Abstract
Therapeutically targeting B cells has received great attention in the treatment of B-cell malignancies and autoimmune diseases. The B-cell activating factor (BAFF) is critical to the survival of normal and neoplastic B cells, and excess production of BAFF contributes to autoimmune diseases. Resveratrol, a natural polyphenolic compound, has a positive effect on the treatment of autoimmune diseases. However, how resveratrol affects BAFF-stimulated B-cell proliferation and survival is poorly understood. Here, we show that resveratrol increased autophagosome formation and ATG5/LC3-II levels and decreased p62 level, promoting autophagic flux/autophagy and thereby suppressing the basal or human soluble BAFF (hsBAFF)-stimulated proliferation and survival of normal and B-lymphoid (Raji) cells. This is supported by the findings that inhibition of autophagy with 3-methyladenine (3-MA, an inhibitor of Vps34) or ATG5 shRNA attenuates resveratrol-induced autophagy and -reduced proliferation/viability in B-cells. Inhibition of mTOR with rapamycin or knockdown of mTOR potentiated resveratrol-induced autophagy and inhibition of hsBAFF-stimulated B-cell proliferation/viability, while overexpression of wild-type mTOR conferred resistance to the actions of resveratrol. Similarly, inhibition of Akt with Akt inhibitor X or ectopic expression of dominant negative Akt reinforced resveratrol-induced autophagy and inhibition of hsBAFF-stimulated B-cell proliferation/viability, whereas expression of constitutively active Akt conferred resistance to the actions of resveratrol. Taken together, these results indicate that resveratrol induces autophagy impeding BAFF-stimulated proliferation and survival via blocking the Akt/mTOR signaling pathway in normal and neoplastic B cells. Our findings highlight that resveratrol has a great potential for prevention and treatment of excessive BAFF-elicited aggressive B-cell disorders and autoimmune diseases.
Keywords: Resveratrol, B-cell activating factor, Autophagy, Akt, B cells, Survival
1. Introduction
The B-cell activating factor (BAFF), also known as BLYS, CD257, DTL, TALL-1, THANK, TNFSF13B, TNFSF20, and ZTNF4, is a cytokine of the tumor necrosis factor (TNF) ligand family, which is expressed in B cells and is important for the proliferation and differentiation of B cells [1–4]. Insufficient production of BAFF links to immunodeficiency, while excessive expression of BAFF leads to autoimmune disorders in mice and humans [5,6]. Many studies have shown that BAFF and its receptors have crucial impacts on peripheral B-cell survival and maturation [4], immune responses and the pathogenesis of autoimmune disorders, including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and Sjogren’s syndrome (SS) [7–9]. Therapeutically targeting B cells has received great attention in fighting against autoimmune diseases and B-cell malignancies such as non-Hodgkin lymphomas and chronic lymphocytic leukemia. Of note, Rituximab, a monoclonal antibody targeting CD20 on the surface of B cells, has been clinically used for treatment of certain autoimmune diseases and cancer [10]. However, how to combat excessive BAFF induction of B cell-related autoimmune diseases and cancer remains a challenge. Hence, it is important to identify new interventions to manage excess BAFF-induced hyper-activation of B cells.
Autophagy is a highly-conserved homeostasis mechanism from yeast to human that delivers damaged organelles and unwanted cellular contents to the lysosome for degradation and recycling [11]. Evidence indicates that autophagy is indispensable in regulating the survival and development of lymphatic cells [12–14]. In certain circumstances, autophagy can be adaptive to stress and promote cell survival, while under other conditions, it can contribute to cell death [15]. The serine/ threonine (Ser/Thr) protein kinase mTOR (mechanistic of mammalian target of rapamycin) controls cell growth, proliferation, survival, migration, and differentiation [16]. mTOR has two complexes, namely mTOR complexes 1/2 (mTORC1/2) [16]. mTORC1 senses cellular stresses, including DNA damage and energy deprivation, regulating cell metabolism [17]. Importantly, as a regulator of autophagy, mTORC1 notably inhibits the initiation of autophagy when growth factors and nutrients are present [18]. Akt is an effector of phosphoinositide-3-kinase (PI3K), and dysfunction of Akt relates to the pathogenesis of a wide range of diseases such as cancer [19], diabetes and autoimmune diseases [20,21]. Recently we have shown that excessive human soluble BAFF (hsBAFF) activates the Akt/mTOR pathway and inhibits autophagy, leading to increased cell proliferation and survival in B cells [22]. Resveratrol is a polyphenolic compound naturally derived from grape, mulberry, nuts and red wine, and its trans isomer is the most biologically active, with anti-inflammatory, antioxidant, and antiaging properties [23,24]. Numerous reports have indicated that resveratrol regulates cell proliferation and survival in various cells by inhibiting Akt/mTOR and/ or activating autophagy [25–27]. Especially, resveratrol has been found to reduce and ameliorate the progression of autoimmune diseases, such as SLE, RA and psoriasis, in animal and human studies [23]. This prompted us to determine whether resveratrol has any impact on BAFF-activated B-cell proliferation and survival by mediating Akt/mTOR-mediated autophagy.
Here we demonstrate that resveratrol induces autophagy impeding hsBAFF-activated cell proliferation and survival via inhibiting the Akt/ mTOR pathway in primary murine B lymphocytes and neoplastic B-lymphoid (Raji) cells. The results provide new insights into the mechanism of how resveratrol executes its action against excessive BAFF-induced B-cell disorders and autoimmune diseases.
2. Materials and methods
2.1. Reagents
Anti-CD19 magnetic fluorobeads-B was obtained from One Lambda (Canoga Park, CA, USA). Refolded human soluble BAFF (hsBAFF), a recombinant form of the extracellular domain of the BAFF synthesized in Escherichia coli, was generated by this group [28]. Resveratrol and 3-methyladenine (3-MA) were bought from Sigma-Aldrich (St. Louis, MO, USA). Rapamycin was supplied by ALEXIS (San Diego, CA, USA), whereas Akt inhibitor X was from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Bafilomycin A1 was from Millipore (Bedford, MA, USA). Fetal bovine serum (FBS) and RPMI 1640 medium were from Gibco (Rockville, MD, USA). One Solution Cell Proliferation Assay kit and Annexin-V-FITC/Propidium Iodide (PI) Apoptosis Detection kit were purchased from Promega (Madison, WI, USA) and Vazyme Biotech Company (Nanjing, China), respectively. Enhanced chemiluminescence solution was procured by Sciben Biotech Company (Nanjing, China). The primary antibodies used were listed as following: phosphorylated-mTOR (p-mTOR) (Ser2448) (SAB4300178), mTOR (T2949), ATG5 (A2859), microtubule-associated protein 1 light chain 3 (LC3, L7543) B, SQSTM1/p62 (P0067) (all from Sigma-Aldrich); p-Akt (Ser473) (# 9271), p-Akt (Thr308) (# 9275), p-S6K1 (Thr389) (# 9234), cleaved-caspase-3 (# 9664), poly (ADP-ribose) polymerase (PARP, #9542) (all from Cell Signaling Technology, Beverly, MA, USA); Akt (Sc-5298), survivin (Sc-17779), S6K1 (sc-230) (all from Santa Cruz Biotechnology); HA (AT16322), FLAG (AT15428), β-actin (AT10100) (all form Sciben Biotech Company). The secondary antibodies used included horseradish peroxidase (HRP)-linked goat anti-rabbit IgG (#32460), HRP-linked goat anti-mouse IgG (#31430) (all from Pierce, Rockford, IL, USA). Other chemicals of analytical grade were bought from local commercial sources.
2.2. Cell culture
Human B-lymphoid Raji cell line (#CCL-86) was obtained from American Type Culture Collection (Manassas, VA, USA), which was used within 10 passages for experiments. The cell line used was certified to be negative for mycoplasma by Mycoblue Mycoplasma Detector Kit (Vazyme Biotech Company) and authenticated by Short Tandem Repeat analysis. For culture, Raji cells were maintained in RPMI 1640 medium supplemented with 10 % FBS, 100 U/ml penicillin, 100 U/ml streptomycin and incubated at 37 °C in a humidified incubator containing 5 % CO2. To verify the data observed from Raji cells, primary B cells were also used in this study. For this, primary murine B lymphocytes were isolated from fresh splenic cells of healthy ICR mice using anti-CD19 magnetic fluorobeads-B as described [29], and seeded in 96-well, 24-well or 6-well plates for experiments. The experimental procedures involving animals in this study were approved by the Institutional Animal Care and Use Committee of Nanjing Normal University (Certificate NO. 200408), and conducted in compliance with the guidelines set forth by the Guide for the Care and Use of Laboratory Animals.
2.3. Recombinant adenoviral vectors and infection of cells
The recombinant adenoviral vectors encoding hemagglutinin (HA)-tagged dominant negative Akt (Ad-dn-Akt, T308A/S473A) and constitutively active Akt (Ad-myr-Akt) were generously provided by Dr. Kenneth Walsh (Boston University, Boston, MA), and adenovirus expressing FLAG-tagged wild-type mTOR (Ad-mTOR) and the control adenovirus expressing β-galactosidase (Ad-LacZ) were described previously [30,31]. Adenoviral vectors encoding GFP-LC3 fusion protein (Ad-GFP-LC3) was bought from Sciben Biotech Company (Nanjing, China). Following infection with the individual adenovirus for 24 h at 5 of multiplicity of infection (MOI = 5), Raji cells were used for experiments. Ad-LacZ served as a control. HA-and FLAG-tagged expressions for dn-Akt/myr-Akt and wt-mTOR were determined by Western blot analysis with antibodies to HA and FLAG, respectively.
2.4. Lentiviral shRNA constructs, production and infection of cells
Lentiviral shRNAs to mTOR and GFP (for control) were produced and used as described previously [32]. To make lentiviral shRNA to ATG5, oligonucleotides containing the target sequences were synthesized, annealed and inserted into FSIPPW lentiviral vector [33] via the EcoR1/ BamH1 restriction enzyme site. Oligonucleiotides used were: ATG5 sense: 5′-AATTCCCGCAGTTGAGGCTCACTTTATGTGCAAGAGACATAAAGTGAGCCTCAACTGCTTTTTG-3′, anti-sense: 5′-GATCCAAAAAGCAGTTGAGGCTCACTTTATGTCTCTTGCACA-TAAAGTGAGCCTCAACTGCGGG-3′. For lentiviral package, the constructed vectors were co-transfected together with pMD2G and psPAX2 (Addgene, Cambridge, MA, USA) to 293TD cells using MegaTran 1.0 reagent (OriGene Technologies, Rockville, MD, USA). The medium containing viral particles was respectively harvested at 48 h and 60 h after transfection. Raji cells (~70% confluence) were infected with above lentivirus-containing medium twice, followed by treatment with puromycin (2 μg/ml) to eliminate uninfected cells, as described previously [32]. After 5 days of culture, cells were used for experiments.
2.5. Assays for cell proliferation and viability
Raji cells and primary murine B cells, or Raji cells infected with Ad-mTOR, Ad-dn-Akt, Ad-myr-Akt and Ad-lacZ, or Raji cells infected with lentiviral shRNAs to mTOR, ATG5 and GFP, respectively, were seeded in 24-well plates at a density of 3 × 105 cells/well (for cell proliferation assay) or 96-well plates at a density of 3 × 104 cells/well (for cell viability assay). After overnight culture, cells were treated with 0–120 μM or 0–20 μM of resveratrol for 48 h, or pretreated with/without 2.5–10 μM or 10 μM of resveratrol for 1 h and then stimulated with/ without 2.5 μg/ml of hsBAFF for 48 h, or pretreated with/without 100 ng/ml of rapamycin for 2 h or with/without 20 nM of bafilomycin A1, 4 mM of 3-MA or 20 μM of Akt inhibitor X for 1 h and then treated with 10 μM of resveratrol for another 1 h, followed by stimulating with/without 2.5 μg/ml of hsBAFF for 48 h. Each treatment had 6 replicates. Finally, cell proliferation and viability were determined with a Coulter Counter (Beckman Coulter, Fullerton, CA, USA) and a Victor X3 Light Plate Reader (PerkinElmer, Waltham, MA, USA), respectively, as described previously [34]. The half maximal inhibitory concentration (IC50) values of resveratrol in Raji cells and primary murine B cells were evaluated using the CCK-8 Assay Kit (Sciben Biotech Company), following the supplier’s instruction.
2.6. Trypan blue exclusion and apoptosis assay
Raji cells and primary murine B cells were seeded in 24-well plates at a density of 3 × 105 cells/well (for trypan blue exclusion) or 6-well plates at a density of 2 × 106 cells/well (for flow cytometry), respectively. After overnight culture, cells were treated with 0–20 μM of resveratrol for 48 h, or pretreated with/without 2.5–10 μM of resveratrol for 1 h and then stimulated with/without 2.5 μg/ml of hsBAFF for 48 h. Afterwards, the number of live cells was determined using trypan blue exclusion, and the percentage of apoptotic cells was assessed by annexin-V-FITC/PI staining, followed by flow cytometry using a fluorescence-activated cell sorter (FACS) Vantage SE flow cytometer (Becton Dickinson, CA, USA).
2.7. GFP-LC3 assay
Raji cells and primary murine B cells, or Raji cells infected with Ad-mTOR, Ad-dn-Akt, Ad-myr-Akt and Ad-lacZ, or Raji cells infected with lentiviral shRNAs to ATG5, mTOR and GFP, respectively, were seeded in 6-well plates at a density of 2 ×106 cells/well and infected with Ad-GFP-LC3. After overnight culture, cells were treated with 0–20 μM of resveratrol for 12 h, or pretreated with/without 2.5–10 μM or 10 μM of resveratrol for 1 h and then stimulated with/without 2.5 μg/ml of hsBAFF for 12 h, or pretreated with/without 100 ng/ml of rapamycin for 2 h or with/without 20 nM of bafilomycin A1, 4 mM of 3-MA or 20 μM of Akt inhibitor X for 1 h, and then treated with 10 μM of resveratrol for another 1 h, followed by stimulating with/without 2.5 μg/ml of hsBAFF for 12 h. Afterwards, the cells were washed 3 times with PBS, followed by photographing under a fluorescence microscope (Leica DMi8, Wetzlar, Germany) and counting the number of LC3 puncta (green) per cell to estimate autophagosome formation.
2.8. Caspase-3/7 activity assay
Raji cells and primary murine B cells were seeded in 96-well plates at a density of 3 × 104 cells/well. The next day, cells were treated with 0–20 μM of resveratrol for 48 h, or pretreated with/without 2.5–10 μM of resveratrol for 1 h and then stimulated with/without 2.5 μg/ml of hsBAFF for 48 h, with 6 replicates of each treatment. The caspase-3/7 activity was then assayed using Caspase-Glo®3/7 Assay Kit (Promega, Madison, WI, USA), following the supplier’s instructions.
2.9. Western blotting
The indicated cells, after treatment, were briefly washed with cold PBS, and lysed on ice in the radioimmunoprecipitation assay buffer. Protein levels in the cell lysates were analyzed by Western blotting as described [32].
2.10. Statistical analysis
All results were expressed as mean values ± standard error (mean ± SE). Student’s t-test for non-paired replicates was used to identify differences between treatment means. Group variability and interaction were compared using either one-way or two-way ANOVA followed by Bonferroni’s post-tests to compare replicate means. Significance was accepted at p < 0.05.
3. Results
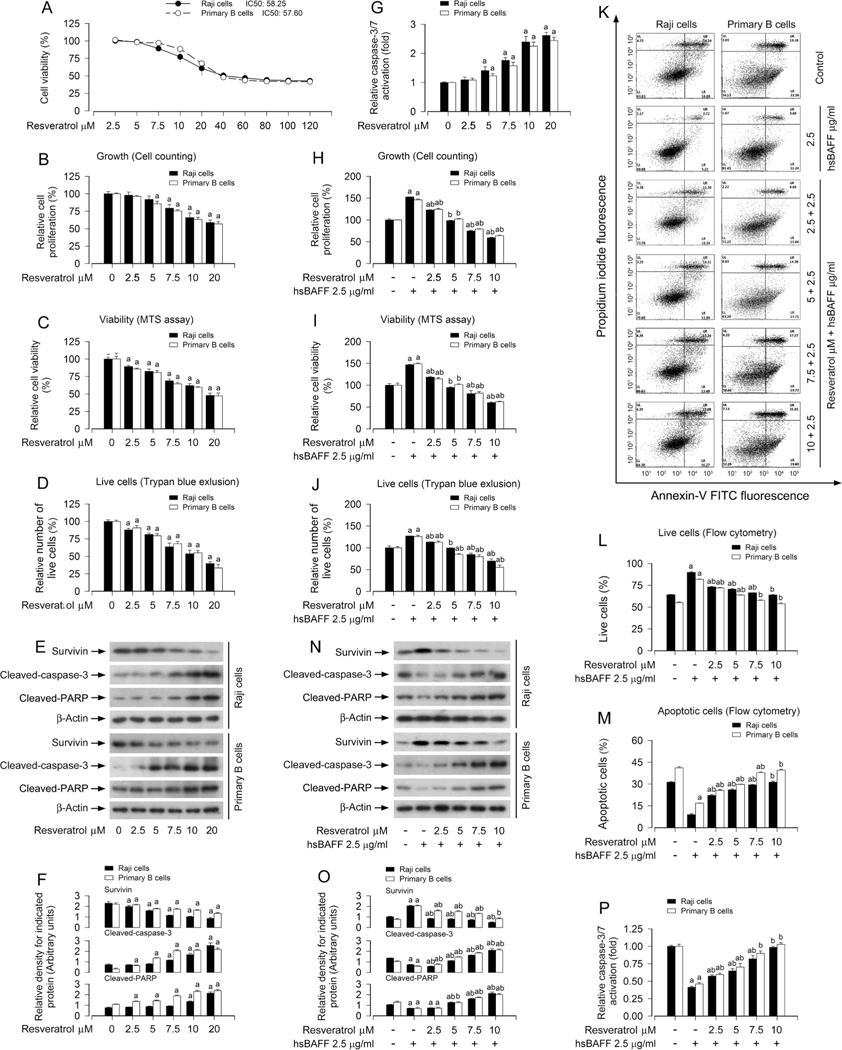
3.1. Resveratrol inhibits hsBAFF-stimulated B-cell proliferation and survival
Our previous studies have shown that excess hsBAFF promotes B-cell proliferation and survival in part by activating the mTOR pathway, which can be blocked by rapamycin (mTOR inhibitor) in Daudi, Raji cells and/or primary murine B cells [29,34]. Here we found that treatment with resveratrol (0–120 μM) alone for 48 h dose-dependently inhibited cell proliferation of Raji cells and primary murine B cells, with IC50 values of 58.25 μM (Raji cells) and 57.60 μM (primary murine B cells) (Fig. 1A). Given that human physiological concentrations of resveratrol and derived metabolites are between 50 nM and 18 μM [35], we chose 0–20 μM of resveratrol to further study the inhibitory effects of resveratrol on B-cell proliferation and survival. The results showed that treatment with resveratrol alone reduced cell proliferation, viability, and live cell number in a concentration-dependent manner, as assessed by cell counting (Fig. 1B), MTS assay (Fig. 1C) and trypan blue exclusion (Fig. 1D), respectively. Western blot results indicated that 12-h treatment with resveratrol dose-dependently decreased the expression of survivin and increased the cleavages of caspase-3 and PARP in the cells (Fig. 1E and F). The caspase3/7 activity assay also revealed a dose-dependent activation of caspases 3/7 by resveratrol in the cells (Fig. 1G). Next, Raji cells and primary murine B cells were pretreated with/without 2.5–10 μM of resveratrol for 1 h and then stimulated with/ without 2.5 μg/ml of hsBAFF for 12 h or 48 h. The results showed that resveratrol also suppressed hsBAFF-stimulated cell proliferation (Fig. 1H), viability (Fig. 1I), and live cell number (Fig. 1J) in a concentration-dependent fashion. To further understand how resveratrol reduces hsBAFF-induced cell survival, we investigated the status of resveratrol-induced apoptosis by FACS using annexin-V-FITC/PI staining. The results indicated that treatment with resveratrol remarkably reduced the relative number of live cells (Fig. 1K and L) and simultaneously elevated the relative number of apoptotic cells (Fig. 1K and M) in hsBAFF-stimulated Raji cells and primary murine B cells concentration-dependently (Fig. 1K–M). Meanwhile, we also noted that resveratrol treatment decreased the expression of survivin, increased the cleavages of caspase-3/PARP (Fig. 1N and O) and activation of caspases 3/7 (Fig. 1P) in the cells exposed to hsBAFF. Collectively, our findings support resveratrol as a potent agent against excess BAFF-stimulated B-cell proliferation and survival. Since 10 μM of resveratrol was able to potently intervene in BAFF-elicited cell proliferation and survival, this dose was chosen for further studies, as described below.
Fig. 1.
Resveratrol attenuates hsBAFF-stimulated B-cell proliferation and survival. Raji cells and primary mouse B cells were treated with resveratrol (0–120 μM) for 48 h (for IC50 and growth inhibition assay), or with resveratrol (0–20 μM) for 12 h (for Western blotting) or 48 h (for cell proliferation, viability and/or apoptosis assays), or pretreated with/without resveratrol (2.5–10 μM or 10 μM) for 1 h and then stimulated with/without hsBAFF (2.5 μg/ml) for 12 h or 48 h. (A) IC50 values and growth inhibition were determined using CCK-8 Assay Kit. (B, H) The relative proliferation was evaluated by cell counting. (C, I) The relative viability was detected by MTS assay. (D, J) The relative number of live cells was estimated by trypan blue exclusion assay. (E and N) Total cell lysates were subjected to Western blotting with indicated antibodies. The blots were probed for β-actin as a loading control. Similar results were obtained in at least five independent experiments. (F, O) The relative densities for survivin, cleaved-caspase-3, cleaved-PARP to β-actin were semi-quantified using NIH image J. (G, P) Caspase-3/7 activity was determined using Caspase-3/7 Assay Kit. (K) The percentages of live (LL), early apoptotic (LR), late apoptotic (UR) and necrotic cells (UL) were determined by FACS using annexin-V-FITC/PI staining. The results from a representative experiment are shown. (L, M) Quantitative analysis of live and apoptotic cells by FACS assay. All data were expressed as mean ± SE (n = 3 for F, L, M. O; n = 6 for A-D, G, H-J, P). Using one-way ANOVA, ap < 0.05, difference vs control group; bp < 0.05, difference vs 2.5 μg/ml hsBAFF group.
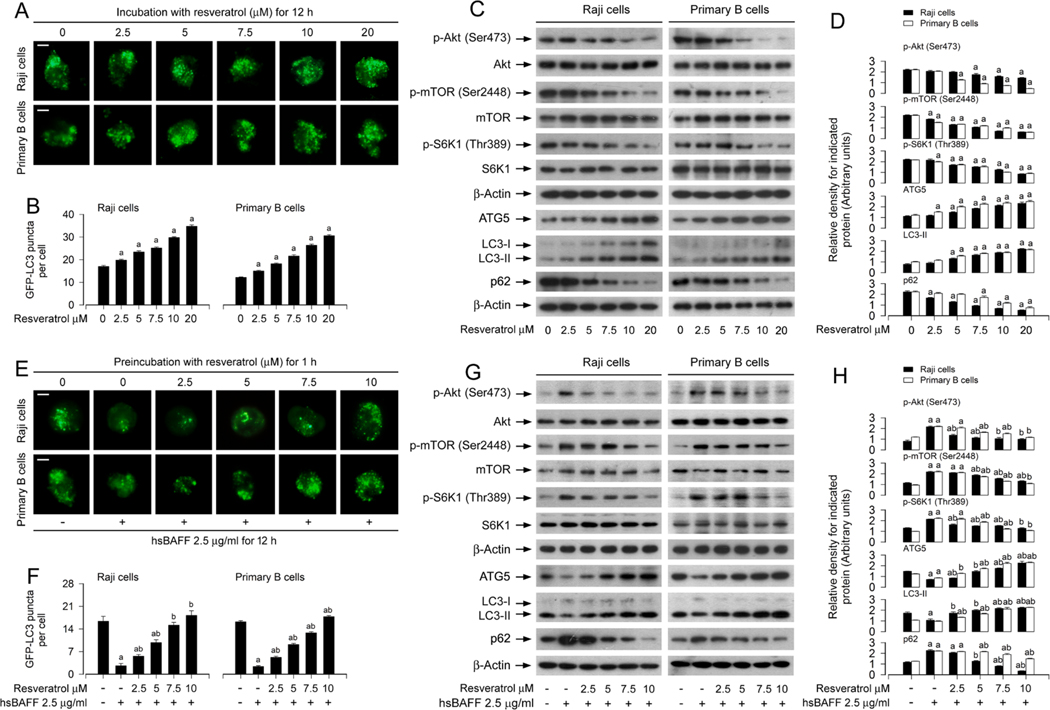
3.2. Resveratrol blocks hsBAFF-induced activation of the Akt/mTOR signaling and inhibition of autophagy in B cells
Our recent study has shown that hsBAFF suppresses autophagy by activating the Akt/mTOR pathway, thereby promoting B-cell proliferation and survival [22]. Next, we investigated whether resveratrol inhibits the basal or hsBAFF-stimulated B-cell proliferation and survival by induction of autophagy. The number of LC3 puncta is commonly used to evaluate the manifestation of autophagosomes [36]. Using GFP-LC3 assay, we observed that in the absence of hsBAFF, autophagic vacuoles with GFP-LC3 (in green) were concentration-dependently increased by resveratrol (Fig. 2A and B), and resveratrol greatly reversed hsBAFF-triggered decrease of autophagic vacuoles (Fig. 2E and F) in Raji cells and primary murine B cells.
Fig. 2.
Resveratrol blocks hsBAFF-induced activation of Akt/mTOR signaling and inhibition of autophagy in B cells. Raji cells and primary mouse B cells infected with/without Ad-GFP-LC3 were treated with resveratrol (0–20 μM) for 12 h, or stimulated with/without hsBAFF (2.5 μg/ml) for 12 h following pretreatment with/ without resveratrol (2.5–10 μM) for 1 h, respectively. (A, E) Representative GFP-LC3 puncta imaging (in green) in the cells was shown by using GFP-LC3 assay. Scale bar: 2 μm. (B, F) The number of GFP-LC3 puncta per cell was quantified. (C, G) Total cell lysates were subjected to Western blotting with indicated antibodies. The blots were probed for β-actin as a loading control. Similar results were obtained in at least five independent experiments. (D, H) The relative densities for p-Akt (Ser473) to Akt, p-mTOR (Ser2448) to mTOR, p-S6K1 (Thr389) to S6K1, and ATG5, LC3-II, p62 to β-actin were semi-quantified using NIH image J. All data were expressed as mean ± SE (n = 3 for D, H; n = 6 for B, F). Using one-way ANOVA, ap < 0.05, difference vs control group; bp < 0.05, difference vs 2.5 μg/ml hsBAFF group.
ATG5 is an essential component for the canonical autophagy [37]. LC3 exists in two molecular forms: LC3-I and LC3-II, and the conversion of LC3-I to LC3-II is frequently used as a surrogate for autophagosome formation, a critical step for autophagy [38]. Additionally, p62 protein, also called sequestosome 1 (SQSTM1), binds to ubiquitinated substrates and LC3, and is degraded along with its cargo, so p62 is commonly considered as a marker for execution of autophagy [39]. To verify the above findings, we further detected the cellular protein levels of ATG5, LC3-II and p62 using Western blotting. The results showed that resveratrol increased ATG5/LC3-II levels and decreased p62 level (Fig. 2C and D), and attenuated hsBAFF-induced decrease of ATG5/LC3-II and increase of p62 dose-dependently in Raji cells and primary murine B cells (Fig. 2G and H). Furthermore, we also noted that resveratrol suppressed the basal or hsBAFF-stimulated phosphorylation of Akt, mTOR and S6K1 dose-dependently in the cells (Fig. 2D, D, G and H), implying that resveratrol inhibits the basal and hsBAFF-activated Akt and mTOR/ S6K1 pathway in B cells. Taken together, the data imply that resveratrol blocks hsBAFF-induced activation of Akt/mTOR signaling pathway and inhibition of autophagy in B cells.
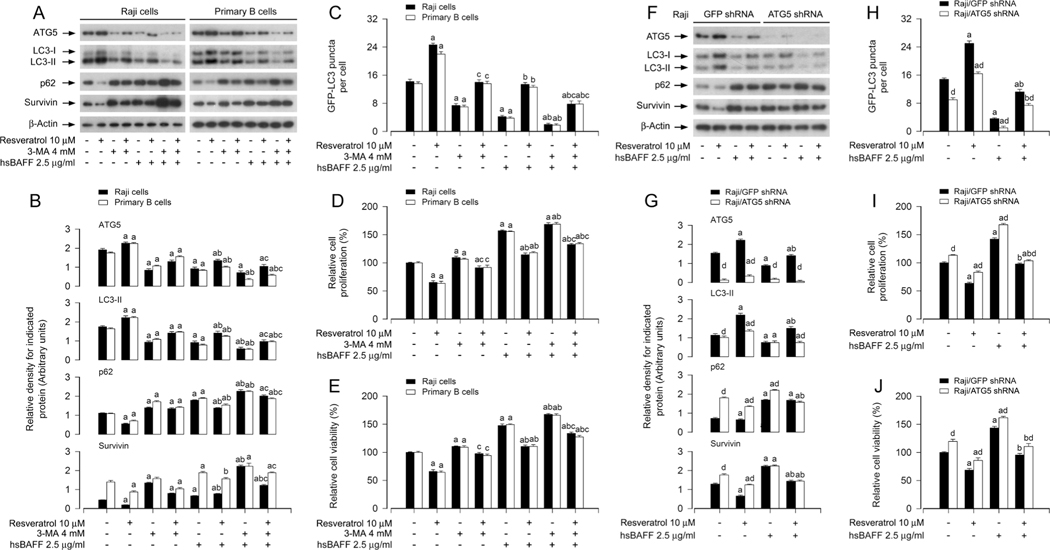
3.3. Resveratrol counteracts hsBAFF-induced inhibition of autophagy, reducing B-cell proliferation/viability
To determine whether the inhibitory effect of resveratrol on hsBAFF-sitmulated B-cell proliferation/viability is attributed to induction of autophagy, firstly, Raji cells and primary murine B cells were pretreated for 1 h with/without 4 mM of 3-methyladenine (3-MA), an inhibitor of class III PI3K Vsp34 essential for autophagosome formation [40], then treated with/without resveratrol (10 μM) for another 1 h, and finally stimulated with/without hsBAFF (2.5 μg/ml) for 12 h or 48 h. As demonstrated in Fig. 3A and B, pretreatment with 3-MA profoundly suppressed resveratrol-induced increase in ATG5 and LC3-II, and decrease in p62 and survivin, regardless of absence or presence of hsBAFF. As a result, 3-MA pretreatment obviously attenuated resveratrol-induced increase of autophagic vacuoles (Fig. 3C) and decrease of cell proliferation/viability (Fig. 3D and E). The results support the notion that resveratrol represses hsBAFF-induced proliferation/ viability in part by inducing autophagy in B cells.
Fig. 3.
Pharmacological inhibition of autophagy with 3-MA or downregulation of ATG5 attenuates the inhibitory effect of resveratrol on hsBAFF-induced B-cell proliferation/viability. Raji cells and primary mouse B cells infected with/without Ad-GFP-LC3, or Raji cells infected with lentiviral shRNA to ATG5 or GFP (as control) and infected with/without Ad-GFP-LC3, respectively, were pretreated with/without resveratrol (10 μM) for 1 h, or pretreated with/without 3-MA (4 mM) for 1 h and then with/without resveratrol (10 μM) for another 1 h, followed by stimulation with/without hsBAFF (2.5 μg/ml) for 12 h (for Western blotting and GFP-LC3 assay) or 48 h (for cell proliferation/viability assay). (A, F) Total cell lysates were subjected to Western blotting using indicated antibodies. The blots were probed for β-actin as a loading control. Similar results were obtained in at least five independent experiments. (B, G) The relative densities for ATG5, LC3-II, p62, survivin to β-actin were semi-quantified using NIH image J. (C, H) The number of GFP-LC3 puncta per cell was quantified by GFP-LC3 assay. (D, I) The relative cell proliferation was evaluated by cell counting. (E, J) The relative cell viability was determined by the MTS assay. All data were expressed as mean ± SE (n = 3 for B, G; n = 6 for C-E, H-J). Using one-way or two-way ANOVA or Student’s t-test, aP < 0.05, difference vs control group; bp < 0.05, difference vs 2.5 μg/ml hsBAFF group; cp < 0.05, difference vs hsBAFF/Resveratrol group or hsBAFF/3-MA group; dp < 0.05, ATG5 shRNA group vs GFP shRNA group.
ATG5 plays a vital role in autophagosome formation, so deletion of ATG5 can completely inhibit autophagy [37]. To validate the role of autophagy induction in resveratrol’s suppression of hsBAFF-stimulated cell proliferation/viability, next, ATG5 expression in Raji cells was silenced by ~ 90% (Fig. 3F and G). As expected, regardless of the absence or presence of hsBAFF, knockdown of ATG5 attenuated resveratrol-induced increase in ATG5 and LC3-II, decrease in p62 and survivin (Fig. 3F and G), increase in autophagic vacuoles (Fig. 3H), and decrease in cell proliferation/viability (Fig. 3I and J). Taken together, our findings demonstrate that resveratrol counteracts hsBAFF-elicited inhibition of autophagy, thereby suppressing B-cell proliferation/ viability.
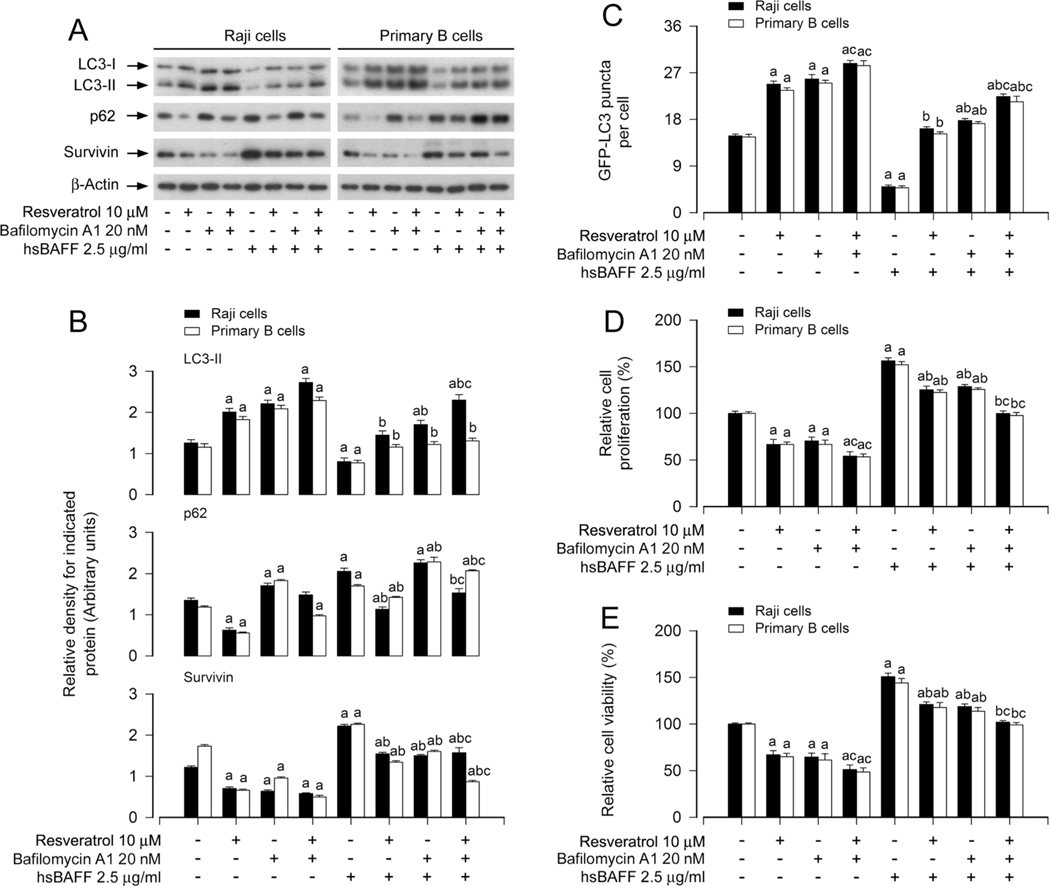
3.4. Resveratrol-induced autophagic flux is critical for its reversing hsBAFF-stimulated B-cell proliferation/viability
Increased p62 protein level is related to reduced autophagy flux [41], while decreased LC3-II level may link to accelerated autophagic flux [42]. We have observed that hsBAFF-decreased LC3-II and -increased p62 were reversed by resveratrol in B cells (Fig. 2). To understand the role of resveratrol-induced autophagic flux in inhibiting hsBAFF-stimulated cell proliferation/viability, bafilomycin A1, an autophagic flux blocker, was employed, which can inhibit autophagosome/lysosome fusion, resulting in defective autophagy [43]. As predicted, pretreatment with bafilomycin A1 inhibited resveratrol-induced autophagic flux, resulting in accumulation of p62 and LC3-II (Fig. 4A and B). Consequently, pretreatment with bafilomycin A1 substantially enhanced the basal and resveratrol-induced increase in autophagic vacuoles, in the absence or presence of hsBAFF (Fig. 4A–C). Interestingly, pretreatment with bafilomycin A1 failed to prevent resveratrol from inhibiting hsBAFF-promoted cell proliferation/viability, but potentiated resveratrol-induced decrease in survivin and cell proliferation/viability in Raji cells and primary murine B cells (Fig. 4A–E). The results suggest that the intact autophagic flux induced by resveratrol is critical for reversing hsBAFF-evoked B-cell proliferation/viability, whereas blocking resveratrol-induced autophagic flux may cause more severe cellular stress, leading to further inhibition of cell proliferation/ viability.
Fig. 4.
Resveratrol-induced autophagic flux is critical for its reversing hsBAFF-stimulated B-cell proliferation/viability. Raji cells and primary mouse B cells infected with/without Ad-GFP-LC3, respectively, were pretreated with/without bafilomycin A1 (20 nM) for 1 h and then with/without resveratrol (10 μM) for another 1 h, followed by stimulation with/without hsBAFF (2.5 μg/ml) for 12 h (for Western blotting and GFP-LC3 assay) or 48 h (for cell proliferation/viability assay). (A) Total cell lysates were subjected to Western blotting using indicated antibodies. The blots were probed for β-actin as a loading control. Similar results were obtained in at least five independent experiments. (B) The relative densities for LC3-II, p62, survivin to β-actin were semi-quantified using NIH image J. (C) The number of GFP-LC3 puncta per cell was quantified by GFP-LC3 assay. (D) The relative cell proliferation was evaluated by cell counting. (E) The relative cell viability was determined by the MTS assay. All data were expressed as mean ± SE (n = 3 for B; n = 6 for C-E). Using one-way or two-way ANOVA, ap < 0.05, difference vs control group; bp < 0.05, difference vs 2.5 μg/ml hsBAFF group; cp < 0.05, difference vs hsBAFF/Resveratrol group or hsBAFF/Bafilomycin A1 group.
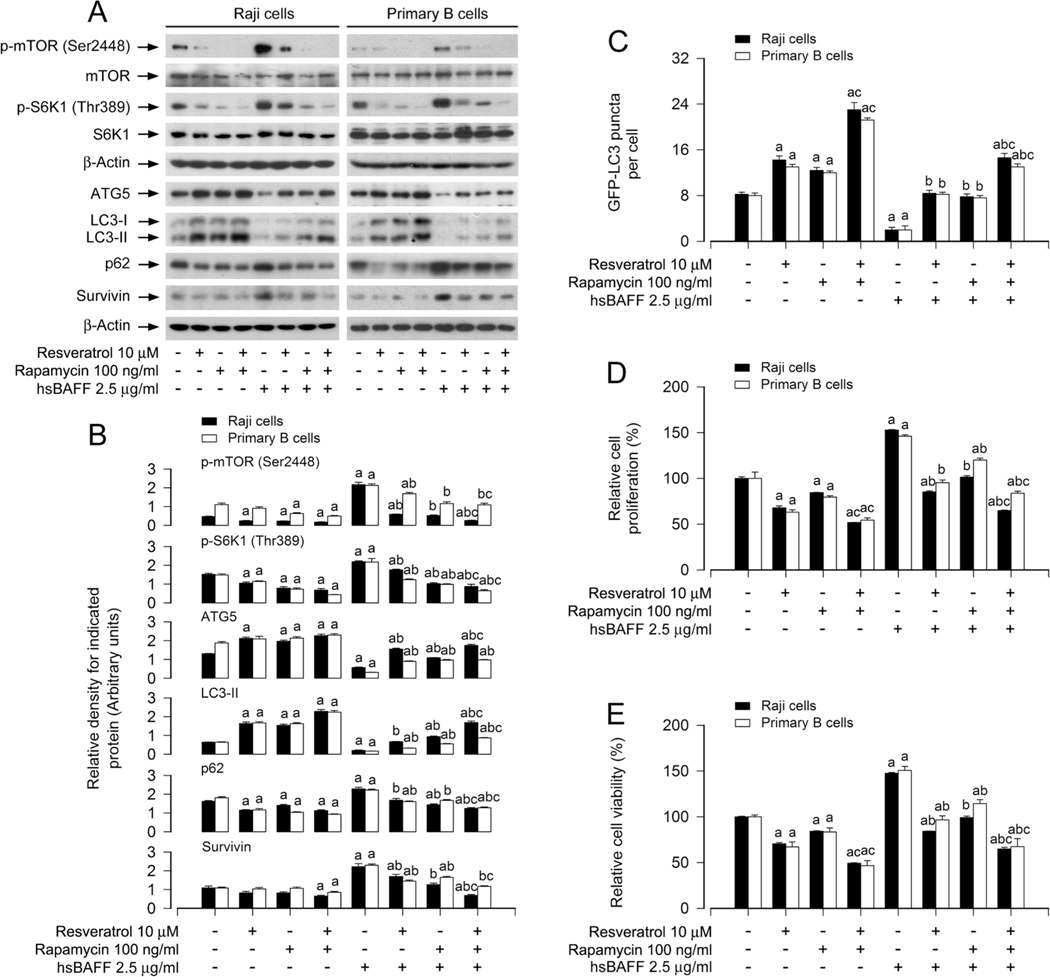
3.5. Resveratrol reverses hsBAFF-induced inhibition of autophagy, suppressing cell proliferation/viability via mTOR-dependent mechanism in B cells
To determine if the inhibitory effect of resveratrol on hsBAFF-stimulated B-cell proliferation and viability is attributed to its promotion of autophagy via mTOR-dependent mechanism, Raji cells and primary murine B cells were pretreated with/without 100 ng/ml of rapamycin (a specific mTOR inhibitor) for 2 h, then treated with/ without 10 μM of resveratrol for another 1 h, and finally stimulated with/without 2.5 μg/ml of hsBAFF for 12 h or 48 h. The results revealed that co-treatment with resveratrol/rapamycin more markedly repressed the basal and hsBAFF-induced expression in p-mTOR, p-S6K1 and survivin in the cells compared to treatment with rapamycin or resveratrol alone (Fig. 5A and B). Of note, the basal or hsBAFF-reduced ATG5 and LC3 II protein levels, GFP-LC3 puncta number, as well as hsBAFF-elevated p62 expression were more significantly reversed by co-treatment with resveratrol/rapamycin than treatment with rapamycin or resveratrol alone (Fig. 5A–C). In agreement with this, co-treatment with resveratrol/rapamycin also inhibited hsBAFF-stimulated B-cell proliferation/viability more potently than treatment with resveratrol or rapamycin alone (Fig. 5D and E).
Fig. 5.
Pharmacological inhibition of mTOR reinforces the inhibitory activity of resveratrol on hsBAFF-induced inhibition of autophagy and increase of cell proliferation/viability in B cells. Raji cells and primary mouse B cells infected with/without Ad-GFP-LC3, respectively, were pretreated with/without rapamycin (100 ng/ml) for 2 h and then with/without resveratrol (10 μM) for another 1 h, followed by stimulation with/without hsBAFF (2.5 μg/ml) for 12 h (for Western blotting and GFP-LC3 assay) or 48 h (for cell proliferation/viability assay). (A) Total cell lysates were subjected to Western blotting using indicated antibodies. The blots were probed for β-actin as a loading control. Similar results were obtained in at least five independent experiments. (B) The relative densities for p-mTOR (Ser2448) to mTOR, p-S6K1 (Thr389) to S6K1, and ATG5, LC3-II, p62, survivin to β-actin were semi-quantified using NIH image J. (C) The number of GFP-LC3 puncta per cell was quantified by GFP-LC3 assay. (D) The relative cell proliferation was evaluated by cell counting. (E) The relative cell viability was determined by the MTS assay. All data were expressed as mean ± SE (n = 3 for B; n = 6 for C-E). Using one-way or two-way ANOVA, ap < 0.05, difference vs control group; bp < 0.05, difference vs 2.5 μg/ml hsBAFF group; cp < 0.05, difference vs hsBAFF/Resveratrol group or hsBAFF/Rapamycin group.
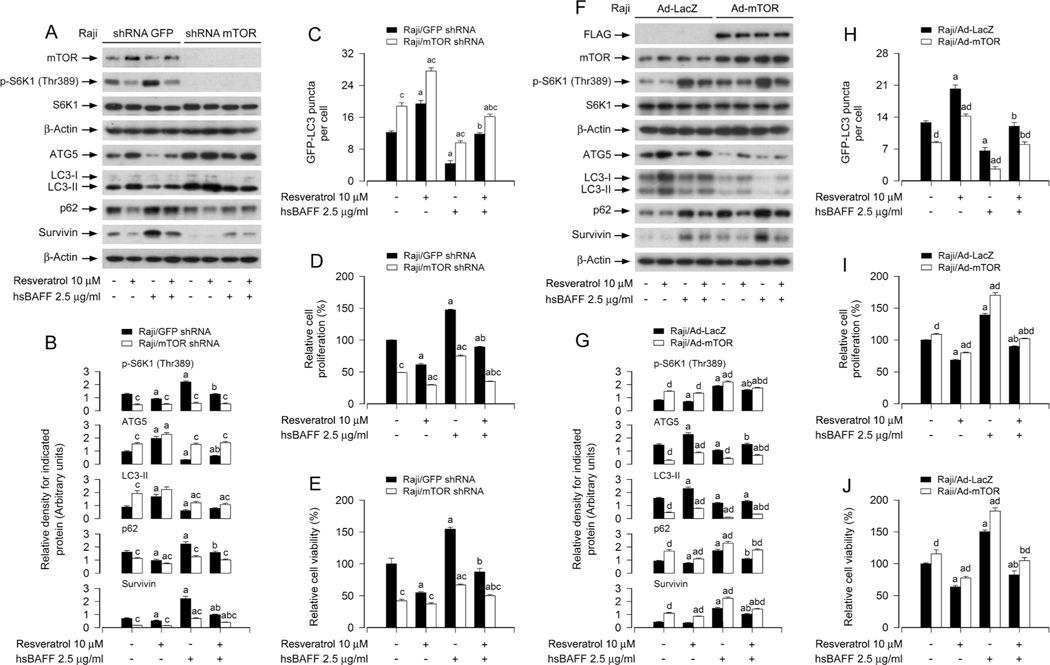
To further verify that mTOR-dependent autophagy is associated with resveratrol’s suppression of hsBAFF-induced B-cell proliferation/ viability, we conducted mTOR gene silencing or overexpressing experiments. Similar to the above treatment with rapamycin, depletion of mTOR (by ~ 90%) in Raji cells (Fig. 6a) inhibited the basal or hsBAFF-induced activation of mTOR signaling, evidenced by non-detectable p-S6K1 (T389) (an indicator of mTOR kinase activity) (Fig. 6A and B). Of importance, knockdown of mTOR markedly hindered the basal and hsBAFF-induced decrease of ATG5 and LC3-II, as well as increase of p62 and survivin in the cells even without pretreatment with resveratrol (Fig. 6A and B). Additionally, as expected, silencing mTOR significantly increased the formation of GFP-LC3 puncta, regardless of the presence or absence of hsBAFF or resveratrol (Fig. 6C). Meanwhile, silencing mTOR attenuated the basal and hsBAFF-stimulated cell proliferation/viability, and enhanced the inhibitory activity of resveratrol (Fig. 6D and E). In contrast, overexpression of FLAG-tagged wild-type mTOR rendered high resistance to the effect of resveratrol (Fig. 6F–J). Together, our observations demonstrate that resveratrol reverses hsBAFF-induced autophagy inhibition, inhibiting cell proliferation/viability through mTOR-dependent mechanism in B cells.
Fig. 6.
Knockdown or overexpression of mTOR interferes with the inhibitory activity of resveratrol on hsBAFF-induced inhibition of autophagy and increase of cell proliferation/viability in B cells. Raji cells, infected with lentiviral shRNA to mTOR or GFP (as control), or infected with Ad-mTOR or Ad-LacZ (for control) and infected with/without Ad-GFP-LC3, respectively, were pretreated with resveratrol (10 μM) for 1 h, followed by stimulation with/without hsBAFF (2.5 μg/ml) for 12 h (for Western blotting and GFP-LC3 assay) or 48 h (for cell proliferation/viability assay). (A, F) Total cell lysates were subjected to Western blotting using indicated antibodies. The blots were probed for β-actin as a loading control. Similar results were obtained in at least five independent experiments. (B, G) The relative densities for p-S6K1 (Thr389) to S6K1, and ATG5, LC3-II, p62, survivin to β-actin were semi-quantified using NIH image J. (C, H) The number of GFP-LC3 puncta per cell was quantified by GFP-LC3 assay. (D, I) The relative cell proliferation was evaluated by cell counting. (E, J) The relative cell viability was determined by the MTS assay. All data were expressed as mean ± SE (n = 3 for B, G; n = 6 for C-E, H-J). Using one-way ANOVA or Student’s t-test, ap < 0.05, difference vs control group; bp < 0.05, difference vs 2.5 μg/ml hsBAFF group; cp < 0.05, mTOR shRNA group vs GFP shRNA group; dp < 0.05, Ad-mTOR group vs Ad-GFP group.
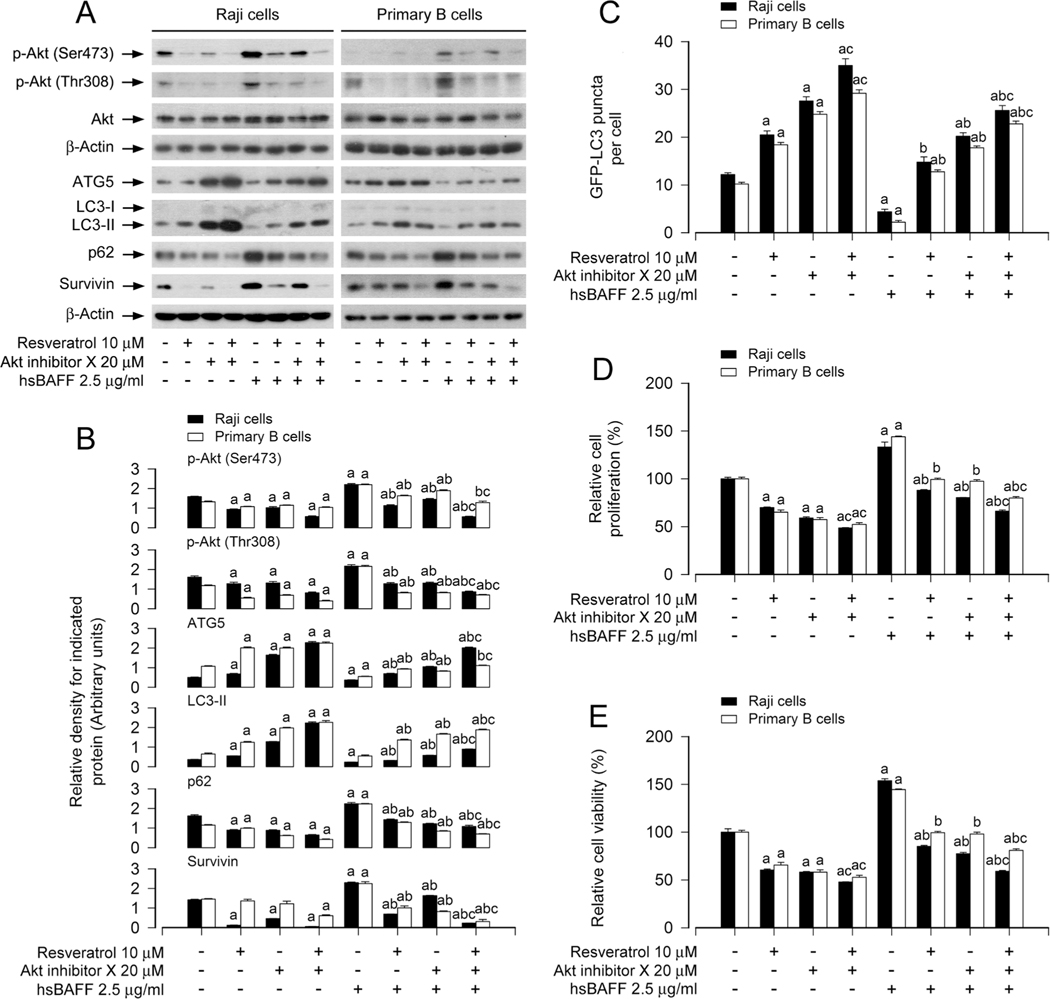
3.6. Resveratrol inhibits Akt activity impeding hsBAFF-induced inhibition of autophagy and increase in cell proliferation/viability in B cells
We have observed that resveratrol blocks the basal and hsBAFF-triggered Akt activation in B cells (Fig. 2C, D, G, H). To understand the significance of Akt in resveratrol’s hindering hsBAFF-induced inhibition of autophagy and increase in cell proliferation/viability in B cells, Raji cells and primary murine B cells were pretreated with/without 20 μM of Akt inhibitor X for 1 h, then treated with/without 10 μM of resveratrol for another 1 h, and finally stimulated with/without 2.5 μg/ ml of hsBAFF for 12 h or 48 h. As demonstrated in Fig. 7A and B, pretreatment with Akt inhibitor X and/or resveratrol substantially declined the levels of p-Akt, p62 and survivin, regardless of the presence or absence of hsBAFF. Meanwhile, the basal or hsBAFF-reduced ATG5 and LC3-II expression, and GFP-LC3 puncta were more markedly reversed in the cells co-pretreated with Akt inhibitor X/resveratrol than in those pretreated with Akt inhibitor X or resveratrol alone (Fig. 7A–C). Moreover, the basal or hsBAFF-promoted cell proliferation/viability was also effectively diminished by Akt inhibitor X or resveratrol, and more significant effects were seen in the cells co-pretreated with Akt inhibitor X/ resveratrol (Fig. 7D and E).
Fig. 7.
Inhibition of Akt enhances the inhibitory activity of resveratrol on hsBAFF-induced inhibition of autophagy and increase of cell proliferation/viability in B cells. Raji cells and primary mouse B cells infected with/without Ad-GFP-LC3, respectively, were pretreated with/without Akt inhibitor X (20 μM) for 1 h and then with/without resveratrol (10 μM) for another 1 h, followed by stimulation with/without hsBAFF (2.5 μg/ml) for 12 h (for Western blotting and GFP-LC3 assay) or 48 h (for cell proliferation/viability assay). (A) Total cell lysates were subjected to Western blotting using indicated antibodies. The blots were probed for β-actin as a loading control. Similar results were obtained in at least five independent experiments. (B) The relative densities for p-Akt (Ser473), p-Akt (Thr308) to Akt, and ATG5, LC3-II, p62, survivin to β-actin were semi-quantified using NIH image J. (C) The number of GFP-LC3 puncta per cell was quantified by GFP-LC3 assay. (D) The relative cell proliferation was evaluated by cell counting. (E) The relative cell viability was determined by the MTS assay. All data were expressed as mean ± SE (n = 3 for B; n = 6 for C-E). Using one-way or two-way ANOVA, ap < 0.05, difference vs control group; bp < 0.05, difference vs 2.5 μg/ml hsBAFF group; cp < 0.05, difference vs hsBAFF/Resveratrol group or hsBAFF/Akt inhibitor X group.
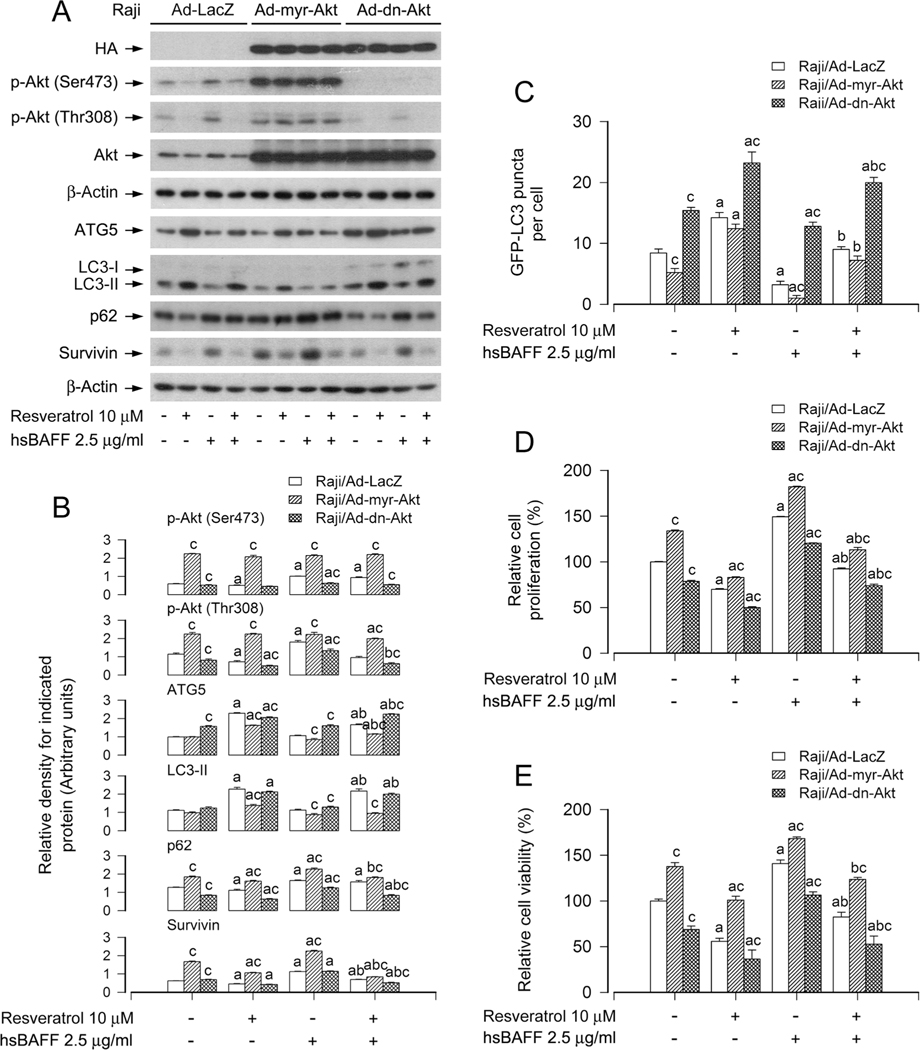
Next, Raji cells, infected with Ad-myr-Akt, Ad-dn-Akt and Ad-LacZ, respectively, were stimulated with/without hsBAFF (2.5 μg/ml) for 12 h or 48 h post pre-treatment with/without resveratrol (10 μM) for 1 h. As expected, high levels of HA-tagged Akt mutants were detected in Ad-myr-Akt-or Ad-dn-Akt-infected cells, but not in Ad-LacZ-infected cells (Fig. 8A). Ectopic expression of myr-Akt or dn-Akt strongly increased or decreased the Akt activity, since the basal or hsBAFF-elevated p-Akt was substantially upregulated or downregulated (Fig. 8A and B), as evidenced by Western blot analysis. Interestingly, expression of dn-Akt reinforced resveratrol’s blocking hsBAFF-evoked activation of Akt, decrease of ATG5, LC3-II and GFP-LC3 puncta, and increase of p62 and proliferation/viability in the cells (Fig. 8A–E). In contrast, expression of myr-Akt rendered great resistance to the effects of resveratrol (Fig. 8A–E). Taken together, our results indicate the importance of resveratrol’s blocking hsBAFF-activated Akt in impeding hsBAFF-induced inhibition of autophagy and increase in cell proliferation/viability in B cells.
Fig. 8.
Ectopic expression of constitutively active or dominant negative Akt affects the inhibitory activity of resveratrol on hsBAFF-induced inhibition of autophagy and increase of cell proliferation/viability in B cells. Raji cells, infected with Ad-myr-Akt, Ad-dn-Akt, or Ad-LacZ (for control) and infected with/without Ad-GFP-LC3, respectively, were pretreated with/without resveratrol (10 μM) for 1 h, followed by stimulation with/without hsBAFF (2.5 μg/ml) for 12 h (for Western blotting and GFP-LC3 assay) or 48 h (for cell proliferation/viability assay). (A) Total cell lysates were subjected to Western blotting using indicated antibodies. The blots were probed for β-actin as a loading control. Similar results were obtained in at least five independent experiments. (B) The relative densities for p-Akt (Ser473), p-Akt (Thr308), ATG5, LC3-II, p62, survivin to β-actin were semi-quantified using NIH image J. (C) The number of GFP-LC3 puncta per cell was quantified by GFP-LC3 assay. (D) The relative cell proliferation was evaluated by cell counting. (E) The relative cell viability was determined by the MTS assay. All data were expressed as mean ± SE (n = 3 for B; n = 6 for C-E). Using one-way ANOVA or Student’s t-test, ap < 0.05, difference vs control group; bp < 0.05, difference vs 2.5 μg/ml hsBAFF group; cp < 0.05, Ad-myr-Akt group or Ad-dn-Akt group vs Ad-GFP group.
4. Discussion
Resveratrol is a natural polyphenolic compound that has positive therapeutic effects on cardiovascular diseases, cancer [44], inflammation, and autoimmune diseases [45]. Some animal and human studies have shown that resveratrol can ameliorate the progression of autoimmune diseases, such as SLE, RA and psoriasis [23]. However, the underlying mechanism is still poorly understood. Here, we present evidence that resveratrol suppresses hsBAFF-induced cell proliferation and survival by promoting autophagic flux and autophagy, which is associated with its inhibition of the Akt/mTOR pathway in normal and neoplastic B-lymphoid cells.
In the current study, we firstly showed that resveratrol decreased cell proliferation/viability and the protein level of survivin, and increased the cleavages of caspase-3/PARP and activation of caspase3/7 dose-dependently (Fig. 1B–G). Subsequently, we identified that resveratrol induced apoptotic cell death and inhibited hsBAFF-activated cell proliferation and survival in primary murine B lymphocytes and neoplastic B-lymphoid (Raji) cells (Fig. 1H–P). Since resveratrol elicits cell growth inhibition and autophagic cell death by blocking the Akt/mTOR signaling in various human tumor cells [46–49], we next tested whether resveratrol suppresses hsBAFF-stimulated B-cell proliferation and survival by inducing autophagy. The experiments demonstrated that resveratrol was able to trigger the increase in autophagosomes/ATG5/ LC3-II and the decrease in p62 dose-dependently, and reverse hsBAFF-elicited diminishment of autophagosome/ATG5/LC3-II and elevation of p62 in Raji cells and purified mouse B lymphocytes, as evidenced by using GFP-LC3 assay and Western blotting (Fig. 2), suggesting that resveratrol indeed induces autophagy and counteracts hsBAFF-induced inhibition of autophagy.
In this study, to elucidate whether resveratrol-induced autophagy plays a vital role in reversing hsBAFF-induced inhibition of autophagy and increase of B-cell proliferation/viability, pharmacological and genetic approaches were utilized. Treatment with 3-MA, an inhibitor of Vsp34 that is essential for autophagosome formation [40], prevented resveratrol from hindering hsBAFF-induced decrease in ATG5/LC3-II protein levels and autophagosomes, as well as increase in p62/survivin and proliferation/viability in Raji cells and primary murine B cells (Fig. 3A–E). Furthermore, knockdown of ATG5 recapitulated the effect of 3-MA (Fig. 3F–J). Taken together, these lines of evidence support that resveratrol promotes autophagy, counteracting hsBAFF-activated cell proliferation/viability in B cells.
When autophagic flux is intact, p62 is degraded in autolysosomes [41]. So, defected autophagic flux reduces p62 degradation, causing accumulation of p62 in the cytosol [50]. In this study, an underscored fact is that both autophagy and autophagic flux were substantially suppressed by excessive hsBAFF stimulation in B cells, as evidenced by obvious decrease in ATG5 and LC3-II levels and abnormal increase in p62 in Raji cells and primary murine B cells in response to hsBAFF (Fig. 2). Interestingly, resveratrol was able to reverse hsBAFF-induced inhibition of autophagy by promoting autophagic flux and inducing autophagy (Fig. 2). To dissect the role of autophagic flux in hsBAFF-stimulated B-cell proliferation/viability inhibited by resveratrol, we employed an autophagic flux blocker bafilomycin A1 [43]. The results showed that bafilomycin A1 could enhance the basal and resveratrol-induced increase in autophagic vacuoles, regardless of the absence or presence of hsBAFF (Fig. 4A–C), but could not prevent resveratrol from inhibiting hsBAFF-stimulated cell proliferation/viability. Instead, bafilomycin A1 potentiated resveratrol-induced decrease in survivin and cell proliferation/viability in Raji cells and purified mouse B lymphocytes (Fig. 4). The results suggest that the intact autophagic flux induced by resveratrol is crucial for its reversing hsBAFF-promoted B-cell proliferation/viability. It has been shown that bafilomycin A1 can inhibit both V-ATPase and Ca-P60A/SERCA-dependent autophagosome/lysosome fusion, thus blocking autolysosome acidification and interfering with degradation of unfolded or misfolded proteins and other cellular debris in the cells, and also greatly increasing the level of intracellular calcium [51]. Likely, blocking autophagic flux with bafilomycin A1 may cause more severe cellular stress, thus enhancing resveratrol’s inhibition of hsBAFF-stimulated B-cell proliferation/viability.
Studies have shown that there exists Akt/mTOR dysfunction with abnormal activity in several autoimmune diseases [20,21,52,53]. Akt and mTOR have been considered as potential therapeutic targets for a variety of lymphoid malignancies and autoimmune diseases [54–56]. The Akt/mTOR pathway negatively regulates autophagy [16]. Especially, resveratrol may alter the activity of Akt and/or mTOR, even PI3K (upstream of Akt/mTOR), inducing autophagy under various conditions, and thus affecting cell proliferation, survival and apoptosis [57–59]. For example, in T-cell acute lymphoblastic leukemia cells, resveratrol promotes cell apoptosis and autophagy by inhibiting Akt/mTOR [57]. In human promyelocytic leukemia HL-60 cells, resveratrol triggers apoptotic cell death by activating autophagy through suppressing the PI3K/Akt/mTOR signaling pathway [58]. However, in contrast, in human nucleus pulposus cells, resveratrol inhibits IL-1β-induced apoptosis by activating the PI3K/Akt/mTOR pathway [59]. It has been reported that BAFF mRNA and protein levels in patients’ kidney tissues with lupus nephritis are positively correlated with the levels of p-Akt and p-mTOR [60]. Our group has shown that BAFF contributes to proliferation and survival in cultured B lymphocytes via activating Akt/ mTOR pathway [29], and unveiled that BAFF inhibits autophagy contributing to cell proliferation and survival by activating the Akt/ mTOR signaling pathway in primary murine B lymphocytes and neoplastic B-lymphoid cells [22]. In the current study, we revealed that resveratrol reversed hsBAFF-evoked autophagy inhibition and concurrently suppressed hsBAFF-induced activation of Akt and mTOR/ S6K1 in Raji cells and purified mouse B lymphocytes (Fig. 2E–H). Pharmacological inhibition of mTOR with rapamycin or depletion of mTOR by RNA interference potentiated resveratrol-induced autophagy and resveratrol-reduced cell proliferation/viability (Figs. 5 and 6), whereas overexpression of wild-type mTOR conferred resistance to the actions of resveratrol (Fig. 6). Also, inhibition of Akt with Akt inhibitor X or ectopic expression of dn-Akt reinforced resveratrol-affected cell autophagy and proliferation/viability, yet expression of myr-Akt conferred resistance to the actions of resveratrol (Figs. 7 and 8). All these observations support that resveratrol reverses hsBAFF-induced inhibition of autophagy, resulting in inhibition of cell proliferation/ viability by suppressing the Akt/mTOR pathway in normal and lymphoma B cells. Since resveratrol deactivates the Akt/mTOR pathway inducing autophagy and elevating autophagic flux, we think that the major player in resveratrol mitigation of hsBAFF-induced autophagy suppression and proliferation/viability in B cells is the Akt/mTOR signaling. This provides a clue to understanding how resveratrol inhibits BAFF-induced aggressive B-cell malignancies and autoimmune diseases.
It has been reported that apart from the function of LC3 in the development of autophagy, the interaction between Beclin 1 and its binding partners (i.e. Bcl-2, Bcl-XL, Mcl-1) also regulates initial steps of autophagy [61,62]. The interaction of Bcl-2 with Beclin 1 suppresses Beclin 1-dependent autophagy by dissociating Beclin 1 from class III PI3K complexes [62]. Our recent work has demonstrated that hsBAFF obviously elevates Bcl-2, Bcl-xL and Mcl-1 expression in Raji cells, Daudi cells and purified mouse B lymphocytes [63]. During this research, we observed that hsBAFF inhibited the expression of p-Beclin 1 (Thr119) in B cells, and overexpression of a point mutant Beclin 1 (T119E) significantly elevated the basal LC3-II expression and autophagosomes’ number, meanwhile Bcl-2 expression, cell proliferation/viability were declined, thereby resisting hsBAFF-inhibited autophagy impeding B-cell proliferation/viability. Further study is needed to address whether or how resveratrol affects the binding of Bcl-2 to Beclin-1 in hsBAFF-stimulated B cells.
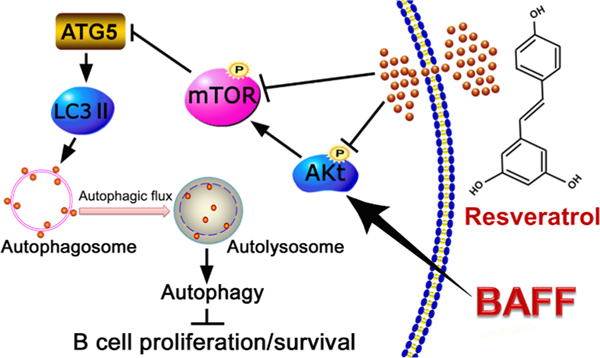
In conclusion, we have demonstrated a role of resveratrol in suppressing excessive hsBAFF-induced cell proliferation and survival in normal and neoplastic B-lymphoid cells. Mechanistically, resveratrol promotes autophagy and autophagic flux impeding hsBAFF-stimulated B-cell proliferation and survival via inhibiting the Akt/mTOR pathway (Fig. 9). Our results suggest that resveratrol may be a promising agent for prevention and treatment of BAFF-elicited aggressive B-cell disorders and autoimmune diseases.
Fig. 9.
A diagram illustrating how resveratrol inhibits hsBAFF-stimulated B-cell proliferation and survival. Resveratrol promotes autophagy and autophagic flux impeding hsBAFF-stimulated cell proliferation and survival via inhibiting the Akt/mTOR pathway in normal and neoplastic B-lymphoid cells. the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31172083 for Long Chen), National Institutes of Health (CA115414 for Shile Huang), Project for the Priority Academic Program Development of Jiangsu Higher Education Institutions of China (PAPD-14KJB180010 for Long Chen), and American Cancer Society (RSG-08-135-01-CNE for Shile Huang).
Abbreviations:
- 3-MA
3-methyladenine
- Akt
protein kinase B (PKB)
- ATG
autophagy-related
- BAFF
B-cell activating factor of the TNF family
- BLyS
B lymphocyte stimulator
- FACS
fluorescence-activated cell sorter
- FBS
fetal bovine serum
- GFP
green fluorescent protein
- IC50
half maximal inhibitory concentration
- LacZ
β-galactosidase
- LC3
microtubule-associated protein 1 light chain 3
- mTOR
mammalian target of rapamycin
- mTORC
mTOR complexes
- PBS
phosphate buffered saline
- PI
propidium iodide
- PI3K
phosphatidylinositol 3′-kinase
- RA
rheumatoid arthritis
- S6K1
ribosomal p70 S6 kinase 1
- SLE
systemic lupus erythematosus
- SS
Sjögren ‘s syndrome
- TALL-1
TNF and apoptosis ligand-related leukocyte-expressed ligand-1
- THANK
TNF homologue that activates apoptosis, nuclear factor κB, and c-Jun NH2-terminal kinase
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence
References
- [1].Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, Feng P, Soppet D, Charters M, Gentz R, Parmelee D, Li Y, Galperina O, Giri J, Roschke V, Nardelli B, Carrell J, Sosnovtseva S, Greenfield W, Ruben SM, Olsen HS, Fikes J, Hilbert DM, BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator, Science 285 (5425) (1999) 260–263. [DOI] [PubMed] [Google Scholar]
- [2].Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N, Ambrose C, Lawton P, Bixler S, Acha-Orbea H, Valmori D, Romero P, Werner-Favre C, Zubler RH, Browning JL, Tschopp J, BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth, J. Exp. Med 189 (11) (1999) 1747–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Batten M, Groom J, Cachero TG, Qian F, Schneider P, Tschopp J, Browning JL, Mackay F, BAFF mediates survival of peripheral immature B lymphocytes, J. Exp. Med 192 (10) (2000) 1453–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E, Scott ML, An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway, Science 293 (5537) (2001) 2111–2114. [DOI] [PubMed] [Google Scholar]
- [5].Mackay F, Browning JL, BAFF: a fundamental survival factor for B cells, Nat. Rev. Immunol 2 (7) (2002) 465–475. [DOI] [PubMed] [Google Scholar]
- [6].Moisini I, Davidson A, BAFF: a local and systemic target in autoimmune diseases, Clin. Exp. Immunol 158 (2) (2009) 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ramanujam M, Davidson A, BAFF blockade for systemic lupus erythematosus: will the promise be fulfilled? Immunol. Rev 223 (2008) 156–174. [DOI] [PubMed] [Google Scholar]
- [8].Cornec D, Devauchelle-Pensec V, Tobon GJ, Pers JO, Jousse-Joulin S, Saraux A, B cells in Sjogren’s syndrome: from pathophysiology to diagnosis and treatment, J. Autoimmun 39 (3) (2012) 161–167. [DOI] [PubMed] [Google Scholar]
- [9].Lahiri A, Pochard P, Le Pottier L, Tobon GJ, Bendaoud B, Youinou P, Pers JO, The complexity of the BAFF TNF-family members: implications for autoimmunity, J. Autoimmun 39 (3) (2012) 189–198. [DOI] [PubMed] [Google Scholar]
- [10].Lee DSW, Rojas OL, Gommerman JL, B cell depletion therapies in autoimmune disease: advances and mechanistic insights, Nat. Rev. Drug Discov 20 (3) (2021) 179–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Feng Y, He D, Yao Z, Klionsky DJ, The machinery of macroautophagy, Cell Res. 24 (1) (2014) 24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cui B, Lin H, Yu J, Yu J, Hu Z, Autophagy and the immune response, Adv. Exp. Med. Biol 1206 (2019) 595–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Clarke AJ, Simon AK, Autophagy in the renewal, differentiation and homeostasis of immune cells, Nat. Rev. Immunol 19 (3) (2019) 170–183. [DOI] [PubMed] [Google Scholar]
- [14].Botbol Y, Guerrero-Ros I, Macian F, Key roles of autophagy in regulating T-cell function, Eur. J. Immunol 46 (6) (2016) 1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Maiuri MC, Zalckvar E, Kimchi A, Kroemer G, Self-eating and self-killing: crosstalk between autophagy and apoptosis, Nat. Rev. Mol. Cell Biol 8 (9) (2007) 741–752. [DOI] [PubMed] [Google Scholar]
- [16].Liu GY, Sabatini DM, mTOR at the nexus of nutrition, growth, ageing and disease, Nat. Rev. Mol. Cell Biol 21 (4) (2020) 183–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ciccia A, Elledge SJ, The DNA damage response: making it safe to play with knives, Mol. Cell 40 (2) (2010) 179–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cao W, Li J, Yang K, Cao D, An overview of autophagy: Mechanism, regulation and research progress, Bull Cancer 108 (3) (2021) 304–322. [DOI] [PubMed] [Google Scholar]
- [19].Trotman LC, Alimonti A, Scaglioni PP, Koutcher JA, Cordon-Cardo C, Pandolfi PP, Identification of a tumour suppressor network opposing nuclear Akt function, Nature 441 (7092) (2006) 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kitz A, de Marcken M, Gautron AS, Mitrovic M, Hafler DA, Dominguez-Villar M, AKT isoforms modulate Th1-like Treg generation and function in human autoimmune disease, EMBO Rep. 17 (8) (2016) 1169–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Duan J, Deng T, Kang J, Chen M, DINP aggravates autoimmune thyroid disease through activation of the Akt/mTOR pathway and suppression of autophagy in Wistar rats, Environ. Pollut 245 (2018) 316–324. [DOI] [PubMed] [Google Scholar]
- [22].Dong X, Qin J, Ma J, Zeng Q, Zhang H, Zhang R, Liu C, Xu C, Zhang S, Huang S, Chen L, BAFF inhibits autophagy promoting cell proliferation and survival by activating Ca2+-CaMKII-dependent Akt/mTOR signaling pathway in normal and neoplastic B-lymphoid cells, Cell. Signal 53 (2019) 68–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Oliveira ALB, Monteiro VVS, Navegantes-Lima KC, Reis JF, Gomes RS, Rodrigues DVS, Gaspar SLF, Monteiro MC, Resveratrol role in autoimmune disease-A mini-review, Nutrients 9 (12) (2017) 1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pervaiz S, Holme AL, Resveratrol: its biologic targets and functional activity, Antioxid. Redox Signal 11 (11) (2009) 2851–2897. [DOI] [PubMed] [Google Scholar]
- [25].Ko JH, Sethi G, Um JY, Shanmugam MK, Arfuso F, Kumar AP, Bishayee A, Ahn KS, The role of resveratrol in cancer therapy, Int. J. Mol. Sci 18 (12) (2017) 2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wu Y, Li X, Zhu JX, Xie W, Le W, Fan Z, Jankovic J, Pan T, Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson’s disease, Neuro-Signals 19 (3) (2011) 163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Liu M, Wilk SA, Wang A, Zhou L, Wang RH, Ogawa W, Deng C, Dong LQ, Liu F, Resveratrol inhibits mTOR signaling by promoting the interaction between mTOR and DEPTOR, J. Biol. Chem 285 (47) (2010) 36387–36394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cao P, Tang XM, Guan ZB, Diao ZY, Zhang SQ, Production and characterization of a bacterial single-chain antibody fragment specific to B-cell- activating factor of the TNF family, Protein Expres. Purif 43 (2) (2005) 157–164. [DOI] [PubMed] [Google Scholar]
- [29].Ke Z, Liang D, Zeng Q, Ren Q, Ma H, Gui L, Chen S, Guo M, Xu Y, Gao W, Zhang S, Chen L, hsBAFF promotes proliferation and survival in cultured B lymphocytes via calcium signaling activation of mTOR pathway, Cytokine 62 (2) (2013) 310–321. [DOI] [PubMed] [Google Scholar]
- [30].Liu L, Luo Y, Chen L, Shen T, Xu B, Chen W, Zhou H, Han X, Huang S, Rapamycin inhibits cytoskeleton reorganization and cell motility by suppressing RhoA expression and activity, J. Biol. Chem 285 (49) (2010) 38362–38373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chen L, Xu B, Liu L, Luo Y, Yin J, Zhou H, Chen W, Shen T, Han X, Huang S, Hydrogen peroxide inhibits mTOR signaling by activation of AMPKα leading to apoptosis of neuronal cells, Lab. Invest 90 (5) (2010) 762–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chen L, Liu L, Luo Y, Huang S, MAPK and mTOR pathways are involved in cadmium-induced neuronal apoptosis, J. Neurochem 105 (1) (2008) 251–261. [DOI] [PubMed] [Google Scholar]
- [33].Kanellopoulou C, Muljo SA, Kung AL, Ganesan S, Drapkin R, Jenuwein T, Livingston DM, Rajewsky K, Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing, Gene. Dev 19 (4) (2005) 489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zeng Q, Zhang H, Qin J, Xu Z, Gui L, Liu B, Liu C, Xu C, Liu W, Zhang S, Huang S, Chen L, Rapamycin inhibits BAFF-stimulated cell proliferation and survival by suppressing mTOR-mediated PP2A-Erk1/2 signaling pathway in normal and neoplastic B-lymphoid cells, Cell. Mol. Life Sci 72 (24) (2015) 4867–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tome-Carneiro J, Larrosa M, Gonzalez-Sarrias A, Tomas-Barberan FA, Garcia-Conesa MT, Espin JC, Resveratrol and clinical trials: the crossroad from in vitro studies to human evidence, Curr. Pharm. Des 19 (34) (2013) 6064–6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ni HM, Bockus A, Wozniak AL, Jones K, Weinman S, Yin XM, Ding WX, Dissecting the dynamic turnover of GFP-LC3 in the autolysosome, Autophagy 7 (2) (2011) 188–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ma T, Li J, Xu Y, Yu C, Xu T, Wang H, Liu K, Cao N, Nie BM, Zhu SY, Xu S, Li K, Wei WG, Wu Y, Guan KL, Ding S, Atg5-independent autophagy regulates mitochondrial clearance and is essential for iPSC reprogramming, Nat. Cell Biol 17 (11) (2015) 1379–1387. [DOI] [PubMed] [Google Scholar]
- [38].Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T, LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing, EMBO J. 19 (21) (2000) 5720–5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yin Y, Sun G, Li E, Kiselyov K, Sun D, ER stress and impaired autophagy flux in neuronal degeneration and brain injury, Ageing Res. Rev 34 (2017) 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wu YT, Tan HL, Shui G, Bauvy C, Huang Q, Wenk MR, Ong CN, Codogno P, Shen HM, Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase, J. Biol. Chem 285 (14) (2010) 10850–10861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sharifi MN, Mowers EE, Drake LE, Macleod KF, Measuring autophagy in stressed cells, Methods Mol. Biol 1292 (2015) 129–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lee JW, Nam H, Kim LE, Jeon Y, Min H, Ha S, Lee Y, Kim SY, Lee SJ, Kim EK, Yu SW, TLR4 (toll-like receptor 4) activation suppresses autophagy through inhibition of FOXO3 and impairs phagocytic capacity of microglia, Autophagy 15 (5) (2019) 753–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Klionsky DJ, Elazar Z, Seglen PO, Rubinsztein DC, Does bafilomycin A1 block the fusion of autophagosomes with lysosomes? Autophagy 4 (7) (2008) 849–850. [DOI] [PubMed] [Google Scholar]
- [44].Petrovski G, Gurusamy N, Das DK, Resveratrol in cardiovascular health and disease, Ann. N Y Acad. Sci 1215 (2011) 22–33. [DOI] [PubMed] [Google Scholar]
- [45].Farris P, Krutmann J, Li YH, McDaniel D, Krol Y, Resveratrol: a unique antioxidant offering a multi-mechanistic approach for treating aging skin, J. Drugs Dermatol 12 (12) (2013) 1389–1394. [PubMed] [Google Scholar]
- [46].Vakana E, Platanias LC, AMPK in BCR-ABL expressing leukemias. Regulatory effects and therapeutic implications, Oncotarget 2 (12) (2011) 1322–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kim DU, Kwak B, Kim SW, Phosphodiesterase 4B is an effective therapeutic target in colorectal cancer, Biochem. Biophys. Res. Commun 508 (3) (2019) 825–831. [DOI] [PubMed] [Google Scholar]
- [48].Jiang H, Shang X, Wu H, Gautam SC, Al-Holou S, Li C, Kuo J, Zhang L, Chopp M, Resveratrol downregulates PI3K/Akt/mTOR signaling pathways in human U251 glioma cells, J. Exp. Ther. Oncol 8 (1) (2009) 25–33. [PMC free article] [PubMed] [Google Scholar]
- [49].He X, Wang Y, Zhu J, Orloff M, Eng C, Resveratrol enhances the anti-tumor activity of the mTOR inhibitor rapamycin in multiple breast cancer cell lines mainly by suppressing rapamycin-induced AKT signaling, Cancer Lett. 301 (2) (2011) 168–176. [DOI] [PubMed] [Google Scholar]
- [50].Zhang XJ, Chen S, Huang KX, Le WD, Why should autophagic flux be assessed? Acta Pharmacol. Sin 34 (5) (2013) 595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Mauvezin C, Neufeld TP, Bafilomycin A1 disrupts autophagic flux by inhibiting both V-ATPase-dependent acidification and Ca-P60A/SERCA-dependent autophagosome-lysosome fusion, Autophagy 11 (8) (2015) 1437–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Laragione T, Gulko PS, mTOR regulates the invasive properties of synovial fibroblasts in rheumatoid arthritis, Mol. Med 16 (9–10) (2010) 352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Fernandez D, Perl A, mTOR signaling: a central pathway to pathogenesis in systemic lupus erythematosus? Discov. Med 9 (46) (2010) 173–178. [PMC free article] [PubMed] [Google Scholar]
- [54].Liu M, Zhang J, Pinder BD, Liu Q, Wang D, Yao H, Gao Y, Toker A, Gao J, Peterson A, Qu J, Siminovitch KA, WAVE2 suppresses mTOR activation to maintain T cell homeostasis and prevent autoimmunity, Science 371 (6536) (2021) eaaz4544. [DOI] [PubMed] [Google Scholar]
- [55].Weichhart T, Saemann MD, The multiple facets of mTOR in immunity, Trends Immunol. 30 (5) (2009) 218–226. [DOI] [PubMed] [Google Scholar]
- [56].Wu T, Mohan C, The AKT axis as a therapeutic target in autoimmune diseases, Endocr. Metab. Immune Disord. Drug Targets 9 (2) (2009) 145–150. [DOI] [PubMed] [Google Scholar]
- [57].Ge J, Liu Y, Li Q, Guo X, Gu L, Ma ZG, Zhu YP, Resveratrol induces apoptosis and autophagy in T-cell acute lymphoblastic leukemia cells by inhibiting Akt/ mTOR and activating p38-MAPK, Biomed. Environ. Sci 26 (11) (2013) 902–911. [DOI] [PubMed] [Google Scholar]
- [58].Fan Y, Chiu JF, Liu J, Deng Y, Xu C, Zhang J, Li G, Resveratrol induces autophagy-dependent apoptosis in HL-60 cells, BMC Cancer 18 (1) (2018) 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Guo X, Bai X, Zhang F, Zheng L, Ding W, Yang S, Resveratrol protects against apoptosis induced by interleukin-1beta in nucleus pulposus cells via activating mTOR/caspase-3 and GSK-3beta/caspase-3 pathways, Biosci. Rep 40 (7) (2020). BSR20202019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [60].Ge F, Wang F, Yan X, Li Z, Wang X, Association of BAFF with PI3K/Akt/mTOR signaling in lupus nephritis, Mol. Med. Rep 16 (5) (2017) 5793–5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B, Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy, Cell 122 (6) (2005) 927–939. [DOI] [PubMed] [Google Scholar]
- [62].Kang R, Zeh HJ, Lotze MT, Tang D, The Beclin 1 network regulates autophagy and apoptosis, Cell Death Differ. 18 (4) (2011) 571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zeng Q, Zhou Z, Qin S, Yao Y, Qin J, Zhang H, Zhang R, Xu C, Zhang S, Huang S, Chen L, Rapamycin inhibits B-cell activating factor (BAFF)-stimulated cell proliferation and survival by suppressing Ca2+-CaMKII-dependent PTEN/Akt- Erk1/2 signaling pathway in normal and neoplastic B-lymphoid cells, Cell Calcium 87 (2020), 102171. [DOI] [PMC free article] [PubMed] [Google Scholar]