Abstract
Background
Sex-related differences in patients with spontaneous, non-traumatic intracerebral hemorrhage (ICH) are poorly investigated so far. This study elucidates whether sex-related differences in ICH care in a neurocritical care setting exist, particularly regarding provided care, while also taking patient characteristics, and outcomes into account.
Methods
This retrospective single center study includes all consecutive patients with spontaneous ICH admitted to the neurocritical care unit in a 10-year period. Patients’ demographics, comorbidities, symptoms at presentation, radiological findings, surgical and medical provided care, intensive care unit mortality and 12 month-mortality, and functional outcome at discharge were compared among men and women.
Results
Overall, 398 patients were included (male = 198 and female = 200). No differences in demographics, Charlson Comorbidity Index (CCI), symptoms at presentation, radiological findings, intensive care unit mortality and 12-month mortality were observed among men and women. Men received an external ventricular drain (EVD) for hydrocephalus-therapy significantly more often than women, despite similar location of the ICH and radiographic parameters. In the multivariate analysis, EVD insertion was independently associated with male sex (odds ratio 2.82, 95% confidence interval 1.61–4.95, P < 0.001) irrespective of demographic or radiological features. Functional outcome after ICH as assessed by the modified Rankin scale, was more favorable for women (P = 0.044).
Conclusions
Sex-related differences in patients with ICH regarding to provided neurosurgical care exist. We provide evidence that insertion of EVD is associated with male sex, disregarding clear reasoning. A sex-bias as well as social factors may play a significant role in decision-making for the insertion of an EVD.
Keywords: Intracerebral hemorrhage, Gender medicine, Stroke, Intensive care
Introduction
Sex-differences in patients with stroke has already been the subject of several studies: Spontaneous intracerebral hemorrhage (ICH) incidence and prevalence has been described to be higher in men than in women, whereas women are older in age then man when spontaneous intracerebral hemorrhage (ICH) occurs [1–5]. Higher prevalence of tobacco and alcohol abuse in men have been discussed as a possible reason for this kind of disparity [3, 6]. On the contrary, subarachnoid hemorrhages and cardioembolic strokes, seem to affect more women than men [7–9]. The comparison of functional outcome between both sexes remains controversial with conflicting data without a clear sex with superior functional outcome, especially if adjusted for age [3, 6, 10]. Parameters of provided care (i.e., interventions, surgeries, medication, etc.) give relevant hints to uncover sex differences in certain pathologies. These aspects of provided care have not yet been thoroughly investigated in spontaneous non traumatic ICH. A better understanding of sex-related differences in patients with ICH may allow clinicians to more accurately advise patients and next-of-kin on the risks and benefits following ICH as well as counterbalance sex-bias in surgical and intensive care treatment.
The present single-center study investigates, whether sex-related differences regarding provided care, patients’ characteristics, and clinical outcomes in this particular cohort of patients with primary non traumatic ICH admitted to a neurocritical care unit exist in a retrospective manner.
Methods
This study was approved by the Ethical Committee in the Kanton of Zurich (IRB approval number KEK 2019-00.713). It was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. For this type of study, formal consent is not required. Strengthening of the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were used to draft the article.
Patients
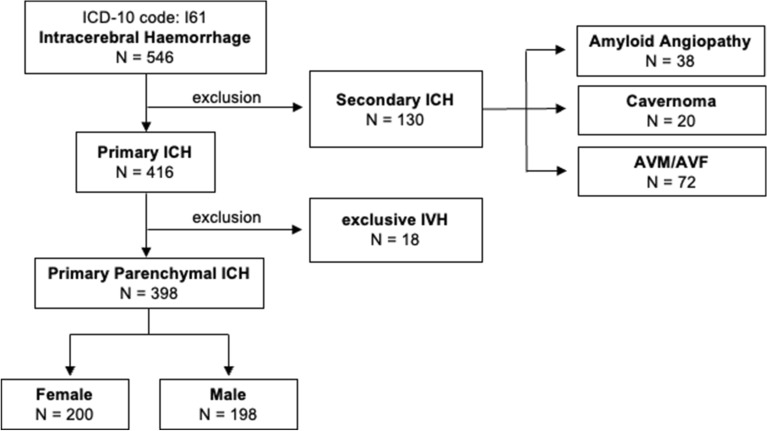
All patients admitted to the neurocritical care unit of the University Hospital of Zurich between January 2009 and December 2019 with spontaneous ICH were eligible for inclusion in this retrospective study. Inclusion criteria of the study were (1) adults (aged > 18 years) and (2) presence of spontaneous ICH. Exclusion criteria were (1) patients’ written or documented oral refusal to have their data analyzed for research projects; (2) ICH secondary to cavernoma, dural arteriovenous fistula, other arteriovenous malformation, or histopathologically confirmed amyloidangiopathy (in autopsies and surgical biopsy during hematoma evacuation); (3) ICH of traumatic etiology; and (4) sole intraventricular hemorrhage with no parenchyma involvement. To rule out ICH of secondary genesis, computed tomography angiography is routinely performed in our department when the patient is admitted.
Data Collection
Data were obtained from the hospitals’ electronic health records (KISIM-TM; Cistec,® Zurich, Switzerland). Demographic data collected were: sex, age, height, weight, and presence of comorbidities, based on the Charlson Comorbidity Index (CCI) (i.e., history of myocardial infarction, congestive heart failure, peripheral vascular disease, history of cerebrovascular event, dementia, chronic pulmonary disease, rheumatologic disease, gastric ulcer, liver disease, diabetes without or with chronic complications, kidney disease, and history of cancer) [11]. Collected symptoms at presentation included hypertension (defined by a systolic blood pressure greater than 160 mmHg), headache, nausea and vomiting, and seizure at onset. Data concerning surgically provided care included insertion of an external ventricular drain (EVD), and surgical hematoma evacuation. At our institution the indication for EVD insertion is made by the treating neurosurgeon on the basis of imaging and their clinical expertise. Additionally, hematoma evacuation is performed by osteoplastic craniotomy only. Endoscopic evacuation is not commonly used at our institution. Moreover, primary decompressive craniectomy is not part of our institution’s treatment protocol. The following intensive care unit (ICU) complications were included: pneumonia (defined as new pulmonary infiltrate by chest X-ray, increased inflammatory blood parameters, and/or positive bacterial culture of tracheal secretion), clinical or electroencephalographic seizure, and blood- and/or platelets transfusion. Radiological findings on the initial cranial computer tomography (CT) scan included the main location of ICH, the presence of a spot sign, perifocal edema, subarachnoid hemorrhage, signs of ventriculomegaly (acute abnormal expansion of the cerebral ventricles), and hemorrhage volume. The hemorrhage volume was calculated using the formula of (abc)/2 (a = maximum length in mm, b = perpendicular width to a in mm, and c = number of slices multiplied by slice thickness in mm) from the initial CT scan. A neuroradiologist (NN) blinded to the clinical presentation and outcome evaluated all imaging. As outcome, ICU-length of stay (in days), hospital-length of stay (in days), mortality (30 days and 1 year after initial ICH), and functional outcome, as evaluated with the modified Rankin scale (mRS) at discharge were collected. Additionally, medical records and family interviews were then searched for indicators for early withdrawal of therapy (within 72 hours) and premorbid functional status (“independent” or “special care”) before the occurence of ICH and noted if it applied. “Independent” refers to autonomy in activities of daily living, life participation, social relationships and occupational performance if the patient is at pre-retirement age. “Special care” refers to any limitations in daily activities that make autonomous living at home impossible and require admission to a nursery home.
Statistical Analysis
Statistical analysis was performed using SPSS V.25. Descriptive statistics are reported as counts/percentages, mean ± standard deviation, or median and interquartile range, as appropriate. For the analysis of associations, patient characteristics were dichotomized depending on either sex or the insertion of an EVD, or the outcome (mRS score 0–3 vs. mRS 4–6). All continuous data were tested for normality using Shapiro–Wilk’s test. Categorical variables were compared using Pearson’s χ2 test or Fisher’s exact test. Continuous/ordinal variables compared using Student’s t-test or Mann–Whitney U-test for parametric and non-parametric data, respectively, as appropriate. Multivariate binomial logistic regression was performed to ascertain the independence of the associations found on the likelihood of insertion of EVD or on the likelihood of unfavorable outcome in the prior univariate analysis. Multivariate binomial logistic regression was performed to ascertain the independence of the associations found on the likelihood of insertion of EVD in the prior univariate analysis. Significance was defined as the probability of a two-sided type 1 error being < 5% (P < 0.05).
Results
Overall, 548 patients were admitted to the neurocritical care unit with International Classification of Diseases, 10th Revision code I.61 for ICH during the study period. Of those, 398 patients fulfilled the inclusion criteria (Fig. 1). Thereof, 198 were male (49%) and 200 (51%) were female. Demographic characteristics, presence of comorbidities, symptoms at presentation, and scores at admission are available in Table 1. No differences in age and symptoms at presentation between both sexes were observed. CCI score did not differ among sexes, but women more often suffered from rheumatic disease (P = 0.049), whereas men more likely had diabetes (P = 0.001) and prior myocardial infarction (P = 0.003).
Fig. 1.
Flowchart of patients’ cohort
Table 1.
Demographic data, initial clinical features, comorbidities
| All (N = 398) | Female (N = 200) | Male (N = 198) | P-value | |
|---|---|---|---|---|
| Age | 66.14 ± 13.53 | 67.19 ± 13.52 | 65.09 ± 13.39 | 0.121 |
| ICH score | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | 0.878 |
| Initial GCS score | 13 (8, 15) | 13 (8, 15) | 13 (8, 15) | 0.782 |
| Admission systolic blood pressure > 160 mmHg | 139 (35%) | 76 (38%) | 63 (32%) | 0.117 |
| Initial headache | 92 (23%) | 44 (22%) | 48 (24%) | 0.504 |
| Inital nausea and vomiting | 106 (27%) | 52 (26%) | 54 (27%) | 0.607 |
| Seizure at onset | 41 (10%) | 18 (9%) | 23 (12%) | 0.312 |
| History of tobacco use | 70 (18%) | 26 (13%) | 44 (22%) | 0.042 |
| Anticoagulation use | 91 (23%) | 41 (20%) | 50 (25%) | 0.26 |
| Charlson Comorbidity index score | 1.78 ± 2.37 | 1.63 ± 1.86 | 1.93 ± 2.78 | 0.201 |
| History of myocardial infarction | 33 (9%) | 8 (4%) | 25 (13%) | 0.003 |
| Congestive heart failure | 41 (10%) | 21 (10%) | 20 (10%) | 1 |
| Peripheral vascular disease | 31 (8%) | 11 (5%) | 20 (10%) | 0.127 |
| History of cerebrovascular event | 56 (14%) | 27 (13%) | 29 (15%) | 0.918 |
| Dementia | 23 (6%) | 12 (6%) | 11 (6%) | 1 |
| Chronic pulmonary disease | 36 (9%) | 13 (6%) | 23 (12%) | 0.109 |
| Rheumatologic disease | 27 (7%) | 19 (9%) | 8 (4%) | 0.049 |
| Gastric ulcer | 3 (1%) | 1 (1%) | 2 (1%) | 0.993 |
| Liver disease | 12 (3%) | 6 (3%) | 6 (3%) | 0.58 |
| Diabetes (all) | 69 (17%) | 21 (10%) | 48 (24%) | < 0.001 |
| Mild-moderate diabetes | 48 (12%) | 13 (6%) | 35 (18%) | 0.001 |
| Diabetes with chronic complications | 5 (1%) | 2 (1%) | 5 (3%) | 0.438 |
| Kidney disease | 78 (20%) | 38 (19%) | 40 (20%) | 0.861 |
| History of Cancer | 40 (10%) | 27 (13%) | 13 (7%) | 0.054 |
Bold values indicate statistical significance at P < 0.05
Values are indicated as number (percentage) mean ± standard deviation, or median interquartile range
GCS Glasgow Coma Scale, ICH intracerebral hemorrhage
Radiological Findings
When we considered the hemorrhage site location, more women than men presented with lobar ICH (P = 0.007). Within lobar ICH, women were more likely to present with frontal and temporal ICH, and men presented more frequently with occipital hemorrhages. In addition, the frequency of deep ICH (basal ganglia or thalamus) did not differ among men and women (P = 0.224). Similarly, the frequency of the infratentorial ICH (e.g. brainstem or cerebellum) did not differ between sexes. Hemorrhage volume, hematoma expansion, presence of subarachnoid hemorrhage, ventriculomegaly, perifocal edema, intraventricular hemorrhage, and midline shift of parenchyma the ventricle on the first head CT-scan were not significantly different between groups (Table 2).
Table 2.
Radiographic features
| All (n = 398) | Female (n = 200) | Male (n = 198) | P-value | |
|---|---|---|---|---|
| Ventriculomegaly | 165 (41) | 88 (44) | 75 (38) | 0.299 |
| Subarachnoid hemorrhage | 211 (53) | 111 (55) | 100 (51) | 0.369 |
| Perifocal edema | 369 (91) | 186 (93) | 177 (89) | 0.365 |
| Hemorrhage volume (mL) | 40.32 ± 47.71 | 39.32 ± 46.54 | 41.33 ± 48.96 | 0.675 |
| Hematoma expansion | 151 (38) | 87 (44) | 64 (32) | 0.243 |
| Midline shift parenchyma (mm) | 7.87 ± 4.76 | 8 ± 4.77 | 7.71 ± 4.76 | 0.664 |
| Midline shift ventricle (mm) | 7.42 ± 4.76 | 7.49 ± 4.99 | 7.34 ± 4.49 | 0.823 |
| Ventricular extension | 246 (62) | 124 (62) | 122 (62) | 0.97 |
| Intraventricular hemorrhage including- third and fourth ventricle | 178 (45) | 93 (47) | 85 (43) | 0.482 |
| Progredient ventricles | 140 (35) | 82 (41) | 58 (29) | 0.275 |
| Localisation | 0.007 | |||
| Deep | 172 (43) | 78 (45) | 94 (55) | |
| Lobar | 140 (35) | 81 (58) | 59 (42) | |
| Infratentorial | 86 (22) | 41 (48) | 45 (52) | |
| Deep | 0.224 | |||
| Basal ganglia | 112 (28) | 48 (43) | 64 (57) | |
| Thalamus | 60 (15) | 30 (50) | 30 (50) | |
| Lobar | 0.002 | |||
| Frontal | 43 (11) | 30 (70) | 13 (30) | |
| Parietal | 40 (10) | 19 (48) | 21 (52) | |
| Temporal | 39 (10) | 25 (64) | 14 (36) | |
| Occipital | 18 (5) | 7 (39) | 11 (61) | |
| Infratentorial | 0.66 | |||
| Cerebellum | 52 (13) | 24 (46) | 28 (54) | |
| Brainstem | 34 (9) | 17 (50) | 17 (50) | |
Bold values indicate statistical significance at P < 0.05
Values are indicated as number and (percentage) and mean ± standard deviation, unless otherwise indicated
Provided Care
Even though, seizures at onset were not more prevalent in women, they received anti-epileptic drugs significantly more often than men (P = 0.007). Among the other conservative medical treatments (such as administration of blood and/or platelet conserves, frequency of intubation and days on mechanical ventilation), no differences were found among men and women (Table 3).
Table 3.
Provided care and outcomes
| All (n = 398) | Female (n = 200) | Male (n = 198) | P-value | |
|---|---|---|---|---|
| EVD | 112 (28) | 45 (23) | 67 (34) | 0.016 |
| Ventriculostomy related infection | 13 (3) | 4 (2) | 9 (5) | 0.153 |
| Hematoma evacuation | 90 (23) | 49 (24) | 41 (21) | 0.433 |
| EC given | 32 (8) | 16 (8) | 16 (8) | 1 |
| TC given | 61 (15) | 33 (16) | 28 (14) | 0.51 |
| Epilepsy during hospital stay | 21 (5%) | 13 (6%) | 8 (4%) | 0.28 |
| Antiepileptic treatment | 40 (10) | 33 (16) | 13 (7) | 0.007 |
| Intubation | 173 (43) | 88 (44) | 85 (42) | 0.75 |
| Days on mechanical ventilation | 4.63 ± 4.70 | 4.92 ± 5.46 | 4.38 ± 3.95 | 0.452 |
| Pneumonia | 88 (22%) | 50 (25%) | 38 (19%) | 0.222 |
| Length of ICU stay (days) | 8.83 ± 21.22 | 6.93 ± 8.58 | 10.76 ± 28.74 | 0.073 |
| Length of hospital stay (days) | 15.08 ± 14.03 | 15.10 ± 12.97 | 15.05 ± 15.06 | 0.972 |
| 30 days mortaility | 115 (29) | 60 (30) | 55 (28) | 0.705 |
| 1 year mortality | 142 (36) | 73 (36) | 69 (35) | 0.575 |
Bold values indicate statistical significance at P < 0.05
Values are indicated as number (percentage) and mean ± standard deviation, unless otherwise indicated
EVD external ventricular drainage, EC Erythrocyte concentrate, ICU intensive care unit, TC Thrombocyte concentrate
When we considered surgical care, an EVD was inserted more often in men than in women (P-value = 0.016), whereas no differences were found in frequency of hematoma evacuation among men and women (P = 0.433) (Table 3). On the basis of the results of the univariate analysis evaluating differences depending on EVD insertion (Table 4), a multivariate analysis was performed (Table 5). Male sex, presence of intraventricular hemorrhage, ventriculomegaly, and volume as well as evacuation of ICH were independently associated with EVD insertion. Male sex in particular increased the odds of EVD insertion by 2.82 (95% confidence interval 1.61–4.95, P < 0.001).
Table 4.
Univariate analysis dichotomized by EVD insertion
| No EVD (n = 286) | EVD (n = 122) | P-value | |
|---|---|---|---|
| Demographic data/clinical features | |||
| Age | 67.15 ± 13.91 | 63.58 ± 12.21 | 0.018 |
| Sex (male) | 131 (45.8) | 67 (59.8) | 0.012 |
| ICH score | 1 (1, 2) | 2 (1, 2) | 0.311 |
| Initial GCS score | 13.5 (8.75, 15) | 12 (6, 14) | 0.008 |
| Admission systolic blood pressure > 160 mmHg | 104 (36.4) | 35 (31.3) | 0.336 |
| Initial headache | 67 (23.4) | 25 (22.3) | 0.814 |
| Inital nausea and vomiting | 73 (25.5) | 33 (29.7) | 0.395 |
| Initial seizure | 29 (10.2) | 13 (11.8) | 0.635 |
| Anticoagulation use | 60 (21) | 31 (28.6) | 0.106 |
| Charlson Comorbidity index score | 1 [0, 3] | 2 [0, 3] | 0.510 |
| Radiographic features | |||
| Ventriculomegaly | 91 (31.8) | 71 (63.4) | < 0.001 |
| Subarachnoid hemorrhage | 149 (52.1) | 62 (55.4) | 0.558 |
| Intraventricular hemorrhage | 147 (51.4) | 99 (88.4) | < 0.001 |
| Intraventricular hemorrhage including- third fourth ventricle | 91 (32) | 87 (71) | < 0.001 |
| Perifocal edema | 260 (90.9) | 102 (91.1) | 0.960 |
| Initial hemorrhage volume | 44.23 ± 52.85 | 30.34 ± 28.81 | 0.001 |
| Hematoma evacuation | 49 (17.1) | 41 (33.6) | < 0.001 |
| Hematoma expansion | 81 (32.5.) | 35 (31.3) | 0.810 |
| Midline shift parenchyma | 148 (51.7) | 63 (56.3) | 0.418 |
| Ventricular progression | 59 (23.6) | 46 (41.1) | 0.001 |
| Localization | |||
| Deep | 96 (33.6) | 72 (64.3) | < 0.001 |
| Lobar | 139 (45.1) | 11 (9.8) | < 0.001 |
| Infraten | 61 (21.3) | 29 (25.9) | 0.328 |
| Deep | |||
| Basal Ganglia | 76 (26.6) | 52 (46.4) | < 0.001 |
| Thalamus | 46 (16.1) | 41 (36.6) | < 0.001 |
| Lobar | |||
| Frontal | 76 (26.6) | 13 (11.6) | 0.001 |
| Parietal | 62 (21.7) | 4 (3.6) | 0.001 |
| Temporal | 69 (24.1) | 12 (10.7) | 0.003 |
| Occipital | 29 (10.1) | 3 (2.7) | 0.014 |
| Infratentorial | |||
| Cerebellum | 38 (13.3) | 22 (19.6) | 0.111 |
| Brainstem | 40 (14.0) | 13 (10.7) | 0.195 |
Bold values indicate statistical significance at P < 0.05
Values are indicated as number (percentage), mean ± standard deviation, or median and (interquartile range)
ICH intracerebral hemorrhage, GCS glasgow coma scale, EVD external ventricular drain
Table 5.
Multivariate analysis
| OR | 95% CI | P-value | |
|---|---|---|---|
| Age (per unit increase) | 0.98 | 0.96–1.00 | 0.058 |
| Sex (male as reference) | 2.82 | 1.61–4.95 | < 0.001 |
| Initial GCS score (per unit increase) | 0.96 | 0.90–1.02 | 0.171 |
| Ventriculomegaly (yes as reference) | 2.94 | 1.63–5.32 | < 0.001 |
| Volume hemorrhage (per mL increase) | 0.98 | 0.97–0.99 | < 0.001 |
| Hematoma evacuation (yes as reference) | 7.97 | 3.70–17.17 | < 0.001 |
| Intraventricular hemorrhage (yes as reference) | 7.45 | 3.57–15.54 | < 0.001 |
| Localization (deep/ lobar/ infratentorial) | 1.25 | 0.83–1.87 | 0.288 |
Bold values indicate statistical significance at P < 0.05
GCS Glasgow Coma Scale
Data are presented as odds ratio (OR) with 95% confidence interval (95%CI)
Outcomes and Survival
Length of ICU-stay, length of hospital stay, 30 day- and 1 year- mortality did not differ between groups (Table 3). When we compared the outcome as evaluated using the mRS, men were more likely to suffer from an unfavorable outcome (Fig. 2). Further predictors of an unfavorable outcome at hospital discharge are shown in Tables 6 and 7. The multivariable analysis (Table 8) showed higher age, male sex, lower initial GCS score, midline shift, insertion of EVD, and higher CCI to be predictive of unfavorable outcome independent of TC transfusion, presence of ventriculomegaly, presence of intraventricular hemorrhage volume of hemorrhage and localization of the hematoma.
Fig. 2.
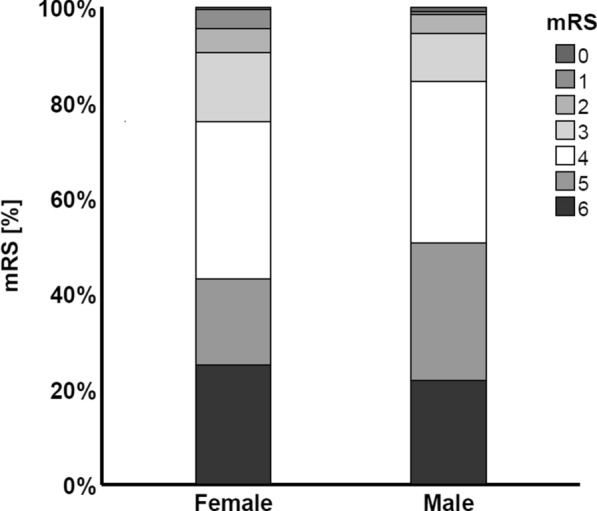
Modified Rankin scale at hospital discharge after the intracerebral hemorrhage
Table 6.
Demographic data, initial clinical features, comorbidities, provided care depending on outcome
| mRS 0–3 (n = 79) | mRS 4–6 (n = 319) | P-value | |
|---|---|---|---|
| Age | 63.00 ± 14.72 | 66.92 ± 13.13 | 0.021 |
| Sex (Male) | 31 (39) | 167 (52) | 0.044 |
| ICH score | 2 (1, 3) | 2 (1, 3) | 0.997 |
| Initial GCS score | 15 (14, 15) | 12 (6, 14) | < 0.001 |
| Admission systolic blood pressure > 160 mmHg | 27 (34) | 112 (35) | 0.896 |
| Initial Headache | 17 (22) | 75 (24) | 0.767 |
| Inital nausea and vomiting | 17 (22) | 89 (28) | 0.260 |
| Initial seizure | 10 (13) | 32 (10) | 0.538 |
| History of tobacco use | 16 (20) | 54 (17) | 0.510 |
| Anticoagulation use | 17 (22) | 75 (24) | 0.767 |
| Charlson Comorbidity Index score | 0 (0, 2) | 2 (0, 3) | 0.001 |
| EVD | 9 (11) | 103 (32) | < 0.001 |
| Ventriculostomy-related infection | 2 (3) | 11 (3) | 0.753 |
| VP-shunt | 0 (0.0) | 18 (6) | 0.030 |
| Ventriculostomy-related infection | 2 (3) | 11 (3) | 0.752 |
| EC given | 7 (9) | 25 (8) | 0.817 |
| TC given | 18 (23) | 42 (13) | 0.035 |
| Antiepileptic treatment | 7 (9) | 39 (12) | 0.440 |
| Intubation | 14 (18) | 157 (49) | < 0.001 |
| Pneumonia | 17 (22) | 71 (22) | 0.882 |
| Length of ICU stay | 4.96 ± 5.97 | 9.79 ± 23.43 | 0.070 |
| Length of hospital stay | 13.22 ± 10.33 | 15.54 ± 14.79 | 0.188 |
Bold values indicate statistical significance at P < 0.05
Values are indicated as number (percentage), mean ± standard deviation, or median (interquartile range)
EC erythrocyte concentrate, EVD external ventricular drain, GCS Glasgow Coma Scale, ICH intracerebral hemorrhage, ICU intensive care unit, mRS modified Rankin scale, TC thrombocyte concentrate
Table 7.
Radiographic Features depending on outcome
| mRS 0–3 (n = 79) | mRS 4–6 (n = 319) | P value | |
|---|---|---|---|
| Ventriculomegaly | 20 (25) | 142 (45) | 0.002 |
| Subarachnoid hemorrhage | 39 (49) | 172 (54) | 0.529 |
| Intraventricular hemorrhage | 38 (48) | 208 (65) | 0.006 |
| Intraventricular hemorrhage including third fourth ventricle | 25 (26) | 153 (48) | 0.011 |
| Perifocal edema | 65 (82) | 297 (93) | 0.005 |
| Initial hemorrhage volume | 20.30 ± 23.18 | 45.28 ± 50.85 | < 0.001 |
| Hematoma evacuation | 22 (28) | 68 (21) | 0.230 |
| Hematoma expansion | 19 (24) | 97 (30) | 0.167 |
| Midline shift parenchyma | 25 (32) | 186 (58) | < 0.001 |
| Ventricular progression | 13 (17) | 92 (29) | 0.010 |
| Localisation | |||
| Deep | 10 (13) | 158 (50) | < 0.001 |
| Lobar | 40 (51) | 100 (31) | < 0.002 |
| Infratentoriel | 29 (37) | 61 (19) | < 0.001 |
| Deep | |||
| Basal ganglia | 6 (8) | 122 (38) | < 0.001 |
| Thalamus | 2 (3) | 85 (27) | < 0.001 |
| Lobar | |||
| Frontal | 11 (14) | 78 (25) | 0.050 |
| Parietal | 12 (15) | 54 (17) | 0.740 |
| Temporal | 22 (28) | 59 (19) | 0.085 |
| Occipital | 10 (13) | 22 (7) | 0.106 |
| Infratentoriel | |||
| Cerebellum | 22 (28) | 38 (12) | 0.001 |
| Brainstem | 9 (11) | 43 (14) | 0.769 |
Bold values indicate statistical significance at P < 0.05
Values are indicated as number (percentage) and mean ± standard deviation, unless otherwise indicated
mRS modified Rankin scale
Table 8.
Multivariable analysis for unfavorable outcome
| P-value | OR | 95% C.I | |
|---|---|---|---|
| Age (per unit increase) | 0.002 | 1.04 | 1.01–1.06 |
| Sex (male as reference) | 0.012 | 2.19 | 1.19–4.03 |
| Initial GCS score (per unit increase) | < 0.001 | 0.74 | 0.65–0.84 |
| CCI (per unit increase) | 0.019 | 1.23 | 1.03–1.45 |
| EVD (yes as reference) | 0.002 | 4.19 | 1.73–10.17 |
| TC given (yes as reference) | 0.560 | 0.80 | 0.38–1.70 |
| Ventriculomegaly (yes as reference) | 0.458 | 0.76 | 0.37–1.57 |
| Intraventricular hemorrhage (yes as reference) | 0.686 | 1.14 | 0.60–2.17 |
| Volume hemorrhage (per mL increase) | 0.102 | 1.01 | 0.99–1.03 |
| Midline shift parenchyma (yes as reference) | 0.010 | 2.56 | 1.25–5.26 |
| Localisation (deep/lobar/infratentoriel) | 0.287 | 0.79 | 0.52–1.22 |
Bold values indicate statistical significance at P < 0.05
Data is presented as OR with 95%CI
CCI Charlson Comorbidity Index, CI confidence interval, EVD external ventricular drain, GCS Glasgow Coma Scale, OR odds ratio, TC thrombocyte concentrate
From all deceased patients n = 115, n = 55 (47,0%) died after early withdrawal of life-sustaining therapy. Of them n = 25 were men (45%) (P = 0.500 compared to female). Premorbid “special care” functional status was comparable in both sexes: n = 6 in women (20%) and n = 6 in men (24%) (P = 0.617).
Discussion
In our cohort of 398 consecutive patients admitted at the ICU following spontaneous ICH, we found that sex-related differences in outcome and provided care existed. In a multivariate analysis, male sex was independently associated with a poorer outcome, and insertion of EVD was significantly associated with male sex disregarding a clear radiographic or clinical reason. To the best of our knowledge, this difference in provided care (EVD insertion) is firstly reported in our study. We suggest that a gender-bias as well as social factors might have played a significant role in decision-making for the insertion of an EVD.
Patients’ demographics and severity scores at presentation and premorbid functional status were similar among men and women. Similarly, absolute CCI scores did not differ among groups. However, as reported in other studies, women more likely suffered from rheumatic disorders, whereas men were more likely to have diabetes and a history of myocardial infarction [12–14]. Despite the similarities in demographics and presentation, men were almost three times as likely to receive an EVD in comparison with women. To understand the reasoning for this difference, we tested the hypothesis that men more frequently suffered from ICH in locations that were very difficult to be reached surgically (i.e., basal ganglia and the brain stem). In patients with such deep ICH, occlusive ventriculomegaly is very common, and the insertion of an EVD might represent the only surgical possibility to control the intracranial pressure. Interestingly, the frequency of deep as well as infratentorial ICH was similar among men and women. Additionally, radiographic signs for hydrocephalus (ventriculomegaly), intraventricular hemorrhage, and progression of ventricular size or ventricular shift–as well-known reasons for an EVD-insertion- were comparable among men and women. Therefore, we conducted a multivariate analysis with insertion of an EVD as outcome, and we found that male sex was independently associated with EVD insertion. Strikingly, even after correction for clinical and radiological features, male sex increased odds of EVD insertion by almost threefold. The same disparity did not apply to the incidence of surgical hematoma evacuation. Additionally, the frequency of limitation of life-sustaining therapy (early withdrawal within 72 hours) was comparable among men and women, and therefore it does not seem to be the reason for this observed difference in provided care in EVD placement.
We can only speculate if the reason for this discrepancy is a form of “gender-bias”. Previous studies, indeed, reported that not only patients’ sex but also physicians’ sex might play a role in the decision-making process in health care, showing a trend for less aggressive treatments for female patients [15–17]. Women, furthermore, commonly have been shown to have less social support than men (often because more women are widowed) while older men are more likely to have a recognized representative (i.e. spouses, partners or children) when a decisions for an invasive medical treatment is necessary [18]. With the available data, we are not able to assess whether these factors, or also additional socioeconomic factors might have played a role in the decision to insert an EVD. The finding that females received anti-epileptic drugs significantly more often than males, despite a similar frequency of clinical or electroencephalographic seizures, also reveals a disposition to provide more conservative treatments for females in comparison to their male counterparts.
Despite the mentioned sex –related differences in provided care in favor of provided hydrocephalus-treatment by EVD in male patients, male sex was associated with significantly poorer outcome. Incidence of ventriculostomy-related infection was the same in both sexes, ruling out that this relevant EVD-related morbidity is causing poorer functional outcome in men. Previous findings concerning sex-related differences in mortality after ICH are conflicting: studies have reported that younger women (< 65 years old) with ICH had a lower mortality than men but also that women suffered from higher mortality and morbidity after ICH [19] [20]. In secondary ICH related to vascular abnormality, female sex was an independent risk factor for poor outcomes [21].
Concerning mortality, we did not observe that women fared worse than men did, probably because our study population differed from those in previous studies, with women not being older than men, suggesting that a combination of both sex and age may contribute to differences in outcome after ICH [10, 20]. Concerning functional outcome at hospital discharge after the ICH, however, female patients achieved a favorable outcome (mRS score 0–3) more often than male patients did, despite less aggressive treatment. This finding suggests that women could have had a favorable functional outcome even more likely, if they had received equal intensity of care as men. Although it is not possible to prove this by our study design, if we considered the efficacy of EVD in treating hydrocephalus, even more women might have had a favorable outcome had they received an EVD. However, because of due to the invasive nature of the treatment the opposite cannot be ruled out and an EVD insertion might carry unknown, outcome-relevant morbidity in men.
Strength and limitations of this Study
The strength of this study lies in the use of a database of consecutive patients with spontaneous ICH during a 10-year period with several parameters collected. However, due to the retrospective nature of our study, our study design bears limitations. Firstly, this is a single center study. Therefore, the generalizability of the results may be limited because we cannot exclude detection and referral biases. While most patients with severe clinical symptoms are admitted to the Neurocritical Care Unit in our institution, some may have also been admitted to the in-house intermediate care unit and thus, not been included in our study. Furthermore, while patients with histopathologically proven cerebral amyloid angiopathy were excluded, due to the small number of biopsies/autopsies we cannot exclude the possibility that some cases have still been included. Furthermore, MRIs were not regularly performed to screen for microbleeds. Moreover, the lack of data regarding the decision-making process of the neurosurgeon to insert an EVD only allows speculations about the reason of the sex-related discrepancy in frequency of EVD-insertion. Moreover, pre-admission status could only be recorded in a retrospective manner and not based on modified Rankin Scale. Due to the retrospective study design and unavailable data on the initial ICP or its course, the effect of EVD insertion on ICP management cannot be judged in this study design.
Conclusion
Sex-related differences in provided care of patients with spontaneous ICH exist. Men were almost three times more likely to receive an EVD in comparison with women. Neither demographical characteristics (including age and clinical presentation) nor imaging features (including ICH location, volume, and existence of ventriculomegaly) explained this discrepancy. These findings support the previously reported disposition to more aggressive treatments for men in comparison with women.
Author Contributions
Conception and design: WB. Acquisition of data: WNW. Analysis and interpretation of data: WBB. Critical revision of the article: Keller. Supervision and approval of final version of submitted manuscript: all authors.
Source of support
Open access funding provided by University of Zurich. This study was not funded.
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This study was approved by the Ethical Committee in the Kanton of Zurich (IRB approval number KEK 2019-00.713).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Haberman S, Capildeo R, Rose FC. Sex differences in the incidence of cerebrovascular disease. J Epidemiol Community Health. 1981;35(1):45–50. doi: 10.1136/jech.35.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appelros P, Stegmayr B, Terent A. Sex differences in stroke epidemiology: a systematic review. Stroke. 2009;40(4):1082–1090. doi: 10.1161/STROKEAHA.108.540781. [DOI] [PubMed] [Google Scholar]
- 3.Roquer J, et al. Sex-related differences in primary intracerebral hemorrhage. Neurology. 2016;87(3):257–262. doi: 10.1212/WNL.0000000000002792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandset EC, et al. Sex differences in treatment, radiological features and outcome after intracerebral haemorrhage: pooled analysis of intensive blood pressure reduction in acute cerebral haemorrhage trials 1 and 2. Eur Stroke J. 2020;5(4):345–350. doi: 10.1177/2396987320957513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galati A, King SL, Nakagawa K. Gender disparities among intracerebral hemorrhage patients from a multi-ethnic population. Hawai'i J Med Public Health. 2015;74(9 Suppl 2):12–15. [PMC free article] [PubMed] [Google Scholar]
- 6.Marini S, et al. Sex differences in intracerebral hemorrhage expansion and mortality. J Neurol Sci. 2017;379:112–116. doi: 10.1016/j.jns.2017.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghods AJ, Lopes D, Chen MJFIN. Gender differences in cerebral aneurysm location. Science. 2012;3:78. doi: 10.3389/fneur.2012.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenørn J, Eskesen V, Schmidt KJBJON. Clinical features and outcome in females and males with ruptured intracranial saccular aneurysms. Science. 1993;7(3):287–290. doi: 10.3109/02688699309023811. [DOI] [PubMed] [Google Scholar]
- 9.Smith DB, et al. Gender differences in the Colorado stroke registry. Science. 2009;40(4):1078–1081. doi: 10.1161/STROKEAHA.108.541730. [DOI] [PubMed] [Google Scholar]
- 10.Gokhale S, Caplan LR, James MLJS. Sex differences in incidence, pathophysiology, and outcome of primary intracerebral hemorrhage. Science. 2015;46(3):886–892. doi: 10.1161/STROKEAHA.114.007682. [DOI] [PubMed] [Google Scholar]
- 11.D'Hoore W, Bouckaert A, Tilquin C. Practical considerations on the use of the Charlson comorbidity index with administrative data bases. J Clin Epidemiol. 1996;49(12):1429–1433. doi: 10.1016/S0895-4356(96)00271-5. [DOI] [PubMed] [Google Scholar]
- 12.Ji J, Sundquist J, Sundquist KJJOA. Gender-specific incidence of autoimmune diseases from national registers. J Clin Epidemiol. 2016;69:102–106. doi: 10.1016/j.jaut.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Kaiser A, et al. Prevalence, awareness and treatment of type 2 diabetes mellitus in Switzerland: the CoLaus study. J Clin Epidemiol. 2012;29(2):190–197. doi: 10.1111/j.1464-5491.2011.03422.x. [DOI] [PubMed] [Google Scholar]
- 14.Mannsverk J, et al. Age and gender differences in incidence and case fatality trends for myocardial infarction: a 30-year follow-up. Tromsø Study. 2012;19(5):927–934. doi: 10.1177/1741826711421081. [DOI] [PubMed] [Google Scholar]
- 15.Raine R, et al. Influence of patient gender on admission to intensive care. J Clin Epidemiol. 2002;56(6):418–423. doi: 10.1136/jech.56.6.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garrouste-Orgeas M, et al. Predictors of intensive care unit refusal in French intensive care units: a multiple-center study. J Clin Epidemiol. 2005;33(4):750–755. doi: 10.1097/01.ccm.0000157752.26180.f1. [DOI] [PubMed] [Google Scholar]
- 17.Bugiardini R et al. (2014) Exploring in-hospital death from myocardial infarction in Eastern Europe: from the International Registry of Acute Coronary Syndromes in Transitional Countries (ISACS-TC). In on the Behalf of the Working Group on Coronary Pathophysiology & Microcirculation of the European Society of Cardiology. 2014. 12(6): p. 903–909 [DOI] [PubMed]
- 18.Hill A, et al. Examining mechanisms for gender differences in admission to intensive care units. J Clin Epidemiol. 2020;55(1):35–43. doi: 10.1111/1475-6773.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ayala C, et al. Sex differences in US mortality rates for stroke and stroke subtypes by race/ethnicity and age, 1995–1998. J Clin Epidemiol. 2002;33(5):1197–1201. doi: 10.1161/01.str.0000015028.52771.d1. [DOI] [PubMed] [Google Scholar]
- 20.Craen A, et al. Gender differences in outcomes after non-traumatic intracerebral hemorrhage. J Clin Epidemiol. 2019;11(10):1008. doi: 10.7759/cureus.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hao Z, et al. Sex-specific differences in clinical characteristics and outcomes among patients with vascular abnormality-related intracerebral hemorrhage. J Clin Epidemiol. 2019;129:e669–e676. doi: 10.1016/j.wneu.2019.05.243. [DOI] [PubMed] [Google Scholar]