Abstract
Myocardial injury as induced by myocardial infarction results in tissue ischemia, which critically incepts cardiomyocyte death. Endothelial cells play a crucial role in restoring oxygen and nutrient supply to the heart. Latest advances in single-cell multi-omics, together with genetic lineage tracing, reveal a transcriptional and phenotypical adaptation to the injured microenvironment, which includes alterations in metabolic, mesenchymal, hematopoietic and pro-inflammatory signatures. The extent of transition in mesenchymal or hematopoietic cell lineages is still debated, but it is clear that several of the adaptive phenotypical changes are transient and endothelial cells revert back to a naïve cell state after resolution of injury responses. This resilience of endothelial cells to acute stress responses is important for preventing chronic dysfunction. Here, we summarize how endothelial cells adjust to injury and how this dynamic response contributes to repair and regeneration. We will highlight intrinsic and microenvironmental factors that contribute to endothelial cell resilience and may be targetable to maintain a functionally active, healthy microcirculation.
Keywords: Endothelial, Plasticity, Endothelial-to-mesenchymal transition, Myocardial infarction, Resilience, Cardiac remodeling, Microenvironment
Introduction
In the adult heart, a tight interplay between multiple cell types ensures tissue integrity throughout lifetime. Fibroblasts are known to orchestra cardiomyocyte function, by providing structural scaffold and electromechanical support [25, 33]. Resident cells of the immune system degrade apoptotic waste and act as sentinel cells to detect small changes in the microenvironment [26, 61]. Proper contraction of the heart requires oxygen and nutrient supply, which is guided by the vasculature and its direct cellular interface to the blood, the endothelium. All these cellular functions are coupled to distinct molecular maintenance programs, which tightly control cell identity. This includes intrinsic mechanisms, which prime the transcriptional landscape of the cells, as well as extrinsic signals by surrounding microenvironmental factors [5, 6, 29]. Tissue damage as induced by myocardial infarction perturbs this steady state and enables individual cells of the heart exiting the maintenance and identity program. While these adaptive responses associated with increase in cellular plasticity are essential for tissue repair, the ability of cells to revert to the homeostatic phenotype is the key for long-term cardiac health (Fig. 1). This ability to return to the original state after a stress response is often referred as cellular resilience.
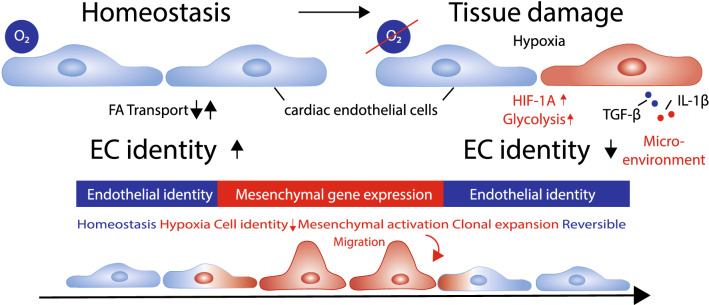
Fig. 1.
Endothelial cells identity changes after myocardial infarction. Cardiac microvascular endothelial cells maintain a tissue dependent cell identity program at baseline level. Tissue damage leading to hypoxic signaling and increase of glycolytic metabolic pathways together with increased concentrations of pro-inflammatory cytokines lead to loss of cell identity and expression of a mesenchymal gene program. While this induction favors migratory and proliferative responses in endothelial cells after infarction, mesenchymal activation is a temporary and reversible mechanism
The era of single-cell multi-omics significantly broadens our understanding of how dynamical stress responses change chromatin and transcriptional landscapes and induced a reconsideration of how we define cellular heterogeneity. Interestingly, studies of cardiovascular tissues rarely identified entirely new cell populations but highlight the presence of multiple states of cellular identities within the heart upon injury [6, 7, 26, 49, 65]. In contrast to previous reports on specialized progenitor pools, increasing evidence suggests that the tissue-resident microvascular endothelial cells (ECs) have a high plasticity and regeneration capacity. Transient changes of the transcriptome after injury contribute to the onset of neovascularization by clonal expansion [78], modulation of the inflammatory response [120], para- and autocrine signaling [116] or metabolic adaptations [62] (Figs. 2, 3, 4).
Fig. 2.
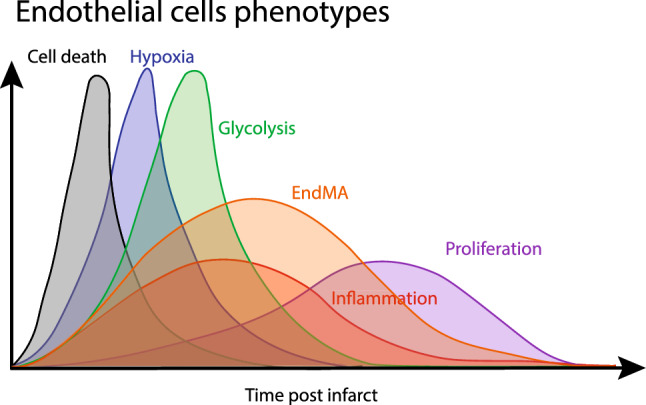
Timeline of endothelial phenotypes after myocardial infarction. Endothelial cells transiently respond and adapt to changes in the cardiac microenvironment. After the first wave of cell death, which occurs in obstructive and infarcted areas, the partial O2 decreases, leading to the onset of hypoxia. Later, cells induce glycolytic genes, which favors plastic phenotypes such as endothelial-to-mesenchymal activation (EndMA). Recently, the emergence of this mesenchymal phenotype has been associated with clonal expansion and proliferation. Endothelial activation and inflammatory signaling can be seen in multiple phases after myocardial infarction, as the endothelium interacts and responds to innate and adaptive immune reactions
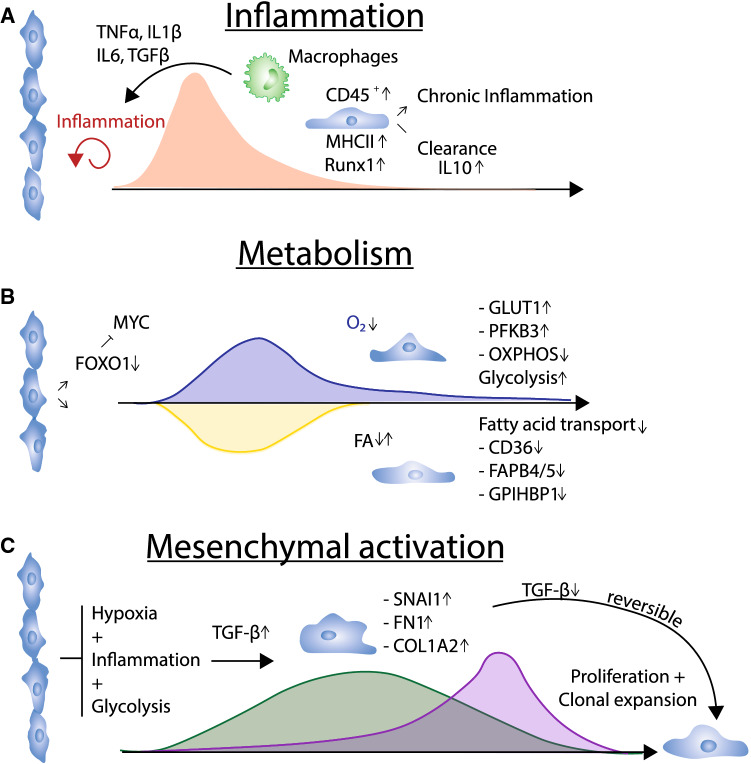
Fig. 3.
Transient phenotypes after myocardial infarction. A The emergance of innate immune reactions favors an inflammatory-induced phenotype which has been described to occur based on increased pro-inflammatory signaling (TNFα, IL-1β, IL-6). Also levels of TGF-β which can origin from multiple sources are associated with this phenotype. Endothelial cells show increased expression of hematopoietic marker CD45 and major histocompatibility complex (MHC) class II molecules. Whether this phenotype is cleared or leads to a chronic inflamed endothelium is debated. B Endothelial metabolic changes after myocardial infarction. Repression of FOXO1 leads to induction of MYC, which initiates a switch from fatty acid transport to glycolytic signaling. This induction is transient and reverts when normoxic conditions are restored. C Mesenchymal activation induces proliferation and clonal expansion. Hypoxia, inflammation and a glycolytic metabolism with increased TGF-β levels induce mesenchymal marker genes, such as Snai1, Fn1 or Col1a2. Mesenchymal activation has been shown to induce clonal expansion in endothelial cells, leading to neovascularization. Upon loss stimulating TGF-β levels, endothelial cells revert back to a naïve phenotype.
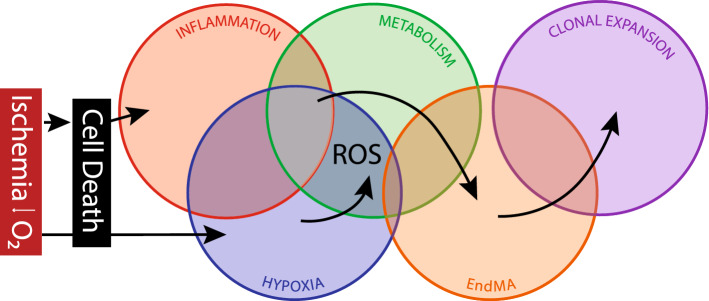
Fig. 4.
Overlapping combinatory signals lead to clonal expansion. The emergence of ischemia leads to a combinatorial reaction of events that favor clonal expansion and neovascularization. Cell death leads to the onset of inflammation which alters endothelial cells phenotype. In combination with hypoxic signaling and altered metabolism, cells can transiently activate a mesenchymal program, which leads to clonal expansion
The ability of ECs to transiently respond, adapt but also recover maintenance functions strongly impacts long-term cardiac health. Its failure leads to endothelial dysfunction which impairs heart functions [146]. Here, we are summarizing why endothelial resilience is the key for cardiovascular repair and we review novel insights into EC plasticity.
Endothelial plasticity after myocardial infarction
Myocardial infarction (MI) results in impaired tissue functionality, which in the long term results in chronic heart failure. Mechanistically, incomplete regeneration of infarcted areas leads to accumulation of fibrotic tissue and microvascular dysfunction. While most patients survive the acute hospitalization [59, 129], 20–30% of these patients develop heart failure within one year post MI [44, 114]. Here, successful reperfusion therapy within the first 90 min is decisive to prevent long-term loss of cardiomyocytes [45]. However, microvascular EC function can substantially suffer, resulting in reduced barrier function, permeability and inflammation [108], ultimately leading to endothelial dysfunction and impaired vascularization. Especially in early phases, ECs have remarkable plasticity and actively contribute to neovascularization and tissue remodeling. Here we are highlighting important adaptive responses of EC, including changes in phenotypes, after myocardial infarct. Of note, major insights derive from mice models in which short-time ischemia/reperfusion (I/R) injury or permanent ligation of left anterior descending coronary artery is used [128]. Both have limitations and may not fully recapitulate human pathophysiology. While I/R procedures are potentially mimicking the situation in patients more precisely, the experimental occlusion time largely determines the outcome, making studies difficult to compare. Permanent ligation models are more broadly used and provided many mechanistic insights into the naturally occurring pathophysiology, which leads to large infarcts and scar tissue. However, micro-embolization based on spontaneous erosion on plaque and obstruction of the capillary network as seen in patients with acute coronary syndrome is difficult to model in rodents [55]. Capillary obstructions lead to no-reflow phenomena and may induce distinct EC responses [39, 41]. Future analysis of human cardiac tissue from patients suffering myocardial infarction by single-cell technologies may help to validate the findings in mice, but will also shed light on how different clinical interventions or infarct size impact endothelial resilience.
Endothelial responses to cell death, hypoxia and inflammation favor interactions with the immune system
Restriction of oxygen and nutrient supply after myocardial infarction represents a challenge for all cells in the damaged tissue, leading either to cell death or induction of survival genes. Cell death is induced in cardiomyocytes and interstitial cells within 30 min post infarction. Cells close to the endocardium are more prone to injury, leading to propagation of cell death toward outer regions at the epicardium [103]. Moreover, I/R with fluctuations in oxygen levels lead to accumulation of ROS. Notably, the levels of ROS in ECs appears to determine whether survival or apoptosis genes are activated [110].
Loss of oxygen not only induces cell death, but activates and stabilizes the transcription factor HIF-1A, which induces survival genes and controls metabolic adaptation. Induction of HIF-1A augments glycolytic metabolism and leads to higher expression of angiogenic factors, such as ANGPT1 and ANGPT2 or VEGF [67, 126]. This initiates neovascularization, which will be discussed in “Angiogenesis and proliferation”.
Inflammation starts when cells of the innate immune system recognize danger-associated patterns, which lead to activation of tissue-resident macrophages and neutrophils and production of pro-inflammatory cytokines and chemokines, such as TGF-β, IL-1β, IL-6, TNF-α. Thereby, damage-induced matrix degradation, production of reactive oxygen species (ROS) and complement activation are the three major drivers in the initiation of immune responses [62, 63]. Interestingly, onset of inflammation largely depends on activation of Toll-like receptor 2 and 4 in the heart [9, 94].
The endothelium responds to the pro-inflammatory environment by supporting immune cell infiltration specifically via degranulation of Weibel–Palade bodies containing P-Selectin [90] and by induced expression of ICAM1 and VCAM1 [26] which promotes leukocyte adherence. ECs additionally produce cytokines and chemokines within 24 h after infarction [124], which fuels the inflammatory activation. TNF-α as well as other paracrine or soluble factors, induce endothelial activation, apoptosis and ROS production, whereas NO biosynthesis is reduced, overall leading to a destabilization of the endothelial monolayer [54, 56, 57]. Moreover, endothelial activation promotes the local adhesion of thrombocytes by von Willebrandt factor [12, 130]. Pro-inflammatory activation and increased thrombocyte adhesion were also shown to occur in remote vessels, such as larger arteries after myocardial infarct. These remote vessel alterations were attributed to systemic activation of the endothelium, leading to higher risk for atherosclerotic after infarction [85].
Recently, ECs were discussed to facilitate immune modulation beyond these classical functions in innate immunity. Evidence of a plastic conversion toward “immune cell-like” phenotype (EndICLT) has been shown on single-cell level when comparing static and disturbed flow conditions in a mouse carotid ligation model [6]. This proatherogenic phenotype was associated with increased expression of typical markers of macrophages, including major histocompatibility complex II (MHCII). Specialized endothelium, like sinusoidal ECs in the liver, has antigen-presenting functions, and can act as sentinel cells, sensing bacterial infections and cross-prime T cells to activation [15, 112, 121]. In the heart, the presence of MHCII-positive ECs with antigen-presenting activities has been described already in the 1990s [106]. In addition, mitral valve ECs were shown to express the hematopoietic marker CD45 post infarction [11], which suggests that these EC may undergo a hematopoietic transition.
The endothelial and hematopoietic fate are tightly linked. In evolution, the emergence of an EC monolayer is hypothesized to occur from specialized circulating blood cells, so called amoebocytes, which epithelized the basal membrane and form a primitive blood tissue barrier [87]. Higher vertebrate ECs share a common progenitor with cells from the hematopoietic system, the hemangioblast, which likewise gives rise to endothelial and hematopoietic progenitors [43, 145]. This explains not only why endothelial-specific signatures, such as PECAM1, CD34 or VEGFR2 [28, 87], are still also found in cells of the hematopoietic system, but also might be the reason that pro-inflammatory cytokines, which have pleiotropic functions on immune cell activity also influence endothelial plasticity. Notably, the transcription factor RUNX1, known to maintain expression of identity genes in hematopoietic stem cells, has been shown to be upregulated in ECs after infarction [82]. While its role in angiogenesis and tissue repair remains controversial, there is evidence that RUNX1 is not only crucial in the formation of hemogenic ECs in development but also has functions in adult heart ECs [19]. Finally, emerging concepts suggest that ECs may fully convert into an immune phenotype after prolonged activation in chronic inflammation [63]. These findings may imply that cardiac ECs are privileged to share functions with hematopoietic cells, allowing to combine forces with immune cells in events of early acute damage but also on the long-run.
EC may not only partially transition to a hematopoietic fate, but myeloid cells may acquire endothelial cell features. Here, the presence of circulating endothelial cells progenitors (cEPCs) has been debated for long time and hint to interesting links between the endothelial and hematopoietic system [123, 127]. Original research dated back to the 1990s and early 2000s showed the existence of bone marrow derived progenitor cells, which were circulating in the blood stream and were directed to injured vasculature [10, 20]. Difficulties in correct identification of these ECs and controversial results on the long-term incorporation into the vasculature questioned the role of cEPCs as true physical building block contributing to new vessels and suggested that they mainly may act as paracrine source to promote vessel growth [144]. In a model of wire-injured carotid artery transplants, virtually all EC regeneration was mediated by migration of adjacent healthy ECs toward injury [32]. Today, the self-renewal and differentiation capacity of ECs upon injury are discussed to be mainly mediated by specialized ECs rather than invading cells. Whether increased endothelial plasticity can lead to shedding of ECs from damaged vasculature to the blood stream, or activation of migratory phenotypes (as it is known in metastasis), which may result in circulating populations of endothelial cells, is not fully clear. Taken together, the contribution of endothelial plasticity to healing processes, such as clonal expansion and migration, could largely be explained by overlapping similarities with cells of the hematopoietic system.
Endothelial metabolic responses
The heart requires high amounts of energy and oxygen for ATP production. In fact, no other organ consumes metabolically so much energy as the heart. In homeostasis, the majority of ATP is generated via mitochondrial oxidative phosphorylation in cardiomyocytes. While the heart utilizes nearly all kind of energy sources, fatty acids are by far the most frequent [69]. Cardiac ECs have uniquely adapted to the organ demands as they express specific signatures of fatty acid transport, such as CD36 and fatty acid-binding proteins (FABP4, FABP5) [49, 95]. CD36 is required for endothelial up-take and transport of fatty acids to the neighboring cardiomyocytes. Partly, fatty acids are also retained and stored in ECs or used for own energy production. ECs, compared to other cells in the heart, have a relatively low number of mitochondria and do not require mitochondrial oxidative phosphorylation for ATP generation. This makes them more resistant to conditions of tissue ischemia and damage compared to cardiomyocytes [16]. Moreover, at high energy demands, ECs can utilize fatty acids, for DNA synthesis and cell division [62]. Under conditions of ischemia, the induction of HIF and oxygen-sensing mechanisms, such as eNOS or NADPH oxidases activity, augments glycolysis to maintain endothelial energy consumption in the low oxygen environment [7], while fatty acid metabolism is reduced [124]. This adaptation is mediated by various changes in the gene expression program leading to higher abundance of GLUT1 or PFKB3 [77] and repression of transcription factors such as FOXO1, which restrict glycolysis in quiescent ECs [136]. However, sufficient ATP generation in low oxygen levels is not the only advantage of a glycolytic program. Byproducts, such as glucose-6-phosphate, are fueled into the pentose-phosphate pathway to generate ribose-5-phosphate, a rate limiting source for nucleotides, which are required for proliferation. Additionally, hypoxia shifts glutamine metabolism from oxidation to reductive carboxylation [115]. In addition to the hypoxia and HIF-dependent changes in EC glycolytic and amino acid metabolism, genes encoding for fatty acid transport are among the strongest differential regulated genes in cardiac endothelial cells at the early stages after injury with the first 24 h infarction [124]. Loss of FABP4 and CD36 and induction of glycolysis-related genes is associated with increased plasticity and proliferation. In addition, loss of oxygen supply and high energetic requirements for angiogenesis promote de novo biogenesis and fusion of mitochondria [8, 91]. Increased demands for energy lead to ATP exhaustion, causing accumulating levels of AMP activates endothelial AMP-activated protein kinase (AMPK), which promote catabolic pathways in ECs in hypoxia after infarction [24, 137]. AMPK-mediated mitochondrial biogenesis, lipid metabolism and fat mobilization were shown to depend largely on SIRT1, another sensor of energy deprivation [75, 97]. Notably, in cardiac ECs, SIRT1 is known to be upregulated in ischemia induced neovascularization [100]. Downstream, SIRT1 represses FOXO1 and activates PGC-1A, which is known to induce mitochondrial biogenesis [14, 51]. De novo formation of mitochondria was associated with an anti-inflammatory phenotype, and suppressed activity of TNF-α and NFκB [51]. However, it is unclear whether and how mitochondrial biogenesis causally contributes to transient EC adaptation and state changes.
Endothelial-to-mesenchymal transition
Reactivation of developmental pathways can induce phenotypical changes and promote endothelial-to-mesenchymal transition (EndMT). This process has initially been described during valve formation, when ECs acquire mesenchymal characteristics by following TGF-β gradients in the cardiac jelly [82, 83]. Here, TGF-β induction leads to a complete and permanent lineage transition, as these cells continue to be fibroblasts. In adulthood, tissue damage following inflammation and hypoxia modulates the perivascular space favoring similar, yet rather incomplete processes. The degree of transition to mesenchymal lineage after myocardial infarction is highly debated. Initially, ECs undergoing EndMT were thought to contribute to myofibroblasts and hence to fibrosis [141]. Recently, however, single-cell sequencing revealed that mesenchymal gene identity at day 7 post infarction is present in ECs but remains rather insignificant [64]. Parallel lineage tracing of endothelial and mesenchymal identities revealed no EndMT after myocardial infarction depicted by α-SMA and ZEB1 expression [142]. Further studies using more detailed time course analysis raveled that ECs have distinct transcriptional response program to TGF-β in very early phases after injury. This transient mesenchymal activation (EndMA) was reversible at later stages when TGF-β level decline, indicative of a temporal and incomplete process [124]. In contrast to EndMT, cells undergoing EndMA showed only modest up-regulation of mesenchymal genes and no complete transition to fibroblasts or other mesenchymal cells [64, 124]
How is this phenotype initiated? Several cytokines in combination with hypoxia initiate EndMA. TGF-β, produced from cells of the innate immune system in response to tissue damage is one of the most potent inducers. Cardiac macrophages selectively express MMP14, which activates latent TGF-β and induce paracrine signaling in ECs post injury [5]. Additionally, many other drivers of EndMA have been identified, including turbulent flow, hypoxia, glycolysis, WNT signaling, NOTCH signaling or reduction in FGF signaling. Interestingly, pro-inflammatory NFκB activation in EC, as it occurs upon IL-1β stimulation augments transition into a mesenchymal phenotype [76, 143] and induces generation of TGF-β1 and TGF-β2 [86].
ECs undergoing mesenchymal activation after infarction were more glycolytic than naïve ECs, which maintain oxidative phosphorylation and fatty acid transport [124]. In fact, reduced fatty acid oxidation by decreased levels of CPT1A has been identified as inducer of EndMT transition [139]. Increased levels of cytoplasmatic acetyl-CoA suppressed induction of EndMT, indicating that plastic transition toward mesenchymal lineage is inhibited in ECs which maintain normal ATP levels and citric acid cycle activity. In addition, genes regulating fatty acid metabolism and mitochondrial biogenesis such as SIRT1 are counteracting TGF-β induction in ECs [68]. In contrast, increased levels of MYC, a main transcription factor which virtually drives expression of all glycolysis-related genes, promotes EndMT under hypoxic conditions [2].
Another mediator of EndMT is mitochondrial dysfunction and oxidative stress. Elevated levels of ROS promote not only the activation of NFκB, but also suppress KLF4 which deregulates endothelial identity and promotes EndMT [88, 107]. Conversely, hydrogen sulfide, a potent inhibitor of EndMT and scavenger of ROS species was shown to suppress TGF-β signaling [119]. Elevated levels of H2S are well known to induce an anti-inflammatory phenotype in ECs. This suggests that ECs, which are close to the injury site, are characterized by high level of ROS, glycolysis and mitochondrial dysfunction and share an inflammatory phenotype, which favors activation of TGF-β and hence EndMT. It seems reasonable that prolonged inflammation could lead to continuous transition to a complete mesenchymal phenotype and eventually fibrosis. Likewise, the reversible nature of EndMA after myocardial infarction might be a consequence of high endothelial resilience against inflammation and an active conversion to a naïve state. However, how ECs can switch back to the endothelial phenotype is poorly understood.
Cardiomyocyte gene expression in endothelial cells
Single cell sequencing studies have consistently reported the expression of cardiomyocyte-specific gene expression signatures in ECs [64, 124]. While it is still debated whether these detected subpopulations are existing or may be the consequence of mRNA contamination during the single cells isolation procedure [1] some hints point to a possible activation of tissue-specific genes in ECs. Interestingly, Ribo-Seq of brain ECs revealed specific expression of synaptic genes, such as pleiotrophin [47]. In the heart, ECs show open chromatin of certain cardiomyocyte signature loci, express myofibrillar genes and cardiac-specific transcription factor MEF2C [70, 140]. Co-culture of human-induced pluripotent stem cell-derived cardiomyocytes with ECs induced MYL7 and MYL4 expression as well as NOTCH and BMP signaling in endothelial cells [35]. The functions of cardiomyocyte gene expression in healthy or injured endothelium however are unclear.
Angiogenesis and proliferation
Neovascularization requires either sprouting angiogenesis of existing vessels or de novo formation of new blood vessels in the damaged area. Several cell types and origins have been discussed to infiltrate and expand in the tissue upon injury and contribute to new blood vessel formation.
Endocardial cells
Lineage tracing in early post-natal stages revealed significant contribution of endocardial cells to coronary endothelial cells [122]. Hence, endocardial cells were discussed to give rise to ECs after infarction too. Here, Cx-40-based lineage tracing revealed increased plasticity of endocardial cells post infarction concomitant with accumulation of arterial foci (endocardial flowers) which share angiogenic capacity [84]. Contrarily, studies using Npr3-Cre lines revealed that adult endocardial cells, unlike their neonatal counterparts, only minimally contribute to coronary EC populations after myocardial infarction [117]. The presence of trabecular myocardium, a feature of the developing heart which gets lost in adulthood might be required for complete endocardial to endothelial transitions [111]. Vice versa, paracrine activities of ECs are required for development of trabecular myocardium. If such paracrine signals are lacking this can result in left ventricular non-compaction pathologies [105, 131].
Fibroblasts as origin of new vessels
Fibroblasts have also been suggested to acquire endothelial cell phenotypes by undergoing mesenchymal-to-endothelial transition, thereby potentially contributing to de novo vascularization of the infarct zone [125]. However, lately the impact on neovascularization by increased plasticity of fibroblasts was questioned as other groups failed to detect endothelial cells of fibroblast origin in the heart, using PDGFRα or COL1A2 genetic tracing systems [34].
Progenitor cells
Single cell sequencing of cardiac endothelial cells described multiple novel subsets of cells, but tissue-resident cardiac microvascular progenitor populations have not been described [49, 64, 96, 124]. Moreover, the contribution of circulating blood-derived cells for generation of de novo formed vessels also has been challenged as discussed before “Endothelial responses to cell death, hypoxia and inflammation favor interactions with the immune system”. However, there is evidence of specialized EC populations, which reside alongside normal ECs and form new colonies upon tissue damage.
CD157 (BST1) was described to mark such a specialized “stem-cell” endothelial subset [132]. Notably, CD157+ CD200+ ECs were found in large vessels in multiple organs, but not in capillaries. Similarly, rare subpopulations of ECs in the damaged aorta show hyper-proliferative activity associated with increased levels of the bZIP activating transcription factors [83]. ATF3 and ATF4 demark angiogenic microvasculature in the skeletal muscle, most likely due to a metabolic priming, which is present in many subtypes of endothelial cells and not restricted to “stem cells” [22]. Whether these specialized cells contribute to neovascularization of the heart upon myocardial infarction remains to be determined.
Endothelial cells
Several studies suggest that cardiac microvascular ECs undergo plastic changes upon myocardial infarction and vascularize the infarct area. This includes degradation of the basal membrane, elongation toward a VEGF gradient and stabilization of the new vessel by pericytes. Based on the local environmental cues and the given vascular bed, two ways of sprouting angiogenesis appear to exist: random/stochastic contribution of ECs to the sprouts or the formation of truly new vessels by specialized hyper-proliferative cells which clonally expand. In contrast to post-natal retina angiogenesis, which was shown to occur in a stochastic manner, a clonal expansion of cardiac endothelial cells around the infarct area was noted [64, 78]. Interestingly, ECs from clonally expanded vessels showed a partial mesenchymal signature, suggesting that these cells have a privileged role within the EC population [78]. Interestingly, the proliferative potential is not uniform in EC subpopulations across the vascular bed. Postnatally, venous ECs reside in an early G1 phase, while arterial cells pause their cell cycle later at G1, which has implications for the proliferative potential and BMP and TGF-β signaling [17, 18, 81]. Interestingly, inducible deletion of the arterial genes ENG, ALK1 or SMAD4 leads to a hyper-proliferative phenotype associated with an up-regulation of venous specific genes [74, 92, 93]. These findings demonstrate that venous EC have a higher proliferation capacity and hint to a possible role of venous ECs as source for clonal expansion.
ECs in fully regenerative models
To understand how efficient vessel growth can occur after injury and how EC resilience is controlled, we can take a look at fully regenerative species, such as zebrafish or at mice during post-natal stages. In zebrafish, re-establishment of the vascular network after cryoinjury occurs quickly but superficial, which may promote rapid oxygen and nutrient supply to the damaged tissue and additionally foster cardiomyocyte proliferation by 3D scaffold guidance [80]. Importantly, the regenerated vascular network was formed by pre-existing coronary vessels, which sprout toward the side of injury and inflammation. ECs around the infarct zone become glycolytic and activate proliferative programs concomitant with increased expression of Apelin, a known guidance molecule for sprouting endothelial cells [66, 80]. Collectively, sprouting guidance is augmented by hypoxia-dependent expression of cxcl12 and cxcr4 [79]. Increased EC paracrine signaling to the surrounding cells promote organ regeneration and drive cardiomyocyte proliferation to fully regenerate the infarcted tissue. Examples of such paracrine signals range from Wnt repression by Notch activation, Fgf signaling or activation of the endocardium via Pdgf. Moreover, epicardial-derived cells promotes the guidance of blood vessel formation by Cxcl12 and other mechanisms [71, 79]. Interestingly, similar increased EC paracrine signals can be also seen in mammalian responses to infarction [71].
While the regenerative capacity is tightly linked to reactivation of the cell cycle in cardiomyocytes, novel studies using single-cell sequencing revealed important differences in the injury response of endothelial cells to infarction in post-natal and adult mice. Compared to adult hearts, less lipocalin-2 (LCN2+) venous EC populations but higher expression of capillary EC markers were found, which could reflect the immature nature of neonatal blood vessels [134]. In addition, postnatally, epicardial-derived multipotent cells still reside in the epicardium and sub-epicardium [3]. It is clear, that ECs in fully regenerative models of myocardial infarction, such as zebrafish or neonatal mice, differ in the ability to induce a fast and efficient neovascularization of the damaged tissue. Specialized more immature cells, which might be absent in adults, may send paracrine signals to support angiogenesis and EC survival, which seems be key for initiate recovery.
Mechanisms of enhanced endothelial resilience?
Translated on human behavior, the term resilience implies long-term successful adaptations to strong stressors or trauma [109]. This phenomenon represents not a personal trait, but rather can be seen as a summary of the individuals’ responses to biological or social factors which allows adaptive and self-protective behavior. The molecular resilience program of cells works indeed similarly. Resilience of ECs can be seen as the long-term successful adaptation to the combinatorial influence of different stressing factors after myocardial infarction. The onset of cell death, hypoxia, inflammation, changes in metabolism followed by plastic fate transitions and clonal expansion provides a selective and challenging pressure on the heart’s endothelium which might be beneficial for combating long-term heart failure.
Is there a way to increase resistance and resilience? Studies of the last decades have shown that ischemic pre-conditioning, an experimental technique with periodically repeated brief ischemia phases followed by extended reperfusion phases, prevent apoptosis and inflammatory responses [23, 98]. In humans, pre-conditioned individuals were less prone to endothelial dysfunction and neutrophil activation [50]. While many of the effects were eradicated after a limited timeframe post conditioning, there is evidence that some longer lasting structural changes in the vasculature take place.
As shown in different animal models, ischemia in the hind limb also led to beneficial and survival-prolonging effects in subsequent cardiac ischemia [135]. This remote pre-conditioning, utilizes humoral and neuronal mechanisms [38] to protect virtually all organs for secondary ischemic injury [13, 40, 42]. The underlying mechanisms are not fully understood but converge to the global induction of early response cascades of cell stress, such as ROS signaling or adenosine, bradycardic agents or cytokines [37, 58]. Downstream, pre-conditioning leads to altered JAK/STAT3 signaling in ECs which in turn has potential effects on survival pathways, apoptosis and proliferation [31, 60]. Ultimately, cells are likely to establish a mitochondrial protection phenotype, characterized by inhibition of the mitochondrial permeability transition pore (mPTP) [30, 36]. Mitochondria protection may be in part mediated by pre-conditioning induced elevation of mitochondrial telomerase reverse transcriptase, which improves EC functions [4].
Likewise, physical exercise has been described to directly modulate endothelial function by modulating NO bioavailability [133] and decreasing mitochondrial-derived oxidative stress [27, 52]. Training can significantly reduce consequences of aging by decreasing ROS production and increasing anti-oxidative protective mechanisms. This is accompanied by augmented levels of PGC-1α [99]. Curiously, moderate exercise levels influence the vascular protective role to oxidative stress by mildly augmenting ROS levels [89]. This triggers the induction of anti-oxidative signaling, e.g., by augmenting Nrf2 and counteracts existing cardiovascular dysfunction [93, 104]. In addition, pharmacological interventions can interfere with oxidative damage and induce mitochondria protection to mitigate the inflammatory response. Inhibition of angiotensin II pathways are known for their anti-inflammatory and vasculo-protective effects [21] and may contribute to EC resilience. In addition, the anti-diabetic drug metformin was shown to prevent the formation of oxidative mitochondrial DNA and reduced inflammation-related dysfunction of vessels and the lung in other disease models [41, 138].
It may be interesting to gain more insights in long-term cardioprotective and the consequences of pre-conditioning mechanisms or exercise on EC plasticity and clonal expansion.
What if resilience of ECs fails?
Myocardial injury has a major impact on EC phenotypes resulting not only in metabolic adaptation but also in partial fate changes. While it is extensively studied how the injury response is regulated, little is known regarding the resolution of the adaptive responses. Certainly, this is of major importance, since a chronically inflamed endothelium would result in microcirculatory dysfunction, a key pathophysiological component of chronic heart failure. While low levels of ROS act as signaling molecules and may allow temporal adaptation, chronic elevation of ROS, together with reduced NO bioavailability is seen as an early pathological step toward long-term dysfunction [48]. Oxidative stress induced loss of mitochondrial DNA integrity impairs vasodilative capabilities [53]. Likewise, a continuous lack of endothelial fatty acid transporters, as it occurs transiently after infarction, would result in insufficient fatty acid availability resulting in profound changes to cardiomyocyte metabolism [46, 72, 73, 118]. Also, failed metabolic feedback may influence how the vascular tree is built and favors functional shunting [101, 102]. Finally, a chronic mesenchymal activation, due to prolonged or permanent mesenchymal induction may lead to complete transition and, hence fuel cardiac fibrosis and microvascular dysfunction.
How is the transition back to a “healthy” endothelium regulated? One possibility is that the transition is mediated by the normalization of stimuli, which induced the changes in endothelial identity. Thus, a reduction of inflammatory cytokines and the restoration of oxygen supply resulting in a normoxic environment may be sufficient to de-activate ECs allowing the return to the original maintenance program and identify. This assumption is supported by in vitro findings, showing that the sole withdrawal of TGF-β reverted EndMT [124]. The limitation of inflammatory responses in ECs might be mediated by a higher abundance of anti-inflammatory cytokines, which induce resolution of inflammation. In support of a role of anti-inflammatory cytokines previous studies showed that IL-10 treatment improved vascular remodeling [113].
However, the resolution of inflammation may not occur efficiently during aging or in the presence of risk factors. Here, transient inflammation might turn into chronic inflammation a condition referred to as “inflammaging”. Moreover, it is unclear whether epigenetic changes that may occur during inflammatory activation can be fully be restored. Certainly, future work is needed to understand the transient nature of endothelial plasticity.
Acknowledgements
The authors thank the German Research Foundation (DFG) for support (SFB1366 Project B4 and EXC 2026-1).
Abbreviations
- AMPK
AMP-activated protein kinase
- cEPCs
Circulatory endothelial progenitor cells
- ECs
Endothelial cells
- EndICLT
Endothelial-to-immune cell-like transition
- EndMA
Endothelial-to-mesenchymal activation
- EndMT
Endothelial-to-mesenchymal transition
- I/R
Ischemia reperfusion
- MI
Myocardial infarction
- NO
Nitric oxide
- mTPT
Mitochondrial permeability transition pore
- ROS
Reactive oxygen species
Funding
Open Access funding enabled and organized by Projekt DEAL.
Declarations
Funding
This study is funded by Deutsche Forschungsgesellschaft DFG, CRC1366.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Abplanalp WT, Tucker N, Dimmeler S. Single-cell technologies to decipher cardiovascular diseases. Eur Heart J. 2022;00:ehac095. doi: 10.1093/eurheartj/ehac095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguiar RS, Pohl F, Morais GL, Nogueira FCS, Carvalho JB, Guida L, Arge LWP, Melo A, Moreira MEL, Cunha DP, Gomes L, Portari EA, Velasquez E, Melani RD, Pezzuto P, de Castro FL, Geddes VEV, Gerber AL, Azevedo GS, Schamber-Reis BL, Gonçalves AL, Junqueira-De-Azevedo I, Nishiyama MY, Ho PL, Schanoski AS, Schuch V, Tanuri A, Chimelli L, Vasconcelos ZFM, Domont GB, Vasconcelos ATR, Nakaya HI. Molecular alterations in the extracellular matrix in the brains of newborns with congenital Zika syndrome. Sci Signal. 2020;13:eaay6736. doi: 10.1126/scisignal.aaz2597. [DOI] [PubMed] [Google Scholar]
- 3.Aguilar-Sanchez C, Michael M, Pennings S. Cardiac stem cells in the postnatal heart: lessons from development. Stem Cells Int. 2018;2018:1247857. doi: 10.1155/2018/1247857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ale-Agha N, Jakobs P, Goy C, Zurek M, Rosen J, Dyballa-Rukes N, Metzger S, Greulich J, von Ameln F, Eckermann O, Unfried K, Brack F, Grandoch M, Thielmann M, Kamler M, Gedik N, Kleinbongard P, Heinen A, Heusch G, Gödecke A, Altschmied J, Haendeler J. Mitochondrial telomerase reverse transcriptase protects from myocardial ischemia/reperfusion injury by improving complex I composition and function. Circulation. 2021;144:1876–1890. doi: 10.1161/circulationaha.120.051923. [DOI] [PubMed] [Google Scholar]
- 5.Alonso-Herranz L, Sahún-Español Á, Paredes A, Gonzalo P, Gkontra P, Núñez V, Clemente C, Cedenilla M, Villalba-Orero M, Inserte J, García-Dorado D, Arroyo AG, Ricote M. Macrophages promote endothelial-to-mesenchymal transition via MT1-MMP/ TGFβ1 after myocardial infarction. Elife. 2020;9:e57920. doi: 10.7554/eLife.57920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andueza A, Kumar S, Kim J, Kang DW, Mumme HL, Perez JI, Villa-Roel N, Jo H. Endothelial reprogramming by disturbed flow revealed by single-cell RNA and chromatin accessibility study. Cell Rep. 2020;33:108491. doi: 10.1016/j.celrep.2020.108491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aragonés J, Fraisl P, Baes M, Carmeliet P. Oxygen sensors at the crossroad of metabolism. Cell Metab. 2009;9:11–22. doi: 10.1016/j.cmet.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1α. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 9.Arslan F, Smeets MB, O’Neill LAJ, Keogh B, McGuirk P, Timmers L, Tersteeg C, Hoefer IE, Doevendans PA, Pasterkamp G, De Kleijn DPV. Myocardial ischemia/reperfusion injury is mediated by leukocytic toll-like receptor-2 and reduced by systemic administration of a novel anti-toll-like receptor-2 antibody. Circulation. 2010;121:80–90. doi: 10.1161/circulationaha.109.880187. [DOI] [PubMed] [Google Scholar]
- 10.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.RES.85.3.221. [DOI] [PubMed] [Google Scholar]
- 11.Bischoff J, Casanovas G, Wylie-Sears J, Kim DH, Bartko PE, Guerrero JL, Dal-Bianco JP, Beaudoin J, Garcia ML, Sullivan SM, Seybolt MM, Morris BA, Keegan J, Irvin WS, Aikawa E, Levine RA. CD45 expression in mitral valve endothelial cells after myocardial infarction. Circ Res. 2016;119:1215–1225. doi: 10.1161/CIRCRESAHA.116.309598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brill A, Fuchs TA, Chauhan AK, Yang JJ, De Meyer SF, Köllnberger M, Wakefield TW, Lämmle B, Massberg S, Wagner DD. Von Willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood. 2011;117:1400–1407. doi: 10.1182/blood-2010-05-287623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Candilio L, Malik A, Hausenloy DJ. Protection of organs other than the heart by remote ischemic conditioning. J Cardiovasc Med. 2013;14:193–205. doi: 10.2459/JCM.0b013e328359dd7b. [DOI] [PubMed] [Google Scholar]
- 14.Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caparrós E, Juanola O, Gómez-Hurtado I, Puig-Kroger A, Piñero P, Zapater P, Linares R, Tarín F, Martínez-López S, Gracia-Sancho J, González-Navajas JM, Francés R. Liver sinusoidal endothelial cells contribute to hepatic antigen-presenting cell function and Th17 expansion in cirrhosis. Cell J. 2020;9:1227. doi: 10.3390/cells9051227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang X, Lochner A, Wang HH, Wang S, Zhu H, Ren J, Zhou H. Coronary microvascular injury in myocardial infarction: perception and knowledge for mitochondrial quality control. Theranostics. 2021;11:6766–6785. doi: 10.7150/thno.60143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chavkin NW, Genet G, Poulet M, Genet N, Marziano C, Vasavada H, Nelson EA, Kour A, McDonnell SP, Huba M, Walsh K, Hirschi KK. Endothelial cell cycle state determines propensity for arterial-venous fate. bioRxiv. 2020 doi: 10.1101/2020.08.12.246512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen D, Schwartz MA, Simons M. Developmental perspectives on arterial fate specification. Front Cell Dev Biol. 2021;9:1495. doi: 10.3389/fcell.2021.691335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457:887–891. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crosby JR, Kaminski WE, Schatteman G, Martin PJ, Raines EW, Seifert RA, Bowen-Pope DF. Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation. Circ Res. 2000 doi: 10.1161/01.RES.87.9.728. [DOI] [PubMed] [Google Scholar]
- 21.Ekholm M, Kahan T. The impact of the renin-angiotensin-aldosterone system on inflammation, coagulation, and atherothrombotic complications, and to aggravated COVID-19. Front Pharmacol. 2021;12:1534. doi: 10.3389/fphar.2021.640185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan Z, Turiel G, Ardicoglu R, Ghobrial M, Masschelein E, Kocijan T, Zhang J, Tan G, Fitzgerald G, Gorski T, Alvarado-Diaz A, Gilardoni P, Adams CM, Ghesquière B, De Bock K. Exercise-induced angiogenesis is dependent on metabolically primed ATF3/4+ endothelial cells. Cell Metab. 2021;33:1793–1807.e9. doi: 10.1016/j.cmet.2021.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng J, Bianchi C, Sandmeyer JL, Sellke FW. Bradykinin preconditioning improves the profile of cell survival proteins and limits apoptosis after cardioplegic arrest. Circulation. 2005;112:I-190–I-195. doi: 10.1161/circulationaha.104.524454. [DOI] [PubMed] [Google Scholar]
- 24.Fisslthaler B, Fleming I. Activation and signaling by the AMP-activated protein kinase in endothelial cells. Circ Res. 2009;105:114–127. doi: 10.1161/circresaha.109.201590. [DOI] [PubMed] [Google Scholar]
- 25.Forte E, Bastos-Furtado M, Rosenthal N. The interstitium in cardiac repair: role of the immune–stromal cell interplay. Nat Rev Cardiol. 2018 doi: 10.1038/s41569-018-0077-x. [DOI] [PubMed] [Google Scholar]
- 26.Frangogiannis NG. The Immune system and the remodeling infarcted heart. J Cardiovasc Pharmacol. 2014;63:185–195. doi: 10.1097/FJC.0000000000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gioscia-Ryan RA, Clayton ZS, Zigler MC, Richey JJ, Cuevas LM, Rossman MJ, Battson ML, Ziemba BP, Hutton DA, VanDongen NS, Seals DR. Lifelong voluntary aerobic exercise prevents age- and Western diet- induced vascular dysfunction, mitochondrial oxidative stress and inflammation in mice. J Physiol. 2021;599:911–925. doi: 10.1113/JP280607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goncharov NV, Nadeev AD, Jenkins RO, Avdonin PV. Markers and biomarkers of endothelium: when something is rotten in the state. Oxid Med Cell Longev. 2017;2017:9759735. doi: 10.1155/2017/9759735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenspan LJ, Weinstein BM. To be or not to be: endothelial cell plasticity in development, repair, and disease. Angiogenesis. 2021;24:251–269. doi: 10.1007/s10456-020-09761-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griffiths EJ, Halestrap AP. Protection by cyclosporin a of ischemia/reperfusion-induced damage in isolated rat hearts. J Mol Cell Cardiol. 1993;25:1461–1469. doi: 10.1006/jmcc.1993.1162. [DOI] [PubMed] [Google Scholar]
- 31.Hadebe N, Cour M, Lecour S. The SAFE pathway for cardioprotection: is this a promising target? Basic Res Cardiol. 2018;113:9. doi: 10.1007/s00395-018-0670-5. [DOI] [PubMed] [Google Scholar]
- 32.Hagensen MK, Raarup MK, Mortensen MB, Thim T, Nyengaard JR, Falk E, Bentzon JF. Circulating endothelial progenitor cells do not contribute to regeneration of endothelium after murine arterial injury. Cardiovasc Res. 2012;93:223–231. doi: 10.1093/cvr/cvr278. [DOI] [PubMed] [Google Scholar]
- 33.Hall C, Gehmlich K, Denning C, Pavlovic D. Complex relationship between cardiac fibroblasts and cardiomyocytes in health and disease. J Am Heart Assoc. 2021;10:e019338. doi: 10.1161/JAHA.120.019338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He L, Huang X, Kanisicak O, Li Y, Wang Y, Li Y, Pu W, Liu Q, Zhang H, Tian X, Zhao H, Liu X, Zhang S, Nie Y, Hu S, Miao X, Wang QD, Wang F, Chen T, Xu Q, Lui KO, Molkentin JD, Zhou B. Preexisting endothelial cells mediate cardiac neovascularization after injury. J Clin Invest. 2017;127:2968–2981. doi: 10.1172/JCI93868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helle E, Ampuja M, Dainis A, Antola L, Temmes E, Tolvanen E, Mervaala E, Kivelä R. HiPS-endothelial cells acquire cardiac endothelial phenotype in co-culture with hiPS-cardiomyocytes. Front Cell Dev Biol. 2021;9:2135. doi: 10.3389/fcell.2021.715093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hentia C, Rizzato A, Camporesi E, Yang Z, Muntean DM, Săndesc D, Bosco G. An overview of protective strategies against ischemia/reperfusion injury: the role of hyperbaric oxygen preconditioning. Brain Behav. 2018;8:e00959. doi: 10.1002/brb3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heusch G. Heart rate in the pathophysiology of coronary blood flow and myocardial ischaemia: benefit from selective bradycardic agents. Br J Pharmacol. 2008;153:1589–1601. doi: 10.1038/sj.bjp.0707673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heusch G. Molecular basis of cardioprotection. Circ Res. 2015;116:674–699. doi: 10.1161/CIRCRESAHA.116.305348. [DOI] [PubMed] [Google Scholar]
- 39.Heusch G. Coronary microvascular obstruction: the new frontier in cardioprotection. Basic Res Cardiol. 2019;114:1–13. doi: 10.1007/s00395-019-0756-8. [DOI] [PubMed] [Google Scholar]
- 40.Heusch G. Myocardial ischaemia–reperfusion injury and cardioprotection in perspective. Nat Rev Cardiol. 2020;17:773–789. doi: 10.1038/s41569-020-0403-y. [DOI] [PubMed] [Google Scholar]
- 41.Heusch G. Coronary blood flow in heart failure: cause, consequence and bystander. Basic Res Cardiol. 2022;117:1. doi: 10.1007/s00395-022-00909-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heusch G, Bøtker HE, Przyklenk K, Redington A, Yellon D. Remote ischemic conditioning. J Am Coll Cardiol. 2015;65:177–195. doi: 10.1016/j.jacc.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432:625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- 44.Hung J, Teng THK, Finn J, Knuiman M, Briffa T, Stewart S, Sanfilippo FM, Ridout S, Hobbs M. Trends from 1996 to 2007 in incidence and mortality outcomes of heart failure after acute myocardial infarction: a population-based study of 20 812 patients with first acute myocardial infarction in Western Australias. J Am Heart Assoc. 2013;2:e000172. doi: 10.1161/JAHA.113.000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Investigators TGA. The effects of tissue plasminogen activator, streptokinase, or both on coronary-artery patency, ventricular function, and survival after acute myocardial infarction. N Engl J Med. 1993;329:1615–1622. doi: 10.1056/nejm199311253292204. [DOI] [PubMed] [Google Scholar]
- 46.Jabs M, Rose AJ, Lehmann LH, Taylor J, Moll I, Sijmonsma TP, Herberich SE, Sauer SW, Poschet G, Federico G, Mogler C, Weis EM, Augustin HG, Yan M, Gretz N, Schmid RM, Adams RH, Gröne HJ, Hell R, Okun JG, Backs J, Nawroth PP, Herzig S, Fischer A. Inhibition of endothelial notch signaling impairs fatty acid transport and leads to metabolic and vascular remodeling of the adult heart. Circulation. 2018;137:2592–2608. doi: 10.1161/CIRCULATIONAHA.117.029733. [DOI] [PubMed] [Google Scholar]
- 47.Jambusaria A, Hong Z, Zhang L, Srivastava S, Jana A, Toth PT, Dai Y, Malik AB, Rehman J. Endothelial heterogeneity across distinct vascular beds during homeostasis and inflammation. Elife. 2020;9:e51413. doi: 10.7554/eLife.51413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Juguilon C, Wang Z, Wang Y, Enrick M, Jamaiyar A, Xu Y, Gadd J, Chen C-LW, Pu A, Kolz C, Ohanyan V, Chen Y-R, Hardwick J, Zhang Y, Chilian WM, Yin L. Mechanism of the switch from NO to H2O2 in endothelium-dependent vasodilation in diabetes. Basic Res Cardiol. 2022;117:2. doi: 10.1007/s00395-022-00910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalucka J, de Rooij LPMH, Goveia J, Rohlenova K, Dumas SJ, Meta E, Conchinha NV, Taverna F, Teuwen LA, Veys K, García-Caballero M, Khan S, Geldhof V, Sokol L, Chen R, Treps L, Borri M, de Zeeuw P, Dubois C, Karakach TK, Falkenberg KD, Parys M, Yin X, Vinckier S, Du Y, Fenton RA, Schoonjans L, Dewerchin M, Eelen G, Thienpont B, Lin L, Bolund L, Li X, Luo Y, Carmeliet P. Single-cell transcriptome atlas of murine endothelial cells. Cell. 2020;180:764–779.e20. doi: 10.1016/j.cell.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 50.Kharbanda RK, Peters M, Walton B, Kattenhorn M, Mullen M, Klein N, Vallance P, Deanfield J, MacAllister R. Ischemic preconditioning prevents endothelial injury and systemic neutrophil activation during ischemia-reperfusion in humans in vivo. Circulation. 2001;103:1624–1630. doi: 10.1161/01.CIR.103.12.1624. [DOI] [PubMed] [Google Scholar]
- 51.Kim HJ, Park KG, Yoo EK, Kim YH, Kim YN, Kim HS, Kim HT, Park JY, Lee KU, Jang WG, Kim JG, Kim BW, Lee IK. Effects of PGC-1α on TNF-α-induced MCP-1 and VCAM-1 expression and NF-κB activation in human aortic smooth muscle and endothelial cells. Antioxidants Redox Signal. 2007;9:301–307. doi: 10.1089/ars.2006.1456. [DOI] [PubMed] [Google Scholar]
- 52.Kirkman DL, Robinson AT, Rossman MJ, Seals DR, Edwards DG. Mitochondrial contributions to vascular endothelial dysfunction, arterial stiffness, and cardiovascular diseases. Am J Physiol Hear Circ Physiol. 2021;320:H2080–H2100. doi: 10.1152/ajpheart.00917.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kiyooka T, Ohanyan V, Yin L, Pung YF, Chen Y-R, Chen C-L, Kang PT, Hardwick JP, Yun J, Janota D, Peng J, Kolz C, Guarini G, Wilson G, Shokolenko I, Stevens DA, Chilian WM. Mitochondrial DNA integrity and function are critical for endothelium-dependent vasodilation in rats with metabolic syndrome. Basic Res Cardiol. 2022;117:3. doi: 10.1007/s00395-021-00908-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kleinbongard P, Böse D, Baars T, Möhlenkamp S, Konorza T, Schöner S, Elter-Schulz M, Eggebrecht H, Degen H, Haude M, Levkau B, Schulz R, Erbel R, Heusch G. Vasoconstrictor potential of coronary aspirate from patients undergoing stenting of saphenous vein aortocoronary bypass grafts and its pharmacological attenuation. Circ Res. 2011;108:344–352. doi: 10.1161/circresaha.110.235713. [DOI] [PubMed] [Google Scholar]
- 55.Kleinbongard P, Heusch G. A fresh look at coronary microembolization. Nat Rev Cardiol. 2022;19:265–280. doi: 10.1038/s41569-021-00632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kleinbongard P, Heusch G, Schulz R. TNFα in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol Ther. 2010;127:295–314. doi: 10.1016/j.pharmthera.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 57.Kleinbongard P, Konorza T, Böse D, Baars T, Haude M, Erbel R, Heusch G. Lessons from human coronary aspirate. J Mol Cell Cardiol. 2012;52:890–896. doi: 10.1016/j.yjmcc.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 58.Kleinbongard P, Skyschally A, Heusch G. Cardioprotection by remote ischemic conditioning and its signal transduction. Pflugers Arch Eur J Physiol. 2017;469:159–181. doi: 10.1007/s00424-016-1922-6. [DOI] [PubMed] [Google Scholar]
- 59.Kuch B, Bolte HD, Hoermann A, Meisinger C, Loewel H. What is the real hospital mortality from acute myocardial infarction? Epidemiological vs clinical view. Eur Heart J. 2002;23:714–720. doi: 10.1053/euhj.2001.2947. [DOI] [PubMed] [Google Scholar]
- 60.Lacerda L, Somers S, Opie LH, Lecour S. Ischaemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res. 2009;84:201–208. doi: 10.1093/cvr/cvp274. [DOI] [PubMed] [Google Scholar]
- 61.Lafuse WP, Wozniak DJ, Rajaram MVS. Role of cardiac macrophages on cardiac inflammation, fibrosis and tissue repair. Cell J. 2020;10:51. doi: 10.3390/cells10010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li X, Sun X, Carmeliet P. Hallmarks of endothelial cell metabolism in health and disease. Cell Metab. 2019;30:414–433. doi: 10.1016/j.cmet.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 63.Li X, Wang L, Fang P, Sun Y, Jiang X, Wang H, Yang XF. Lysophospholipids induce innate immune transdifferentiation of endothelial cells, resulting in prolonged endothelial activation. J Biol Chem. 2018;293:11033–11045. doi: 10.1074/jbc.RA118.002752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Z, Solomonidis EG, Meloni M, Taylor RS, Duffin R, Dobie R, Magalhaes MS, Henderson BEP, Louwe PA, D’Amico G, Hodivala-Dilke KM, Shah AM, Mills NL, Simons BD, Gray GA, Henderson NC, Baker AH, Brittan M. Single-cell transcriptome analyses reveal novel targets modulating cardiac neovascularization by resident endothelial cells following myocardial infarction. Eur Heart J. 2019;40:2507–2520. doi: 10.1093/eurheartj/ehz305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Litviňuková M, Talavera-López C, Maatz H, Reichart D, Worth CL, Lindberg EL, Kanda M, Polanski K, Heinig M, Lee M, Nadelmann ER, Roberts K, Tuck L, Fasouli ES, DeLaughter DM, McDonough B, Wakimoto H, Gorham JM, Samari S, Mahbubani KT, Saeb-Parsy K, Patone G, Boyle JJ, Zhang H, Zhang H, Viveiros A, Oudit GY, Bayraktar OA, Seidman JG, Seidman CE, Noseda M, Hubner N, Teichmann SA. Cells of the adult human heart. Nature. 2020;588:466–472. doi: 10.1038/s41586-020-2797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Q, Hu T, He L, Huang X, Tian X, Zhang H, He L, Pu W, Zhang L, Sun H, Fang J, Yu Y, Duan S, Hu C, Hui L, Zhang H, Quertermous T, Xu Q, Red-Horse K, Wythe JD, Zhou B. Genetic targeting of sprouting angiogenesis using Apln-CreER. Nat Commun. 2015;6:6020. doi: 10.1038/ncomms7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Y, Cox SR, Morita T, Kourembanas S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5’ enhancer. Circ Res. 1995;77:638–643. doi: 10.1161/01.RES.77.3.638. [DOI] [PubMed] [Google Scholar]
- 68.Liu ZH, Zhang Y, Wang X, Fan XF, Zhang Y, Li X, Gong YS, Han LP. SIRT1 activation attenuates cardiac fibrosis by endothelial-to-mesenchymal transition. Biomed Pharmacother. 2019;118:109227. doi: 10.1016/j.biopha.2019.109227. [DOI] [PubMed] [Google Scholar]
- 69.Lopaschuk GD, Karwi QG, Tian R, Wende AR, Abel ED. Cardiac energy metabolism in heart failure. Circ Res. 2021;128:1487–1513. doi: 10.1161/CIRCRESAHA.121.318241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lother A, Bergemann S, Deng L, Moser M, Bode C, Hein L. Cardiac endothelial cell transcriptome. Arterioscler Thromb Vasc Biol. 2018;38:566–574. doi: 10.1161/ATVBAHA.117.310549. [DOI] [PubMed] [Google Scholar]
- 71.Lowe V, Wisniewski L, Pellet-Many C. The zebrafish cardiac endothelial cell—roles in development and regeneration. J Cardiovasc Dev Dis. 2021;8:49. doi: 10.3390/jcdd8050049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luxán G, Dimmeler S. The vasculature: a therapeutic target in heart failure? Cardiovasc Res. 2022;118:53–64. doi: 10.1093/cvr/cvab047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luxán G, Stewen J, Díaz N, Kato K, Maney SK, Aravamudhan A, Berkenfeld F, Nagelmann N, Drexler HCA, Zeuschner D, Faber C, Schillers H, Hermann S, Wiseman J, Vaquerizas JM, Pitulescu ME, Adams RH. Endothelial EphB4 maintains vascular integrity and transport function in adult heart. Elife. 2019;8:e45863. doi: 10.7554/eLife.45863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mahmoud M, Allinson KR, Zhai Z, Oakenfull R, Ghandi P, Adams RH, Fruttiger M, Arthur HM. Pathogenesis of arteriovenous malformations in the absence of endoglin. Circ Res. 2010;106:1425–1433. doi: 10.1161/circresaha.109.211037. [DOI] [PubMed] [Google Scholar]
- 75.Majeed Y, Halabi N, Madani AY, Engelke R, Bhagwat AM, Abdesselem H, Agha MV, Vakayil M, Courjaret R, Goswami N, Ben HH, Elrayess MA, Rafii A, Graumann J, Schmidt F, Mazloum NA. SIRT1 promotes lipid metabolism and mitochondrial biogenesis in adipocytes and coordinates adipogenesis by targeting key enzymatic pathways. Sci Rep. 2021;11:8177. doi: 10.1038/s41598-021-87759-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maleszewska M, Moonen JRAJ, Huijkman N, van de Sluis B, Krenning G, Harmsen MC. IL-1β and TGFβ2 synergistically induce endothelial to mesenchymal transition in an NFκB-dependent manner. Immunobiology. 2013;218:443–454. doi: 10.1016/j.imbio.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 77.Maltepe E, Schmidt JV, Baunoch D, Bradfield CA, Simon MC. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature. 1997;386:403–407. doi: 10.1038/386403a0. [DOI] [PubMed] [Google Scholar]
- 78.Manavski Y, Lucas T, Glaser SF, Dorsheimer L, Günther S, Braun T, Rieger MA, Zeiher AM, Boon RA, Dimmeler S. Clonal expansion of endothelial cells contributes to ischemia-induced neovascularization. Circ Res. 2018;122:670–677. doi: 10.1161/CIRCRESAHA.117.312310. [DOI] [PubMed] [Google Scholar]
- 79.Marín-Juez R, El-Sammak H, Helker CSM, Kamezaki A, Mullapuli ST, Bibli SI, Foglia MJ, Fleming I, Poss KD, Stainier DYR. Coronary revascularization during heart regeneration is regulated by epicardial and endocardial cues and forms a scaffold for cardiomyocyte repopulation. Dev Cell. 2019;51:503–515.e4. doi: 10.1016/j.devcel.2019.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marín-Juez R, Marass M, Gauvrit S, Rossi A, Lai SL, Materna SC, Black BL, Stainier DYR. Fast revascularization of the injured area is essential to support zebrafish heart regeneration. Proc Natl Acad Sci U S A. 2016;113:11237–11242. doi: 10.1073/pnas.1605431113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marziano C, Genet G, Hirschi KK. Vascular endothelial cell specification in health and disease. Angiogenesis. 2021;24:213–236. doi: 10.1007/s10456-021-09785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McCarroll CS, He W, Foote K, Bradley A, McGlynn K, Vidler F, Nixon C, Nather K, Fattah C, Riddell A, Bowman P, Elliott EB, Bell M, Hawksby C, MacKenzie SM, Morrison LJ, Terry A, Blyth K, Smith GL, McBride MW, Kubin T, Braun T, Nicklin SA, Cameron ER, Loughrey CM. Runx1 deficiency protects against adverse cardiac remodeling after myocardial infarction. Circulation. 2018;137:57–70. doi: 10.1161/circulationaha.117.028911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McDonald AI, Shirali AS, Aragón R, Ma F, Hernandez G, Vaughn DA, Mack JJ, Lim TY, Sunshine H, Zhao P, Kalinichenko V, Hai T, Pelegrini M, Ardehali R, Iruela-Arispe ML. Endothelial regeneration of large vessels is a biphasic process driven by local cells with distinct proliferative capacities. Cell Stem Cell. 2018;23:210–225.e6. doi: 10.1016/j.stem.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miquerol L, Thireau J, Bideaux P, Sturny R, Richard S, Kelly RG. Endothelial plasticity drives arterial remodeling within the endocardium after myocardial infarction. Circ Res. 2015;116:1765–1771. doi: 10.1161/CIRCRESAHA.116.306476. [DOI] [PubMed] [Google Scholar]
- 85.Moccetti F, Brown E, Xie A, Packwood W, Qi Y, Ruggeri Z, Shentu W, Chen J, López JA, Lindner JR. Myocardial infarction produces sustained proinflammatory endothelial activation in remote arteries. J Am Coll Cardiol. 2018;72:1015–1026. doi: 10.1016/j.jacc.2018.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Montorfano I, Becerra A, Cerro R, Echeverría C, Sáez E, Morales MG, Fernández R, Cabello-Verrugio C, Simon F. Oxidative stress mediates the conversion of endothelial cells into myofibroblasts via a TGF-b1 and TGF-b2-dependent pathway. Lab Investig. 2014;94:1068–1082. doi: 10.1038/labinvest.2014.100. [DOI] [PubMed] [Google Scholar]
- 87.Munoz-Chapuli R, Carmona R, Guadix JA, Macias D, Perez-Pomares JM. The origin of the endothelial cells: an evo-devo approach for the invertebrate/vertebrate transition of the circulatory system. Evol Dev. 2005;7:351–358. doi: 10.1111/j.1525-142X.2005.05040.x. [DOI] [PubMed] [Google Scholar]
- 88.Murdoch CE, Chaubey S, Zeng L, Yu B, Ivetic A, Walker SJ, Vanhoutte D, Heymans S, Grieve DJ, Cave AC, Brewer AC, Zhang M, Shah AM. Endothelial NADPH oxidase-2 promotes interstitial cardiac fibrosis and diastolic dysfunction through proinflammatory effects and endothelial- mesenchymal transition. J Am Coll Cardiol. 2014;63:2734–2741. doi: 10.1016/j.jacc.2014.02.572. [DOI] [PubMed] [Google Scholar]
- 89.Narasimhan M, Rajasekaran NS. Exercise, Nrf2 and antioxidant signaling in cardiac aging. Front Physiol. 2016;7:241. doi: 10.3389/fphys.2016.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Naß J, Terglane J, Gerke V. Weibel palade bodies: unique secretory organelles of endothelial cells that control blood vessel homeostasis. Front Cell Dev Biol. 2021;9:3621. doi: 10.3389/fcell.2021.813995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 92.Ola R, Dubrac A, Han J, Zhang F, Fang JS, Larrivée B, Lee M, Urarte AA, Kraehling JR, Genet G, Hirschi KK, Sessa WC, Canals FV, Graupera M, Yan M, Young LH, Oh PS, Eichmann A. PI3 kinase inhibition improves vascular malformations in mouse models of hereditary haemorrhagic telangiectasia. Nat Commun. 2016;7:13650. doi: 10.1038/ncomms13650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ola R, Künzel SH, Zhang F, Genet G, Chakraborty R, Pibouin-Fragner L, Martin K, Sessa W, Dubrac A, Eichmann A. SMAD4 prevents flow induced arteriovenous malformations by inhibiting casein kinase 2. Circulation. 2018;138:2379–2394. doi: 10.1161/circulationaha.118.033842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oyama JI, Blais C, Liu X, Pu M, Kobzik L, Kelly RA, Bourcier T. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation. 2004;109:784–789. doi: 10.1161/01.CIR.0000112575.66565.84. [DOI] [PubMed] [Google Scholar]
- 95.Paik DT, Tian L, Williams IM, Rhee S, Zhang H, Liu C, Mishra R, Wu SM, Red-Horse K, Wu JC. Single-cell RNA sequencing unveils unique transcriptomic signatures of organ-specific endothelial cells. Circulation. 2020;142:1848–1862. doi: 10.1161/circulationaha.119.041433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pasut A, Becker LM, Cuypers A, Carmeliet P. Endothelial cell plasticity at the single-cell level. Angiogenesis. 2021;24:311–326. doi: 10.1007/s10456-021-09797-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, De Oliveira RM, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-γ. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pickard JMJ, Bøtker HE, Crimi G, Davidson B, Davidson SM, Dutka D, Ferdinandy P, Ganske R, Garcia-Dorado D, Giricz Z, Gourine AV, Heusch G, Kharbanda R, Kleinbongard P, MacAllister R, McIntyre C, Meybohm P, Prunier F, Redington A, Robertson NJ, Suleiman MS, Vanezis A, Walsh S, Yellon DM, Hausenloy DJ. Remote ischemic conditioning: from experimental observation to clinical application: report from the 8th Biennial Hatter Cardiovascular Institute Workshop. Basic Res Cardiol. 2015;110:453. doi: 10.1007/s00395-014-0453-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pierce GL, Donato AJ, Larocca TJ, Eskurza I, Silver AE, Seals DR. Habitually exercising older men do not demonstrate age-associated vascular endothelial oxidative stress. Aging Cell. 2011;10:1032–1037. doi: 10.1111/j.1474-9726.2011.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, Haendeler J, Mione M, Dejana E, Alt FW, Zeiher AM, Dimmeler S. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007;21:2644–2658. doi: 10.1101/gad.435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pries AR, Reglin B. Coronary microcirculatory pathophysiology: can we afford it to remain a black box? Eur Heart J. 2017;38:478–488. doi: 10.1093/eurheartj/ehv760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reglin B, Pries AR. Metabolic control of microvascular networks: oxygen sensing and beyond. J Vasc Res. 2014;51:376–392. doi: 10.1159/000369460. [DOI] [PubMed] [Google Scholar]
- 103.Reimer KA, Lowe JE, Rasmussen MM, Jennings RB. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977;56:786–794. doi: 10.1161/01.CIR.56.5.786. [DOI] [PubMed] [Google Scholar]
- 104.Reuland DJ, McCord JM, Hamilton KL. The role of Nrf2 in the attenuation of cardiovascular disease. Exerc Sport Sci Rev. 2013;41:162–168. doi: 10.1097/JES.0b013e3182948a1e. [DOI] [PubMed] [Google Scholar]
- 105.Rhee S, Paik DT, Yang JY, Nagelberg D, Williams I, Tian L, Roth R, Chandy M, Ban J, Belbachir N, Kim S, Zhang H, Phansalkar R, Wong KM, King DA, Valdez C, Winn VD, Morrison AJ, Wu JC, Red-Horse K. Endocardial/endothelial angiocrines regulate cardiomyocyte development and maturation and induce features of ventricular non-compaction. Eur Heart J. 2021;42:4264–4276. doi: 10.1093/eurheartj/ehab298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rose ML, Page C, Hengstenberg C, Yacoub MH. Identification of antigen presenting cells in normal and transplanted human heart: importance of endothelial cells. Hum Immunol. 1990;28:179–185. doi: 10.1016/0198-8859(90)90017-J. [DOI] [PubMed] [Google Scholar]
- 107.Rosencrans WM, Walsh ZH, Houerbi N, Blum A, Belew M, Liu C, Chernak B, Brauer PR, Trazo A, Olson A, Hagos E. Cells deficient for Krüppel-like factor 4 exhibit mitochondrial dysfunction and impaired mitophagy. Eur J Cell Biol. 2020;99:151061. doi: 10.1016/j.ejcb.2019.151061. [DOI] [PubMed] [Google Scholar]
- 108.Rudd MA, Johnstone MT, Rabbani LRE, George D, Ware JA, Loscalzo J. Thrombolytic therapy causes an increase in vascular permeability that is reversed by 1-deamino- 8-D-vasopressin. Circulation. 1991;84:2568–2573. doi: 10.1161/01.CIR.84.6.2568. [DOI] [PubMed] [Google Scholar]
- 109.Russo SJ, Murrough JW, Han M-H, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15:1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Santoro MM. Fashioning blood vessels by ROS signalling and metabolism. Semin Cell Dev Biol. 2018;80:35–42. doi: 10.1016/j.semcdb.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 111.Sedmera D, Pexieder T, Vuillemin M, Thompson RP, Anderson RH. Developmental patterning of the myocardium. Anat Rec. 2000;258:319–337. doi: 10.1002/(SICI)1097-0185(20000401)258:4. [DOI] [PubMed] [Google Scholar]
- 112.Shetty S, Lalor PF, Adams DH. Liver sinusoidal endothelial cells—gatekeepers of hepatic immunity. Nat Rev Gastroenterol Hepatol. 2018;15:555–567. doi: 10.1038/s41575-018-0020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stumpf C, Seybold K, Petzi S, Wasmeier G, Raaz D, Yilmaz A, Anger T, Daniel WG, Garlichs CD. Interleukin-10 improves left ventricular function in rats with heart failure subsequent to myocardial infarction. Eur J Heart Fail. 2008;10:733–739. doi: 10.1016/j.ejheart.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 114.Sulo G, Igland J, Vollset SE, Nygård O, Ebbing M, Sulo E, Egeland GM, Tell GS. Heart failure complicating acute myocardial infarction; burden and timing of occurrence: A nation-wide analysis including 86,771 patients from the cardiovascular disease in Norway (CVDNOR) project. J Am Heart Assoc. 2016;5:e002667. doi: 10.1161/JAHA.115.002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sun RC, Denko NC. Hypoxic regulation of glutamine metabolism through HIF1 and SIAH2 supports lipid synthesis that is necessary for tumor growth. Cell Metab. 2014;19:285–292. doi: 10.1016/j.cmet.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Talman V, Kivelä R. Cardiomyocyte—endothelial cell interactions in cardiac remodeling and regeneration. Front Cardiovasc Med. 2018;5:101. doi: 10.3389/fcvm.2018.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tang J, Zhang H, He L, Huang X, Li Y, Pu W, Yu W, Zhang L, Cai D, Lui KO, Zhou B. Genetic fate mapping defines the vascular potential of endocardial cells in the adult heart. Circ Res. 2018;122:984–993. doi: 10.1161/circresaha.117.312354. [DOI] [PubMed] [Google Scholar]
- 118.Taylor J, Fischer A. Endothelial cells dictate cardiac fuel source. Aging (Albany NY) 2019;11:1083–1084. doi: 10.18632/aging.101825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Testai L, Brancaleone V, Flori L, Montanaro R, Calderone V. Modulation of EndMT by hydrogen sulfide in the prevention of cardiovascular fibrosis. Antioxidants. 2021;10:910. doi: 10.3390/antiox10060910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Theofilis P, Sagris M, Oikonomou E, Antonopoulos AS, Siasos G, Tsioufis C, Tousoulis D. Inflammatory mechanisms contributing to endothelial dysfunction. Biomedicines. 2021;9:781. doi: 10.3390/biomedicines9070781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 2010;10:753–766. doi: 10.1038/nri2858. [DOI] [PubMed] [Google Scholar]
- 122.Tian X, Pu WT, Zhou B. Cellular origin and developmental program of coronary angiogenesis. Circ Res. 2015;116:515–530. doi: 10.1161/CIRCRESAHA.116.305097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Timmermans F, Plum J, Yöder MC, Ingram DA, Vandekerckhove B, Case J. Endothelial progenitor cells: identity defined? J Cell Mol Med. 2008;13:87–102. doi: 10.1111/j.1582-4934.2008.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tombor LS, John D, Glaser SF, Luxán G, Forte E, Furtado M, Rosenthal N, Baumgarten N, Schulz MH, Wittig J, Rogg E-M, Manavski Y, Fischer A, Muhly-Reinholz M, Klee K, Looso M, Selignow C, Acker T, Bibli S-I, Fleming I, Patrick R, Harvey RP, Abplanalp WT, Dimmeler S. Single cell sequencing reveals endothelial plasticity with transient mesenchymal activation after myocardial infarction. Nat Commun. 2021;12:681. doi: 10.1038/s41467-021-20905-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ubil E, Duan J, Pillai ICL, Rosa-Garrido M, Wu Y, Bargiacchi F, Lu Y, Stanbouly S, Huang J, Rojas M, Vondriska TM, Stefani E, Deb A. Mesenchymal-endothelial transition contributes to cardiac neovascularization. Nature. 2014;514:585–590. doi: 10.1038/nature13839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ullah K, Wu R. Hypoxia-inducible factor regulates endothelial metabolism in cardiovascular disease. Front Physiol. 2021;12:832. doi: 10.3389/fphys.2021.670653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Urbich C, Dimmeler S. Endothelial progenitor cells. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 128.de Villiers C, Riley PR. Mouse models of myocardial infarction: Comparing permanent ligation and ischaemia-reperfusion. DMM Dis Model Mech. 2020;13:dmm046565. doi: 10.1242/dmm.046565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Volmink JA, Newton JN, Hicks NR, Sleight P, Fowler GH, Neil HAW. Coronary event and case fatality rates in an English population: results of the Oxford myocardial infarction incidence study. HEARFR. 1998;80:40–44. doi: 10.1136/hrt.80.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wagner DD, Olmsted JB, Marder VJ. Immunolocalization of von willebrand protein in weibei-palade bodies of human endothelial cells. J Cell Biol. 1982;95:355–360. doi: 10.1083/jcb.95.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wagner JUG, Dimmeler S. The endothelial niche in heart failure: from development to regeneration. Eur Heart J. 2021;42:4277–4279. doi: 10.1093/eurheartj/ehab304. [DOI] [PubMed] [Google Scholar]
- 132.Wakabayashi T, Naito H, Suehiro J, Lin Y, Kawaji H, Iba T, Kouno T, Ishikawa-Kato S, Furuno M, Takara K, Muramatsu F, Weizhen J, Kidoya H, Ishihara K, Hayashizaki Y, Nishida K, Yoder MC, Takakura N. CD157 marks tissue-resident endothelial stem cells with homeostatic and regenerative properties. Cell Stem Cell. 2018;22:384–397.e6. doi: 10.1016/j.stem.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 133.Walther C, Gielen S, Hambrecht R. The effect of exercise training on endothelial function in cardiovascular disease in humans. Exerc Sport Sci Rev. 2004;32:129–134. doi: 10.1097/00003677-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 134.Wang Z, Cui M, Shah AM, Tan W, Liu N, Bassel-Duby R, Olson EN. Cell-type-specific gene regulatory networks underlying murine neonatal heart regeneration at single-cell resolution. Cell Rep. 2020;33:108472. doi: 10.1016/j.celrep.2020.108472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wei M, Xin P, Li S, Tao J, Li Y, Li J, Liu M, Li J, Zhu W, Redington AN. Repeated remote ischemic postconditioning protects against adverse left ventricular remodeling and improves survival in a rat model of myocardial infarction. Circ Res. 2011;108:1220–1225. doi: 10.1161/CIRCRESAHA.110.236190. [DOI] [PubMed] [Google Scholar]
- 136.Wilhelm K, Happel K, Eelen G, Schoors S, Oellerich MF, Lim R, Zimmermann B, Aspalter IM, Franco CA, Boettger T, Braun T, Fruttiger M, Rajewsky K, Keller C, Brüning JC, Gerhardt H, Carmeliet P, Potente M. FOXO1 couples metabolic activity and growth state in the vascular endothelium. Nature. 2016;529:216–220. doi: 10.1038/nature16498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wu S, Zou M-H. AMPK, mitochondrial function, and cardiovascular disease. Int J Mol Sci. 2020;21:4987. doi: 10.3390/ijms21144987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xian H, Liu Y, Rundberg Nilsson A, Gatchalian R, Crother TR, Tourtellotte WG, Zhang Y, Aleman-Muench GR, Lewis G, Chen W, Kang S, Luevanos M, Trudler D, Lipton SA, Soroosh P, Teijaro J, de la Torre JC, Arditi M, Karin M, Sanchez-Lopez E. Metformin inhibition of mitochondrial ATP and DNA synthesis abrogates NLRP3 inflammasome activation and pulmonary inflammation. Immunity. 2021;54:1463–1477.e11. doi: 10.1016/J.IMMUNI.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xiong J, Kawagishi H, Yan Y, Liu J, Wells QS, Edmunds LR, Fergusson MM, Yu ZX, Rovira II, Brittain EL, Wolfgang MJ, Jurczak MJ, Fessel JP, Finkel T. A metabolic basis for endothelial-to-mesenchymal transition. Mol Cell. 2018;69:689–698.e7. doi: 10.1016/j.molcel.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yucel N, Axsom J, Yang Y, Li L, Rhoades JH, Arany Z. Cardiac endothelial cells maintain open chromatin and expression of cardiomyocyte myofibrillar genes. Elife. 2020;9:e55730. doi: 10.7554/eLife.55730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 142.Zhang S, Li Y, Huang X, Liu K, Wang QD, Chen AF, Sun K, Lui KO, Zhou B. Seamless genetic recording of transiently activated mesenchymal gene expression in endothelial cells during cardiac fibrosis. Circulation. 2021;144:2004–2020. doi: 10.1161/CIRCULATIONAHA.121.055417. [DOI] [PubMed] [Google Scholar]
- 143.Zhao P, Yao Q, Zhang PJ, The E, Zhai Y, Ao L, Jarrett MJ, Dinarello CA, Fullerton DA, Meng X. Single-cell RNA-seq reveals a critical role of novel pro-inflammatory EndMT in mediating adverse remodeling in coronary artery-on-a-chip. Sci Adv. 2021;7:eabg1694. doi: 10.1126/sciadv.abg1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, Schaper W. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004;94:230–238. doi: 10.1161/01.RES.0000110419.50982.1C. [DOI] [PubMed] [Google Scholar]
- 145.Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y, Becker MS, Zanetta L, Dejana E, Gasson JC, Tallquist MD, Iruela-Arispe ML. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell. 2008;3:625–636. doi: 10.1016/J.STEM.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zuchi C, Tritto I, Carluccio E, Mattei C, Cattadori G, Ambrosio G. Role of endothelial dysfunction in heart failure. Heart Fail Rev. 2020;25:21–30. doi: 10.1007/s10741-019-09881-3. [DOI] [PubMed] [Google Scholar]