Abstract
Ergosterol is a membrane component specific to fungi that can be used to estimate fungal biomass using appropriate factors of conversion. Our objectives were to determine the limits of use of ergosterol content as a measure of biomass for aquatic hyphomycetes, and to evaluate a previously established ergosterol-to-biomass conversion factor. We varied inoculum quality, growth medium, and degree of shaking of four aquatic hyphomycete species. In cultures inoculated with homogenized mycelium, we found a significant effect of shaking condition and culture age on ergosterol content. In liquid cultures with defined medium, ergosterol content reached 10 to 11 μg/mg of mycelium (dry mass) and varied by factors of 2.2 during exponential growth and 1.3 during stationary phase. The increase in ergosterol content during exponential phase could be attributed, at least in part, to rapid depletion of glucose. Oxygen availability to internal hyphae within the mycelial mass is also responsible for the differences found between culture conditions. Ergosterol concentration ranged from 0.8 to 1.6 μg/mg in static cultures inoculated with agar plugs. Ergosterol content varied by a factor of 4 in two media of different richnesses. For different combinations of these parameters, strong (r2 = 0.83 to 0.98) and highly significant (P ≪ 0.001) linear relationships between ergosterol and mycelial dry mass (up to 110 mg) were observed. Overall, the ergosterol content varied by a factor of 14 (0.8 to 11 mg/g). These results suggest that care must be taken when the ergosterol content is used to compare data generated in different field environments.
Ergosterol is a membrane component of most fungi but is absent from vascular plants and metazoan animals (13). Its relative chemical instability suggests that it is rapidly degraded upon cell death and therefore provides a good quantitative measure of viable cells (19). Hence, ergosterol levels are commonly used to estimate fungal biomass on various substrates under a wide range of field and environmental conditions (4, 6, 9, 10, 11, 20).
Estimates of the importance of aquatic hyphomycetes have increased. Based on ergosterol and ATP assays, the biomass of these fungi is estimated to constitute up to 16% of the detrital dry mass of decomposing leaf litter (6). These estimates rely on conversion factors to estimate the amount of fungal biomass and are based on the amount of ergosterol measured. The accuracy of these conversion factors is critical, with values for aquatic hyphomycetes estimated to range from 2 to 16 μg/mg (dry weight) (10). Based on liquid cultures of various species grown under laboratory conditions, a mean conversion factor of 5.5 μg/mg of mycelium (dry mass) has been proposed (8).
Ergosterol content depends on the physiological state and general growth conditions of the fungus. In several species of aquatic hyphomycete cultured in liquid media with different C/N ratios, ergosterol content was only slightly affected by culture age and medium composition (8). However, other factors also can influence ergosterol synthesis (18, 19). In particular, ergosterol synthesis requires molecular oxygen, and low oxygen tensions can dramatically reduce ergosterol concentration (14, 15). The use of ergosterol as an indicator of fungal biomass has also been questioned in one study involving static cultures (1), but the unusual results obtained have been primarily attributed to the experimental procedures employed (5, 6). The low ergosterol contents reported in reference 1 suggest that culture conditions other than those previously used (8) could significantly affect ergosterol content.
It thus appeared that ergosterol content in aquatic hyphomycetes could vary depending on the growth conditions. Such variations would limit its use as an indicator of biomass when applied to different field environments. Our objectives in this study were (i) to determine how shaking, inoculum type, and growth medium altered the relationship between ergosterol and mycelial biomass of aquatic hyphomycetes and (ii) to determine the conditions under which the ergosterol assay can be reliably used to quantifying fungal biomass in decomposing leaf litter.
MATERIALS AND METHODS
Fungal isolates.
We used four aquatic hyphomycetes species: Alatospora acuminata Ingold (ALAC 79–170), Clavariopsis aquatica De Wild (CLAQ 28–185 and CLAQ 102–299), Tetrachaetum elegans Ingold (THEL R4–9), and Tetracladium marchalianum De Wild (TEMA 28–144). All were monosporic isolates obtained from either river foam (A. acuminata, C. aquatica CLAQ 28–185, and T. marchalianum) or submerged leaf litter (C. aquatica CLAQ 102–299 and T. elegans). Spores were collected from various streams in southwestern France, except for C. aquatica CLAQ 102–299, which was isolated from a stream in western Canada. Cultures were maintained on solid MEA medium (2% [wt/vol] malt extract [Merck 1.11929], 2% [wt/vol] agar [Merck 1614]) at 16°C. These strains were isolated in 1992 to 1994 and stored in liquid nitrogen (2). From this frozen stock, we used an aliquot cultured on MEA about 1 month before each experiment.
Chemicals.
All chemicals were of at least reagent grade. Dichloromethane and methanol were of high-performance liquid chromatography (HPLC) grade.
Cultures from homogenized mycelium.
Inoculum was prepared from a preculture grown at 16°C in a defined medium (GMS-VAA), by homogenization using a T 25 Ultra-Turrax operating with a scatter tool (S25-10G; Ika-Labortechnik, Staufen, Germany) as described previously (2). Mycelial fragments obtained under these conditions and measured on a microscope slide were 330 ± 92 μm in length by 240 ± 54 μm in width (mean ± standard deviation; n = 30). The concentration of mycelium in the inoculum was estimated using absorption at 600 nm of a 10-ml aliquot which was sonicated (90 s; duty cycle, 50%; output, 6) with a Sonifier 250 (Branson Ultrasonic Corp., Danbury, Conn.) with a 12.7-mm horn and a flat tip (catalog no. 101-148-013). A calibration curve was established using aliquots of dilutions of the same mycelial suspension that had been either analyzed at 600 nm or filtered on lyophilized, preweighed, 47-mm-diameter GF/C filters (Whatman International Ltd, Maidstone, United Kingdom) and then lyophilized and weighed. Optical density at 600 nm (OD600) was a linear function of the dry mass up to 1.1 mg ml−1 (OD600 = 1.16 × dry mass + 0.0003; r2 = 0.99; 1 OD600 unit = 0.91 mg ml−1). The concentration of mycelium in the suspension used for the experiments was 1.2 mg ml−1.
Two milliliters of the suspension was used to inoculate a 250-ml Erlenmeyer flask containing 50 ml of GMS-VAA. The mycelial suspension was stirred between samplings. Three series of culture flasks were prepared. The first series (BP series) consisted of baffle-plate flasks which were designed to increase turbulence and were placed on a rotary shaker (75 rpm, 25.4-mm orbital path) along with the second series of 30 conventional flasks (SC series). The third series consisted of conventional flasks that were not shaken (UC series). Three replicates of 2 ml of inoculum correponding to the day 0 samples were randomly filtered and washed during the course of inoculation of each series. Cultures were incubated at 16°C in darkness, and mycelial pellets were collected on filters.
Cultures from agar plugs.
Cultures were grown as described by Bermingham et al. (1) with modifications. A. acuminata ALAC 79–170 and C. aquatica CLAQ 28–185 and CLAQ 102–199 were cultured at 16°C on five 85-mm petri dishes each containing 30 ml of MEA (5.5-mm thickness) inoculated with one 25-mm octagonal agar plug. After 20 to 25 days, 15 250-ml Erlenmeyer flasks each containing 40 ml of 2% (wt/vol) malt extract (Merck 1.11929) broth (MEB) were inoculated with one 9-mm-diameter plug from the leading edge of the culture. For T. marchalianum, the petri dishes contained 15 ml of MEA (2.7-mm thickness) and growth in MEB and GMS-VAA (2) was examined. For all four strains, flasks were incubated statically at 16°C in darkness and mycelium was harvested after 3 to 14 days by collection on lyophilized preweighed 47-mm-diameter GF/C filters. At each sampling time, three replicate cultures were harvested. Filters were washed twice with 30 ml of deionized water. Each filter was placed in an aluminum pastry boat, flash frozen with liquid nitrogen, and stored at −80°C. Agar plugs on filters were lyophilized overnight before ergosterol extraction. The filters were weighed (accuracy, ±0.05 mg), and ergosterol was extracted from the mycelium within 30 min after weighing. For agar plugs grown in MEB, the mass of mycelium was obtained by determining the difference from that of agar plugs without mycelium. For agar plugs grown in GMS-VAA, because the malt in the agar plug had diffused into the liquid medium, the difference was calculated from plugs without mycelium which were maintained in GMS-VAA medium during the same time.
Ergosterol extraction and quantification.
The standard extraction procedure previously described by Gessner et al. (7) was used with modifications. Each lyophilized sample (mycelium and filter) was saponified by refluxing at 80°C for 30 min in 25 ml of methanol-ethanol (3:2, vol/vol) containing 2 g of KOH and 0.75 mg of 2,6-di-tert-butyl-4-methylphenol (Aldrich, St. Quentin Fallavier, France) as an antioxidant. After filtration of the saponified solution, the glassware was rinsed three times with 2 ml of methanol-ethanol (3:2, vol/vol), and 5 ml of deionized water was added to each filtrate. After partitioning with petroleum ether, the ether fraction was concentrated, transferred to an HPLC vial, evaporated to dryness, and then washed three times with 2 ml of petroleum ether under a flow of nitrogen. The vial was capped tightly and stored at −20°C for up to 3 days.
Before HPLC analysis, samples were dissolved in 1 ml of dichloromethane-isopropanol (100:1, vol/vol). Ergosterol was separated by reverse-phase HPLC using a LiChrospher C18 ODS2 5-μm column (Interchim, Montluçon, France), maintained at 38°C and connected to a Kontron 422M pump and a 432 UV/visible detector. Samples (10 μl) were injected using a Kontron autosampler 360. Isocratic elution was performed with methanol at a flow rate of 1.5 ml min−1. Ergosterol was detected by absorbance at 282 nm with a retention time of 7.8 min. The linear relationship between peak height (y) and ergosterol concentration (x) was determined using 10-μl injections of six standards (0.868 to 27.8 μg ml−1): the equation was y = 0.654x, with an r2 value of 0.998.
Glucose determination.
Glucose concentration in liquid defined medium was determined for the unshaken cultures and both shaken culture conditions. A 200-μl aliquot of medium was taken and centrifuged at 20,800 × g at room temperature for 5 min to remove mycelial fragments. A glucose–oxidase-peroxidase method was used following the recommendations of the kit's manufacturer (catalog no. A02486; Biotrol Diagnostic, Chennnevières-les-Louvres, France).
Oxygen saturation index.
Oxygen saturation was measured using a microprocessor oximeter (Oxi 320; WTW, Weilheim, Germany) coupled to a CellOx 325 oxygen sensor (self consumption at 20°C was 0.008 μg h−1). The electrode was equilibrated at 16°C before calibration. The sensor tip was placed 5 mm below the medium surface during measurements.
Statistical analysis.
Two-way analysis of variance was used to test for the effects of liquid culture condition and time on the arcsine √ transformed ergosterol/dry mass ratio. Linear regression analysis was used to relate the ergosterol and dry mass of each strain. Linearity was tested by visual inspection of plots of residuals against the predicted values (3). Slopes were compared with analysis of covariance. These analyses were completed with the SYSTAT software package, version 5.2 (Systat, Inc., Evanston, Ill).
RESULTS
Cultures from homogenized mycelium.
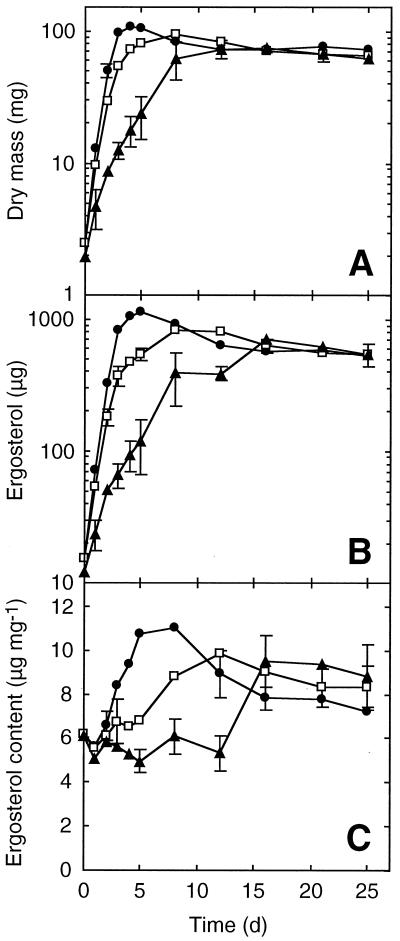
The ergosterol content in the first experiment with static cultures of T. elegans grown on GMS-VAA varied between 5 and 6 μg/mg over the 12-day course. The maximum ergosterol content observed in the BP series was between 11 and 11.5 μg/mg, and the value at day 12 in SC cultures was 9 μg/mg. Few differences were observed in the kinetics when the experiment was repeated; data from the second experiment are shown (Fig. 1), as it was run over a longer period of time. Mycelia in BP flasks grew as beads 1.5 to 2 mm in diameter, mycelia in SC flasks occurred in irregular aggregates with a maximum length of 0.3 to 1 cm, and mycelia in UC flasks grew as a macroscopically heterogeneous jelly-like mass.
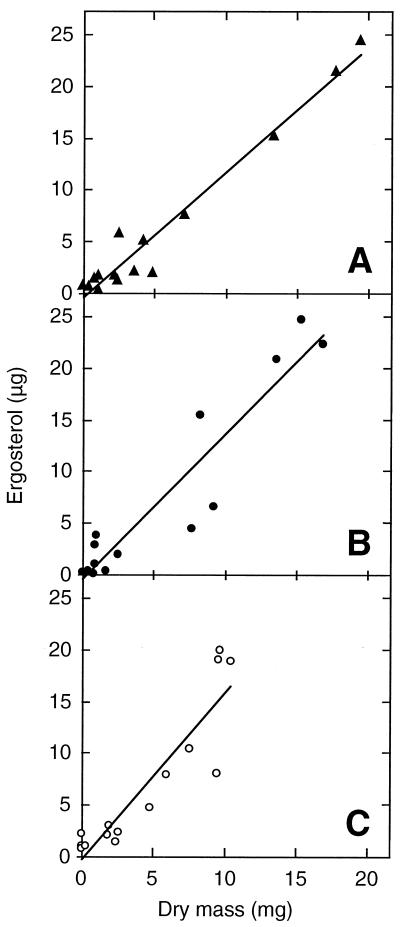
FIG. 1.
Mycelial dry mass (A), ergosterol (B), and ergosterol content (C) of T. elegans in liquid cultures as a function of time. Cultures were inoculated with homogenized mycelium in BP(●), SC (□), and UC (▴) series. Each point is the mean of three replicates, and bars denote 1 standard deviation.
Ergosterol and dry mass growth curves for the three incubation conditions were similar (Fig. 1A and B). The growth rates were significantly greater in shaking cultures (doubling time [Td] = 0.6 and 0.7 day in the BP and SC series, respectively) than in the static cultures (UC) (Td = 1.7 day), but the same plateau was reached at day 15 for all three incubation conditions. This plateau corresponds to 65 to 75 mg of dry mass and 550 to 650 μg of ergosterol.
The ergosterol/dry mass ratios from days 0 to 8 (Fig. 1C) were almost constant (mean values from 5 to 6 μg/mg) for the static flasks, but they varied from 5.5 to 10 and 11 μg/mg for the SC and BP series, respectively. The ergosterol content almost doubled at the beginning of the plateau phase between days 12 and 16 in the UC series. The mean values of all series converged on day 16, remaining almost constant within the overall range of 7 to 9.5 μg/mg. Both culture condition and time had a significant effect on ergosterol content (analysis of variance, P < 0.001).
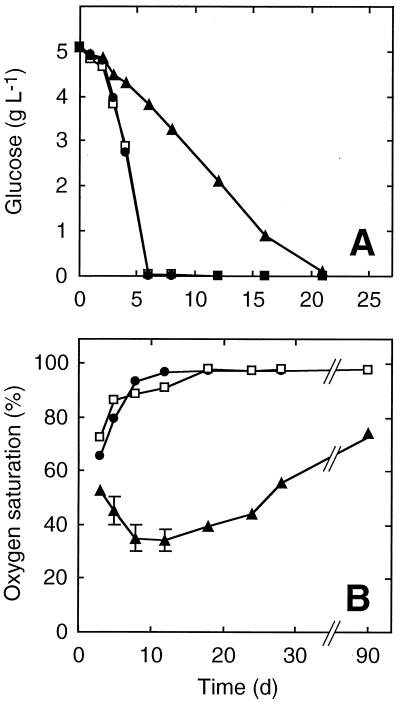
Glucose concentration decreased rapidly during the first days in the SC and BP flask series and was 0 on day 5 (Fig. 2A). The decrease was slower in the UC flasks; at day 5 the glucose concentration was still above 4 g/liter but was nearly exhausted by day 21.
FIG. 2.
Glucose concentration (A) and oxygen saturation (B) in liquid cultures as a function of time. For details, see the legend to Fig. 1.
Oxygen saturation decreased in BP and SC flasks during the first three days and then progressively increased until days 12 and 18, respectively, to reach a value close to the initial percentage (Fig. 2B). In UC cultures, oxygen saturation decreased to about 34% at day 12 and then slowly increased; at day 90, the value was 74%.
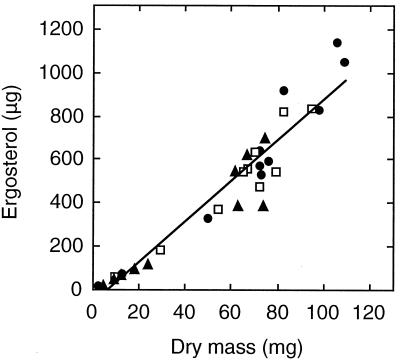
There was a linear relationship between ergosterol and mycelial dry mass (Fig. 3) (P ≪ 0.001). The linear regression slopes were within 10% of the mean slope plotted in Fig. 3.
FIG. 3.
Ergosterol as a function of dry mass in liquid cultures. Data are from Fig. 1A and B. The regression line was calculated from the combined data of the three series: BP (●), SC (□), and UC (▴) (y = 9.3x − 62; r2 = 0.92). The equations for the individual series were y = 10x − 91 with r2 = 0.92 (BP), y = 8.8x − 47 with r2 = 0.93 (SC), and y = 8.2x − 29 with r2 = 0.89 (UC).
Cultures from agar plugs.
The agar plug technique of inoculation produced three types of mycelium in the same culture flask: (i) inside the plug; (ii) outside the plug, including both mycelia growing around the plug and those from tiny fragments of agar that detached from the plug during cutting and inoculation; and (iii) aerial.
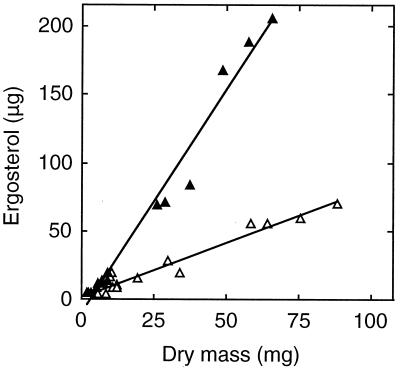
Using inoculum plugs (2.7-mm thickness) of T. marchalianum incubated in either MEB or GMS-VAA, we observed a strong linear relationship between ergosterol and mycelial dry mass (up to 60 to 90 mg) (P ≪ 0.001) (Fig. 4). Ergosterol contents of cultures in GMS-VAA and MEB were significantly different (P < 0.001), as indicated by the slopes of 3.3 and 0.81 μg/mg, respectively. Growth rate (Td = 3.1 and 2.4 days in MEB and GMS-VAA, respectively) and dry mass were greater in MEB (data not shown).
FIG. 4.
Relationship between ergosterol amount and mycelial dry mass for T. marchalianum grown in MEB (open symbols) or GMS-VAA (closed symbols) inoculated with agar plugs. The equations were y = 0.81x − 3 with r2 = 0.95 (MEB) and y = 3.3x − 9.8 with r2 = 0.98 (GMS-VAA).
We also found a significant linear relationship between ergosterol content and dry mass (up to 10 to 20 mg) for each of the three strains inoculated as 5.5-mm-thick agar plugs and grown in MEB medium (P ≪ 0.001) (Fig. 5). Average ergosterol content ranged from 1.2 to 1.6 μg/mg, as indicated by the slopes of these regressions. The experiment with A. acuminata was repeated and gave a ratio of 1.3 μg/mg. The Td of A. acuminata was 2.5 days, while those of C. aquatica isolates CLAQ 28–185 and CLAQ 102–299 were very similar (1.9 and 1.8 days, respectively; data not shown).
FIG. 5.
Relationship between ergosterol amount and mycelium dry mass for three strains of aquatic hyphomycetes, A. acuminata (A), C. aquatica CLAQ 28–185 (B), and C. aquatica CLAQ 102–299 (C), grown in MEB inoculated with agar plugs. The equations were y = 1.2x − 0.44 with r2 = 0.96 (A), y = 1.4x − 0.34 with r2 = 0.89 (B), and y = 1.6x − 0.25 with r2 = 0.83 (C).
DISCUSSION
Culture conditions significantly affect the ergosterol content of aquatic hyphomycetes. This conclusion differs from that of Gessner and Chauvet (8), who tested 12 aquatic hyphomycete species in three different liquid media using the same agitation mode as in our shaken cultures. These authors did not find a trend of higher or lower ergosterol content in any of the three media tested (including MEB and GMS). However, in that study, mycelium was harvested 6 to 10 days after inoculation, i.e., a period during which mean ergosterol varied about two fold in our shake-flask cultures. Consequently, differences in mycelial ergosterol contents due to medium composition may have been masked by differences attributable to growth stages. The previous authors also did not find a significant influence of culture age (7 to 28 days) on ergosterol content in the four species tested. This 7- to 28-day period also exhibited the minimum variation in ergosterol content in our shaken cultures.
Our study shows that the ergosterol content progressively increased in shaken cultures during exponential and very early stationary phase (Fig. 1). Under these conditions, cell growth was rapid and resulted in rapid consumption of glucose. Johnson and McGill (12) reported a threefold increase in ergosterol content in mycelium of the basidiomycete Hebeloma crustuliniforme in d-glucose-deficient cultures. Similarly, in our shake flasks, increased ergosterol content during exponential phase could be attributed, at least in part, to progressive glucose deprivation. However, although glucose consumption was identical in both sets of shake flasks, the increase in ergosterol concentration was slower in the unbaffled flasks. Ergosterol synthesis requires molecular oxygen (18), and low oxygen tensions can dramatically reduce ergosterol content in Mucor rouxii (15) and Rhizopus oligosporus (14). In our study, oxygen levels were similar in both baffled and unbaffled shake flasks. We hypothesize that the difference in ergosterol content during the exponential phase between the two types of shake-flask cultures is due to reduced oxygen availability to the internal hyphae of the larger mycelial aggregates in the unbaffled flasks. In static cultures, the ergosterol content almost doubled between days 12 and 16. As the oxygen saturation was 35 to 40% from days 8 to 18, we attribute the increase in ergosterol content to decreasing glucose concentration. We think that the decrease in ergosterol content during stationary phase probably resulted from the degradation of ergosterol in dead cells (19).
The lowest ergosterol contents were found in static cultures in MEB inoculated with agar plugs (Fig. 4 and 5). This result may be partially explained by reduced oxygen availability to the internal hyphae in a large mycelium mass, but similar cultures in MEB had one quarter the amount of ergosterol found in cultures grown in GMS-VAA medium. These media differ only slightly in nitrogen and free glucose content. MEB contains high levels of other carbohydrates (8) (e.g., maltose) not found in GMS-VAA that can be readily broken down to glucose by aquatic hyphomycetes (21). In particular, such breakdown by α-d-glucoside glucohydrolase (EC 3.2.1.20) would result in about fourfold-higher glucose levels in MEB than in GMS-VAA (data not shown). Thus, we attribute the differences in ergosterol content in these media to differences in effective glucose levels.
Bermingham et al. (1) did not find a significant relationship between ergosterol and fungal dry mass of A. acuminata and C. aquatica (coefficients of determination [r2] < 0.15), whereas we found highly significant linear relationships when using very similar culture conditions. This discrepancy could be due to the use of different strains or to the method of Bermingham et al. (1), in which fungal mass and ergosterol were determined from separate samples. Fell and Newell (5) pointed out that much of the variation in ergosterol conversion factors in the study by Bermingham et al. could be a consequence of imprecise dry mass determination. In our study, we used the same lyophilized sample for determination of both dry mass and ergosterol, a process that eliminates the wet-to-dry mass conversion and the errors resulting therefrom.
The ergosterol content varied by a factor of 14 (0.8 to 11 mg/g) in the four aquatic hyphomycete species and culture conditions tested. Gessner and Chauvet (8) tested 12 species in three different media using the same culture condition as in our SC series and found that ergosterol content varied by a factor of 5 (2.3 to 11.5 mg/g). The larger range obtained in the present study was clearly due to the low values generated by the static cultures inoculated with agar plugs in MEB. Bermingham et al., who initially described these culture conditions, reported similar low ergosterol contents (1). In our static cultures, growth was slower (Td = 1.8 to 3.1 days) than in shaken cultures inoculated with homogenized mycelium (Td = 0.6 or 0.7 day). Doubling times of fungal growth reported from studies on leaf litter decomposing in streams generally range from 5 to 14 days (5). This makes static cultures inoculated with agar plugs a potential model system that must still be improved in order to generate growth rates in the range of values prevailing under natural conditions.
The general ergosterol-to-biomass conversion factor of 182 (f = 1,000/5.5) (8) is acceptable when aquatic hyphomycete biomass is determined in comparable environments, e.g., on leaf litter in flowing water. However, using this conversion factor would underestimate the aquatic hyphomycete biomass by a factor of 6 to 7 if low values of ergosterol content, such as those reported here, occur under natural conditions. Thus, it is important to determine the range of ergosterol contents that can occur in field situations. Special care should be taken before a simple ergosterol-to-biomass factor is applied to samples from different field conditions; e.g., oxygen concentration is different in litter buried in sediment than it is in litter exposed to running water. We think that ergosterol content can provide reasonably robust estimates of aquatic hyphomycete biomass across different species and external conditions, but the results obtained should be interpreted in light of the availability of the oxygen and nutrients.
ACKNOWLEDGMENTS
This work was supported by the Centre National de la Recherche Scientifique and the Université Paul Sabatier-Toulouse III.
We thank Michèle Escautier, Patrick Sarthe, and Anne-Marie Jean-Louis for technical assistance and Mark O. Gessner for helpful comments on an early draft of the manuscript.
REFERENCES
- 1.Bermingham S, Maltby L, Cooke R C. A critical assessment of the validity of ergosterol as an indicator of fungal biomass. Mycol Res. 1995;99:479–484. [Google Scholar]
- 2.Charcosset J Y, Gardes M. Infraspecific genetic diversity and substrate preference in the aquatic hyphomycete Tetrachaetum elegans. Mycol Res. 1999;103:736–742. [Google Scholar]
- 3.Dowdy S, Wearden S. Statistics for research. New York, N.Y: John Wiley; 1991. [Google Scholar]
- 4.Ekblad A, Wallander H, Näsholm T. Chitin and ergosterol combined to measure total and living fungal biomass in ectomycorrhizas. New Phytol. 1998;138:143–149. [Google Scholar]
- 5.Fell J W, Newell S Y. Biochemical and molecular methods for the study of marine fungi. In: Cooksey K E, editor. Molecular approaches to the study of the ocean. London, United Kingdom: Chapman and Hall; 1998. pp. 259–283. [Google Scholar]
- 6.Gessner M O. Fungal biomass, production and sporulation associated with particulate organic matter in streams. Limnetica. 1997;13:33–44. [Google Scholar]
- 7.Gessner M O, Bauchrowitz M A, Escautier M. Extraction and quantification of ergosterol as a measure of fungal biomass in leaf litter. Microb Ecol. 1991;22:285–291. doi: 10.1007/BF02540230. [DOI] [PubMed] [Google Scholar]
- 8.Gessner M O, Chauvet E. Ergosterol-to-biomass conversion factors for aquatic hyphomycetes. Appl Environ Microbiol. 1993;59:502–507. doi: 10.1128/aem.59.2.502-507.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gessner M O, Chauvet E. Growth and production of aquatic hyphomycetes in decomposing leaf litter. Limnol Oceanogr. 1997;42:496–505. [Google Scholar]
- 10.Gessner M O, Newell S Y. Bulk quantitative methods for the examination of eukaryotic organoosmotrophs in plant litter. In: Hurst C J, editor. Manual of environmental microbiology. Washington, D. C.: ASM Press; 1997. pp. 295–308. [Google Scholar]
- 11.Gessner M O, Suberkropp K, Chauvet E. Decomposition of plant litter by fungi in marine and freshwater ecosystems. In: Wicklow D T, Söderström B, editors. The Mycota: a comprehensive treatise on fungi as experimental systems for basic and applied research. IV. Environmental and microbial relationships. Berlin, Germany: Springer-Verlag; 1997. pp. 303–322. [Google Scholar]
- 12.Johnson B N, McGill W B. Ontological and environmental influences on ergosterol content and activities of polyamine biosynthesis enzymes in Hebeloma crustuliniforme mycelia. Can J Microbiol. 1990;36:682–689. [Google Scholar]
- 13.Newell S Y. Estimating fungal biomass and productivity in decomposing litter. In: Carroll G C, Wicklow D T, editors. The fungal community. Its organization and role in the ecosystem. New York, N.Y: Marcel Dekker; 1992. pp. 521–561. [Google Scholar]
- 14.Nout M J R, Bonants-Van Laarhoven T M G, de Jongh P, de Koster P G. Ergosterol content of Rhizopus oligosporus NRRL 5905 grown in liquid and solid substrates. Appl Microbiol Biotechnol. 1987;26:456–461. [Google Scholar]
- 15.Safe S. The effect of environment on the free and hydrosoluble sterols of Mucor rouxii. Biochim Biophys Acta. 1973;326:471–475. doi: 10.1016/0005-2760(73)90147-1. [DOI] [PubMed] [Google Scholar]
- 16.Suberkropp K. The influence of nutrients on fungal growth, productivity, and sporulation during leaf breakdown in streams. Can J Bot. 1995;73:1361–1369. [Google Scholar]
- 17.Suberkropp K, Gessner M O, Chauvet E. Comparison of ATP and ergosterol as indicators of fungal biomass associated with decomposing leaves in streams. Appl Environ Microbiol. 1993;59:3367–3372. doi: 10.1128/aem.59.10.3367-3372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van den Bossche H. Importance of sterols in fungal membranes. In: Kuhn P J, Trinci A P J, Jung M J, Goosey M W, Copping L G, editors. Biochemistry of cell walls and membranes in fungi. Berlin, Germany: Springer-Verlag; 1990. pp. 135–157. [Google Scholar]
- 19.Weete J D, Weber D J. Lipid biochemistry of fungi and other organisms. New York, N.Y: Plenum Press; 1980. [Google Scholar]
- 20.West A W, Grant W D, Sparling G P. Use of ergosterol, diaminopimelic acid and glucosamine contents of soils to monitor changes in microbial populations. Soil Biol Biochem. 1987;19:607–612. [Google Scholar]
- 21.Zemek J, Marvanová L, Kuniak L, Kadlecíková B. Hydrolytic enzymes in aquatic hyphomycetes. Folia Microbiol. 1985;30:363–372. [Google Scholar]