Abstract
Purpose:
To examine postpartum depressive symptom trajectories from birth to age 5 and their risk factors in a national sample of mothers of preterm and full-term infants.
Methods:
The racially and ethnically diverse sample comprised 11,320 maternal participants (Mage=29; SD=5.9) in the Environmental influences on Child Health Outcomes (ECHO) Program in the United States with data on newborn gestational age at birth (≥22 weeks) and maternal depression symptoms during the first 5 years following childbirth. Growth mixture models determined the number and trajectory of postpartum depression classes among women in the preterm and full-term groups, and we examined predictors of class membership.
Results:
Five trajectories described depressive symptoms for both groups; however, notable differences were observed. One in 5 mothers of preterm infants developed clinically relevant depressive symptoms over time compared with 1 in 10 mothers of full-term infants. Among women who delivered preterm compared with those who delivered full-term, symptoms were more likely to increase over time and become severe when offspring were older.
Conclusions:
Distinct subgroups describe mothers’ depressive symptom trajectories through 5 years following childbirth. Mild to moderate depressive symptoms may onset or persist for many women beyond the initial postpartum period regardless of newborn gestational age at birth. For women with preterm infants, initially mild symptoms may increase to high levels of severity during the preschool and toddler years.
Keywords: Pregnancy, maternal depression, postpartum period, newborn, infant, premature
INTRODUCTION
Preterm birth, defined as birth before 37 completed weeks of gestation is the leading cause of neonatal and childhood mortality worldwide and is a significant public health concern (Hug et al., 2017; Walani, 2020). In the United States (U.S.), one in 10 women experience a preterm birth (Martin et al., 2021). While one in seven mothers experience postpartum depression overall (Wisner et al., 2006), the experience of a preterm birth and challenges associated with the long-term development of infants born prematurely may increase women’s risk (Carson et al., 2015). Despite the clear risk for women, very little is known about the long-term developmental trajectories of maternal depression in the context of preterm birth, particularly in socioeconomically, racially, and ethnically diverse samples.
The course of postpartum depressive symptoms is not uniform. Rather, there appear to be unique subgroups of maternal depressive symptoms, although studies of heterogeneity generally examine symptom trajectories across all mothers without regard to the timing of birth. Such studies typically reveal stable low, moderate, and high depressive symptom trajectories (Santos et al., 2017), with some exceptions (Baron et al., 2017). For example, a study of predominantly White women revealed four profiles of maternal depressive symptoms through 3 years postpartum: 1) low, stable symptoms; 2) low, increasing symptoms; 3) moderate, decreasing symptoms; and 4) high, persistent symptoms (Putnick et al., 2020). Mothers with a history of a mood disorder or gestational diabetes and mothers younger in age were more likely to be characterized by a profile of high, persistent depressive symptoms (Putnick et al., 2020). In a large cohort study of middle-income women in Brazil, analyses revealed four profiles comparable to the aforementioned study (with the addition of a fifth, “moderate-low” symptom profile): prenatal depression, prenatal alcohol/tobacco use, younger children in the household, and older maternal age were associated with severe, persistent symptoms (Matijasevich et al., 2015).
Both preterm birth and maternal postpartum depression represent common and significant health concerns, yet their association remains largely unexplored, particularly over time. Large-scale longitudinal studies are crucial to describe longer-term postpartum adjustment and the optimal timing of screening, prevention, and intervention among women at risk. Within a large, diverse sample of women assessed repeatedly across the first 5 years following delivery, the current longitudinal study examined the onset, course, and risk factors for depressive symptoms as a function of birth timing (preterm versus full-term) and across the sample overall.
MATERIALS AND METHODS
Participants
The Environmental influences on Child Health Outcomes (ECHO) Program was funded by the National Institute of Health (NIH) in 2016 to investigate the influence of early life exposures on child health and development. ECHO is a consortium of 69 extant observational and intervention studies from academic and related institutions across the United States that comprise a singular ECHO-wide cohort. Studies within the ECHO Program include children and their mothers; many studies began recruitment during the prenatal period and all studies are following children long-term (Gillman & Blaisdell, 2018). For more information, please see https://www.nih.gov/echo/about-echo-program. Of the 69 cohorts in the ECHO Program, 35 were included in this study. Cohort eligibility criteria for these analyses included possessing information about gestational age at birth to derive prematurity status and at least one measure of postpartum depressive symptoms during the 5 years following childbirth (note that of the analytic sample in the current study, 55% provided data on depression at more than one time point). Mothers were at least 18 years of age at delivery, and the pregnancy was a singleton, live birth with a gestational age of ≥22 weeks (see eTable 1 in the Online Resource for a description of each included cohort and eTable 2 for comparisons of the ECHO-wide cohort and the analytic sample).
Depressive Symptom Measures
Postpartum depressive symptoms were captured from self-report measures that included one of the following: (1) the Patient-Reported Outcomes Measurement Information System (PROMIS) v1.0, (2) the Edinburgh Postnatal Depression Scale, (3) the Achenbach System of Empirically Based Assessment Adult Self-Report (ASR) Depression Problems Syndrome Scale, (4) the Brief Symptom Inventory, (5) the Center for Epidemiological Studies Depression Scale, (6) the Patient Health Questionnaire, (7) the Beck Depression Inventory, and (8) the Kessler 6 Mental Health Scale. All assessments reflected current symptoms (i.e., no retrospective reports) and were harmonized to the PROMIS T-score metric using a previously constructed and validated crosswalk table (Blackwell et al., 2018). The average PROMIS T-score (normed for the general U.S. population) is 50 (SD=10). The following T-score cut-offs are used to indicate depressive symptom severity: 55=mild, 60=moderate, 65=moderately severe, and ≥70=severe (Kroenke et al., 2020). To harmonize the data across cohorts with varying measurement schedules, 12-month periods of depressive symptom assessment were created across the first 5 postpartum years (0–12 months postpartum, 13–24 months postpartum, 25–36 months postpartum, 37–48 months postpartum, and 49–60 months postpartum).
Risk Factors
Risk factors were considered for inclusion based on previous empirical studies of maternal postpartum depression and on availability (i.e., data had been harmonized across ECHO cohorts). Variables included maternal age at delivery, maternal education (less than high school, a high school degree or General Educational Development degree or equivalent, some college, master’s degree, or above), and maternal race (White, Black, Asian, Native Hawaiian or other Pacific Islander, American Indian or Alaska Native, Other race, or more than one race). The following variables were dichotomized: maternal ethnicity (Hispanic versus not Hispanic); marital status (single or married/living with partner during the prenatal period); health insurance (no insurance versus any insurance); alcohol, tobacco, or marijuana use during pregnancy (any use versus no use); preeclampsia (present versus absent during pregnancy); and prenatal depression diagnosis.
Statistical Analysis
To explore trajectories of postpartum depression, a growth mixture model (GMM) was conducted within the preterm and the full-term groups separately. GMM analyses identify multiple unobserved subpopulations and describe change over time within each subpopulation (Muthén & Muthén, 2000; Ram & Grimm, 2009). For this study, we estimated an intercept and slope measured by maternal depression (harmonized to the PROMIS T-score) in 12-month bins across 5 years postpartum (i.e., 0–12 months postpartum, 13–24 months postpartum, etc.). Analysis began with class enumeration whereby models were fit with an increasing number of classes. The number of trajectories within each structure was evaluated using standard fit indices, including Bayesian Information Criteria (BIC) and the Lo-Mendell-Rubin adjusted likelihood ratio test (LMR), as well as substantive interpretation (Masyn, 2013; Nylund et al., 2007). The model with the smallest BIC value and substantively interpretable classes was selected. Once the class solution was chosen for both the preterm and the full-term groups, the form and function of trajectories were compared.
The impact of risk factors was explored utilizing the manual three-step approach, which allows for the final model to account for measurement error. This approach begins with identifying the latent trajectory classes and saving the latent class probabilities. In a new model, logits are captured and used alongside the most likely latent class variable to model the impact of covariates on the latent trajectory classes (Nylund-Gibson et al., 2014). Current research standards indicate data can be treated as missing at random when the missing data are unrelated to the outcome of interest. Missing data on the depression trajectory indicators were accounted for using full information maximum likelihood estimation. Missing data on the risk factors were addressed with multiple imputation (Asparouhov, 2010) using Mplus (Muthén & Muthén, 1998) version 8.1.6. Standard errors were adjusted for clustering at the cohort level via sandwich estimators.
RESULTS
Sample Characteristics
The full analytic sample (N=11,320) included 10,064 mothers of full-term infants (89%) and 1,256 mothers of preterm infants (11%; eFigure 1 in the Online Resource). Among women who delivered preterm, the gestational ages at delivery were as follows: 22 weeks to less than 24 weeks (N=26, 2.1%), 24 weeks to less than 28 weeks (N=206, 16.4%), 28 weeks to less than 32 weeks (N=216, 17.2%), and 32 weeks to less than 37 weeks (N=808, 64.3%). Sample demographics and information on risk factors can be found in Table 1. Compared with women who delivered full-term infants, a higher proportion of women who delivered preterm infants had preeclampsia and gave birth to offspring who were subsequently admitted to the neonatal intensive care unit following birth (see Table 1).
Table 1.
Sample demographics and risk factors
| Study population | Preterm sample | Full-term sample | Chi-square p-value | |
|---|---|---|---|---|
|
| ||||
| Number of pregnancies | 11,320 | 1,256 | 10,064 | |
| Maternal demographics | ||||
| Age at delivery, N (%) with data | 11,320 (100%) | 1,256 (100%) | 10,064 (100%) | <0.05 |
| Mean (SD) | 29 (18–52, 5.9) | 29 (18–52, 6.3) | 29 (18–51, 5.8) | |
| Race, N (%) with data | 10,561 (93%) | 1,166 (93%) | 9,395 (93%) | <0.001 |
| White, N (%) | 5,719 (54%) | 537 (46%) | 5,182 (55%) | |
| Black or African American, N (%) | 2,719 (26%) | 343 (29%) | 2,376 (25%) | |
| Asian, N (%) | 478 (5%) | 60 (5%) | 418 (4%) | |
| Native Hawaiian or other Pacific Islander, N (%) | 68 (<1%) | 9 (<1%) | 59 (<1%) | |
| American Indian or Alaska Native, N (%) | 215 (2%) | 25 (2%) | 190 (2%) | |
| More than one race, N (%) | 729 (7%) | 108 (9%) | 621 (7%) | |
| Other race, N (%) | 633 (6%) | 84 (7%) | 549 (6%) | |
| Ethnicity, N (%) with data | 11,206 (99%) | 1,228 (98%) | 9,978 (99%) | <0.05 |
| Hispanic, N (%) | 2,968 (26%) | 336 (27%) | 2,632 (26%) | |
| Non-Hispanic, N (%) | 8,238 (74%) | 892 (73%) | 7,346 (74%) | |
| Socioeconomic status | ||||
| Prenatal education, N (%) with data | 9,172 (81%) | 1,027 (82%) | 8,145 (81%) | <0.001 |
| < High school, N (%) | 917 (10%) | 124 (12%) | 793 (10%) | |
| High school degree, GED or equivalent, N (%) | 1,930 (21%) | 244 (24%) | 1,686 (21%) | |
| Some college (no degree); associates degree; trade school, N (%) | 2,010 (22%) | 295 (29%) | 1,715 (21%) | |
| Bachelor’s degree, N (%) | 2,282 (25%) | 203 (20%) | 2,079 (26%) | |
| Master’s degree, N (%) | ||||
| Professional or doctorate degree, N (%) | 2,033 (22%) | 161 (16%) | 1,872 (23%) | |
| Prenatal employment, N (%) with data | 4,572 (40%) | 389 (31%) | 4,183 (42%) | <0.001 |
| Employed, N (%) | 3,603 (79%) | 298 (77%) | 3,305 (79%) | |
| Prenatal marital status, N (%) with data | 9,094 (80%) | 995 (79%) | 8,099 (80%) | Not significant |
| Married or living with a partner, N (%) | 6,779 (75%) | 708 (71%) | 6,071 (75%) | |
| Insurance type, N (%) with data | 5,203 (46%) | 464 (37%) | 4,739 (47%) | <0.05 |
| Any private, market, work insurance, N (%) | 2,765 (53%) | 223 (48%) | 2,542 (54%) | |
| Medicare/Medicaid, N (%) | 2,452 (47%) | 241 (52%) | 2,211 (47%) | |
| Other, N (%) | 1,176 (23%) | 97 (21%) | 1,079 (23%) | |
| No Insurance, N (%) | 96 (2%) | 14 (3%) | 82 (2%) | |
| Substance use during pregnancy | ||||
| Alcohol, N (%) with data | 9,696 (86%) | 1,113 (89%) | 8,583 (85%) | <0.001 |
| Yes, N (%) | 1,368 (14%) | 101 (9%) | 1,267 (15%) | |
| Tobacco, N (%) with data | 10,329 (91%) | 1,177 (94%) | 9,152 (91%) | <0.001 |
| Yes, N (%) | 865 (8%) | 124 (11%) | 741 (8%) | |
| Marijuana, N (%) with data | 8,073 (71%) | 996 (79%) | 7,077 (70%) | <0.001 |
| Yes, N (%) | 345 (4%) | 68 (7%) | 277 (4%) | |
| Complications during pregnancy | ||||
| Gestational diabetes, N (%) with data | 8,109 (72%) | 848 (68%) | 7,261 (72%) | <0.001 |
| Yes, N (%) | 689 (8%) | 93 (11%) | 596 (8%) | |
| Preeclampsia, N (%) with data | 8,451 (75%) | 988 (79%) | 7,463 (74%) | <0.001 |
| Yes, N (%) | 462 (5%) | 206 (21%) | 256 (3%) | |
| Neonatal Intensive Care Unit admission, N (%) with data | 2,023 (18%) | 516 (41%) | 1,507 (15%) | <0.001 |
| Yes, N (%) | 565 (28%) | 441 (85%) | 124 (8%) | |
| Prenatal depression, N (%) with data | 4,531 (40%) | 663 (53%) | 3,868 (38%) | <0.05 |
| Yes, N (%) | 565 (12%) | 77 (12%) | 488 (13%) | |
| Prenatal anxiety, N (%) with data | 2,657 (23%) | 510 (41%) | 2,147 (21%) | <0.05 |
| Yes, N (%) | 201 (8%) | 34 (7%) | 167 (8%) | |
| Parity, N (%) with data | 5,178 (46%) | 576 (46%) | 4,602 (46%) | Not significant |
| 0, N (%) | 2,307 (45%) | 266 (46%) | 2,041 (44%) | |
| 1, N (%) | 1,663 (32%) | 170 (30%) | 1,493 (32%) | |
| 2, N (%) | 784 (15%) | 85 (15%) | 699 (15%) | |
| >2, N (%) | 424 (8%) | 55 (10%) | 369 (8%) | |
| Calendar year of child’s birth, N (%) with data | 11,320 (100%) | 1,256(100%) | 10,064 (100%) | <0.001 |
| 1980–2000, N (%) | 19 (<1%) | <5 (<1%) | <19 (1%) | |
| 2001–2010, N (%) | 2,304 (20%) | 182 (14%) | 2,122 (21%) | |
| 2011–2015, N (%) | 2,959 (26%) | 337 (27%) | 2,622 (26%) | |
| 2016–2020, N (%) | 3,733 (33%) | 338 (31%) | 3,345 (33%) | |
Postpartum Depressive Symptoms Trajectories
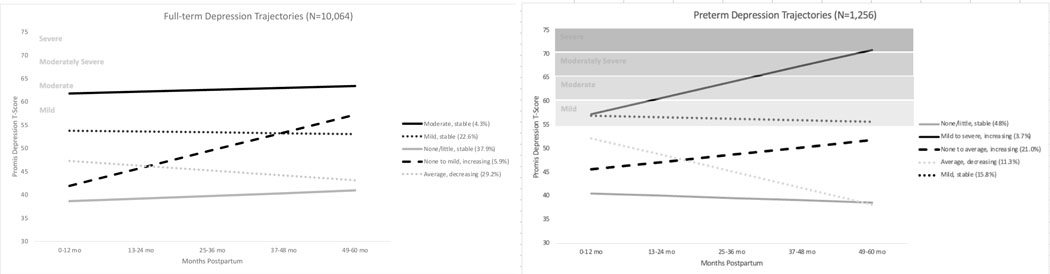
A series of GMMs ranging from 1 to 6 classes were estimated within the preterm and full-term groups separately. In both groups, the BIC, sample size-adjusted BIC, and LMR supported 5-class solutions; however, examination of the solutions indicated unique group differences that necessitated different models for preterm versus full-term birth (Figure 1). Fit statistics for class enumeration are shown in Table 2.
Fig. 1.
Depression class trajectories among mothers of full-term and preterm infants
Table 2.
Class enumeration fit statistics
| Log likelihood | # of free parameters | BIC | ssaBIC | LMR | LMR p-value | Smallest class | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Preterm sample (N=1,256) | |||||||
|
| |||||||
| 1 class | −8911.917 | 7 | 17837.833 | 17851.548 | |||
| 2 class | −8614.417 | 10 | 17248.835 | 17268.427 | 568.44 | 0 | 28% |
| 3 class | −8565.943 | 13 | 17157.887 | 17183.357 | 92.62 | 0.012 | 12.70% |
| 4 class | −8552.489 | 16 | 17219.150 | 17168.326 | 25.7 | 0.633 | 3.80% |
| 5 class | −8531.556 | 19 | 17101.112 | 17138.338 | 38.981 | 0.38 | 3.7% |
| 6 class | −8514.568 | 22 | 17186.121 | 17116.239 | 32.46 | 0.0356 | 1% |
|
| |||||||
| Full-term sample (N=10,064) | |||||||
|
| |||||||
| 1 class | −68625.495 | 7 | 137315.508 | 137293.263 | |||
| 2 class | −66846.452 | 10 | 133785.071 | 133753.293 | 3433.896 | 0 | 32% |
| 3 class | −66563.773 | 13 | 133247.363 | 133206.05 | 545.626 | 0 | 7.00% |
| 4 class | −66476.365 | 16 | 133100.198 | 133049.352 | 168.713 | 0 | 4.60% |
| 5 class | −66422.324 | 19 | 133019.766 | 132959.387 | 104.31 | 0.01 | 4.3% |
| 6 class | −66385.385 | 22 | 132903.538 | 132903.626 | 71.299 | 0.0145 | 1% |
Bold indicates the chosen class solution for each group.
BIC, Bayesian Information Criteria; LMR, Lo-Mendell-Rubin test, ssaBIC, sample size-adjusted Bayesian Information Criteria.
For the preterm sample, the largest class (N=603, 48%) was characterized by little to no symptoms that remained stable across the study period (None/Little, Stable). Another class was characterized by a somewhat higher intercept and negative slope; symptoms began at an average level (relative to other classes) and declined over time (Average, Decreasing, N=143, 11.3%). Approximately one-fifth of the sample was characterized by a lower intercept but a statistically significant positive slope; symptoms began below average and increased to average levels (None to Average, Increasing, N=264, 21%). The Mild, Stable class was characterized by a higher intercept and a small, non-significant slope indicating persistently mild symptoms over time (N=199, 15.8%). Finally, a small but clinically relevant class was characterized by a higher intercept and a statistically significant positive slope. Within this class, symptoms began mildly and reached severe levels by 5 years postpartum (Mild to Severe, Increasing, N=48, 3.7%). For descriptive purposes, we display the preterm classes with the percentage of women in each class by gestational age at delivery (eFigure 2 in the Online Resource).
For the full-term group, the largest class was similarly characterized by little to no depression symptoms that were largely stable across the study period (N=3,819, 37.9%; None/Little, Stable). A second trajectory was characterized by a lower intercept and statistically significant positive slope; symptoms began below average and increased to mild levels as children approached age 5 (None to Mild, Increasing; N=597, 5.9%). A third trajectory was characterized by a somewhat higher intercept and a significant negative slope; symptoms began in the average range and decreased over time to below average levels (Average, Decreasing; N=2,939, 29.2%). A class labeled Mild, Stable (N=2,271, 22.6%) was characterized by a higher intercept and a non-significant slope. Note, the label of Mild, Stable was applied to this full-term class given its similarity to the class of the same name in the preterm group; however, average symptom levels were slightly below the cut-off for mild symptom scores (T=55). Finally, a Moderate, Stable class (N=438, 4.3%) was characterized by the highest intercept and a non-significant but positive slope; symptoms remained in the moderate range across the 5 years following the birth of the child.
Risk Factors for Depressive Symptom Trajectories
For women who delivered prematurely, prenatal depression was a significant predictor when comparing the Mild, Stable class to the None/Little, Stable class (OR=4.59, 95% CI 1.53, 13.76; see Table 3). The greatest prevalence of women who displayed prenatal depression symptoms was observed in the Mild to Severe, Increasing class (25.5% of women in this class reported prenatal depression) and the Mild, Stable class (26.1% of women in this class reported prenatal depression). In comparison, 7.6% of women in the None/Little, Stable class reported prenatal depression. No other comparisons reached statistical significance.
Table 3.
Predictors of class membership
| Mild to Severe, Increasing (3.7%, n=48) | Mild, Stable (15.8%, n=199) | Average, Decreasing (11.3%, n=143) | None to Average, Increasing (21%, n=264) | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Variable | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI |
|
| ||||||||
| Preterm sample (N=1,256) | ||||||||
|
| ||||||||
| Education, less than high school | 2.98 | 0.44, 20.49 | 1.52 | 0.23, 10.19 | 0.94 | 0.17, 5.26 | 1.87 | 0.54, 6.51 |
| Education, some college or more | 1.44 | 0.30, 6.87 | 2.46 | 0.36, 16.65 | 0.59 | 0.27, 1.29 | 1.08 | 0.43, 2.69 |
| Marital status, not married or living with a partner | 1.32 | 0.40, 4.35 | 5.47 | 0.48, 62.17 | 0.91 | 0.43, 1.96 | 1.96 | 0.83, 4.62 |
| Prenatal substance use, any | 1.06 | 0.23, 4.96 | 1.02 | 0.28, 3.76 | 0.97 | 0.28, 3.30 | 1.59 | 0.75, 3.41 |
| White race vs. other | 0.32 | 0.07, 1.48 | 1.89 | 0.58, 6.11 | 0.60 | 0.25, 1.46 | 1.22 | 0.58, 2.57 |
| Preeclampsia | 2.19 | 0.69, 6.92 | 0.59 | 0.18, 1.94 | 0.86 | 0.16, 4.56 | 0.77 | 0.25, 2.42 |
| Prenatal depression | 3.89 | 0.37, 41.39 | 4.59 | 1.53, 13.76 | 3.39 | 0.46, 25.05 | 1.10 | 0.13, 9.34 |
|
| ||||||||
| Full-term sample (N=10,064) | ||||||||
|
| ||||||||
| Average, Decreasing (29.2%, n=2,939) | Moderate, Stable (4.3%, n=438) | None to Mild, Increasing (5.9%, n=597) | Mild, Stable (22.6%, n=2,271) | |||||
|
| ||||||||
| White race | 1.46 | 0.96, 2.20 | 0.63 | 0.31, 1.28 | 0.37 | 0.11, 1.22 | 1.47 | 0.96, 2.24 |
| Other race | 1.17 | 0.73, 1.88 | 0.75 | 0.40, 1.40 | 0.44 | 0.09, 2.24 | 1.57 | 0.99, 2.48 |
| Hispanic | 0.32 | 0.13, 0.78 | 0.57 | 0.19, 1.71 | 0.68 | 0.19, 2.50 | 2.26 | 0.74, 6.89 |
| Education, less than high school | 0.92 | 0.61, 1.40 | 2.59 | 1.29, 5.21 | 2.06 | 0.98, 4.30 | 0.86 | 0.41, 1.81 |
| Education, some college or more) | 0.74 | 0.49, 1.10 | 0.81 | 0.41, 1.59 | 0.76 | 0.30, 1.96 | 2.33 | 1.52, 3.55 |
| Marital status, not married or living with a partner | 0.77 | 0.50, 1.18 | 0.89 | 0.38, 2.09 | 0.53 | 0.22, 1.28 | 3.02 | 1.68, 5.43 |
| Health insurance, any | 2.49 | 1.15, 5.36 | 2.32 | 1.00, 5.41 | 3.11 | 0.57, 16.82 | 0.90 | 0.40, 2.02 |
| Prenatal alcohol use | 1.43 | 0.86, 2.37 | 1.17 | 0.48, 2.84 | 0.83 | 0.19, 3.69 | 3.06 | 1.06, 8.80 |
| Prenatal tobacco use | 1.32 | 0.69, 2.51 | 1.90 | 1.11, 3.24 | 2.12 | 0.75, 5.98 | 1.19 | 0.63, 2.26 |
| Prenatal marijuana use | 1.35 | 0.65, 2.78 | 2.99 | 1.02, 8.76 | 2.33 | 0.38, 14.26 | 1.04 | 0.29, 3.78 |
| Preeclampsia | 1.06 | 0.54, 2.09 | 0.75 | 0.26, 2.20 | 0.80 | 0.08, 7.58 | 1.60 | 0.97, 2.61 |
| Prenatal depression | 1.40 | 0.67, 2.90 | 4.41 | 1.71, 11.36 | 2.19 | 0.38, 12.61 | 2.10 | 1.16, 3.81 |
The None/Little, Stable class served as the referent for both the preterm and full-term samples. Black race served as the reference group for race in the full-term sample. Due to the smaller sample size, any race other than White served as the reference group for the preterm sample. High school diploma served as the reference for education for both the preterm and full-term samples. Due to limited endorsement in the preterm sample, prenatal alcohol, tobacco, and marijuana use was collapsed into a binary response (yes/no), reflecting any substance use. CI, confidence interval; OR, odds ratio. Bold indicates significance.
In the full-term group, prenatal depression increased the odds of being in the Moderate, Stable class (OR=4.41, 95% CI 1.71, 11.36) and the Mild, Stable (OR=2.10, 95% CI 1.16, 3.81) class compared with the None/Little, Stable reference class (Table 3). Prenatal tobacco (OR=1.90, 95% CI 1.11, 3.24) and marijuana use (OR=2.99, 95% CI 1.02, 8.76) increased the odds for being in the Moderate, Stable class, and prenatal alcohol use (OR=3.06, 95% CI 1.06, 8.80) increased the odds for being in the Mild, Stable class compared with the None/Little, Stable class. Women with health insurance were more likely to be in the Moderate, Stable class (OR=2.32, 95% 1.00, 5.41) or the Average, Decreasing class (OR = 2.49, 95% 1.15, 5.36) relative to the None/Little, Stable class. Relative to women who earned a high school diploma, those with less than a high school education were more likely to be in the class of Moderate, Stable symptoms (OR=2.59, 95% CI 1.29, 5.21); however, those with more years of education (some college or more) showed increased odds of being in the Mild, Stable group (OR=2.33, 95% CI 1.52, 3.55). Finally, women who were not married/partnered were more likely to be in the Mild, Stable symptoms class compared with the None/Little, Stable class (OR = 3.02, 95% CI 1.68, 5.43).
Although the primary objective of the present study was to examine the long-term course of postpartum depressive symptoms among women who delivered preterm infants compared with those who delivered full-term infants, we also examined trajectories across the sample as a whole to enhance the evidence base regarding women’s postpartum adjustment. Supplemental growth mixture models in the overall sample revealed a 5-class solution (see eFigure 3) that was nearly identical to the 5 classes that were derived among only those women who delivered full-term infants, with similar class proportions across samples. Preterm birth did not emerge as a significant predictor of class membership in the overall model (OR range: 1.02 to 1.55); however, all other predictors of class membership were consistent across the overall sample and the sample with only full-term births (data not shown).
DISCUSSION AND CONCLUSIONS
The postpartum period is a vulnerable time for the development of maternal depressive symptoms, which have significant implications for both maternal and child health. Women who deliver prematurely may be at elevated risk, but limited research has examined their postpartum adjustment over time and whether it differs from the experience of mothers who deliver full-term infants. Leveraging a large, national consortium sample of 11,320 women, we document the course of postpartum depressive symptoms and their risk factors among mothers of preterm versus full-term infants.
Among mothers of preterm infants, a substantial proportion evidenced little or no depressive symptoms over time, suggestive of generally positive adjustment in the context of a significant life stressor. However, we observed a small (but clinically meaningful) group of mothers of preterm infants with initially mild depressive symptoms that increased to a severe level by the time offspring were 5 years of age. Given that clinical guidelines do not typically recommend a follow-up of this duration, these results are quite notable: It is possible that the development of such severe maternal depressive symptoms may otherwise go undetected by health care providers, particularly when they are of mild severity in the initial postpartum period. An additional group of women exhibited mild depressive symptoms following preterm birth that remained stable into early childhood. Across these two classes (Mild to Severe, Increasing and Mild, Stable), the prevalence and persistence of symptoms (approximately 20% of the sample) is important though difficult to contextualize within extant empirical evidence. In a meta-analysis of preterm birth and postpartum depression, only 19% of studies collected repeated measures of maternal depressive symptoms, and none extended beyond the first postpartum year (de Paula Eduardo et al., 2019). Our novel findings highlight the utility of extending the monitoring of maternal well-being throughout the first 5 years following childbirth.
Akin to mothers of infants born preterm, the largest proportion of women of full-term infants evidenced none or very limited depressive symptoms that appeared stable over time. Such a “low-risk” class has consistently emerged in prior growth curve mixture modeling studies (Baron et al., 2017) and is typically the largest class (Santos et al., 2017). We also observed stable, mild, and moderate symptom trajectories among mothers of full-term offspring, suggesting that unremitting, chronic symptom trajectories are more common than increasing or decreasing patterns. Notably, studies of heterogeneity of maternal depressive symptoms typically conclude their assessments by 2 years postpartum and are composed of samples ranging from approximately 120 to 600 women (Baron et al., 2017). The larger sample size and long-term follow-up in the present study is an important advance on extant research and suggests that some of the symptom patterns that emerge earlier in the postpartum period persist well into early childhood.
Interesting differences emerged in the patterns of depressive symptoms between women with full-term versus preterm infants. First, three of the five trajectories observed among full-term pregnancies represented stable symptom patterns (ie, None/Little, Mild, Moderate), whereas only two stable trajectories emerged among mothers following premature births. The unpredictability associated with prematurity-related health and developmental concerns may lead to more variable symptom patterns. Second, a severe depressive symptom class emerged only among women with preterm infants; overall, more women in the preterm group compared with the full-term group belonged to classes with at least mild (or more severe) depressive symptoms when children were 5 years of age (19.5% versus 10.2%). Finally, the distribution of increasing and decreasing symptom courses was substantially different across groups: more women with full-term infants were classified in a decreasing trajectory rather than an increasing trajectory (29.2% versus 5.9%), whereas more women with preterm infants were classified in an increasing, rather than a decreasing, trajectory (24.7% versus 11.3%). Although the severity of maternal depressive symptoms typically decreases over time in general population studies (Vliegen et al., 2014), our results indicate that this general trend may be less common among women with preterm infants.
Few sociodemographic characteristics were associated with class membership among women who delivered prematurely. Only prenatal depression increased the risk for Mild, Stable depressive symptoms relative to None/Little, Stable symptoms. It may be the case that depressive symptoms following a preterm birth may be more closely tied to the birth experience (eg, trauma, ‘loss’ of a full-term pregnancy), neonatal care challenges, and ongoing concerns about the infant’s health and development rather than sociodemographic risk factors. In contrast, women who delivered full-term infants were at greater risk for exhibiting trajectories of more severe and persistent symptoms if they reported prenatal substance use, were single/unmarried, did not have health insurance, or experienced prenatal depression. Interestingly, a recent systematic review found that sociodemographic factors did not consistently differentiate among depression trajectory groups, an effect at least partially attributed to the studies’ small sample sizes (Baron et al., 2017). Leveraging a much larger sample than any prior research, our study results may help identify women with full-term infants at higher risk for poor postpartum psychological adjustment.
In recognition of their divergent birth and postpartum experiences, the present study was purposeful in its modeling approach: we compared the symptom courses between women who delivered full-term infants versus those who delivered preterm infants. However, analyses across the total sample of women were informative as they revealed depression trajectories that were nearly identical to those derived among only those mothers of full-term infants. Such results may be expected given the tendency for a much larger sample (women who delivered full-term) to mask the nuances of a smaller sample (women who delivered preterm), but these findings also suggest that analyses that do not carefully consider birth timing may overlook important differences in women’s postpartum adjustment. For example, the overall sample model did not reveal a severe depressive symptom class that was unique to women of preterm infants. Moreover, the stark differences in the proportion of women with increasing and decreasing symptom courses by birth timing were only evident when separate group analyses were conducted. These disparate findings highlight the clinical importance of both population-wide and targeted subgroup analyses.
The current study has several limitations. First, missing and non-harmonizable data across the cohorts was a barrier to including certain important risk factors (e.g., household income, child neurodevelopmental disability diagnosis, birth complications). Moreover, some predictors of class membership were less frequently endorsed, particularly within the smallest classes of women with infants born prematurely. By definition, it is the case that rare exposures generally yield wide CIs, suggesting less precise estimates (Brockhaus, Grouven, & Bender, 2016); replication of these findings is needed to yield more robust support. Second, differences in the timing of maternal depression assessments across the 35 cohorts necessitated the use of 12-month time bins. Future research using shorter timeframes may provide a more nuanced accounting of trajectory shape. Third, while post hoc adjustments accounted for the nesting of participants within cohorts, such an approach may not fully account for heterogeneity in design across studies. All data in the present study were collected from women in relation to a single pregnancy/live birth and data on women’s subsequent reproductive histories or births were not available. It is thus possible that depressive symptoms measured during the 5 postpartum years following the target pregnancy/live birth were influenced by subsequent pregnancies and birth outcomes.
The present study can inform clinical practices. The American Academy of Pediatrics recommends perinatal depression screenings through the 6-month well-child visit (Earls & The Committee on Psychosocial Aspects of Child and Family Health, 2010). However, our findings showed longer-term risk for depressive symptoms, which supports the extension of depression screenings beyond the 6-month well-child visit, particularly among mothers of preterm infants. Additionally, mothers who delivered prematurely face challenges to their postpartum well-being related to the risk of short- and long-term comorbidities in their child; however, extant clinical policies may be more focused on offspring than parental well-being (Winter et al., 2018). Holistic interventions supporting families with young children, particularly those with special needs, may help prevent the onset of severe parental depression.
In a large, regionally, racially, and ethnically diverse multi-cohort study, we examined the 5-year course of maternal depressive symptoms. Severe depressive symptoms emerged when offspring were older only among women who delivered prematurely. Regardless of the timing of delivery, prenatal depression was associated with persistent, more severe symptoms following childbirth, and in women of full-term infants, prenatal depression operated alongside sociodemographic factors to increase risk. Our results highlight the necessity of maternal depression screenings and health policies to deliver supportive interventions during and beyond the initial postpartum period, particularly for mothers of preterm infants and those with a history of depression in pregnancy. Given the global burden of depression and the critical influence of maternal mental health on children’s development, the implications of such efforts could be far reaching.
Supplementary Material
STATEMENTS AND DECLARATIONS
Sources of Funding and Support
Research reported in this publication was supported by the Environmental influences on Child Health Outcomes (ECHO) program, Office of The Director, National Institutes of Health, under award numbers U2COD023375 (Coordinating Center), U24OD023382 (Data Analysis Center), U24OD023319 (PRO Core), and 4UH3OD023271 and 4UH3OD023305 (NB), UH3OD023286 (SF), UH3OD023244 (AEH), UH3OD023320 (AS), UH3OD023251 (AA, JM, JFC), UH3OD023279 (AJE), UH3OD023288 (CTM), UH3OD023347 (BL), 5UH3OD023282, UH3OD023348 (HS), UH3 OD-023349 (CB), UH3OD023249. The first author (DR) is also supported by K23MH113709 from the National Institute of Mental Health.
Role of the Funder
The funder had no role in the study design, data collection, and analysis. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Additional Acknowledgments
The authors wish to thank our ECHO colleagues; the medical, nursing and program staff; and the children and families participating in the ECHO cohorts. We also acknowledge the contribution of the following ECHO program collaborators: ECHO Components—Coordinating Center: Duke Clinical Research Institute, Durham, North Carolina: Smith PB, Newby KL; Data Analysis Center: Johns Hopkins University Bloomberg School of Public Health, Baltimore, Maryland: Jacobson LP; Research Triangle Institute, Durham, North Carolina: Parker CB; Person-Reported Outcomes Core: Northwestern University, Evanston, Illinois: Gershon R, Cella D; Children’s Health and Exposure Analysis Resource: Icahn School of Medicine at Mount Sinai, New York City, New York: Teitelbaum S; Wright RO; Wadsworth Center, Albany, New York: Aldous, KM, RTI International, Research Triangle Park, North Carolina: Fennell T; University of Minnesota, Minneapolis, Minnesota: Hecht SS, Peterson L; Westat, Inc., Rockville, Maryland: O’Brien, B; IDeA States Pediatric Trials Network: University of Arkansas for Medical Sciences, Little Rock: Lee JY, Snowden J; ECHO Awardees and Cohorts: Albert Einstein College of Medicine, Bronx, New York: Aschner, JL; Brigham & Women’s Hospital, Boston, Massachusetts: Litonjua, AA, Weiss, ST; Dartmouth College, Hanover, New Hampshire: Karagas, MR; Drexel University, Philadelphia, Pennsylvania: Newschaffer, CJ; Emory University, Atlanta, Georgia: Dunlop, AL, Brennan, PA, Corwin, EJ; Harvard Pilgrim Health Care, Boston, Massachusetts: Oken, E, Kleinman, KP; Icahn School of Medicine at Mount Sinai, Boston, Massachusetts: Wright, RJ, Wright, RO; Kaiser Permanente, Oakland, California: Ferrara, A, Croen, LA; Memorial Hospital of Rhode Island, Pawtucket, Rhode Island: Deoni, S, D’Sa, VA; New York State Psychiatric Institute at Columbia University, New York: Duarte, CS, Canino, GJ, Monk, CE, Posner, JE; New York University School of Medicine, New York, New York: Trasande, L; Northeastern University, Boston, Massachusetts: Alshawabkeh, AN; University of Colorado Anschutz Medical Campus, Aurora, Colorado: Dabelea, D; University of Rochester, New York: O’Connor, TG, Miller RK, Wadhwa, PD; University of Pittsburgh, Magee-Women’s Hospital, Pennsylvania: Simhan, HN; University of Utah, Salt Lake City: Stanford, JB, Clark EB, Porucznik, C; University of Washington, Seattle: Karr, C, Sathyanarayana, S; University of Tennessee Health Sciences Center: Mason, A; University of Wisconsin, Madison: Gern, J; Washington University, St. Louis: Bacharier, L.
Footnotes
Competing Interests
The authors of this manuscript have no conflicts of interest to disclose.
Ethics approval
Each cohort obtained approval to conduct the study from the institutional review board at the home institution in which the study was conducted.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
REFERENCES
- Asparouhov T, Muthén B (2010) Multiple imputation with Mplus. Mplus Web Notes. http://www.statmodel.com/download/Imputations7.pdf. Accessed August 1, 2021. [Google Scholar]
- Baron E, Bass J, Murray SM, Schneider M, Lund C (2017) A systematic review of growth curve mixture modelling literature investigating trajectories of perinatal depressive symptoms and associated risk factors. J Affect Disord 223:194–208. 10.1016/j.jad.2017.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell CK, Wakschlag LS, Gershon RC, Cella D; ECHO PRO Core (2018) Measurement framework for the Environmental influences on Child Health Outcomes research program. Curr Opin Pediatr 30(2):276–284. 10.1097/MOP.0000000000000606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhaus AC, Grouven U, & Bender R (2016). Performance of the Peto odds ratio compared to the usual odds ratio estimator in the case of rare events. Biomedical Journal, 58, 1428–1444. [DOI] [PubMed] [Google Scholar]
- Carson C, Redshaw M, Gray R, Quigley MA (2015) Risk of psychological distress in parents of preterm children in the first year: Evidence from the UK Millennium Cohort Study. BMJ Open 5(12):e007942. 10.1136/bmjopen-2015-007942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paula Eduardo JAF, de Rezende MG, Menezes PR, Del-Ben CM (2019) Preterm birth as a risk factor for postpartum depression: A systematic review and meta-analysis. J Affect Disord 259:392–403. 10.1016/j.jad.2019.08.069 [DOI] [PubMed] [Google Scholar]
- Earls MF, Committee on Psychosocial Aspects of Child and Family Health (2010) Incorporating recognition and management of perinatal and postpartum depression into pediatric practice. Pediatrics 126:1032–1039. 10.1542/peds.2010-2348 [DOI] [PubMed] [Google Scholar]
- Gillman MW, Blaisdell CJ (2018) Environmental influences on Child Health Outcomes, a research program of the National Institutes of Health. Curr Opin Pediatr 30(2):260–262. 10.1097/MOP.0000000000000600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug L, Sharrow D, You D (2017) Levels & trends in child mortality: report. Estimates developed by the UN Inter-agency Group for Child Mortality Estimation. New York, NY. [Google Scholar]
- Kroenke K, Stump TE, Chen CX, Kean J, Bair MJ, Damush TM, Krebs EE, Monahan PO (2020) Minimally important differences and severity thresholds are estimated for the PROMIS depression scales from three randomized clinical trials. J Affect Disord 266:100–108. 10.1016/j.jad.2020.01.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Osterman MJ (2021) Births in the United States, National Vital Statistics; Report, 2019. https://www.cdc.gov/nchs/products/databriefs.htm [Google Scholar]
- Masyn KE (2013) Latent class analysis and finite mixture modeling. In: The Oxford handbook of quantitative methods, vol. 2. Oxford University Press, Oxford, pp. 551–611 [Google Scholar]
- Matijasevich A, Murray J, Cooper PJ, Anselmi L, Barros AJD, Barros FC, Santos IS (2015) Trajectories of maternal depression and offspring psychopathology at 6 years: 2004 Pelotas cohort study. J Affect Disord 174:424–431. 10.1016/j.jad.2014.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén B, Muthén LK (2000) Integrating person-centered and variable-centered analyses: growth mixture modeling with latent trajectory classes. Alcohol Clin Exp Res 24:882–891. 10.1111/j.1530-0277.2000.tb02070.x [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO (1998) Mplus user’s guide, 8th ed. Los Angeles, CA, Muthén & Muthén. [Google Scholar]
- Nylund KL, Asparouhov T, Muthén BO (2007) Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Struct Equ Modeling 14:535–569. 10.1080/10705510701575396 [DOI] [Google Scholar]
- Nylund-Gibson K, Grimm R, Quirk M, & Furlong M (2014). A latent transition mixture model using the three-step specification. Structural Equation Modeling: A Multidisciplinary Journal, 21(3), 439–454. [Google Scholar]
- Putnick DL, Sundaram R, Bell EM, Ghassabian A, Goldstein RB, Robinson SL, Vafai Y, Gilman SE, Yeung E (2020) Trajectories of maternal postpartum depressive symptoms. Pediatrics 146:e20200857. 10.1542/peds.2020-0857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram N, Grimm KJ (2009) Methods and Measures: Growth mixture modeling: A method for identifying differences in longitudinal change among unobserved groups. Int J Behav Dev 33:565–576. 10.1177/0165025409343765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos H, Tan X, Salomon R (2017) Heterogeneity in perinatal depression: How far have we come? A systematic review. Arch Womens Ment Health 20:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vliegen N, Casalin S, Luyten P (2014) The course of postpartum depression: a review of longitudinal studies. Harv Rev Psychiatry 22:1–22. 10.1097/HRP.0000000000000013 [DOI] [PubMed] [Google Scholar]
- Walani SR (2020) Global burden of preterm birth. Int J Gynaecol Obstet 150:31–33. 10.1002/ijgo.13195 [DOI] [PubMed] [Google Scholar]
- Winter L, Colditz PB, Sanders MR, Boyd RN, Pritchard M, Gray PH, Whittingham K, Forrest K, Leeks R, Webb L (2018) Depression, posttraumatic stress and relationship distress in parents of very preterm infants. Arch Womens Ment Health 21:445–451. 10.1007/s00737-018-0821-6 [DOI] [PubMed] [Google Scholar]
- Wisner KL, Chambers C, Sit DKY (2006) Postpartum depression: a major public health problem. JAMA 296:2616–2618. 10.1001/jama.296.21.2616 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.