Abstract
Objective
To investigate the effect of P2X7R on MSU crystal-induced acute gouty arthritis in rats and its mechanism on inflammatory responses.
Methods
In vivo activation or inhibition of P2X7R was examined in the ATP group or the BBG group of rats, and the control group were injected with PBS. All three groups of rats were injected with MSU in the right joint cavity. The development of acute gouty arthritis was observed and evaluated at 6h, 12h, 24h, 48h and 72h. The clinical manifestations of acute arthritis, the expression level of P2X7R in spleen macrophages, the ability of macrophages to take up YO-PRO-1, and the level of Tregs, Th17 cells and inflammatory cytokines were assessed. Besides, mRNA expression levels of P2X7R, NLRP3 and IL-1β were also detected.
Results
After 12h and 24h administration, P2X7R agonist ATP significantly accelerated the development of acute gouty arthritis, while the P2X7R inhibitor BBG had the opposite effect on this process. Activation of P2X7R significantly aggravated the ankle joint arthritis of the rat and promoted the infiltration of neutrophils and macrophages in the synovial tissue. In addition, the expression of P2X7R in macrophages of ATP group, the uptake of YO-PRO-1 and the expression of NLRP3 mRNA were significantly higher than that in other two groups. At 12h or 24h, activation or inhibition P2X7R had a significant effect on the IL-1β, IL-6, IL-17, IL-10 and TGF-β1. The ratios of Treg/Th17 gradually decreased in the First three time points, it was the lowest at 24h.
Conclusion
Activation of P2X7R by ATP aggravated the development of acute gouty arthritis through P2X7R/NLRP3 pathway, promoted the secretion of related inflammatory cytokines, which affected radio of Tregs/Th17 cells. The whole pathogenesis process appeared a pattern from acute attack to remission in time-dependent trend.
Keywords: purinergic receptor P2X ligand-gated ion channel 7, adenosine triphosphate, acute gouty arthritis, rat
Introduction
Acute gouty arthritis is triggered by monosodium urate (MSU) crystal deposition in and around joints that activates resident macrophages into an inflammatory state with production of proinflammatory cytokines including IL-1β,1 TNF-ɑ and IL-6, which further contribute to neutrophils recruitment.2,3 It is generally believed that MSU crystals stimulate IL-1β secretion and causes joint swelling and pain to be the major mechanism in pathogenesis of gout, but this mechanism does not explain why the majority of patients with hyperuricemia do not have gout attacks. Previous studies had found that adenosine triphosphate (ATP) stimulated production of IL-1β in peripheral blood leukocytes of patients with gout was higher than that of the patients with hyperuricemia without gout, and the gene polymorphism of ATP receptor (purinergic receptor P2X ligand-gated ion channel 7, P2X7R) is significantly associated with the incidence of gout.4 Therefore, ATP has been considered as the second pathogenic signal of gout, and P2X7R function may determines which patients with hyperuricemia will have gout attack.5
ATP is a direct source of energy in organisms. When the internal environment is stable, the extracellular concentration is low, however, under pathological conditions, ATP acts as a Danger Associated Molecular Patterns (DAMPs) and can bind to purinergic receptors (P2 receptors) and initiate a signal transduction cascade to induce inflammation.6–8 Previous studies had found that the release of ATP and purine signaling pathways were related to the activation of crystal-mediated inflammasomes.9
P2X7R, as an important member of the P2X family, is an ATP-gated cation channel. Moreover, P2X7R has unique dual functionality: 1) it is activated by ATP to form a non-selective cation channel, allowing sodium, potassium, calcium and other cations to flow across the membrane; 2) under the stimulation of high concentrations of ATP, P2X7R has the ability to form a wide range of non-selective membrane pores in macrophages, which is sufficient to allow passage of substances with a molecular weight of 900 kDa.10 P2X7R is ubiquitously expressed in immune cells of all tissues and organs of the human body, especially on monocytes or macrophages, and plays an important role in innate immunity.11 P2X7R is the most relevant to the inflammatory response in the P2X family. It activates the Nod-like receptor family proteins to regulate the activation of nucleotide-binding oligomerization domain, leucine rich repeat and pyrin domain containing (NLRP3) inflammatory bodies and promotes the release of IL-1β. Therefore, it is speculated that P2X7R may be involved in the occurrence of acute gouty arthritis.
The aim of this study was to investigate the role of P2X7R in the pathogenesis of acute gouty arthritis induced by MSU crystals. We found that activation of P2X7R by ATP aggravated the progress of acute gouty arthritis through P2X7R/NLRP3 pathway, promoted the secretion of related cytokines, which affected Tregs and Th17 cell balance.
Materials and Methods
Rat
Male Sprague–Dawley rats (220±20 g) were obtained from Anhui Experimental Animal Center (Anhui, China). Before the experiments started, the rats were acclimated for one week in temperature and humidity-controlled room with a 12h alternating light-dark cycle at Animal Care Commission of Anhui Provincial Hospital. All rats were given standard chow and water was freely available for the duration of the study. This study was approved by the Animal Care and Use Committee of the First Affiliated Hospital of University of Science and Technology of China (Approval No: 20160222). All animal experiments were conducted in accordance with the ethical standards of the First Affiliated Hospital of University of Science and Technology of China.
MSU Crystals Preparation
Dissolve 0.4g of NaOH in 100mL of distilled water to make a 0.1mol/L NaOH solution, and place it on a heating electric furnace. Slowly add 1.5g of uric acid (Sigma, St Louis, MO, USA) to the beaker, and keep stirring until the uric acid is completely dissolved to obtain a clear liquid (alkaline solution). After being placed at room temperature at 4°C overnight, crystals can be seen to precipitate, and slowly titrated with dilute hydrochloric acid to pH 7.2–7.4, a large number of crystals can be seen (length 5–25um, needle-tip rod-shaped, with birefringence).12 Before the experiment, dissolve the MSU crystals in a certain amount of PBS solution according to the experimental concentration requirements, and use the limulus test (Sigma, St Louis, MO, USA) to detect whether it contains endotoxin.
The Rat Model of Acute Gout Arthritis and Grouping
After weighed and labeled, the rats were divided into 4 groups based on the randomized table: Normal group (n=8), ATP group (n=40), Brilliant Blue G group (BBG group, n=40) and control group (n=40), the latter three groups were called experimental group (The rats in the experimental group were all model rats, namely the model group). The rat models of acute gouty arthritis were induced as follows: MSU crystals were mixed in PBS, and a 100μL volume of 4 mg of MSU suspension was injected into the right ankle joint of each rat through a 1mL syringe with its tip beveled to 45° while they were under 10% chloral hydrate anesthesia.13 After modeling, rats in ATP group, BBG group and Control group were injected with equal dose (500 ul) of P2X7R agonist ATP (10mmol/L) (Sigma, St. Louis, MO, USA), P2X7R inhibitor BBG (50 mg/kg of body weight) (Sigma, St. Louis, MO, USA) and PBS (China) via tail vein, respectively. At 6h, 12h, 24h, 48h and 72h after drugs injection, 8 rats were randomly selected from ATP group, BBG group and control group at each time point to be sacrificed. Rats in Normal group were injected with equal solution without MSU crystals at the same site and sacrificed together with rats at 6h.
Assessment of Acute Gout Arthritis
Before each group (ATP group, BBG group or control group) of rats was sacrificed at each time point (6h, 12h, 24h, 48h and 72h), the circumference of the joints at different time points before and after the inflammation was measured, and the clinical manifestations of the right ankle joints of the rats were observed. The assessment of acute gouty arthritis as mentioned earlier, mainly includes swelling index and clinical score.14 Briefly, Swelling index= (Measurement of time point of joint circumference - initial circumference)/initial circumference. Clinical changes were scored on a scale of 0–3:15 0=Normal,1=erythema and mild swelling confined to the ankle joint, visible signs of bone, mild limping, 2=erythema and obvious swelling confined to the ankle joint, disappearance of bony signs, obvious limping, 3=erythema and severe swelling extending from the ankle to limb, foot completely off the ground, three-legged gait.
Histologic Examination
Rats were sacrificed at each time point and their right ankle joints were fixed in 10% formalin and then decalcified overnight in decalcified solution. The ankle joint was cut open from the sagittal plane and embedded in paraffin. The sections were cut to 2μm, deparaffinized and stained with Hematoxylin and eosin (H&E). The HE slides were observed and photographed by light microscope (LeicaDM4000, Solms, Germany). The degree of synovial infiltration of neutrophils was assessed by manual counting,16 scored 0–4:0 = normal, 1 = minimal infiltration, 2 = mild infiltration 3 = moderate infiltration, 4 = strong infiltration. Image J software was used to assess the degree of mononuclear infiltration of the synovium.
Blood Collection
Before the rats were sacrificed at each time point (6h, 12h, 24h, 48h and 72h), blood samples were taken from their intraocular veins. The samples were allowed to stand at room temperature for 30 minutes and then centrifuged for 5 minutes (10,000rpm). The serum was collected and stored at −80°C for the analysis of cytokine level.
Enzyme-Linked Immunosorbent Assay
The level of IL-1β, IL-6, TNF-α, IL-10, TGF-β1 and IL-17 in rats’ serum at each time point (6h, 12h,24h,48h and 72h) were measured by Enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minnesota, USA) and IL-17 ELISA kit (Nuvos, Littleton, USA) according to the manufacturer’s instructions. The absorbance was read at 450 nm using a microplate reader.
Spleen Treatment
When rats were sacrificed at each time point (6h, 12h,24h,48h and 72h), the spleen was removed from the abdominal cavity. The spleen was fully grounded and spleen cell suspensions were prepared by filtration through a 40μm filter. Then, the red cells in the spleen cell suspension were lysed to obtain a white cell suspension, and the entire procedure was performed under aseptic conditions.
YO-PRO-1 Uptake Experiment
The treated spleen cells (1x106) were suspended, adding 1ul of YO-PRO-1 (Invitrogen Carlsbad, CA, USA), and after standing for 30 minutes, cells were washed twice with PBS, and the intake of YO-PRO-1 was detected by flow cytometry.
Flow Cytometry
The treated spleen cells (1×10^6cells) were stained with anti-P2X7 receptor (extracellular)-FITC antibody (Alomone, Jerusalem, Israel) and CD68 antibody (NOVUS, Littleton, USA), and the white cell suspension obtained from the spleen was stained with FITC conjugated mouse anti-rat CD4, PE conjugated mouse anti-rat CD25 (BD Bioscience, MD, USA), PE conjugated anti-IL-17A and APC conjugated anti-Foxp3 antibodies (eBioscience, San Jose, CA, USA). Data was acquired by a Calibur (BD BioSciences) flow cytometer and data analysis was performed using Flow Jo software version 7.6 (Tree Star, Ashland, OR, USA).
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
The RNA was extracted from cultured macrophages and spleen white cells by an RNeasy Micro Kit (QIAGEN, Duesseldorf, Germany) and reverse-transcribed to cDNA with primers using the cDNA Reverse Transcription Kit (QIAGEN, Duesseldorf, Germany). All primers (P2X7R, NLRP3, IL-1β, Foxp3, IL-17 and β-actin) were designed and produced by Takara Biotechnology (Dalian, China).Real-time PCR amplification was performed with SYBR® Green PCR kit (Qiagen) according to the manufacturer’s instructions. The concentration of RNA was detected by NanoDrop2000 (Thermo Fisher Scientific, Wilmington, DE, USA). All cDNA samples were amplified in the ABI 7500 Fast Real-Time PCR System (Applied Biosystems, California, USA) and relative expression was calculated using the ∆∆Ct standardization method.
Statistical Analysis
At each time point, the three groups (ATP group, BBG group, and control group) were compared with each other. The data of the experimental groups were analyzed by one-way ANOVA with repeatable measurement data. All the values are expressed as the mean ± standard error of measurement (SEM). Statistical analyses were performed with IBM SPSS 23.0 statistical software. P<0.05 was considered as statistical significance.
Results
P2X7R Regulates the Development of Acute Gouty Arthritis
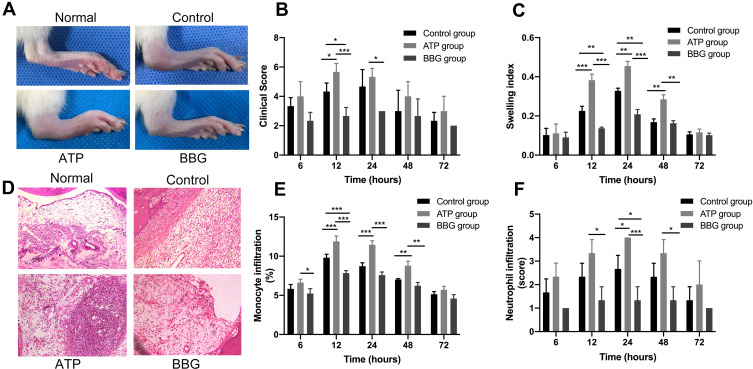
To investigate whether the functional changes of P2X7R affect the clinical manifestations of model rats, we performed an in vivo injection of ATP or BBG to active or inhibit P2X7R in model rats, respectively (Figure 1A). At each time point (6h, 12h, 24h, 48h and 72h), the swelling index and clinical score were used to evaluate the three groups of rat ankle joints. At 12h, clinical scores of ATP group were significantly higher than those of BBG group and control group, and the control group was higher than BBG group. The swelling index of ATP group began to increase at 12h, and reach the highest at 24h, The difference among three groups was statistically significant, afterwards there were no significant difference among them, at 48h and 72h (Figure 1B and C).
Figure 1.
P2X7R regulates the development of acute gouty arthritis in rats. (A) At 12h, clinical manifestations of right ankle joint in rats among ATP group, BBG group and control group. (B and C) Clinical score and swelling index of three groups of rats at each time point. (D) Inflammatory cells infiltrated the synovial tissue of the right ankle joint in three groups of rats, at 24h. (E and F) Infiltration of mononuclear cells and neutrophils in the synovial tissue of the ankle joint among the three groups. * P<0.05, **P< 0.01, ***P< 0.001.
Furthermore, there was a large infiltration of inflammatory cells in the synovial tissue of the right ankle joint of rats at 24h (Figure 1D). As shown in Figure 1E, at 12h, 24h and 48h, the infiltration of mononuclear cell in ATP group was the highest in three groups. Which in the BBG group was the lowest at 12h. However, the level of neutrophil infiltration among the three groups were significantly different at 24h, and it was later than the time point of macrophage infiltration (Figure 1F).
The Expression Changes of P2X7R on Macrophages Affect Their Ability to Take Up YO-PRO-1
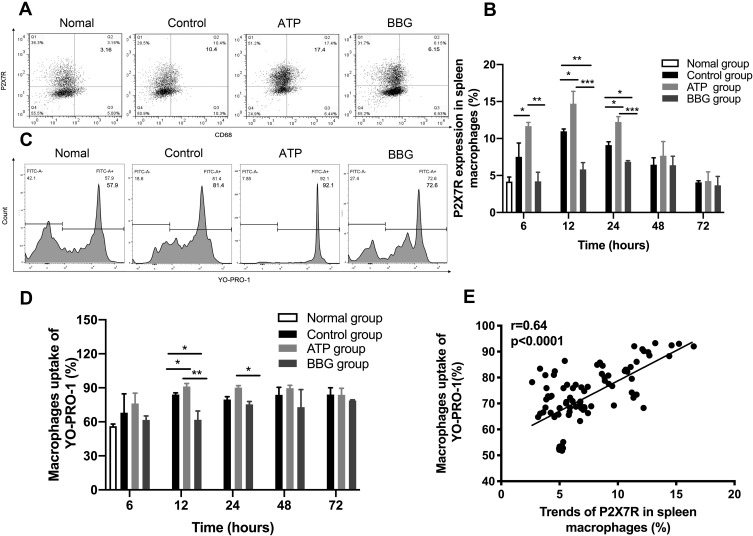
To explore the expression level of P2X7R in acute gouty arthritis, we isolated macrophages from the spleen of ATP, BBG and control groups at different time points (Figure 2A). At 6h, 12h and 24h, the expression of P2X7R in ATP group was obviously the highest among the three groups, and BBG group was lower than control group at 12h and 24h (Figure 2B). At each time point, the ability of each group of macrophages to take up YO-PRO-1 was different (Figure 2C), as shown in Figure 2D, at 12h and 24h, the macrophages ability of ATP group was the highest, followed by control group, BBG group was the lowest. At the same time point, changes in P2X7R expression levels also affected macrophage uptake of YO-PRO-1, and there was a positive correlation between them (Figure 2E).
Figure 2.
The expression changes of P2X7R on macrophages affect their ability to take up YO-PRO-1. (A) At 12h, the expression level of P2X7R in each group of macrophages was detected by flow cytometry. (B) Expression of P2X7R among the three groups at different time points. (C) At 12h, the percentage of YO-PRO-1 uptake by macrophages in each group was detected by flow cytometry. (D) The ability of macrophages to uptake YO-PRO-1 among the three groups at each time point. (E) The expression level of P2X7R was positively correlated with the ability of macrophages to uptake YO-PRO-1. * P<0.05, ** P< 0.01, *** P< 0.001.
P2X7R Mediates NLRP3 Inflammatory-Dependent IL-1β Secretion on Macrophages
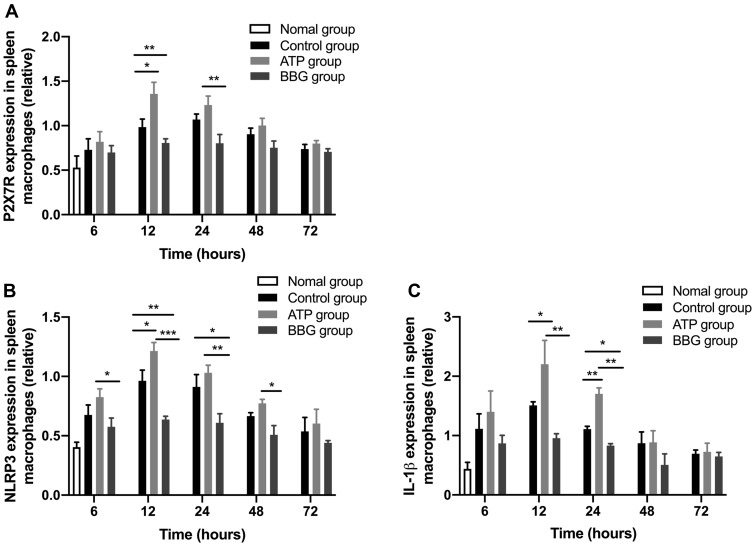
To examine whether ATP-actived P2X7R on macrophages promotes IL-1β secretion through activating NLRP3 inflammasome, the expressions of P2X7R, NLRP3 and IL-1β mRNA in rat spleen macrophages were detected by qRT-PCR. The P2X7R, NLRP3 and IL-1β mRNA in ATP group were significantly higher than that in other two groups at 12h. Yet, the expression of NLRP3 in BBG group was the significantly lowest among the three groups. Notably, at 24h, the expression of IL-1β was obviously highest in ATP group, followed by the control group, then the BBG group, and the difference among the three groups were significant (Figure 3A-C).
Figure 3.
P2X7R mediated NLRP3 inflammatory-dependent IL-1β secretion on macrophages. The expressions of P2X7R (A), NLRP3 (B) and IL-1β (C) in rat spleen macrophages were analyzed by qRT-PCR. *P<0.05, **P< 0.01, ***P< 0.001.
P2X7R Promote Th17 Cells and Pro-Inflammatory Cytokines Production
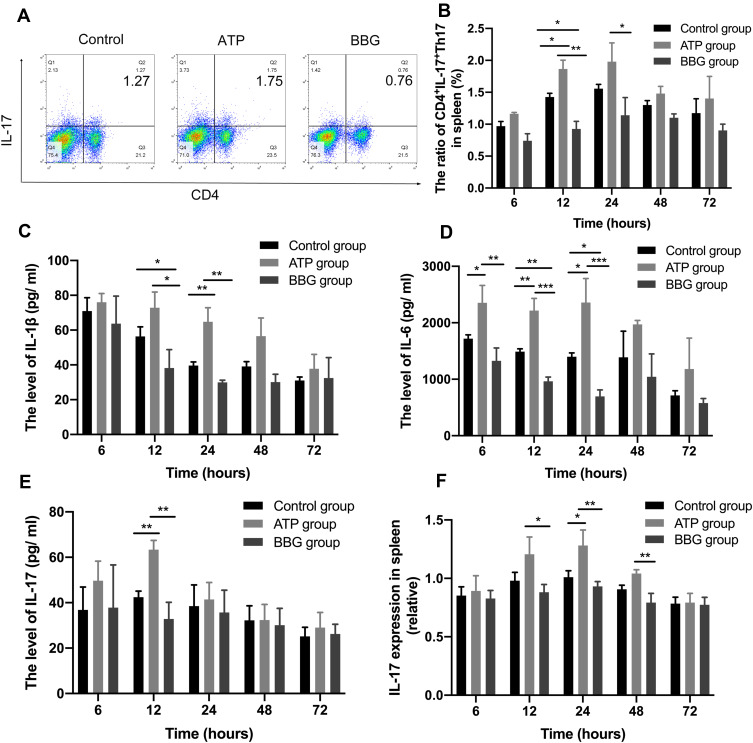
To further investigate whether P2X7R affects the expression of acute inflammation cells in rats with acute gouty arthritis, we also detected the expressions of CD4+IL-17+Th17 cells in three groups at each time point by flow cytometry (Figure 4A). At 12h, the ATP group showed the highest expression of CD4+IL-17+Th17 cells in the spleen, and the BBG group displayed the lowest (Figure 4B).
Figure 4.
P2X7R promote Th17 cells and pro-inflammatory cytokines production. (A) The expressions of CD4+IL-17+Th17 cells among the three groups at 12h. (B) The levels of CD4+IL-17+Th17 cells in ATP group, BBG group and Control group at each time point. (C-E) The serum levels of IL-1β, IL-6 and IL-17 of the three groups at each time point. (F) The expression of IL-17 mRNA among three groups at each time point. * P<0.05, ** P< 0.01, *** P< 0.001.
To determine whether P2X7R regulates the production of pro-inflammatory cytokines in acute gouty arthritis, we measured the levels of IL-1β, IL-6 and IL-17 in serum. We found the peaks of pro-inflammatory cytokines mainly at 12h-24h. At 12h, IL-1β of ATP group was the highest among the three groups, soon after 24h IL-1β mainly showed a downward trend during the process (Figure 4C).
At 6h, 12h and 24h, the highest level of IL-6 in ATP group, and the BBG group was lowest in three groups at 12h, 24h (Figure 4D). The IL-6 levels of ATP group reached the highest level at 24h, then tend to the lowest level at 72h.
At 12h, the IL-17 level of ATP group was the highest in three groups (Figure 4E). In addition, at 24h, the expression of IL-17 mRNA in ATP group was the highest (Figure 4F).
The Changes of Tregs and Anti-Inflammatory Cytokines in P2X7R-Regulated Acute Gouty Arthritis
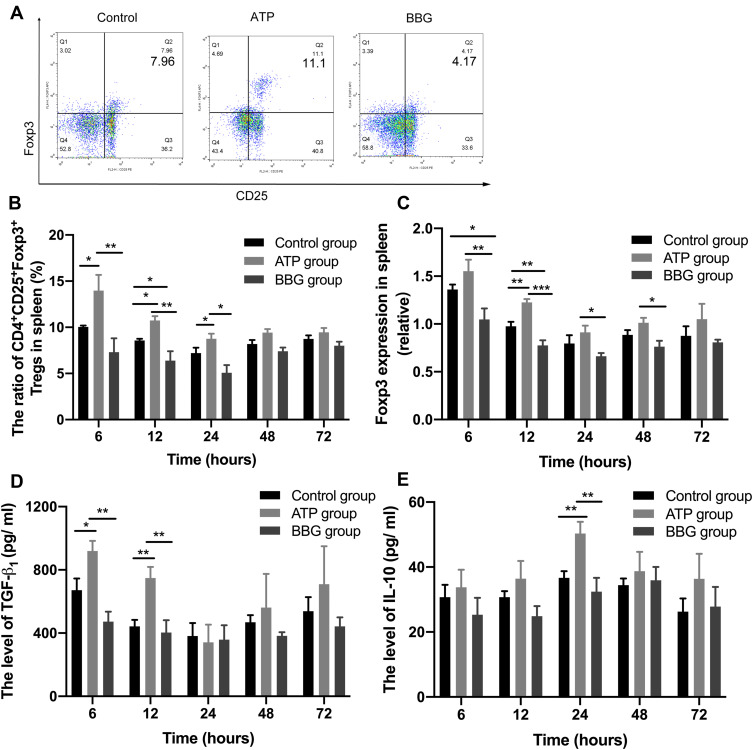
To assess whether P2X7R affects the Tregs expression of acute gouty arthritis, we measured the levels of CD4+CD25+Foxp3+Tregs in the three groups at each time point (Figure 5A). At 6h,12h and 24h, the expression level of Tregs in ATP group was significantly the highest in three groups, while the BBG group was reversed at 12h (Figure 5B). Furthermore, we confirmed that similar results have also been displayed in Foxp3 mRNA expressions among the three groups at 12h (Figure 5C).
Figure 5.
Tregs and anti-inflammatory cytokines in P2X7R-regulated acute gouty arthritis. (A) The expressions of Tregs among the three groups at 12h. (B) The levels of CD4+CD25+FOXP3+Tregs in three groups at each time point. (C) The expression of FOXP3 mRNA among three groups at each time point. (D-E) The expressions of TGF-β1 and IL-10 in serum among the three groups. *P<0.05, **P< 0.01, ***P< 0.001.
In addition, the expression of TGF-β1 in ATP group was highest among three groups at 6h and 12h (Figure 5D), but IL-10 lever of ATP group was significantly the highest in three groups at 24h (Figure 5E). The variation trend of TGF-β levels presented from high to lowest at 24h, then to high level again at 72h in three groups.
The Changing Trend of ATP-Activated P2X7R-Regulated Inflammation Process Matched with the Acute Attack to Remission Form in Acute Gouty Arthritis
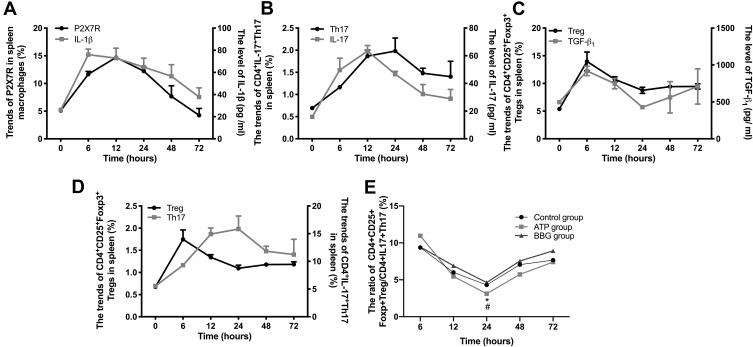
We further have explored the correlations in key time points between P2X7R and Treg/Th17 cells as well as related cytokines. Here, we took the ATP group as an example (Due to the similar trends among the three groups): At 6h and 12h, P2X7R and IL-1β, Th17 and IL-17 mainly showed an upward trend. Th17 cells reached the highest level at 24h (Figure 6A and B). But Treg and TGF-β1 both were at low levels at 24h (Figure 6C and D). After that, Th17 cells declined, while Treg cells risen gradually after 24h. The ratios of Treg/Th17 gradually decreased to the lowest level at 24h, then increased. Till 72h, the ratio nearly up to normal. Moreover, the ratio of Treg/Th17 in ATP group was the lowest in three groups at 24h, with significant difference (Figure 6E).
Figure 6.
The changing trend of cytokines and Treg/Th17 cells matched with the pathogenesis process of ATP-activated P2X7R-regulated acute gouty arthritis. (A) The trends of P2X7R and IL-1β in ATP group. (B) The trends of CD4+IL-17+Th17 cells and IL-17 in ATP group. (C) The trends of CD4+CD25+Foxp3+Treg cells and TGF-β1 in ATP group. (D) The trends of CD4+CD25+Foxp3+Treg cells and CD4+IL-17+Th17 cells in ATP group. (E) The ratios of Treg/Th17 among ATP group, BBG group and Control group. * P<0.05, ATP group vs BBG group, # P<0.05, ATP group vs Control group.
Discussion
Gout is an inflammatory disease, which characterized by the deposition of MSU crystals. The main clinical manifestation of gout is recurrent acute gouty arthritis. However, most patients with hyperuricemia have MSU deposition, but gout will not occur, suggesting that other factors are involved in the onset of gout.17 Under physiological and pathological conditions, multiple factors such as ATP, hyperglycemia, and blood lipids affect NLRP3 inflammasome activation.18–20 As a DAMPs, ATP can act in a variety of environments. When the concentration of extracellular ATP increases locally, P2X7R can be activated in the course of disease.21 P2X7 is a receptor for ATP, which is mainly highly expressed on the membrane of monocytes/macrophages. It is most related to inflammation in the P2X family and can promote the release of IL-1β by activating the NLRP3 inflammasome.1,22 Therefore, we believe that ATP and MSU synergistically participate in the onset of gout, and P2X7R plays a regulatory role in the onset of gout.5 In the present study, we found that activation of P2X7R significantly increased the swelling index and clinical score of the ankle joint in early inflammation, and abundant infiltration of macrophages and neutrophils occur in the synovial tissue of the joint, while inhibition of P2X7R has alleviated cell infiltration. Notably, the swelling index was consistent with the peak of neutrophil infiltration in the synovium of the joint, at 24h, which indicated that P2X7R regulates the inflammatory process of acute gouty arthritis in rats directly.
Activation of P2X7R by ATP promotes membrane pore formation, which in turn increases the uptake of the impermeable fluorescent dye YO-PRO-1, while the P2X7R antagonist significantly inhibits the uptake of the dye.23 Our results suggested a positive correlation between P2X7R expression and macrophage uptake of YO-PRO-1, here a large amount of ATP in the early stage of acute inflammatory (12h and 24h) have been found to promote the increase of P2X7R expression, which in turn to increase the uptake rate of YO-PRO-1, while inhibitor BBG suppressed this process. In addition, the expression of P2X7R and dye uptake reached the highest level at 12h and began to decrease slightly at 24h. P2X7R is also activated by ATP to induce NLRP3 inflammasome assembly and release of proinflammatory IL-1β and IL-18.18,24 In the present study, we found that ATP/P2X7R/NLRP3 inflammasome axis also engaged in development of acute gouty arthritis. However, the expression of NLRP3 and IL-1β at the gene levels were not always consistent with the expression of P2X7R. This suggested that P2X7R might also regulate inflammatory responses through other pathways, and the expression of inflammation-related factors in vivo was the result of the interaction of multiple factors.
In the inflammation of acute gout attacks, MSU crystals promoted macrophage activation and various pro-inflammatory cytokines production including IL-1β, IL-6 and IL-17.3,25 In addition, P2X7R signaling pathway promotes IL-1β release through activation of NLRP3 inflammasome. Yue et al have found that the levels of extracellular ATP, IL-1β and TNF-ɑ in the hippocampus of rats increased under acute stress with increase of NLRP3 inflammasome. However, treatment with the P2X7R inhibitor reduced IL-1β, TNF-ɑ and NLRP3 inflammasome, indicating that ATP-P2X7R-NLRP3 inflammasome axis has been involved in the regulation of proinflammatory cytokine production under stress.26 In the current study, we identified that ATP-activated P2X7R significantly promoted the secretion of IL-1β and IL-6 at 6h, and started to decrease slightly at 12h and 24h, but the overall level was still higher than the BBG group and control group. Moreover IL-17 production was significantly increased after activation of P2X7R at 12h, but its level began to decrease at 24h, indicating that the secretion of IL-17 was not only affected by P2X7R, but might also be related to the secretion of other anti-inflammatory factors (such as the peak of IL-10 secretion at 24h).
Besides, studies had shown that IL-6 acted as a potent pro-inflammatory cytokine in T cells by promoting Th17 differentiation, and its effect was concomitant with TGF-β1. IL-6 also inhibited TGF-β1-induced Treg differentiation, suggesting that IL-6 was a key factor in determining Treg/Th17 balance.27 It is generally believed that TGF-β1 was the main cytokine that promotes the relief of acute inflammation.28 Our previous study found that the level of TGF-β1 in the serum of model rat was positively correlated with the expression of CD4+CD25+Foxp3+ Tregs, suggesting that TGF-β1 was related to the remission of acute gouty arthritis.14 In this study, the level of IL-6 sustained at a high level in the early stage from 6h to 24h, meanwhile the lever of TGF-β1 turned to the lowest at 24h, which might inhibit the TGF-β1-induced Treg differentiation. Soon after, the level of IL-6 decreased to nearly normal level at 72h, while TGF-β1 raised again, which coincided with the process of acute gouty arthritis. That indicated that the TGF-β1 may be the important recovery role in gout.
Previously we have found an alteration of the ratio of Tregs/Th17 cells in MSU crystal-induced acute gouty arthritis rats.14 During acute inflammation, ATP activates P2X7R on Tregs and cause the reduction of the anti-inflammatory capacity and promotion apoptosis of Tregs.29,30 Th17 cells represent a pro-inflammatory T cell subset, which contributes to autoimmunity and tissue damage. Additionally, acute tissue damage leads not only to the release of ATP, but also to the release of IL-6, resulting in an increase of Th17 differentiation from T cells facilitated by P2X7R-mediated signaling.31,32 These data indicated that P2X7R is able to regulate differentiation of Tregs and Th17 cells. Here we try to define the alteration between Tregs and Th17 cells in the development of acute gouty arthritis. Interestingly we identified that 12h and 24h were the key time points, P2X7R come with Treg /Th17 cells and related cytokines, as well as the peak ankle arthritis response had obvious changes in these key points. Besides, at the key time points 24h, the activation of P2X7R significantly increased the amplitude of Treg/Th17 and decreased its ratio, suggesting that ATP-activated P2X7R led to Treg/Th17 cells imbalance, which may be the important role on occurrence of acute gout.
In this study, various indicators tended to decrease after 24 hours, which was consistent with the relief of clinical manifestations, and perfectly reflected the process of inflammation from acute gout to self-relief. The whole pathogenesis process appeared a pattern from acute attack to remission in time-dependent trend. These data provide new insights that ATP-activated P2X7R regulates the development of acute gouty arthritis through activating NLRP3 inflammasome and regulating Treg/Th17 balance, suggesting that high level of ATP as a trigger play an important role, while P2X7R may be a potential target for the treatment of acute gouty arthritis.
Conclusion
Activation of P2X7R by ATP significantly increased the severity of acute gouty arthritis, but inhibition of P2X7R by BBG significantly reduced the severity of acute gouty arthritis. The mechanisms underlying P2X7R regulating the development of acute gouty arthritis through activating NLRP3 Inflammasome and inflammatory cytokine production, which regulated Treg/Th17 balance. These data suggest that P2X7R play a key regulatory role in MSU crystal-induced acute gouty arthritis in rats.
Acknowledgment
Thanks to Yu Ning for helping to revise the article.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (81671601, 81771774), and the Anhui Key Research and Development Foundation (201904a07020103).
Author Contributions
Xiaojuan Dai wrote the manuscript. Xiaojuan Dai and Xuan Fang performed the experiment, analyzed and interpreted the data. Yuan Xia, Manyun Li, Xiaomei Li, and Yiping Wang contributed to validation of the methods. Xiangpei Li and Jinhui Tao acquired Funding and supervised this study. All authors read, edited, approved the final manuscript and contributed to study conception and design. In addition, all authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–241. [DOI] [PubMed] [Google Scholar]
- 2.Martin WJ, Harper JL. Innate Inflammation and Resolution in Acute Gout. Immunol Cell Biol. 2010;88(1):15–19. [DOI] [PubMed] [Google Scholar]
- 3.Martin WJ, Walton M, Harper J. Resident macrophages initiating and driving inflammation in a monosodium urate monohydrate crystal-induced murine peritoneal model of acute gout. Arthritis Rheum. 2009;60(1):281–289. [DOI] [PubMed] [Google Scholar]
- 4.Tao JH, Chen M, Tang JP, et al. Single nucleotide polymorphisms associated with P2X7R function regulate the onset of gouty arthritis. PLoS One. 2017;12(8):e0181685. doi: 10.1371/journal.pone.0181685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tao JH, Zhang Y, Li XP. P2X7R: a potential key regulator of acute gouty arthritis. Semin Arthritis Rheu. 2013;43(3):376. doi: 10.1016/j.semarthrit.2013.04.007 [DOI] [PubMed] [Google Scholar]
- 6.Barbera-Cremades M, Baroja-Mazo A, Gomez AI, Machado F, Di virgilio F, Pelegrı´n P. P2X7 receptor-stimulation causes fever via PGE2 and IL-1b release. FASEB J. 2012;26:2951–2962. [DOI] [PubMed] [Google Scholar]
- 7.Iyer SS, Pulskens WP, Sadler JJ, et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci U S A. 2009;106(48):20388–20393. doi: 10.1073/pnas.0908698106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacob F, Perez Novo C, Bachert C, Van Crombruggen K. Purinergic signaling in inflammatory cells: P2 receptor expression, functional effects, and modulation of inflammatory responses. Purinergic Signal. 2013;9(3):285e306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riteau N, Baron L, Villeret B, et al. ATP release and purinergic signaling: a common pathway for particIe—mediated inflammasome activation. Cell Death Dis. 2012;3(10):e403. doi: 10.1038/cddis.2012.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hechler B, Gachet C. Purinergic receptors in thrombosis and inflammation. Arterioscler Thromb VascBiol. 2015;35:2307–2315. [DOI] [PubMed] [Google Scholar]
- 11.Morandini AC, Savio LE, Coutinho-Silva R. The role of P2X7 receptor in infectious inflammatory diseases and the influence of ectonucleotidases. Biomed J. 2014;37(4):169–177. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmeister C, Trevisan G, Rossato MF, de Oliveira SM, Gomez MV, Ferreira J. Role of TRPV1 in nociception and edema induced by monosodium urate crystals in rats. Pain. 2011;152(8):1777–1788. [DOI] [PubMed] [Google Scholar]
- 13.Coderre TJ, Wall PD. Ankle joint urate arthritis (AJUA) in rats: an alternative animal model of arthritis to that produced by Freund’s adjuvant. Pain. 1987;28(3):379–393. [DOI] [PubMed] [Google Scholar]
- 14.Dai XJ, Tao JH, Xuan F, et al. Changes of Treg/Th17 Ratio in Spleen of Acute Gouty Arthritis Rat Induced by MSU Crystals. Inflammation. 2018;41(5):1–10. [DOI] [PubMed] [Google Scholar]
- 15.Park JS, Oh Y, Park O, et al. PEGylated TRAIL ameliorates experimental inflammatory arthritis by regulation of Th17 cells and regulatory T cells. J Controlled Release. 2017;1:731. [DOI] [PubMed] [Google Scholar]
- 16.Hartmann P, Szabó A, Eros G, et al. Anti-inflammatory effects of phosphatidylcholine in neutrophil leukocyte-dependent acute arthritis in rats. Eur J Pharmacol. 2009;622(1–3):58. [DOI] [PubMed] [Google Scholar]
- 17.Rouault T, Caldwell DS, Holmes EW. Aspiration of the asymptomatic metatarsophalangeal joint in gout patients and hyperuricemic controls. Arthritis Rheum. 1982;25(2):209–212. [DOI] [PubMed] [Google Scholar]
- 18.Gombault A, Baron L, Couillin I. ATP release and purinergic signaling in NLRP3 inflammasome activation. Front Immunol. 2013;3:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee H, Lim Y. Gamma-tocopherol ameliorates hyperglycemia-induced hepatic inflammation associated withNLRP3 inflammasome in alloxan-induced diabetic mice. Nutr Res Pract. 2019;13(5):377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajamäki K, Lappalainen J, Oörni K, et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5(7):e11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lenertz LY, Gavala ML, Zhu Y, et al. Transcriptional Control Mechanisms Associated with the Nucleotide Receptor P2X7, a Critical Regulator of Immunologic, Osteogenic and Neurologic Functions. Immunol Res. 2011;50(1):22–38. doi: 10.1007/s12026-011-8203-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrari D, Pizzirani C, Adinolfi E, et al. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. [DOI] [PubMed] [Google Scholar]
- 23.Aga M, Johnson CJ, Hart AP, et al. Modulation of monocyte signaling and pore formation in response to agonists of the nucleotide receptor P2X(7). J Geophysical Res Space Phys. 2002;93(A11):12893–12900. [PubMed] [Google Scholar]
- 24.Gicquel T, Robert S, Loyer P, et al. IL-1β production is dependent of the activation of purinergic receptors and NLRP3 pathway in human macrophages. FASEB J. 2015;29:4162–4173. [DOI] [PubMed] [Google Scholar]
- 25.Schett AG, Dayer JM, Manger B, et al. Mechanisms of inflammation in gout. Rheumatology. 2015;12(2):1090–1096. [Google Scholar]
- 26.Yue N, Huang H, Zhu X, et al. Activation of P2X7 receptor and NLRP3 inflammasome assembly in hippocampal glial cells mediates chronic stress-induced depressive-like behaviors. Neuroinflammation. 2017;14(1):102. doi: 10.1186/s12974-017-0865-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40(7):1830–1835. [DOI] [PubMed] [Google Scholar]
- 28.Scanu A, Oliviero F, Ramonda R, Frallonardo P, Dayer JM, Punzi L. Cytokine levels in human synovial fluid during the different stages of acute gout: role of transforming growth factor β1 in the resolution phase. Ann Rheum Dis. 2012;71(4):621–624. [DOI] [PubMed] [Google Scholar]
- 29.Schenk U, Frascoli M, Proietti M, et al. ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors. Sci Signal. 2011;4(162):ra12. [DOI] [PubMed] [Google Scholar]
- 30.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediatesimmune suppression. J Exp Med. 2007;204:1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trautmann A. Extracellular ATP in the immune system: more than just a ”danger signal”. Sci Signal. 2009;2(56):pe6. [DOI] [PubMed] [Google Scholar]
- 32.Piconese S, Gri G, Tripodo C, et al. Mast cells counteract regulatory T-cell suppression through interleukin-6 and OX40 /OX40L axis toward Th17-cell differentiation. Blood. 2009;114:2639–2648. [DOI] [PubMed] [Google Scholar]