Abstract
The ongoing pandemic of severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2) and subsequently, coronavirus disease 2019 (COVID‐19), has led to the deaths of over 6.1 million people and sparked a greater interest in virology to expedite the development process for antivirals. The US Food and Drug Administration (FDA) granted emergency use authorization for three antivirals: remdesivir, molnupiravir, and nirmatrelvir. Remdesivir and molnupiravir are nucleoside analogs that undergo biotransformation to form active metabolites that incorporate into new viral RNA to stall replication. Unlike remdesivir or molnupiravir, nirmatrelvir is a protease inhibitor that covalently binds to the SARS‐CoV‐2 3C‐like protease to interrupt the viral replication cycle. A recent study identified that remdesivir and the active metabolite of molnupiravir, EIDD‐1931, are substrates of equilibrative nucleoside transporters 1 and 2 (ENT1 and 2). Despite the ubiquitous expression of the ENTs, the preclinical efficacy of remdesivir and molnupiravir is not reflected in wide‐scale SARS‐CoV‐2 clinical trials. Interestingly, downregulation of ENT1 and ENT2 expression has been shown in lung epithelial and endothelial cells in response to hypoxia and acute lung injury, although it has not been directly studied in patients with COVID‐19. It is possible that the poor efficacy of remdesivir and molnupiravir in these patients may be partially attributed to the repression of ENTs in the lungs, but further studies are warranted. This study investigated the interaction between nirmatrelvir and the ENTs and found that it was a poor inhibitor of ENT‐mediated [3H]uridine uptake at 300 μM. Unlike for remdesivir or EIDD‐1931, ENT activity is unlikely to be a factor for nirmatrelvir disposition in humans; however, whether this contributes to the similar in vitro and clinical efficacy will require further mechanistic studies.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Remdesivir and molnupiravir show poor clinical efficacy despite positive results in preclinical models and early clinical trials. Additionally, remdesivir and EIDD‐1931 (an active metabolite of molnupiravir) are substrates of the ubiquitously expressed equilibrative nucleoside transporter 1 and 2 (ENT1 and 2), which are downregulated in the lungs of patients with acute lung injury and/or hypoxia. Severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2) can cause acute lung injury and hypoxia. Nirmatrelvir is effective in patients with coronavirus disease 2019 (COVID‐19), but its interaction with ENT1/2 is unknown.
WHAT QUESTION DID THIS STUDY ADDRESS?
What is the mechanistic basis for the comparable preclinical versus clinical efficacy of the SARS‐CoV‐2 antiviral, nirmatrelvir, compared to remdesivir or molnupiravir?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Nirmatrelvir and other antivirals that are not substrates of the ENTs may be more effective in patients with COVID‐19 with acute lung injury and/or hypoxia. SARS‐CoV‐2 infection and subsequent development into COVID‐19 causes acute lung injury and pulmonary hypoxia. In vivo models for acute lung injury and hypoxia have been shown to repress pulmonary ENT1 and ENT2 expression.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Repurposing drugs is an effective strategy for use in SARS‐CoV‐2 infection; however, their clinical efficacy may be hindered by changes in transporter expression at the primary site of action despite positive in vitro readouts. Understanding the basic mechanisms for drug disposition in certain tissues and how they are changed by certain disease states will improve the design of preclinical studies and their translatability to clinical trials.
INTRODUCTION
Coronaviruses are a group of enveloped, positive‐sense single‐stranded RNA viruses with four genera that cause disease in mammals and/or birds. Six species of human coronavirus are known, of which three species can cause severe symptoms. The recent severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) emerged as a highly transmissible and pathogenic human coronavirus in late 2019. 1 SARS‐CoV‐2 infection causes the ongoing coronavirus disease 2019 (COVID‐19) pandemic, which has led to 492 million positive cases and over 6.1 million deaths as of April 2022. 2 Patients with COVID‐19 are typically afflicted with acute lung injury and hypoxemia, which is partially managed by ventilators. 3 Some patients that recover from severe COVID‐19 retain long‐term complications, including pulmonary fibrosis. Consequently, there have been significant efforts to discover or design prophylactics and other therapies to manage symptoms and disease progression.
Of these efforts, small molecules, such as nucleoside analogs and protease inhibitors, are common treatments for SARS‐CoV‐2 infection and COVID‐19. Many of these are repurposed drugs, having shown promising results in in vitro and non‐human in vivo models. 4 , 5 Despite showing promise, these drugs were less effective than expected in full clinical trials. 6 , 7 , 8 , 9 , 10 The first COVID‐19 antiviral granted emergency use authorization (EUA) by the US Food and Drug Administration (FDA) was remdesivir in 2020. 11 Remdesivir is a nucleoside analog prodrug designed in 2009 to treat hepatitis C and the respiratory syncytial virus, then later repurposed to treat Ebola virus disease and Marburg virus infection. 12 Despite its suitable safety profile, remdesivir was significantly less effective than monoclonal antibody treatments for Ebola virus disease and further studies were ceased. Remdesivir can diffuse across cell membranes where it is metabolized into its active antiviral metabolite: GS‐443902 5′‐triphosphate. Because coronavirus replication involves an RNA‐dependent RNA polymerase encoded by the viral genome, GS‐443902 is incorporated into the new viral genome during the replication cycle and RNA synthesis will terminate after three additional nucleotides. Whereas early clinical trials (NCT04280705, NCT04292899, and NCT04292730) for the treatment of COVID‐19 with remdesivir showed promising results in treated groups compared to placebo, 7 results of later trials (NCT04257656 and NCT04315948) suggested that the drug was largely ineffective. 8 , 10 Consequently, the widespread clinical efficacy of remdesivir remains controversial.
The second repurposed antiviral granted FDA EUA in 2021 to treat COVID‐19 was molnupiravir for patients with mild‐to‐moderate symptoms. 9 , 13 Molnupiravir is a nucleoside analog designed to treat Venezuelan equine encephalitis virus infections, repurposed to treat influenza, and later abandoned due to concerns about mutagenicity. 12 Molnupiravir is also a prodrug that undergoes metabolism to generate the active metabolite: EIDD‐1931 5′‐triphosphate. EIDD‐1931 is also incorporated into new viral RNA, which causes early termination of the viral replication cycle. Despite early signs in reducing the risk of hospitalization or death due to COVID‐19 by 48%, full clinical trial results (NCT04575597) for molnupiravir were less favorable, which indicated a risk reduction of only 30%. 6 , 9
Although remdesivir and molnupiravir can diffuse across membranes, these two drugs may also interact with uptake and/or efflux transporters, which will affect their tissue disposition. A recent study explored the interaction among remdesivir, molnupiravir, and EIDD‐1931, with the ubiquitously expressed equilibrative nucleoside transporters 1 and 2 (ENT1 and 2). Remdesivir and EIDD‐1931 uptake into ENT1 or ENT2 knockout HeLa S3 (hereinafter referred to as HeLa S3‐ENT2 or HeLa S3‐ENT1 for the transporter that is still expressed) cells was partially inhibited by the ENT inhibitor, NBMPR, suggesting that, in addition to passive diffusion, other carrier‐mediated pathways are involved in the uptake of these two drugs. 14 On the other hand, molnupiravir uptake into the same cell lines was unaffected by co‐incubation with NBMPR despite showing a modest inhibitory interaction on ENT‐mediated [3H]uridine uptake. 14 These findings indicate that remdesivir and EIDD‐1931 are substrates of ENT1 and ENT2, but molnupiravir is not, implying the chemical structures of these drugs are a determining factor in their carrier‐mediated tissue disposition. Therefore, understanding the chemical features of these drugs and the transport mechanisms that are involved is necessary to inform the development of future drugs for COVID‐19 and other viral diseases.
In December 2021, Pfizer’s Paxlovid (nirmatrelvir and ritonavir) oral treatment for COVID‐19 was granted FDA EUA. 15 Unlike remdesivir and molnupiravir, nirmatrelvir was designed as a 3C‐like protease (3CLpro) inhibitor that covalently binds to the cysteine 145 residue of the viral 3CLpro to inhibit the SARS‐CoV‐2 replication cycle. Early preclinical studies with nirmatrelvir were highly promising with in vitro and in vivo models, although these models are not completely representative of human infection and disease progression. 16 However, these findings were reflected in a clinical trial (NCT04960202) for nirmatrelvir, which revealed a risk reduction of hospitalization or death by 89.1% at the interim analysis and 88.9% by the end of the trial. 17 Another recent study showed nirmatrelvir does not readily diffuse and strongly interacted with several pharmacologically relevant transporters, including OATP1B1 and P‐gp, but was only a substrate for P‐gp. 18 Because ENTs are currently not included in the list of pharmacologically important transporters for unwanted drug–drug interactions (DDIs) published by the FDA, they were untested in that study. Although the chemical structure of nirmatrelvir is a stark contrast to those of remdesivir and molnupiravir, suggesting it may not interact with the ENTs, the present study investigated this issue directly to better understand the mechanism behind the similar preclinical versus clinical efficacy of nirmatrelvir compared to remdesivir and molnupiravir. The inhibitory effect of nirmatrelvir on [3H]uridine uptake was evaluated in HeLa S3‐ENT1 and ‐ENT2 cells. The results indicated that nirmatrelvir does not interact with the ENTs at pharmacologically relevant concentrations, which may help explain the comparable clinical efficacy of this drug in patients with COVID‐19 seen in preclinical studies.
METHODS
Reagents
All reagents were purchased from ThermoFisher Scientific unless otherwise noted. PF‐07321332 (Nirmatrelvir) was purchased from Selleck Chemicals (Catalog #S9866). Nirmatrelvir was solubilized in DMSO and further diluted to working concentrations in the transport buffer. The [3H]uridine (35.2 Ci/mmol) was purchased from PerkinElmer (Catalog #NET367001MC).
HeLa S3 ENT1 and ENT2 knockout cell cultures
HeLa S3‐ENT1 and HeLa S3‐ENT2 cells were maintained as previously described. 19 Briefly, cells were grown in F12K (Sigma‐Aldrich; Catalog #N3520) supplemented with 10% fetal bovine serum, and 1% penicillin–streptomycin in a 37°C humidified 5% CO2 incubator. All cells were split once reaching 80–90% confluence and washed with standard phosphate‐buffered saline (PBS) during routine maintenance.
Transport assay by radioactive liquid scintillation counting
Confluent monolayers of HeLa S3‐ENT1 and HeLa S3‐ENT2 cells cultured in 96‐well plates were used to assess total [3H]uridine uptake. Transport experiments were performed as previously described with minor modifications. 19 Cells were plated into Nunc MicroWell 96‐well optical bottom plates (ThermoFisher Scientific; Catalog #165306) and grown to confluence before each experiment. Test compounds were diluted into Waymouth’s buffer (WB; 135 mM NaCl, 28 mM D‐glucose, 13 mM HEPES, 5 mM KCl, 2.5 mM CaCl2•2H2O, 1.2 mM MgCl2, 0.8 mM MgSO4•7H2O, pH 7.4). Confluent cells plated were washed twice with room temperature WB before incubating wells with 50 μl WB transport buffer supplemented with 1 μCi/ml (~15–20 nM) [3H]uridine with or without nirmatrelvir. Transport was terminated after 2 min by washing the cells twice with ice‐cold WB using a Biotek 405 LS Microplate Washer (BioTek). After washing, ~ 200 μl of MicroScint‐20 scintillation cocktail (PerkinElmer; Catalog #6013621) was added to each well before sealing the plate with microplate film. Plates were incubated at room temperature for at least 2 h before measuring total accumulated radioactivity using a Wallac 1450 MicroBeta TriLux liquid scintillation counter (PerkinElmer).
Statistical analysis
Competitive transport experiments were completed with cells cultured from three separate passages, each with four replicates. All data were analyzed using GraphPad Prism 8 (GraphPad Software). A multiple t‐test with Bonferroni’s multiple comparison correction was used to determine statistical significance between the control and inhibitor groups. Significance is indicated as *p ≤ 0.05 and ****p ≤ 0.0001. All data are presented as mean ± SD unless otherwise indicated.
RESULTS
Nirmatrelvir interaction with ENT1 and ENT2
The interaction between nirmatrelvir and the ENTs was investigated due to the reported interactions between SARS‐CoV‐2 antivirals remdesivir and EIDD‐1931 with ENT1 and ENT2. After co‐incubating ~ 15–20 nM [3H]uridine with the tested compounds in HeLa S3‐ENT1 or HeLa S3‐ENT2 cells for 2 min, total accumulation of [3H]uridine was measured by liquid scintillation counting (Figure 1). Statistically significant inhibition of [3H]uridine was observed when concomitantly incubated with a molar excess of unlabeled uridine or with the ENT‐specific inhibitor, NBMPR. Approximately 100 nM and 100 μM NBMPR inhibit ENT1 and ENT2, respectively. Therefore, 100 μM NBMPR was used to inhibit [3H]uridine for both cell lines. The remaining [3H]uridine signal is attributed to the combined influence of diffusion, nonspecific binding, and incomplete rinsing of the substrate‐containing buffer. The [3H]uridine inhibition by nirmatrelvir was not observed in either cell line, indicating that it does not interact with ENT1 or ENT2 as a substrate or inhibitor.
FIGURE 1.
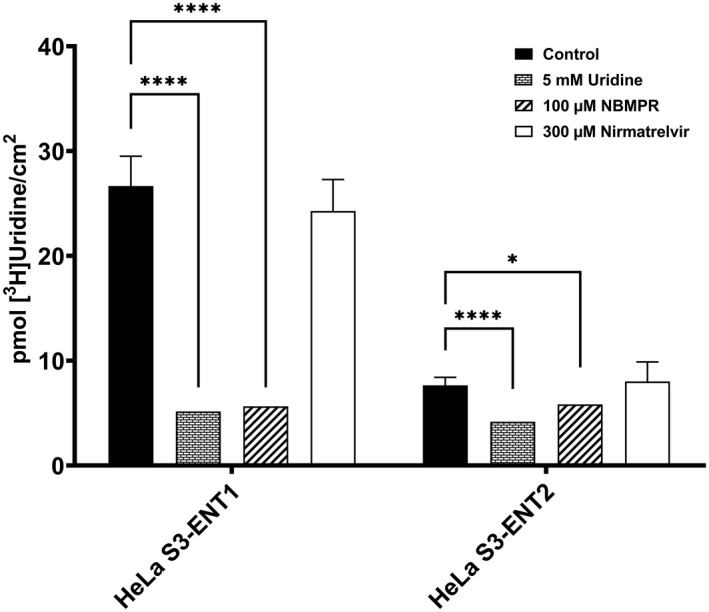
Inhibition of total transporter‐mediated uptake of [3H]uridine by unlabeled uridine, NBMPR, or nirmatrelvir in HeLa S3‐ENT1 or HeLa S3‐ENT2 cells. Cellular accumulation of [3H]uridine was measured after 2 min in all experiments. Data are represented as mean ± SD. Significance is indicated as *p ≤ 0.05 and ****p ≤ 0.0001.
DISCUSSION
The repurposing of old drugs for new applications is a powerful strategy to quickly identify molecules that are highly effective in in vitro or in vivo models and has been the most common approach for identifying new SARS‐CoV‐2 antivirals. Remdesivir and molnupiravir were originally designed to target other viruses, although with little success. Early studies identified that these two drugs had antiviral activity against SARS‐CoV‐2 infection with human in vitro models. 4 , 5 , 12 However, these models cannot account for the complex pharmacokinetic and pharmacodynamic properties of drugs in human patients. Following these observations, non‐human in vivo studies and preliminary clinical trials revealed promising results that led to their FDA EUA. 7 , 9 , 11 , 13 In later, full clinical trials, remdesivir and molnupiravir were found to be significantly less effective in treating SARS‐CoV‐2 infection than expected. 6 , 8 , 9 , 10 These observations raise major concerns regarding the translatability of early preclinical models for identifying SARS‐CoV‐2 antivirals.
Remdesivir and molnupiravir are nucleoside analog prodrugs that must enter cells to be metabolized into their main active metabolites, GS‐443902 and EIDD‐1931. Although remdesivir can diffuse across membranes, it is a substrate of OATP1B1 and P‐gp, and an inhibitor of OATP1B3 and MATE1. 20 As a result, these interactions put remdesivir at higher risk of unwanted DDIs and increase the likelihood of CYP3A4‐mediated metabolism in the liver. 20 Comparatively, molnupiravir has more favorable pharmacokinetic properties because it is not a substrate or inhibitor of OATP1B1, OATP1B3, OCT1, OCT2, OAT1, OAT3, MATE1, MATE2K, MRP2, P‐gp, and BCRP. 21 Interestingly, a recent study showed that remdesivir and EIDD‐1931 are substrates of ENT1 and ENT2, but molnupiravir was not. 14 Due to the ubiquitous expression of ENT1/2, it is expected that remdesivir and EIDD‐1931 would have favorable tissue distribution patterns for the treatment of SARS‐CoV‐2. However, the clinical efficacy of remdesivir and EIDD‐1931 diverge from this hypothesis, and the mechanism for this phenomenon may be explained by SARS‐CoV‐2‐induced acute lung injury 22 and hypoxia, which may reduce pulmonary ENT expression and function.
HIF‐1α levels are significantly elevated during tissue hypoxia, which ultimately lead to ENT repression. 23 Moreover, NF‐κB also represses ENT expression during acute lung injury. 24 SARS‐CoV‐2 infection can cause acute lung injury and tissue hypoxia with a drastic increase in HIF‐1α, NF‐κB, and other cytokine levels. 22 , 25 Consequently, ENT repression may lead to a decrease in remdesivir disposition to the lungs, which would partially explain the poor clinical efficacy versus preclinical observations. Although molnupiravir is not a substrate of the ENTs, EIDD‐1931 (and remdesivir) may be effectively sequestered in the cells where they were metabolized due to decreased ENT expression. Remdesivir and EIDD‐1931 sequestration in these cells will have a favorable effect on internal SARS‐CoV‐2 replication, but local tissue or systemic distribution of the drugs may be attenuated. Additionally, extracellular adenosine levels are elevated during acute lung injury as an anti‐inflammatory signal to suppress inflammation. 24 , 26 Consequently, adenosine can competitively inhibit remdesivir and EIDD‐1931 uptake, leading to lower drug concentrations in the lungs. However, further studies in in vivo models are necessary to confirm this mechanism.
Unlike remdesivir or molnupiravir, nirmatrelvir was newly designed based on an existing scaffold to target the SARS‐CoV‐2 3CLpro. Due to differences in the viral protein target and chemical structure of nirmatrelvir, it was not expected to be a substrate of the ENTs. To test this, the interaction between nirmatrelvir and ENT1 or ENT2 was assessed in novel ENT‐knockout HeLa S3 cell lines. 14 , 19 As expected, nirmatrelvir did not inhibit ENT1‐ or ENT2‐mediated [3H]uridine uptake even at 300 μM, which is over 60‐fold higher than peak plasma concentrations in humans (Figure 1). 27 Therefore, if nirmatrelvir does not interact with the ENTs at this concentration, it is unlikely to interact at lower physiologically relevant concentrations. In addition to ENT1 and ENT2, nirmatrelvir is not a substrate for OATP1B1, OATP1B3, OATP2B1, OATP4C1, OCT1, OCT2, OAT1‐3, MATE1, MATE2K, NTCP, BCRP, or PEPT1. 18 , 27 Therefore, nirmatrelvir may be a substrate of an unknown uptake transport pathway and necessitates further testing. These observations may help explain the high efficacy of nirmatrelvir in patients with COVID‐19, although other factors such as metabolic stability play an important role. Nirmatrelvir exhibits low passive permeability and is a substrate of P‐gp, indicating that tissue disposition may be challenging. 18 However, nirmatrelvir is co‐administered with ritonavir to inhibit CYP3A4 activity and boost plasma concentrations. 15 , 18 , 27 Ritonavir is also a protease inhibitor and is unlikely to interact with the ENTs, although further studies are necessary to confirm this assumption. Consequently, pulmonary tissue concentrations of nirmatrelvir/ritonavir may be higher than remdesivir, molnupiravir, or their metabolites, although extensive comparative studies among these three drugs have not been published. These observations suggest that unknown carrier‐mediated pathway(s) is(are) responsible for intracellular accumulation of nirmatrelvir and further work is needed to identify them.
In summary, the findings presented in this study indicate that the lack of interaction between nirmatrelvir and the ENTs may contribute to the similar clinical efficacy of nirmatrelvir in patients with COVID‐19 as observed in preclinical models versus those observed with remdesivir and molnupiravir. Despite demonstrating positive in vitro results, clinical trials for remdesivir and molnupiravir are more contentious. The data presented here show that nirmatrelvir does not interact with ENT1 or ENT2, unlike remdesivir or EIDD‐1931, and is unlikely to be affected by variable ENT expression and activity that may be caused by COVID‐19 in humans. Furthermore, increased extracellular adenosine levels competing for ENT‐mediated uptake due to SARS‐CoV‐2‐induced acute lung injury and hypoxia is unlikely to affect nirmatrelvir disposition, whereas remdesivir and EIDD‐1931 uptake would be susceptible to this mechanism. Future mechanistic studies with in vivo models to assess this hypothesis are warranted. Nevertheless, these data offer insight into the unforeseen mechanisms by which certain antivirals such as nirmatrelvir exhibit comparable efficacy in clinical trials versus early preclinical studies.
CONFLICT OF INTEREST
The authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
R.K.H., S.H.W., and N.J.C. wrote the manuscript. R.K.H., S.H.W., and N.J.C. designed the research. R.K.H. performed the research. R.K.H., S.H.W., and N.J.C. analyzed the data.
Hau RK, Wright SH, Cherrington NJ. PF‐07321332 (Nirmatrelvir) does not interact with human ENT1 or ENT2: Implications for COVID‐19 patients. Clin Transl Sci. 2022;15:1599‐1605. doi: 10.1111/cts.13292
Funding information
This work was supported by funding from the National Institutes of General Medical Sciences (Grants R01 GM123643 and R01 GM129777) and National Institute of Environmental Health Sciences (R01 Grants ES028668 and T32 ES007091).
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. COVID‐19 CORONAVIRUS PANDEMIC . Worldometer. https://www.worldometers.info/coronavirus/. Accessed April 4, 2022.
- 3. Li L, Huang Q, Wang DC, Ingbar DH, Wang X. Acute lung injury in patients with COVID‐19 infection. Clin Transl Med. 2020;10:20‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sheahan TP, Sims AC, Zhou S, et al. An orally bioavailable broad‐spectrum antiviral inhibits SARS‐CoV‐2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med. 2020;12:eabb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res. 2020;30:269‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Merck and Ridgeback Biotherapeutics Provide Update on Results from MOVe‐OUT Study of Molnupiravir, an Investigational Oral Antiviral Medicine, in At Risk Adults With Mild‐to‐Moderate COVID‐19. MerckMerck & Co., Inc. https://www.merck.com/news/merck‐and‐ridgeback‐biotherapeutics‐provide‐update‐on‐results‐from‐move‐out‐study‐of‐molnupiravir‐an‐investigational‐oral‐antiviral‐medicine‐in‐at‐risk‐adults‐with‐mild‐to‐moderate‐covid‐19/. Accessed April 4, 2022. [Google Scholar]
- 7. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid‐19 – final report. N Engl J Med. 2020;383:1813‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Consortium WHOST, Pan H, Peto R, et al. Repurposed antiviral drugs for Covid‐19 – interim WHO solidarity trial results. N Engl J Med. 2021;384:497‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of Covid‐19 in nonhospitalized patients. N Engl J Med. 2022;386:509‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID‐19: a randomised, double‐blind, placebo‐controlled, multicentre trial. Lancet. 2020;395:1569‐1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. FDA Approves First Treatment for COVID‐19. US FDA. https://www.fda.gov/news‐events/press‐announcements/fda‐approves‐first‐treatment‐covid‐19. Accessed April 4, 2022. [Google Scholar]
- 12. Cully M. A tale of two antiviral targets – and the COVID‐19 drugs that bind them. Nat Rev Drug Discov. 2022;21:3‐5. [DOI] [PubMed] [Google Scholar]
- 13. Coronavirus (COVID‐19) Update: FDA Authorizes Additional Oral Antiviral for Treatment of COVID‐19 in Certain Adults. US FDA. https://www.fda.gov/news‐events/press‐announcements/coronavirus‐covid‐19‐update‐fda‐authorizes‐additional‐oral‐antiviral‐treatment‐covid‐19‐certain. Accessed April 4, 2022. [Google Scholar]
- 14. Miller SR, McGrath ME, Zorn KM, Ekins S, Wright SH, Cherrington NJ. Remdesivir and EIDD‐1931 interact with human Equilibrative nucleoside transporters 1 and 2: implications for reaching SARS‐CoV‐2 viral sanctuary sites. Mol Pharmacol. 2021;100:548‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coronavirus (COVID‐19) Update: FDA Authorizes First Oral Antiviral for Treatment of COVID‐19. US FDA. https://www.fda.gov/news‐events/press‐announcements/coronavirus‐covid‐19‐update‐fda‐authorizes‐first‐oral‐antiviral‐treatment‐covid‐19. Accessed April 4, 2022. [Google Scholar]
- 16. Owen DR, Allerton CMN, Anderson AS, et al. An oral SARS‐CoV‐2 M(pro) inhibitor clinical candidate for the treatment of COVID‐19. Science. 2021;374:1586‐1593. [DOI] [PubMed] [Google Scholar]
- 17. Hammond J, Leister‐Tebbe H, Gardner A, et al. Oral Nirmatrelvir for high‐risk, nonhospitalized adults with Covid‐19. N Engl J Med. 2022;386:1397‐1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eng H, Dantonio AL, Kadar EP, et al. Disposition of PF‐07321332 (Nirmatrelvir), an orally bioavailable inhibitor of SARS‐CoV‐2 3CL protease, across animals and humans. Drug Metab Dispos. 2022;50:576‐590. [DOI] [PubMed] [Google Scholar]
- 19. Miller SR, Zhang X, Hau RK, et al. Predicting drug interactions with human equilibrative nucleoside transporters 1 and 2 using functional knockout cell lines and Bayesian modeling. Mol Pharmacol. 2021;99:147‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Remdesivir EUA Fact Sheet for Healthcare Providers ‐ updated January 21, 2022. US FDA. https://www.fda.gov/media/137566/download. Accessed April 4, 2022. [Google Scholar]
- 21. Molnupiravir HCP FS 2112022. US FDA. https://www.fda.gov/media/155054/download. [Google Scholar]
- 22. Leist SR, Dinnon KH 3rd, Schafer A, et al. A mouse‐adapted SARS‐CoV‐2 induces acute lung injury and mortality in standard laboratory mice. Cell. 2020;183:1070‐1085. e1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eltzschig HK, Abdulla P, Hoffman E, et al. HIF‐1‐dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J Exp Med. 2005;202:1493‐1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morote‐Garcia JC, Kohler D, Roth JM, et al. Repression of the equilibrative nucleoside transporters dampens inflammatory lung injury. Am J Respir Cell Mol Biol. 2013;49:296‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taniguchi‐Ponciano K, Vadillo E, Mayani H, et al. Increased expression of hypoxia‐induced factor 1alpha mRNA and its related genes in myeloid blood cells from critically ill COVID‐19 patients. Ann Med. 2021;53:197‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eckle T, Koeppen M, Eltzschig HK. Role of extracellular adenosine in acute lung injury. Physiology (Bethesda). 2009;24:298‐306. [DOI] [PubMed] [Google Scholar]
- 27. Paxlovid HCP FS 02232022. US FDA. https://www.fda.gov/media/155050/download. Accessed April 4, 2022. [Google Scholar]


