Abstract
Klotho is a life extension factor that has the ability to regulate the function of GluN2B-containing N-methyl-d-aspartate receptors (NMDARs), whose dysfunction in the nucleus accumbens (NAc) underlies critical aspects of the pathophysiology of major depression. Here, we study the functional relevance of klotho in the pathogenesis of depression. A chronic social defeat stress paradigm, in which mice are categorized as either susceptible or unsusceptible based on their performance in a social interaction test, was used in this study. We found that the expression of klotho was largely decreased in the NAc of susceptible mice compared to control or unsusceptible mice. Genetic knockdown of klotho in the NAc induced behavioral alterations relevant to depression in naive mice, while overexpression of klotho produced an antidepressive effect in normal mice and ameliorated the behavioral responses to stress in susceptible mice. Molecularly, knockdown of klotho in the NAc resulted in selective decreases in total and synaptic GluN2B expression that were identical to those in susceptible mice. Elevation of klotho in the NAc reversed the reductions in GluN2B expressions and altered synaptic transmission and spine density in the NAc of susceptible mice. Furthermore, blockade of GluN2B with a specific antagonist abolished the beneficial effects of klotho elevation in susceptible mice. Collectively, we demonstrated that klotho in the NAc modulates behavioral responses to stress by regulating the function of GluN2B-containing NMDARs. These results reveal a novel role for klotho in the pathogenesis of depression, providing new insights into the molecular basis of major depression.
Subject terms: Striatum, Depression
Introduction
Depression is a common type of affective disorder that is characterized by marked and persistent low mood and loss of interest, leading to serious functional impairments in patients [1]. The Global Burden of Disease Study demonstrated that depression was the second leading cause of disability-adjusted life-years in 2020 [2]. Numerous antidepressants are currently used in clinical practice; however, it still takes weeks for patients to obtain therapeutic effects, and some patients are poorly tolerant to antidepressants [3]. Understanding the pathophysiological mechanisms underlying major depression would shed light on the treatment of major depressive disorders.
The nucleus accumbens (NAc), a brain region located in the ventral aspect of the basal ganglia, is widely recognized as the center of reward and motivation [4]. NAc medium spiny neurons display a two-state membrane potential controlled by active channels and synaptic input [5], thereby affecting the functional activity in networks that underlie cognition and behavior [6]. The NAc receives glutamatergic inputs from the medial prefrontal cortex (mPFC), basolateral amygdala and hippocampus. Accumulating evidence has shown that altered glutamatergic transmission in the NAc contributes importantly to the pathophysiology of depression [7–10]. Synaptic glutamate release in the mPFC-NAc is decreased in mice exhibiting behaviors relevant to depression [9], and the decreased activity at glutamatergic synapses obstructs the later induction of long-term depression (LTD) in the NAc of these mice [11, 12]. Our previous study revealed that chronic stress caused persistent downregulation of total and synaptic GluN2B, an N-methyl-D-aspartate receptor (NMDAR) subunit with key functions in learning and memory, in the NAc and disrupted the induction of NMDAR-dependent LTD of cortico-accumbal glutamatergic synapses, while restoration of GluN2B loss reversed the stress-induced LTD deficit and alleviated behavioral responses to stress in chronic social defeat stress (CSDS)-susceptible mice, indicating that downregulation of GluN2B function in the NAc underlies the synaptic and behavioral adaptations to chronic stress [8].
The klotho protein is a recently discovered protein that is associated with life extension. Overexpression of klotho extends lifespan, whereas loss of klotho leads to accelerated aging and a short life [13–15]. In addition to lifespan extension, klotho has also been linked to cognition and other neuropsychiatric disorders. For example, knockout of klotho resulted in memory retention deficits in mice [16], while elevation of klotho expression can enhance hippocampus-dependent learning and memory in normal rodents and protect against cognitive decline in animal models of Alzheimer’s disease (AD) [17–19]. In a previous clinical observational study, AA Prather [20] found that women under high chronic stress displayed significantly lower levels of serum klotho than low-stress controls. KLOTH gene variants have been found to influence the response to selective serotonin reuptake inhibitors (SSRIs) in late-life major depressive disorder [21]. Electroconvulsive therapy (ECT), a highly effective antidepressant treatment, significantly enhanced the levels of klotho in the cerebrospinal fluid of geriatric patients with major depression [22]. However, whether klotho is involved in the pathogenesis of major depression remains unclear.
Klotho is highly expressed in the choroid plexus and neurons, as well as in the kidney and reproductive organs. Its transmembrane form can be released by sheddases and circulate in serum and cerebrospinal fluid throughout life [23]. Studies have demonstrated that klotho can enhance the function of the GluN2B subunit [18, 19]. Upregulation of klotho expression was found to promote hippocampal synaptic plasticity and cognition by enriching synaptic GluN2B in the hippocampus of mice, while blocking GluN2B abolished the beneficial effects of klotho elevation [18]. Elevating klotho expression in human amyloid precursor protein (hAPP) transgenic mice increased the abundance of GluN2B in postsynaptic densities to improve spatial learning and memory [24]. In view of the important role of accumbal GluN2B loss in the pathophysiology of depression [8], as well as the ability of klotho to regulate GluN2B function, it is possible that klotho in the NAc also fulfills important functions in the pathogenesis of major depression. To test this hypothesis, we first investigated whether the expression of klotho was changed in the NAc from mice displaying behaviors relevant to depression and then explored the influences of modulating accumbal klotho expression on behavioral responses to stress in mice. By constructing a stable adeno-associated virus vector system to regulate the expression of klotho in the NAc of mice, we demonstrated that klotho in the NAc modulates behavioral responses to stress in mice by regulating the surface stability of the GluN2B subunit.
Materials and methods
Animals
Adult male C57BL/6 J mice (8–10 weeks old) and male CD1 mice (6 months old) were purchased from Hunan SJA Laboratory Animal Co., Ltd. (Changsha, Hunan, China). Mice were fed under standard conditions (12 h light/dark cycle; lights on from 07:00 to 19:00; 23 ± 1 °C ambient temperature; 55 ± 10% relative humidity), with free access to food and water. All behavioral experiments were performed in the day and conducted in compliance with the Guide for the Care and Use of Laboratory Animals (8th edition, Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences, Washington DC). This research was approved by the Review Committee for the Use of Human or Animal Subjects of Jiangxi Mental Hospital. Experimenters were blinded to the experimental groups, and the order of testing was counterbalanced during behavioral experiments.
Social interaction test (SIT)
Social avoidance behavior was assessed with novel CD1 mice in a two-stage social interaction test. In the first 2.5-min test (target absent), the defeated mice were allowed to freely explore an arena (44 × 44 cm) containing a plexiglass and wire mesh enclosure (10 × 6 cm) against one wall of the arena. In the second 2.5-min test (target present), the experimental mice were returned to the area with a novel CD1 mice enclosed in the plexiglass wire mesh cage. This allowed the animal to see, hear, and smell but not physically contact the other mouse. Time spent in the ‘interaction zone’ (14 × 26 cm) surrounding the plexiglass wire mesh cage and ‘distance traveled’ within the arena were recorded by ANY-maze tracking software (ANY-maze, Wood Dale, IL). The segregation of susceptible and unsusceptible mice was based on the social interaction ratio, which was calculated as the time in the interaction zone when the target was present/time in the interaction zone when the target was absent × 100%. Defeated mice with a social interaction ratio < 100% were defined as ‘susceptible’, while those with a social interaction ratio ≥ 100% were defined as ‘unsusceptible’.
Additional experimental procedures and statistics are described in the Supplemental Information.
Results
CSDS significantly decreased klotho expression in the NAc of susceptible mice
The CSDS has good predictive validity for modeling the symptomatology of depression; thus, we adopted this model to investigate the role of klotho in depression in this study. C57BL/6 J mice were exposed to 10 consecutive days of stress and then designated as susceptible or unsusceptible mice based on social interaction ratios (Fig. S1a). Compared to control and unsusceptible mice, susceptible mice displayed a significant decrease in sucrose preference (Fig. S1b, p < 0.01) and a significant increase in immobility time in both the FST and the TST (Fig. S1c and d, p < 0.01). Reduced social interaction and sucrose preference and increased immobility time in the FST and TST are important indicators of depression-related behaviors in mice [8]. The above behavioral results demonstrated that CSDS could successfully induce behavioral alterations relevant to depression in mice, thus CSDS-susceptible mice were used as the model mice of major depression in subsequent experiments.
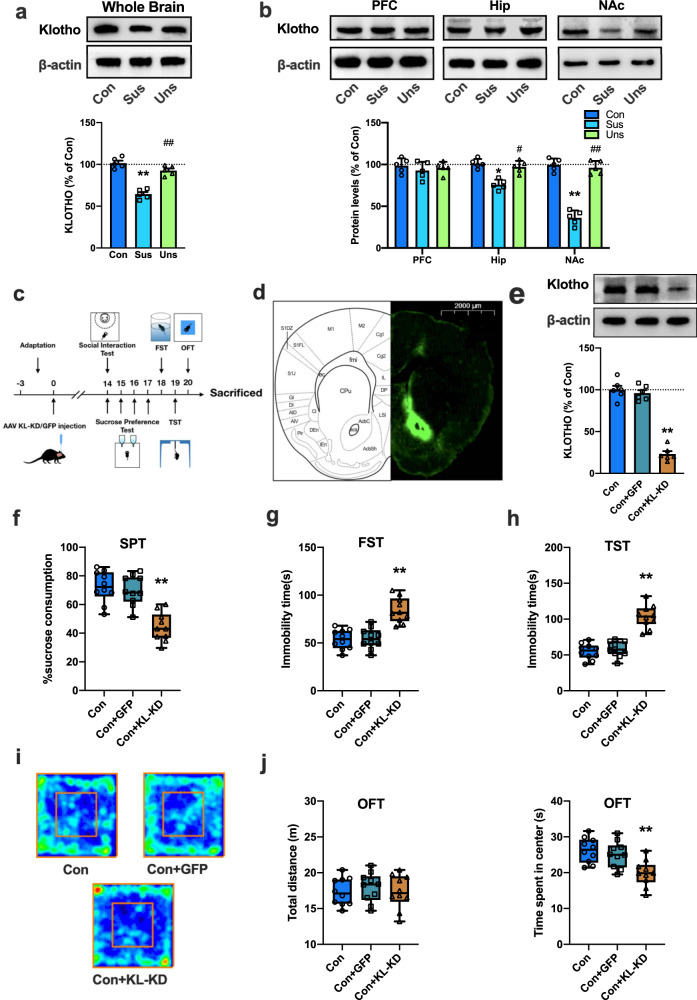
To explore whether CSDS results in changes in klotho expression in the brain, the protein level of klotho was determined by western blot analysis. As shown in Fig. 1a, klotho protein expression in the whole brain was significantly decreased in susceptible mice compared with control or unsusceptible mice (p < 0.01). Then, the expression of klotho in brain regions closely related to depression, including the PFC, hippocampus and NAc, was detected. As shown in Fig. 1b, there was no significant difference in klotho expression in the PFC among the control, unsusceptible and susceptible groups. However, compared to control or unsusceptible mice, the levels of klotho in the hippocampus and NAc of susceptible mice were significantly decreased. Klotho proteins in the hippocampus and NAc of susceptible mice were reduced by 25.2 ± 3.7% (p < 0.05 vs. control) and 63.2 ± 5.2% (p < 0.01 vs. control), respectively. Quantitative PCR with reverse transcription (qRT-PCR) analyses of mRNA extracts showed that the expression of klotho mRNA in the NAc of susceptible mice did not differ from that in control or unsusceptible mice (Fig. S2, p > 0.05), indicating that the downregulation of klotho in the NAc of susceptible mice was not due to a change in gene expression.
Fig. 1. Downregulation of klotho in the NAc contributes to the behavioral responses to stress in mice.
a Representative images of western blotting and histograms showing the level of klotho protein in whole brains from the control, susceptible and unsusceptible mice (n = 5 mice per group). b The expression of klotho protein in the PFC, Hip, and NAc from the control, susceptible and unsusceptible mice (n = 5 mice per group). c Timeline of the experimental procedures. d Fluorescence image of a fixed brain section which expressed AAV-KL-KD in the NAc 14 days after stereotactic injection (Scale bar = 2000 μm). e Representative western blotting images and histograms showing the protein expression of klotho in the NAc in each group (n = 6 mice per group). f KL-KD in the NAc significantly decreased sucrose preference in mice. g, h KL-KD in the NAc increased the immobility time in FST (g) and TST (h) in mice. i Representative images of movement tracks in the OFT. j KL-KD in the NAc did not affect total traveled distance (left), but significantly decreased the time spent in the central area (right) in the OFT in mice. For (a), (b) and (e), data are presented as normalized mean ± SEM. For (f–j), all box and whisker plot displays the median, first and third quartiles (boxes), and the min-max (whiskers) (n = 10 mice per group). *p < 0.05 and **p < 0.01 vs. control. #p < 0.05 and ##p < 0.01 vs. susceptible group or GFP group.
Genetic knockdown of klotho in the NAc induced behavioral alterations relevant to depression in mice
Given that the level of klotho protein was largely decreased in the NAc of mice after CSDS, we presumed that abnormal accumbal klotho signaling might be involved in the pathogenesis of depression. To test this hypothesis, we investigated whether downregulation of klotho expression in the NAc would result in behavioral alterations relevant to depression in mice (Fig. 1c). We used an adeno-associated viral vector (AAV) to specifically reduce klotho expression in the NAc. AAV-klotho knockdown-GFP (KL-KD) or AAV-Null-GFP (GFP) was stereotaxically infused into the NAc of naive mice. The expression of klotho in the NAc was detected 2 weeks after injection. We found numerous GFP-positive cells in the NAc region after injection (Fig. 1d), and the protein expression of klotho in the NAc of KL-KD mice was significantly decreased compared to that in control or GFP group mice (Fig. 1e, p < 0.01 vs. control), indicating that AAV-mediated knockdown of klotho in the NAc was successful.
Next, we examined the behavioral performance of mice with klotho knockdown in the NAc. As expected, compared to control mice, KL-KD mice had a significantly lower level of sucrose consumption (Fig. 1f, p < 0.01 vs. control group). The immobility time of mice in the KL-KD group was also significantly increased in the FST and TST (Fig. 1g, h, p < 0.01 vs. control group). The open field test showed no significant difference among groups in the distance traveled (p > 0.05), while the time spent in the center square was significantly decreased in KL-KD mice (Fig. 1i and j, p < 0.01 vs. control group), indicating that the behavioral alterations in KL-KD mice were not due to a change in spontaneous locomotor activity but rather behavioral changes relevant to depression. AAV-control-GFP (GFP) mice did not display any behavioral differences from the control group. These results suggest that a reduction in klotho in the NAc may result in behavioral alterations relevant to depression in mice.
Genetic overexpression of klotho in the NAc produces antidepressive effects in both normal and CSDS susceptible mice
To verify the role of klotho signaling in depression, we investigated whether elevation of klotho expression in the NAc would affect behavioral responses to stress. We first tested the influence of elevating accumbal klotho on behaviors relevant to depression in normal mice. AAV was employed to enhance klotho expression in the NAc. AAV-klotho overexpression-GFP (KL-OE) or AAV-Null-GFP (GFP) was stereotaxically infused into the NAc of normal mice. Two weeks after injection, the protein expression of klotho in the NAc of KL-OE mice was significantly increased compared to the expression level in control or GFP group mice (Fig. S3a, p < 0.01 vs. control or GFP group). Increased sucrose consumption in the SPT and decreased immobility time in the FST were observed in these KL-OE mice (Fig. S3b, c, both p < 0.05 vs. the control group), indicating that elevation of accumbal klotho can exert an antidepressive effect in normal mice.
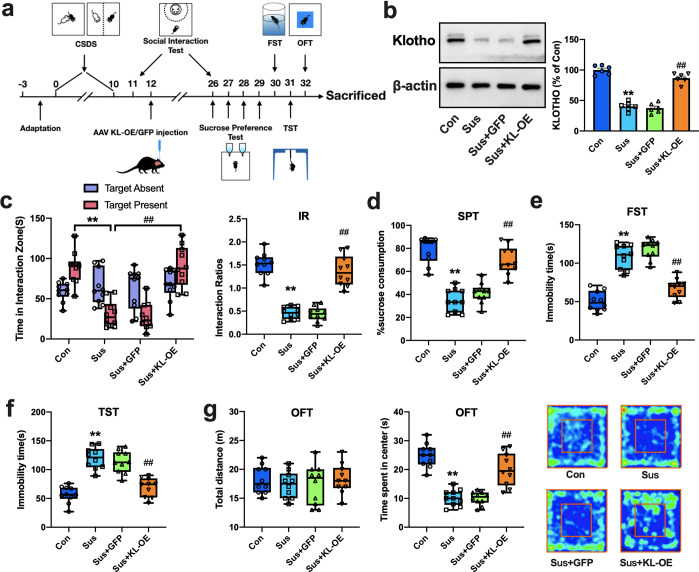
Then, we further explored whether elevation of klotho expression in the NAc would attenuate the behavioral responses to stress in CSDS susceptible mice (Fig. 2a). As shown in Fig. 2b, KL-OE increased the protein expression of klotho in the NAc of susceptible mice to a level comparable to that in control group mice (p < 0.01 vs. susceptible or susceptible-GFP group). The results from the social interaction test revealed that accumbal KL-OE significantly increased the exploration time in the interaction zone and the interaction ratio in susceptible mice (Fig. 2c, p < 0.01 vs. susceptible or susceptible-GFP group). The decreased sucrose preference in susceptible mice was also significantly reversed by KL-OE (Fig. 2d, p < 0.01 vs. susceptible or susceptible-GFP group). Furthermore, accumbal KL-OE in susceptible mice shortened the immobility time in the FST (Fig. 2e) and TST (Fig. 2f) to levels comparable to those in the control group (p < 0.01 vs. susceptible or susceptible-GFP group). The open field test showed that accumbal KL-OE in susceptible mice did not affect the distance traveled by mice but increased the time spent in the center (Fig. 2g, p < 0.01 vs. susceptible or susceptible-GFP group). These results demonstrated that elevation of klotho in the NAc ameliorated behavioral responses to stress in susceptible mice, confirming the critical modulatory effects of accumbal klotho in depression-related behaviors in mice.
Fig. 2. Genetic overexpression of klotho in the NAc ameliorates the behavioral responses to stress in CSDS susceptible mice.
a Timeline of the experimental procedures. b Representative western blotting images and histograms showing the protein expression of klotho in the NAc in each group (n = 6 mice per group). c KL-OE in the NAc significantly increased the time in interaction zone (left) and social interaction ratios (right) in susceptible mice. d–f KL-OE in the NAc significantly reversed the decreased sucrose preference (d) and the increased immobility time in FST (e) and TST (f) in susceptible mice. g KL-OE in the NAc did not affect total traveled distance (left), but increased the time spent in the central area (middle) in OFT in susceptible mice. Representative images of movement tracks in OFT were shown in the right. For b, data were presented as normalized mean ± SEM. For c–g, all box and whisker plot display the median, first and third quartiles (boxes), and the min-max (whiskers) (n = 10 mice per group). **p < 0.01 vs. control; ##p < 0.01 vs. susceptible or susceptible-GFP group.
Knockdown of klotho in the NAc resulted in selective decreases in total and synaptic GluN2B that were identical to those in CSDS susceptible mice
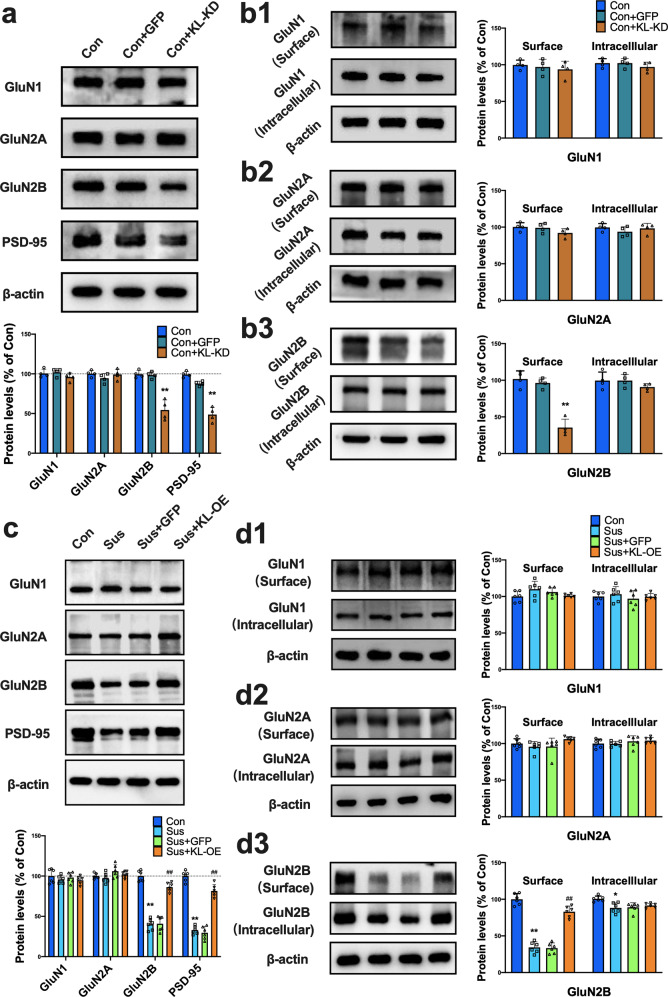
To explore the potential mechanisms underlying the modulatory effects of klotho on behavioral responses to stress, we turned our attention to NMDARs, whose dysfunction has been demonstrated to mediate behavioral and synaptic adaptations to chronic stress [8]. We performed western blotting to examine the levels of NMDAR subunits and postsynaptic density protein 95 (PSD-95) in total protein homogenates of the NAc 10 days after CSDS. As previously reported [8], we showed that the total protein expression levels of GluN1 and GluN2A in the NAc were not changed after CSDS (p > 0.05 vs. control), while the level of the GluN2B subunit in the NAc of susceptible mice was significantly decreased compared to the level in the control group (Fig. S4a, p < 0.01). PSD-95 is a neuronal PDZ protein that associates with NMDARs at synapses to facilitate downstream intracellular signaling and modulate synaptic plasticity [25, 26]. Similar to GluN2B, PSD-95 displayed a significant decrease in the NAc of susceptible mice (Fig. S4a, p < 0.01 vs. control). The results from qRT-PCR analyses showed that the mRNA levels of GluN2A, GluN2B and PSD-95 in the NAc of susceptible mice were not different from those in control or unsusceptible mice (Fig. S5, p > 0.05), indicating that downregulation of klotho, GluN2B and PSD-95 protein expression in the NAc of susceptible mice did not result from changes in gene expression. Then, we investigated whether knockdown of klotho in the NAc would result in similar changes in NMDAR subunit expression. As shown in Fig. 3a, KL-KD in the NAc caused a specific decrease in total protein levels of the GluN2B subunit and PSD-95 but did not alter total protein levels of GluN1 and GluN2A (p < 0.01 vs. control or GFP group).
Fig. 3. Modulation of klotho level in the NAc regulates the total and surface expression of GluN2B in both the naive and susceptible mice.
a Knockdown of accumbal klotho did not affect the total GluN1 and GluN2A protein levels, while it significantly decreased the total GluN2B and PSD-95 protein expression in the NAc (n = 4 mice per group; **p < 0.01 vs. control). b1-2 Western blotting analysis revealed that genetic knockdown of accumbal klotho produced no effect on the expressions of GluN1 (b1) and GluN2A (b2) in both the surface and intracellular pools in the NAc of mice. (b3) Genetic knockdown of accumbal klotho significantly reduced GluN2B expression in the surface pool but not in the intracellular pool (n = 4 mice per group; **p < 0.01 vs. control). c Representative immunoblots showing the total protein expressions of GluN1, GluN2A, GluN2B and PSD-95 in the NAc of mice from each group. Statistical results show that CSDS decreased the protein expressions of GluN2B and PSD-95 in NAc, while klotho overexpression reversed the effects of CSDS (n = 6 mice per group; **p < 0.01 vs. control; ##p < 0.01 vs. GFP group or susceptible group). d1-3 Representative immunoblots showing the expressions of GluN1 (d1), GluN2A (d2) and GluN2B (d3) in both the surface and intracellular pool in NAc. Statistical results show that CSDS caused a reduction of GluN2B in the surface pool of NAc, while klotho overexpression reversed the decline of surface GluN2B expression (n = 6 mice per group; **p < 0.01 vs. control; ##p < 0.01 vs. GFP group or susceptible group). All data were presented as normalized mean ± SEM.
The biological consequences of NMDAR activation mainly depend on whether the receptors are located in synaptic or extrasynaptic sites [27]. We therefore detected the surface expression of NMDAR subunits in the NAc of mice using a protein cross-linking assay that specifically detects synaptic proteins. Similar to a previous report [8], we showed that there was no difference in the levels of GluN1 and GluN2A in either the surface pool or the intracellular pool between control and susceptible mice (Fig. S4b, c, p > 0.05 vs. control group). However, a robust decrease in GluN2B was observed in the surface pool in susceptible mice (Fig. S4d, p < 0.01 vs. control group). These results demonstrated selective GluN2B downregulation in a specific subcellular compartment (surface membranes) in the NAc of depressive model mice. We next explored the influence of klotho downregulation on the expression of NMDARs in the synapses of the NAc. Similarly, there was no difference in the levels of GluN1 and GluN2A in either the surface or intracellular pool between control and KL-KD mice (Fig. 3b1, b2, p > 0.05), while a significant decrease was observed in GluN2B in the surface pool in KL-KD mice (Fig. 3b3, p < 0.01 vs. control or GFP group). These results demonstrated that knockdown of klotho produced a change in GluN2B expression identical to that observed in susceptible mice, suggesting that a decrease in accumbal klotho resulted in behavioral alterations relevant to depression in mice via selective downregulation of GluN2B at NAc synapses.
Genetic overexpression of klotho in the NAc upregulated the expression level of accumbal GluN2B in both normal and CSDS susceptible mice
To further confirm the action target of klotho in modulating behavioral responses to stress, we explored the influence of elevated accumbal klotho on GluN2B expression in the NAc of mice. We first detected the total and surface expression of GluN2B in the NAc in mice with klotho overexpression. As shown in Fig. S6a, genetic overexpression of klotho in the NAc in normal mice did not affect the total expression levels of GluN1 and GluN2A subunits but significantly increased the levels of total GluN2B and PSD-95 (p < 0.01 vs. control group). A protein cross-linking assay revealed that the surface expression of GluN2B in the NAc was significantly increased by klotho expression (Fig. S6b, p < 0.01 vs. susceptible group).
We next investigated whether elevation of klotho could restore the total and surface expression of GluN2B in the NAc of susceptible mice. As shown in Fig. 3c, genetic overexpression of klotho in the NAc in susceptible mice had no effect on the expression levels of GluN1 and GluN2A subunits but significantly increased the total expression of GluN2B and PSD-95 to levels comparable to those in the control group (p < 0.01 vs. susceptible group). The results from BS3 cross-linking experiments showed that genetic overexpression of klotho in the NAc in susceptible mice had no effect on the levels of GluN1 and GluN2A subunits in either the surface or intracellular pool (Fig. 3d1, d2) but significantly increased the surface expression of GluN2B in the NAc (Fig. 3d3, p < 0.01 vs. susceptible group). Together with the above data, these results demonstrated that klotho in the NAc can modulate the behavioral responses to stress by regulating the stability of surface GluN2B.
Genetic overexpression of accumbal klotho reversed altered synaptic and structural plasticity in CSDS susceptible mice
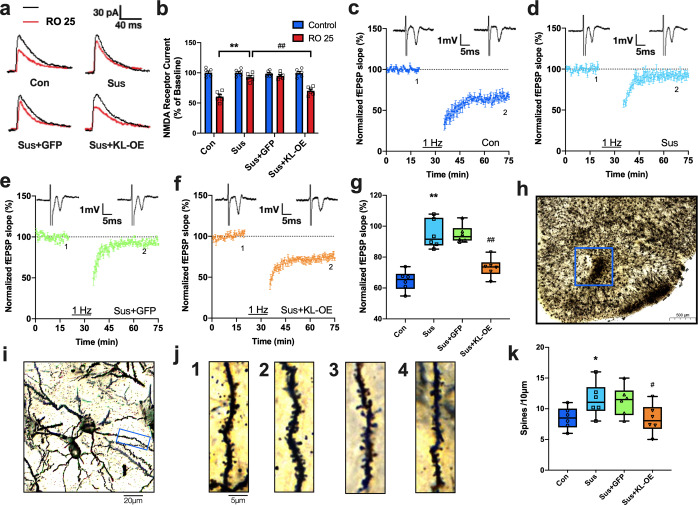
Previous studies have verified that synaptic molecular adaptations occurring in the neurons of the NAc underlie susceptible and resilient responses to chronic stress [8]. We next performed a set of electrophysiological experiments to investigate the modulatory effects of klotho on synaptic plasticity at cortico-accumbal glutamatergic synapses. We first conducted whole-cell voltage-clamp recordings of synaptically evoked NMDAR-mediated excitatory postsynaptic currents (EPSCs) in NAc slices to examine the modulation of GluN2B-NMDARs. The relative contribution of GluN2B to EPSCs was determined by measuring the sensitivity of EPSCs to Ro 25-6981, a second-generation NMDAR blocker that displays a 3000-fold higher specificity to the GluN2B subunit than to other subunits [28]. Compared to the control group, Ro 25–6981-sensitive EPSCs were substantially reduced in the NAc neurons of susceptible mice (control: 34.5 ± 5.9% of baseline, susceptible: 5.8 ± 3.1% of baseline; p < 0.01), and overexpression of klotho in the NAc significantly increased Ro 25–6981-sensitive EPSCs in susceptible mice to a level comparable to that in control mice (31.7 ± 5.9% of baseline, p < 0.01 vs. susceptible group) (Fig. 4a, b). These results indicate that elevation of klotho can reverse the detrimental effect of CSDS on GluN2B-mediated function.
Fig. 4. Genetic overexpression of klotho in NAc restores the CSDS-induced disruption of NMDAR-dependent LTD and dendritic spines density.
a, b Representative traces (a) and quantitation (b) of isolated NMDAR EPSCs in the presence of NBQX (10 µM) at baseline (black) and following perfusion with Ro 25–6981 (Ro 25; 0.1 µM) (red) in the same slices. Five slices from three mice in each group were analyzed. c–f Averaged data show the LTD induced by LFS in control (c), Susceptible (d), Sus + GFP (e) and Sus + KL-OE group (f). The LFS consisting of a 1-Hz, 900-pulse trains at stimulus intensity. g The box and whiskers show the levels of normalized fEPSP slope 40 min after LFS in each group (n = 5 per group). h, i Representative micro-photograph show stained dendrites in NAc: (h) Scale bar = 500 μm, (i) Scale bar = 20 μm. j Representative dendritic spine images from control (j1), Sus (j2), Sus + GFP (j3) and Sus + KL-OE group (j4). Scale bar = 5 μm. k Quantification of dendritic spine density in the NAc of mice from each group (n = 6 mice per group). Each point was the normalized mean ± SEM. *p < 0.05 and **p < 0.01 vs. control; #p < 0.05 and ##p < 0.01 vs. susceptible group.
The PPF, a sensitive measure of the probability of transmitter release, is a common form of short-term presynaptic plasticity. As shown in Fig. S7a, b, CSDS did not affect the PPF in the cortico-accumbal pathway, and klotho overexpression in susceptible mice also had no effect on PPF, suggesting a lack of gross change in presynaptic function. Then, the input-output relationships for field excitatory postsynaptic potentials amplitude, an indicator of synaptic efficacy, were compared among groups. We found a slight decrease in the amplitude of field excitatory postsynaptic potentials in the cortico-accumbal pathway in susceptible mice, while genetic overexpression of accumbal klotho reversed the decreased basal synapse transmission in these mice (Fig. S7c, d, p < 0.05 vs. susceptible group).
Persistent impairment in NMDAR-dependent LTD in the NAc has been associated with behavioral adaptations to chronic stress [8]. Thus, NMDAR-LTD in the NAc was compared among the control, susceptible, and KL-OE susceptible groups. Consistent with a previous study, NMDAR-LTD was disrupted in susceptible mice (Fig. 4d and g, p < 0.05 vs. control group). Genetic overexpression of accumbal klotho significantly reversed the disruption of NMDAR-LTD in the NAc of susceptible mice (Fig. 4f and g, p < 0.05 vs. susceptible and GFP group). This result indicates that regulation of accumbal klotho can normalize the impaired synaptic plasticity that is associated with stress-related behavioral responses.
To further characterize the mechanisms underlying the modulation of klotho in behavioral responses to stress, we investigated the effects of klotho elevation on structural plasticity in susceptible mice. Golgi staining was employed to determine dendrite spine density in neurons of the NAc. Consistent with a previous report [29], the dendrite spine density in neurons of the NAc was significantly increased in susceptible mice (Fig. 4j and k, p < 0.05 vs. control group). Genetic overexpression of accumbal klotho in susceptible mice significantly reversed the alteration in dendrite spine density in the NAc (Fig. 4j and k, p < 0.05 vs. susceptible mice), demonstrating that elevation of accumbal klotho normalized structural plasticity in susceptible mice.
Blockade of GluN2B abolished the beneficial effects of klotho elevation in CSDS susceptible mice
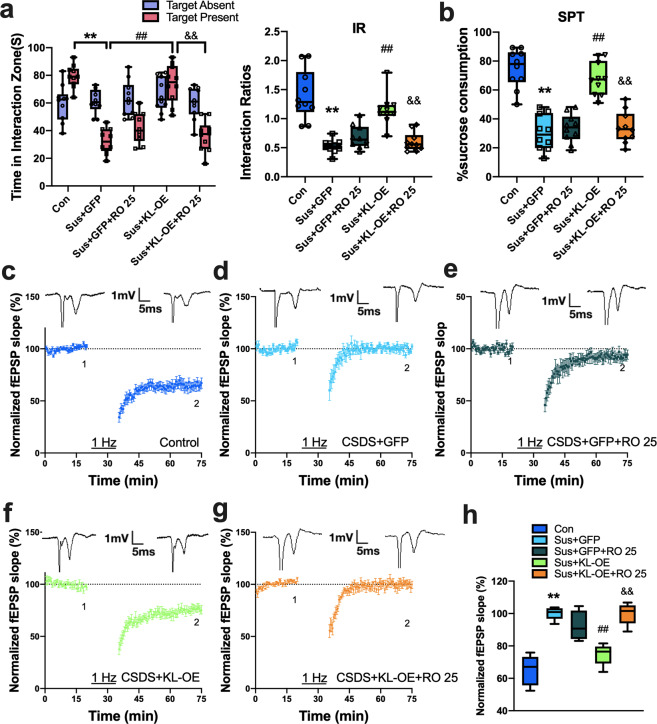
We then investigated whether blocking GluN2B-containing NMDARs would eliminate the beneficial effects of klotho elevation in susceptible mice. Ro 25–6981 is usually used in the 0.1–1 μM range in the brain in vitro [30]. Given that blockade of GluN2B may have impacts on synaptic plasticity and behavioral responses to stress, a low dose of Ro 25–6981 was used in this study to avoid this possibility as much as possible. We showed that bilateral intra-NAc infusion of a low dose of Ro 25–6981 (0.1 μM, 0.5 μl) 20 min before the social interaction or sucrose preference test had no significant effect on the exploration time in the interaction zone, the interaction ratio or sucrose preference in susceptible mice, while it significantly abolished the increased social interaction and sucrose preference induced by klotho overexpression in these mice (Fig. 5a, b, p < 0.01 vs. KL-OE susceptible mice). Post hoc comparisons using Bonferroni’s test showed that both the interaction ratio and the sucrose consumption in klotho-overexpressing susceptible mice that were also treated with Ro 25–6981 were not different from those in GFP-treated susceptible mice (p > 0.05). In a separate set of experiments, we investigated the influence of acute administration of Ro 25–6981 on NMDAR-dependent LTD in the NAc of mice. Similar to the behavioral results, bath application of Ro 25–6981 (0.1 μM) for 20 min did not affect LFS-induced LTD in slices from susceptible mice (Fig. 5e) but significantly eliminated the benefit of klotho overexpression on LTD in these mice (p < 0.01 vs. KL-OE susceptible mice; Fig. 5g). Post hoc comparisons showed that the level of LTD in klotho-overexpressing susceptible mice with bath application of Ro 25–6981 was comparable to that in susceptible mice (p > 0.05). These data indicate that upregulation of GluN2B-NMDAR function mediated the beneficial effects of klotho elevation in susceptible mice.
Fig. 5. Treatment with GluN2B antagonist abolishes the beneficial effects of klotho elevation on behavioral responses to stress and synaptic plasticity in CSDS susceptible mice.
a, b Intra-NAc infusion of Ro 25–6981 (0.1 μM, 0.5 μl) 20 min before test did not affect the time in interaction zone and social interaction ratios (a) and sucrose preference (b) in susceptible mice, while it obviously abolished the enhancement effect of klotho overexpression on time in interaction zone and social interaction ratios (a) and sucrose preference (b) in these mice (n = 10 mice per group). c–g NMDAR-dependent LTD recorded in the NAc in control (c), Sus + GFP (d), Sus + GFP + Ro-25 (e), Sus + KL-OE (f) and Sus + KL-OE + Ro-25 group (g). Bath application of Ro 25–6981 (0.1 μM) for 20 min did not affect LFS-induced NMDAR-dependent LTD in slices from susceptible mice, but clearly abolished the beneficial effects of klotho overexpression on LTD in these mice. h The box and whiskers show the level of normalized fEPSP slope 40 min after LFS from each group (n = 6 per group). For (a–d) and (j), all box and whisker plot display the median, first and third quartiles (boxes), and the min-max (whiskers), and other data were presented as normalized mean ± SEM. **p < 0.01 vs. control; ##p < 0.01 vs. Sus + GFP group; &&p < 0.01 vs. Sus + KL-OE group.
Discussion
In the present study, we demonstrated a critical role of accumbal klotho in the pathogenesis of major depression. Persistent low mood, anhedonia, social-behavior withdrawal and hopelessness are the core symptoms of depression [1]. The social interaction ratio, sucrose preference and immobility time in the FST and TST are commonly used indicators of depression-related behaviors in mice [8, 31]. We found that exposure to chronic stress led to a significant downregulation of klotho in the NAc of mice and that genetic knockdown of klotho in the NAc induced changes in sucrose preference and immobility time in the FST and TST, indicating that downregulation of accumbal klotho contributed to behavioral alterations relevant to depression. Overexpression of klotho in the NAc produced an antidepressive effect in normal mice, ameliorated behavioral responses to stress and reversed the alterations in synaptic plasticity and structural morphology in CSDS susceptible mice. The molecular effects of klotho might be correlated with the regulation of GluN2B-containing NMDAR function because klotho knockdown in the NAc resulted in selective decreases in total and synaptic GluN2B expression, which were identical to those observed in susceptible mice, and elevation of accumbal klotho reversed the changes in GluN2B expression. Moreover, a GluN2B-specific antagonist abolished the benefits of klotho elevation in behavioral responses to stress and accumbal LTD in susceptible mice. These findings demonstrate that klotho in the NAc modulates behavioral responses to stress by regulating the function of GluN2B-containing NMDARs.
Klotho is a single-pass membrane-bound protein that can be alternatively spliced to a membrane-bound form (m-KL) and secreted form (s-KL) [32]. It is released and cleaved in cerebrospinal fluid (CSF) and plasma and influences longevity and susceptibility to multiple complex disorders, including atherosclerosis, stroke and depression [33]. Klotho mutant mice display an increased level of oxidative stress in the hippocampus at 5 weeks of age and impaired cognitive function at 7 weeks [16]. Women under high chronic stress were found to have significantly lower levels of serum klotho than low-stress controls [20]. The levels of klotho in the CSF were also significantly increased in geriatric patients with severe depression after electroconvulsive therapy, a highly effective antidepressant treatment strategy [22]. In this study, we found that chronic stress resulted in a significant decrease in klotho expression in the NAc. Previous studies have demonstrated that blunted responses in the NAc to gain were observed in depressed individuals [34] as well as the offspring and first-degree relatives of depressed individuals [35]. Rappaport et al. reported that current depression severity was associated with hyporeactivity in the NAc in response to the anticipation of a reward [36]. We showed that genetic knockdown of klotho in the NAc induced behavioral alterations relevant to depression in mice and that genetic overexpression of accumbal klotho obviously ameliorated behavioral responses to stress in susceptible mice. These data indicate that reduced klotho in the NAc may be implicated in the pathophysiology of major depression. However, the molecular mechanism underlying the expression changes in klotho, GluN2B and PSD-95 in susceptible mice is still unclear. The results from qRT-PCR analyses showed that the mRNA levels of klotho, GluN2A, GluN2B and PSD-95 did not change in the NAc of susceptible mice, suggesting that the downregulation of these proteins could not be due to changes in gene expression but might be attributed to protein instability. Protein instability has been shown to contribute to changes in protein expression in the NAc under pathological conditions. For instance, chronic exposure to amphetamine induced selective downregulation of GluN2B subunits in rat striatal neurons by accelerating ubiquitination and degradation of crucial GluN2B-anchoring proteins [37]. Restoration of NR2B loss in the NAc of susceptible mice by the proteasome inhibitor MG132 prevented the behavioral sensitization of mice to chronic stress [8]. Nevertheless, further studies are still needed to elucidate the exact molecular mechanism underlying the expression changes in klotho, GluN2B and PSD-95 in stressed mice.
Synaptic plasticity is the activity-dependent modification of the strength or efficacy of synaptic transmission at synapses and has been demonstrated to play a central role in the capacity of the brain to incorporate transient experiences into persistent memory traces [38]. It has been proposed that activity-dependent remodeling of excitatory synapses and associated dendritic spines is impaired during chronic stress, leading to neurological circuit disorders in the brain and the onset of depression symptoms [39]. NAc neurons receive glutamatergic inputs arising from limbic and cortical regions. Stress-induced dysfunction in the synaptic plasticity of cortico-accumbal glutamatergic synapses is implicated in the symptomology of depression [40]. Consistent with a previous report [8], we showed that there was a significant decrease in excitatory postsynaptic responses and an impairment of NMDAR-dependent LTD in the NAc of susceptible mice. Overexpression of klotho in the NAc significantly reversed the reduced postsynaptic responses and impaired LTD in susceptible mice, indicating that klotho elevation can restore the synaptic function in the NAc of susceptible mice. Alterations in synaptic strength or connectivity of neurons are responsible for the long-lasting behavioral symptoms induced by chronic stress [29, 41–43]. After chronic social defeat stress, medium spiny neurons (MSNs) of the NAc exhibit increased spine density that is correlated with enhanced behavioral responses to stress [43, 44]. We found that the dendrite spine density in the MSNs of the NAc was significantly increased in susceptible mice, while genetic overexpression of accumbal klotho substantially reversed the alteration in dendrite spine density in these mice, demonstrating that elevation of accumbal klotho normalized the structural plasticity in depressed mice. These findings that klotho elevation reversed the disruptions in synaptic and structural plasticity in susceptible mice provide supporting evidence of the benefit of elevating klotho to improve behavioral responses to stress.
Data from our previous study showed that a reduction in GluN2B in the NAc can aggravate the behavioral sensitization of mice to stress [8]. It is worth noting that systemic administration of NMDA receptor antagonists, such as Ro 25–6981 and ketamine, is capable of exerting significant antidepressant effects in both model animals and depressive patients [45]. This fact seems to conflict with our findings. However, there are some possible explanations for this discrepancy. First, the mechanisms by which ketamine exerts its antidepressant effects are complex. Recent studies suggest that the effects of ketamine on depression include not only NMDAR antagonism but also a glutamate surge, reduced inhibitory GABAergic transmission, an AMPAR-mediated increase in mTOR-dependent neuroplasticity and BDNF release [46–50]. On the other hand, different brain regions might exert different effects due to their different structural components. A major proportion of NAc neurons are MSNs [51]. During the course of depression, the NAc undergoes certain changes in protein expression and functional connectivity that are different from those in other brain regions. For example, chronic stress causes a reduction in hippocampal BDNF expression and spine density [52, 53], while increased BDNF expression and dendrite spine density were observed in the NAc after chronic stress [54]. Our previous study also demonstrated that chronic stress results in a long-lasting reduction in GluN2B in the NAc, which could be restored by fluoxetine treatment, and unsusceptible mice showed patterns of GluN2B regulation that overlapped dramatically with those seen with fluoxetine treatment [8]. In the present study, we showed that chronic stress causes a parallel change in GluN2B and klotho expression in the NAc and that the total and surface expression of GluN2B in the NAc can be regulated by altering klotho levels via AAV-mediated knockdown or overexpression. Furthermore, treatment with a low dose of Ro 25–6981 had no significant effect on the behavioral responses to stress or NMDAR-LTD in susceptible mice but significantly abolished the beneficial effects of klotho overexpression in these mice, indicating that GluN2B is the action target of klotho in the modulation of behavioral responses to stress. However, how klotho regulates the levels of total GluN2B protein and enriches GluN2B within synapses, directly or indirectly, remains to be determined but may involve regulation of translation, posttranslational modification, recycling, or trafficking of the subunit. It also remains to be determined whether the effects of klotho elevation on GluN2B are mediated by the transmembrane or secreted form of klotho. In addition to neurons, klotho can regulate ERK phosphorylation and aerobic glycolysis in astrocytes [55, 56]. Klotho also has antioxidative stress, anti-inflammatory, and antiapoptotic effects on vascular endothelial cells via regulation of PI3K/AKT pathways [57]. Mounting evidence has shown that astrocytic and endothelial dysfunction are involved in the progression of depression [58, 59]. Therefore, the ‘antidepressant’ effect of klotho may also be (partially) mediated by other cell types, such as astrocytes or endothelial cells.
Taken together, we preliminarily demonstrated that klotho in the NAc modulates behavioral responses to stress by regulating the function of GluN2B-containing NMDARs. This finding provides novel insights into the pathogenesis of major depression, and regulation of klotho in the NAc might be a strategy for depression treatment. However, how chronic stress causes a change in klotho expression in the NAc still needs further study.
Supplementary information
Author contributions
Y.-J.Y., B.W. and W.W. designed the research; H.-J.W., W.-N.W., H.F., L.-E.L., J.-Q.Z., C.-N.C., Y.-H.L., S.-Z.J., J.-W.X. and Z.-M.Y. performed the research; H.-J.W., L.-E.L., and Y.-J.Y. analyzed data; and H.-J.W. and Y.-J.Y. wrote the paper.
Funding
This study was supported by grants from the National Natural Science Foundation of China (82060258, 81760254, 81760256 and 81960256). It was also supported by the Jiangxi Provincial Natural Science Foundation (20202BAB206026, 20202BAB216012 and 20202BBG73022). The study also received support by grants for the Jiangxi Provincial Clinical Research Center (2020BCG74002) and the Academic and Technical Leaders of Major Disciplines Foundation (20204BCJL22049).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Han-jun Wu, Wen-ning Wu.
Contributor Information
Wei Wang, Email: wuhansy@126.com.
Yuan-jian Yang, Email: yuanjimyang@yeah.net.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-022-01323-3.
References
- 1.Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. 2014;140:774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349:1498–504.. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 3.Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, et al. Major depressive disorder. Nat Rev Dis Prim. 2016;2:16065. doi: 10.1038/nrdp.2016.65. [DOI] [PubMed] [Google Scholar]
- 4.Zhou Y, Zhu H, Liu Z, Chen X, Su X, Ma C, et al. A ventral CA1 to nucleus accumbens core engram circuit mediates conditioned place preference for cocaine. Nat Neurosci. 2019;22:1986–99. doi: 10.1038/s41593-019-0524-y. [DOI] [PubMed] [Google Scholar]
- 5.Steephen JE, Manchanda R. Differences in biophysical properties of nucleus accumbens medium spiny neurons emerging from inactivation of inward rectifying potassium currents. J Comput Neurosci. 2009;27:453–70. doi: 10.1007/s10827-009-0161-7. [DOI] [PubMed] [Google Scholar]
- 6.Cho S, Hachmann JT, Balzekas I, In MH, Andres-Beck LG, Lee KH, et al. Resting-state functional connectivity modulates the BOLD activation induced by nucleus accumbens stimulation in the swine brain. Brain Behav. 2019;9:e01431. doi: 10.1002/brb3.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Jiang B, Wang W, Wang F, Hu ZL, Xiao JL, Yang S, et al. The stability of NR2B in the nucleus accumbens controls behavioral and synaptic adaptations to chronic stress. Biol Psychiatry. 2013;74:145–55.. doi: 10.1016/j.biopsych.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 9.Bagot RC, Parise EM, Pena CJ, Zhang HX, Maze I, Chaudhury D, et al. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat Commun. 2015;6:7062. doi: 10.1038/ncomms8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muir J, Tse YC, Iyer ES, Biris J, Cvetkovska V, Lopez J, et al. Ventral hippocampal afferents to nucleus accumbens encode both latent vulnerability and stress-induced susceptibility. Biol Psychiatry. 2020;88:843–54.. doi: 10.1016/j.biopsych.2020.05.021. [DOI] [PubMed] [Google Scholar]
- 11.van Beugen BJ, Qiao X, Simmons DH, De Zeeuw CI, Hansel C. Enhanced AMPA receptor function promotes cerebellar long-term depression rather than potentiation. Learn Mem. 2014;21:662–7. doi: 10.1101/lm.035220.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siddoway B, Hou H, Xia H. Molecular mechanisms of homeostatic synaptic downscaling. Neuropharmacology. 2014;78:38–44. doi: 10.1016/j.neuropharm.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 14.Masuda H, Chikuda H, Suga T, Kawaguchi H, Kuro-o M. Regulation of multiple ageing-like phenotypes by inducible klotho gene expression in klotho mutant mice. Mech Ageing Dev. 2005;126:1274–83. doi: 10.1016/j.mad.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Torbus-Paluszczak M, Bartman W, Adamczyk-Sowa M. Klotho protein in neurodegenerative disorders. Neurol Sci. 2018;39:1677–82. doi: 10.1007/s10072-018-3496-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagai T, Yamada K, Kim HC, Kim YS, Noda Y, Imura A, et al. Cognition impairment in the genetic model of aging klotho gene mutant mice: a role of oxidative stress. FASEB J. 2003;17:50–2. doi: 10.1096/fj.02-0448fje. [DOI] [PubMed] [Google Scholar]
- 17.Li D, Jing D, Liu Z, Chen Y, Huang F, Behnisch T. Enhanced expression of secreted alpha-klotho in the hippocampus alters nesting behavior and memory formation in mice. Front Cell Neurosci. 2019;13:133. doi: 10.3389/fncel.2019.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubal DB, Yokoyama JS, Zhu L, Broestl L, Worden K, Wang D, et al. Life extension factor klotho enhances cognition. Cell Rep. 2014;7:1065–76. doi: 10.1016/j.celrep.2014.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubal DB, Zhu L, Sanchez PE, Worden K, Broestl L, Johnson E, et al. Life extension factor klotho prevents mortality and enhances cognition in hAPP transgenic mice. J Neurosci. 2015;35:2358–71. doi: 10.1523/JNEUROSCI.5791-12.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prather AA, Epel ES, Arenander J, Broestl L, Garay BI, Wang D, et al. Longevity factor klotho and chronic psychological stress. Transl Psychiatry. 2015;5:e585. doi: 10.1038/tp.2015.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paroni G, Seripa D, Fontana A, D’Onofrio G, Gravina C, Urbano M, et al. Klotho gene and selective serotonin reuptake inhibitors: response to treatment in late-life major depressive disorder. Mol Neurobiol. 2017;54:1340–51. doi: 10.1007/s12035-016-9711-y. [DOI] [PubMed] [Google Scholar]
- 22.Hoyer C, Sartorius A, Aksay SS, Bumb JM, Janke C, Thiel M, et al. Electroconvulsive therapy enhances the anti-ageing hormone Klotho in the cerebrospinal fluid of geriatric patients with major depression. Eur Neuropsychopharmacol. 2018;28:428–35. doi: 10.1016/j.euroneuro.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, et al. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565:143–7. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- 24.Leon J, Moreno AJ, Garay BI, Chalkley RJ, Burlingame AL, Wang D, et al. Peripheral elevation of a klotho fragment enhances brain function and resilience in young, aging, and alpha-synuclein transgenic mice. Cell Rep. 2017;20:1360–71. doi: 10.1016/j.celrep.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–8. doi: 10.1126/science.290.5495.1364. [DOI] [PubMed] [Google Scholar]
- 26.Mello R, Marchand F, Pezet S, McMahon SB, Dickenson AH. Perturbing PSD-95 interactions with NR2B-subtype receptors attenuates spinal nociceptive plasticity and neuropathic pain. Mol Ther. 2011;19:1780–92. doi: 10.1038/mt.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–14. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- 28.Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharm. 2007;7:39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, et al. IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci. 2011;31:314–21. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Volianskis A, Bannister N, Collett VJ, Irvine MW, Monaghan DT, Fitzjohn SM, et al. Different NMDA receptor subtypes mediate induction of long-term potentiation and two forms of short-term potentiation at CA1 synapses in rat hippocampus in vitro. J Physiol. 2013;591:955–72. doi: 10.1113/jphysiol.2012.247296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Z, Tang Z, Zou K, Huang Z, Liu L, Yang Y, et al. D-Serine produces antidepressant-like effects in mice through suppression of BDNF signaling pathway and regulation of synaptic adaptations in the nucleus accumbens. Mol Med. 2021;27:127. doi: 10.1186/s10020-021-00389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masso A, Sanchez A, Gimenez-Llort L, Lizcano JM, Canete M, Garcia B, et al. Secreted and transmembrane alphaKlotho isoforms have different spatio-temporal profiles in the brain during aging and Alzheimer’s disease progression. PLoS ONE. 2015;10:e0143623. doi: 10.1371/journal.pone.0143623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pavlatou MG, Remaley AT, Gold PW. Klotho: a humeral mediator in CSF and plasma that influences longevity and susceptibility to multiple complex disorders, including depression. Transl Psychiatry. 2016;6:e876. doi: 10.1038/tp.2016.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keren H, O’Callaghan G, Vidal-Ribas P, Buzzell GA, Brotman MA, Leibenluft E, et al. Reward processing in depression: a conceptual and meta-analytic review across fMRI and EEG studies. Am J Psychiatry. 2018;175:1111–20. doi: 10.1176/appi.ajp.2018.17101124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luking KR, Pagliaccio D, Luby JL, Barch DM. Depression risk predicts blunted neural responses to gains and enhanced responses to losses in healthy children. J Am Acad Child Adolesc Psychiatry. 2016;55:328–37. doi: 10.1016/j.jaac.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rappaport BI, Kandala S, Luby JL, Barch DM. Brain reward system dysfunction in adolescence: current, cumulative, and developmental periods of depression. Am J Psychiatry. 2020;177:754–63. doi: 10.1176/appi.ajp.2019.19030281. [DOI] [PubMed] [Google Scholar]
- 37.Mao LM, Wang W, Chu XP, Zhang GC, Liu XY, Yang YJ, et al. Stability of surface NMDA receptors controls synaptic and behavioral adaptations to amphetamine. Nat Neurosci. 2009;12:602–10. doi: 10.1038/nn.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33:18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- 39.Vose LR, Stanton PK. Synaptic plasticity, metaplasticity and depression. Curr Neuropharmacol. 2017;15:71–86. doi: 10.2174/1570159X14666160202121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma K, Zhang H, Wei G, Dong Z, Zhao H, Han X, et al. Identification of key genes, pathways, and miRNA/mRNA regulatory networks of CUMS-induced depression in nucleus accumbens by integrated bioinformatics analysis. Neuropsychiatr Dis Treat. 2019;15:685–700. doi: 10.2147/NDT.S200264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–58. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 42.Khibnik LA, Beaumont M, Doyle M, Heshmati M, Slesinger PA, Nestler EJ, et al. Stress and cocaine trigger divergent and cell type-specific regulation of synaptic transmission at single spines in nucleus accumbens. Biol Psychiatry. 2016;79:898–905. doi: 10.1016/j.biopsych.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qiao H, Li MX, Xu C, Chen HB, An SC, Ma XM. Dendritic spines in depression: what we learned from animal models. Neural Plast. 2016;2016:8056370. doi: 10.1155/2016/8056370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fox ME, Figueiredo A, Menken MS, Lobo MK. Dendritic spine density is increased on nucleus accumbens D2 neurons after chronic social defeat. Sci Rep. 2020;10:12393. doi: 10.1038/s41598-020-69339-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–5. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abdallah CG, Sanacora G, Duman RS, Krystal JH. The neurobiology of depression, ketamine and rapid-acting antidepressants: is it glutamate inhibition or activation? Pharm Ther. 2018;190:148–58. doi: 10.1016/j.pharmthera.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–64. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cavalleri L, Merlo Pich E, Millan MJ, Chiamulera C, Kunath T, Spano PF, et al. Ketamine enhances structural plasticity in mouse mesencephalic and human iPSC-derived dopaminergic neurons via AMPAR-driven BDNF and mTOR signaling. Mol Psychiatry. 2018;23:812–23. doi: 10.1038/mp.2017.241. [DOI] [PubMed] [Google Scholar]
- 49.Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23:801–11. doi: 10.1038/mp.2017.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gerhard DM, Pothula S, Liu RJ, Wu M, Li XY, Girgenti MJ, et al. GABA interneurons are the cellular trigger for ketamine’s rapid antidepressant actions. J Clin Invest. 2020;130:1336–49. doi: 10.1172/JCI130808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soares-Cunha C, de Vasconcelos NAP, Coimbra B, Domingues AV, Silva JM, Loureiro-Campos E, et al. Nucleus accumbens medium spiny neurons subtypes signal both reward and aversion. Mol Psychiatry. 2020;25:3241–55. doi: 10.1038/s41380-019-0484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamani C, Machado DC, Hipolide DC, Dubiela FP, Suchecki D, Macedo CE, et al. Deep brain stimulation reverses anhedonic-like behavior in a chronic model of depression: role of serotonin and brain derived neurotrophic factor. Biol Psychiatry. 2012;71:30–5. doi: 10.1016/j.biopsych.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Miguel Z, Haditsch U, Palmer TD, Azpiroz A, Sapolsky RM. Adult-generated neurons born during chronic social stress are uniquely adapted to respond to subsequent chronic social stress. Mol Psychiatry. 2019;24:1178–88. doi: 10.1038/s41380-017-0013-1. [DOI] [PubMed] [Google Scholar]
- 54.Walsh JJ, Friedman AK, Sun H, Heller EA, Ku SM, Juarez B, et al. Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nat Neurosci. 2014;17:27–9. doi: 10.1038/nn.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Landry T, Li P, Shookster D, Jiang Z, Li H, Laing BT, et al. Centrally circulating alpha-klotho inversely correlates with human obesity and modulates arcuate cell populations in mice. Mol Metab. 2021;44:101136. doi: 10.1016/j.molmet.2020.101136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mazucanti CH, Kawamoto EM, Mattson MP, Scavone C, Camandola S. Activity-dependent neuronal Klotho enhances astrocytic aerobic glycolysis. J Cereb Blood Flow Metab. 2019;39:1544–56. doi: 10.1177/0271678X18762700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cui W, Leng B, Wang G. Klotho protein inhibits H2O2-induced oxidative injury in endothelial cells via regulation of PI3K/AKT/Nrf2/HO-1 pathways. Can J Physiol Pharm. 2019;97:370–76. doi: 10.1139/cjpp-2018-0277. [DOI] [PubMed] [Google Scholar]
- 58.Morris G, Puri BK, Olive L, Carvalho A, Berk M, Walder K, et al. Endothelial dysfunction in neuroprogressive disorders-causes and suggested treatments. BMC Med. 2020;18:305. doi: 10.1186/s12916-020-01749-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Leary LA, Mechawar N. Implication of cerebral astrocytes in major depression: a review of fine neuroanatomical evidence in humans. Glia. 2021;69:2077–99. doi: 10.1002/glia.23994. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.