Abstract
Phosphorus (P) deficiency is the main hurdle in achieving sustainable crop production ps especially in calcareous soils. Using bio-fertilizers like phosphate solubilizing bacteria (PSB) could be a useful approach for sustainable P management as they improve P availability in soil via dissolution, desorption and mineralization reactions. In addition, application of organic amendments with PSB could further ameliorate soil conditions for sustainable management of immobilized nutrients in calcarious soils. Therefore, we performed pot experiment to study the role of PSB in nullifying antagonistic effects of liming (4.78, 10, 15 and 20%) on P availability from poultry manure (PM), farm yard manure (FYM), single super phosphate (SSP) and rock phosphate (RP) in alkaline soils. PSB inoculation improved wheat growth, P availability and stimulated soil acidification over control regardless of P sources and lime levels. Soil calcification adversely affected plant growth, P nutrition, induced soil salinity and alkalinity, however, PSB and manures application potentially nullified such harmful effects over mentioned traits. Individually, organic sources were superior than mineral sources however, the performance of mineral fertilizers with PSB was at par to sole application of manures. Furthermore, application of RP with PSB proved as effective as sole SSP. Therefore, using PSB as bio-fertilizer has huge potential for improving P availability in calcareous soils.
Subject terms: Ecology, Plant sciences, Environmental sciences
Introduction
Optimum availability of Phosphorus (P) for humans, plants and animals on sustainable basis is a fundamental prerequisite to meet the worldwide hunger by 20301. Sustainable management of P at global scale is a fundamental aspect for the achievement of global food. Phosphorus performs vital functions in plant metabolism, growth and development. It cannot be manufactured by plants and has no substitute, thus management of phosphorus require sustainable measures to improve its crops use efficiency. Globally, deficiency of P is a main yield reducing nutrient next to nitrogen2. It also cannot be fixed from atmosphere biologically as N dose, that’s why mineralization of P in soils is a key factor that can enhanced its bioavailability for agricultural crops on sustainable basis3. Primary and secondary ortho-phosphate, and phosphate are the bio-available mineral forms of P in soil. These are subjected to losses due to adsorption on clay surfaces4, and/or through precipitation with cations like Ca+2 and Mg+2 at high pH or Fe+2 and Al+3 at low pH. Consequently, the bio-available P in the soil is as low as 0.1 mg kg−15 and nearly, 30–40% cultivable land across the globe suffers from P deficiency. Khan et al.6 reported that, the reserved P in soil is sufficient to support optimum plant growth for 100 years, if it is made bio-available by certain means.
Calcareous soils are the most abundant (800 million hectares worldwide) in arid and semi-arid areas7. These soils contain high quantity of calcite that fixes significant quantities of P either by precipitation with Ca+2 and Mg+2 and/or by sorption on calcite surfaces (16 to 200 m2 g−1)8. Consequently, 90% of calcareous soils are scarce in bio-available P9. To fulfill plant P requirements, these soils are regularly supplemented with phosphatic fertilizers10. According to Goldstein11 almost 30 million tons (MT) of P fertilizers (worth 4 billion USD) are added to the soils throughout the world. About 20% of added P is used by the plants while the rest of 80% is lost through different processes12. Such losses not only rise cost of production but also cause environmental pollution13. Organic supplements added to calcareous soils affect soil P chemistry by forming insoluble complexes like Ca-phytates14. Rock phosphate (RP) which contains 17% P can be used as an economical alternative for substituting expensive chemical P fertilizers5,15, however, it solubility is very low in calcareous soils16. Thus, these circumstances have compelled the scientists to find eco-friendly and economically feasible substitutes of these sources to improve crop yield and P nutrition in P deficient soils17.
Using phosphate solubilizing bacteria (PSB) as an alternative to expensive mineral P fertilizers could be an environmental-friendly approach for improving crop yield in calcareous soil15. The PSB may increase the solubility of precipitated P like Ca3-(PO4)2 through the release of protons, phenolic compounds, and siderophores18, organic19 and mineral acids20–22. The may promote the process of biological nitrogen fixation (BNF) and may increase the availability of micro nutrients like Fe+2 and Zn+2 etc.23. They may also prevent P losses by immobilizing it in the presence of labile carbon24 and may re-add it into soil by it decomposition. They may also improve bio availability of P through liberation of extracellular enzymes25. The PSB may also improve crop growth and P availability in calcareous soils by the production of gibberellins26, cytokinins27, IAA, Alkaline phosphatases, hydroxyl ions and CO228 and by H+ protonation29, anion exchange and chelation30. Therefore, PSB have a main role in regulating soil P cycle like sorption–desorption, dissolution–precipitation, and mineralization–immobilization process. Jalili et al.31 reported that, integration of PSB and PGPR could decrease the use of P fertilizer by 50% without having any adverse effect on crop yield. Many scientists observed increase in crop yield like rice32, maize33 and other cereals34 with PSB inoculation. Similarly, Bolan35 observed long term improvement in P availability and crop yield with combined use of organic and mineral P supplements than sole mineral P fertilizations in calcareous soils.
Application of PSB can reduce dependence on expensive chemical P fertilizers either by solubilizing the preserved insoluble soil P or by substituting them with environment friendly and economical natural P sources like RP. However, their density, performance and P solubilizing ability vary with different soils and production system21. Furthermore, limited research has been conducted on exploring the role of PSB under low organic matter containing calcareous soils. Thus, this study was conducted to explore potential of PSB for optimizing P supply and improving wheat yield in alkaline soil amended with different P supplements under different levels of lime.
Results
PGPR features and composition of used PSB
The peat based inoculum was composed of Arthrobacter (9%), Burkholderia (10%), Bacillus (16%), Enterobacter (3%), Mycobacterium (14%), Pseudomonas (13%), Pantoea (10%) and Rhizobia (9%) while, 16% of the colonies were unidentified (Table 1). The PSB was capable of phosphate solubilization (8.4 diameter of halo in mm), Axine (4.01 mg ml−1), IAA (8.3 µg ml−1), organic acids (10.6 g l−1) and siderpores (5.6 diameter of halo in mm) production (Table 2). The inoculum contained 1.75 × 108 cfu PSB g−1 on average basis (wet weight basis).
Table 1.
Percent bacterial composition of PSB inoculum.
| Bacterial species | Percent composition |
|---|---|
| Arthrobacter | 9 |
| Bacillus | 16 |
| Burkholderia | 10 |
| Enterobacter | 3 |
| Mycobacterium | 14 |
| Pantoea | 10 |
| Pseudomonas | 13 |
| Rhizobia | 9 |
| Unidentified | 16 |
Table 2.
Auxine, indole acetic acid (IAA), organic acid and siderophore production, phosphate-solubilization and population by/of PSB.
| PGPR characteristics | Quantity | Unit |
|---|---|---|
| Auxin production | 4.0 ± 0.39 | mg ml−1 |
| IAA production | 8.3 ± 0.59 | µg ml−1 |
| Phosphate-solubility | 8.4 ± 0.52 | Diameter of halo in mm |
| Siderophores production | 5.6 ± 0.80 | Diameter of halo in mm |
| Total organic acid | 10.6 ± 0.74 | g l−1 |
| Population | 1.75 × 108 | cfu g−1 inoculum (wet weight basis) |
± Values represent SE of mean (n = 3).
Tillers, plant height, grains per spike and 100 grains weight of wheat
Results exhibiting the effects of PSB inoculation and P sources on wheat tillers per pot (TP), plant height (PH), grains per spike (GS) and 100 grains weight (HGW) in soil with varying lime contents are presented in Table 3. Seed inoculation with PSB significantly improved TP, PH, GS and HGW by 10.7, 12.7, 10.3 and 7.0%, correspondingly, compared to without PSB pots. Similarly, among the sources poultry manure (applied @ of 45 mg P2O5 kg−1) produced significantly higher TP (2.29), PH (54.9 cm), GS (39.4) and HWG (4.3 g) which were statistically at par with farmyard manure (FYM) except HGW where PM performed better than FYM. The minimum values of these tested traits were noted where RP was used however, its performance was statistically similar to SSP for all traits except PH where SSP (47.9 cm) produced taller plants than RP (42.9 cm) as presented in Table 3. Liming adversely affected all the yield attributes. With increasing lime content a significant decline of 10.9, 23.7 and 39% was observed in TP, 5.3, 16.2 and 31.3% in PH, 5.6, 11 and 21.4% in GS and 3.3, 7.5 and 16.6% in HGW at 10, 15 and 20% lime over control (4.78%), respectively. However, PH responded statistically similar to control and 10% lime.
Table 3.
Impact of PSB inoculation and P sources on wheat tillers per pot, plant height, grains per spike and 100 grains weight in soil with varying lime content.
| Inoculation | Tillers plant−1 | Plant height (cm) | Grains spike−1 | 100 grains weight (g) |
|---|---|---|---|---|
| Without PSB | 1.84 | 46.52 | 34.71 | 3.85 |
| With PSB | 2.03 | 52.43 | 38.29 | 4.12 |
| LSD (α = 0.05) | 0.096 | 2.25 | 1.115 | 0.077 |
| P sources | ||||
| SSP | 1.64b | 47.88b | 34.95b | 3.75c |
| Rock phosphate | 1.57b | 42.87c | 33.83b | 3.79c |
| FYM | 2.25a | 52.25a | 37.83a | 4.07b |
| PM | 2.29a | 54.91a | 39.37a | 4.30a |
| LSD (α = 0.05) | 0.136 | 3.195 | 1.577 | 0.109 |
| Lime (%) | ||||
| Control (4.78%) | 2.38a | 57.00a | 40.33a | 4.27a |
| 10 | 2.12b | 54.00a | 38.08b | 4.13b |
| 15 | 1.81c | 47.75b | 35.87c | 3.95c |
| 20 | 1.45d | 39.16c | 31.70d | 3.56d |
| LSD (α = 0.05) | 0.136 | 3.195 | 1.577 | 0.109 |
| Interaction | ||||
| L × I | ns | Figure 1 | ns | Figure 2 |
| L × PS | Figure 3 | Figure 4 | ns | ns |
| I × PS | ns | ns | Figure 5 | ns |
| L × I × PS | ns | ns | ns | ns |
| CV | 12.21 | 11.20 | 7.49 | 4.76 |
PSB, LSD, SSP, FYM, PM and ns denote phosphate solubilizing bacteria, least significant difference, single super phosphate, farmyard manure, poultry manure and non-significant interaction, respectively.
Means sharing letter in each category are statistically at par at α ≤ 0.05.
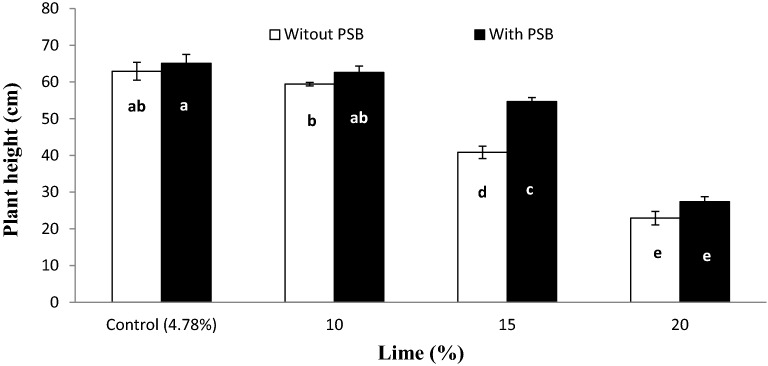
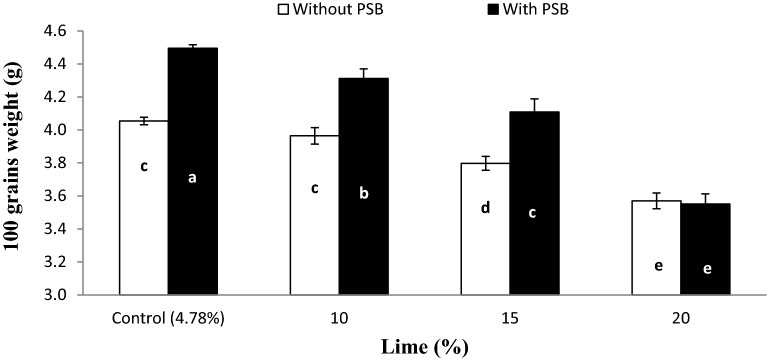
The interactive effect of lime × inoculum (L × I) was significant for PH (Fig. 1) and HGW (Fig. 2). The PH gradually decreased with increasing lime content from 10 to 20% both in inoculated and un-inoculated pots while, pots treated with 10% lime produced taller plants which were statistically comparable to control (4.78%) lime both with and without PSB inoculation. Inoculated and un-inoculated plants performed statistically similar at all lime contents except 15% where PSB inoculation significantly increased PH over un-inoculated. Inoculated treatments produced heavier grains than un-inoculated soil at all lime contents except 20% where inoculation didn’t affect the 100 grains weight. Furthermore, 15% lime with PSB and 10% lime without PSB produced 100 grains with similar weight (Fig. 2).
Figure 1.
Interactive effect of lime and P sources on plant height per pot. Graph bars having different letters are significantly different at α = 0.05. Errors bar represent standard error for the mean of three values.
Figure 2.
Interactive effect of lime and PSB on wheat 100 grains weight. Graph bars having different letters are significantly different at α = 0.05. Errors bar represent standard error for the mean of three values.
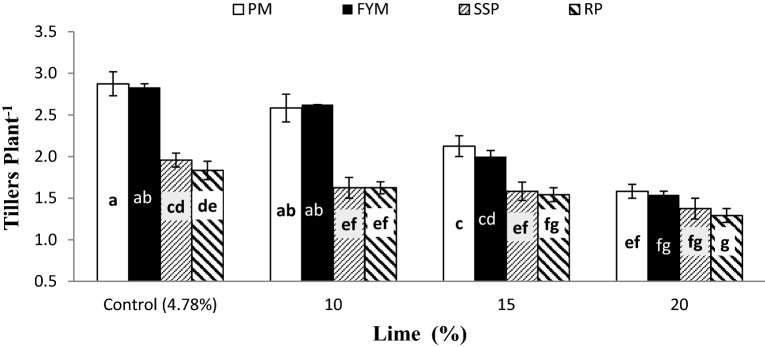
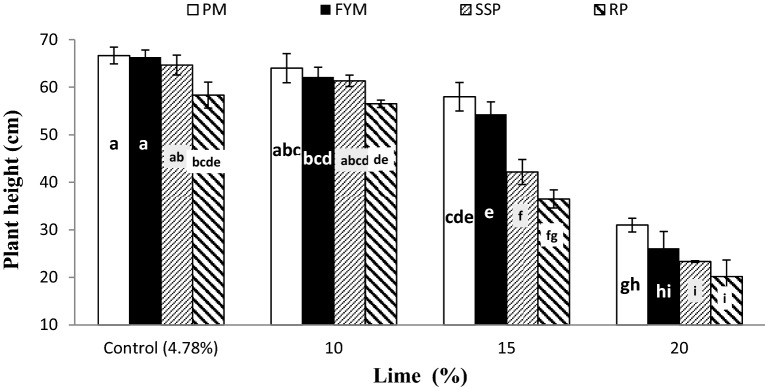
Data regarding the significant interaction of lime × P sources (L × PS) for TP and PH are presented in Figs. 3 and 4, respectively. Organic sources (PM and FYM) produced significantly higher TP than mineral sources (SSP and RP) at all levels of lime except 20%. At 20% lime PM, FYM and SSP showed similar effect on TP however, RP performance was notably poor than PM and at par to FYM and SSP. Likewise, the TP responded alike to PM and FYM at 20% lime and SSP and RP with 15% lime. Non-significant intra source difference were observed on both the organic (between PM and FYM) and mineral (between SSP and RP) P supplements at all lime contents. Liming negatively affected PH at all P sources, however its adverse effect was more prominent in mineral sources than organic sources at 15 and 20% lime (Fig. 4). Soil calcification up to 10% did not show any adverse effect on PH over control (4.78%), but addition of lime beyond 10% resulted in dwarf plants both at organic and mineral P application. Poultry manure acted more potentially for harmonizing the harmful effect of lime than the other sources at all lime content however its performance was similar to FYM and SSP at control and 10% lime, and FYM at 15 and 20% lime. Phosphorus applied as RP produced shorter plants at all lime contents. Furthermore, similar stature plants were observed both at 20% lime + organic sources (PM and FYM) and 15% lime + mineral P sources (SSP and RP) and 15% lime with organic sources and 10% lime with mineral P sources.
Figure 3.
Interactive effect of lime and PSB on wheat tillers per plant. Graph bars having different letters are significantly different at α = 0.05. Errors bar represent standard error for the mean of three values.
Figure 4.
Interactive effect of lime and P sources on plant height. Graph bars having different letters are significantly different at α = 0.05. Errors bar represent standard error for the mean of three values.
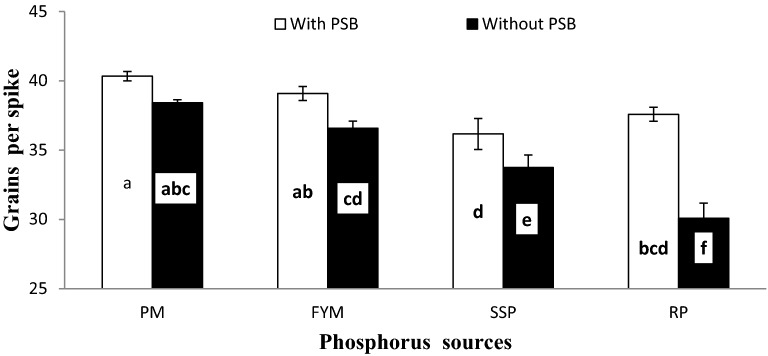
Significant (p ≤ 0.01) interactive effect of inoculation and P sources (I × PS) for grains per spike (GS) showed that inoculation produced denser spike than un-inoculated plants at all P sources except PM (Fig. 5). Generally, organic sources produced significantly higher GS than mineral sources. Phosphorus applied as PM along with PSB produced statistically at par GS to PM without PSB and FYM with PSB which was considerably higher than FYM without PSB. The SSP and RP with PSB responded similar with respect to GS however, the performance of SSP alone (without PSB) was potentially better than RP without inoculation. Furthermore, RP with PSB produced statistically more filled spike than sole SSP.
Figure 5.
Interactive effect of P sources and PSB on wheat grains per spike. Graph bars having different letters are significantly different at α = 0.05. Errors bar represent standard error for the mean of three values.
Root dry weight, grain and biological of wheat
Grain yield (GY), biological yield (BY) and root dry weight (RDW) of wheat were significantly influenced by PSB inoculation, soil calcification and phosphorus sources (Table 4). Inoculation with PSB inoculation improved GY, BY and RDW by 14.1, 16.3 and 8.1% over un-inoculated control. Generally, organic sources (PM and FYM) performed well than mineral sources (SSP and RP). Poultry manure produced maximum GY, BY and RDW which was significantly higher than FYM for GY and BY and at par with FYM for RDW. Similarly, poor performance was observed for RP in all traits which was comparable to SSP in case of GY and RDW and significantly lower than SSP for BY. On the basis of performance, the P sources could be ranked as PM ≥ FYM > SSP ≥ RP. The GY, BY and RDW gradually decreased with increasing lime content from control to 20%. With increasing level of lime wheat grain, biological and root dry weight were significantly decreased (Table 4).
Table 4.
Wheat root dry weight, harvest index, grain, biological and straw yield as influenced by PSB inoculation, soil calcification and phosphorus application from different sources.
| Inoculation | Grain yield (g) pot−1 | Biological yield (g) pot−1 | Root dry weight (g) |
|---|---|---|---|
| Without PSB | 6.40 | 18.07 | 3.70 |
| With PSB | 7.30 | 21.02 | 4.00 |
| LSD (α = 0.05) | 0.249 | 0.467 | 0.150 |
| P sources | |||
| SSP | 6.28c | 17.55c | 3.47b |
| Rock phosphate | 5.88cd | 16.71d | 3.48b |
| FYM | 7.39b | 21.46b | 4.12a |
| PM | 7.86a | 22.47a | 4.30a |
| LSD (α = 0.05) | 0.353 | 0.660 | 0.212 |
| Lime (%) | |||
| Control (4.78%) | 9.29a | 22.40a | 4.46a |
| 10 | 7.68b | 20.86b | 4.09b |
| 15 | 6.03c | 19.80c | 3.70c |
| 20 | 4.40d | 15.13d | 3.14d |
| LSD (α = 0.05) | 0.353 | 0.660 | 0.212 |
| Interaction | |||
| L × I | Figure 6 | ns | ns |
| L × PS | Figure 7 | ns | ns |
| I × PS | ns | Figure 8 | Figure 9 |
| L × I × PS | ns | ns | ns |
| CV | 8.93 | 5.86 | 9.59 |
PSB, LSD, SSP, FYM, PM and ns denote phosphate solubilizing bacteria, least significant difference, single super phosphate, farmyard manure, poultry manure and non-significant interaction, respectively.
Means with different letter in each column are significantly different at p ≤ 0.05.
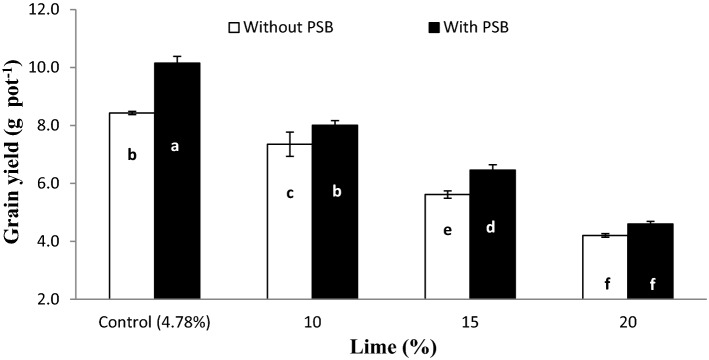
The interaction of lime × inoculum (L × I) was significant for GY at p ≤ 0.01 (Fig. 6). Wheat grain yield decreased with addition of lime at each lime level both in inoculated and un-inoculated pots. Similarly, PSB inoculation significantly improved GY over un-inoculated pots at all lime contents except 20% lime. Furthermore, it was also evident that 10% lime with inoculation resulted similar quantity of grains as control lime without inoculation. Highest GY was noticed for control lime with inoculation while the lowest was observed at both 20% lime with and without inoculation.
Figure 6.
Interactive effect of lime and PSB on grain yield (g pot−1). Graph bars having different letters are significantly different at α = 0.05. Errors bar represent standard error for the mean of three values.
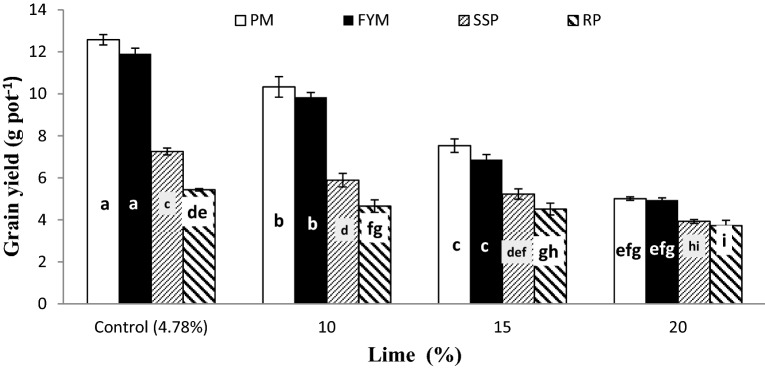
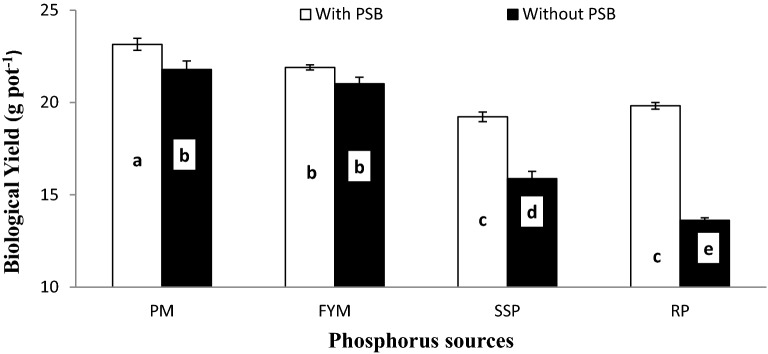
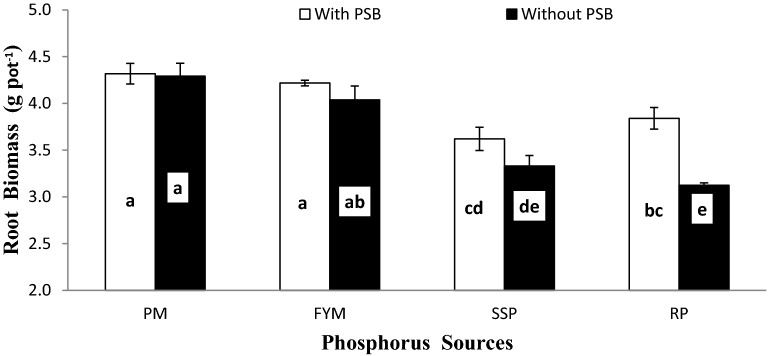
Wheat grain yield was also considerably affected by lime × P sources (L × P) as presented in Fig. 7. The L × P interaction showed a similar pattern for GY. The grain yield significantly decreased with addition of lime into soil at all P sources. Organic sources (PM and FYM) performed better than mineral sources (SSP and RP). There was no significant difference among the response of PM and FYM, and SSP and RP for GY regardless of soil lime concentration. Soil treated with organic sources at 15% lime significantly improved GY over those treated with mineral sources at 10% lime. Wheat BY and RDW were also notably affected by inoculum × P sources (I × PS) as presented in Figs. 8 and 9, respectively. Generally, seed inoculation with PSB markedly improved all mentioned traits over un-inoculated. The response of inoculation was more prominent in mineral (SSP and RP) sources than organic (PM and FYM). In either case, organic sources performed potentially better than mineral sources. There was no difference between inoculated and un-inoculated treatments for PM and FYM in all traits except BY where inoculated PM produced significantly more biomass than un-inoculated. Except biological yield where PM + PSB produced more biomass than FYM + PSB while for the rest of the mentioned traits the performance of PM and FYM were statistically similar irrespective of the inoculation. There was no significant difference among SSP and RP with inoculation in BY and RDW but without inoculation SSP produced more BY than RP whereas, for RDW their differences were un-noticeable.
Figure 7.
Interactive effect of lime and P sources on grain yield (g pot−1). Graph bars having different letters are significantly different at α = 0.05. Errors bar represent standard error for the mean of three values.
Figure 8.
Interactive effect of P sources and PSB on wheat biological yield (g pot−1). Graph bars having different letters are significantly different at α = 0.05. Errors bar represent standard error for the mean of three values.
Figure 9.
Interactive effect of PSB and P sources on root biomass (g). Graph bars having different letters are significantly different at α = 0.05. Errors bar represent standard error for the mean of three values.
Wheat P concentration, uptake and post-harvest soil Olsen P
Effects of PSB, P sources and soil calcification on wheat P concentration (PPC), uptake (PPU) and post-harvest soil Olsen P (PSP) are presented in Table 5. Inoculation, liming and P sources distinctly affected all mentioned traits. Inoculation with PSB increased PPC (7.1%), PPU (24.3%) and PSP (3.4%) over un-inoculated control. Poultry manure proved to be the most potential source for improving these traits and its effect was statistically similar to FYM for PPC and PSP. Rock phosphate performed poorer than the rest of P sources for these traits however its effect was similar to SSP for PSP. Liming adversely affected PPC, PPU and PSP and a gradual decrease in these was observed with increasing lime content from control to 20%. Addition of lime at the rate of 10, 15 and 20% declined PPC, PPU and PSP by 4.1, 21.5 and 36.1% (PPC), 10.7, 30.7 and 56.7% (PPU) and 6.7, 13.6 and 23.6% (PSP), respectively.
Table 5.
Role of PSB and P sources in improving wheat P concentration, uptake and post-harvest Olsen P content in soil under varying lime levels.
| Inoculation | Plant P (%) | P uptake (mg pot−1) | Post-harvest soil Olsen P (mg kg−1) |
|---|---|---|---|
| Without PSB | 0.141 | 26.3 | 5.93 |
| With PSB | 0.151 | 32.6 | 6.14 |
| LSD (α = 0.05) | 0.0032 | 0.984 | 0.090 |
| P sources | |||
| SSP | 0.140b | 25.4c | 5.70b |
| Rock phosphate | 0.135c | 23.2d | 5.58b |
| FYM | 0.153a | 33.4b | 6.45a |
| PM | 0.156a | 35.8a | 6.41a |
| LSD (α = 0.05) | 0.0047 | 1.391 | 0.127 |
| Lime (%) | |||
| Control (4.78%) | 0.172a | 35.8a | 6.78a |
| 10 | 0.165b | 33.4b | 6.33b |
| 15 | 0.135c | 27.1c | 5.86c |
| 20 | 0.110d | 16.9d | 5.18d |
| LSD (α = 0.05) | 0.0047 | 1.391 | 0.127 |
| Interaction | |||
| L × I | ns | Figure 10** | ns |
| L × PS | ns | Figure 11*** | Figure 12* |
| I × PS | Figure 13* | Figure 14*** | ns |
| L × I × PS | ns | ns | ns |
| CV | 5.12 | 8.20 | 3.65 |
PSB, LSD, SSP, FYM, PM and ns denote phosphate solubilizing bacteria, least significant difference, single super phosphate, farmyard manure, poultry manure and non-significant interaction, respectively.
Means with different letter in each column are significantly different at p ≤ 0.05.
Single asterisk stands for significant, Double asterisk stands for higly significant, Triple asterisk stands for very higly significant.
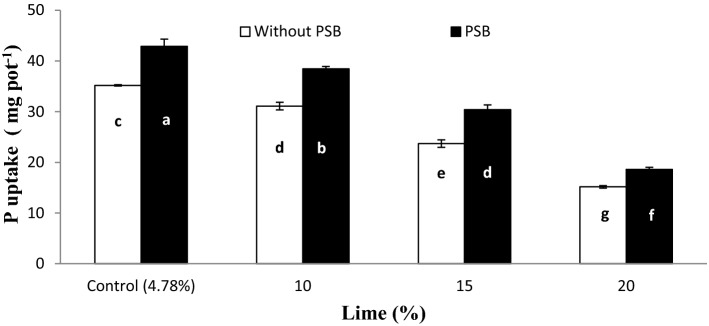
Significant interactive effect of lime and inoculums (L × I) was observed for PPU (Fig. 10). Inoculation with PSB significantly improved PPU over without inoculation at each lime content. PPU decreased with increasing lime content both with and without inoculation pots. Maximum P uptake was recorded for pots amended with control + PSB while the minimum was observed for 20% lime + no PSB. Moreover, the uptake was at par in pots treated with 15% lime with inoculation and 10% lime without inoculation and 10% lime with PSB even performed better than control lime without inoculation.
Figure 10.
Plant P uptake (mg kg−1) affected by lime and PSB. Errors bar represent standard error for the mean of three values. Graph bars sharing letters are statistically comparable at p ≤ 0.01.
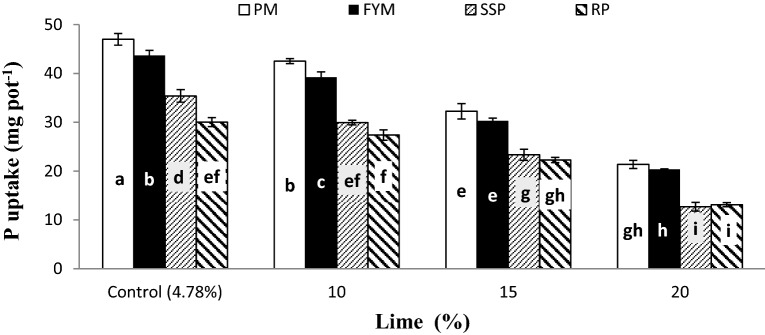
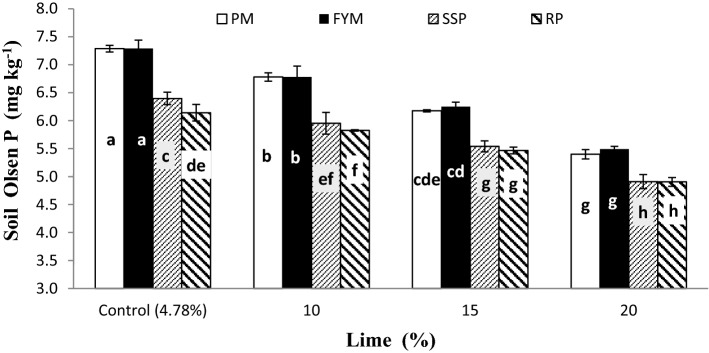
Both PPU and PSP were significantly improved by the interaction of lime and P sources (L × PS) as presented in Figs. 11 and 12, respectively. Application of lime decreased both PPU and PSP regardless of P sources though, FYM and PM played well than RP and SSP. Non-significant variations were noticed between PM and FYM for both PPU and PSP at all lime content excluding control and 10% lime where significantly increased PPU compared to FYM. In non-calcareous soils (4.78% lime) SSP performed statistically better than RP in both PPU and PSP whereas there were no differences in either case among these at the rest of lime. Both PPU and PSP were statistically greater in pots treated with 15% lime and organic sources than 10% lime and mineral sources.
Figure 11.
Plant P uptake (mg kg−1) as affected by the interaction of P sources and Lime. Graph bars having different letters are significantly different at α = 0.05. Errors bar represent standard error for the mean of three values.
Figure 12.
Soil post-harvest P (mg kg−1) as affected by P sources and Lime. Graph bars having different letters are significantly different at α = 0.05. Errors bar represent standard error for the mean of three values.
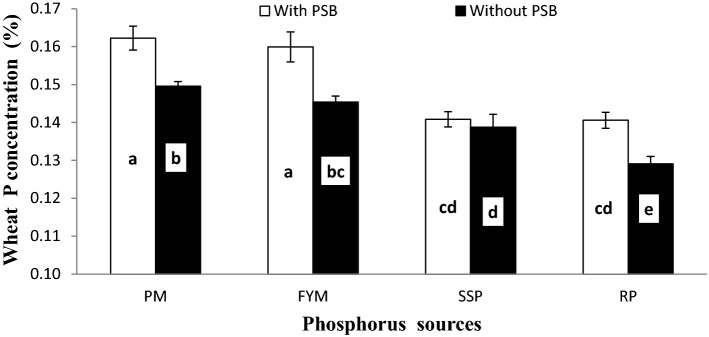
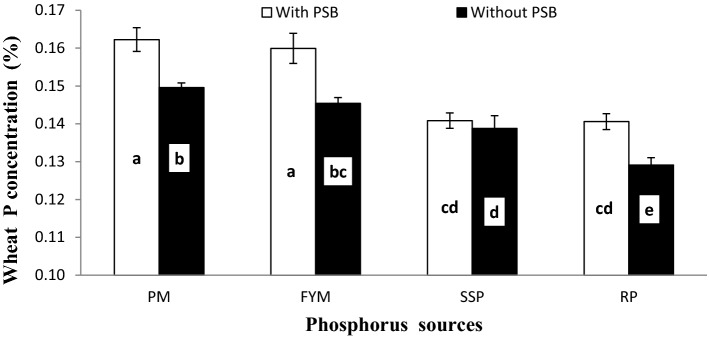
The response of PPC and PPU was also significant to the interaction of inoculum and P sources (I × PS) as presented in Figs. 13 and 14, respectively. PSB inoculation improved both PPC and PPU over no-inoculation at corresponding P sources except for SSP where there was no effect of inoculation on PPC. Both PPC and PPU were higher in soil treated with Pm and FYM than RP and SSP irrespective of PSB inoculation. Non-significant variation was observed among PM and FYM with and without inoculation for PPC while in case of PPU, PM performed superior than FYM both with and without PSB. Similarly, there were no differences among SSP and RP when inoculated with PSB for PPC and PPU whereas, without inoculation SSP was better than RP. In addition, SSP and RP with PSB responded alike to FYM without PSB in PPC.
Figure 13.
Interactive effect of PSB and P sources on plant P concentration (%).Graph bars having different letters are significantly different at α = 0.05. Errors bar represent standard error for the mean of three values.
Figure 14.
Interactive effect of PSB and P sources on wheat P uptake (mg kg−1). Graph bars having different letters are significantly different at α = 0.05. Errors bar represent standard error for the mean of three values.
Post-harvest soil EC, pH, organic matter and lime
Findings regarding post-harvest soil EC, pH, soil organic matter (SOM) and lime content as affected by PSB, phosphorus sources and lime are presented in Table 6. There was no statistical difference between inoculated and un-inoculated pots for post-harvest soil EC, pH and lime while, SOM was significantly decreased by about 0.83% in inoculated treatments over control. Similarly, P sources didn’t affect soil EC and lime. A significant decrease in soil pH was observed with the application of organic sources (PM and FYM), however, their effect was not statistically different from SSP. The highest pH was observed at RP which was also similar to SSP. Significant increase in after harvest SOM was observed with addition of P as FYM which was higher than PM and the lowest SOM was observed where P was supplemented through SSP and RP. Liming significantly augmented post-harvest soil EC, pH, SOM and lime content (Table 6). With increasing lime application, all mentioned attributes were gradually increased. Liming increased post-harvest soil EC by 42, 82 and 111, pH by 3, 5 and 6, SOM by 1, 3 and 5 and lime 106,210 and 314% over control (4.78%) at 10, 15 and 20% lime, respectively.
Table 6.
Post-harvest (wheat) soil EC, pH, organic matter and lime contents as influenced by PSB, phosphorus sources under different lime levels.
| Inoculation | Soil EC (dS m−1) | Soil pH | Organic matter (%) | Total lime (%) |
|---|---|---|---|---|
| Without PSB | 0.97 | 8.99 | 0.843 | 12.33 |
| With PSB | 0.97 | 8.11 | 0.836 | 12.28 |
| LSD (α = 0.05) | ns | 0.031 | 0.0059 | ns |
| P sources | ||||
| SSP | 0.96 | 8.07ab | 0.816c | 12.33 |
| Rock phosphate | 0.95 | 8.08a | 0.813c | 12.31 |
| FYM | 0.94 | 8.05b | 0.872a | 12.30 |
| PM | 0.94 | 8.05b | 0.856b | 12.29 |
| LSD (α = 0.05) | ns | 0.028 | 0.0083 | ns |
| Lime (%) | ||||
| Control (4.78%) | 0.59d | 7.75d | 0.820c | 4.77d |
| 10 | 0.85c | 8.05c | 0.828c | 9.88c |
| 15 | 1.09b | 8.15b | 0.848b | 14.82b |
| 20 | 1.27a | 8.29a | 0.861a | 19.76a |
| LSD (α = 0.05) | 0.30 | 0.028 | 0.0083 | 0.058 |
| Interaction | ||||
| L × I | ns | ns | ns | ns |
| L × PS | ns | ns | Figure 15*** | ns |
| I × PS | ns | ns | ns | ns |
| L × I × PS | ns | ns | ns | ns |
| CV | 5.40 | 0.62 | 1.73 | 0.82 |
PSB, LSD, SSP, FYM, PM and ns denote phosphate solubilizing bacteria, least significant difference, single super phosphate, farmyard manure, poultry manure and non-significant interaction, respectively.
Means with different letter in each column are significantly different at p ≤ 0.05.
Triple asterisk stands for very higly significant.
None of the interactions were significant for post-harvest soil EC, pH, SOM and lime except lime and P sources (L × PS) which significantly altered post-harvest SOM (Fig. 15). Addition of lime didn’t affect SOM in pots treated with SSP and RP. Pots where P was applied as organic sources like PM and FYM, SOM significantly varied with liming. Maximum SOM was recorded for FYM at 20% lime followed by PM at 20 and 15% which was at par to FYM at 15% lime. SOM responded alike to PM and FYM at control, 10 and 15% lime while at 20% lime FYM significantly improved soil OM over PM. Furthermore, at each lime level the response of SOM was significantly higher to organic sources than mineral sources.
Figure 15.
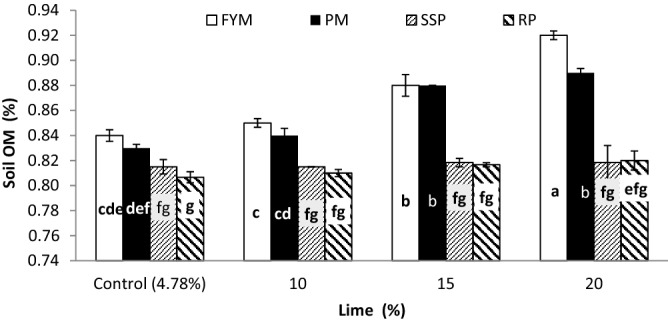
Post-harvest Soil organic matter (%) as affected by interaction of P sources and lime. Graph bars having different letters are significantly different at α = 0.05. Errors bar represent standard error for the mean of three values.
Discussion
Over use of mineral phosphorus fertilizer is deteriorating the environment, i.e., biodiversity loss. Continuous use of inorganic fertilizers is also depleting the soil organic matter that is directly associated with water infiltration, microbial proliferation and soil fertility i.e., mineralized P. For sustainable management of soil health and achievement of optimum crop productivity use of organic amendments and rhizobacteria are prime in importance. Organic amendments not only improve soil physical and chemical attributes but also facilitate in microbial proliferation which is beneficial for crop productivity24. That’s why for sustainable management of soil nutrients especially phosphorus we examined the potential of PSB under different P supplements in improving wheat yield, P availability and soil properties in artificially calcified soils. Researchers reported Aspergillus, Bacillus (B. subtilis, B. polymyxa, B. sircalmous, Bacillus megaterium and B. circulans), Penicillium, Enterobacter, Pseudomonas and Rhizobium as the most efficient P solubilizer and could be used as the main strains of PSB36–38. We also observed that PSB inoculation improved wheat growth, yield, soil and plant P concentration, and uptake and decreased post-harvest soil organic matter and pH over un-inoculated control. The findings of Tawaraya et al.39 supported our results regarding the yield improvement via PSB inoculation in agricultural crops. In this study PSB × lime demonstrated that, PSB inoculation nullified antagonistic effect of liming on plant growth and soil and plant P nutrition which further confirms the findings of Islam and Hossain40 who reported enhanced Psolubilizationand improved crop P nutrition in various crops by PSB in calcareous soils. Presence of PSB modified the soil physio-chemical properties which facilitate in solubilization of Fixed/immobilized P in soil. We noticed improvement in germination with PSB inoculation which is in conformity to Amruthesh et al.41 who observed increase in germination and seedling vigor of different crop plants with PSB inoculation. Betterment of plant height and tillers per pot by PSB in our study are in confirmation to Kumar et al.42 who reported significant increase in plant height and plant density by Azotobacter chroococcum inoculation in sorghum. This could be ascribed to better absorption of nutrients, mainly P due to change in soil pH through secretions of organic acid and phosphatase enzyme activity. Rhizobacteria secretes growth regulators, i.e., IAA that significantly enhance root surface area, adventitious and lateral root length. This increase in root length is mainly attributes as improvement in the cell division especially in hypocotyls and less accumulation of dead cell in the cortex region of root43,44. In current study, wheat grain, straw and biological yield were improved with PSB inoculation regardless of the P sources used however, the increase was relatively more in pots amended with PM and FYM than SSP and RP. Dwivedi et al.45 who observed increase in wheat yield with seed inoculation of PSB. According to Saad and Hammad46 application of PSB with calcium superphosphate resulted in maximum grain yield of wheat. Similarly, it is also confirmed by Islam and Hossain40 who observed maximum biological yield of wheat crop at rock phosphate inoculated with P solubilizing fungi like Aspergillus niger and Pseudomonas titrinum. Increase in biological yield of sorghum, maize and rice with PSB inoculation in non-calcareous soils have also been observed by Chabot and Antoun47 and Kundu et al.48. As like our findings, Kumar et al.42 obtained enhanced wheat straw yield by PSB inculcation. According to Sharma et al.49 PSB can improve soil productivity via the syntheses of beneficial metabolites, such as, antibiotics, phyto-hormones and siderophores. Afzal et al.50 obtained better seed P concentration, tillers, grain and biological yields of wheat. One of the possible reasons for mentioned improvement may be their acting as PGPR. According to Jalili et al.51 PSB may improve crop nutrition and growth through production of auxin, ACC-deaminase, root colonization, P solubilization, chitinase activity, siderophores production, and antibiotic production46.
We found that, PSB inoculation also significantly increased P availability regardless of whatever P sources was used. Sharma and Prasad52 and Vyas and Gulati53 also found enhanced cereal growth and nutrition due to PSB inoculation with and without fertilizers application. Our findings are in agreement to Mukherjee and Rai54 who also observed increased P uptake in wheat and cotton55 due to PSB inoculation over without PSB pots. This improvement in crop nutrition and yield could probably be due to the production of phytohormones52 and organic acids such as, acetic, citric gluconic, lactic, isovaleric, 2-ketogluconic, isobutyric, oxalic acid by PSB56,57 which acidify soil and enhance P availability to crop. It was also evident from the interaction of PS × I that RP performed comparable to SSP when inoculated with PSB for most of the agronomic and soil parameters. It could be because of enhanced P release from RP by PSB due to production of organic acids which enhances P solubalization58. It can be attributed to reduction of soil pH by PSB through the release of organic acids which increase P solubalization from RP as cited by Banik and Dey59. The PSB promoted soil acidification and enhanced crop P concentration and uptake over without PSB treatments irrespective of lime levels and P sources however, the organic sources were superior to mineral sources. This could be attributed to chelation of cations like (Al, Fe and Ca) and decreases of soil pH by the hydroxyl and carboxyl groups of acids produced by PSB and organic manures60. Ekin61 and Zabihi et al.62 indicated that PSB inoculation increases the efficiency of P fertilizers. Gulati et al.63 reported improved crop P nutrition by seed inoculation with PSB. Improvement in root biomass due to PSB inoculation may probably be due to syntheses of growth regulators at the root interface by PSB, which stimulates root development and promotes water and nutrients absorption by plants from the soil64. Our results demonstrate that, P must be applied as organic sources like PM and FYM both to calcareous and non-calcareous soils for lowering soil pH and improving soil OM contents. Sharma and Prasad52 also observed enhanced P availability with PSB inoculation which was further advanced with addition of crop residues. It may be attributed to the improvement of soil physical conditions, microbial growth, extra nutrient supplementation and soil acidification by organic sources65. The relative inferior performance of mineral sources may be due to quick fixation of available P form these sources which renders its availability to plant as reported by Biswas66. In our case organic manures (PM and FYM) application decreased soil pH, improved wheat P concentration, and uptake and nullified antagonistic effect of lime due to solubilization of native nutrients as also reported by Mitra et al.67. Similar findings were also reported by Dwivedi et al.45 in non-calcareous soils. The acidification of soil by PM and FYM may be attributed to release of organic acids during the process of their decomposition. Mitra et al.67 and Laxminarayana68 also found similar increase in P availability and uptake by integrated nutrient management in sun hemp.
Generally, liming of alkaline soils adversely affected overall soil and plant parameters; however organic manures and PSB were capable of neutralizing/minimizing this harmfulness up to some extent. It could be due to precipitation of available P with Ca+2 ion of lime in alkaline soil which render P availability in soil and its uptake by plants65. Sanyal and De Datta69 reported that P precipitates as a range of mono-(CaHPO4), di- and tri-Ca phosphates [e.g. Ca3(PO4)2] and hydrates in alkaline calcareous soils. In contrast to our finding Briedis et al.70 and Bronick and Lal71 reported that liming of an acid soils neutralize soil pH, improve root, shoot and soil organic carbon storage. We noticed that liming increased post-harvest SOM. The PSB may substitute the costly mineral fertilizers by natural, economical and eco-friendly P sources like phosphate rocks. PSB may also reduce the exogenous application of costly phosphatic fertilizers by enhancing fertilizers use efficiency through effective utilization of insoluble reserved phosphorus in calcareous alkaline soils.
Conclusions
The inoculation (PSB) was effective in improving crop growth, P nutrition and soil acidification when compared to un-inoculated control (without PSB), irrespective of P sources and varying level of lime. Individually, liming antagonized plant growth, P availability and induced soil salinity and alkalinity that’s why, non-calcareous soil is the best soil for optimum crop growth. However, PSB inoculation along with manures (PM and FYM) application potentially minimized the adverse effects of liming over mentioned traits. Solely, organic P supplements (PM and FYM) performed significantly better than mineral supplements (SSP and RP) in advancing wheat growth and soil condition but when mineral sources were inoculated with PSB its performance were mostly comparable to organic sources. Rock Phosphate with PSB has been shown as effective as sole SSP. Therefore, P application as organic manures in conjugation to PSB inoculation can be an environmental friendly and sustainable approach for improving plant growth and properties of calcareous soils. Furthermore, RP can be used as potential substitute to SSP if inoculated with PSB. It is highly recommended that more research and investigation can be done for other crops with the combine application of organic manures and PSB for sustainable agriculture. However, these findings shall be verified under diverse agro-climatic conditions on variety of crops before formulating large scale recommendations.
Materials and methods
Soil description
A surface (0–20 cm) soil (Gulyana soil series) was obtained from field under wheat–maize cultivation at Agricultural Research Station, Bajabamkhel, District Swabi, KPK-Pakistan (34° 7′ 12′′ North and 72° 28′ 20′′ East). The soil was shade dried and sieved (2 mm). It was alkaline (pH 7.56) and non-saline (0.76 d Sm−1), non-calcareous (4.78% lime) in nature and silt loam in texture. The soil was low in organic matter (0.82%), and deficient in Olsen extractable P (5.28 mg kg−1), K (78 mg kg−1) and total N (0.08 g kg−1) as shown in Table 7. According to the USDA classification system the soil was classified as Inceptisols soil with Ochric surface horizon.
Table 7.
Physico-chemical properties of soil used in experiment before cultivation.
| Property | Quantity |
|---|---|
| Bulk density | 1.24 g cm−3 |
| Textural class | Silt loam |
| Soil pH | 7.56 |
| ECe 1:2 | 0.76 dSm−1 |
| CEC | 36.1 cmole kg−1 |
| Total lime | 4.78% |
| Organic matter | 8.2 g kg−1 |
| Total nitrogen | 0.08 g kg−1 |
| Olsen P | 5.28 mg kg−1 |
| Potassium (K) | 78 mg kg−1 |
Experimental materials
The poultry and farm yard manures were purchased from nearby dairy and poultry farms, respectively, and were analyzed for their nitrogen, phosphorus and potassium (NPK) concentration (Table 8). The powdered RP was acquired from Nuclear Institute of Food and Agriculture (NIFA), Peshawar and was analysed for its P concentration. The PSB was obtained from National Agriculture Research Center and was examined for its composition, population and plant growth promoting rhizobacteria (PGPR) characterization.
Table 8.
NPK composition of P sources.
| Source | Total N | P | K |
|---|---|---|---|
| (%) | |||
| RP | – | 17.1 | – |
| PM | 2.26 | 1.4 | 1.28 |
| FYM | 1.35 | 0.88 | 1.03 |
RP, PM and FYM stands for rock phosphate, poultry manure and farm yard manure.
Characterization of used PSB
The bacterial composition of the inoculum was examined using Bergeys manual of systematic bacteriology72–74 on modified Pikovskaya’s agar medium amended with Ca3(PO4)2 as an insoluble P. Phosphate solubilization75 and P content in the culture supernatant was measured by the procedure of Nelson and Sommrs76. The PSB were tested for alkaline phosphatase activity77, siderophores78, and IAA and organic acid production79.
Experimental procedures
This pot experiment was consisted of two forms of inoculation (without PSB and with PSB), four types of P supplements [poultry manure (PM), farm yard manure (FYM), single super phosphate (SSP) and rock phosphate (RP)] applied @ of 45 mg P2O5 kg−1 soil under varying (4) lime (4.78, 10, 15 and 20%) content making 32 treatments, in pots containing 7 kg soil (including natural/added lime). Factorial (3) complete randomized design (CRD) with three replications was used. Lime was applied 30 days before sowing while, P sources and PSB were supplemented at the time of sowing. Phosphorus was supplemented as SSP, RP, PM and FYM at the rate of 45 mg P2O5 kg−1 on the basis of P contents (Table 2) as per proposed treatments. Urea and SOP were applied in solution form to all pots at the rate of 60 mg N kg−1 and 30 mg K2O kg−1 soil (including N and K added from organic sources) as a basal dose. PSB inoculum containing 1.75 × 108, cfu of PSB g−1 (wet weight), was applied as seed inoculation @ of 2 kg PSB inoculum per 120 kg seed80. Post inoculation PSB per seeds were 1.36 × 105 CFU81. Ten seeds were sown in each pot (30 cm diameter) which was later thinned to 6 plants. The pots were sited in open atmosphere followed by periodical randomization. The pots were retained at around 60% of field capacity (FC) by irrigation with tap water on daily basis as per procedure Wu et al.82. The pots were kept outside in a netted enclosure so that the pots would reflect the outside air temperature and the environment. The cultural practices recommended for pot experiments were followed during the study.
Agronomic parameters of wheat
Days to emergence for wheat were calculated by counting the days taken from date of sowing till 75% emergence occurred in all pots. Emergence per pot was taken by totaling the seedlings emerged in each pot after germination. Tillers plant−1 were recorded at maturity by counting the tillers of two randomly selected plants in each pot and then averaged. Plant height (cm) was calculated by measuring height of two randomly selected plants from base to the tip of the plants excluding awns with the help of meter rod in each pot and then averaged. Days to maturity were calculated by counting the number of days from date of planting to 75% physiological maturity. Grains spike−1 were counted by threshing two randomly selected spikes from each pot and averaged. A sample of hundred grains was taken randomly from each pot and weighed using a sensitive electronic balance to record 100 grains weight. For biological yield, all plants of each pot were harvested and allowed to dry under sun for five days and then weighed. Grain yield was recorded by weighing the grains obtained from whole pot after threshing and cleaning. The roots collected from each pot were washed, dried and their biomass was recorded via electronic balance.
Soil and plant analysis
Soil pH and EC in 1: 5 soil water saturation extract were measured by the procedure of Thomas83 and Rhoades84, correspondingly. N and K contents in soil were quantified by Kjeldhal method85 and Ryan et al.86, respectively. Olsen NaHCO3 protocol was adopted for determination of P in soil87. The lime content was calculated by titration method88, texture by Bahadur et al.89 while, soil OM content was quantified by the method of Nelson and Sommer90. Polemio and Rhoades88 protocol was used for determination of soil cation exchange capacity. Acid digestion method used by Richards91 was applied for determination of plant P concentration. P uptake was taken as a product of the plant biomass and its respective concentration in each pot.
Statistical analysis
For PGPR characterization descriptive statistics was applied for calculating standard error. Data regarding plant parameters and post-harvest soil properties were subjected to Fisher’s (F) test for analysis of variance92 by using Statistix 8.1. The results were further run for least significant difference (LSD) test to find the difference among the means.
Complies with international, national and/or institutional guidelines
Experimental research and field studies on plants (either cultivated or wild), comply with relevant institutional, national, and international guidelines and legislation. Experimental studies were carried out in accordance with relevant institutional, national or international guidelines or regulation.
Permissions or licenses
The experiment was started, after taking permission from The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan.
Identification of the plant material
Before collection, the plant was identified by Dr. Hanif Khan (Taxonomist), using the standard protocol at the Department of Soil Science, Agricultural University, Peshawar, Pakistan.
Ethics approval and consent to participate
We all declare that manuscripts reporting studies do not involve any human participants, human data, or human tissue. So, it is not applicable.
Author contributions
Conceptualization, M.A., S.F.; Data curation, M.A., M.H.S.; Formal analysis, B.A., C.C.M., R.A.M., C.W., D.W.; Investigation, M.A.; Methodology, S.S.; Project administration, M.M., R.U., A.J., M.A.; Resources, M.A., W.A.S., S.D., R.D., M.R.; Supervision, S.F.; Validation, F.W., K.L.; Funding, C.C.M., R.A.M.; Writing—original draft, M.A., R.A.M.; Writing—review & editing, K.L., M.T.H., R.A.M., S.F.
Funding
The study was supported by the National Research Development Projects to finance excellence (PFE)-14/2022-2024 Granted by the Romanian Ministry of Research and Innovation.
Data availability
The datasets generated and/or analysed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Muhammad Adnan, Email: madnan@uoswabi.edu.pk.
Shah Fahad, Email: shah_fahad80@yahoo.com.
Shah Saud, Email: saudhort@gmail.com.
Romina Alina Marc, Email: romina.vlaic@usamvcluj.ro.
References
- 1.United Nations . Transforming Our World: The 2030 Agenda for Sustainable Development. United Nations; 2015. [Google Scholar]
- 2.Salimpour S, Khavazi K, Nadian H, Besharati H, Miransari M. Enhancing phosphorous availability to canola (Brassica napus L.) using P solubilizing and sulfur oxidizing bacteria. Plant Biol. 2010;6:629–642. [Google Scholar]
- 3.Ezawa T, Smith SE, Smith FA. P metabolism and transport in AM fungi. Plant Soil. 2002;244:221–230. doi: 10.1023/A:1020258325010. [DOI] [Google Scholar]
- 4.Halajnia A, Haghnia GH, Fotovat A, Khorasani R. Phosphorus fractions in calcareous soils amended with P fertilizer and cattle manure. Geoderma. 2009;150:209–213. doi: 10.1016/j.geoderma.2009.02.010. [DOI] [Google Scholar]
- 5.Adnan M, Fahad S, Zamin M, Shah S, Mian IA, Danish S, et al. Coupling phosphate-solubilizing bacteria with phosphorus supplements improve maize phosphorus acquisition and growth under lime induced salinity stress. Plants. 2020;9:900. doi: 10.3390/plants9070900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan AA, Jilani G, Akhtar MS, Naqvi SMS, Rasheed M. Phosphorus solubilizing bacteria, occurrence, mechanisms and their role in crop production. J. Agric. Biol. Sci. 2009;1:48–58. [Google Scholar]
- 7.Torrent J, Barron V, Schwertmann U. Phosphate adsorption and desorption by goethites differing in crystal morphology. Soil Sci. Soc. Am. J. 1990;54:1007–1012. doi: 10.2136/sssaj1990.03615995005400040012x. [DOI] [Google Scholar]
- 8.Rehim A. Band-application of phosphorus with farm manure improves phosphorus use efficiency, productivity, and net returns of wheat on sandy clay loam soil. Turk. J. Agric. For. 2016;40:319–326. doi: 10.3906/tar-1505-133. [DOI] [Google Scholar]
- 9.Bieleski RL. Phosphate pools, phosphate transport and phosphate availability. Annu. Rev. Plant Physiol. 1973;24:225–252. doi: 10.1146/annurev.pp.24.060173.001301. [DOI] [Google Scholar]
- 10.Goldstein AH. Recent progress in understanding the molecular genetics and biochemistry of calcium phosphate solubilization by gram negative bacteria. Biol. Agric. Hortic. 1995;12:185–193. doi: 10.1080/01448765.1995.9754736. [DOI] [Google Scholar]
- 11.Lopez-Bucio J, Vega OM, Guevara-Garcıa A, Herrera-Estrella L. Enhanced phosphorus uptake in transgenic tobacco plants that overproduce citrate. Nat. Biotechnol. 2000;18:450–453. doi: 10.1038/74531. [DOI] [PubMed] [Google Scholar]
- 12.Tilman D, Fargione J, Wol BD, Antonio C, Dobson A, Howarth R, Schindler WH, Schlesinger D, Simberlof D, Wackhamer D. Forecasting agriculturally driven global environmental change. Science. 2001;292:281–284. doi: 10.1126/science.1057544. [DOI] [PubMed] [Google Scholar]
- 13.Sato S, Solomon D, Hyl C, Ketterings QM, Lehmann J. Phosphorus speciation in manure and manure-amended soils using XANES spectroscopy. Environ. Sci. Technol. 2000;39:7485–74919. doi: 10.1021/es0503130. [DOI] [PubMed] [Google Scholar]
- 14.Brady NC, Weil RR, Weil RR. The Nature and Properties of Soils. Prentice Hall; 2008. pp. 662–710. [Google Scholar]
- 15.Adnan M, Shah F, Zamin M, Shah S, Mian IA, Danish S, Zafar-ul-Hye M, Battaglia ML, Naz RMM, Saeed B, Saud S, Ahmad I, Yue Z, Brtnicky M, Holatko J, Datta R. Coupling phosphate solubilizing bacteria with Phosphorus supplements improve maize phosphorus acquisition and growth under lime induced salinity stress. Plants. 2020;9:900. doi: 10.3390/plants9070900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caravaca F, Alguacil MM, Azcon R, Diaz G, Roldan A. Comparing the effectiveness of mycorrhizal inoculum and amendment with sugar beet, rock phosphate and Aspergillus niger to enhance field performance of the leguminous shrub Dorycnium pentaphyllum L. Appl. Soil Ecol. 2004;25:169–180. doi: 10.1016/j.apsoil.2003.08.002. [DOI] [Google Scholar]
- 17.Zaidi A, Khan M, Ahemad MS, Oves M, Wani PA. Recent advances in plant growth promotion by phosphate-solubilizing microbes. In: Khan MS, Zaidi A, Musarrat J, editors. Microbial Strategies for Crop Improvement. Springer; 2009. pp. 23–50. [Google Scholar]
- 18.Illmer P, Barbato A, Schinner F. Solubilization of hardly-soluble AlPO4 with P-solubilizing microorganism. Soil Biol. Biochem. 1995;27:265–270. doi: 10.1016/0038-0717(94)00205-F. [DOI] [Google Scholar]
- 19.Ryan PR, Delhaize E, Jones DL. Function and mechanism of organic anion exudation from plant roots. Annu. Rev. Plant Biol. 2001;52:527–560. doi: 10.1146/annurev.arplant.52.1.527. [DOI] [PubMed] [Google Scholar]
- 20.Chen YP, Rekha PD, Arun AB, Shen FT, Lai WA, Young CC. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl. Soil Ecol. 2006;34:33–41. doi: 10.1016/j.apsoil.2005.12.002. [DOI] [Google Scholar]
- 21.Adnan M, Fahad S, Khan IA, Saeed M, Saud S, Ihsan MZ, Raiz M, Wang D, Wu C. Integration of poultry manure and phosphate solubilizing bacteria improved availability of Ca bound P in calcareous soils. 3 Biotech. 2019;9:368. doi: 10.1007/s13205-019-1894-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He Z, Zhu J. Microbial utilization and transformation of phosphate adsorbed by variable charged minerals. Soil Biol. Biochem. 1988;30:917–923. doi: 10.1016/S0038-0717(97)00188-0. [DOI] [Google Scholar]
- 23.Kucey RMN. Effect of Penicillium bilajion the solubility and uptake of P and micronutrients from soil by wheat. Can. J. Soil Sci. 1988;68:261–270. doi: 10.4141/cjss88-026. [DOI] [Google Scholar]
- 24.Bünemann EK, Bossio DA, Smithson PC, Frossard E, Oberson A. Microbial community composition and substrate use in a highly weathered soil as affected by crop rotation and P fertilization. Soil Biol. Biochem. 2004;36:889–901. doi: 10.1016/j.soilbio.2004.02.002. [DOI] [Google Scholar]
- 25.McGill WB, Cole CV. Comparative aspects of cycling of organic C, N, S and P through soil organic matter. Geoderma. 1981;26:267–268. doi: 10.1016/0016-7061(81)90024-0. [DOI] [Google Scholar]
- 26.Chaiharn M, Lumyong S. Screening and optimization of indole-3-acetic acid production and phosphate solubilization from rhizobacteria aimed at improving plant growth. Curr. Microbiol. 2011;62:173–181. doi: 10.1007/s00284-010-9674-6. [DOI] [PubMed] [Google Scholar]
- 27.Kucey RMN, Janzen HH, Legett ME. Microbially mediated increases in plant-available phosphorus. Adv. Agron. 1989;42:198–228. [Google Scholar]
- 28.Rodriguez H, Fraga R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999;17:319–339. doi: 10.1016/S0734-9750(99)00014-2. [DOI] [PubMed] [Google Scholar]
- 29.Xiao Y, Wang X, Chen W, Huang Q. Isolation and identification of three potassium-solubilizing bacteria from rape rhizospheric soil and their effects on ryegrass. Geomicrobiol. J. 2017;34:873–880. doi: 10.1080/01490451.2017.1286416. [DOI] [Google Scholar]
- 30.Sugihara S, Funakawa S, Kilasara M, Kosaki T. Dynamics of microbial biomass nitrogen in relation to plant nitrogen uptake during the crop growth period in a dry tropical cropland in Tanzania. Soil Sci. Plant Nutr. 2010;56:105–114. doi: 10.1111/j.1747-0765.2009.00428.x. [DOI] [Google Scholar]
- 31.Jalili F, Khavazi K, Pazira E, Nejati A, Rahmani AH, Rasuli SH, Miransari M. Isolation and characterization of ACC deaminase producing fluorescent pseudomonads, to alleviate salinity stress on canola (Brassica napus L.) growth. J. Plant Physiol. 2009;166:667–674. doi: 10.1016/j.jplph.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Tiwari VN, Lehri LK, Pathak AN. Effect of inoculating crops with phospho-microbes. Exp. Agric. 1989;25:47–50. doi: 10.1017/S0014479700016434. [DOI] [Google Scholar]
- 33.Pal SS. Interaction of an acid tolerant strain of phosphate solubilizing bacteria with a few acid tolerant crops. Plant Soil. 1999;213:221–230. doi: 10.1023/A:1004539502221. [DOI] [Google Scholar]
- 34.Afzal A, Ashraf M, Asad SA, Faroog M. Effect of phosphate solubilizing microorganism on phosphorus uptake, yield and yield traits of wheat (Triticum aestivum L.) in rainfed area. Int. J. Agric. Biol. 2005;7:207–209. [Google Scholar]
- 35.Bolan NS, Naidu R, Mahimairajaand S, Baskaran S. Influence of low-molecular-weight organic acids on the solubilization of phosphates. Biol. Fertil. Soils. 1994;18:311–319. doi: 10.1007/BF00570634. [DOI] [Google Scholar]
- 36.Mihoub A, Amin AEEAZ, Motaghian HR, Saeed MF, Naeem A. Citric acid (CA)–modified biochar improved available phosphorus concentration and its half-life in a P-fertilized calcareous sandy soil. J. Soil Sci. Plant Nutr. 2022;22(1):465–474. doi: 10.1007/s42729-021-00662-2. [DOI] [Google Scholar]
- 37.Adnan M, Shah Z, Sharif M, Rahman H. Liming induces carbon dioxide (CO2) emission in PSB inoculated alkaline soil supplemented with different phosphorus sources. Environ. Sci. Pollut. Res. 2018;25(10):9501–9509. doi: 10.1007/s11356-018-1255-4. [DOI] [PubMed] [Google Scholar]
- 38.Amin AEEAZ, Mihoub A. Effect of sulfur-enriched biochar in combination with sulfur-oxidizing bacterium (Thiobacillus spp.) on release and distribution of phosphorus in high calcareous p-fixing soils. J. Soil Sci. Plant Nutr. 2021;21(3):2041–2047. doi: 10.1007/s42729-021-00500-5. [DOI] [Google Scholar]
- 39.Tawaraya K, Hirose R, Wagatsuma T. Inoculation of arbuscularmycorrhizal fungi can substantially reduce phosphate fertilizer application to Alliumfis-tulosum L. and achieve marketable yield underfield condition. Biol. Fertil. Soils. 2012;48:839–843. doi: 10.1007/s00374-012-0669-2. [DOI] [Google Scholar]
- 40.Islam MT, Hossain MM. Plant probiotics in phosphorus nutrition in crops, with special reference to rice. In: Maheshwari DK, editor. Bacteria in Agrobiology, Plant Probiotics. Springer; 2012. pp. 325–363. [Google Scholar]
- 41.Amruthesh, K. N., Raj, S. N., Kiran, B., Shetty, H. S. & Reddy, M. S. Growth promotion by plant growth-promoting rhizobacteria in some economically important crop plants. In Sixth International PGPR Workshop, 5–10 October, Calicut, India, 97–103 (2003).
- 42.Kumar S, Lai L, Kumar P, Feliciano YMV, Battaglia ML, Hong CO, Owens VN, Fike J, Farris R, Galbraith J. Impacts of nitrogen rate and landscape position on soils and switchgrass root growth parameters. Agron. J. 2019;111:1046–1059. doi: 10.2134/agronj2018.08.0483. [DOI] [Google Scholar]
- 43.Mihoub A, Boukhalfa-Deraoui N. Performance of different phosphorus fertilizer types on wheat grown in calcareous sandy soil of El-Menia, Southern Algeria. Asian J. Crop Sci. 2014;6:383–391. doi: 10.3923/ajcs.2014.383.391. [DOI] [Google Scholar]
- 44.Piccini D, Azcon R. Effect of phosphate solubilizing bacteria and vesicular-arbuscular mycorrhizal fungi on the utilization of Bayovar rock phosphate by alfalfa plants using a sand-vermiculite medium. Plant Soil. 1987;50:45–50. doi: 10.1007/BF02371029. [DOI] [Google Scholar]
- 45.Dwivedi BS, Singh VK, Dwivedi V. Application of phosphate rock, with or without Aspergillus awamori inoculation, to meet phosphorus demands of rice–wheat systems in the Indo Gangetic plains of India. Aus. J. Exp. Agric. 2004;44:1041–1050. doi: 10.1071/EA03208. [DOI] [Google Scholar]
- 46.Saad OAO, Hammad AMM. Fertilizing wheat plants with rock phosphate combined with phosphate dissolving bacteria and V.A mycorrhiza as alternate for ca–superphosphate. Ann. Agric. Sci. Cairo. 1998;43:445–460. [Google Scholar]
- 47.Chabot R, Antoun H. Growth promotion of maize and lettuce by phosphate solubilizing Rhizobium leguminosarum. Plant Soil. 1996;184:311–321. doi: 10.1007/BF00010460. [DOI] [Google Scholar]
- 48.Kundu BS, Gaur AC. Rice response to inoculation with N2 fixing and P solubilizing microorganisms. Plant Soil. 1984;79:227–234. doi: 10.1007/BF02182344. [DOI] [Google Scholar]
- 49.Sharma GD, Thakur R, Raj S, Kauraw DL, Kulhare PS. Impact of integrated nutrient management on yield, nutrient uptake, protein content of wheat (Triticum aestivum) and soil fertility in a typic Haplustert. Bioscan. 2013;8:1159–1164. [Google Scholar]
- 50.Afzal A, Asghari B. Rhizobium and phosphate solubilizing bacteria improve the yield and phosphorus uptake in wheat (Triticum aestivum) Int. J. Agric. Biol. 2008;10:85–88. [Google Scholar]
- 51.Jalili G, Akram A, Ali RM, Hafeez FY, Shamsi IH, Chaudhry AN, Chaudhry AG. Enhancing crop growth, nutrients availability, economics and beneficial rhizosphere micro flora through organic and bio fertilizers. Ann. Microbiol. 2007;57(2):177–183. doi: 10.1007/BF03175204. [DOI] [Google Scholar]
- 52.Sharma SN, Prasad R. Yield and P uptake by rice and wheat grown in a sequence as influenced by phosphate fertilization with diammonium phosphate and Mussoorie rock phosphate with or without crop residues and phosphate solubilizing bacteria. J. Agric. Sci. 2003;141:359–369. doi: 10.1017/S0021859603003678. [DOI] [Google Scholar]
- 53.Vyas P, Gulati A. Organic acid production in vitro and plant growth promotion in maize under controlled environment by phosphate-solubilizing fluorescent Pseudomonas. BMC Microbiol. 2009;9:174. doi: 10.1186/1471-2180-9-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mukherjee PK, Rai RK. Sensitivity of P uptake to change in root growth and soil volume as influenced by VAM, PSB and P levels in wheat and chickpeas. Ann. Agric. Res. 1999;20:528–530. [Google Scholar]
- 55.Egamberdiyeva, D. Proc. Inst. Microbiol. Tashkent, Uzekistan (2004).
- 56.Mihoub A, Daddi Bouhoun M, Naeem A, Saker ML. Low-molecular weight organic acids improve plant availability of phosphorus in different textured calcareous soils. Arch. Agron. Soil Sci. 2017;63:1023–1034. doi: 10.1080/03650340.2016.1249477. [DOI] [Google Scholar]
- 57.Thakuria D, Talukdar NC, Goswami C, Hazarika S, Boro RC, Khan MR. Characterization and screening of bacteria from rhizosphere of rice grown in acidic soils of Assam. Curr. Sci. 2004;86:978–985. [Google Scholar]
- 58.Mamta P, Praveen G, Vijaylata S, Arving KB, Bikram T, Ravinder R. Stimulatory effect of phosphate solubilizing bacteria on plant growth, stevioside and rebaudioside-A content of Stevia rebaudiana Bertoni. Appl. Soil Ecol. 2010;46:222–229. doi: 10.1016/j.apsoil.2010.08.008. [DOI] [Google Scholar]
- 59.Banik SBK. Solubilization of inorganic phosphate and production of organic acids by micro-organisms isolated in sucrose tricalcium phosphate agar plate. Zentralblat. Bakterol. Parasilenkl. Infektionskr. Hyg. 1981;136:478–486. [Google Scholar]
- 60.Stevenson FJ. Cycles of Soil: Carbon, Nitrogen, Phosphorus, Sulfur, Micro-nutrients. Wiley; 2005. [Google Scholar]
- 61.Ekin Z. Performance of phosphorus solubilizing bacteria for improving growth and yield of sun flower (Helianthus annuus L.) in the presence of phosphorus fertilizer. Afr. J. Biotechnol. 2010;9:3794–3800. [Google Scholar]
- 62.Zabihi HR, Savaghebi GR, Khavazi K, Ganjali A, Miransari M. Pseudomonas bacteria and phosphorus fertilization, affecting wheat (Triticum aestivum L.) yield and P uptake under green house and field conditions. Acta Physiol. Plant. 2010;33:145–152. doi: 10.1007/s11738-010-0531-9. [DOI] [Google Scholar]
- 63.Gulati A, Rahi P, Vyas P. Characterization of phosphate-solubilizing fluorescent Pseudomonas from the rhizosphere of seabuckthorn growing in the cold deserts of Himalayas. Curr. Microbiol. 2007;56:73–79. doi: 10.1007/s00284-007-9042-3. [DOI] [PubMed] [Google Scholar]
- 64.Kloepper JW, Lifshitz R, Zablotowicz RM. Free-living bacterial inocula for enhancing crop productivity. Trends Biotechnol. 1989;7:39–44. doi: 10.1016/0167-7799(89)90057-7. [DOI] [Google Scholar]
- 65.Satchell JE. Ecology and environment in the United Arab Emirates. J. Arid. Environ. 1978;1:201–226. doi: 10.1016/S0140-1963(18)31724-5. [DOI] [Google Scholar]
- 66.Biswas DR. Nutrient recycling potential of rock phosphate and waste mica enriched compost on crop productivity and changes in soil fertility under potato–soybean cropping sequence in an Inceptisol of Indo-Gangetic Plains of India. Nutr. Cycl. Agroecosyst. 2011;89:15–30. doi: 10.1007/s10705-010-9372-6. [DOI] [Google Scholar]
- 67.Mitra S, Roy A, Saha AR, Maitra DN, Sinha MK, Mahapatra BS, Saha S. Effect of integrated nutrient management on fiber yield, nutrient uptake and soil fertility in jute (Corchorus olitorius) Indian J. Anim. Sci. 2010;80(9):801–804. [Google Scholar]
- 68.Laxminarayana K. Effect of integrated use of inorganic and organic manures on soil properties, yield and nutrient uptake of rice in Ultisols of Mizoram. J. Indian Soc. Soil Sci. 2006;54:120–123. [Google Scholar]
- 69.Sanyal SK, De Datta SK. Chemistry of phosphorus transformations in soil. Adv. Soil Sci. 1991;16:1–120. [Google Scholar]
- 70.Briedis C, Sá JCM, Caires EF, Navarro JF, Inagaki TM, Boer A, Neto CQ, Ferreira AO, Canalli LB, Santos JB. Soil organic matter pools and carbon-protection mechanisms in aggregate classes influenced by surface liming in a no-till system. Geoderma. 2012;170:80–88. doi: 10.1016/j.geoderma.2011.10.011. [DOI] [Google Scholar]
- 71.Bronick CJ, Lal R. Soil structure and management: A review. Geoderma. 2005;124:3–22. doi: 10.1016/j.geoderma.2004.03.005. [DOI] [Google Scholar]
- 72.Krieg NR, Holt JG. Bergey’s Manual of Systemetic Bacteriology. Williams & Wilkin; 1984. p. 984. [Google Scholar]
- 73.Holt JG, Krieg NR, Sneath PHA, Staley JT, Williams ST, editors. Bergey’s Manual of Determinative Bacteriology. 9. The Williams & Wilkin; 1994. p. 787. [Google Scholar]
- 74.Gordon RE, Haynes WC, Pang CN. The Genus Bacillus. Agricultural Handbook. No. 427. Department of Agriculture; 1973. p. 283. [Google Scholar]
- 75.Nautiyal CS. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999;170(1):265–270. doi: 10.1111/j.1574-6968.1999.tb13383.x. [DOI] [PubMed] [Google Scholar]
- 76.Nelson DW, Sommers LE. Total carbon, organic carbon, and organic matter. In: Page AL, editor. Methods of Soil Analysis, Part 2. 2. Wiley; 1996. pp. 961–1010. [Google Scholar]
- 77.Eivazi F, Tabatabai M. Phosphatases in soils. Soil Biol. Biochem. 1977;9:167–172. doi: 10.1016/0038-0717(77)90070-0. [DOI] [Google Scholar]
- 78.Alexander DB, Zuberer DA. Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol. Fertil. Soils. 1991;12:39–45. doi: 10.1007/BF00369386. [DOI] [Google Scholar]
- 79.Vincet JMA. Manual for the Practical Study of the Root-Nodule Bacteria; IBPH and Book No. 15. Blackwell Scientific Publication; 1970. [Google Scholar]
- 80.Alagawadi AR, Gaur AC. Associative effect of Rhizobium and phosphate solubilizing bacteria on the yield and nutrient uptake of chickpea. Plant Soil. 1988;105:241–246. doi: 10.1007/BF02376788. [DOI] [Google Scholar]
- 81.Satyaprakash M, Nikitha T, Reddi EUB, Sadhana B, Vani SS. Phosphorous and phosphate solubilising bacteria and their role in plant nutrition. Int. J. Curr. Microbiol. Appl. Sci. 2017;6:2133–2144. doi: 10.20546/ijcmas.2017.604.251. [DOI] [Google Scholar]
- 82.Wu SC, Cao ZH, Li ZG, Cheung KC, Wong MH. Effects of biofertilizer containing N-fixer, P and K solubilizers and AM fungi on maize growth: A greenhouse trial. Geoderma. 2005;125:155–166. doi: 10.1016/j.geoderma.2004.07.003. [DOI] [Google Scholar]
- 83.Thomas GW. Soil pH and soil acidity. In: Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA, Johnston CT, Sumner ME, editors. Methods of Soil Analysis, Part 3, Chemical Methods. Wiley; 1996. pp. 475–490. [Google Scholar]
- 84.Rhoades JD. Salinity, electrical conductivity and total dissolved solids. In: Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA, Johnston CT, Sumner ME, editors. Methods of Soil Analysis, Part 3, Chemical Methods. Soil Science Society of America; 1996. pp. 417–435. [Google Scholar]
- 85.Bremner JM, Breitenbeck GA. A simple method for determination of ammonium in semi-micro Kjeldahl analysis of soil and plant material using a block digestor. Commun. Soil Sci. Plant Anal. 1983;14:905–913. doi: 10.1080/00103628309367418. [DOI] [Google Scholar]
- 86.Ryan J, Estefan G, Rashid A. Soil and Plant Analysis Laboratory Manual. 2. The National Agricultural Research Center (NARC); 2001. p. 172. [Google Scholar]
- 87.Olsen SR, Cole CV, Watanabe FS, Dean LA. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate (No. 939) Department of Agriculture Circular; 1954. [Google Scholar]
- 88.Loeppert RH, Suarez DL. Carbonate and gypsum. In: Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA, Johnston CT, Sumner ME, editors. Methods of Soil Analysis, Part 3, Chemical Methods. Soil Science Society of America; 1996. pp. 181–197. [Google Scholar]
- 89.Bahadur L, Tiwari DD, Mishra J, Gupta BR. Effect of integrated nutrient management on yield, microbial population and changes in soil properties under rice-wheat cropping system in sodic soil. J. Indian Soc. Soil Sci. 2012;60(4):326–329. [Google Scholar]
- 90.Nelson DW, Sommers LE, et al. Total carbon, organic carbon, and organic matter. In: Sparks DL, et al., editors. Methods of Soil Analysis, Part 2. 2. Soil Science Society of America; 1996. pp. 961–1010. [Google Scholar]
- 91.Richards LA. Diagnosis and improvement of saline and alkali soils. LWW. 1954;78(2):154. [Google Scholar]
- 92.Steel RGD, Torrie JH. Principles and Procedures of Statistics, a Biometrical Approach. McGraw Hill; 1996. pp. 195–233. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available, but are available from the corresponding author on reasonable request.