difficult to interpret, and dimensionality-reduction methods are needed to efficiently interpret them. In our study, we first used PCA as an unsupervised exploratory method to investigate potential differences in cytokine profiles between men and women. We found that the first and second components accounted for 27% and 19% of the variance in the cytokine profile respectively. Although PCA is an unsupervised analysis and therefore no information about sex of each study participant was provided here in the method, we found that participants clustered by sex in PCA score plots (Figure 1A).
Figure 1: Projection of multivariate dimensionality reduction.

(A) Principal component analysis of cytokine profiles in women compared to men with HIV on suppressive ART. PCA analysis on cytokine profile shows distinct clustering of men (orange triangles) and women (Blue circles). The first and second principal components explain 27% and 19% of the variance respectively. (B) Two-dimensional PLS-DA projections of cytokines in blood plasma of men and women with HIV on suppressive ART. Shown are PLS-DA projections spanned by the first two components of the PLS-DA model with ellipses representing Hotelling’s 2-samples T2 with 95% confidence intervals in blood plasma of men (orange triangles) and women (Blue circles). The E-statistic was used to test the statistical differences in the separation between the cytokine profiles in the 2 sex-based groups. The multivariate distance between men and women observations was significant in (p = <0.001).
PLS-DA projections showed distinct cytokine profiles in men compared to women
To use a supervised learning method to predict sex, we fit a PLS-DA model and were able to achieve a minimum balanced error rate ≈ 13% with 2 components using k-fold cross validation to estimate model performance. When participants were projected into the subspace spanned by the first two components of the PLS-DA model, we found that the projections showed a distinct clustering of men and women. (Figure 1B). The two components explained 100% and 43% of the variance in sex and 24% and 18% of the variance in cytokines respectively. The separation between the two groups, measured as the energy (E) statistic, was statistically different (E-statistic = 5.83, p = < 0.001).
Pro-inflammatory cytokines and IL-7 were deemed important in predicting sex
To identify what features are driving the differences in the cytokines between men and women, we calculated VIP scores, which reflect the relative importance of each cytokine with each variate in predicting sex in the PLS-DA model. Cytokines with VIP larger than 1 were considered the most relevant for explaining the differences observed in PLS-DA projections. As a result, cytokines deemed the most important in discriminating men from women (from more to less important) were CXCL1 (Gro-α), IL-7, CCL4 (MIP-1β), CCL3 (MIP-1α), TNF-α, CXCL-13, CCL2 (MCP-1), TRAIL, and CCL5 (RANTES). (Table 2).
Table 2:
VIP scores for each cytokine
| Cytokine | VIP |
|---|---|
| CXCL1 (Gro-α) | 1.33 |
| IL-7 | 1.27 |
| CCL4 (MIP-1β) | 1.26 |
| CCL3 (MIP-1α) | 1.11 |
| TNF-α | 1.06 |
| CXCL13 | 1.05 |
| CCL2 (MCP-1) | 1.04 |
| TRAIL | 1.04 |
| CCL5 (RANTES) | 1.01 |
| IL-18 | 0.92 |
| CXCL10 (IP-10) | 0.89 |
| CXCL11 (I-TAC) | 0.78 |
| CXCL9 (MIG) | 0.78 |
| CCL19 (MIP-3β) | 0.74 |
| IL-16 | 0.70 |
| IL-8 | 0.67 |
All cytokines that had VIP scores exceeding 1 were individually tested for differences between men and women using Wilcoxon Rank Sum Tests (Table 3). False Discovery Rate (FDR) correction to p-values were applied to adjust for multiple comparisons.
Table 3:
Results from Wilcoxon Rank Sum
| Cytokine | p-value | adj, p-value |
|---|---|---|
| CCL3 (MIP-1α) | <.001*** | <0.001*** |
| CCL4 (MIP-1β) | <.001*** | <0.001*** |
| CCL5 (RANTES) | <.001*** | <0.001*** |
| CXCL1 (Gro-α) | <.001*** | <0.001*** |
| IL-7 | <.001*** | <0.001*** |
| TRAIL | 0.060 | 0.090 |
| TNF- α | 0.128 | 0.164 |
| CXCL13 | 0.817 | 0.917 |
| CCL2 (MCP-1) | 0.917 | 0.917 |
Tested individually, 5 cytokines remained significantly different between men and women (CXCL1, p < 0.001; CCL5, p < 0.001; CCL4, p < 0.001; IL-7, p < 0.001; CCL3, p < 0.001).
The median concentrations of the 5 cytokines that remained statistically different in the Wilcoxon test were then log-transformed and plotted in Figure 2. All cytokines were found in higher concentrations in women compared to men.
Figure 2: Effect of sex on chemokine/cytokine in blood plasma.
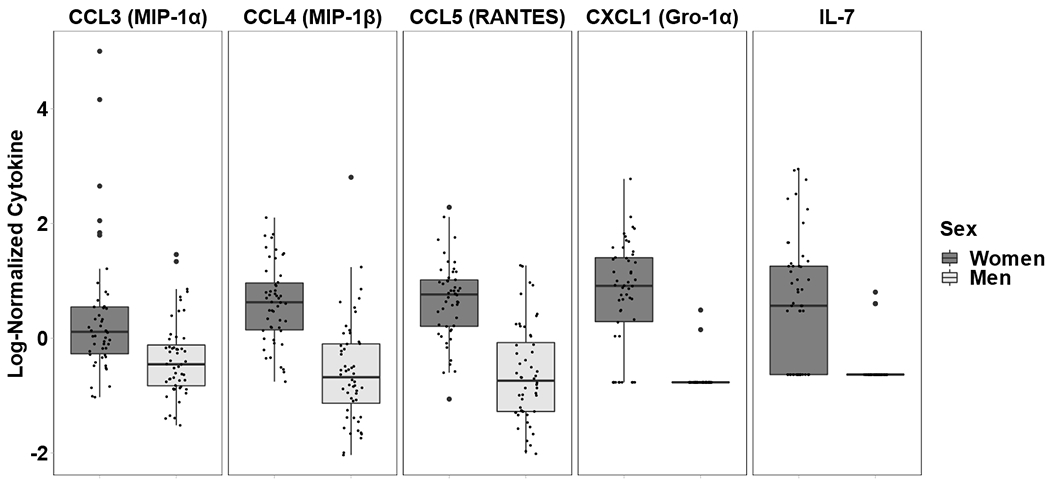
Shown is the difference of the concentrations for 5 chemokine/cytokines (log10 normalized) in women and men infected with HIV on suppressive ART. The statistical significance of 9 cytokines with VIP>1 identified in the PLS-DA model were further tested for by Wilcoxon Rank Sum test. Five out of 9 remained significant.
The concentrations of these 5 Chemokine/cytokine were log10-transformed and plotted as boxplots. For each cytokine and each boxplot, each point represents a participant’s cytokine concentration, the box represents the interquartile range (IQR), the middle line represents the median, while the points beyond the whiskers are outliers. All chemokine/cytokines had higher concentrations in women compared to men.
Five variables were significantly different between our cohorts of men and women (i.e. age, race, ethnicity, ART regimen, and CD4 T cell count). To control for differences in baseline characteristics between men and women, we fit multivariate linear regression models for each cytokine log-transformed concentration as the outcome and sex as a predictor. We individually included each baseline characteristic as a covariate and examined if the effect of sex remained significant. All cytokines remained significant for each of the five variables except for CCL3 and race (p = 0.088) (Table S2).
DISCUSSION
Sex (at birth) represents one of the most evolutionarily well-conserved differences in biology. Yet, it remains one of the most underappreciated differences in biomedical research [21]. Efforts from journals and research funding agencies, including NIH have led to new policies to consider sex as an important biological variable [22].
In HIV infection, there is evidence that pathogenesis differs between sex. Many studies report that in contrast to men, women have lower viral loads [3–8], lower HIV reservoirs [12, 19, 23] and higher CD4 cell counts [7, 9]. Despite this, women seem to progress to AIDS faster than men when adjusting for viral load differences [2, 24]. The exact mechanism behind this phenomenon is still unclear, but some differences in cell activation has been previously reported [10, 11]. The contribution of cytokines in the persistence of elevated immune activation in people with HIV has been widely documented, even in presence of ART [25, 26]. However, the specific differences in cytokine profiles between women and men with HIV have not been thoroughly studied. Here we investigated some of these differences by measuring the concentrations of 36 cytokine/chemokines in blood plasma of women and men with HIV on suppressive ART.
Using PCA initially, as an unsupervised method where no information about sex for each subject was provided, we found that the cytokine profiles were indeed different between women and men. Projections from the PLS-DA model confirmed these differences and we identified 9 cytokines with VIP>1 that were driving these differences. Wilcoxon Rank Sum/Fisher’s exact confirmed the statistical significance for 5 of these 9 cytokines. All 5 cytokines were found to be in higher concentrations in plasma of women compared to men. Four of these cytokines belong to a group of pro-inflammatory cytokines with chemoattractant properties, namely CXCL1 (Gro-α), CCL5 (RANTES), CCL3 (MIP-1α), and CCL4 (MIP-1β). Interestingly, CCL3, CCL4 and CCL5, which are β chemokines that share the same receptor, CCR5, have been identified as strong HIV-suppressive factors produced by CD8 T cells [27]. This observation could be a possible reason for the differences of viral loads previously reported between women and men [3–8].
Moreover, increased concentrations of inflammatory chemokines, which are specialized in the recruitment of immune cells to the inflamed regions, are in agreement with previously reported higher immune activation in women with HIV, despite ART. Sex differences in inflammatory biomarkers in HIV have mostly been studied in relation to cardiovascular disease and monocyte activation [28, 29]. For example, sCD163 concentrations, a marker of macrophage activation, are higher in women versus men, both before and after 24 months of suppressive ART [13]. Women with HIV were reported to have a smaller decrease in CRP and sCD14 and more pronounced increase in TNFα after ART compared to men [9]. Krebs et al., (2016) reported that levels of sCD14, another marker of microbial translocation, decreased in men following 48 weeks of ART, but not in women [30].
Increased expression of markers of activation on cells have also been reported in women with HIV. For example, Meier et al reported higher CD8+ T cell activation in women compared to men. They found that an average of 4.6% more CD38+HLA-DR+CD8+ T cells corresponds to the effect of approximately one log10 higher plasma HIV-1 RNA copy numbers. Santinelli et al. (2020) found that markers of immune activation expressed on CD4+ and CD8+ T cells were significantly higher in women than in men both in the gut and in PBMCs in the setting of ART-treated HIV infection [11]. In contrast, one study found higher proportions of CD4 T cells HLA-DR and CD38 positive in men compared to women HIV infected and virologically suppressed [12].
The outcome of HIV disease seems to be determined by the early inflammatory response to the virus, with higher inflammation being associated with worse outcome (reviewed in [31]). The sex-based difference in inflammation seems to be also set very early in the course of infection. In a nonhuman primate model, George et al (2019) not only showed that pathogenic sequelae seen during chronic infection was shaped early during the course of HIV infection but that female macaques already had significantly higher levels of pro-inflammatory cytokines as compared to their male counterparts day 4 post-inoculation [32]. Ultimately these female animals progressed faster to SHIV than their male counterparts [33].
Our PLS-DA model also identified IL-7 as significantly higher in women compared to men living with HIV on suppressive ART. IL-7 is essential for normal T-cell production and homeostasis. Together with a cross-sectional study showing that circulating IL-7 levels were found to be 40% higher in women than in men [34], our results suggest that the sex-based difference in IL-7 levels might contribute to differences in CD4 counts between women and men with HIV. A recent study from our group found that total HIV reservoir declines more slowly in women compared to men, while the inducible HIV RNA+ reservoir, which is highly enriched in replication competent virus, increases in women after menopause [35]. Potential mechanisms altering cell proliferation during reproductive aging include alterations in homeostasis due to changes in IL-7 expression, which is the central regulator of homeostatic T cell proliferation in HIV infection [36, 37]. In fact, exogenous administration of IL-7 to people living with HIV in clinical trials was associated with CD4+ T-cell proliferation and expansion of cells with integrated HIV DNA without disrupting latency programs [37, 38]. Taken together, our data suggests that the elevated IL-7 seen in women with HIV, despite suppressive ART, could also be related to increased immune activation and disease progression.
What are the reasons behind sex-based differences observed in HIV infection? Sex steroid hormones most likely and logically play a pivotal role. Menstrual cycle and levels of sex hormones modulate HIV acquisition and HIV viral load [39, 40]. For example, estradiol has recently been shown to contribute to better viral control despite higher CD4+ T Cell activation during acute HIV-1 infection in Zambian women [41]. Sex hormones also have a variety of direct effects on immune cell function. Both estrogen and progesterone have been reported to modulate the secretion of IFNα, a major prognostic indicator for HIV-1 clinical progression [42–44]. In particular, 2 studies showed that plasmacytoid dendritic cells pDCs derived from women produce markedly more IFNα in response to HIV-1–mediated stimulation of TLR7 than pDCs derived from men [10, 45], resulting in stronger expression of interferon stimulated genes [46] and stronger secondary activation of CD8+ T cells in women living with HIV [10].
Interestingly, lower viral loads are also seen in prepubescent girls with HIV compared with boys [47, 48], suggesting that other mechanisms than differential expression in sex hormones, including the inherent imbalance in the expression of genes encoded by the X and Y chromosomes may be involved. The X chromosome is known to harbor majority of the immune-related genes, such as TLR7, TLR8, FOXP3, and CD40L (CD154) (reviewed in [1]). Importantly, 10-20% of the X-chromosome escapes inactivation [49], which may lead to an over-expression of certain gene products in women as shown for TLR7 [50]. Finally, sex-specificity composition of the microbiome may be involved as well. Proteobacteria, which has been associated with markers of mucosal immune disruption, T cell activation, and chronic inflammation in HIV patients has been shown to be significantly higher in females rhesus macaques than males [33]. Our study has some limitations that warrant consideration. Our groups were not perfectly matched as there were geographic differences (New York for women versus Southern California for men) as well as some differences in CD4 count, age, ethnicity, ART regimen, and race. Multivariate linear regression models performed for each significant cytokine after individually adjusting for each baseline characteristic that was significantly different between the two cohorts showed that for each cytokine, sex remained significant as predictor after adjustment for all 5 covariates except for CCL3 and race. Moreover, no information on potential differences in terms of chemokine receptor, virus tropism, or co-infections, which could potentially imbalance the profiles of pro-inflammatory cytokine between the two cohorts, was available in this study.
The use of a single time point was another limitation, as plasma inflammatory marker concentrations may change over time. Overall, longitudinal matched studies are needed to better address the cytokine differences in men and women. Future studies may also include an HIV control group to see whether similar differences are also found between men and women without HIV.
In summary, we found that inflammatory cytokines remain higher in virologically suppressed women compared to men living with HIV, suggesting that cytokines contribute to maintaining higher immune activation in women despite suppressive therapy with ART. Our results may explain why women progress faster to AIDS compared to men at a given viral load. As more studies including sex as a variable are being published, it is becoming clear that mechanisms by which mammals achieve and maintain sex differences can directly and indirectly influence host-pathogen interactions at the cellular and molecular level [18]. Careful experimental design and systemic inclusion of sex as a research variable is required to gain further insights into sex-based differences in the immune pathogenesis of HIV-1 infection to provide optimal care for both sexes.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to all the study participants and all the nurses at all the enrollment sites.
TW, JM, SM: enrollment of clinical participants, CV, AW, TW, JM, SM and SG: data generation. CV, AW, SM, LM, and SG: data analysis and interpretation. CV, AW, TW, JM, LM, and SG: original draft writing, review and final editing.
FUNDING STATEMENT
This work was supported by the Department of Veterans Affairs and the James B. Pendleton Charitable Trust, and grants AI036214, AI147821, DA051915, AI158293.
Footnotes
CONFLICT OF INTEREST
Authors declare no conflict of interest.
MEETING
These findings have not been presented to any meeting.
References
- 1.Fischer J, Jung N, Robinson N, Lehmann C. Sex differences in immune responses to infectious diseases. Infection 2015; 43(4):399–403. [DOI] [PubMed] [Google Scholar]
- 2.Farzadegan H, Hoover DR, Astemborski J, Lyles CM, Margolick JB, Markham RB, et al. Sex differences in HIV-1 viral load and progression to AIDS. Lancet 1998; 352(9139):1510–1514. [DOI] [PubMed] [Google Scholar]
- 3.Evans JS, Nims T, Cooley J, Bradley W, Jagodzinski L, Zhou S, et al. Serum levels of virus burden in early-stage human immunodeficiency virus type 1 disease in women. J Infect Dis 1997; 175(4):795–800. [DOI] [PubMed] [Google Scholar]
- 4.Lyles CM, Dorrucci M, Vlahov D, Pezzotti P, Angarano G, Sinicco A, et al. Longitudinal human immunodeficiency virus type 1 load in the italian seroconversion study: correlates and temporal trends of virus load. J Infect Dis 1999; 180(4):1018–1024. [DOI] [PubMed] [Google Scholar]
- 5.Sterling TR, Vlahov D, Astemborski J, Hoover DR, Margolick JB, Quinn TC. Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. N Engl J Med 2001; 344(10):720–725. [DOI] [PubMed] [Google Scholar]
- 6.Gandhi M, Bacchetti P, Miotti P, Quinn TC, Veronese F, Greenblatt RM. Does patient sex affect human immunodeficiency virus levels? Clin Infect Dis 2002; 35(3):313–322. [DOI] [PubMed] [Google Scholar]
- 7.Collazos J, Asensi V, Carton JA. Sex differences in the clinical, immunological and virological parameters of HIV-infected patients treated with HAART. AIDS 2007; 21(7):835–843. [DOI] [PubMed] [Google Scholar]
- 8.Cuzin L, Pugliese P, Saune K, Allavena C, Ghosn J, Cottalorda J, et al. Levels of intracellular HIV-DNA in patients with suppressive antiretroviral therapy. AIDS 2015; 29(13):1665–1671. [DOI] [PubMed] [Google Scholar]
- 9.Mathad JS, Gupte N, Balagopal A, Asmuth D, Hakim J, Santos B, et al. Sex-Related Differences in Inflammatory and Immune Activation Markers Before and After Combined Antiretroviral Therapy Initiation. J Acquir Immune Defic Syndr 2016; 73(2):123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meier A, Chang JJ, Chan ES, Pollard RB, Sidhu HK, Kulkarni S, et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med 2009; 15(8):955–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santinelli L, Ceccarelli G, Borrazzo C, Innocenti GP, Frasca F, Cavallari EN, et al. Sex-related differences in markers of immune activation in virologically suppressed HIV-infected patients. Biol Sex Differ 2020; 11(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scully EP, Gandhi M, Johnston R, Hoh R, Lockhart A, Dobrowolski C, et al. Sex-Based Differences in Human Immunodeficiency Virus Type 1 Reservoir Activity and Residual Immune Activation. J Infect Dis 2019; 219(7):1084–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ticona E, Bull ME, Soria J, Tapia K, Legard J, Styrchak SM, et al. Biomarkers of inflammation in HIV-infected Peruvian men and women before and during suppressive antiretroviral therapy. AIDS 2015; 29(13):1617–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi T, Ellingson MK, Wong P, Israelow B, Lucas C, Klein J, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 2020; 588(7837):315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi T, Iwasaki A. Sex differences in immune responses. Science 2021; 371(6527):347–348. [DOI] [PubMed] [Google Scholar]
- 16.Mathad JS, Lee MH, Chalem A, Frey MK, Chapman-Davis E, Kopparam RV, et al. Sex-Related Differences in Clinical Presentation and Risk Factors for Mortality in Patients Hospitalized With Coronavirus Disease 2019 in New York City. Open Forum Infect Dis 2021; 8(8):ofab370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanpouille C, Introini A, Morris SR, Margolis L, Daar ES, Dube MP, et al. Distinct cytokine/chemokine network in semen and blood characterize different stages of HIV infection. AIDS 2016; 30(2):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh S, Klein RS. Sex Drives Dimorphic Immune Responses to Viral Infections. J Immunol 2017; 198(5):1782–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gianella S, Tran SM, Morris S, Vargas M, Porrachia M, Oliveira MF, et al. Sex Differences in CMV Replication and HIV Persistence During Suppressive ART. Open Forum Infect Dis 2020; 7(8):ofaa289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fichorova RN, Richardson-Harman N, Alfano M, Belec L, Carbonneil C, Chen S, et al. Biological and technical variables affecting immunoassay recovery of cytokines from human serum and simulated vaginal fluid: a multicenter study. Anal Chem 2008; 80(12):4741–4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein SL, Schiebinger L, Stefanick ML, Cahill L, Danska J, de Vries GJ, et al. Opinion: Sex inclusion in basic research drives discovery. Proc Natl Acad Sci U S A 2015; 112(17):5257–5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature 2014; 509(7500):282–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prodger JL, Capoferri AA, Yu K, Lai J, Reynolds SJ, Kasule J, et al. Reduced HIV-1 latent reservoir outgrowth and distinct immune correlates among women in Rakai, Uganda. JCI Insight 2020; 5(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prins M, Meyer L, Hessol NA. Sex and the course of HIV infection in the pre- and highly active antiretroviral therapy eras. AIDS 2005; 19(4):357–370. [DOI] [PubMed] [Google Scholar]
- 25.Keating SM, Golub ET, Nowicki M, Young M, Anastos K, Crystal H, et al. The effect of HIV infection and HAART on inflammatory biomarkers in a population-based cohort of women. AIDS 2011; 25(15):1823–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taiwo B, Matining RM, Zheng L, Lederman MM, Rinaldo CR, Kim PS, et al. Associations of T cell activation and inflammatory biomarkers with virological response to darunavir/ritonavir plus raltegravir therapy. J Antimicrob Chemother 2013; 68(8):1857–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 1995; 270(5243):1811–1815. [DOI] [PubMed] [Google Scholar]
- 28.Fitch KV, Srinivasa S, Abbara S, Burdo TH, Williams KC, Eneh P, et al. Noncalcified coronary atherosclerotic plaque and immune activation in HIV-infected women. J Infect Dis 2013; 208(11):1737–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanna DB, Lin J, Post WS, Hodis HN, Xue X, Anastos K, et al. Association of Macrophage Inflammation Biomarkers With Progression of Subclinical Carotid Artery Atherosclerosis in HIV-Infected Women and Men. J Infect Dis 2017; 215(9):1352–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krebs SJ, Slike BM, Sithinamsuwan P, Allen IE, Chalermchai T, Tipsuk S, et al. Sex differences in soluble markers vary before and after the initiation of antiretroviral therapy in chronically HIV-infected individuals. AIDS 2016; 30(10):1533–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paiardini M, Muller-Trutwin M. HIV-associated chronic immune activation. Immunol Rev 2013; 254(1):78–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.George J, Johnson RC, Mattapallil MJ, Renn L, Rabin R, Merrell DS, et al. Gender differences in innate responses and gene expression profiles in memory CD4 T cells are apparent very early during acute simian immunodeficiency virus infection. PLoS One 2019; 14(9):e0221159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren W, Ma Y, Yang L, Gettie A, Salas J, Russell K, et al. Fast disease progression in simian HIV-infected female macaque is accompanied by a robust local inflammatory innate immune and microbial response. AIDS 2015; 29(10):F1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Napolitano LA, Burt TD, Bacchetti P, Barron Y, French AL, Kovacs A, et al. Increased circulating interleukin-7 levels in HIV-1-infected women. J Acquir Immune Defic Syndr 2005; 40(5):581–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gianella SAR S, Nakazawa M, Chaillon A, Strain M, Layman L, Caballero G, Scully E, Scott B, Pacis C, Weber KM, Landay A, Anderson C, Karn J. Sex differences in HIV Persistence and Reservoir Size during Aging. Clin Infect Dis 2021:In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barata JT, Durum SK, Seddon B. Flip the coin: IL-7 and IL-7R in health and disease. Nat Immunol 2019; 20(12):1584–1593. [DOI] [PubMed] [Google Scholar]
- 37.Vandergeeten C, Fromentin R, DaFonseca S, Lawani MB, Sereti I, Lederman MM, et al. Interleukin-7 promotes HIV persistence during antiretroviral therapy. Blood 2013; 121(21):4321–4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sereti I, Dunham RM, Spritzler J, Aga E, Proschan MA, Medvik K, et al. IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood 2009; 113(25):6304–6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sodora DL, Gettie A, Miller CJ, Marx PA. Vaginal transmission of SIV: assessing infectivity and hormonal influences in macaques inoculated with cell-free and cell-associated viral stocks. AIDS Res Hum Retroviruses 1998; 14 Suppl 1:S119–123. [PubMed] [Google Scholar]
- 40.Addo MM, Altfeld M. Sex-based differences in HIV type 1 pathogenesis. J Infect Dis 2014; 209 Suppl 3:S86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El-Badry E, Macharia G, Claiborne D, Brooks K, Dilernia DA, Goepfert P, et al. Better Viral Control despite Higher CD4(+) T Cell Activation during Acute HIV-1 Infection in Zambian Women Is Linked to the Sex Hormone Estradiol. J Virol 2020; 94(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eyster ME, Goedert JJ, Poon MC, Preble OT. Acid-labile alpha interferon. A possible preclinical marker for the acquired immunodeficiency syndrome in hemophilia. N Engl J Med 1983; 309(10):583–586. [DOI] [PubMed] [Google Scholar]
- 43.Fahey JL, Taylor JM, Detels R, Hofmann B, Melmed R, Nishanian P, et al. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N Engl J Med 1990; 322(3):166–172. [DOI] [PubMed] [Google Scholar]
- 44.Mildvan D, Machado SG, Wilets I, Grossberg SE. Endogenous interferon and triglyceride concentrations to assess response to zidovudine in AIDS and advanced AIDS-related complex. Lancet 1992; 339(8791):453–456. [DOI] [PubMed] [Google Scholar]
- 45.Berghofer B, Frommer T, Haley G, Fink L, Bein G, Hackstein H. TLR7 ligands induce higher IFN-alpha production in females. J Immunol 2006; 177(4):2088–2096. [DOI] [PubMed] [Google Scholar]
- 46.Chang JJ, Woods M, Lindsay RJ, Doyle EH, Griesbeck M, Chan ES, et al. Higher expression of several interferon-stimulated genes in HIV-1-infected females after adjusting for the level of viral replication. J Infect Dis 2013; 208(5):830–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.European Collaborative S. Level and pattern of HIV-1-RNA viral load over age: differences between girls and boys? AIDS 2002; 16(1):97–104. [DOI] [PubMed] [Google Scholar]
- 48.Ruel TD, Zanoni BC, Ssewanyana I, Cao H, Havlir DV, Kamya M, et al. Sex differences in HIV RNA level and CD4 cell percentage during childhood. Clin Infect Dis 2011; 53(6):592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carrel L, Brown CJ. When the Lyon(ized chromosome) roars: ongoing expression from an inactive X chromosome. Philos Trans R Soc Lond B Biol Sci 2017; 372(1733). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science 2006; 312(5780):1669–1672. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


