Abstract
Limited information exists about survival outcomes after second primary cancers (SPCs) among breast cancer survivors. Studies suggest that mortality after certain SPCs may be higher than mortality after first primary cancers (FPCs) of the same type. A cohort study was conducted among 63,424 US women using the Surveillance, Epidemiology, and End Results 18 database (2000–2016) to compare mortality after a SPC among breast cancer survivors to mortality among women after a FPC using Cox proportional hazard regression. Propensity scores were used to match survivors with SPCs to women with FPCs 1:1 based on cancer type and prognostic factors. During a median follow-up of 42 months, 11,532 cancer deaths occurred after SPCs among survivors compared to 9305 deaths after FPCs. Cumulative cancer mortality was 44.7% for survivors with SPCs and 35.2% for women with FPCs. Survivors with SPCs had higher risk of cancer death (hazard ratio (HR): 1.27, 95% CI: 1.23–1.30) and death overall (HR: 1.18, 95% CI: 1.15–1.21) than women with FPCs. Increased risk of cancer death after SPCs compared to FPCs was observed for cancer in breast, lung, colon and/or rectum, uterus, lymphoma, melanoma, thyroid, and leukemia. Estrogen receptor status and treatment of the prior breast cancer as well as time between prior breast cancer and SPC significantly modified the mortality difference between women with SPC and FPC. A more tailored approach to early detection and treatment could improve outcomes from second cancer in breast cancer survivors.
Subject terms: Cancer epidemiology, Outcomes research
Introduction
In the US, the number of breast cancer survivors is estimated to reach close to 5 million by 20301. Second primary cancer (SPC) of all types can be a life-threatening event for survivors. The estimated cumulative incidence of SPC among women with breast cancer is 20% at 25 years2,3. The incidence of SPC in breast cancer survivors is 4–40% higher than the incidence of developing a first primary cancer (FPC) among women in the general population2,4–7. Breast cancer survivors are at elevated risk for multiple cancer types including second breast cancer, lung cancer, endometrial cancer, ovarian cancer, and leukemia2,4. Shared genetic and environmental risk factors, as well as the toxicant effects from cancer treatments, are hypothesized to contribute to the elevated risk2,4,8–12.
Some studies suggest that breast cancer survivors diagnosed with a SPC may have a worse prognosis compared to a FPC of the same type13,14. There is, however, limited data on survival outcomes after a second cancer. More information is needed to determine whether survivors require a more tailored approach to early detection and treatment of second cancers. To address this knowledge gap, we used data from Surveillance, Epidemiology, and End Results (SEER) program to compare cancer and all-cause mortality after a SPC in female breast cancer survivors to women who developed a FPC over the same time period (see study design schema in Fig. 1).
Fig. 1. Study design using second colorectal cancer as an example.
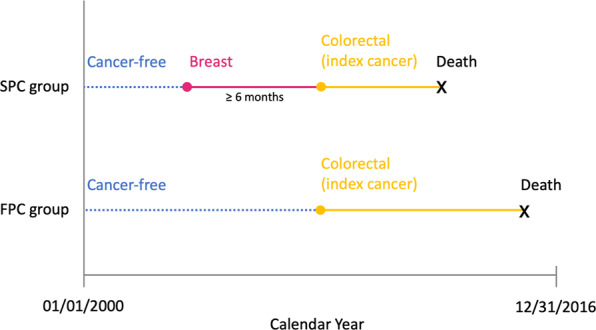
Colorectal cancer is one of the top 10 cancers we studied. FPC first primary cancer, SPC second primary cancer.
Results
Descriptive characteristics
The FPC and SPC groups were well matched based on propensity scores as shown in Table 1. Half of the second cancers were diagnosed within 5 years of the prior breast cancer. Prior breast cancers were primarily ER-positive (69.1%) and diagnosed at local stage (66.8%). Night-five percent of the prior breast cancers had tumor size ≤50 mm, 54% of them had negative lymph node status, and 96.4% of them received surgery. In a subgroup of 3184 breast cancer survivors diagnosed after 2010, when HER2 status was available, 68.4% of their prior breast cancers were luminal A molecular subtype (ER-positive and/or PR-positive and HER2-negative). The median follow-up time was 44 and 40 months for FPC and SPC group respectively. A comparison of FPC and SPC in the original unmatched cohort is shown in Supplementary Table 1.
Table 1.
Demographic and clinical characteristics of propensity score-matched study population identified from the SEER database.
| Characteristics | 1:1 PS-matched FPC (N = 31,712), No. (%) |
SPC among breast cancer survivors (N = 31,712), No. (%) |
|---|---|---|
| Age of diagnosis, mean (SD), years | 66.5 (13.2) | 66.6 (13.1) |
| Race | ||
| White | 26279 (82.9%) | 26094 (82.3%) |
| Black | 3442 (10.9%) | 3501 (11.0%) |
| Other | 1991 (6.3%) | 2117 (6.7%) |
| Primary site | ||
| Breast | 13903 (43.8%) | 13931 (43.9%) |
| Lung | 4900 (15.5%) | 4850 (15.3%) |
| Colorectal | 3424 (10.8%) | 3351 (10.6%) |
| Uterus | 2335 (7.4%) | 2392 (7.5%) |
| Lymphoma | 1202 (3.8%) | 1191 (3.8%) |
| Thyroid | 1237 (3.9%) | 1268 (4.0%) |
| Melanoma | 1240 (3.9%) | 1261 (4.0%) |
| Ovary | 1113 (3.5%) | 1095 (3.5%) |
| Pancreas | 1093 (3.4%) | 1130 (3.6%) |
| Leukemia | 1265 (4.0%) | 1243 (3.9%) |
| Tumor stage | ||
| Local | 16760 (52.9%) | 16739 (52.8%) |
| Regional | 7579 (23.9%) | 7591 (23.9%) |
| Distant | 7373 (23.2%) | 7382 (23.3%) |
| Year of diagnosis | ||
| 2000–2004 | 3397 (10.7%) | 3393 (10.7%) |
| 2005–2009 | 10600 (33.4%) | 10592 (33.4%) |
| 2010–2014 | 17715 (55.9%) | 17727 (55.9%) |
| Surgerya | ||
| No/Unknown | 8682 (27.4%) | 8755 (27.6%) |
| Yes | 23030 (72.6%) | 22957 (72.4%) |
| Chemotherapya | ||
| No/Unknown | 21497 (67.8%) | 21476 (67.7%) |
| Yes | 10215 (32.2%) | 10236 (32.3%) |
| Radiotherapya | ||
| No/Unknown | 24792 (78.2%) | 24585 (77.5%) |
| Yes | 6920 (21.8%) | 7127 (22.5%) |
| Characteristics of the prior breast cancer | ||
| Age of diagnosis (year) | ||
| ≤50 | – | 1922 (22.3%) |
| >50 | – | 29790 (77.7%) |
|
Time interval between prior breast cancer and SPC |
||
| 6 months-5 years | – | 17077 (53.9%) |
| >5 years | – | 14635 (46.1%) |
| Tumor stage | ||
| Local | – | 21176 (66.8%) |
| Regional | – | 9441 (29.8%) |
| Distant | – | 658 (2.1%) |
| Unknown | – | 437 (1.4%) |
| Tumor grade | ||
| Grade 1 | – | 6481 (20.4%) |
| Grade 2 | – | 12279 (38.7%) |
| Grade 3&4 | – | 10289 (32.4%) |
| Unknown | – | 2663 (8.4%) |
| Tumor size (mm) | ||
| ≤10 | – | 7843 (24.7%) |
| >10 and ≤20 | – | 11286 (35.6%) |
| >20 and ≤50 | – | 8362 (26.4%) |
| >50 | – | 1694 (5.3%) |
| Unknown | – | 2527 (8.0%) |
| Lymph node status | ||
| Negative | – | 19582 (61.7%) |
| Positive | – | 8785 (27.7%) |
| Unknown | – | 3345 (10.5%) |
| ER status | ||
| Negative | – | 6190 (19.5%) |
| Positive | – | 21920 (69.1%) |
| Unknown | – | 3602 (11.4%) |
| PR status | ||
| Negative | – | 9088 (28.7%) |
| Positive | – | 18563 (58.5%) |
| Unknown | – | 4061 (12.8%) |
| Molecular subtypeb | ||
| ER+ or PR+/HER2− (Luminal A) | – | 2178 (68.4%) |
| ER+ or PR+/HER2+ (Luminal B) | – | 256 (8.0%) |
| ER- and PR−/HER2+ (HER2 Enriched) | – | 120 (3.8%) |
| ER- and PR−/HER2− (Triple Negative) | – | 359 (11.3%) |
| Unknown | – | 271 (8.5%) |
| Surgerya | ||
| No/Unknown | – | 1134 (3.6%) |
| Yes | – | 30578 (96.4%) |
| Chemotherapya | ||
| No/Unknown | – | 19567 (61.7%) |
| Yes | – | 12145 (38.3%) |
| Radiotherapya | ||
| No/Unknown | – | 14617 (46.1%) |
| Yes | – | 17095 (53.9%) |
FPC first primary cancer, SPC second primary cancer, ER estrogen receptor, PR progesterone receptor, HER2 human epidermal growth factor receptor 2, +: positive, −: negative.
aThis indicates initial treatment.
bThis variable is limited to data from 2010 and onwards (N = 3184), because HER2 status is only available after 2010.
Relative difference in the risk of death comparing SPC to FPC
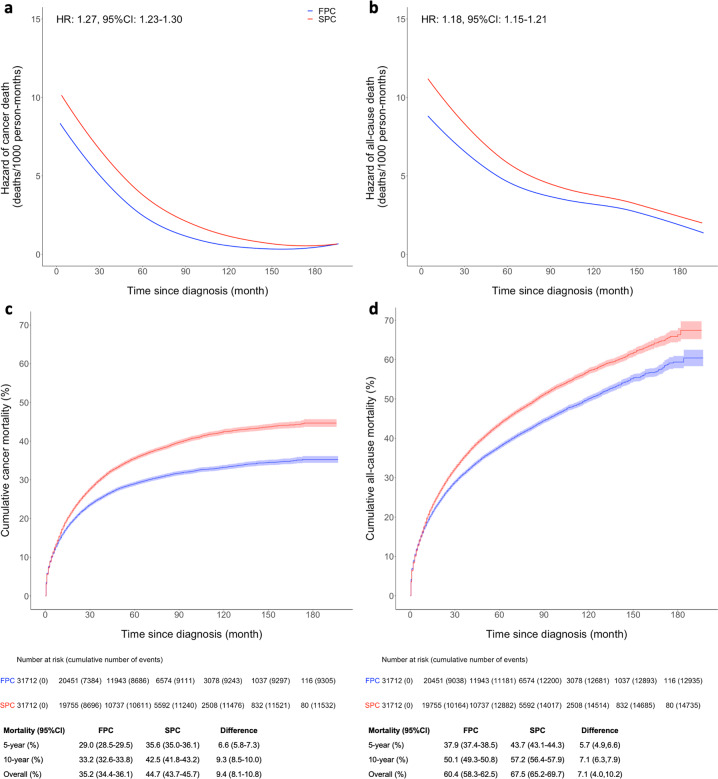
During the follow-up of 197 months, 12,935 deaths (9305 from cancer) and 14,735 deaths (11,532 from cancer) occurred in FPC and SPC groups respectively. The hazard of death for SPC was greater than that for FPC for both cancer and all-cause death, although the hazard functions of two groups did begin to converge with increasing follow-up time (Fig. 2a, b). Breast cancer survivors with SPC experienced 27% increased risk of cancer death (HR:1.27, 95% CI: 1.23, 1.30) and 18% increased risk of all-cause death (HR:1.18, 95% CI: 1.15, 1.21), compared with their first cancer counterparts. Further adjustment for five racial/ethnic groups did not change the estimates. Subdistribution HR of cancer death was consistent with the HR from the Cox regression (Supplementary Table 2).
Fig. 2. Hazard function and cumulative mortality comparing SPC to matched FPC.
Non-parametric hazard functions with hazard ratios (HRs) and 95% confidence intervals (CIs) for cancer mortality (a) and all-cause mortality (b). Cumulative mortality functions for cancer mortality (c) and all-cause mortality (d). SPC and FPC were matched by propensity scores calculated from race, age at diagnosis, cancer type, year of diagnosis, surgery, chemotherapy, and radiotherapy. Shaded areas in c and d show the 95% CIs of cumulative mortality. The absolute differences in 5 year, 10 year and overall mortality are provided below the figures. FPC first primary cancer, SPC second primary cancer.
The risk of dying from cancer comparing SPC to FPC for the top 10 cancer types is shown in Table 2. Increased risk of death was observed for second breast cancer (HR: 1.82, 95% CI: 1.71, 1.94), colorectal cancer (HR: 1.11, 95% CI: 1.02, 1.21), uterine cancer (HR: 1.40, 95% CI: 1.24, 1.58), lymphoma (HR: 1.15, 95% CI: 1.00, 1.32), thyroid cancer (HR: 3.09, 95% CI: 2.06, 4.61), melanoma (HR: 1.51, 95% CI: 1.18, 1.92), and leukemia (HR: 1.53, 95% CI: 1.37, 1.70). Decreased risk of death was observed for second lung cancer (HR: 0.95, 95% CI: 0.91, 1.00) even after adjustment of subtype. There was no difference in risk of death for second ovarian (HR: 1.02, 95% CI: 0.92, 1.14) and pancreatic cancer (HR: 0.97, 95% CI: 0.89, 1.06).
Table 2.
Hazard ratios (HRs) comparing cancer mortality after the second primary cancer (SPC) in breast cancer survivors to cancer mortality after the first primary cancer (FPC) for different types of cancer.
| Number of cases | Person-months | Number of deaths | HR (95% CI)a | |
|---|---|---|---|---|
| Breast cancer | 13,903 | 903004.5 | 1815 | 1.00 (Reference) |
| BC + BC | 13,931 | 825131.5 | 3198 | 1.82 (1.71, 1.94) |
| ER-positive BC + BC | 9069 | 534883.5 | 1842 | 1.77 (1.65, 1.90) |
| ER-negative BC + BC | 3184 | 183208 | 889 | 1.98 (1.81, 2.17) |
| Lung cancer | 4900 | 121576 | 3283 | 1.00 (Reference) |
| BC + Lung cancer | 4850 | 120105 | 3339 | 0.95 (0.91, 1.00) |
| ER-positive BC + Lung cancer | 3414 | 87507 | 2260 | 0.92 (0.87, 0.97) |
| ER-negative BC + Lung cancer | 880 | 20057.5 | 659 | 1.10 (1.01, 1.20) |
| Colorectal cancer | 3424 | 181289.5 | 1079 | 1.00 (Reference) |
| BC + Colorectal cancer | 3351 | 173083 | 1171 | 1.11 (1.02, 1.21) |
| ER-positive BC + Colorectal cancer | 2466 | 128772 | 838 | 1.07 (0.98, 1.18) |
| ER-negative BC + Colorectal cancer | 515 | 24758.5 | 186 | 1.17 (1.00, 1.36) |
| Uterine cancer | 2335 | 147537.5 | 433 | 1.00 (Reference) |
| BC + Uterine cancer | 2392 | 144851.5 | 669 | 1.40 (1.24, 1.58) |
| ER-positive BC + Uterine cancer | 1752 | 104264 | 507 | 1.43 (1.26, 1.63) |
| ER-negative BC + Uterine cancer | 380 | 23595 | 88 | 1.30 (1.03, 1.64) |
| Lymphoma | 1202 | 60396.5 | 391 | 1.00 (Reference) |
| BC + Lymphoma | 1191 | 55653 | 448 | 1.15 (1.00, 1.32) |
| ER-positive BC + Lymphoma | 921 | 42804.5 | 335 | 1.09 (0.95, 1.27) |
| ER-negative BC + Lymphoma | 153 | 7048 | 66 | 1.51 (1.16, 1.97) |
| Thyroid cancer | 1237 | 86451.5 | 32 | 1.00 (Reference) |
| BC + Thyroid cancer | 1268 | 85812.5 | 107 | 3.09 (2.06, 4.61) |
| ER-positive BC + Thyroid cancer | 926 | 61235 | 66 | 2.44 (1.59, 3.76) |
| ER-negative BC + Thyroid cancer | 217 | 15688 | 24 | 5.05 (2.93, 8.68) |
| Melanoma | 1240 | 81894.5 | 106 | 1.00 (Reference) |
| BC + Melanoma | 1261 | 83854 | 181 | 1.51 (1.18, 1.92) |
| ER-positive BC + Melanoma | 956 | 62150.5 | 129 | 1.34 (1.03, 1.74) |
| ER-negative BC + Melanoma | 197 | 13689.5 | 30 | 2.06 (1.36, 3.10) |
| Ovarian cancer | 1113 | 49886 | 631 | 1.00 (Reference) |
| BC + Ovarian cancer | 1095 | 48083.5 | 667 | 1.02 (0.92, 1.14) |
| ER-positive BC + Ovarian cancer | 684 | 28563 | 437 | 1.03 (0.91, 1.16) |
| ER-negative BC + Ovarian cancer | 275 | 13461.5 | 145 | 1.00 (0.83, 1.20) |
| Pancreatic cancer | 1093 | 12472.5 | 959 | 1.00 (Reference) |
| BC + Pancreatic cancer | 1130 | 13716 | 999 | 0.97 (0.89, 1.06) |
| ER-positive BC + Pancreatic cancer | 832 | 9953 | 742 | 0.97 (0.88, 1.06) |
| ER-negative BC + Pancreatic cancer | 161 | 2012 | 139 | 0.99 (0.82, 1.18) |
| Leukemia | 1265 | 53258 | 576 | 1.00 (Reference) |
| BC + Leukemia | 1243 | 38144 | 753 | 1.53 (1.37, 1.70) |
| ER-positive BC + Leukemia | 900 | 28404.5 | 533 | 1.45 (1.29, 1.63) |
| ER-negative BC + Leukemia | 228 | 6510.5 | 144 | 1.79 (1.49, 2.16) |
Estrogen receptor (ER) status is missing for some breast cancers.
HRs for overall, ER-positive, and ER-negative breast cancer survivors are presented separately.
FPC first primary cancer, SPC second primary cancer, HR hazard ratio, CI confidence interval, BC breast cancer, ER estrogen receptor.
aModels adjusted for race, year of diagnosis, age at diagnosis, tumor stage, and treatments (surgery, chemotherapy, and radiotherapy). For breast cancer, we further adjusted for ER status. For leukemia, we omitted surgery (it was not a treatment option) and tumor stage (all leukemia were distant stage).
The risk of cancer death differed by tumor characteristics of the prior breast cancer. The increased risk of cancer death after a SPC was accentuated in ER-negative vs ER positive breast cancer survivors when compared to FPC except for uterine cancer, which had a greater association in ER-positive survivors (Table 2). The decreased risk after second lung cancer was only observed in ER-positive survivors. In a subgroup of women diagnosed after 2010, we found a greater risk of cancer death in survivors with a second breast cancer diagnosed initially with a luminal A (HR: 2.16, 95% CI: 1.73, 2.69), luminal B (HR: 3.29, 95% CI: 1.98, 5.48), and triple negative (HR: 2.57, 95% CI: 1.85, 3.57) prior breast cancers, as compared to women with only one breast cancer (Supplementary Table 3).
We observed similar increased risk of cancer death for second breast cancers diagnosed ≥1 year (HR: 1.78, 95% CI: 1.67, 1.89), particularly for contralateral breast cancer (HR: 1.90, 95% CI: 1.78, 2.03) (Supplementary Table 4).
Relative difference in the risk of death comparing SPC to FPC restricted to survivors with local stage breast cancer
The increased risk of cancer death persisted when limited to breast cancer survivors diagnosed with local stage disease and who received surgery (N = 20,820). The followings are the type specific HRs of cancer death: second breast cancer (HR: 1.30, 95% CI: 1.20, 1.40), uterine cancer (HR: 1.27, 95% CI: 1.10, 1.45), thyroid cancer (HR: 1.87, 95% CI: 1.15, 3.04), and leukemia (HR: 1.29, 95% CI: 1.14, 1.47) (Table 3). A stronger association was observed for SPC in women initially diagnosed with an ER-negative cancer (Table 3). The associations also varied by time between prior breast cancer and SPC (Supplementary Table 5). When further restricted to survivors treated by chemotherapy (N = 5139), a greater increase in risk of cancer death was observed for second breast cancer (HR: 1.57, 95% CI: 1.40, 1.75), colorectal cancer (HR: 1.27, 95% CI: 1.06, 1.54), uterine cancer (HR: 1.82, 95% CI: 1.44, 2.29), thyroid cancer (HR: 5.18, 95% CI: 2.74, 9.82), and leukemia (HR: 1.96, 95% CI: 1.62, 2.38) (Table 4). Sensitivity analysis assuming all ER-negative survivors received chemotherapy yielded similar results (Supplementary Table 6). Among survivors treated by chemotherapy, the increased risk of cancer death became similar for ER-positive and ER-negative survivors for second breast cancer, thyroid cancer, and leukemia, but not for second lung cancer and lymphoma (Table 4). Among survivors treated by radiotherapy alone, we did not observe a greater increase in risk of cancer death after SPC. The risk of cancer death after second breast cancer, uterine cancer, and lymphoma increased further among survivors who previously received both radiotherapy and chemotherapy (Supplementary Table 7).
Table 3.
Hazard ratios (HRs) comparing cancer mortality after the second primary cancer (SPC) in breast cancer survivors (restricted to survivors with prior breast cancer of local stage and received surgery) to cancer mortality after the first primary cancer (FPC) for different types of cancer.
| Number of cases | Person-months | Number of deaths | HR (95% CI)a | |
|---|---|---|---|---|
| Breast cancer | 13,903 | 903004.5 | 1815 | 1.00 (Reference) |
| BC + BC | 9000 | 571431 | 1338 | 1.30 (1.20, 1.40) |
| ER-positive BC + BC | 6076 | 378583 | 798 | 1.24 (1.13, 1.35) |
| ER-negative BC + BC | 1936 | 122156.5 | 373 | 1.50 (1.33, 1.70) |
| Lung cancer | 4900 | 121576 | 3283 | 1.00 (Reference) |
| BC + Lung cancer | 3358 | 83597.5 | 2281 | 0.93 (0.89, 0.99) |
| ER-positive BC + Lung cancer | 2435 | 62588.5 | 1590 | 0.90 (0.85, 0.95) |
| ER-negative BC + Lung cancer | 556 | 12672.5 | 420 | 1.11 (1.00, 1.23) |
| Colorectal cancer | 3424 | 181289.5 | 1079 | 1.00 (Reference) |
| BC + Colorectal cancer | 2172 | 118342 | 672 | 0.97 (0.88, 1.07) |
| ER-positive BC + Colorectal cancer | 1607 | 88011.5 | 483 | 0.91 (0.82, 1.02) |
| ER-negative BC + Colorectal cancer | 347 | 17424 | 116 | 1.17 (0.97, 1.42) |
| Uterine cancer | 2335 | 147537.5 | 433 | 1.00 (Reference) |
| BC + Uterine cancer | 1631 | 102150.5 | 403 | 1.27 (1.10, 1.45) |
| ER-positive BC + Uterine cancer | 1200 | 73089 | 304 | 1.25 (1.07, 1.45) |
| ER-negative BC + Uterine cancer | 250 | 16398 | 55 | 1.40 (1.05, 1.86) |
| Lymphoma | 1202 | 60396.5 | 391 | 1.00 (Reference) |
| BC + Lymphoma | 849 | 40474.5 | 308 | 1.09 (0.94, 1.27) |
| ER-positive BC + Lymphoma | 662 | 31506.5 | 227 | 1.02 (0.87, 1.21) |
| ER-negative BC + Lymphoma | 102 | 4598 | 47 | 1.48 (1.09, 2.01) |
| Thyroid cancer | 1237 | 86451.5 | 32 | 1.00 (Reference) |
| BC + Thyroid cancer | 772 | 53027.5 | 43 | 1.87 (1.15, 3.04) |
| ER-positive BC + Thyroid cancer | 567 | 37790 | 27 | 1.52 (0.87, 2.64) |
| ER-negative BC + Thyroid cancer | 127 | 9265 | 8 | 2.93 (1.31, 6.54) |
| Melanoma | 1240 | 81894.5 | 106 | 1.00 (Reference) |
| BC + Melanoma | 824 | 54725 | 92 | 1.02 (0.76, 1.37) |
| ER-positive BC + Melanoma | 633 | 40854.5 | 67 | 0.87 (0.63, 1.21) |
| ER-negative BC + Melanoma | 127 | 8920.5 | 14 | 1.47 (0.83, 2.61) |
| Ovarian cancer | 1113 | 49886 | 631 | 1.00 (Reference) |
| BC + Ovarian cancer | 737 | 33072 | 434 | 0.95 (0.84, 1.08) |
| ER-positive BC + Ovarian cancer | 479 | 20143 | 295 | 0.96 (0.83, 1.10) |
| ER-negative BC + Ovarian cancer | 177 | 8923.5 | 92 | 0.94 (0.75, 1.17) |
| Pancreatic cancer | 1093 | 12472.5 | 959 | 1.00 (Reference) |
| BC + Pancreatic cancer | 777 | 8898 | 696 | 0.96 (0.87, 1.06) |
| ER-positive BC + Pancreatic cancer | 587 | 6597 | 531 | 0.97 (0.88, 1.08) |
| ER-negative BC + Pancreatic cancer | 108 | 1317 | 94 | 0.97 (0.78, 1.20) |
| Leukemia | 1265 | 53258 | 576 | 1.00 (Reference) |
| BC + Leukemia | 700 | 23418.5 | 396 | 1.29 (1.14, 1.47) |
| ER-positive BC + Leukemia | 512 | 17983.5 | 279 | 1.20 (1.04, 1.38) |
| ER-negative BC + Leukemia | 127 | 3350 | 80 | 1.74 (1.37, 2.20) |
Estrogen receptor (ER) status is missing for some breast cancers.
HRs for overall, ER-positive, and ER-negative breast cancer survivors are presented separately.
FPC first primary cancer, SPC second primary cancer, HR hazard ratio, CI confidence interval, BC breast cancer, ER estrogen receptor.
aModels adjusted for race, year of diagnosis, age at diagnosis, tumor stage, and treatments (surgery, chemotherapy, and radiotherapy). For breast cancer, we further adjusted for ER status. For leukemia, we omitted surgery (it was not a treatment option) and tumor stage (all leukemia were distant stage).
Table 4.
Hazard ratios (HRs) comparing cancer mortality after the second primary cancer (SPC) in breast cancer survivors (restricted to survivors with prior breast cancer of local stage and received surgery and chemotherapy) to cancer mortality after the first primary cancer (FPC) for different types of cancer.
| Number of cases | Person-months | Number of deaths | HR (95% CI)a | |
|---|---|---|---|---|
| Breast cancer | 13,903 | 903004.5 | 1815 | 1.00 (Reference) |
| BC + BC | 2483 | 156591.5 | 451 | 1.57 (1.40, 1.75) |
| ER-positive BC + BC | 1194 | 73886 | 210 | 1.63 (1.40, 1.91) |
| ER-negative BC + BC | 1103 | 69579.5 | 207 | 1.57 (1.34, 1.84) |
| Lung cancer | 4900 | 121576 | 3283 | 1.00 (Reference) |
| BC + Lung cancer | 661 | 17218 | 470 | 1.05 (0.95, 1.15) |
| ER-positive BC + Lung cancer | 352 | 9948 | 244 | 1.02 (0.89, 1.16) |
| ER-negative BC + Lung cancer | 260 | 5967 | 190 | 1.12 (0.97, 1.30) |
| Colorectal cancer | 3424 | 181289.5 | 1079 | 1.00 (Reference) |
| BC + Colorectal cancer | 395 | 21137.5 | 130 | 1.27 (1.06, 1.54) |
| ER-positive BC + Colorectal cancer | 211 | 11318 | 69 | 1.34 (1.05, 1.72) |
| ER-negative BC + Colorectal cancer | 159 | 8238.5 | 53 | 1.16 (0.87, 1.53) |
| Uterine cancer | 2335 | 147537.5 | 433 | 1.00 (Reference) |
| BC + Uterine cancer | 412 | 26563 | 97 | 1.82 (1.44, 2.29) |
| ER-positive BC + Uterine cancer | 248 | 15667 | 59 | 1.77 (1.34, 2.35) |
| ER-negative BC + Uterine cancer | 137 | 8734 | 32 | 1.86 (1.29, 2.68) |
| Lymphoma | 1202 | 60396.5 | 391 | 1.00 (Reference) |
| BC + Lymphoma | 132 | 7611.5 | 39 | 1.27 (0.90, 1.78) |
| ER-positive BC + Lymphoma | 84 | 4966.5 | 19 | 0.97 (0.61, 1.56) |
| ER-negative BC + Lymphoma | 42 | 2213 | 19 | 1.88 (1.17, 3.02) |
| Thyroid cancer | 1237 | 86451.5 | 32 | 1.00 (Reference) |
| BC + Thyroid cancer | 259 | 17760.5 | 17 | 5.18 (2.74, 9.82) |
| ER-positive BC + Thyroid cancer | 157 | 10312 | 8 | 4.58 (2.01, 10.42) |
| ER-negative BC + Thyroid cancer | 87 | 6364 | 7 | 4.68 (1.97, 11.13) |
| Melanoma | 1240 | 81894.5 | 106 | 1.00 (Reference) |
| BC + Melanoma | 223 | 15083.5 | 17 | 1.30 (0.74, 2.28) |
| ER-positive BC + Melanoma | 143 | 9516 | 10 | 1.11 (0.54, 2.29) |
| ER-negative BC + Melanoma | 72 | 4967.5 | 6 | 1.56 (0.67, 3.62) |
| Ovarian cancer | 1113 | 49886 | 631 | 1.00 (Reference) |
| BC + Ovarian cancer | 219 | 10552.5 | 121 | 1.01 (0.82, 1.23) |
| ER-positive BC + Ovarian cancer | 87 | 3924.5 | 54 | 1.18 (0.89, 1.56) |
| ER-negative BC + Ovarian cancer | 118 | 5963 | 61 | 0.94 (0.72, 1.23) |
| Pancreatic cancer | 1093 | 12472.5 | 959 | 1.00 (Reference) |
| BC + Pancreatic cancer | 133 | 2042.5 | 119 | 1.05 (0.86, 1.28) |
| ER-positive BC + Pancreatic cancer | 76 | 1208 | 68 | 1.06 (0.82, 1.36) |
| ER-negative BC + Pancreatic cancer | 45 | 558.5 | 41 | 1.09 (0.79, 1.50) |
| Leukemia | 1265 | 53258 | 576 | 1.00 (Reference) |
| BC + Leukemia | 222 | 6548.5 | 141 | 1.96 (1.62, 2.38) |
| ER-positive BC + Leukemia | 127 | 4093.5 | 77 | 1.80 (1.41, 2.31) |
| ER-negative BC + Leukemia | 83 | 2253.5 | 54 | 2.00 (1.50, 2.66) |
Estrogen receptor (ER) status is missing for some breast cancers.
HRs for overall, ER-positive, and ER-negative breast cancer survivors are presented separately.
FPC first primary cancer, SPC second primary cancer, HR hazard ratio, CI confidence interval, BC breast cancer, ER estrogen receptor.
aModels adjusted for race, year of diagnosis, age at diagnosis, tumor stage, and treatments (surgery, chemotherapy, and radiotherapy). For breast cancer, we further adjusted for ER status. For leukemia, we omitted surgery (it was not a treatment option) and tumor stage (all leukemia were distant stage).
Absolute difference in cumulative mortality comparing SPC to FPC
Cumulative cancer and all-cause mortality were 44.7% and 67.5% for SPC vs 35.2% and 60.4% for FPC during entire follow-up (Fig. 2c, d). For both cancer and all-cause mortality, the curves begin to diverge at 6 months post diagnosis. A greater overall cancer mortality was observed for all types of SPCs except lung and pancreatic cancer, with the absolute difference between SPC and FPC ranging from 3.7 to 15.1% (Table 5). Cumulative 5-year and 10-year cancer mortality and the absolute mortality difference between SPC and FPC by cancer type are also shown in Table 5.
Table 5.
Cumulative cancer mortality of second primary cancer (SPC) compared to first primary cancer (FPC) at 5 years, 10 years, and end of follow-up since diagnosis for different types of cancer.
| 5-year mortality (95% CI) (%) | 10-year mortality (95% CI) (%) | Overall mortality (95% CI) (%) | |
|---|---|---|---|
| BC + BC | 21.7 (20.9, 22.4) | 30.7 (29.6, 31.7) | 33.1 (31.4, 34.8) |
| Breast cancer | 12.1 (11.6, 12.7) | 17.0 (16.2, 17.8) | 20.0 (18.6, 21.3) |
| Absolute mortality differencea | 9.5 (8.6, 10.5) | 13.7 (12.8, 14.6) | 13.1 (11.0, 15.3) |
| BC + Lung cancer | 69.7 (68.3, 71.0) | 73.9 (72.3, 75.4) | 75.4 (73.2, 77.7) |
| Lung cancer | 67.9 (66.5, 69.3) | 71.5 (70.0, 73.0) | 72.7 (70.1, 75.3) |
| Absolute mortality difference | 1.8 (−0.2, 3.7) | 2.4 (0.4, 4.3) | 2.7 (−0.7, 6.1) |
| BC + Colorectal cancer | 33.7 (32.0, 35.4) | 40.2 (38.2, 42.1) | 40.6 (38.5, 42.6) |
| Colorectal cancer | 30.7 (29.1, 32.3) | 34.9 (33.1, 36.7) | 36.9 (34.3, 39.5) |
| Absolute mortality difference | 3.0 (0.7, 5.3) | 5.3 (3.0, 7.6) | 3.7 (0.4, 7.0) |
| BC + Uterine cancer | 27.1 (25.2, 29.0) | 32.3 (30.1, 34.6) | 36.3 (32.3, 40.3) |
| Uterine cancer | 18.9 (17.2, 20.5) | 20.7 (18.9, 22.5) | 21.2 (19.2, 23.1) |
| Absolute mortality difference | 8.2 (5.7, 10.7) | 11.6 (9.1, 14.1) | 15.1 (10.7, 19.5) |
| BC + Lymphoma | 36.4 (33.5, 39.2) | 43.3 (39.8, 46.7) | 47.8 (41.5, 54.0) |
| Lymphoma | 32.0 (29.3, 34.7) | 36.4 (33.2, 39.6) | 37.6 (33.7, 41.6) |
| Absolute mortality difference | 4.4 (0.4, 8.3) | 6.9 (2.9, 10.8) | 10.1 (2.7, 17.6) |
| BC + Thyroid cancer | 7.6 (6.1, 9.2) | 12.0 (9.4, 14.7) | 14.2 (10.1, 18.3) |
| Thyroid cancer | 2.3 (1.4, 3.2) | 3.3 (1.9, 4.7) | 4.2 (2.0, 6.4) |
| Absolute mortality difference | 5.3 (3.5, 7.1) | 8.7 (6.9, 10.5) | 10.0 (5.3, 14.6) |
| BC + Melanoma | 14.0 (11.9, 16.0) | 19.0 (16.3, 21.7) | 19.0 (16.3, 21.7) |
| Melanoma | 8.4 (6.7, 10.0) | 10.7 (8.6, 12.7) | 12.1 (8.7, 15.5) |
| Absolute mortality difference | 5.6 (2.9, 8.2) | 8.3 (5.6, 11.0) | 6.9 (2.5, 11.3) |
| BC + Ovarian cancer | 57.3 (54.2, 60.5) | 70.6 (67.2, 74.0) | 73.1 (69.3, 76.9) |
| Ovarian cancer | 55.1 (52.0, 58.2) | 64.6 (61.1, 68.1) | 66.2 (62.5, 69.8) |
| Absolute mortality difference | 2.3 (−2.1, 6.7) | 6.0 (1.6, 10.4) | 6.9 (1.7, 12.2) |
| BC + Pancreatic cancer | 89.6 (87.7, 91.5) | 89.8 (87.9, 91.7) | 92.4 (89.5, 95.3) |
| Pancreatic cancer | 88.7 (86.8, 90.7) | 89.7 (87.7, 91.6) | 89.7 (87.7, 91.6) |
| Absolute mortality difference | 0.9 (−1.9, 3.6) | 0.1 (−2.6, 2.8) | 2.7 (−0.8, 6.2) |
| BC + Leukemia | 59.9 (57.1, 62.7) | 63.9 (60.9, 67.0) | 66.7 (62.4, 71.0) |
| Leukemia | 44.4 (41.6, 47.3) | 50.7 (47.4, 53.9) | 52.4 (48.4, 56.5) |
| Absolute mortality difference | 15.4 (11.5, 19.4) | 13.2 (9.3, 17.2) | 14.2 (8.4, 20.1) |
FPC first primary cancer, SPC second primary cancer, CI confidence interval, BC breast cancer.
aAbsolute mortality difference = Mortality of SPC – Mortality of FPC.
Discussion
This large diverse population-based study examined cancer and all-cause mortality after a second cancer in breast cancer survivors and compared these risks with mortality after a first cancer matched on cancer type, race, and prognostic factors. The cumulative cancer mortality and all-cause mortality after SPCs among breast cancer survivors was 9% and 7% higher than the comparable FPCs over the same time period. The cumulative mortality curves are together at diagnosis but begin to diverge ~6 months later. Increases in cumulative mortality for survivors with SPC by cancer type ranged from 3.7 to 15.1%. Based on Cox proportional hazard models, up to a threefold elevation in risk of cancer death was observed for second cancers in the breast, lung, colon and/or rectum, uterus, lymphoma, melanoma, thyroid, and leukemia. Chemotherapy and radiotherapy treatment of the prior breast cancer, ER status, and the time between prior breast cancer and SPC significantly modified the mortality difference between women with SPC and FPC for specific cancer types.
Several prior studies have demonstrated an increased mortality among individuals with two vs one cancer. However, these studies did not stratify by type of first cancer. Zhou et al. reported an increase in all-cause mortality between 17 and 56% for second cancers with a prior history of any adulthood cancer compared to their first cancer counterparts14. Keegan et al. reported a higher mortality after SPC than FPC, with the largest increase in adolescent and young adult cancer survivors15. Studies among breast cancer survivors have been limited to second breast cancers in the contralateral breast. Consistent with our observations, some studies13,16–18, although not all19–22, showed that women who developed contralateral breast cancer (CBC) had increased mortality compared to those with a unilateral breast cancer, particularly if the second cancer occurred close in time to the first cancer diagnosis.
In our study, the difference in cancer mortality between second and first cancer was not observed for second ovarian and pancreatic cancer. This is likely due to the fact that patients with these two cancers often survive <6 months which is when we begin to observe a mortality difference. Zhou et al. reported a similar result for all-cause mortality14. Zhou et al. also observed that second thyroid cancer, uterine cancer, breast cancer, melanoma, and colorectal cancer had a greater all-cause mortality compared to their FPCs counterparts, which is consistent with our findings.
Treatment of the first breast cancer is one factor that could contribute to the higher mortality observed after second cancers. In our study, mortality difference between second and first breast cancer, uterine cancer, colorectal cancer, thyroid cancer, and leukemia was even larger among survivors who received chemotherapy for their first breast cancer. Radiotherapy alone however was not associated with a higher mortality difference between SPC and FPC. Further, the largest mortality difference between second and first breast cancer, uterine cancer, and lymphoma was observed among women who received both chemotherapy and radiotherapy. For second breast cancer, thyroid cancer, and leukemia, the receipt of chemotherapy explained the greater mortality difference between FPC and SPC among ER-negative survivors than ER-positive survivors, while for second lymphoma and lung cancer, there will likely be additional factors.
There are several biological explanations for a chemotherapy-associated increase in cancer mortality. Chemotherapy-related neoplasms can present with a more aggressive phenotype than sporadic cancer. A prior population-based study that compared chemotherapy and/or radiotherapy-induced acute myeloid leukemia (AML) to sporadic AML found that patients with treatment-induced AML were more likely to have adverse cytogenetics, worse response to treatment, and poor prognosis23. Clonal hematopoiesis can occur as a direct result of both chemotherapy and radiotherapy and is also associated with an increase in mortality24,25. An increased mortality was also found after a second uterine cancer among ER-positive survivors compared to a first uterine cancer. ER-positive survivors likely received hormone treatment including tamoxifen, which has been observed to cause uterine cancers that have unfavorable tumor characteristics (i.e., p53-positive, ER-negative, advanced FIGO stage, and higher grade) and a worse prognosis compared to sporadic uterine cancer26–28.
In addition to direct treatment effects, other potential explanations for the mortality disparity we observed include the fact that patients diagnosed with a second cancer could receive less intensive therapy and/or for shorter duration due to worry about their health status. It is plausible that women with SPCs have greater cumulative exposure to environmental/lifestyle risk factors of cancer such as obesity or smoking that can impact both cancer incidence and mortality29. Some of the women who developed SPCs may also have an inherited genetic susceptibility associated with more aggressive cancer phenotypes30. Interestingly, our results indicate that ER-positive survivors diagnosed with a second lung cancer had a reduced mortality than women with a first lung cancer. Laccetti et al. also found that prior cancer history was associated with improved survival among advanced stage lung cancer31. This reduction in mortality could be due to the fact that cancer survivors are more likely to stop smoking compared to cancer-free individuals32.
This study has several limitations. We were not able to adjust for or stratify by lifestyle factors such as body mass index, smoking, and alcohol, that could be different between FPC and SPC. Since there could be significant misclassification among patients classified as receiving no/unknown chemotherapy, we did not compare groups with and without chemotherapy. We chose not to present cancer-specific hazard ratios (HRs), given the potential for misclassification to have occurred in recording the cause of death among women with two cancers33.
The strengths of this national study using the SEER database include the large sample size, long-term follow-up, high-quality ascertainment of cancer diagnosis and mortality, and use of propensity-score matching. This study demonstrates that breast cancer survivors with a SPC have worse survival outcomes compared to women with a FPC. Treatment for the prior breast cancer appear to only partially contribute to the worse prognosis after SPC, suggesting that there are other yet unrecognized factors that impact the survival disparity. Future studies are needed to identify those novel drivers of this large absolute difference in mortality between SPC and FPC and possibly to test different early detection and treatment strategies among subgroups of survivors based on ER status and previous treatment.
Methods
Study population and study design
The study population was identified from the SEER 18 database34. SEER is a US national program that has been in existence since 1973 that collects patient data from cancer registries. Data on race/ethnicity, multiple primary cancers, tumor characteristics, and first course of treatment was extracted from medical records by experienced cancer registrars at each registry site. Starting from 2000, SEER has expanded its coverage from 13 to 18 cancer registries across the country which represents 27.8% of the population (SEER 18).
A cohort study was conducted to compare cancer and all-cause mortality between breast cancer survivors who developed a second cancer (SPC group) and individuals who developed only one primary cancer of the same type (FPC group). Figure 1 describes the study design. The SPC group included women 18 years or older diagnosed with incident breast cancer followed by a second cancer between January 1st, 2000 and December 31st, 2014. Second cancer was defined as the diagnosis of one of ten cancers at least 6 months after the initial breast cancer. Prior studies have used varying time intervals between first and second cancer diagnosis ranging from 2 months to 1 year2,7,35. The ten cancers are the most frequent types and represent more than 80% of second cancers diagnosed in breast cancer survivors. They include breast cancer, lung cancer, colorectal cancer, uterine cancer, lymphoma, melanoma, thyroid cancer, pancreatic cancer, ovarian cancer, and leukemia. The FPC group included women diagnosed with one primary invasive cancer during the same time period. The end of follow-up for both groups was December 31st, 2016, 2 years after the date of last cancer diagnosis. Women who developed another cancer after 2014 were excluded from the analysis.
Ascertainment of cancer and tumor characteristics
Data on cancer was extracted from pathology records based on the North American Association of Central Cancer Registries’ (NAACCR) Data Standards. SEER variable “behavior code” was used to identify all invasive cancers diagnosed between January 1st, 2000 and December 31st, 2014, followed by “site recode” to classify cancer type. The “site recode” variable was created based on International Classification of Diseases for Oncology, Third Edition (ICD-O-3) histology36. The variable “sequence number” was used to determine the number of cancers.
Information was available on age, year of diagnosis, and the following tumor characteristics: stage, grade, size, lymph node status, estrogen receptor (ER) status (for breast cancer), progesterone receptor (PR) status (for breast cancer), human epidermal growth factor receptor 2 (HER2) status (for breast cancer after year of 2010), surgery (yes vs no/unknown), initial chemotherapy (yes vs no/unknown), and initial radiotherapy (yes vs no/unknown). Of note, the SEER database cannot distinguish between patients who did not have treatment and those in whom the data on treatment was missing for chemotherapy and radiotherapy, thus the original variable was classified as “no/unknown”.
Ascertainment of vital status and cause of death
SEER obtained vital status, survival time, as well as cause of death from the National Center for Health Statistics37. We excluded 6% of patients with missing data on FPC/SPC race, ethnicity, tumor stage, cause of death, or survival time for analysis.
Statistical analysis
Propensity-score matching was conducted to balance the distribution of known prognostic factors between SPC and FPC. Propensity scores were generated based on race (White, Black, and Other), age at diagnosis (continuous), calendar year of diagnosis (2000–2014), cancer types, summary tumor stage (local, regional, and distant), and treatments (surgery, chemotherapy, and radiotherapy) in women with personal breast cancer history compared to women without. Nearest-neighbor matching was conducted to match one SPC to one FPC that has the closest propensity score38. The distribution of propensity score in each group and the standardized mean difference of matched variables were generated to check the matching. Similar propensity score distribution between groups and a standardized mean difference below 0.1 indicated excellent matching.
Means (standard deviation) and proportions were calculated to summarize the demographic and tumor characteristics for the SPC group, compared with the FPC group. Among breast cancer survivors, tumor characteristics of the prior breast cancer were also described.
Time-to-event analyses were conducted to compare the mortality after SPC to FPC. The outcome variable was person-time in months from time of diagnosis of the index cancer (second cancer in the SPC group and first cancer in the FPC group) to the date of death from cancer (any type of cancer), which could be censored by date of death from other conditions, date of last contact, or December 31st, 2016, whichever came first. A half month of follow-up time was added to women with survival time of 0 month. In the matched cohort, we used R package “bshazard” to generate the non-parametric hazard functions for cancer and all-cause death comparing SPC with FPC. Hazard ratio (HR) for cancer and all-cause death with 95% confidence intervals (CI) comparing SPC with FPC were estimated from Cox proportional hazard regression. The proportional hazard assumption was checked by graphing the Schoenfeld residuals, and we did not observe major violations.
HRs for cancer death were calculated for each of the top 10 cancers (breast cancer, lung cancer, colorectal cancer, uterine cancer, lymphoma, melanoma, thyroid cancer, pancreatic cancer, ovarian cancer, and leukemia). In addition, to address potential residual confounding we also adjusted for the matching variables [race, age at diagnosis, calendar year of diagnosis, cancer type, summary tumor stage, treatments, and ER status (only in regression for second breast cancer)]. A similar analysis was completed stratified by ER status of the prior breast cancer. For second breast cancer alone in women diagnosed after 2010 we also evaluated differences by molecular subtype of their prior breast cancer (luminal A, luminal B, triple negative, and HER2 enriched). For second lung cancer, we further adjusted for tumor histology (small cell vs non-small cell lung cancer) in the Cox model.
In order to minimize the impact of the prior breast cancer on mortality outcomes, similar analyses as described above were conducted limited to breast cancer survivors diagnosed with local stage disease and received surgery. Additional analysis was also conducted by time between prior breast cancer and SPC (≤5 vs >5 years). To explore the effect that chemotherapy for their prior breast cancer may have on mortality, we further limited to survivors with local stage disease who received surgery and chemotherapy for their prior breast cancer. We could not directly compare survivors with and without chemotherapy, because patients in the “no/unknown” chemotherapy group could have received chemotherapy but this was missed by the registry. To explore the additional effect of prior radiotherapy after surgery, we conducted separate analyses among survivors with local stage disease who received surgery and radiotherapy for their first breast cancer and those who received surgery, radiotherapy, and chemotherapy.
To understand the cumulative risk of death after SPC, we graphed the cumulative mortality curves and quantified the cumulative cancer and all-cause mortality (5-year, 10-year, and overall) and mortality difference comparing SPC to FPC. Competing risk of death from other conditions was considered for cancer mortality.
The following sensitivity analyses were performed. (1) In the matched cohort, categorical variable of race/ethnicity (Non-Hispanic White, Non-Hispanic Black, Non-Hispanic American Indian or Alaska Native, Non-Hispanic Asian or Pacific Islander, and Hispanic) and locations of 18 cancer registries were further adjusted for in the model. (2) Fine and Gray model was used to obtain subdistribution HR for cancer death. (3) To evaluate the possible misclassification of recurrence as a second primary, we conducted analysis among women with second breast cancer diagnosed ≥1 year, and for ipsilateral and contralateral breast cancer separately. (4) Considering that chemotherapy is underreported in SEER, we assumed all survivors with a first ER-negative breast cancer received chemotherapy and repeated the subgroup analysis for survivors with local stage disease who received surgery and chemotherapy.
All analyses were performed in software R (version 3.6.1). Two-sided p values < 0.05 were considered statistically significant in hypothesis testing.
Ethical approval
Ethical approval is not required as data used for this study were taken from the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program, which is a public database.
Supplementary information
Acknowledgements
This work was supported by the Breast Cancer Research Foundation. K.V. has received research funds from Cepheid Inc. for work unrelated to this paper and also has an ongoing research collaboration with Optra Health Inc. for work unrelated to this paper. K.V. and M.R.J are supported by P30CA006973.
Author contributions
Z.D. and K.V. conceived and designed the study. Z.D. prepared the database and conducted the analysis. Z.D. and K.V. drafted the paper. All authors contributed to the interpretation of the results and critical revision of the paper. All authors finally approved the paper.
Data availability
The data analyzed in this study were obtained from the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program at https://seer.cancer.gov/.
Code availability
Codes used to generate the data are available upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41523-022-00447-5.
References
- 1.Miller KD, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019;69:363–385. doi: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 2.Curtis R. E. et al. (eds). New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973–2000. (National Cancer Institute, NIH Publ. No. 05-5302. Bethesda, MD, 2006).
- 3.Nsouli-Maktabi HH, Henson DE, Younes N, Young HA, Cleary SD. Second primary breast, endometrial, and ovarian cancers in Black and White breast cancer survivors over a 35-year time span: Effect of age. Breast Cancer Res. Treat. 2011;129:963–969. doi: 10.1007/s10549-011-1560-9. [DOI] [PubMed] [Google Scholar]
- 4.Molina-Montes E, et al. Risk of second cancers cancer after a first primary breast cancer: a systematic review and meta-analysis. Gynecol. Oncol. 2015;136:158–171. doi: 10.1016/j.ygyno.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 5.Schaapveld M, et al. Risk of new primary nonbreast cancers after breast cancer treatment: a dutch population-based study. J. Clin. Oncol. 2008;26:1239–1246. doi: 10.1200/JCO.2007.11.9081. [DOI] [PubMed] [Google Scholar]
- 6.Mellemkjær L. et al. Risk of second cancer among women with breast cancer. Int. J. Cancer. 10.1002/ijc.21651 (2006). [DOI] [PubMed]
- 7.Sung H, Hyun N, Leach CR, Yabroff KR, Jemal A. Association of first primary cancer with risk of subsequent primary cancer among survivors of adult-onset cancers in the United States. JAMA - J. Am. Med. Assoc. 2020;324:2521–2535. doi: 10.1001/jama.2020.23130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trentham-Dietz A, Newcomb PA, Nichols HB, Hampton JM. Breast cancer risk factors and second primary malignancies among women with breast cancer. Breast Cancer Res. Treat. 2007;105:195–207. doi: 10.1007/s10549-006-9446-y. [DOI] [PubMed] [Google Scholar]
- 9.De Gonzalez BA, et al. Second solid cancers after radiotherapy for breast cancer in SEER cancer registries. Br. J. Cancer. 2010;102:220–226. doi: 10.1038/sj.bjc.6605435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirova YM, et al. Second malignancies after breast cancer: The impact of different treatment modalities. Br. J. Cancer. 2008;98:870–874. doi: 10.1038/sj.bjc.6604241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuchenbaecker KB, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA - J. Am. Med. Assoc. 2017;317:2402–2416. doi: 10.1001/jama.2017.7112. [DOI] [PubMed] [Google Scholar]
- 12.Wolff AC, et al. Risk of marrow neoplasms after adjuvant breast cancer therapy: the national comprehensive cancer network experience. J. Clin. Oncol. 2015;33:340–348. doi: 10.1200/JCO.2013.54.6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartman M, et al. Incidence and prognosis of synchronous and metachronous bilateral breast cancer. J. Clin. Oncol. 2007;25:4210–4216. doi: 10.1200/JCO.2006.10.5056. [DOI] [PubMed] [Google Scholar]
- 14.Zhou H, et al. Impact of prior cancer history on the overall survival of patients newly diagnosed with cancer: a pan-cancer analysis of the SEER database. Int J. Cancer. 2018;143:1569–1577. doi: 10.1002/ijc.31543. [DOI] [PubMed] [Google Scholar]
- 15.Keegan THM, Bleyer A, Rosenberg AS, Li Q, Goldfarb M. Second primary malignant neoplasms and survival in adolescent and young adult cancer survivors. JAMA Oncol. 2017;3:1554–1557. doi: 10.1001/jamaoncol.2017.0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langballe R, et al. Mortality after contralateral breast cancer in Denmark. Breast Cancer Res. Treat. 2018;171:489–499. doi: 10.1007/s10549-018-4846-3. [DOI] [PubMed] [Google Scholar]
- 17.Vichapat V, et al. Prognosis of metachronous contralateral breast cancer: Importance of stage, age and interval time between the two diagnoses. Breast Cancer Res. Treat. 2011;130:609–618. doi: 10.1007/s10549-011-1618-8. [DOI] [PubMed] [Google Scholar]
- 18.Font-Gonzalez A, et al. Inferior survival for young patients with contralateral compared to unilateral breast cancer: A nationwide population-based study in the Netherlands. Breast Cancer Res. Treat. 2013;139:811–819. doi: 10.1007/s10549-013-2588-9. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi H, Watanabe K, Takahashi M, Taguchi K, Sasaki F, Todo S. The impact of bilateral breast cancer on the prognosis of breast cancer: A comparative study with unilateral breast cancer. Breast Cancer. 2005;12:196–202. doi: 10.2325/jbcs.12.196. [DOI] [PubMed] [Google Scholar]
- 20.Verkooijen HM, et al. Survival after bilateral breast cancer: Results from a population-based study. Breast Cancer Res. Treat. 2007;105:347–357. doi: 10.1007/s10549-006-9455-x. [DOI] [PubMed] [Google Scholar]
- 21.Kheirelseid EAH, et al. Bilateral breast cancer: Analysis of incidence, outcome, survival and disease characteristics. Breast Cancer Res. Treat. 2011;126:131–140. doi: 10.1007/s10549-010-1057-y. [DOI] [PubMed] [Google Scholar]
- 22.Jobsen JJ, van der Palen, J, Ong F, Riemersma S, Struikmans H. Bilateral breast cancer, synchronous and metachronous; differences and outcome. Breast Cancer Res. Treat. 2015;153:277–283. doi: 10.1007/s10549-015-3538-5. [DOI] [PubMed] [Google Scholar]
- 23.Østgård LSG, et al. Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: a national population-based cohort study. J. Clin. Oncol. 2015;33:3641–3649. doi: 10.1200/JCO.2014.60.0890. [DOI] [PubMed] [Google Scholar]
- 24.Bolton KL, et al. Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat. Genet. 2020;52:1219–1226. doi: 10.1038/s41588-020-00710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaiswal S, et al. Age-Related Clonal Hematopoiesis Associated with Adverse Outcomes. N. Engl. J. Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones M. E. et al. Endometrial cancer survival after breast cancer in relation to tamoxifen treatment: Pooled results from three countries. Breast Cancer Res. 14, 10.1186/bcr3206 (2012). [DOI] [PMC free article] [PubMed]
- 27.Hoogendoorn WE, et al. Prognosis of uterine corpus cancer after tamoxifen treatment for breast cancer. Breast Cancer Res. Treat. 2008;112:99–108. doi: 10.1007/s10549-007-9823-1. [DOI] [PubMed] [Google Scholar]
- 28.Deng, Z., Jones, M.R., Visvanathan, K. Comparison of tumor profiles of second cancers among breast cancer survivors to first cancers in the SEER registries: a nationwide study [abstract]. Proc Am Assoc Cancer Res Annu Meet 2021; 2021 Apr 10–15 May 17–21 Philadelphia AACR; Cancer Res 2021;81(13_Suppl) Abstract nr 896.
- 29.Petrelli F, et al. Association of obesity with survival outcomes in patients with cancer: a systematic review and meta-analysis. JAMA Netw. Open. 2021;4:1–30. doi: 10.1001/jamanetworkopen.2021.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan Z, et al. Germline mutation in DNA-repair genes is associated with poor survival in BRCA1/2-negative breast cancer patients. Cancer Sci. 2019;110:3368–3374. doi: 10.1111/cas.14175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laccetti, A. L., Pruitt, S. L., Xuan, L., Halm, E. A. & Gerber, D. E. Effect of prior cancer on outcomes in advanced lung cancer: Implications for clinical trial eligibility and accrual. J Natl Cancer Inst. 107, 1–9 (2015). [DOI] [PMC free article] [PubMed]
- 32.Westmaas JL, Newton CC, Stevens VL, Flanders W, Gapstur SM, Jacobs EJ. Does a recent cancer diagnosis predict smoking cessation? An analysis from a large prospective US cohort. J. Clin. Oncol. 2015;33:1647–1652. doi: 10.1200/JCO.2014.58.3088. [DOI] [PubMed] [Google Scholar]
- 33.Lund JL, Harlan LC, Yabroff KR, Warren JL. Should cause of death from the death certificate be used to examine cancer-specific survival a study of patients with distant stage disease. Cancer Investig. 2010;28:758–764. doi: 10.3109/07357901003630959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Registries Custom Data (with additional treatment fields), Nov 2018 Sub (1975–2016 varying) - Linked To County Attributes - Total U.S., 1969. National Cancer Institute, DCCPS, Surveillance Research Program, released April 2019, based on the November 2018 submission.
- 35.Brown AL, et al. Survival disparities for second primary malignancies diagnosed among childhood: A population-based assessment. Cancer. 2019;125:3623–3630. doi: 10.1002/cncr.32356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.SEER. Site Recode ICD-O-3/WHO 2008 Definition. https://seer.cancer.gov/siterecode/icdo3_dwhoheme/index.html.
- 37.SEER. U.S. Mortality Data, 1969–2018. https://seer.cancer.gov/mortality/.
- 38.Stuart EA. Matching Methods for Causal Inference: A Review and a Look Forward. Statist. Sci. 2010;25:1–21. doi: 10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analyzed in this study were obtained from the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program at https://seer.cancer.gov/.
Codes used to generate the data are available upon reasonable request.