Abstract
Vaccination against COVID-19 is one of the critical tools to provide herd immunity, reduce mortality, and control the pandemic worldwide. Despite the safety of vaccination against SARS-CoV-2 in the healthy population, a minority of people may develop rare post-vaccine adverse reactions such as autoimmune syndromes. The current study aimed to identify and present a series of patients with de-novo autoimmune rheumatic diseases (ARDs) associated with COVID-19 vaccines. Inclusion criteria were the onset of ARDs symptoms at ∼3–4 weeks post-vaccination, age ≥ 16, no previous history of ARDs, meeting the classification criteria for one of the ARDs, and staying in the follow-up. The most commonly used vaccines in patients were Sinopharm [7 cases (50%)] and AstraZeneca [6 cases (42.9%)]. ARDs were significantly more common in subjects who received the AstraZeneca vaccine than in those who received other vaccines. Based on the results, patients were diagnosed with rheumatoid arthritis or one of its subtypes (5 cases), vasculitis (4 cases), systemic lupus erythematosus (3 cases), and peripheral seronegative spondyloarthritis (2 cases). Except for one patient with self-limitation of ARD, others were treated with disease-modifying antirheumatic drugs, and one case developed irreversible neurological complications. Indeed, our data can warn physicians about the possibility of ARDs post-vaccination, lead to faster diagnosis, prevent loss of window of opportunity for treatment, and prevent irreversible organ damage. Based on the published literature, autoimmune phenomena post-COVID‐19 vaccination may be related to the overstimulation of mediators and cytokines due to complicated antigen-specific/non-specific immunological responses and mechanisms.
Keywords: Autoimmune rheumatic diseases, COVID-19, Post-vaccination, Vaccine
1. Introduction
The most recent COVID-19 (Coronavirus disease 2019) pneumonia caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has seriously threatened public health worldwide [1], [2], [3]. Despite the tremendous advantages of developed vaccines against COVID-19 in providing herd immunity and contributing to global health, their long-term safety and effectiveness in human subjects remain a serious concern. Various immune adverse reactions to vaccination may occur in the mild to the severe range after vaccination. It has been suggested that viral vaccination may trigger autoimmunity and lead to autoimmune rheumatic diseases (ARDs) and inflammatory syndromes [4], [5]. Multisystem ARDs are a heterogeneous group of autoimmune inflammatory disorders mainly affecting musculoskeletal and connective tissue systems identified by a series of self-reactive, antigen-driven immune responses. The ARDs' occurrence can be related to several factors, including genetic features, environmental problems, infections, hormonal alterations, and life events stressors [6]; however, the main reasons for ARD in a specific individual are not fully understood. The early most common clinical manifestations of ARDs include joint aches, swelling, redness over the joints, stiffness or limited range of motion, fatigue, and mild fever. Using different classification criteria in different regions makes it difficult to estimate the global burden of ARDs and accurate data on their incidence and prevalence; thus, the epidemiology of ARDs in the world remains a challenging issue [7]. Several inflammatory conditions such as arthritis, vasculitis, and central or peripheral nervous system symptoms after vaccination against tetanus, rubella, hepatitis B, and influenza have been reported [8], [9]. These phenomena may be associated with complicated immunologic mechanisms induced by non-target or target antigens of vaccines, leading to unwanted overstimulation of immune responses against self-antigens. The most common non-target antigens include (i) proteins which used to grow the viruses in the cell culture medium, such as fetal bovine serum, (ii) proteins shed from the mammalian cells into the culture medium, and (iii) adjuvants and stabilizers, such as gelatin to be used for vaccine developments. Commonly immune response against vaccine-associated non-target allergens is usually tolerable and harmless, except in populations that are often allergic, and IgE-mediated responses may lead to potential problems [10]. Despite several reports of inflammatory diseases following widespread vaccination against COVID-19 [11], [12], [13], [14], [15], [16], [17], there is still controversy over recognizing these vaccines as a rare etiologic agent of autoimmune diseases. Here, we present fourteen cases of de-novo ARDs following COVID-19 vaccines, indicating a possible association between vaccination and autoimmunity.
2. Methods
This observational two-center study was conducted in the rheumatology clinics of the Connective Tissue Diseases Research Center at Tabriz University of Medical Sciences and Kashan University of Medical Sciences. All patients were referred to clinics with ARDs symptoms after implementing the COVID-19 vaccination program in Iran from April 2021 and were considered for enrollment in the study. Overall, 1011 patients were registered with different ARDs, including rheumatoid arthritis (RA, no. 474), undifferentiated arthritis (UIA, no. 58), adult-onset Still's disease (AOSD, no. 7), palindromic rheumatism (PR, no. 37), and other collagen vascular diseases containing systemic lupus erythematosus (SLE, no. 59), inflammatory myopathies (no. 12), systemic sclerosis (SS, no. 37), Sjögren's syndrome (SS, no. 29), mixed connective tissue disease (MCTD, no. 1), seronegative spondyloarthropathies (SpA) (no. 167), vasculitis (no. 59), and Behcet's disease (no. 71). Inclusion criteria were the onset of ARDs symptoms at ∼3–4 weeks post-vaccination [16], age ≥ 16, no previous history of ARDs, meeting the classification criteria of one of the ARDs, and staying in the follow-up. Information about COVID-19 vaccination and demographic and clinical characteristics of participants were obtained from patient records, direct interviews, and clinical examinations. Venous blood samples were collected after a 12-h overnight of fasting for laboratory characteristics. The serum samples were separated from whole blood and were kept at −70 °C until biochemical analysis. The SPSS version 22 (SPSS, Inc., USA) was used for statistical analyses. The rate of ARD in patients receiving different types of vaccines was compared to the proportion of vaccines used for vaccination against COVID-19 in Iran by cross-tabulation analysis. The local ethics committee approved the study protocol and written informed consent was obtained from all participants. The study was performed according to the Helsinki humanity research declaration, 2008.
3. Case presentations
Between April 2021 and January 2022, 22 adult patients with symptoms of ARDs after COVID-19 vaccination were considered for eligibility. Eventually, 14 patients were diagnosed with ARDs based on classification criteria, and whose symptoms had started within approximately four weeks after vaccination were included in the study. The ARDs symptoms were not self-limiting or transient reactions, and the patients received disease-modifying antirheumatic drugs (DMARDs) to control their clinical manifestations, and they still suffered from the illness's symptoms in the follow-up tests. The duration of follow-up was 2–10 months. The vaccines used in these patients were Sinopharm [7 cases (50%)], AstraZeneca [6 cases (42.9%)], and COVIran Barakat [1 case (7.1%)]. It should be noted that vaccines for public vaccination in Iran until January 2022 were Sinopharm (78.9%), AstraZeneca (11.7%), COVIran Barekat (8.1%), and Sputnik (1.3%). Based on the cross-tabulation analysis, ARD was significantly more common in subjects who received the AstraZeneca vaccine than in subjects who received other vaccines (P < 0.001). Based on the results, the involved patients were diagnosed with RA or one of its subtypes (five cases), vasculitis (four cases), SLE (three cases), and peripheral SpA (pSpA) (two cases). The following results briefly represent the main clinical characteristics of patients and related treatment procedures for controlling their disease activity and symptoms. The detailed information on participants' clinical and paraclinical characteristics is shown in Table 1 .
Table 1.
The detailed information on participants' clinical and paraclinical characteristics.
| patients | Age | Sex | Familial history of AD | History of allergy | Smoking status | Type of vaccine | Interval between vaccination and first symptoms | Symptoms | Duration of symptoms up to diagnosis | Blood indices, inflammatory markers and HLA typing | Immunologic tests | Imagings | Biopsy | Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 52 | F | Yes | No | Never smoker | AstraZeneca | 14 days after the second dose | Inflammatory pain and tenderness in the neck and shoulder and pelvic girdle | 33 | Hb, 10.9 gr/dl; MCV, 81 fl; WBC, 6300 per µL; ESR, 65 mm/h; CRP, 36 (<6) mg/L | All were negative | No findings | NA | PMR |
| 2 | 74 | F | No | No | Never smoker | AstraZeneca | 24 days after the first dose | Inflammatory pain and tenderness in the neck and shoulder and pelvic girdles | 30 days | Hb, 11.1 gr/dl; MCV, 83 fl; WBC, 5800 per µL; ESR, 77 mm/h; CRP, 36 (<6) mg/L | All were negative | Subacromial bursitis in US of shoulders | NA | PMR |
| 3 | 85 | M | No | No | Never smoker | AstraZeneca | 1 day after the first dose | Inflammatory pain and tenderness in the neck and shoulder and pelvic girdles | 35 days | Hb, 11.0 gr/dl; MCV, 85 fl; WBC, 35 × 103 per µL (Neut, 30%; Lymph, 60%); Plt, 650 × 103 per µL; ESR, 97 mm/h; CRP, 26 (<6) mg/L | All were negative | No findings | NA | PMR |
| 4 | 43 | F | No | No | Never smoker | COVIran Barakat | 2 days after the first dose | Weight loss, palpable purpura, abdominal pain, mononeuritis multiplex | 10 days | Hb, 9.2 gr/dl; MCV, 80 fl; WBC, 10,200 per µL; Eos, 8%; Plt, 142 × 103 per µL; ESR, 95 mm/h; CRP, 10 (<6) mg/L | All were negative | Normal chest radiography | Necrotizing vasculitis | PAN |
| 5 | 85 | F | No | No | Never smoker | Sinopharm | 14 days after the first dose | Symmetric polyarthritis with involvement of small joints | 7 days | Hb, 11.1 gr/dl; MCV, 82 fl; WBC, 8200 per µL; ESR, 89 mm/h; CRP, 28 (<6) mg/L | RF, 76 (<20) IU/mL; anti-CCP, 15 (<20); ANA, 1/80; anti-dsDNA, 0.7 (<1.2) IU/mL | Normal chest, hands and knees radiographs | NA | RA |
| 6 | 58 | F | No | No | Never smoker | AstraZeneca | 10 days after the first dose | Symmetric polyarthritis with involvement of small and large joints | 78 days | Hb, 11.6 gr/dl; WBC, 12500; Neut, 84%; ESR, 74 mm/h; CRP, 21 (<6) mg/L | Anti-CCP, 58 (<18) IU/mL; anti-MCV, 319 (<18) IU/mL; others were negative | Normal hand and knee radiographs | NA | RA |
| 7 | 39 | F | No | No | Never smoker | Sinopharm | 26 days after the first dose | Attacks of periarthritis lasting 1–2 days | 60 days | Hb, 12.8 gr/dl; WBC, 7500; Neut, 74%; ESR, 45 mm/h; CRP, 56 (<6) mg/L; uric acid, 6.2 mg/L | All were negative | Normal hand and knee radiographs | NA | PR |
| 8 | 39 | M | No | No | Heavy smoker | Sinopharm | 3 days after the first dose | Fever, chills, myalgia, and macular pink rash on the trunk and thighs | 29 days | Hb, 13.2 gr/dl; WBC, 21,100 per µL; Neut 85%, Eos, 4.2%; ESR, 60 mm/h; CRP, 39 (<6) mg/L; ferritin, 180 mg/dl | All were negative | Normal chest X ray and CT scan of chest, abdomen and pelvis | NA | AOSD |
| 9 | 56 | M | No | Asthma | Never smoker | Sinopharm | 20 days after the second dose | Oligoarthritis | 21 days | Hb, 12.6 gr/dl; WBC, 8200; ESR, 72 mm/h; CRP, 12 (<6) mg/L; HLA B27− | All were negative | Normal knee and ankle radiographs and MRI of thoracic and lumbar spine | NA | UIA |
| 10 | 71 | F | No | No | Never smoker | Sinopharm | 1 day after the first dose | Symmetric polyarthritis with involvement of small joints, photosensitivity, Raynaud’s phenomenon | 38 days | Hb, 10.7 gr/dl; MCV, 81 fl; WBC, 6700 per µL ESR, 25 mm/h; CRP, 12.3 (<6) mg/L | ANA, 1/640; speckled pattern; others were negative | Normal chest and wrists radiographs | NA | SLE |
| 11 | 46 | F | No | No | Never smoker | Sinopharm | 15 days after the first dose | Fever, constitutional symptoms, hair loss, discoid plaques on the face and upper limbs | 58 days | Hb, 10.6 gr/dl; MCV, 85 fl; WBC, 2600 per µL; Lymph, 860 per µL; Plt, 142 × 103 per µL; ESR, 77 mm/h; CRP, 2 (<6) mg/L | ANA, 1.7 (<1.1) IU/mL; anti-ds DNA, 0.8 (<1.1) IU/mL; C3, 0.41 (0.9–1.8) g/L; C4, 0.06 (0.1–0.4) g/L | – | Discoid lesions biopsy was consistent with discoid lupus | SLE |
| 12 | 55 | M | No | No | Never smoker | AstraZeneca | 7 days after the first dose | Additive oligoarthritis | 3 days | Hb, 12.8 gr/dl; WBC, 8500 per µL; Lymph, 950 per µL; ESR, 100 mm/h; CRP, 53 (<6) mg/L | ANA, 1/160; anti-dsDNA, 85 (<40) IU/mL; C3, 1.05 (0.9–1.8) g/L; C4, 0.25 (0.1–0.4) g/L; others were negative | Normal chest, wrists and ankles radiographs, and MRI of the sacroiliac joints | NA | SLE |
| 13 | 45 | M | No | No | Never smoker | AstraZeneca | 6 days after the second dose | Additive arthritis predominantly in the lower limb | 14 days | Hb, 12.7 gr/dl; WBC, 8500; Neut, 85%; ESR, 85 mm/h; CRP, 49 (<6) mg/L; HLA B27− | All were negative | Bone marrow edema on MRI of the left sacroiliac joint | NA | pSpA |
| 14 | 61 | F | No | No | Never smoker | Sinopharm | 15 days after the second dose | Oligoarthritis of lower limb, inflammatory LBP, enthesitis and unilateral anterior uveitis | 5 days | Hb, 10.4 gr/dl; MCV, 83 fl; ESR, 58 mm/h; CRP, 21 (<6) mg/L; HLA B27+ | All were negative | Normal chest, knees and pelvis radiographs | NA | pSpA |
AD, autoimmune diseases; F, female; RA, rheumatoid arthritis; Hb, hemoglobin; MCV, mean corpuscular volume; fl, femtoliters; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; WBC, white blood cell; PMR, polymyalgia rheumatica; US, ultrasound; M, male; Neut, neutrophil; Lymph, lymphocyte; Eos, eosinophil; Plt, platelet; PAN, polyarteritis nodosa; RA, rheumatoid arthritis; RF, rheumatoid arthritis; anti-CCP, anti-citrullinated C peptide; ANA, antinuclear antibody; anti-ds DNA, anti-double stranded DNA; PR, palindromic rheumatism; AOSD, adult-onset Still’s disease; UIA, undifferentiated inflammatory arthritis; SLE, systemic lupus erythematosus; MRI, magnetic resonance imaging; pSpA, peripheral seronegative spondyloarthritis; LBP, low back pain; NA, not applicable.
3.1. Vasculitis
3.1.1. Polymyalgia rheumatica (PMR)
PMR is a large vessel vasculitis characterized by inflammatory muscle pain and stiffness in the shoulders, neck, and hips [18]. A 52-year-old female was presented on Oct 11, 2021 (patient #1) with a 33-day history of inflammatory pain in the neck, shoulder, pelvic girdles, and morning stiffness lasting 60 min. Based on the family history report, her grandmother was suffering from RA. Her clinical complaints appeared two weeks after receiving the second dose of the vaccine. Physical examination revealed 2+ tenderness in the shoulder and pelvic girdles muscles. Laboratory tests showed normochromic normocytic anemia, elevated C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR). Antinuclear antibodies (ANA), rheumatoid factor (RF), anti-citrullinated protein antibodies (ACPA), and antineutrophil cytoplasmic antibodies (ANCA) were negative. Chest X-ray and Doppler sonography of temporal arteries were normal. The patient met the European League Against Rheumatism (EULAR) and American College of Rheumatology (ACR) provisional criteria for the classification of polymyalgia rheumatica (PMR). Treatment with 15 mg/d prednisolone was started, and the response was dramatic. In subsequent visits, methotrexate (MTX) 10 mg/week was added due to recurrence of symptoms following a reduction in prednisolone dose to <10 mg/d.
Another case was a 74-year-old female presented on Jul 10, 2021 (patient #2) with a 30-day history of inflammatory pain in the neck, shoulder, pelvic girdles, and morning stiffness lasting 60 min. Her complaints appeared 24 days after receiving the first dose of the vaccine, and the physical examination revealed 2+ tenderness in the shoulder and pelvic girdles muscles. Based on laboratory tests, normochromic normocytic anemia was detected, CRP and ESR were significantly elevated, and the ANA, RF, ACPA, and ANCA values were negative. Ultrasonography of the shoulders revealed bilateral subacromial bursitis, and chest X-ray and Doppler sonography of temporal arteries were normal. She met the EULAR and ACR provisional criteria for the classification of PMR. Treatment with 20 mg/d prednisolone was started, and the response was dramatic. In subsequent visits, the prednisolone dose was reduced to 7.5 mg/d. She received the second and third doses of the same vaccine without further problems.
In addition to the earlier cases, an 85-year-old male was presented on Jun 10, 2021 (patient #3) with a 35-day history of inflammatory pain in the neck and shoulder, pelvic girdles, and morning stiffness lasting 90 min, one day after the first dose of the vaccine. In physical examination, we noticed tenderness in the shoulder and pelvic girdles muscles and pain in the range of motion (ROM) of shoulder motions. Laboratory tests showed significant leukocytosis, thrombocytosis, normochromic normocytic anemia, and elevated CRP and ESR. A hematologist's peripheral blood smear examination did not show atypical cells. ANA, RF, ACPA, and ANCA were negative. Chest X-ray, doppler sonography of temporal arteries, and bone scan were normal. He met the EULAR and ACR provisional criteria for the classification of PMR. Treatment with prednisolone 15 mg/d was started. The response was dramatic. In subsequent visits, the dose of prednisolone was reduced to 10 mg/d. White blood cell (WBC) and platelet counts gradually decreased over four months. In Oct 2022, he was given the Sinopharm vaccine as recommended by his doctor to continue vaccination, but ten days later, he developed a fever, malaise, cough, and dyspnea and was admitted to the intensive care unit. He was diagnosed with COVID-19 and unfortunately expired due to respiratory failure.
3.1.2. Polyarteritis nodosa (PAN)
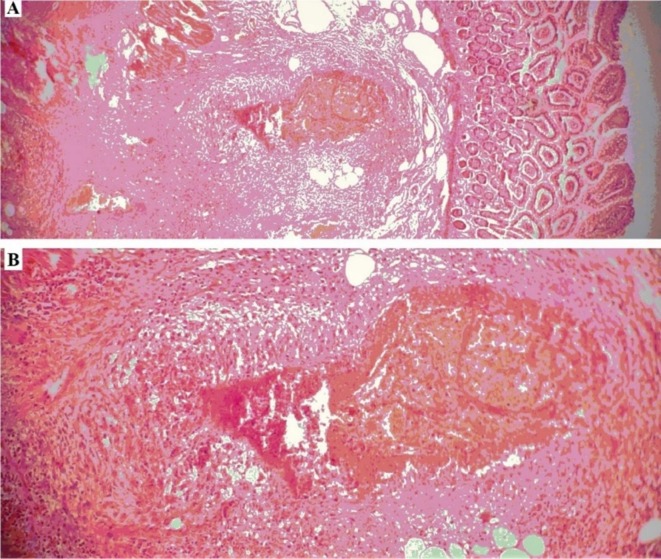
PAN is a medium-sized vessel vasculitis characterized by constitutional symptoms, limb ischemia, mononeuritis, multiplex, bowel ischemia, and musculoskeletal involvement [19]. A 43-year-old female was presented on Aug 12, 2021 (patient #4) with a 10-day history of malaise, weight loss (10 Kg), and abdominal pain, which started 2 days after the first dose of the vaccine. With a diagnosis of acute abdomen, laparotomy was performed. Due to mesenteric ischemia, the gross pathology diagnosis was bowel perforation. She developed palpable purpura in the lower limb and left-sided wrist and foot drop during the hospitalization. A nerve conduction study showed mononeuritis multiplex. Intestinal biopsy demonstrated infiltration of inflammatory cells in the intestinal wall and fibrinoid necrosis of mesenteric vessels (Fig. 1 ). Laboratory tests showed normochromic normocytic anemia, leukocytosis, eosinophilia, and high ESR and CRP. All of the autoantibodies and viral markers were negative. She met the ACR classification criteria for polyarteritis nodosa (PAN). Induction therapy with high-dose steroids and monthly pulses of cyclophosphamide (1000 mg) was started. At the time of writing, she received the fifth dose of cyclophosphamide, and the disease was under control, but moderate weakness continued in the dorsiflexion of the left wrist and ankle.
Fig. 1.
H&E stained section of small bowel in patient #4 shows Vasculitis in the submucosa; A: 40× magnification, and B: 100× magnification.
3.2. RA and its subtypes
3.2.1. RA
RA is the most common form of inflammatory arthritis characterized by chronic symmetric polyarthritis involving small and large peripheral joints [20]. An 85-year-old female was presented on Jun 4, 2021 (patient #5) with additive inflammatory arthralgia, which started 14 days after the first dose of the vaccine. Her medical history was positive for hypertension and heart failure. In physical examination, 2+ swelling and 3+ tenderness in the wrists, knees, and ankles were detected. Laboratory tests showed normochromic normocytic anemia, high ESR and CRP, and positive RF and ANA. Chest, hand, and knee radiographs were normal. She met RA's EULAR/ACR 2010 classification criteria based on clinical features. Treatment with 10 mg/week MTX, 5 mg/kg/d hydroxychloroquine (HCQ), and 7.5 mg/d prednisolone was started. Symptoms were controlled after two weeks, and prednisolone was tapered. However, at the time of writing, she had arthralgia and morning stiffness lasting 2 h and was being treated with MTX 15 mg/week, HCQ 5 mg/kg/d, and prednisolone 10 mg/d.
Another case was a 58-year-old female who presented with inflammatory arthralgia on Nov 15, 2021 (patient #6). Symptoms started 10 days after the first dose of the vaccine. In physical examination, we observed +2 swelling and tenderness in the third metacarpophalangeal (MCP) joints of both hands, the left wrist, and the MTPs of both feet. Laboratory tests showed mild leukocytosis, high ESR and CRP, and positive ACPA. She met the EULAR/ACR 2010 classification criteria for RA. Treatment was started with MTX 10 mg/week, HCQ 5 mg/kg/d, and prednisolone 10 mg/d. Symptoms were controlled after three weeks, and prednisolone was tapered.
3.2.2. PR
PR is an autoimmune inflammatory syndrome characterized by intermittent flares of pain, swelling, and erythema around the joint, often mono-articular, severe, and unpredictable. As a recurring form of arthritis, PR may progress to chronic rheumatic disease in many patients, mainly RA [21]. A 39-year-old female was presented on Nov 3, 2022 (patient #7) with attacks of pain, swelling, and erythema in the periarticular region of the hand, knee, shoulder, and ankle lasting 1–2 days, which started 26 days after the first dose of the vaccine. Attacks occurred at least once a week. In each attack, only one area was involved. On physical examination, periarthritis of the left wrist was observed. Other laboratory tests and imaging were routine except for a high ESR and CRP during an attack. She met the Hannonen criteria for diagnosis of palindromic rheumatism. Treatment with HCQ 5 mg/kg/day and prednisolone 7.5 mg/day was started. Symptoms were controlled, and prednisolone was tapered < 5 mg/day.
3.2.3. AOSD
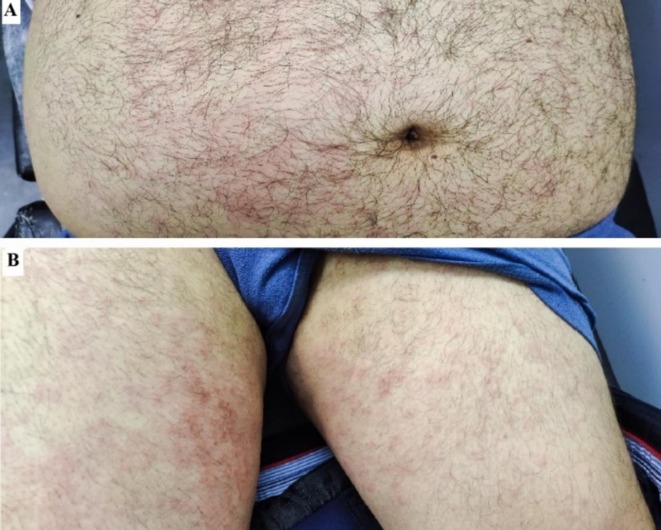
AOSD is a rare systemic inflammatory disorder generally occurring in young adults with clinical symptoms such as spiking fever, arthritis, lymphadenopathy, hepatosplenomegaly, and evanescent macular rash during the fever spikes [22]. A 39-year-old male was presented on Oct 18, 2021 (patient #8) with a 4-week history of fever, chills, malaise, myalgia, and skin lesions which started three days after the first dose of the vaccine. His physical examination revealed a high fever (T = 39.2) and macular nonpruritic pink rash on the trunk and thighs (Fig. 2 ). Laboratory tests showed marked leukocytosis and high acute phase reactants. Other laboratory tests and imaging were normal. He met the Yamaguchi criteria for adult-onset still’s disease (AOSD). Treatment with prednisolone 0.5 mg/kg/d and HCQ 5 mg/kg/d was started. His symptoms had resolved at the time of writing, and he was being treated with HCQ 5 mg/kg/d, MTX 15 mg/week, and prednisolone 20 mg/d.
Fig. 2.
Macular salmon-colored rash on the abdomen and thighs of patient #8; A: abdomen, and B: tighs.
3.2.4. UIA
UIA describes patients with inflammatory oligoarthritis or polyarthritis that do not fully meet the criteria for a specific rheumatic disease [23]. A 56-year-old male was presented on Oct 31, 2021 (patient #9) with a 3-week history of oligoarthritis of the left ankle and left knee, which started 20 days after the second dose of the vaccine. There were no systemic symptoms. Other laboratory tests and imaging were routine except for a high ESR and CRP. With a diagnosis of UIA, naproxen 1500 mg/d was started. His symptoms were resolved during the next two weeks, and naproxen was tapered and discontinued. He received the third dose of the same vaccine on Oct 16, 2021, and a week later developed arthritis of the left knee, which resolved within a few days. He was asymptomatic at the time of writing, and acute phase reactants were in a normal range.
3.3. SLE
SLE is a classic auto-antibody mediated disease characterized by immune complexes deposition in the various organs, which leads to joints, skin, brain, lungs, kidneys, and other organs inflammation and damage with significant potential morbidity and mortality [24]. A 71-year-old female was presented on Jun 23, 2021 (patient #10) with a 38-day history of inflammatory arthralgia in the small joints, which started one day after the first dose of the vaccine. In physical examination, we detected 2+ swelling and tenderness in the MCP 2, 3, and proximal interphalangeal (PIP) 1–5 joints of both hands and wrists. Autoantibodies and viral markers at presentation were negative, and chest and hand radiographs were normal. Treatment with MTX 10 mg/week and prednisolone 7.5 mg/d was started. However, during the follow-up, she reported sensitivity to light and Raynaud's phenomenon and developed a highly positive ANA (speckled pattern). She met the Systemic Lupus International Collaborating Clinics (SLICC) classification criteria for SLE. At the time of writing, her rheumatic disease was active, and she was being treated with azathioprine 100 mg/d, HCQ 5 mg/kg/d, and prednisolone 7.5 mg/d.
Another case was a 46-year-old female who presented on Aug 17, 2021 (patient #11) with a 2-months history of fever, constitutional symptoms, hair loss, discoid plaques on the face and upper limbs, which started 15 days after the first dose of the vaccine. Laboratory tests showed leukopenia, lymphopenia, high ESR and CRP, positive ANA, and low complement. She met the SLICC classification criteria for SLE. Treatment with HCQ 5 mg/kg/d and prednisolone 10 mg/d was started. Symptoms were controlled after four weeks.
In addition to earlier cases, a 55-year-old male was presented on Jun 18, 2021 (patient #12) with a 3-days history of oligoarthritis of the wrists, ankle, and left knee, which started 7 days after the first dose of the vaccine. Laboratory tests showed lymphopenia, high ESR and CRP, positive ANA, and anti-dsDNA. She met the SLICC classification criteria for SLE. Treatment with HCQ 5 mg/kg/d and meloxicam 15 mg/d was started. Symptoms were controlled after two weeks. During follow-up, arthralgia recurred, and he developed photosensitivity and proteinuria. His disease was active at the time of writing, and he was treated with HCQ 5 mg/kg/d, mycophenolate mofetil 2000 mg/d, and prednisolone 7.5 mg/d.
3.4. pSpA
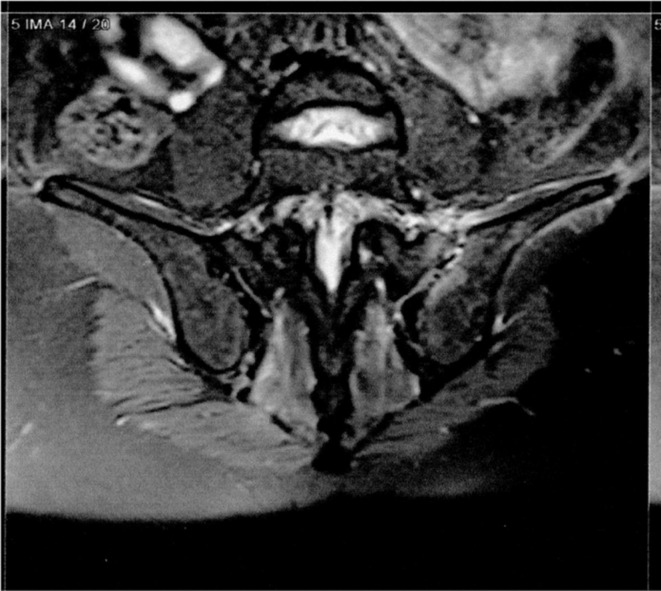
pSpA is a subtype of SpA with predominantly peripheral joint involvement and characterized by asymmetric large joints arthritis and enthesitis [25]. A 45-year-old male was presented on Sept 22, 2021 (patient #13) with a 14-day history of inflammatory arthralgia in the lower limb joints, which started 6 days after the second dose of the vaccine. In physical examination, we detected 2–3+ swelling and tenderness in the metatarsophalangeal joints (MTPs) 1–5 on both sides, left ankle and right elbow. Autoantibodies and HLA B27 were negative. Magnet resonance imaging (MRI) of sacroiliac joints showed bone marrow edema in the left sacroiliac joint (Fig. 3 ). He met the Assessment of SpondyloArthritis International Society (ASAS) classification criteria for pSpA. Treatment with indomethacin 150 mg/d and then naproxen 1500 mg/d could not control symptoms. After three weeks, etanercept 50 mg/week was started, and the response was dramatic.
Fig. 3.
Magnetic resonance imaging (MRI) of sacroiliac joints in patient #13 shows bone marrow edema on the left sacroiliac joint.
Another case was a 61-year-old female who presented on Jul 27, 2021 (patient #14) with a 5-day history of arthralgia, inflammatory low back pain, heel pain, and red eye, which started 15 days after the second dose of the vaccine. On physical examination, anterior uveitis of the left eye and 3+ swelling and tenderness in the knees, 2+ tenderness of both Achilles' tendons, and pain and limitation of motion in spinal movements were detected. The HLA B27 was detected positive. He met the ASAS classification criteria for pSpA. Treatment was started with diclofenac 150 mg/d and sulfasalazine (SSZ) 2000 mg/d. After two weeks, symptoms significantly decreased (∼80%). His disease was in remission at the time of writing, and he was being treated with SSZ 2000 mg/d and diclofenac 75 mg/d. She received the second dose of the vaccine on November 2021 and developed transient pain at the injection site, fever, and arthralgia lasting one day.
4. Discussion
Based on the medical resources, rheumatic diseases caused by infectious agents are defined as diseases that usually develop within ∼4 to 8 weeks after infection, such as rheumatic fever, post-streptococcal reactive arthritis [26], and reactive arthritis [27]. In addition, several reports of immune-mediated inflammatory diseases following COVID-19 vaccination have occurred during ∼4–8 weeks after the vaccine [11], [12], [28]. Thus, we hypothesized that rheumatic diseases developed within this time interval after vaccination might be related to the vaccine. Herein, we reported 14 patients with de-novo symptoms related to the ARDs, which commonly developed after the first dose of the COVID-19 vaccine (10 cases). ARDs following the AstraZeneca vaccine were more common than vaccination with Sinopharm, COVIran Barekat, and Sputnik. In most patients (11 cases), symptoms started two weeks after the vaccination; however, diagnosis in 8 patients was delayed for more than four weeks. Autoantibodies were detected positive in 5 patients. Except for one patient whose ARD was self-limited, others required treatment with anti-inflammatory drugs and DMARDs, and even one patient developed irreversible neurological complications.
Autoimmunity following vaccination is a multifactorial phenomenon related to genetic risk factors, environmental factors, and overstimulation of the immune responses. It has been suggested that different immunological mechanisms, including molecular mimicry (antigen-specific) and bystander activation (non-specific), could be associated with developing autoimmune diseases or autoinflammatory-like conditions after viral vaccines [29]. Molecular mimicry represents the amino acid similarity between the host's organs and foreign specific antigens, leading to autoimmune reactions against these organs. The molecular mimicry hypothesis may be essential in acquired autoimmunity development post-vaccination [4]. Almost 4% of monoclonal antibodies against the virus could also cross-react with host self-proteins. In addition to the molecular mimicry with an epitope to auto-reactive T-cell clones, the contemporary presence of an actual infection or a potent adjuvant is required for inducing autoimmune diseases. It has also been suggested that for occurring an autoimmune disease, the cross-reaction between virus and host should probably happen at “disease-related” epitopes, those peptides of self-antigens that MHC Class II molecules (major histocompatibility complex) can present on APCs (antigen-presenting cells) to auto-reactive CD4+ T-cells [29].
Bystander activation is a non-specific stimulation of pre-primed dormant auto-reactive T-cells, particularly CD8+ T-cells, which can be initiated following signals (e.g., high level of cytokines) derived from the antigen-specific response against the vaccine-antigens or adjuvant. The proliferation of CD8+ T-cells can be mediated by IL-15 production, which is stimulated via releasing the interferons (INFs α/β- INF-γ) and INF-inducer cytokines (IL-12 and IL-18). In this pathway, production and presentation of IL-15 bounded to its specific IL-15 receptor-α (IL-15R-α) on the surface of the APC induce bystander stimulation of memory-phenotype CD8+ T cells expressing high levels of CD122. In addition, IL-2 cytokine may be involved in bystander activation of CD4+ T cells; however, the signals inducing this pathway are less well-known [30]. It has been suggested that CD4+ T-cell bystander activation affects immune homeostasis differently compared to bystander activation of CD8+ T cells [31]. Therefore, bystander activation can be induced through vaccine injection as a possible result of immune system overstimulation. Overall, the impacts of infection-induced bystander activation are not fully understood, and future studies are needed to address these outstanding issues [32].
Autoimmunity induction by vaccine adjuvant is also a highly debated issue; however, there is insufficient evidence related to this topic. The most frequently reported manifestations of adjuvanted vaccines are local reactions such as redness, swelling, and pain at the injection site. It has been suggested that vaccine adjuvants could be triggered in different autoimmune reactions by stimulating inflammatory products leading to bystander activation of irrelevant T cells; however, the exact mechanism is still unclear. Limited information on systemic reactions following vaccination shows that this matter has been largely neglected [33].
It has also been proposed that occurring autoimmunity may be related to specific genetic factors such as human leukocyte antigen (HLA) patterns in the case of multiple sclerosis (MS) following the hepatitis B vaccine, which the HLA-DR2 haplotype or B7 or other HLA patterns may be associated with autoimmunity [4], [34]. Therefore, evaluating personalized approaches, including genetic risk factors for autoimmunity (e.g., familial autoimmunity), must also be considered in developing new vaccines [32].
The appearance of immune thrombocytopenia (an autoimmune bleeding disorder) [35], flare of RA after 2 years of sustained clinical remission [36], RA [37], and ReA [2] have been reported following COVID-19 vaccines by other researchers. The American College of Rheumatology issued a theoretical risk of flare in autoimmune disease after COVID-19 vaccination on Feb 8, 2021, with moderate consensus. Based on provided data, it seems that the similarity between SARS-CoV-2 viral peptides and self-antigens may be related to stimulating inflammatory response with autoinflammatory and autoimmune conditions [32]; however, this phenomenon has not been approved yet in large part due to the low incidence of identified individuals and lack of high level of evidence.
There are several limitations related to the current study. Due to the small sample size, bias may have been created in assessing the incidence of ARD in subjects receiving different types of vaccines. The association between the ARDs severity and vaccine dose/types cannot be discussed due to the low incidence of the patients. The present study was conducted on patients referred to rheumatology clinics at Tabriz University of Medical Sciences and Kashan University of Medical Sciences; thus, some patients may be missed by referring to other related centers. Moreover, the incidence of the patients may be affected by the genetic background in different populations, environmental factors, and population size. In case (#3), who is expired due to respiratory failure, the patient's incomplete clinical history may raise the possibility that his inflammatory symptoms were caused by COVID-19 infection rather than vaccination.
Overall, the current data alone cannot provide sufficient evidence to support a strong conclusion, and deep insight into the people's incidental and idiopathic diseases is needed to develop this hypothesis. It should be noted that the current study was based on a suggestion, and we require a comprehensive case-control study to prove and compare the incidence of rheumatic disease in vaccinated and unvaccinated subjects.
5. Conclusion
In summary, SARS-CoV-2 viral peptides may be related to ARDs stimulation; thus, avoiding the molecular mimicry and bystander activation of auto-reactive T-cells should be considered in developing new vaccines as a crucial safety issue. However, this phenomenon has not been approved yet due to the low incidence of patients and lack of a high level of evidence. Indeed, our data can warn physicians about the possibility of ARDs post-vaccination, lead to faster diagnosis, prevent loss of window of opportunity for treatment, and prevent irreversible organ damages. It should be noted that the valuable benefits of COVID-19 vaccination in controlling the pandemic worldwide are not deniable, and ARDs may occur with lower and milder clinical symptoms than those associated with severe COVID-19; thus, immunization should not be avoided. However, extensive studies are warranted to monitor possible predisposed individuals and clarify induced autoimmunological mechanisms by COVID-19 vaccines.
Funding
No specific funding was received from any public, commercial or not-for-profit bodies to carry out the work described in this article.
Ethical approval
This study was approved by the Institutional Review Board and Research Ethics Committees of Tabriz University of Medical Sciences (IR.TBZMED.REC.1400.461).
Patients consent
Informed consent was obtained from all patients included in the study.
Data availability statements
The data supporting this study's findings are available on request from the corresponding author.
CRediT authorship contribution statement
Azam Safary: Conceptualization, Writing – original draft, Visualization, Investigation, Validation, Writing – review & editing, Supervision. Kamal Esalatmanesh: Methodology, Investigation, Validation, Data curation. Amir Taher Eftekharsadat: Methodology, Investigation, Data curation. Mohammadreza Jafari Nakjovani: Methodology, Investigation, Data curation. Alireza Khabbazi: Conceptualization, Methodology, Software, Data curation, Formal analysis, Investigation, Visualization, Writing – review & editing, Supervision, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to acknowledge the Connective Tissue Diseases Research Center at Tabriz University of Medical Sciences.
Data availability
Data will be made available on request.
References
- 1.Lotfi F., Akbarzadeh-Khiavi M., Lotfi Z., Rahbarnia L., Safary A., Zarredar H., Baghbanzadeh A., Naghili B., Baradaran B. Micronutrient therapy and effective immune response: a promising approach for management of COVID-19. Infection. 2021:1–15. doi: 10.1007/s15010-021-01644-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akbarzadeh-Khiavi M., Torabi M., Rahbarnia L., Safary A. Baricitinib combination therapy: a narrative review of repurposed Janus kinase inhibitor against severe SARS-CoV-2 infection. Infection. 2021:1–14. doi: 10.1007/s15010-021-01730-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masoudi-Sobhanzadeh Y. Computational-based drug repurposing methods in COVID-19. Bioimpacts. 2020;10(3):205–206. doi: 10.34172/bi.2020.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waisbren B.A., Sr Acquired autoimmunity after viral vaccination is caused by molecular mimicry and antigen complimentarity in the presence of an immunologic adjuvant and specific HLA patterns. Med. Hypotheses. 2008;70(2):346–348. doi: 10.1016/j.mehy.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y., Xu Z., Wang P., Li X.M., Shuai Z.W., Ye D.Q., Pan H.F. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology. 2022;165(4):386–401. doi: 10.1111/imm.13443. [DOI] [PubMed] [Google Scholar]
- 6.Moutsopoulos H.M. Autoimmune rheumatic diseases: One or many diseases? J. Transl. Autoimmun. 2021;4:100129. doi: 10.1016/j.jtauto.2021.100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldblatt F., O'Neill S.G. Clinical aspects of autoimmune rheumatic diseases. Lancet. 2013;382(9894):797–808. doi: 10.1016/S0140-6736(13)61499-3. [DOI] [PubMed] [Google Scholar]
- 8.Toussirot É., Bereau M. Vaccination and Induction of Autoimmune Diseases. Inflamm. Allergy Drug Targets. 2015;14(2):94–98. doi: 10.2174/1871528114666160105113046. [DOI] [PubMed] [Google Scholar]
- 9.Salemi S., D'Amelio R. Could autoimmunity be induced by vaccination? Int. Rev. Immunol. 2010;29(3):247–269. doi: 10.3109/08830181003746304. [DOI] [PubMed] [Google Scholar]
- 10.Gershwin L.J. Adverse reactions to vaccination: from anaphylaxis to autoimmunity. Vet. Clin. North Am. Small Anim. 2018;48(2):279. doi: 10.1016/j.cvsm.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillion V., Jadoul M., Demoulin N., Aydin S., Devresse A. Granulomatous vasculitis after the AstraZeneca anti-SARS-CoV-2 vaccine. Kidney Int. 2021;100(3):706–707. doi: 10.1016/j.kint.2021.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shakoor M.T., Birkenbach M.P., Lynch M. ANCA-Associated Vasculitis Following Pfizer-BioNTech COVID-19 Vaccine. Am. J. Kidney Dis. 2021;78(4):611–613. doi: 10.1053/j.ajkd.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen S.R., Prussick L., Kahn J.S., Gao D.X., Radfar A., Rosmarin D. Leukocytoclastic vasculitis flare following the COVID-19 vaccine. Int. J. Dermatol. 2021;60(8):1032–1033. doi: 10.1111/ijd.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villa M., Díaz-Crespo F., Pérez de José A., Verdalles Ú., Verde E., Almeida Ruiz F., Acosta A., Mijaylova A., Goicoechea M. A case of ANCA-associated vasculitis after AZD1222 (Oxford-AstraZeneca) SARS-CoV-2 vaccination: casualty or causality? Kidney Int. 2021;100(4):937–938. doi: 10.1016/j.kint.2021.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izzedine H., Bonilla M., Jhaveri K.D. Nephrotic syndrome and vasculitis following SARS-CoV-2 vaccine: true association or circumstantial? Nephrol. Dial. Transplant. 2021;36(9):1565–1569. doi: 10.1093/ndt/gfab215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishay Y., Kenig A., Tsemach-Toren T., Amer R., Rubin L., Hershkovitz Y., Kharouf F. Autoimmune phenomena following SARS-CoV-2 vaccination. Int. Immunopharmacol. 2021;99:107970. doi: 10.1016/j.intimp.2021.107970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jara L.J., Vera-Lastra O., Mahroum N., Pineda C., Shoenfeld Y. Autoimmune post-COVID vaccine syndromes: does the spectrum of autoimmune/inflammatory syndrome expand? Clin. Rheumatol. 2022:1–7. doi: 10.1007/s10067-022-06149-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camellino D., Giusti A., Girasole G., Bianchi G., Dejaco C. Pathogenesis, Diagnosis and Management of Polymyalgia Rheumatica. Drugs Aging. 2019;36(11):1015–1026. doi: 10.1007/s40266-019-00705-5. [DOI] [PubMed] [Google Scholar]
- 19.Hočevar A., Tomšič M., Perdan Pirkmajer K. Clinical Approach to Diagnosis and Therapy of Polyarteritis Nodosa. Curr. Rheumatol. Rep. 2021;23(3):14. doi: 10.1007/s11926-021-00983-2. [DOI] [PubMed] [Google Scholar]
- 20.Lin Y.-J., Anzaghe M., Schülke S. Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis. Cells. 2020;9(4):880. doi: 10.3390/cells9040880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mankia K., Emery P. Palindromic rheumatism as part of the rheumatoid arthritis continuum. Nat. Rev. Rheumatol. 2019;15(11):687–695. doi: 10.1038/s41584-019-0308-5. [DOI] [PubMed] [Google Scholar]
- 22.Giacomelli R., Ruscitti P., Shoenfeld Y. A comprehensive review on adult onset Still's disease. J. Autoimmun. 2018;93:24–36. doi: 10.1016/j.jaut.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 23.Wevers-de Boer K.V., Heimans L., Huizinga T.W., Allaart C.F. Drug therapy in undifferentiated arthritis: a systematic literature review. Ann. Rheum. Dis. 2013;72(9):1436–1444. doi: 10.1136/annrheumdis-2012-203165. [DOI] [PubMed] [Google Scholar]
- 24.Narváez J. Systemic lupus erythematosus 2020. Med. Clin. (Barc) 2020;155(11):494–501. doi: 10.1016/j.medcli.2020.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Ziade N., Rassi J., Elzorkany B., Lopez-Medina C., Gamal S.M., Hlais S., Dougados M., Baraliakos X. What is peripheral spondyloarthritis? Identifying proportion, phenotype and burden in post hoc analysis of the ASAS-PerSpA study. Semin. Arthritis. Rheum. 2022;55:152012. doi: 10.1016/j.semarthrit.2022.152012. [DOI] [PubMed] [Google Scholar]
- 26.A.G. Andrew Steer, Acute rheumatic fever: Clinical manifestations and diagnosis, 2022. https://www.uptodate.com/contents/acute-rheumatic-fever-clinical-manifestations-and-diagnosis?search=acute-rheumatic-fever-clinical-manifestations-anddiagnosis&source=search_result&selectedTitle=1~92&usage_type=default&display_rank=1#H12. 2022).
- 27.A.v.T. David T Yu, Reactive arthritis, 2022. https://www.uptodate.com/contents/reactive-arthritis?search=reactive%20arthritis&source=search_result&selectedTitle=1~142&usage_type=default&display_rank=1.
- 28.Prabhahar A., Naidu G., Chauhan P., Sekar A., Sharma A., Sharma A., Kumar A., Nada R., Rathi M., Kohli H.S., Ramachandran R. ANCA-associated vasculitis following ChAdOx1 nCoV19 vaccination: case-based review. Rheumatol. Int. 2022;42(4):749–758. doi: 10.1007/s00296-021-05069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olivieri B., Betterle C., Zanoni G. Vaccinations and Autoimmune Diseases. Vaccines (Basel) 2021;9(8):815. doi: 10.3390/vaccines9080815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyman O. Bystander activation of CD4+ T cells. Eur. J. Immunol. 2010;40(4):936–939. doi: 10.1002/eji.201040466. [DOI] [PubMed] [Google Scholar]
- 31.van Aalst S., Ludwig I.S., van der Zee R., van Eden W., Broere F., Cohen I.R. Bystander activation of irrelevant CD4+ T cells following antigen-specific vaccination occurs in the presence and absence of adjuvant. PLoS ONE. 2017;12(5):e0177365. doi: 10.1371/journal.pone.0177365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodríguez Y., Novelli L., Rojas M., De Santis M., Acosta-Ampudia Y., Monsalve D.M., Ramírez-Santana C., Costanzo A., Ridgway W.M., Ansari A.A. Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. J. Autoimmun. 2020;114:102506. doi: 10.1016/j.jaut.2020.102506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Batista-Duharte A., Portuondo D., Perez O., Carlos I.Z. Systemic immunotoxicity reactions induced by adjuvanted vaccines. Int. Immunopharmacol. 2014;20(1):170–180. doi: 10.1016/j.intimp.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 34.Shoenfeld Y., Aron-Maor A. Vaccination and autoimmunity—‘vaccinosis’: a dangerous liaison? J. Autoimmun. 2000;14(1):1–10. doi: 10.1006/jaut.1999.0346. [DOI] [PubMed] [Google Scholar]
- 35.Guetl K., Gary T., Raggam R.B., Schmid J., Wölfler A., Brodmann M. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia treated with immunoglobulin and argatroban. Lancet (London, England) 2021;397(10293):e19. doi: 10.1016/S0140-6736(21)01238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terracina K.A., Tan F.K. Flare of rheumatoid arthritis after COVID-19 vaccination. Lancet Rheumatol. 2021;3(7):e469–e470. doi: 10.1016/S2665-9913(21)00108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Unal Enginar A. Arthritis following COVID-19 vaccination: report of two cases. Int. Immunopharmacol. 2021;101(Pt B):108256. doi: 10.1016/j.intimp.2021.108256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.