Abstract
We aimed to evaluate the relationship between cumulative endogenous estrogen exposure and fracture risk in 150,682 postmenopausal women (aged 50 to 79 years at baseline) who participated in the Women’s Health Initiative. We hypothesized that characteristics indicating lower cumulative endogenous estrogen exposure would be associated with increased fracture risk. We determined ages at menarche and menopause as well as history of irregular menses from baseline questionnaires and calculated years of endogenous estrogen exposure from ages at menarche and menopause. Incident clinical fractures were self-reported over an average 16.7 years of follow-up. We used multivariable proportional hazards models to assess the associations between the estrogen-related variables and incidence of any clinical fracture. In fully adjusted models, those with the fewest years of endogenous estrogen exposure (<30) had an 11% higher risk of developing central body fractures and a 9% higher risk of lower extremity fractures than women with 36 to 40 years of endogenous estrogen exposure (the reference category). In contrast, women with the most years of endogenous estrogen exposure (more than 45 years) had a 9% lower risk of lower extremity fractures than the reference category. Women with irregular (not monthly) menstrual cycles were 7% to 8% more likely to experience lower extremity fractures than women with regular monthly cycles. Our findings support the hypothesis that characteristics signifying lower cumulative endogenous estrogen exposure are associated with higher fracture risk.
Keywords: FRACTURE, MENOPAUSE, MENARCHE, IRREGULAR MENSES
Introduction
The female reproductive system regulates bone acquisition and loss throughout the life span. Estrogen secretion from puberty through young adulthood is the dominant factor responsible for increased bone density, which peaks between 25 and 35 years of age. Continued estrogen secretion then helps to maintain peak bone density until menopause, when decreased gonadal sex steroid production leads to rapid bone loss.(1)
Estrogen-related factors, including ages at first and final menstrual period and history of long or irregular menstrual cycles, can serve as proxies for endogenous estrogen exposure, and therefore can serve as potential risk factors for clinical fracture. However, associations between these reproductive characteristics and fracture risk have been mixed in previous studies,(2–5) most of which had small sample sizes and lacked longitudinal ascertainment of fractures. The Women’s Health Initiative (WHI) provides prospectively ascertained fracture data on a large sample, with robust description of participants’ years of endogenous estrogen exposure over a long period. Accordingly, the WHI provides an ideal opportunity to evaluate the association between reproductive characteristics and fracture risk. Here we test the hypothesis that those with reproductive characteristics consistent with lower lifelong endogenous estrogen exposure (ie, shorter period between menarche and menopause and history of irregular menses) have an increased risk of fractures.
Materials and Methods
Study population
The multicenter WHI consists of several randomized trials and an observational study cohort designed to evaluate how postmenopausal hormone therapy, diet modification, and calcium and vitamin D supplementation impacted risk of heart disease, fractures, and breast and colorectal cancer in older women.(6) Postmenopausal women aged 50 to 79 years of age were recruited at 40 clinical sites between September 1, 1993, and December 31, 1998.(7) The WHI enrolled 68,132 women in at least one clinical trial (CT) and 93,676 women in the observational study (OS) for a total of 161,808 participants. In 2005, 93,567 women reconsented to ongoing follow-up in the WHI Extension Study 1, which was designed to continue to examine risk factors that contribute to cardiovascular disease, cancer, and osteoporosis. Institutional review board approval was obtained at all clinical centers, and all participants provided written informed consent.
For the current analyses, data from all CT and OS participants were included in the primary analyses if they provided at least one follow-up assessment of any clinical fracture (the primary outcome) and the designated estrogen-related variables (ie, menstrual history) (n = 150,682, Fig. 1). We followed women for incidence of clinical fracture until March 1, 2019.
Fig. 1.
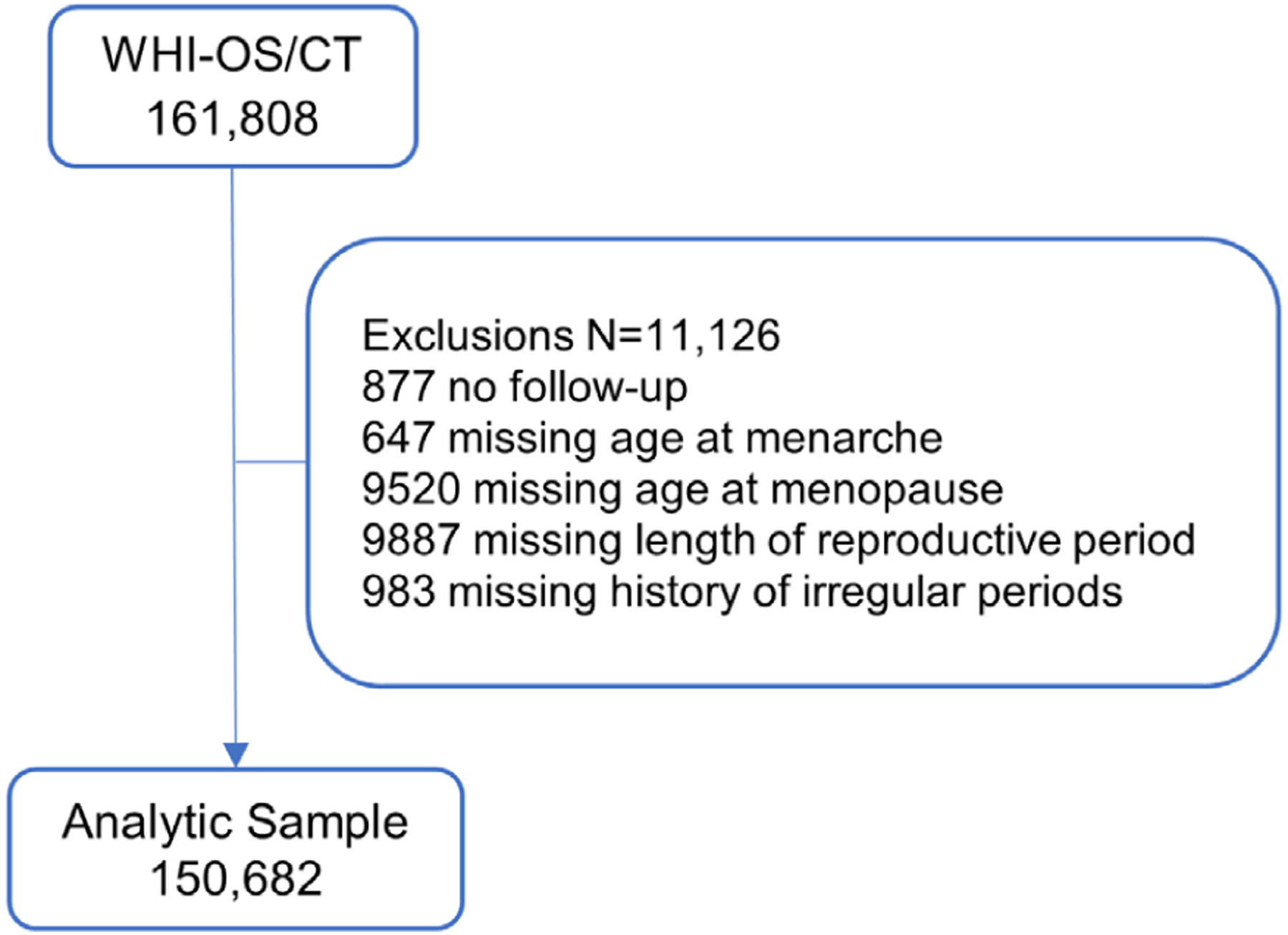
Consort diagram.
Data collection
WHI data collection procedures have been published in detail elsewhere.(6) Briefly, participants completed baseline questionnaires that sought demographic information, including self-identified race and ethnicity; medical and family history information; and information on lifestyle characteristics, including physical activity and dietary intake. Trained clinic staff measured height, weight, and waist circumference at baseline following standardized protocols; height and weight were used to calculate body mass index (BMI, wt/ht2). Baseline questionnaires also asked participants to report whether they had experienced a previous fracture and if so, to what part(s) of the body. We collapsed responses into a binary (yes/no) variable indicating whether participants reported any history of fractures. Participants were also asked to bring all medications, both prescription and over-the-counter, that they were taking regularly to their baseline visit. Interviewers entered each medication name and strength from its packaging into the Medi-Span software program (First DataBank, Inc., San Bruno, CA, USA), which assigned drug codes.
Exposure variables
Estrogen-related variables were collected by self-report at baseline. Participants reported age at menarche (“how old were you when you had your first menstrual period [menses]?”), age at final menstrual period (“how old were you when you last had regular menstrual bleeding [a period]?”), previous history of irregular periods (“during most of your life, were your periods regular; that is, did they occur about once a month? [Do not include any time when you were pregnant or taking birth control pills]”), history of hysterectomy and oophorectomy, and number of pregnancies.
Age at menopause was defined as age at final menstrual period, bilateral oophorectomy, or initiation of menopausal hormone therapy. If a woman had a hysterectomy without bilateral oophorectomy, her age at menopause was defined as age at initiation of hormone therapy or experience of vasomotor symptoms (hot flashes, night sweats). For those who had a hysterectomy without bilateral oophorectomy at 50 years of age or older but did not initiate hormone therapy or have menopausal symptoms, age at menopause was defined as the age when the hysterectomy was performed. If age at menopause was calculated to be 60 years or older, it was recoded as 60 years.(8)
The primary exposure for this study was cumulative years of endogenous estrogen exposure (age at menopause minus age at menarche), categorized into five groups (<30, 30–35, 36–40, 41–45, >45 years). These categories are consistent with previous WHI work on the association between reproductive characteristics and health outcomes,(9) and are based on the expected average time between menarche and menopause for WHI participants (38 years, given expected average age of menarche [13 years] and expected average age of menopause [51 years]). We considered dividing the data into equal quartiles or quintiles but did not in order to ensure the categories would be clinically translatable. We also wanted to examine the “extremes” of endogenous estrogen exposure (<30 and >45 years). Prior work on this topic has used similar categories.(10)
Secondary exposures included regularity of periods (not regular, sometimes regular, regular/monthly), menarchal age (<12, 12, 13, 14, ≥15 years),(11,12) and age at menopause onset (<45, 45–55, and >55 years).(11,13)
Outcome
The outcome of interest was incidence of fracture reported by March 1, 2019. Participants reported fracture events each year for those in the observational study and every 6 months for the trial participants. Specifically, participants were asked, “Has a doctor told you for the first time that you have a new broken, crushed, or fractured bone? Which bone did you break?” Responses included: hip, upper leg (not hip), pelvis, knee (patella), lower leg or ankle, foot (not toe), tailbone, spine or back (vertebra), lower arm or wrist, hand (not finger), elbow, and upper arm or shoulder. Responses were grouped into the following mutually exclusive categories: upper limb (elbow, hand except fingers, lower arm/wrist, upper arm/humerus or shoulder), lower limb (foot except toes, knee/patella, upper leg except hip, lower leg/ankle), and central body (hip, pelvis, and spine). During the main WHI study period, women in the clinical trials and a subset of women in the observational study had all fractures adjudicated. Only hip fractures continued to be centrally adjudicated through the end of the WHI Extension Study 1 (2010). After that, a subset continued to be adjudicated while the remaining cohort fractures were self-reported.
Covariates
Covariates included baseline information on BMI, self-identified race and ethnicity, smoking, alcohol intake, education, marital status, bilateral oophorectomy, hormone therapy use (pills or patches), oral contraceptive use (data are skewed to higher use in younger women given that oral contraceptives were not available during the period of endogenous estrogen exposure for women over age 60 years at WHI baseline visit), number of term pregnancies, months breastfeeding,(14) total physical activity (MET h/week), fracture history before WHI study enrollment, number of falls in the previous 12 months, scores on the RAND-36 physical functioning subscale,(15) calcium and vitamin D intake (dietary and supplement use), and oral glucocorticoid use. We also controlled for comorbidities at baseline that might impact both reproductive factors and fracture risk, including treated diabetes, multiple sclerosis, Parkinson’s disease, heart disease (coronary heart disease or heart failure), chronic obstructive lung disease, inflammatory bowel disease (Crohn’s disease and ulcerative colitis), and rheumatoid arthritis.(10,16–21) Only 2% of women were taking bisphosphonates, so we did not control for bisphosphonate use in our analysis.
Statistical analysis
Means and standard deviations or frequency distributions for baseline variables were calculated for each estrogen exposure category.
Cox proportional hazards models were used to estimate associations between number of years of endogenous estrogen exposure (<30, 30–35, 36–40, 41–45, >45) and fracture incidence by body region of clinical fracture (upper extremity, lower extremity, central); we also separately examined hip fractures (also considered in central fractures). The baseline hazard in all models was stratified by study assignment (observational study/clinical trial, hormone therapy arm, diet modification arm, calcium and vitamin D arm) and 5-year age groups. Events were recorded as the years from enrollment to first fracture of each type; participants’ follow-up was defined as time to first event, time of last follow-up, or death.
Multivariable models were fit to obtain effects adjusted for the baseline covariates listed above (see “Covariates”). We separately conducted secondary analyses to assess the associations between risk of fracture and age of menarche, age at menopause, and cycle regularity. We used exposure × time to assess proportional hazards assumption; no violations were found. All statistical tests were conducted at alpha = 0.05.
We evaluated three effect modifiers, specified a priori, that had the potential to modify the association between years of endogenous estrogen exposure and fracture risk: self-identified race (Asian, Black, and White; other races had too few participants to include in analyses); baseline hormone therapy use (ever versus never); and bilateral oophorectomy (yes versus no). We added a multiplicative interaction term between each potential effect modifier and the exposure of interest to the fully adjusted models. We did not adjust for multiple comparisons, as these analyses were exploratory in nature.
All analyses were performed with SAS software (version 9.4, SAS Institute, Inc., Cary, NC, USA).
Results
Description of cohort
The CT and OS cohorts had a combined 161,808 subjects; 11,126 were excluded from this analysis because of missing outcome or exposure data, resulting in an analytic cohort of 150,682 participants for the primary analysis (Fig. 1), with median follow-up of 16.7 years (interquartile range 9 to 21 years).
Baseline characteristics
Table 1 shows baseline characteristics by categorized years of endogenous estrogen exposure. Women with both the most and fewest years of endogenous estrogen exposure had higher mean BMIs than women in the mid-range group. Those with the fewest years of endogenous estrogen exposure were more likely to have ever used hormone therapy or oral contraceptives and to have had a bilateral oophorectomy. Women with more years of endogenous estrogen exposure were more likely than those with shorter exposures to have had one or more term pregnancies, five or more term pregnancies, or to have breastfed for more than 12 months. As expected, women with more years of endogenous estrogen exposure were more likely than those with less exposure to have had an early menarche and a late menopause, and women with more endogenous estrogen exposure had been in menopause for a shorter time before enrollment in the study.
Table 1.
Baseline Characteristics by Endogenous Estrogen Exposure
| Sample size | Endogenous estrogen exposure (years) | ||||
|---|---|---|---|---|---|
| <30 | 30–35 | 36–40 | 41–45 | >45 | |
| 25,784 | 38,383 | 55,644 | 25,013 | 5858 | |
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| Age (years), mean (SD) | 63.5 (7.2) | 62.8 (7.6) | 62.5 (7.3) | 64.0 (6.3) | 68.0 (5.2) |
| Ethnicity | |||||
| Hispanic/Latino | 1245 (4.8) | 1701 (4.4) | 2226 (4.0) | 928 (3.7) | 240 (4.1) |
| Not Hispanic/Latino | 24,320 (94.3) | 36,352 (94.7) | 52,986 (95.2) | 23,896 (95.5) | 5578 (95.2) |
| Unknown/not reported | 219 (0.8) | 330 (0.9) | 432 (0.8) | 189 (0.8) | 40 (0.7) |
| Race | |||||
| American Indian or Alaska Native | 127 (0.5) | 119 (0.3) | 138 (0.2) | 62 (0.2) | 14 (0.2) |
| Asian | 477 (1.8) | 1028 (2.7) | 1548 (2.8) | 640 (2.6) | 140 (2.4) |
| Native Hawaiian or other Pacific Islander | 27 (0.1) | 32 (0.1) | 48 (0.1) | 14 (0.1) | 5 (0.1) |
| Black | 3451 (13.4) | 3169 (8.3) | 3831 (6.9) | 1732 (6.9) | 460 (7.9) |
| White | 20,729 (80.4) | 32,843 (85.6) | 48,543 (87.2) | 21,952 (87.8) | 5098 (87.0) |
| More than one race | 383 (1.5) | 470 (1.2) | 602 (1.1) | 247 (1.0) | 51 (0.9) |
| Unknown/not reported | 590 (2.3) | 722 (1.9) | 934 (1.7) | 366 (1.5) | 90 (1.5) |
| Body mass index (kg/m2), mean (SD) | 28.5 (6.1) | 27.7 (5.8) | 27.6 (5.8) | 28.1 (6.0) | 28.5 (6.2) |
| <25 | 8009 (31.3) | 14,060 (37.0) | 20,969 (38.0) | 8467 (34.1) | 1852 (31.9) |
| 25–<30 | 8950 (35.0) | 13,300 (35.0) | 19,022 (34.5) | 8570 (34.5) | 2031 (35.0) |
| ≥30 | 8621 (33.7) | 10,673 (28.1) | 15,121 (27.4) | 7776 (31.3) | 1922 (33.1) |
| Education | |||||
| High school or less | 7066 (27.6) | 8689 (22.8) | 10,927 (19.8) | 4933 (19.8) | 1278 (22.0) |
| Some college or vocational training | 11,097 (43.4) | 14,876 (39.1) | 20,013 (36.2) | 8589 (34.5) | 2183 (37.5) |
| College grad or more | 7423 (29.0) | 14,514 (38.1) | 24,290 (44.0) | 11,340 (45.6) | 2354 (40.5) |
| Marital status | |||||
| Never married | 959 (3.7) | 1793 (4.7) | 2630 (4.7) | 1008 (4.0) | 203 (3.5) |
| Divorced or separated | 4601 (17.9) | 6216 (16.3) | 8603 (15.5) | 3485 (14.0) | 821 (14.1) |
| Widowed | 4841 (18.9) | 6525 (17.1) | 8595 (15.5) | 4156 (16.7) | 1406 (24.1) |
| Married/living as married | 15,261 (59.5) | 23,675 (62.0) | 35,579 (64.2) | 16,256 (65.3) | 3396 (58.3) |
| Total physical activity (MET h/week), mean (SD) | 11.3 (13.3) | 12.2 (13.5) | 12.9 (13.8) | 13.2 (14.1) | 12.8 (13.7) |
| Rand-36 physical functioning subscale, mean (SD) | 76.6 (22.2) | 81.1 (19.9) | 83.1 (18.7) | 82.6 (18.9) | 78.3 (21.0) |
| Alcohol servings per week | |||||
| <1 | 17,662 (68.7) | 23,964 (62.6) | 33,095 (59.6) | 15,158 (60.8) | 3743 (64.1) |
| 1–3 | 3075 (12.0) | 5409 (14.1) | 8365 (15.1) | 3751 (15.0) | 788 (13.5) |
| >3 | 4979 (19.4) | 8925 (23.3) | 14,036 (25.3) | 6034 (24.2) | 1312 (22.5) |
| Smoking status | |||||
| Never smoked | 12,302 (48.3) | 18,732 (49.4) | 28,005 (50.9) | 13,413 (54.2) | 3176 (54.9) |
| Past smoker | 10,785 (42.4) | 16,237 (42.8) | 23,502 (42.7) | 10,212 (41.3) | 2373 (41.0) |
| Current smoker | 2361 (9.3) | 2970 (7.8) | 3516 (6.4) | 1124 (4.5) | 236 (4.1) |
| Current oral glucocorticoid use | |||||
| Yes | 230 (0.9) | 286 (0.7) | 344 (0.6) | 149 (0.6) | 60 (1.0) |
| Fracture at age 55+ years | |||||
| No | 16,595 (71.4) | 23,829 (67.8) | 35,405 (69.3) | 18,149 (80.3) | 3989 (79.0) |
| Yes | 3348 (14.4) | 4772 (13.6) | 6628 (13.0) | 3367 (14.9) | 1058 (21.0) |
| Age <55 years | 3289 (14.2) | 6525 (18.6) | 9043 (17.7) | 1088 (4.8) | 1 (0.0) |
| Times fell down in last 12 months | |||||
| <2 | 21,496 (85.9) | 32,680 (87.4) | 47,992 (88.3) | 21,567 (88.4) | 4847 (87.2) |
| 2 or more | 3516 (14.1) | 4692 (12.6) | 6382 (11.7) | 2842 (11.6) | 709 (12.8) |
| Hormone therapy (HT) study arm | |||||
| Not randomized to HT | 21,420 (83.1) | 32,324 (84.2) | 46,914 (84.3) | 20,940 (83.7) | 5038 (86.0) |
| Randomized to HT | 2144 (8.3) | 3060 (8.0) | 4390 (7.9) | 2038 (8.1) | 409 (7.0) |
| Randomized to placebo | 2220 (8.6) | 2999 (7.8) | 4340 (7.8) | 2035 (8.1) | 411 (7.0) |
| HT usea | |||||
| Never used | 6610 (25.7) | 13,402 (35.0) | 25,334 (45.6) | 13,010 (52.0) | 2339 (40.0) |
| Past user | 6502 (25.3) | 7350 (19.2) | 7947 (14.3) | 3015 (12.1) | 809 (13.8) |
| Current user | 12,630 (49.1) | 17,591 (45.9) | 22,329 (40.2) | 8973 (35.9) | 2705 (46.2) |
| Oral contraceptive use, ever | |||||
| Yes | 9646 (37.4) | 16,742 (43.6) | 25,117 (45.1) | 9949 (39.8) | 1696 (29.0) |
| Diet modification (DM) arm | |||||
| Not randomized to DM | 17,851 (69.2) | 26,860 (70.0) | 39,337 (70.7) | 17,588 (70.3) | 4160 (71.0) |
| Intervention | 3111 (12.1) | 4651 (12.1) | 6511 (11.7) | 2985 (11.9) | 709 (12.1) |
| Control | 4822 (18.7) | 6872 (17.9) | 9796 (17.6) | 4440 (17.8) | 989 (16.9) |
| Calcium daily intake (diet and supplement, mg), mean (SD) | 1095 (698.9) | 1164 (708.3) | 1203 (708.9) | 1218 (716.6) | 1244 (755.9 |
| Vitamin D daily intake (diet and supplement; mcg), mean (SD) | 8.8 (6.9) | 9.2 (6.9) | 9.4 (6.9) | 9.6 (6.9) | 9.9 (7.4) |
| History of bilateral oophorectomy | |||||
| Yes | 11,832 (47.5) | 10,168 (27.0) | 6955 (12.6) | 1915 (7.8) | 320 (5.7) |
| No. of term pregnancies | |||||
| None | 3567 (13.9) | 4846 (12.7) | 6572 (11.9) | 2396 (9.6) | 518 (8.9) |
| 1–2 | 8913 (34.7) | 13504 (35.3) | 19,161 (34.6) | 7893 (31.7) | 1700 (29.2) |
| 3–4 | 9660 (37.6) | 14,693 (38.5) | 22,020 (39.7) | 10,556 (42.4) | 2471 (42.4) |
| 5 or more | 3529 (13.7) | 5166 (13.5) | 7667 (13.8) | 4059 (16.3) | 1133 (19.5) |
| Total no. of months breastfed | |||||
| Never | 13450 (52.9) | 18,931 (49.9) | 26,355 (47.9) | 11,306 (45.7) | 2537 (44.0) |
| 1–6 months | 6822 (26.9) | 9876 (26.0) | 14068 (25.6) | 6420 (25.9) | 1593 (27.6) |
| 7–12 months | 2501 (9.8) | 4145 (10.9) | 6389 (11.6) | 2884 (11.6) | 693 (12.0) |
| >12 months | 2633 (10.4) | 4997 (13.2) | 8215 (14.9) | 4154 (16.8) | 947 (16.4) |
| Cycle regularity | |||||
| Regular | 20,244 (78.5) | 31,902 (83.1) | 46,904 (84.3) | 20,704 (82.8) | 4905 (83.7) |
| Sometimes regular | 2813 (10.9) | 3644 (9.5) | 5082 (9.1) | 2516 (10.1) | 508 (8.7) |
| Not regular | 2727 (10.6) | 2837 (7.4) | 3658 (6.6) | 1,793 (7.2) | 445 (7.6) |
| Age at menarche (years) | |||||
| <12 | 4668 (18.1) | 6650 (17.3) | 11,481 (20.6) | 8303 (33.2) | 2022 (34.5) |
| 12 | 5714 (22.2) | 9716 (25.3) | 15,318 (27.5) | 6984 (27.9) | 1660 (28.3) |
| 13 | 7753 (30.1) | 10,891 (28.4) | 17,283 (31.1) | 6245 (25.0) | 1504 (25.7) |
| 14 | 3797 (14.7) | 5549 (14.5) | 7642 (13.7) | 2295 (9.2) | 672 (11.5) |
| ≥15 | 3852 (14.9) | 5577 (14.5) | 3920 (7.0) | 1186 (4.7) | 0 (0.0) |
| Age at menopause (years) | |||||
| <45 | 25,348 (98.3) | 8910 (23.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 45–55 | 436 (1.7) | 29,473 (76.8) | 55,458 (99.7) | 18,797 (75.1) | 86 (1.5) |
| >55 | 0 (0.0) | 0 (0.0) | 186 (0.3) | 6216 (24.9) | 5772 (98.5) |
| Years since menopause | |||||
| <5 | 1 (0.0) | 1931 (5.0) | 10,657 (19.2) | 6388 (25.5) | 1296 (22.1) |
| 5–10 | 574 (2.2) | 7531 (19.6) | 15,089 (27.1) | 8317 (33.3) | 2475 (42.2) |
| >10 | 25,209 (97.8) | 28,921 (75.3) | 29,898 (53.7) | 10,308 (41.2) | 2087 (35.6) |
Data expressed as n (%) unless otherwise noted.
Missing data are not included in the table; <6% of data were missing.
Self-reported unopposed estrogen and/or estrogen plus progesterone including pills or patches.
Those with the fewest years of endogenous estrogen exposure had more irregular cycles, and those with the most years of endogenous estrogen exposure were older than those with fewer years of exposure at the baseline visit and less likely to self-identify as Black. Those with the most years of endogenous estrogen exposure had higher education levels on average and were also less likely to be current smokers and have experienced a fracture before age 55 years than those with less exposure.
Years of endogenous estrogen exposure and fracture incidence
Our analysis found statistically significant associations between years of endogenous estrogen exposure and total fractures (p < 0.001) (Fig. 2). Women with the fewest years of endogenous estrogen exposure (<30 years) had an 8% to 9% higher risk of experiencing a central body fracture or lower extremity fracture relative to women whose years of endogenous estrogen exposure were in the middle quintile (36 to 40 years). In contrast, women with the most years of endogenous estrogen exposure (more than 45 years) had a 9% lower risk of lower extremity fracture compared with those whose years of endogenous estrogen exposure were in the middle (36 to 40 years). We did not note an association with upper extremity fractures. When we examined hip fractures separately (Supplemental Fig. SS1), the risk estimates were similar to those for central body fractures, although the associations were no longer statistically significant.
Fig. 2.
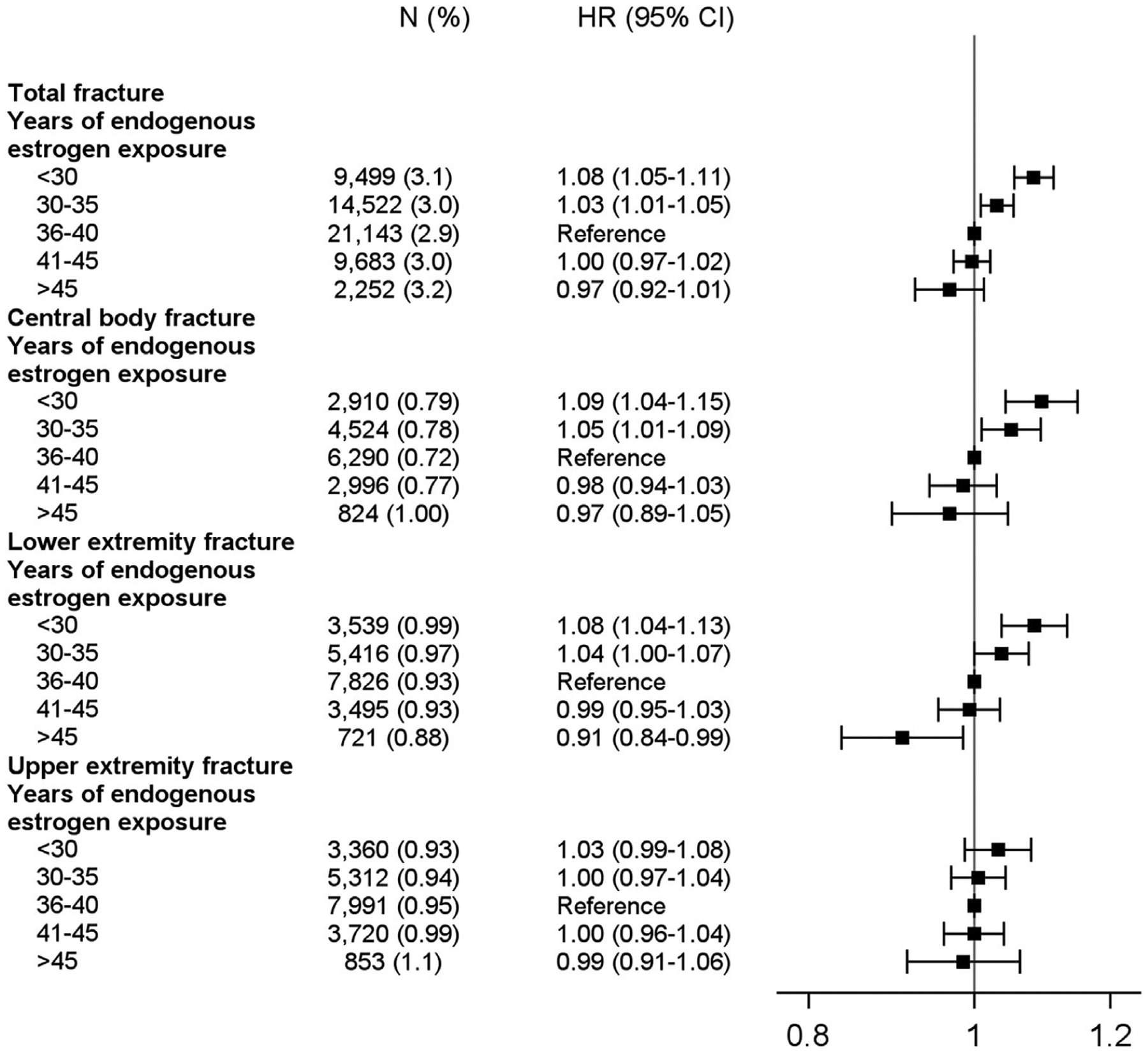
Risk of fracture according to years of endogenous estrogen exposure.
Age at menopause and fracture incidence
Earlier age at onset of menopause was associated with higher risk of total fractures (p < 0.001; Fig. 3). Women who experienced menopause before 45 years of age had a 4% to 8% higher likelihood of experiencing central body, lower extremity, or upper extremity fracture compared with those experiencing menopause between the ages of 46 and 55. When we examined the association between age of menopause and hip fractures separately, risk estimates were similar to those for central body fractures, although they were no longer statistically significant (Supplemental Fig. SS1).
Fig. 3.
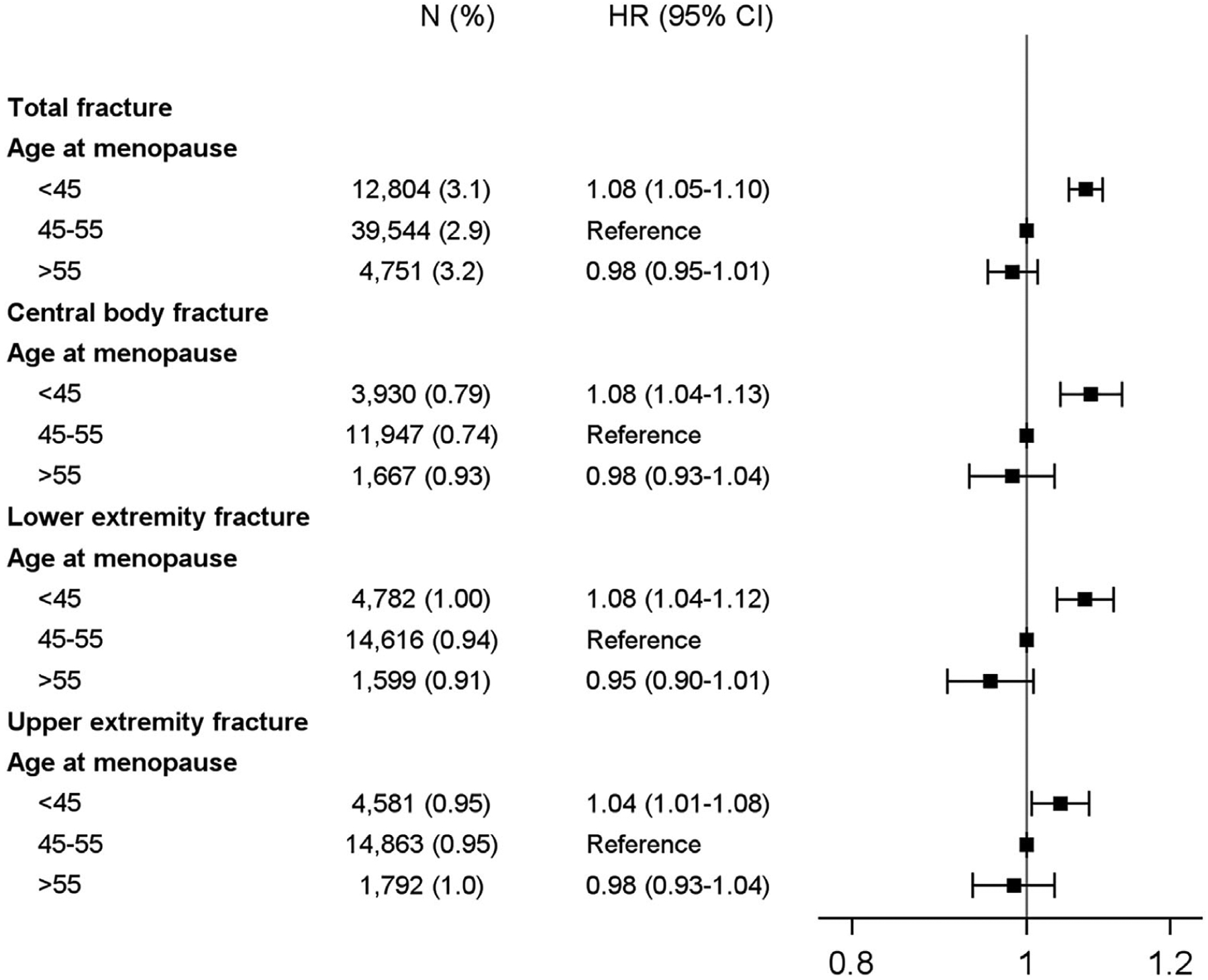
Risk of fracture according to age at menopause.
Age at menarche and fracture incidence
When we examined menarchal age, we found a statistically significant association between older age at menarche and total fractures (p < 0.001; Fig. 4). Women who had their first period at 15 years of age or older had a 10% increased risk of lower extremity fracture compared with those whose first period was at 12 years of age. However, there was no significant association between age of menarche and central body or upper extremity fractures.
Fig. 4.
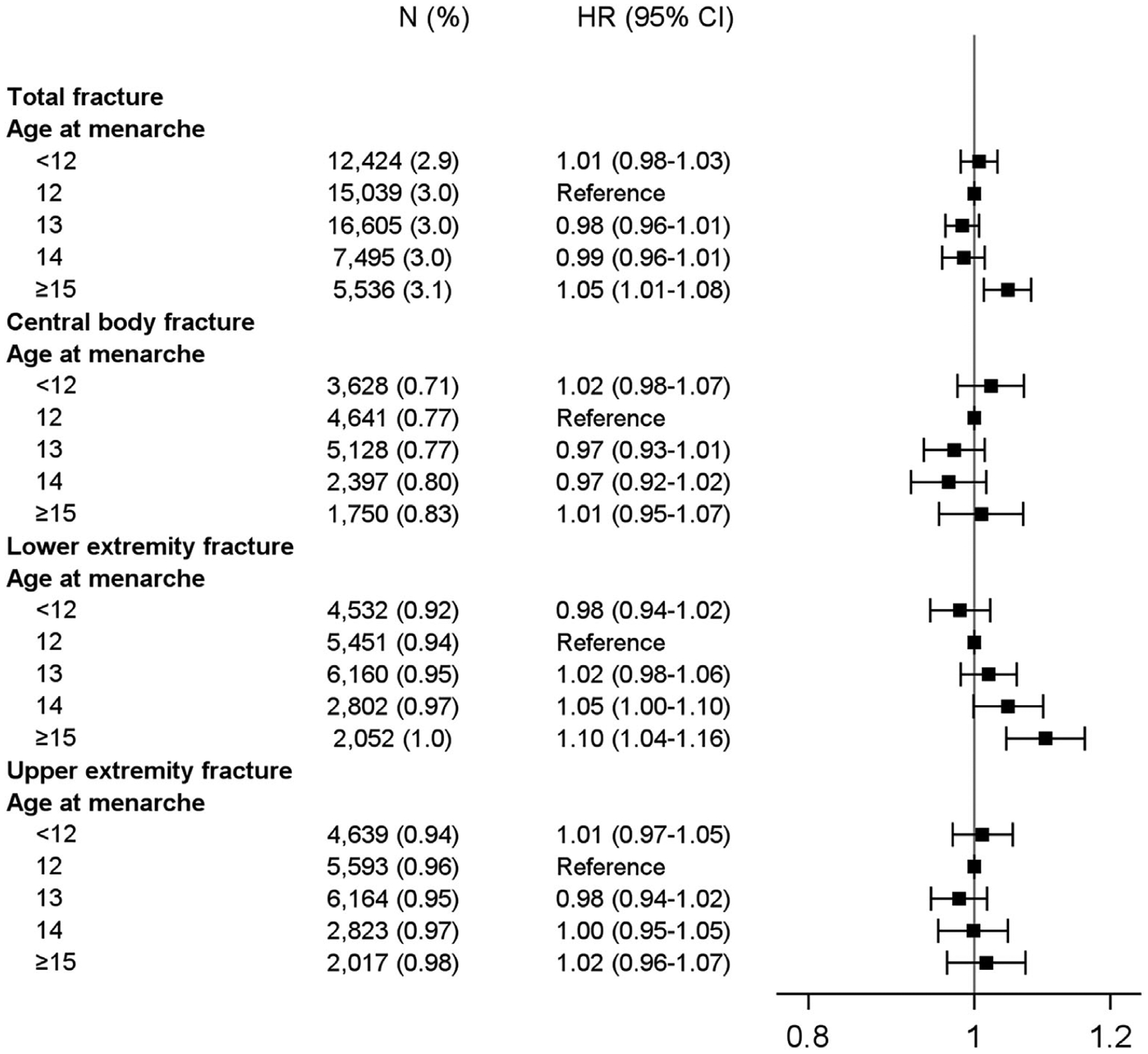
Risk of fracture according to age at menarche.
Cycle regularity and fracture incidence
We found that greater cycle irregularity was significantly associated with a higher risk of total fractures (p < 0.001; Fig. 5); women with regular cycles had the lowest risk of fractures. Women with sometimes regular or not regular cycles had a 6% to 7% increased risk of lower extremity fracture compared with women who had regular cycles; there was no association with central body or upper extremity fractures.
Fig. 5.
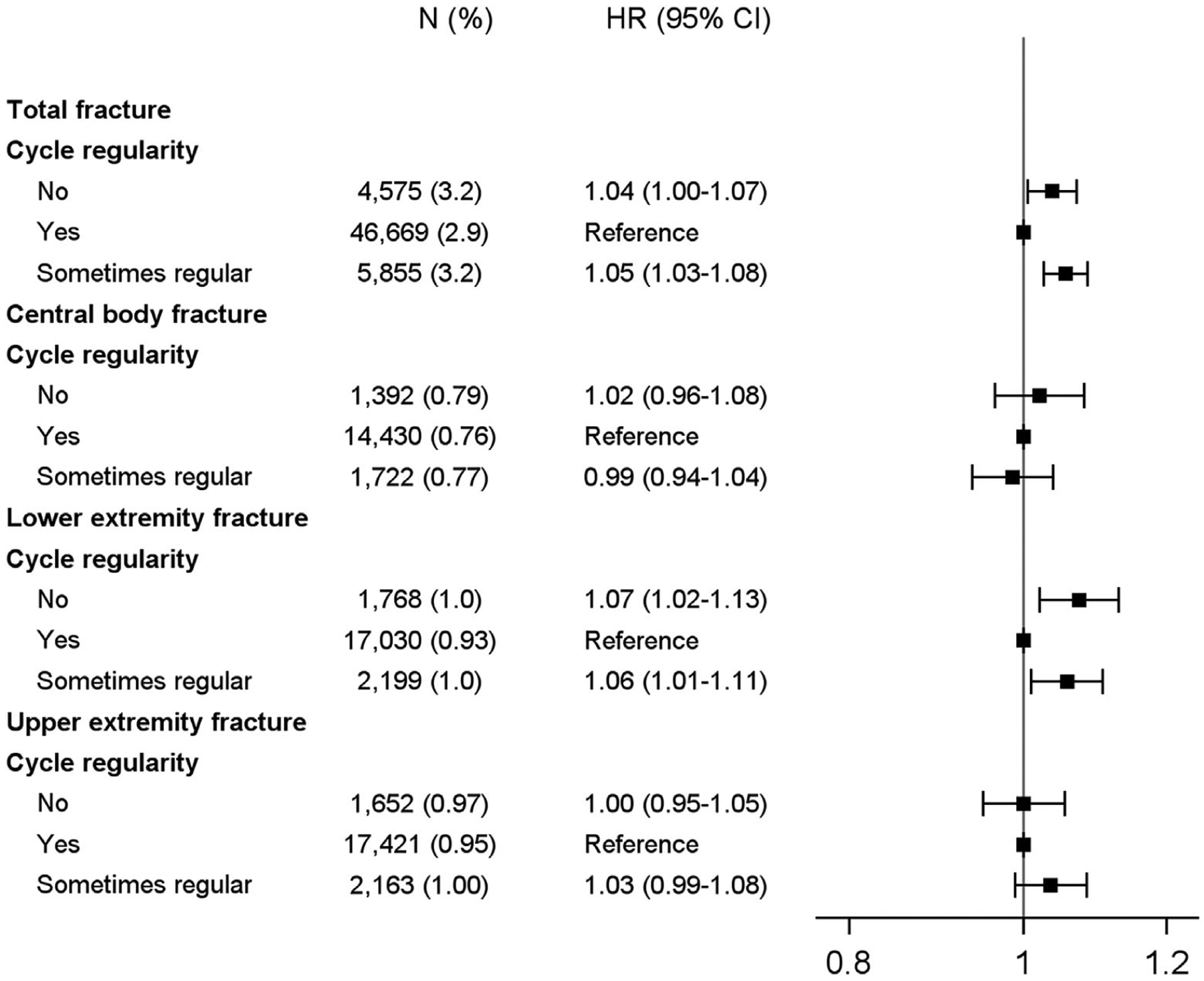
Risk of fracture according to cycle regularity.
Interactions with race, hormone therapy use, and bilateral oophorectomy
We also examined whether associations differed by self-identified race (Asian, Black, and White). There were no statistical differences in the association between years of endogenous estrogen exposure, age of menopause, age of menarche, and fracture risk among women who self-reported being Asian, Black, or White. Associations also did not differ consistently according to hormone therapy use (ever versus never) or history of bilateral oophorectomy (Supplemental Table SS1).
Discussion
Relative to women whose years of endogenous estrogen exposure were in the mid-range (36 to 40 years), women with the fewest years of endogenous estrogen exposure (<30 years) had an increased risk of developing fractures, specifically central body and lower extremity fractures. Those with the most exposure (more than 45 years) had a decreased risk of lower extremity fracture. When we examined components of endogenous estrogen exposure separately, younger age at menopause was associated with a significantly increased risk of both central body and lower extremity fracture risk. Older age at menarche was associated with an increased risk of lower extremity fracture. When hip fractures were examined separately, the risk estimates were similar to those for central body fractures but were no longer statistically significant, likely due to decreased statistical power.
The association between fracture risk and reproductive characteristics has been understudied. If future studies confirm our findings, women might benefit from counseling regarding a potential influence of reproductive factors on fracture risk. The findings might also help to inform future development of strategies to ensure such women receive the indicated osteoporosis screening in a timely fashion and to identify at-risk women for counseling about dietary, physical activity, and other measures to reduce fracture risk.(22,23)
These results confirm our hypothesis that a longer duration of exposure to endogenous estrogen is associated with lower fracture risk. Our hypothesis was based on evidence that estrogen has a positive impact on bone structural integrity. A later menarche may result in less estrogen exposure during adolescent bone development,(24,25) and an earlier menopause results in an earlier decline in bone density.(26) Results of previous cohort studies examining these associations have been mixed, possibly due to the modest associations not being detected by smaller studies (due to inadequate statistical power) as well as inaccuracy in recall of age of menopause and menarche. In our data, higher ages of menarche (15 and older) were significantly associated with fracture risk, possibly because menarche at an older age was more accurately recalled.
Women with irregular cycles were more likely to experience lower extremity fracture compared with women with regular cycles. This is consistent with our hypothesis because women with irregular cycles spend a larger amount of time in the early part of the follicular phase, during which endogenous estrogen levels are relatively low.(27)
Strengths of our study include the large number of participants (>150,000), the large number of racial and ethnic minorities, the long duration of longitudinal follow-up, and availability of detailed data about osteoporotic risk factors. However, there were also limitations. Fracture incidence by self-report is not as accurate as incidence based on medical records. In the Women’s Health Initiative, itself-report validity is higher for hip fractures (78%) and forearm/wrist fractures (81%) than for clinical spine fractures (51%).(28) Recall of ages of menopause and menarche may also be inaccurate, although categorizing into categories may improve validity.(29–32) Also, WHI participants, like other studies that rely on volunteers, tend to be healthier than the general population, so the associations between years of endogenous estrogen exposure and subsequent fracture may be stronger than reported here. Finally, although we adjusted for multiple potential covariates, there may be other lifestyle-related factors we did not include that are related to fracture risk.
Although the associations are modest, given the high incidence of fractures among the aging population of US women, our results have both public health and clinical implications by advancing knowledge about individual risk factors. Future studies should investigate the utility of including timing of menopause and menarche and menstrual regularity into routine osteoporotic risk-screening tools and clinical counseling. Including such factors into risk screening could improve strategies for primary prevention of fractures in women.
Supplementary Material
Acknowledgments
We thank the following people at the Kaiser Permanente Center for Health Research (paid for by direct and indirect funding from a grant from NIDDK, R01/R56 KD099882): Neon Brooks, PhD, and Katherine Essick, who assisted with editing, and Cassandra Angus, who contributed to paper formatting.
The list of WHI investigators is available online at https://www.whi.org/doc/WHI-Investigator-Short-List.pdf.
The WHI program is funded by the US Department of Health and Human Services through the National Heart, Lung, and Blood Institute, which is a part of the National Institutes of Health. The following contracts support this work: HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Footnotes
Disclosures
All authors state that they have no conflicts of interest.
Additional Supporting Information may be found in the online version of this article.
Data Availability Statement
Data are publicly available thru BioLINCC. The link is https://biolincc.nhlbi.nih.gov/home/.
References
- 1.Clarke BL, Khosla S. Female reproductive system and bone. Arch Biochem Biophys. 2010;503(1):118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paganini-Hill A, Atchison KA, Gornbein JA, Nattiv A, Service SK, White SC. Menstrual and reproductive factors and fracture risk: the Leisure World Cohort Study. J Womens Health (Larchmt). 2005; 14(9):808–819. [DOI] [PubMed] [Google Scholar]
- 3.Cooper GS, Sandler DP. Long-term effects of reproductive-age menstrual cycle patterns on peri- and postmenopausal fracture risk. Am J Epidemiol. 1997;145(9):804–809. [DOI] [PubMed] [Google Scholar]
- 4.Nicodemus KK, Folsom AR, Anderson KE. Menstrual history and risk of hip fractures in postmenopausal women. The Iowa Women’s Health Study. Am J Epidemiol. 2001;153(3):251–255. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen TV, Jones G, Sambrook PN, White CP, Kelly PJ, Eisman JA. Effects of estrogen exposure and reproductive factors on bone mineral density and osteoporotic fractures. J Clin Endocrinol Metab. 1995;80(9):2709–2714. [DOI] [PubMed] [Google Scholar]
- 6.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13(9 Suppl):S5–S17. [DOI] [PubMed] [Google Scholar]
- 7.Hays J, Hunt JR, Hubbell FA, et al. The Women’s Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13(9 Suppl): S18–S77. [DOI] [PubMed] [Google Scholar]
- 8.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297(13):1465–1477. [DOI] [PubMed] [Google Scholar]
- 9.LeBlanc ES, Kapphahn K, Hedlin H, et al. Reproductive history and risk of type 2 diabetes mellitus in postmenopausal women: findings from the Women’s Health Initiative. Menopause. 2017;24(1):64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ley SH, Li Y, Tobias DK, et al. Duration of reproductive life span, age at menarche, and age at menopause are associated with risk of cardiovascular disease in women. J Am Heart Assoc. 2017;6(11):e006713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinstein M, Gorrindo T, Riley A, et al. Timing of menopause and patterns of menstrual bleeding. Am J Epidemiol. 2003;158(8):782–791. [DOI] [PubMed] [Google Scholar]
- 12.Yermachenko A, Dvornyk V. Nongenetic determinants of age at menarche: a systematic review. Biomed Res Int. 2014;2014:371583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas. 1992;14(2):103–115. [DOI] [PubMed] [Google Scholar]
- 14.Chao S The effect of lactation on ovulation and fertility. Clin Perinatol. 1987;14(1):39–50. [PubMed] [Google Scholar]
- 15.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-item health survey 1.0. Health Econ. 1993;2(3):217–227. [DOI] [PubMed] [Google Scholar]
- 16.Rankin K, Bove R. Caring for women with multiple sclerosis across the lifespan. Curr Neurol Neurosci Rep. 2018;18(7):36. [DOI] [PubMed] [Google Scholar]
- 17.Kusters CDJ, Paul KC, Duarte Folle A, et al. Increased menopausal age reduces the risk of Parkinson’s disease: a Mendelian randomization approach. Mov Disord. 2021;36(10):2264–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall PS, Nah G, Howard BV, et al. Reproductive factors and incidence of heart failure hospitalization in the Women’s Health Initiative. J Am Coll Cardiol. 2017;69(20):2517–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim JH, Kang D, Hong YS, et al. Association between reproductive lifespan and lung function among postmenopausal women. J Thorac Dis. 2020;12(8):4243–4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dennison EM, Compston JE, Flahive J, et al. Effect of co-morbidities on fracture risk: findings from the Global Longitudinal Study of Osteoporosis in Women (GLOW). Bone. 2012;50(6):1288–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walsh LJ, Lewis SA, Wong CA, et al. The impact of oral corticosteroid use on bone mineral density and vertebral fracture. Am J Respir Crit Care Med. 2002;166(5):691–695. [DOI] [PubMed] [Google Scholar]
- 22.Viswanathan M, Reddy S, Berkman N, et al. Screening to prevent osteoporotic fractures: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319(24):2532–51. [DOI] [PubMed] [Google Scholar]
- 23.Merlijn T, Swart KMA, van der Horst HE, Netelenbos JC, Elders PJM. Fracture prevention by screening for high fracture risk: a systematic review and meta-analysis. Osteoporos Int. 2020;31(2):251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCormick DP, Ponder SW, Fawcett HD, Palmer JL. Spinal bone mineral density in 335 normal and obese children and adolescents: evidence for ethnic and sex differences. J Bone Miner Res. 1991;6(5):507–513. [DOI] [PubMed] [Google Scholar]
- 25.Ott SM. Bone density in adolescents. N Engl J Med. 1991;325(23): 1646–1647. [DOI] [PubMed] [Google Scholar]
- 26.Svejme O, Ahlborg HG, Nilsson J, Karlsson MK. Early menopause and risk of osteoporosis, fracture and mortality: a 34-year prospective observational study in 390 women. BJOG. 2012;119(7):810–816. [DOI] [PubMed] [Google Scholar]
- 27.Harlow SD, Ephross SA. Epidemiology of menstruation and its relevance to women’s health. Epidemiol Rev. 1995;17(2):265–286. [DOI] [PubMed] [Google Scholar]
- 28.Chen Z, Kooperberg C, Pettinger MB, et al. Validity of self-report for fractures among a multiethnic cohort of postmenopausal women: results from the Women’s Health Initiative observational study and clinical trials. Menopause. 2004;11(3):264–274. [DOI] [PubMed] [Google Scholar]
- 29.Colditz GA, Stampfer MJ, Willett WC, et al. Reproducibility and validity of self-reported menopausal status in a prospective cohort study. Am J Epidemiol. 1987;126(2):319–325. [DOI] [PubMed] [Google Scholar]
- 30.den Tonkelaar I Validity and reproducibility of self-reported age at menopause in women participating in the DOM-project. Maturitas. 1997;27(2):117–123. [DOI] [PubMed] [Google Scholar]
- 31.Lundblad MW, Jacobsen BK. The reproducibility of self-reported age at menarche: the Tromsø study. BMC Womens Health. 2017;17(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper R, Blell M, Hardy R, et al. Validity of age at menarche self-reported in adulthood. J Epidemiol Community Health. 2006;60(11): 993–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are publicly available thru BioLINCC. The link is https://biolincc.nhlbi.nih.gov/home/.


