Abstract
Background
Periosteum is a vascularized tissue membrane covering the bone surface and plays a decisive role in bone reconstruction process after fracture. Various artificial periosteum has been developed to assist the allografts or bionic bone scaffolds in accelerating bone healing. Recently, the biomimicking design of artificial periosteum has attracted increasing attention due to the recapitulation of the natural extracellular microenvironment of the periosteum and has presented unique capacity to modulate the cell fates and ultimately enhance the bone formation and improve neovascularization.
Methods
A systematic literature search is performed and relevant findings in biomimicking design of artificial periosteum have been reviewed and cited.
Results
We give a systematical overview of current development of biomimicking design of artificial periosteum. We first summarize the universal strategies for designing biomimicking artificial periosteum including biochemical biomimicry and biophysical biomimicry aspects. We then discuss three types of novel versatile biomimicking artificial periosteum including physical-chemical combined artificial periosteum, heterogeneous structured biomimicking periosteum, and healing phase-targeting biomimicking periosteum. Finally, we comment on the potential implications and prospects in the future design of biomimicking artificial periosteum.
Conclusion
This review summarizes the preparation strategies of biomimicking artificial periosteum in recent years with a discussion of material selection, animal model adoption, biophysical and biochemical cues to regulate the cell fates as well as three types of latest developed versatile biomimicking artificial periosteum. In future, integration of innervation, osteochondral regeneration, and osteoimmunomodulation, should be taken into consideration when fabricating multifunctional artificial periosteum.
The Translational Potential of this Article: This study provides a holistic view on the design strategy and the therapeutic potential of biomimicking artificial periosteum to promote bone healing. It is hoped to open a new avenue of artificial periosteum design with biomimicking considerations and reposition of the current strategy for accelerated bone healing.
Keywords: Artificial periosteum, Biomimicking design, Biochemical biomimicry, Biophysical biomimicry, Bone regeneration
1. Introduction
Periosteum is a dense and highly vascularized connective tissue membrane with fibroblasts constituting the outer layer and a multipotent mesenchymal stem cell (MSC)/osteogenic progenitor cell constructing the inner layer [1,2]. It covers the surface of the majority of the bone tissues to serve as the attachment site for muscles, ligaments, and tendons, and delivers essential blood, nutrition, and regenerative cells to the cambium layer of the bone cortex to mediate the bone growth, bone modeling, and remodeling process [3,4]. The remarkable regenerative potential of periosteum affirms its decisive role in bone regeneration after fracture. As reported, the absence of periosteum can lead to a drastically decreased new bone formation and up to 10-fold reduced vascularization, leading to compromised bone regeneration [5,6].
Because of the periosteum's superior regenerative capacity in terms of bone repair, natural periosteum transplantation has been adopted clinically to assist the allografts or bionic bone scaffolds in accelerating bone healing. However, their therapeutic efficacy is significantly impeded due to the limited availability, donor-site morbidity, and possible immune rejection [7,8]. To this end, artificial periosteum is developed as a substitute for the natural periosteum [[9], [10], [11], [12], [13]]. Recently, the biomimicking design of artificial periosteum has attracted increasing attention due to the recapitulation of the natural extracellular microenvironment of the periosteum (e.g., cell types, biochemical factors, and biophysical structures) to facilitate cell proliferation, differentiation, and maturation. Specifically, exogenous stem cell sheet mimicking the giant osteogenic progenitor cells in the periosteum has presented favorable effectiveness in bone formation and neovascularization [14]. Moreover, researchers also incorporated biomimicking biochemical elements of natural periosteum (e.g., growth factors) to modulate the cell behaviors after implantation [15]. In terms of the structurally biomimicking artificial periosteum, topographic design (e.g., grooves and surface patterns) with enhanced mechanical strength and adhesion ability can emulate the periosteum functions, addressing poor graft localization, tissue segment detachment, and limited cell survival [16].
To date, reviews of artificial periosteum mainly emphasize the material selection (e.g., natural or synthetic materials) [9] or the construction of periosteum with specific structures (e.g., microstructures and nanostructures) [17]. Recently, there is a review focusing on the histological structure, osteogenic function and application of periosteum [18]. However, it overlooked the critical role of biomimicking design in artificial periosteum construction including both the biochemical and biophysical considerations. Such biomimicking artificial periosteum has been widely recognized with promising therapeutic efficacy and great clinical application potential. In this review, we first provide an overview of the universal strategies for designing biomimicking artificial periosteum from two aspects: biochemical biomimicry and biophysical biomimicry. We then discuss three types of biomimicking artificial periosteum: physical-chemical combined artificial periosteum, healing phase-targeting biomimicking periosteum and heterogeneous structured biomimicking periosteum. We finally comment on the future direction of designing artificial periosteum. We aim to equip researchers with better understanding to improve biomimicking designs to enhance the efficacy of the artificial periosteum.
2. Strategies for design of biomimicking artificial periosteum
2.1. Materials for fabrication of biomimicking artificial periosteum
In the design of biomimicking artificial periosteum, materials with distinct chemical and physical properties should resemble the properties of natural periosteum. For example, the materials should possess suitable mechanical properties since the periosteum serves as the functional interface between bone and muscle which undergoes dynamic mechanical loadings [19,20]. In addition, after in vivo implantation, the biodegradability of artificial periosteum is also critical. Ideally, the degradation kinetics of artificial periosteum should coordinate with process of bone healing. It has been reported that about 62% of the natural periosteum would be generated between 14 and 35 days after the fracture. To this end, the materials should remain its intact properties for at least 14 days to provide sufficient mechanical support and various biological functions to stimulate the initial bone reconstruction. Then, it could degrade slowly to allow the neo-tissue ingrowth and completely degrade after 7 weeks to be replaced by the generated natural periosteum [21,22]. To date, various biocompatible biomaterials have been used to fabricate artificial periosteum. The most commonly used materials can be mainly divided into natural polymers, synthetic polymers, and composite materials with inorganic additives (Table 1), yet bulk materials alone cannot recapitulate the inherent functions of the native periosteum [[23], [24], [25], [26]].
Table 1.
Commonly used biomaterials for fabrication of artificial periosteum.
| Classification | Materials | Pros | Cons | Ref. | |
|---|---|---|---|---|---|
| Natural polymer | Collagen | Hemostatic; permeability | Poor mechanical property; fast degradation | [[28], [29], [30], [31], [32],50] | |
| Fibrin | Promotion of the release of biological factors and osteogenic differentiation | Poor mechanical property; fast degradation | [25,33,51] | ||
| Chitosan | Slow biodegradation; antibacterial activity; | Pathogenic impurities | [34,35] | ||
| Gelatin | Provide nutrition; Promotion of cell proliferation |
Poor mechanical properties; fast degradation | [13,49,52] | ||
| Synthetic polymer | PLGA | Biocompatibility; non-immunogenicity; easy to control the degradation rate | Cause inflammation | [36,39] | |
| PLA | Biocompatibility; non-immunogenicity | Difficult to control the degradation rate | [37] | ||
| PCL | Biocompatibility; hydrophobicity | Poor mechanical properties | [11,38] | ||
| Inorganic additives | HA | Biocompatibility; promote osteogenic differentiation and angiogenesis | Adverse effects on the immune system | [41,53] | |
| TCP | Biocompatibility; promote osteogenic differentiation and angiogenesis | Adverse effects on the immune system | [16,56] | ||
| BGN | Biocompatibility; promote osteogenic differentiation and angiogenesis | Haemolysis | [42,46] | ||
| GO | Electroactivity | Cytotoxicity | [43,44] | ||
| MnO2 | Anti-inflammation | Cytotoxicity | [47] | ||
2.1.1. Natural polymer
The widely used natural polymers include collagen, fibrin, chitosan, gelatin, etc. They have excellent biocompatibility and can significantly improve the interaction between materials and tissue cells due to cell-binding moieties [27]. For example, collagen has been adopted to prepare the artificial periosteum because of its low immunogenicity and similar composition of natural periosteum. It has demonstrated superior capacity to promote the new bone formation. However, it can be fast degraded by the collagen enzymes into peptide segments and amino acids within 3–5 weeks [[28], [29], [30]]. Moreover, the mechanical property of collagen (∼0.42 kPa) is substantially lower than natural periosteum even with the regulation of the cross-linking degree [31,32]. Fibrin is a protein synthesized by the liver with coagulation function and is treated as traumatic healing materials. Compared with the collagen-based periosteum, the fibrin-based periosteum could not only act as the hemostatic gauze but enhance the metabolic activity and the proliferation level of periosteal cells [33]. However, the relatively poor mechanical property and uncontrollable degradation performance impede application of fibrin-based periosteum [25]. Chitosan, a kind of acetyl enriched derivative of polysaccharide, has shown antibacterial activity and slow biodegradation rate [34]. The chitosan-based periosteum presented slow degradation for 25 days and could accelerate the bone healing [35]. Although chitosan has robust mechanical properties compared to collagen and fibrin owing to its chemical structure, it is still far from the mechanical properties of natural periosteum. Moreover, the possible pathogenic impurity of chitosan is a critical concern for its clinical application [9].
In all, natural polymers possess the advantages of wide sources and high biocompatibility, but the poor mechanical properties and uncontrolled degradability limit their clinical transition. Scientists therefore explored synthetic polymers for further applications.
2.1.2. Synthetic polymer
Compared with natural polymers, synthetic polymers such as poly (lactide-co-glycolide) (PLGA), polylactic acid (PLA), polycaprolactone (PCL) demonstrate excellent controllability in the mechanical and degradation properties [[36], [37], [38]]. Thus, these synthetic polymers have been adopted for engineering artificial periosteum. Although PLGA and PLA have relatively poor bioactivity compared with natural polymers, their mechanical properties could be readily modulated to match the natural periosteum. Moreover, degradation of PLGA and PLA can be regulated to around 2 months which is compatible to the bone healing process. In addition, it has been demonstrated that their degradation product (e.g., lactic acid) can induce osteogenic differentiation of mouse calvaria MC3T3-E1 cells by acting on G protein-coupled receptor 81 [36,37,39,40]. The slow degradation further enables their application as long-term drug delivery vehicles. The drug-loaded PLGA nanofibers could achieve sustained release of vancomycin, lidocaine, and ceftazidime around 35 days to increase the bone union rate [36].
Despite the excellent controllability over mechanical and degradation properties, the synthetic materials have faced challenges in terms of biocompatibility, osteoconductivity and osteoinductivity. Therefore, further modulation of the synthetic materials for enhanced bioactivity is highly sought after for their clinical implementation.
2.1.3. Inorganic additives
With the deepening of research on bionic periosteum, novel composite materials with inorganic additives have received extensive attention to improve the low mechanical strength of natural polymers and the bioactivity of synthetic polymers [[23], [24], [25], [26], [27]]. Currently, inorganic additives like nano-hydroxyapatite (HA) [41], tricalcium phosphate (TCP) [16], mesoporous bioactive glass nanoparticles (BGN) [42], graphene oxide (GO) [43], and manganese dioxide (MnO2) nanoparticles have been introduced into the tissue engineered periosteum [44]. The HA has the chemical similarity to the natural bone and is an essential osteogenic element. It has been found that incorporation of HA into natural polymer (e.g., gelatin) could substantially improve the mechanical performance and prolong the degradation rate of the composite material to more than 28 days. Moreover, the released ions (e.g., Ca2+) could also mediate the microenvironment to promote the osteogenesis and angiogenesis process resulting in boosted bone healing [45]. Similarly, the BGN has been reported to induce the migration, maturation, and organization of endothelial progenitors to enhance bone regeneration [46]. Incorporation of BGN in the synthetic poly (vinylidene fluoride-trifluoroethylene) (PVFT) polymer significantly enhanced the attachment as well as proliferation of bone cells, and promoted the angiogenesis of the endothelium cells to accelerate bone tissue reconstruction [42]. Moreover, the conductive GO could induce the osteogenesis of stem cells and is also beneficial for the innervation during bone regeneration [43,44]. Anti-inflammatory MnO2 has also been incorporated in the biomimicking periosteum fabrication to suppress the initial inflammation after bone injury and regulate the osteoimmunomodulation process in bone reconstruction [47]. However, the possible cytotoxicity, accumulated side effect (e.g., haemolysis, inflammation) and adverse effects on the immune system of the nano sized additives may need more systematic evaluation [48,49].
Except for rational adoption of biomaterials, there are some emerging strategies to design the biomimicking artificial periosteum conveying the biochemical cues (e.g., cells, growth factors) and biophysical cues (e.g., topological and mechanical stimulation) to modulate the bone healing process [15,[23], [24], [25], [26],56]. We here briefly summarize notable strategies in biomimicking artificial periosteum design in terms of biochemical mimicry and biophysical mimicry (Fig. 1).
Figure 1.
Illustration of design strategies of biomimicking artificial periosteum to enhance bone healing, including biochemical mimicry and biophysical mimicry.
2.2. Biochemical mimicry
Except for the basal materials, the natural periosteum embodies various biochemical factors including regenerative cells, osteogenic and angiogenic factors, and small biomolecules, which all play imperative role in their regenerative performance. Therefore, these biomimicking elements should be considered in the design of the periosteum to promote bone fracture healing.
2.2.1. Cells
As a highly vascularized tissue, the periosteum possesses multiple cell types including osteoblasts, endothelial cells, or MSCs and osteoprogenitor cells that can differentiate into osteoblasts [57]. These cells play active roles in new bone formation or neovascularization, and direct implantation of these viable cells as biomimetic periosteum has presented exceptional efficacy in bone regeneration (Table 2). For instance, transplanting osteoblasts can improve the expression of alkaline phosphate (ALP) and osteocalcin of native cells, two essential markers in bone formation and bone mineralization respectively, accelerating bone regeneration [58,59]. Incorporating MSCs into artificial periosteum also elevates ALP activity and up-regulates osteogenic gene expression with improved bone-forming capability [24]. Additionally, the native periosteum consists of well-organized extracellular matrix (ECM) fibers, and therefore using human dermal fibroblasts to fabricate ECM sheets results in aligned collagen fiber arrays, benefiting the bone repair and remodeling process [60]. In addition, MSCs could also release soluble factors that play autocrine, paracrine, and systemic roles to reduce inflammation and reduce apoptosis of tissue cells, promote the proliferation of progenitor cells of endogenous tissue organs, and achieve the effect of repairing tissues and organs [61]. Meanwhile, as a natural periosteum contains a high number of vascular tissues, the introduction of human umbilical vascular endothelial cells (HUVECs) is beneficial for forming new blood vessels, which significantly improves the structural and functional properties of MSC sheets as well as its therapeutic effect on the grafted femurs with bone defect [62]. Incorporating specific cell types can be used to rebuild the particular function of the native microenvironment to promote bone healing; however, the limited cell source, decreased cell viability after implantation, and possible immune reactions should be considered during the application.
Table 2.
Biochemical factors in biomimetic periosteum fabrication.
| Classification | Biochemical elements | Pros | Cons | Ref. |
|---|---|---|---|---|
| Cells | Osteoblasts | Promote new bone formation; Accelerate bone mineralization |
Insufficient sources, long expansion cycle | [58] |
| MSCs | Self-renewal ability; Osteogenesis capacity |
Limited vascularization; | [[74], [75], [76]] | |
| Human dermal fibroblasts | Mimic ECM structure | Limited vascularization; | [60] | |
| HUVECs | Angiogenesis capacity | Limited osteoinduction; | [77] | |
| Growth factors | TGF | Promote collagen production; Anti-inflammation |
High cost; short half-life Possible oncogenesis |
[69] |
| PDGF | Promote the proliferation of osteoprogenitor cells | [78] | ||
| VEGF | Promote angiogenesis | [74,79,80] | ||
| FGF | Promote tissue repair and periosteal chondrogenesis | [[81], [82], [83]] | ||
| IGF | Promote fracture healing; Increase the bone mineral density |
[84,85] | ||
| BMP | Induce MSC osteogenic differentiation; Promote fracture healing; |
[67,86] | ||
| Small biomolecules | Dexamethasone | Promote cell proliferation and bone formation | Difficult to control the dose | [87,88] |
| Glycerophosphate | Induce mineralized bone formation | Short drug effect | [89] | |
| RGD | Enhance cell adhesion to non-adhesive surfaces | High cost | [90] | |
| Icariin | Enhance the proliferation of periosteal cells | Abnormal coagulation | [11,91] |
2.2.2. Growth factors
Biomimicking artificial periosteum calls for the smart incorporation of growth factors, including bone morphogenetic protein (BMP), vascular endothelial growth factor (VEGF), transforming growth factor (TGF), and fibroblast growth factor (FGF), to regulate the cellular behaviors and modulate the interrelated signaling pathway at the defects for enhanced bone formation (Table 2) [[63], [64], [65], [66]]. For example, BMPs are promising osteoinductive factors in bone reconstruction. It promotes new bone reconstruction by mediating the proliferation and differentiation of osteoblasts, thereby benefiting bone defect healing [67,68]. On the other hand, TGF is critical in regulating chondrogenic differentiation of MSCs and expands the redifferentiation of human articular chondrocytes (HACs), promoting the formation of new bone and neocartilage [69]. However, despite the high efficiency of these factors, the high cost and short half-life of growth factors also impede their clinical transformation.
2.2.3. Small biomolecules
In addition to cells and growth factors, some small biomolecules (Table 2) can also regulate cell recruitment, stimulate cell differentiation and activate specific signaling pathways during bone healing, thereby replicating functions of the native periosteum [70]. For example, dexamethasone in artificial periosteum contributes to the stem cell differentiation into osteoblasts, with high expression of osteogenic proteins and genes [71]. The incorporation of icariin, the main component of epimedium, a traditional Chinese medicine herb, effectively promotes osteoblast proliferation and differentiation and works as an inhibitor of osteoclast formation [72]. Meanwhile, the modification with adhesive peptides Arg-Gly-Asp (RGD) enables enhanced adhesion and spreading of osteoblasts on periosteum surfaces, thereby promoting bone regeneration [73]. Furthermore, lidocaine, vancomycin, and moxifloxacin hydrochloride can also be added to the biomimetic periosteal scaffolds for anti-bacteria and anti-inflammation capacity [11,36].
2.2.4. Temporal manifestation and interaction
Bone healing is a dynamic process. At the beginning of the bone healing, the hematoma forms after fracture, which can lead to the higher expression of inflammatory growth factors including tumor necrosis factor-α (TNF-α) and Interleukin-1 (IL-1) [[92], [93], [94], [95]]. Thus, anti-inflammatory small biomolecules (e.g., diacerein) are usually adopted. For example, the diacerein is the IL-1 inhibitor, which can inhibit cartilage degradation, periosteum inflammation and promote cartilage synthesis [96,97]. At the same time, the activated platelets can release platelet-derived growth factors (PDGF) which can enhance the proliferation and differentiation of local regenerative cells [98,99]. Therefore, the initial implantation of high-density of regenerative cells (e.g., stem cells) could benefit from the microenvironment with PDGF expression. Then, after the inflammation, multipotent mesenchymal stem cells start to proliferate in large quantities. Meanwhile, the periosteal cells could secrete a series of osteogenic cytokines (e.g., BMP-2) to induce MSCs to chondrocytes or osteocytes. However, the large bone defects are always accompanied with deficient osteogenic factor secretion. Thus, higher concentration of osteogenic small biomolecular (e.g., dexamethasone, Icarin) can play critical roles at this stage to enhance the stem cell differentiation [82,83]. For instance, the dexamethasone can stimulate the stem cells with upregulated BMP-2 to promote the osteogenesis. In the late stage of bone healing, the local concentration of transforming growth factor-β (TGF-β) in natural periosteum is increased, which could promote the differentiated osteoblasts to synthesize type I collagen and boost the mineralization to finally restore original shape and structure of the defected bone [100]. However, it should be noted that the temporal manifestation and interaction among the biochemical factors are still in its infancy and more research should be conducted to elucidate the underlined mechanism.
2.3. Biophysical mimicry
Recently, animal experiments and clinical practice have shown that biophysical factors (e.g., topological, mechanical and electrical signals) could influence the effectiveness of the artificial periosteum and the bone reconstruction [101]. These biophysical signals aid artificial periosteum reconstruction by physical imitation of the natural periosteum to achieve the biological functions for enhanced bone healing.
2.3.1. Topological structure biomimicry
It has been widely reported that topography can induce specific cell responses including the adhesion, proliferation and differentiation [102]. During the dynamic bone healing process, topological structure of periosteum also undergoes certain changes. Upon injury, the periosteum gets thickened especially the inner cambium layer because of the periosteal reactions [[103], [104], [105]]. Afterwards, the cambium would show specific beam structure due to the proliferated bone progenitor cells. Then, the periosteum callus is formed with a wavy topology, followed by the formation of woven bone, which will be replaced by mature lamellar bone eventually. The generated periosteum has a delicate topographic surface composed of longitudinally oriented cells and ECM fibers, which could control and regulate the alignment of cells and formation of collagenous fibers as well as the bone development direction [106]. That is, reconstruction of periosteum-mimicking topography present great potential to synchronously modulate angiogenesis and osteogenesis for enhanced bone regeneration by guiding the cell behaviors of HUVECs and stem cells. For instance, a Janus artificial periosteum embodied with interior adhesiveness and exterior microgrooved patterns were fabricated to closely adhere to the natural bones [107]. At the same time, it effectively supported cell adhesion and regulated cell alignment. Such anatomical patterns of the bionic periosteum significantly modulated the osteogenesis of stem cells and angiogenesis of HUVECs without the use of biological macromolecules. In addition, this type of periosteum can prevent scar tissues from growing inwards. These unique features (e.g., surface topography) regulate the spatial arrangement of cells to mimic the cell alignment in natural periosteum and accelerate the bone healing [108]. That is, the periosteum's characteristic structure (e.g., surface topography) creates an outstanding niche to induce bone regeneration, which plays a critical role in the healing and reconstruction of bone defects.
2.3.2. Mechanical biomimicry
The periosteum is located outside the bone with appropriate viscoelastic behavior (920–1930 kPa) [13], which stabilizes bones mechanically, especially after bone fracture [109]. As a functional interface between bones and muscles, the periosteum bears mechanical loads to meet daily activities. Equipping the artificial periosteum with the mechanical microenvironment corresponding to the natural periosteum provides excellent biophysical cues to improve bone repair. For example, Matsumoto et al. found that mechanical unloading can cause the excess bone resorption of the rat tails and lead to the substantial reduction of bone mineral density (BMD) after 14 days. On the other hand, mechanical overloading can increase the expression of c-fos (a gene expressed in bone cells) to regulate the development, differentiation, and growth of periosteal cells with increased BMD [110,112]. Furthermore, during the mechanical loading, changes in interstitial fluid flow are observed, which can cause shear stress in the actin cytoskeleton of periosteal cells, modulating bone formation and mineralization [113]. Meanwhile, pulsating fluid flow enhances the activity of cyclo-oxygenase (COX) −2, which leads to the production of prostaglandin (PG) E2, an important biomolecule for regulating bone growth and remodeling of osteocytes [114,115]. To conclude, the biomimicry of mechanical microenvironment can modulate the cell fate and promote bone repair.
2.3.3. Electrical stimulation
The electrical properties of bone tissues have been revealed starting from the piezoelectric properties since the 1950s. Since then, researchers have discovered the endogenous electrical properties of bone and proposed that such property is important for the feedback control mechanism of bone modeling and remodeling. According to the nature of the electrical signals provided, the electrical stimulation systems can be divided into three main groups: capacitive stimulation, inductive stimulation, and direct current stimulation. For example, the capacitively coupled electric field activates charged transmembrane receptors involved in the calcium/calmodulin pathway, promoting osteogenic differentiation of stem cells to boost bone formation [116]. However, after bone trauma with periosteum damage, the electrical signal transduction can be interfered [117]. In other words, re-construction of the electric potential changes in the artificial periosteum can help modulate the bone repair.
Studies have shown that electroactive piezoelectric materials such as piezoelectric collagen fibers [77,114] and poly (vinylidene-fluoroethylene-trifluoroethylene) (PVFT) could generate electrical signals under deformations [117] and induce stem cells to differentiate into osteoblasts by restoring the local osteogenic electrical microenvironment. In one study, Zhang et al. found that by controlling its β phase content, the surface potential of PVFT membranes can be modulated around the range of the physiological bone electric potential (∼10 pC/N) [118]. Such electrical environment of PVFT membranes could modulate the osteogenic differentiation of MSCs with surface potential of −53 mV showing better osteogenic properties compared with the membranes with −78 mV surface potential. These results suggested that using these materials with electrical stimulation to fabricate artificial periosteum which can mimic the electrical properties of natural periosteum can achieve enhanced bone recovery.
As discussed above, there are diversified biochemical and biophysical mimicry strategies in biomimicking artificial periosteum design. The general guideline of biomimicking artificial periosteum design is to comprehensively replicate the defected natural periosteum based on the patient clinical situation, skeletal segment involved, and bone defect location. For example, the biomechanics of load bearing tibia bone defect and non-load bearing cranial defect are substantially different; thus, the mechanical properties of artificial periosteum under these conditions should be dedicatedly designed [119]. Moreover, the integration of multiple considerations (e.g., chemical compositions and physical properties) on demand of patient conditions could further generate artificial periosteum with enhanced therapeutic efficacy. For the patients with movable joint bone defect and impaired neovascularization capacity (e.g., cancer patients receiving angiogenesis inhibitors), artificial periosteum with excellent flexibility and angiogenesis capacity will be particularly beneficial [120].
2.4. Animal models for biomimicking artificial periosteum evaluation
Artificial periosteum can be designed to simulate native periosteum from biochemical and biophysical aspects. In addition to in vitro studies, it is critical to validate the effectiveness of these strategies in animal models as bone repair involves a series of in vivo signal cascade responses, cellular behavior regulation and microenvironment interactions [121]. The animal models for in vivo therapeutic performance evaluation of the biomimicking artificial periosteum can be generally classified into bone defect models and bone fracture models (please see summary of animal models in Table 3) [122]. The bone defect models usually simulate the large bone defects with the removal of critical-sized bones on skull, femur or tibia of animals. For critical sized calvarial defect model which are usually performed on small animals such as mice or rats, the bone defects are created onto skull by a dental trephine and are directly covered with artificial periosteal samples [13,47]. The skull defect models are more widely used due to the high throughout evaluation for the bone regeneration process including osteogenesis and angiogenesis with saved time and cost [123]. However, such non-load bearing models ignore the biomechanics of the artificial periosteum and are unable to simulate the dynamic mechanical microenvironment of artificial periosteum after implantation. In contrast to the skull defect models, femur or tibia defect models with the implantation of bone grafts (e.g., autografts, allografts) and artificial periosteum are closer to recapitulate the clinical practice of artificial periosteum [124]. These models are suitable for systematical evaluation of the therapeutic efficacy of the artificial periosteum especially for the biomechanics evaluation; nevertheless, such animal models are time consuming, and the choice of bone grafts undoubtedly have an impact on the experimental results. In bone fracture models, a controlled fracture is made by surgical scissors and fixed with Kirschner-wire or intramedullary pin. Then, the artificial periosteum is used to cover the fixed bone fracture [36,125]. Although this model is relatively less used, it is more conducive to evaluate osteointegration of artificial periosteum and could give more insight of the interaction between the artificial periosteum and the surrounding tissues.
Table 3.
Animal models for biomimicking artificial periosteum evaluation.
| Animal models | Species | Applications | Pros | Cons | Ref. | |
|---|---|---|---|---|---|---|
| Bone defect models | Critical sized calvarial defect | Rat, mouse | Artificial periosteal alone for defect coverage | Easy to conduct, high throughout, save time and cost | Damage to the brain, hard to represent clinical conditions | [123,127] |
| Femur/tibia bone defect | Rabbit, ovine, rat, sheep | Bone grafting with artificial periosteum | Closer to the clinical practices, biomechanics evaluation | Complicated surgical procedures, careful selection of bone grafting | [72,[128], [129], [130]] | |
| Bone fracture models | Femoral fracture model | Rat, rabbit | Artificial periosteal alone with fixation | Evaluation of osteointegration | Complicated surgical procedures | [36,131] |
In addition to careful consideration of the experimental models, it is also important to choose the species. Different animal species may have different therapeutic effects. For example, rabbits have higher intrinsic healing ability of cartilage defects than goats or humans [126]. Thus, the rabbits are more suitable for the evaluation of osteochondral defects. Moreover, the size of the animals also has a certain influence. For example, the goats and monkeys allowing for the creation of much larger critical-size bone defects could better resemble the clinical large bone defect due to severe trauma. However, performing high-precision surgical procedures on these animals is too difficult and the time, manpower as well as animal costs are prohibitive. Therefore, the small size animals (e.g., mice or rats) are easy to perform with large numbers of experiments. Altogether, the therapeutic performance differences between animal models and human trials are still unclear and need more exploration to facilitate the translation from pre-clinical evaluations in animals to clinical applications in human. Moreover, investigating the artificial periosteum in at least two different species will lead to a better understanding of the preclinically relevant applications.
3. Novel versatile biomimicking artificial periosteum
The current design of biomimicking periosteum calls for the recapitulation of the natural periosteum with versatile strategies and enhanced regenerative performance [11,51]. For example, the microenvironment of the natural periosteum embodies various biochemical (e.g., growth factors) and biophysical (e.g., surface topology) factors. It has been increasingly recognized that the facile combination of these biochemical and biophysical factors could replicate the effects of natural periosteum with significant breakthroughs [132]. Except for the biochemical and biophysical stimuli, the natural periosteum undergoes a series of dynamic healing phases after bone fracture such as inflammatory response, cell recruitment and differentiation. The smart recapitulation of the healing phases has also presented fascinating effectiveness to modulate the bone healing process [133]. Thus, the biomimicking periosteum with the simulation of the phased-healing process has attracted increasing attention in recent years. Additionally, from the structural aspect, the natural periosteum demonstrates inherent heterogeneous structures from top to bottom with different biological functions [17]. Therefore, the reproduction of the anisotropic structure of the natural periosteum could bestow the artificial periosteum with heterogeneous biological performance to accelerate the bone reconstruction. In this section, we categorize the latest periosteum into physical-chemical combined artificial periosteum, heterogeneous structured biomimicking periosteum, and healing phase-targeting biomimicking periosteum.
3.1. Physical-chemical combined artificial periosteum
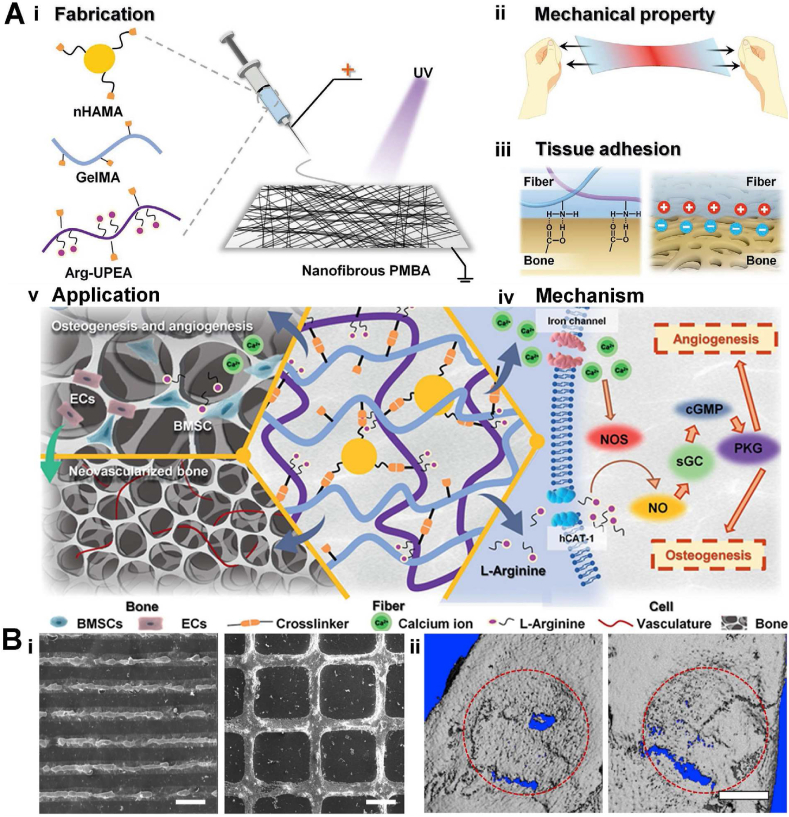
Compatible mechanical strength, flexibility, and excellent tissue adhesion are the basic physical properties of artificial periosteum, which can provide better surgery handleability as well as the biophysical stimulation. However, physical properties alone are not enough to regulate bone repair, as bone repair also needs chemical regulation to modulate cellular responses. Here, an ideal artificial periosteum should possess superior physical properties and embody chemical stimulation to promote osteogenesis and angiogenesis. To this end, Yang et al. designed a periosteum mimicking bone aid (PMBA) with appropriate mechanical strength and adhesion ability to promote osteogenesis and angiogenesis [13]. The PMBA was fabricated by electrospinning a mixture of methacrylate gelatin (GelMA), methacrylate hydroxyapatite nanoparticles (nHAMA), and l-arginine (L-Arg)-based unsaturated poly (ester amide) (Arg-UPEA). In this design, photocrosslinkable GelMA possessed excellent biocompatibility and tissue adhesiveness, nHAMA endowed the PMBA better mechanical performances and worked as a calcium ion (Ca2+) reservoir, while Arg-UPEA further increased the adhesion strength of PMBA and achieved the long-term release of bioactive gas nitric oxide (NO) precursor L-Arg (Fig. 2A|i). Upon light exposure, those components formed an organic-inorganic crosslinked double network for enhanced mechanical strength (∼ 1 MPa), and microscopy measurements confirmed the nanofibers had a similar structure to natural periosteum. The introduction of Ca2+ and Arg into the PBMA chemically simulated the original chemical components of the bone tissue. After implantation in a rat critical-sized calvarial defect model, the PMBA released L-Arg and Ca2+ and regulated a series of the periosteum-mimicking physiological process (e.g., activation of the NO/cGMP cell signaling pathway), reproducing the biological effect (osteogenesis and angiogenesis) of natural periosteum. This work successfully fabricated the bio-mimicking periosteum from both physical, chemical, and biological aspects: the initial materials components equipped the scaffolds with suitable mechanical and adhesion ability as natural periosteum (Fig. 2Aii, iii, and iv), while its degradation products were consistent with the original bone chemical components, which could long-term mediate the bone and vascular regeneration. This strategy has been proven successful in in vivo application, where both the micro-CT scan and bone mineral density analysis have demonstrated outstanding bone regeneration potential. It is envisioned that this strategy will have great potential in bone tissue engineering (Fig 2Av). In addition to endowing the artificial periosteum with compatible mechanical properties, reasonable design of microstructures on periosteum is also an effective strategy for biophysical stimulation. For instance, it has been reported the nano-grooved morphology or ridges could regulate the osteogenic differentiation of stem cells and promote the biomineralization [134]. Therefore, the combination of such biophysical micropatterns and the osteogenic biochemical factors could further enhance the bone regeneration. In one study, Yang et al. designed a biomimicking periosteum with micropatterns for site-specific mineralization. Mineralized hydroxyapatite nanoparticles (HANPs) were incorporated into the periosteum as micropatterns, and apatite as well as BMP-2 were subsequently co-precipitated on the microstructures by site-specific mineralization [41]. In vitro experiments verified the scaffold's continuous release of Ca2+ and BMP-2 within 14 days, which could provide a simulated periosteal microenvironment for cell recruitment and cell differentiation. Meanwhile, the linear and grid structure of the micropatterns (Fig. 2Bi) could guide the cell alignment and promote the differentiation of stem cells. The in vivo results in a rat cranial defect model have demonstrated that the biomimetic periosteum had the ability to promote the formation of blood vessels and ossification at the bone defect sites. Micro-CT scan data showed less ectopic bone formation and more regenerated bone in the biomimicking periosteum's group (Fig. 2Bii).
Fig. 2.
A. Periosteum mimicking bone aid (PMBA) made by Yang et al. (i): Nanofibers prepared by biomimetic natural periosteal structure. (ii): Stretchable properties similar to natural periosteum (biophysical stimulation) (iii): Tissue adhesion mechanism. (iv) Synergistic effect of calcium and l-arginine (biophysical stimulation). (v) Combination of biophysical and biochemical stimulation can significantly promote osteogenesis and vascularization of the defect. Figures are reproduced from Ref. [13] with permission. B. A bioinspired membrane fabricated by Yang et al. (i) SEM images of the line (left) and grid (right) pattern. (ii) Micro-CT 3D reconstruction of the line pattern (left) and grid pattern (right). Figures are reproduced from Ref. [41] with permission.
Apart from the structure of periosteum, the inherent piezoelectric property of natural periosteum has also attracted increasing attention in the design of novel biomimicking artificial periosteum. Recent studies have shown that electroactive piezoelectric biomaterials can promote the recruitment of stem cells, enhance cell migration and induce their osteogenic differentiation. Thus, the artificial periosteum with facile integration of piezoelectric property and proper biochemical cues has presented superior efficacy [118,135]. For example, Zhao et al. developed a novel biomimicking periosteum made of piezoelectric PVFT membrane loaded with BGN [42]. The PVFT could not only provide a piezoelectric signal to induce stem cell migration and differentiation, but also capture the positive-charged Ca2+ released from BGN to enhance the surface mineralization. The high surface area, high porosity, and high curvature of the BGN further provided an ECM-like environment for the growth and differentiation of osteoblasts. The micro-CT results showed the PVFT/BGN group exhibited significantly increased bone formation compared with the PVFT only group or BGN only group using a rat critical-sized calvarial defect model. This design integrated electrical stimulation and biochemical stimulation to achieve a comprehensive simulation of the function of the natural periosteum.
Surface modification is a useful strategy to regulate the biophysical and biochemical properties of the biomaterials. Parafilm is a flexible thermoplastic film with excellent cohesion and flexibility, yet the water resistance and bio-inertness limited its application. To this end, Shi et al. deposited dopamine fibronectin on parafilm periosteum to improve its hydrophilicity and biocompatibility and generated various micropatterns on the modified parafilm by lithography [108]. Then, adipose mesenchymal stem cells (ADMSCs) were cultured onto the surface followed by mechanical stretching of the membranes. In this film, the cells were elongated by mechanical stretching of the periosteum along the pattern direction, simulating the extrusion and stretching effect of the long and narrow natural bone structure on the cells, while the dopamine fiber binding protein simulated the periosteum biological microenvironment for enhanced cell attachment and differentiation. In vitro, the expression of smooth-muscle-specific gene (e.g., SM22-α) and α-actin in the stretching group was much higher than the results found in the non-stretching group. In addition, ALP activity, type I collagen expression, and BMP-2 expression were significantly up-regulated in the stretching group, which proved that this periosteum could promote substantially osteogenic differentiation of ADMSCs. This new strategy successfully simulated mechanical stimulation in natural bone tissues.
In summary, the physical-chemical combined artificial periosteum considers the interactions of biophysical and biochemical stimulation during periosteum design to promote bone repair from biomolecules and cells to tissues. It can enrich the functionality of the biomimicking periosteum like anti-inflammation and tissue adhesion, promoting vascularization and osteogenesis, and is likely to see extensive application in clinic. These studies set a good illustration of physical-chemical combined artificial periosteum, but there are still potential pitfalls. For some artificial periosteum with micropatterns or surface modification which can recruit cells for osteogenesis or angiogenesis, the number of cells and competitive adhesion should be explored as insufficient targeted cells may lead to inadequate cell recruitment. Additionally, when using electrical, thermal, magnetic and/or other physical signals, a controllable approach is needed to regulate these signals to avoid other disturbance. For the therapeutic molecule-loaded artificial periosteum, how to maintain the activity of bioactive factors is a concern for its long-term effect. Meanwhile, extra addition of therapeutic molecules may lead to burst release, resulting in excessive osteogenesis or ectopic osteogenesis.
3.2. Healing phase-targeting biomimicking periosteum
In the complex processes of bone healing, periosteum makes significant contribution from the beginning to the end. At early stage after the injury (24–48 h), an acute inflammatory reaction can be found at the periosteum [133]. The periosteum secretes various cytokines or growth factors to initiate the repair cascade, which involves the recruitment of cells at the injury site, angiogenesis stimulation and ECM synthesis [136,137]. Afterwards, the progenitor cells in periosteum begin to proliferate. Cell tracing showed that nearly 90% of neo-cartilage and woven bone in callus of early fractures originated from periosteum [6]. Subsequently, the bridging of the bony periosteal callus forms a new cortical shell under which the cells differentiate into chondrocytes and osteoblasts [133]. Eventually, mature lamellar bone will replace the woven bone by the cooperation of osteoclasts and osteoblasts, gradually restoring the original bone shape. Hence, from clinical aspect, design of artificial periosteum from the aspect of simulating bone healing phases will biologically mimic the natural periosteum to restore the periosteal function and accelerate bone regeneration. For example, Li et al. reported a functional periosteum composed of electrospun PLA scaffolds with BMP-2 loaded hollow MnO2 (h-MnO2) nanoparticle encapsulation and leptin receptor antibody (LEPR) coating [43]. This biomimetic periosteum effectively recruited skeletal stem cells (SSCs) through LEPR antibody on the scaffold surface. h-MnO2 nanoparticles could further stabilize SSCs, inhibit early inflammation and improve overall cell viability and proliferation. Subsequently, BMP-2 released from the h-MnO2 nanoparticles promoted the osteogenesis of SSCs. The high osteogenic capacity of the scaffolds in vivo in a mice cranial defect model verified the potential application value.
After periosteum implantation, potential infection and initial inflammation happen immediately, while osteogenesis is a long-term pathological process after inflammation. A severe early infection or inflammation can greatly compromise the efficiency of treatment and even increase the morbidity and mortality. Therefore, appropriate and orderly release of antibacterial, anti-inflammation and osteogenic agents would be an ideal solution to cooperate with the process of bone tissue regeneration. In a study of Gong et al., a core–shell structured electrospun PCL membrane with programmable release behavior according to the repair process was designed [138]. They introduced icariin (ICA) and moxifloxacin hydrochloride (MOX) into PCL core and gelatin shell, respectively (Figure.3Ai). The rapid release of MOX in the fibrous shell could inhibit bacterial colonization and played an auxiliary role in anti-infection and anti-inflammation in the initial stage of bone repair; while the slow and sustained release of ICA provided favorable bone induction cues for the adhesion, proliferation, and differentiation of bone-related cells on the materials and could continuously promote osteogenic differentiation in the whole process of bone repair (Fig. 3Ai). In the in vivo rabbit segmental defect models, after three months of implantation, normal trabecular bone, mature bone marrow cavity, and cartilage were observed, and the volume of regenerated bones using the core–shell structured electrospun fibers was more than that in the blank group or the non-controlled drug release group (Fig. 3Aii, iii). This phased release strategy was a collection of multiple treatment methods, which could realize the in-situ release of various drugs through one implantation, assist the early antibacterial stage through MOX, and improve the whole stage of osteogenic differentiation by ICA, demonstrating the potential of phased release strategy in bone regeneration.
From the aspect of molecular level, the bioactive factors secreted from periosteum undergoes dynamic change to regulate different cell behaviors at varied bone repairing period. Thus, smart control of the phased release behavior of the bioactive factors from artificial periosteum can also simulate healing process and regulate bone regeneration. In a study, Yin et al. fabricated an electrofoun fibrous chitosan collagen (CS/COL) scaffold for the phased release of BMP-2 and FGF-2. In this design, BMP-2 was encapsulated in poly (lactic acid)-poly (ethylene glycol)-poly (lactic acid) (PELA) microcapsules and FGF-2 was immobilized onto the surface of PELA to achieve the phased release of BMP-2 and FGF-2 [139]. The results proved this staged release strategy could improve cell proliferation and promote differentiation of periosteum derived cells (PDCs). Additionally, the bone volume and BMD of new bone tissue in groups with PELA-encapsuled BMP-2 and PELA-attached FGF-2 were higher than those groups without growth fator, indicating the phased release of FGF-2 and BMP-2 helped bone regeneration. In rat skull defect models, immunohistochemical observation and computed tomography data showed that the phased release group had more bone regeneration than the non-controlled releasing group. This strategy simulated the release order of growth factors of natural periosteum and bridged the natural bone regeneration cycle, which could significantly improve the drug utilization and therapeutic efficacy.
Previous studies have demonstrated that BMP-2 is the initial factor during fracture healing, while it is typical to see BMP-7 being expressed two weeks after fracture [140]. Based on this, Jo et al. prepared a heparinized collagen membrane as artificial periosteum to achieve the phased release of BMP-2 and BMP-7. The programmed release of BMP-2 at an early stage and long-term BMP-7 release simulated the phased growth factor needs of natural periosteum, which is beneficial to bone repair (Fig. 3Bi, ii) [141]. In vitro, enzyme-linked immunosorbent assay (ELISA) confirmed that BMP-2 would be released in a few days, while BMP-7 could be released slowly for a few months Fig. 3Biii). The in vivo data of rat calvarial defect models demonstrated that heparin-BMP-7 + BMP-2 group had the highest bone mass and connective tissue maturity (Fig. 3Biv, v). This strategy was biomimetic of the periodic release (rapid BMP-2 release at initial period and subsequent BMP-7 release) of growth factors from natural periosteum, which not only overcame the risk of postoperative infection caused by multiple injections of drugsm but also avoided the potential risk of abnormal differentiation and repair caused by the disproportion of growth factors at different regeneration stages within the physiological bone regeneration cycle.
Fig. 3.
A. Icariin (ICA) moxifloxacin hydrochloride (MOX) loaded multi-phase release poly ε-caprolactone (PCL) scaffolds. (i) Manufacture and effect of electrospun multi-phase release PCL scaffolds. (ii) In vitro antibacterial activity and biocompatibility evaluation of the electrospun core–shell fibrous membranes. (iii) 3D reconstruction images obtained by Micro-CT three months later. Figures are reproduced from Ref. [138] with permission. B. Heparin-BMP-7 & BMP-2 loaded biomimetic collagen scaffolds. (i) Image of collagen heparin/collagen scaffolds. (ii) Quantitative adsorption of toluidine blue O (TBO) on collagen heparin/collagen scaffolds in vitro. (iii) In vitro release of BMP-2 and BMP-7 from scaffolds. (iv) Bone tissue section staining of biomimetic periosteum at eight weeks. (v) Quantitative bone repair results of biomimetic periosteum at eight weeks. Figures are reproduced from Ref. [141] with permission. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The healing phase-targeting biomimicking periosteum can resemble the dynamic physiological bone repair process with staged drug release to regulate the anti-inflammatory, anti-bacterial, angiogenic and osteogenic process. This strategy is a great advance of biomimetic design of the natural periosteum. However, there are still some concerns. The precise modulation of the phased drug release is difficult. The high local concentration caused by burst release in the initial stage can cause bio-toxic side effects. Moreover, the bioactivity of the late released drugs should be more clearly evaluated since most of them are released after weeks and may be deactivated or degraded by pH, or oxidative environment of the healing process. Additionally, dynamic monitoring of bone repair at different stages is still challenging in the current strategy and the integration of monitoring setting could better elaborate the effect of healing phase-targeting biomimicking periosteum.
3.3. Heterogeneous structured biomimicking periosteum
Despite the successful biomimicry of natural periosteum with physical-chemical combined stimulation and targeted healing-phases, the highly hierarchical structure of natural periosteum coherently composed of two or more layers with different composition and functions should also be taken into consideration. To this end, design of biomimicking heterogeneous periosteum with different biological functions can facilitate its clinical application and give new insight to the novel artificial periosteum design.
One of the critical roles of the artificial periosteum is to prevent the invasiveness of epidermal cells and connective tissue cells interfering with osteoblasts, and to selectively promote osteogenesis and angiogenesis [142]. Therefore, Delia et al. designed a double-sided bionic periosteum by immersing one side of the alginate (ALG) membrane into HANPs (Fig. 4Ai) [143]. The inner side containing HANPs could provide Ca2+ to promote osteogenesis, while the outer side of ALG (without HANPs) provided porous fibrous structure to promote epithelial cell adhesion and angiogenesis (Fig. 4 Aii). In vitro data demonstrated that the mineral-rich side could selectively promote the proliferation and osteogenesis of osteoblasts. In contrast, the fibrous side had a positive effect on epithelial cells (Fig. 4Aii, iii). Such design of anisotropic structure and chemical characteristics of the bionic periosteum successfully and selectively promoted the specific cells to enhance bone healing. Although the Janus hydrogel periosteum can simulate the different functions of the inner and outer membranes of the natural periosteum and display spatial segmentation, the migration of osteoblasts from outside to inside was hindered, which may lead to uneven and discontinuous new tissue formation. To simulate the uniform and continuous layered structure of natural periosteum, Wang et al. designed a multilayered cell loaded PCL/collagen/HANP electrospun scaffolds (Fig. 4Bi, ii) [38]. The electrospun fiber sheets have ECM alike structure that benefit cell attachment and drug loading but limit cellular infiltration and vascular ingrowth. In this study, a layer-by-layer assembly strategy was adopted for uniform cell seeding and tissue construction. The multilayered fibrous membrane seeded with BMSCs was fabricated, which possessed porous structure similar to that of natural bone tissue. In vitro experiments demonstrated the biocompatibility of the scaffolds (Fig. 4Biii). In the mice segmental femoral bone allograft model, the volume of new bone formed increased significantly after implantation. Histological analysis showed that the scaffolds promoted the formation of both intramembranous and endochondral bone (Fig. 4Biv). The layer-by-layer strategy allowed for the controlled insertion of multiple functional components, providing flexibility and multifunctionality for artificial periosteum.
Fig. 4.
A. Double-sided biomimetic periosteum. (i) Morphology of the double-sided biomimetic periosteum by scanning electron microscope (SEM), and the selectivity of fibroblasts and osteoblasts in different surfaces. (ii) Effect of different porosity on cell proliferation rate. (iii) The alkaline phosphatase (ALP) activity of sodium alginate with different porosity. Figures are reproduced from Ref. [143] with permission. B. Electrospun multilayered PCL/collagen/nano hydroxyapatite scaffolds. (i) SEM images of scaffolds and fluorescence images of BMSCs cultured on the scaffolds. (ii) The process of stacking a plurality of electrospun scaffolds with cells into multilayered scaffolds. (iii) Distribution of fluorescent BMSCs in multilayered scaffolds. (iv) Immunofluorescence staining of CD31 (red) and endomycin (blue) vessels at five weeks in vivo. Figures are reproduced from Ref. [38] with permission. C. Hyaluronic acid microsol with VEGF loaded PLLA electrospun fibres. (i) Manufacturing process of the heterogeneous structured scaffolds. (ii) Immunofluorescence expression of cell adhesion related protein: integrin β1. (iii) Expression of cell adhesion related protein integrin β1. (iv) Comparative experiment of bone regeneration. (v) Reconstructed 3D model of bone regeneration by micro-CT. Figures are reproduced from Ref. [80] with permission. D. Adhesive janus periosteum with fine patterns. (i) Schematic illustration of the janus periosteum. (ii) Shear (left) and normal (right) adhesion strength under dry conditions. (iii) Relative HUVEC angiogenetic marker genes of VEGF-A (left) and eNOS (right). (iv) Representative Actin/DAPI staining images of rMSCs on different Janus periosteum for cell morphology observation (top) and cell alignment evaluation (bottom). Figures are reproduced from Ref. [107] with permission. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
As mentioned above, the macro hierarchy structures realized physical separation and promoted migration and differentiation. At the same time, the layered structure of microtubules can better simulate the periosteal microenvironment. In a study by Liang et al. they electrospun multilayered poly lactide (PLLA) fibers and collagen I nanofibers. The PLLA fibers contained hyaluronic acid microsol loading VEGF (Fig. 4Ci) [80]. This strategy simulated the multilayered fiber structure of the periosteum at the micro-level, which could provide biochemical and structural stimulation for cell attachment, growth and differentiation. The fibrous layer can be generated by the formation of exogenous vascularized fibrous membrane under the effect of angiogenesis, while the endogenous cambium is formed by the proliferation and differentiation of MSCs on the collagen layer, which ultimately leads to bone reconstruction. The in vitro data showed that the release of VEGF was controllable and continuous, which ensured the continuous promotion of angiogenesis. By culturing BMSCs on this artificial periosteum, the collagen nanofibers enhanced cell adhesion and proliferation (Fig. 4Cii, iii). The designed biomimetic periosteum showed higher ALP activity and more bone crystal formation in vitro. After eight weeks of the implantation, the authors found that there existed a transparent and dense periosteum-like connective tissue above the defect area in a rat calvarial critical size defect model, while a bare hole invaded by soft tissue with a nonunion case was found within the pure VEGF control group (Fig. 4 Civ, v). In addition to chemical compositional heterogeneity, structural heterogeneity was also investigated. Inspired by the multilayered structure of the periosteum with interior surface adhesion and exterior anatomical patterns for cell regulation, Yang et al. designed a bionic double-sided periosteum (Fig. 4 Di) [107]. Photo-crosslinkable poly (lactide-co-propylene glycol-co-lactide) dimethacrylates (PGLADMA) material was used as the substrate, and the inner side was engraved into a gecko-inspired pattern (GP) to improve the adhesion of the inner side (Fig. 4Dii). In vitro experiments have proved its excellent dry and wet adhesion ability, with shear and normal adhesion strengths being 3.6 and 2.8 N cm−2 under the dry condition, respectively (Fig. 4Diii). The outer side was carved into microgrooved arrays of different widths to promote osteogenesis and angiogenesis by mimicking the natural structure of natural periosteum. By co-culturing HUVECs and MSCs on the material's outer surface in vitro, the groove structure presented the best ability of angiogenesis and osteogenesis (Fig. 4Div), which could be attributed to the transmission contraction stress and mechanical balance structure in the aligned morphology. This double-sided bionic periosteum could adhere to bone tissue and enabled simultaneous osteogenesis and angiogenesis in a critical-sized calvarial defect model, establishing its unique advantages in bone healing.
In all, the artificial periosteum with heterogeneous structures can mimic the multilayered structure of the natural periosteum and meet the different needs of the microenvironments on both sides of bone fracture/defect. In terms of the continuous structural changes in artificial periosteum, researchers should pay attention to its thickness and permeability, as overlapped dense structure may lead to limited oxygen and nutrient transportation as well as cell migration, and even cause necrosis. In terms of large-scale production, the fabrication of heterogeneous structured periosteum is quite complicated with high cost. This is because the heterogeneous structures need to be fabricated separately and must avoid the interference of layers during preparation. In addition, in most heterogeneous structured periosteum, each layer only considers the function of a single cell type; however, the artificial periosteum is micro-scaled thin film and the multitype cells may involve in each layer of the heterogeneous periosteum, leading to the uncertainty of the anisotropic biological functions of the heterogeneous periosteum. Furthermore, for certain bone diseases (e.g., osteoporosis), the bone status and structure keep changing, heterogeneous structured periosteum for normal bones may not be suitable in this case, so the future design may perform independent studies of these cases.
4. Conclusion and future prospects
This review summarizes the preparation strategies of biomimicking artificial periosteum in recent years, the biophysical and biochemical factors that affect the functionality of artificial periosteum, and some latest developed versatile biomimicking artificial periosteum.
In future, in addition to the osteogenesis and angiogenesis design of artificial periosteum, the anatomic structure and function of nerve fibers should also be incorporated when fabricating artificial periosteum since it has been revealed that the innervation plays a significant role in human periosteum and the interaction between the blood vessels and the nerve fibers could promote the development and function of each other, enhance the osteogenesis process and accelerate the bone regeneration [144,145]. For example, Qin's group have extensively researched the interplay between innervation and bone regeneration. They found the sensory nerve played a crucial role in critical size bone fracture healing and the blockade of sensory nerve could defer the bone regeneration [146]. They also presented that the magnesium (Mg) ions could induce the expression of local neuronal calcitonin gene-related polypeptide-α (CGRP) in the sensory nerves, leading to the increased osteogenic differentiation of stem cells and promoted migration of endothelial cells [147]. They have further prepared the magnesium-pretreated periosteum with Mg accumulation and demonstrated such magnesium-pretreated periosteum could upregulate the neuronal CGRP expression after implantation, resulting in increased osteogenic differentiation of periosteum-derived stem cells [148]. However, such magnesium mediated bone regeneration relied on the well-functionalized local nerves in the native periosteum and there has been limited artificial periosteum with the consideration of innervation with damaged nerve fibers. This is attributed to the dilemma of modulating multiple cell types, and the complexity of pre-establishing highly connective peripheral nerve networks in engineered periosteum. In this regard, a better understanding of the coupling mechanisms among innervation, angiogenesis, and osteogenesis is highly sought after. Besides, the biomimetic approach should also be implemented to design and fabricate novel neurovascularized engineered periosteum integrating the material development, structure design, and advanced fabrication techniques to fully recapitulate the microenvironment of natural periosteum for accelerating bone healing.
In addition, since the bone defects close to joint are always accompanied with the cartilage damage; thus, the potential role of artificial periosteum for osteochondral regeneration should be noticed. Actually, some studies have claimed that MSCs could differentiate into stable articular cartilage phenotypes in vitro [149]. Because of the abundant progenitor cells in the periosteum, scientists have studied the possibility of periosteal graft transplantation to repair osteochondral defects [150,151]. The bionic artificial periosteum can form a stable periosteal layer to promote cartilage production and a layer of neo-endochondral after implantation in a suitable environment. The cartilage remodeling and endochondral ossification may contribute to the stability of the newly formed cartilage layer because early subchondral bone loss can contribute to the occurrence of osteoarthritis.
Furthermore, due to the exogenous nature of the engineered artificial periosteum, they would inevitably activate the human immune system and elicit significant effects on immune cells, which will ultimately affect subsequent immune responses [152]. Such osteoimmune environments around the implants could significantly influence bone dynamics, and the immunomodulation of the bone implants has been recognized as a vital biological property for mediating osteogenic performance. It could provide a valuable and beneficial strategy for developing advanced bone biomaterials with multifactorial effects on regulating osteoclastogenesis, manipulating immune response, and mediating osteogenesis [[153], [154], [155]]. Nevertheless, such immunomodulatory effect has rarely been incorporated in the present artificial periosteum. This calls for developing facile engineered periosteum with delicately designed osteoimmunomodulation effect to regulate the host immune response, release osteogenic factors, and balance osteogenesis and osteoclastogenesis for enhanced bone regeneration.
Moreover, bone healing is a complicated process that could be influenced by many different factors which further complicate the healing process. Therefore, it is essential to design a multifunctional artificial periosteum, which can not only meet the requirement of the specific injury condition but also assist the process of bone defect regeneration. The smart design of anti-bacteria and bioactive artificial periosteum is highly desirable to achieve success in bone implantation prone to infection. Moreover, for bone defects originating from malignant bone tumor resection, it is necessary for the engineered periosteum to eliminate the remaining tumor cells and to improve bone regeneration [156]. Therefore, multifunctional artificial periosteum with antitumor therapeutic capacity should also be developed in future studies.
Finally, despite the critical role of biomimicking artificial periosteum in bone repair has been highlighted, their clinical application is in the initial stage. To date, the fixation or adhesion between the artificial periosteum and the bone graft is still challenging. This could cause the protruding edges which may pierce the surrounding soft tissues or empty cavity formation with excess fibrous tissue invasion [157]. Moreover, for the cell-laden artificial periosteum, the potential ethical concerns, low cell retention rate and insufficient nutrition supply has significantly hindered their clinical application [158]. More importantly, the customized artificial periosteum for patients with different ages, clinical conditions and bone defect locations should be carefully designed to meet their specific clinical situation (e.g., load-bearing site, the elderly with osteoporosis) [159]. Altogether, more clinical-use orientated consideration and strategies should be implemented in the future design of artificial periosteum to facilitate its clinical translation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work is supported by the National Excellent Young Scientists Fund (82122002) from the National Natural Science Foundation of China and Guangdong-Foshan Joint Fund (2020B151530002) from Guangdong Basic and Applied Basic Research Foundation.
References
- 1.Allen M.R., Hock J.M., Burr D.B. Periosteum: biology, regulation, and response to osteoporosis therapies. Bone. 2004;35:1003–1012. doi: 10.1016/j.bone.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Chang H., Knothe Tate M.L. Concise review: the periosteum: tapping into a reservoir of clinically useful progenitor cells. Stem Cells Transl Med. 2012;1:480–491. doi: 10.5966/sctm.2011-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts S.J., van Gastel N., Carmeliet G., Luyten F.P. Uncovering the periosteum for skeletal regeneration: the stem cell that lies beneath. Bone. 2015;70:10–18. doi: 10.1016/j.bone.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Seeman E. The periosteum—a surface for all seasons. Osteoporos Int. 2007;18:123–128. doi: 10.1007/s00198-006-0296-6. [DOI] [PubMed] [Google Scholar]
- 5.Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res. 2009;24:274–282. doi: 10.1359/jbmr.081003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X., Xie C., Lin A.S.P., Ito H., Awad H., Lieberman J.R., et al. Periosteal progenitor cell fate in segmental cortical bone graft transplantations: implications for functional tissue engineering. J Bone Miner Res. 2005;20:2124–2137. doi: 10.1359/JBMR.050806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie J., Hou Y., Yao Y., Fu N., Cai X., Li G., et al. Regulation of extracellular matrix remodeling proteins by osteoblasts in titanium nanoparticle-induced aseptic loosening model. J Biomed Nanotechnol. 2015;11:1826–1835. doi: 10.1166/jbn.2015.2119. [DOI] [PubMed] [Google Scholar]
- 8.Dhivya S., Ajita J., Selvamurugan N. Metallic nanomaterials for bone tissue engineering. J Biomed Nanotechnol. 2015;11:1675–1700. doi: 10.1166/jbn.2015.2115. [DOI] [PubMed] [Google Scholar]
- 9.Wang Q., Xu J., Jin H., Zheng W., Zhang X., Huang Y., et al. Artificial periosteum in bone defect repair—a review. Chin Chem Lett. 2017;28:1801–1807. [Google Scholar]
- 10.Hoffman M.D., Benoit D.S.W. Emerging ideas: engineering the periosteum: revitalizing allografts by mimicking autograft healing. Clinical Clin Orthop Relat Res. 2013;471:721–726. doi: 10.1007/s11999-012-2695-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong M., Chi C., Ye J., Liao M., Xie W., Wu C., et al. Icariin-loaded electrospun PCL/gelatin nanofiber membrane as potential artificial periosteum. Colloids Surf, B. 2018;170:201–209. doi: 10.1016/j.colsurfb.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Bei H.P., Hung P.M., Yeung H.L., Wang S., Zhao X. Bone-a-petite: engineering exosomes towards bone, osteochondral, and cartilage repair. Small. 2021;17 doi: 10.1002/smll.202101741. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y., Xu T., Zhang Q., Piao Y., Bei H.P., Zhao X. Biomimetic, stiff, and adhesive periosteum with osteogenic–angiogenic coupling effect for bone regeneration. Small. 2021;17 doi: 10.1002/smll.202006598. [DOI] [PubMed] [Google Scholar]
- 14.Syed-Picard F.N., Shah G.A., Costello B.J., Sfeir C. Regeneration of periosteum by human bone marrow stromal cell sheets. J Oral Maxillofac Surg. 2014;72:1078–1083. doi: 10.1016/j.joms.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Emans P.J., Caron M.M.J., van Rhijn L.W., Shastri V.P., Welting T.J.M. Cartilage tissue engineering; lessons learned from periosteum. Tissue Sci Eng S. 2011;2 [Google Scholar]
- 16.Guo H., Li X., Yuan X., Ma X. Reconstruction of radial bone defects using the reinforced tissue-engineered periosteum: an experimental study on rabbit weight-bearing segment. J Trauma Acute Aare. 2012;72 doi: 10.1097/ta.0b013e3182196a54. [DOI] [PubMed] [Google Scholar]
- 17.Li N., Song J., Zhu G., Li X., Liu L., Shi X., et al. Periosteum tissue engineering—a review. Biomater Sci. 2016;4:1554–1561. doi: 10.1039/c6bm00481d. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W., Wang N., Yang M., Sun T., Zhang J., Zhao Y., et al. Periosteum and development of the tissue-engineered periosteum for guided bone regeneration. J Orthop Transl. 2022;33:41–54. doi: 10.1016/j.jot.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elucidating Multiscale Periosteal Mechanobiology A Key to Unlocking the smart properties and regenerative capacity of the periosteum? Tissue Eng B. 2013;19(2):147–159. doi: 10.1089/ten.teb.2012.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans S.F., Parent J.B., Lasko C.E., Zhen X., Knothe U.R., Lemaire T., et al. Periosteum, bone's “smart” bounding membrane, exhibits direction-dependent permeability. J Bone Miner Res. 2013;28(3):608–617. doi: 10.1002/jbmr.1777. [DOI] [PubMed] [Google Scholar]
- 21.Tagliamento M., Boutros C., Viansone A., Brau J.-J., Descols P. Denosumab related osteonecrosis of the jaw: unusual pattern with periosteal reaction. Eur J Cancer. 2022;166:33–37. doi: 10.1016/j.ejca.2022.01.026. [DOI] [PubMed] [Google Scholar]
- 22.Venkateswaran V., Mallya S. The biology of periosteal reactions. Oral Surg Oral Med Oral Pathol Oral Radiol. 2021;132(3):e117. [Google Scholar]
- 23.Chen C., Liu F., Tang Y., Qu J., Cao Y., Zheng C., et al. Book-shaped acellular fibrocartilage scaffold with cell-loading capability and chondrogenic inducibility for tissue-engineered fibrocartilage and bone–tendon healing. ACS Appl Mater Interfaces. 2019;11:2891–2907. doi: 10.1021/acsami.8b20563. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman M.D., Xie C., Zhang X., Benoit D.S.W. The effect of mesenchymal stem cells delivered via hydrogel-based tissue engineered periosteum on bone allograft healing. Biomaterials. 2013;34:8887–8898. doi: 10.1016/j.biomaterials.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffman M.D., Benoit D.S.W. Emulating native periosteum cell population and subsequent paracrine factor production to promote tissue engineered periosteum-mediated allograft healing. Biomaterials. 2015;52:426–440. doi: 10.1016/j.biomaterials.2015.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baldwin J.G., Wagner F., Martine L.C., Holzapfel B.M., Theodoropoulos C., Bas O., et al. Periosteum tissue engineering in an orthotopic in vivo platform. Biomaterials. 2017;121:193–204. doi: 10.1016/j.biomaterials.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 27.Zhang M., Matinlinna J.P., Tsoi J.K.H., Liu W., et al. Recent developments in biomaterials for long-bone segmental defect reconstruction: a narrative overview. J Orthop Transl. 2020;22:26–33. doi: 10.1016/j.jot.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chattopadhyay S., Raines R.T. Collagen-based biomaterials for wound healing. Biopolymers. 2014;101(8):821–833. doi: 10.1002/bip.22486. 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hockers T., Abensur D., Valentini P., Legrand R., Hämmerle C.H.F. The combined use of bioresorbable membranes and xenografts or autografts in the treatment of bone defects around implants. A study in beagle dogs. 1999;10(6):487–498. doi: 10.1034/j.1600-0501.1999.100607.x. [DOI] [PubMed] [Google Scholar]
- 30.Guda T., Walker J.A., Singleton B.M., Hernandez J.W., Son J.-S., Kim S.-G., et al. Guided Bone Regeneration in Long-Bone Defects with a structural hydroxyapatite graft and collagen membrane. Tissue Eng. 2012;19(17–18):1879–1888. doi: 10.1089/ten.tea.2012.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ber S., Torun Köse G., Hasırcı V. Bone tissue engineering on patterned collagen films: an in vitro study. Biomaterials. 2005;26(14):1977–1986. doi: 10.1016/j.biomaterials.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Oechsle A.M., Häupler M., Weigel F., Gibis M., Kohlus R., Weiss J. Modulation of extruded collagen films by the addition of co-gelling proteins. J Food Eng. 2016;171:164–173. [Google Scholar]
- 33.Gassling V., Douglas T., Warnke P.H., Açil Y., Wiltfang J., Becker S.T. Platelet-rich fibrin membranes as scaffolds for periosteal tissue engineering. Clin Oral Implants Res. 2010;21(5):543–549. doi: 10.1111/j.1600-0501.2009.01900.x. [DOI] [PubMed] [Google Scholar]
- 34.Almodóvar J., Kipper M.J. Coating electrospun chitosan nanofibers with polyelectrolyte multilayers using the polysaccharides heparin and n,n,n-trimethyl chitosan. Macromol Biosci. 2011;11(1):72–76. doi: 10.1002/mabi.201000261. [DOI] [PubMed] [Google Scholar]
- 35.Bombaldi de Souza R.F., Bombaldi de Souza F.C., Thorpe A., Mantovani D., Popat K.C., Moraes Â.M. Phosphorylation of chitosan to improve osteoinduction of chitosan/xanthan-based scaffolds for periosteal tissue engineering. Int J Biol Macromol. 2020;143:619–632. doi: 10.1016/j.ijbiomac.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Chou Y.-C., Cheng Y.-S., Hsu Y.-H., Yu Y.-H., Liu S.-J. A bio-artificial poly([D,L]-lactide-co-glycolide) drug-eluting nanofibrous periosteum for segmental long bone open fractures with significant periosteal stripping injuries. Int J Nanomed. 2016;11:941–953. doi: 10.2147/IJN.S99791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawase T., Tanaka T., Nishimoto T., Okuda K., Nagata M., Burns D.M., et al. Improved adhesion of human cultured periosteal sheets to a porous poly(L-lactic acid) membrane scaffold without the aid of exogenous adhesion biomolecules. J Biomed Mater Res. 2011;98A(1):100–113. doi: 10.1002/jbm.a.33074. [DOI] [PubMed] [Google Scholar]
- 38.Wang T., Zhai Y., Nuzzo M., Yang X., Yang Y., Zhang X. Layer-by-layer nanofiber-enabled engineering of biomimetic periosteum for bone repair and reconstruction. Biomaterials. 2018;182:279–288. doi: 10.1016/j.biomaterials.2018.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyu S., Huang C., Yang H., Zhang X. Electrospun fibers as a scaffolding platform for bone tissue repair. J Orthop Res. 2013;31(9):1382–1389. doi: 10.1002/jor.22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu W., Shen H., Zhang J.G., Zhang L., Zeng Y., Huang H.L., et al. Cytosolic proteome profiling of monocytes for male osteoporosis. Osteoporos Int. 2017;28(3):1035–1046. doi: 10.1007/s00198-016-3825-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang G., Liu H., Cui Y., Li J., Zhou X., Wang N., et al. Bioinspired membrane provides periosteum-mimetic microenvironment for accelerating vascularized bone regeneration. Biomaterials. 2021;268 doi: 10.1016/j.biomaterials.2020.120561. [DOI] [PubMed] [Google Scholar]
- 42.Zhao F., Zhang C., Liu J., Liu L., Cao X., Chen X., et al. Periosteum structure/function-mimicking bioactive scaffolds with piezoelectric/chem/nano signals for critical-sized bone regeneration. Chem Eng J. 2020;402 [Google Scholar]
- 43.Ardjomandi N., Henrich A., Huth J., Klein C., Schweizer E., Scheideler L., et al. Coating of ß-tricalcium phosphate scaffolds—a comparison between graphene oxide and poly-lactic-co-glycolic acid. Biomed Mater. 2015;10 doi: 10.1088/1748-6041/10/4/045018. [DOI] [PubMed] [Google Scholar]
- 44.Li H., Wang H., Pan J., Li J., Zhang K., Duan W., et al. Nanoscaled bionic periosteum orchestrating the osteogenic microenvironment for sequential bone regeneration. ACS Appl Mater Interfaces. 2020;12:36823–36836. doi: 10.1021/acsami.0c06906. [DOI] [PubMed] [Google Scholar]
- 45.Park J.-W., Park K.-B., Suh J.-Y. Effects of calcium ion incorporation on bone healing of Ti6Al4V alloy implants in rabbit tibiae. Biomaterials. 2007;28(22):3306–3313. doi: 10.1016/j.biomaterials.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 46.Aguirre A., González A., Planell J.A., Engel E. Extracellular calcium modulates in vitro bone marrow-derived Flk-1+ CD34+ progenitor cell chemotaxis and differentiation through a calcium-sensing receptor. Biochem Biophys Res Commun. 2010;393(1):156–161. doi: 10.1016/j.bbrc.2010.01.109. [DOI] [PubMed] [Google Scholar]
- 47.Nie W., Peng C., Zhou X., Chen L., Wang W., Zhang Y., et al. Three-dimensional porous scaffold by self-assembly of reduced graphene oxide and nano-hydroxyapatite composites for bone tissue engineering. Carbon. 2017;116:325–337. [Google Scholar]
- 48.Mena I., Marin O., Fuenzalida S., Cotzias G.C.J.N. Chronic manganese poisoning. Clinical picture and manganese turnover. Neurology. 1967;17(2):128–136. doi: 10.1212/wnl.17.2.128. [DOI] [PubMed] [Google Scholar]
- 49.Kim E.A., Cheong H.-K., Joo K.-D., Shin J.-H., Lee J.S., Choi S.-B., et al. Effect of manganese exposure on the neuroendocrine system in welders. Neurotoxicology. 2007;28(2):263–269. doi: 10.1016/j.neuro.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 50.Sun Y., Liu S., Fu Y., Kou X.-X., He D.-Q., Wang G.-N., et al. Mineralized collagen regulates macrophage polarization during bone regeneration. J Biomed Nanotechnol. 2016;12:2029–2040. doi: 10.1166/jbn.2016.2296. [DOI] [PubMed] [Google Scholar]
- 51.Choukroun J., Diss A., Simonpieri A., Girard M.-O., Schoeffler C., Dohan S.L., et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. part v: histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg Oral Med O. 2006;101:299–303. doi: 10.1016/j.tripleo.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 52.Chun Y.Y., Wang J.K., Tan N.S., Chan P.P.Y., Tan T.T.Y., Choong C. A periosteum-inspired 3d hydrogel-bioceramic composite for enhanced bone regeneration. Macromol Biosci. 2016;16:276–287. doi: 10.1002/mabi.201500258. [DOI] [PubMed] [Google Scholar]
- 53.Kurashina K., Kurita H., Takeuchi H., Hirano M., Klein C.P.A.T., de Groot K. Osteogenesis in muscle with composite graft of hydroxyapatite and autogenous calvarial periosteum: a preliminary report. Biomaterials. 1995;16:119–123. doi: 10.1016/0142-9612(95)98273-h. [DOI] [PubMed] [Google Scholar]
- 56.El Backly R.M., Zaky S.H., Muraglia A., Tonachini L., Brun F., Canciani B., et al. A platelet-rich plasma-based membrane as a periosteal substitute with enhanced osteogenic and angiogenic properties: a new concept for bone repair. Tissue Eng. 2012;19:152–165. doi: 10.1089/ten.TEA.2012.0357. [DOI] [PubMed] [Google Scholar]
- 57.Augustin G., Antabak A., Davila S. RETRACTED: the periosteum: part 1: anatomy, histology and molecular biology. Injury. 2007;38:1115–1130. doi: 10.1016/j.injury.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 58.Uchiyama H., Yamato M., Sasaki R., Sekine H., Yang J., Ogiuchi H., et al. In vivo 3D analysis with micro-computed tomography of rat calvaria bone regeneration using periosteal cell sheets fabricated on temperature-responsive culture dishes. J Tissue Eng Regener Med. 2011;5:483–490. doi: 10.1002/term.340. [DOI] [PubMed] [Google Scholar]
- 59.Shi X., Chen S., Zhao Y., Lai C., Wu H. Enhanced osteogenesis by a biomimic pseudo-periosteum-involved tissue engineering strategy. Adv Healthcare Mater. 2013;2:1229–1235. doi: 10.1002/adhm.201300012. [DOI] [PubMed] [Google Scholar]
- 60.Xing Q., Qian Z., Kannan B., Tahtinen M., Zhao F. Osteogenic differentiation evaluation of an engineered extracellular matrix based tissue sheet for potential periosteum replacement. ACS Appl Mater Interfaces. 2015;7:23239–23247. doi: 10.1021/acsami.5b07386. [DOI] [PubMed] [Google Scholar]
- 61.Sid-Otmane C., Perrault L.P., Ly H.Q. Mesenchymal stem cell mediates cardiac repair through autocrine, paracrine and endocrine axes. J Transl Med. 2020;18:336. doi: 10.1186/s12967-020-02504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang C., Hu K., Liu X., Reynolds M.A., Bao C., Wang P., et al. Novel hiPSC-based tri-culture for pre-vascularization of calcium phosphate scaffold to enhance bone and vessel formation. Mater Sci Eng C. 2017;79:296–304. doi: 10.1016/j.msec.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 63.Mendes L.F., Katagiri H., Tam W.L., Chai Y.C., Geris L., Roberts S.J., et al. Advancing osteochondral tissue engineering: bone morphogenetic protein, transforming growth factor, and fibroblast growth factor signaling drive ordered differentiation of periosteal cells resulting in stable cartilage and bone formation in vivo. Stem Cell Res Ther. 2018;9:42. doi: 10.1186/s13287-018-0787-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murphy M.K., Huey D.J., Hu J.C., Athanasiou K.A. TGF-β1, GDF-5, and BMP-2 stimulation induces chondrogenesis in expanded human articular chondrocytes and marrow-derived stromal cells. Stem Cell. 2015;33:762–773. doi: 10.1002/stem.1890. [DOI] [PubMed] [Google Scholar]
- 65.Gonzalez-Fernandez T., Tierney E.G., Cunniffe G.M., O'Brien F.J., Kelly D.J. Gene delivery of tgf-β3 and bmp2 in an msc-laden alginate hydrogel for articular cartilage and endochondral bone tissue engineering. Tissue Eng. 2016;22:776–787. doi: 10.1089/ten.TEA.2015.0576. [DOI] [PubMed] [Google Scholar]
- 66.Toh W.S., Lee E.H., Guo X.-M., Chan J.K.Y., Yeow C.H., Choo A.B., et al. Cartilage repair using hyaluronan hydrogel-encapsulated human embryonic stem cell-derived chondrogenic cells. Biomaterials. 2010;31:6968–6980. doi: 10.1016/j.biomaterials.2010.05.064. [DOI] [PubMed] [Google Scholar]
- 67.Rasubala L., Yoshikawa H., Nagata K., Iijima T., Ohishi M. Platelet-derived growth factor and bone morphogenetic protein in the healing of mandibular fractures in rats. Br J Oral Maxillofac Surg. 2003;41:173–178. doi: 10.1016/s0266-4356(03)00075-5. [DOI] [PubMed] [Google Scholar]
- 68.Schönmeyr B., Clavin N., Avraham T., Longo V., Mehrara B.J. Synthesis of a tissue-engineered periosteum with acellular dermal matrix and cultured mesenchymal stem cells. Tissue Eng. 2009;15:1833–1841. doi: 10.1089/ten.tea.2008.0446. [DOI] [PubMed] [Google Scholar]
- 69.Mara CS de, Sartori A.R., Duarte A.S., Andrade A.L.L., Pedro M.A.C., Coimbra I.B. Periosteum as a source of mesenchymal stem cells: the effects of TGF-β3 on chondrogenesis. Clinics. 2011;66:487–492. doi: 10.1590/S1807-59322011000300022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pittenger Mark F., Mackay Alastair M., Beck Stephen C., Jaiswal Rama K., Douglas R., Mosca Joseph D., et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 71.Su W.-T., Chiou W.-L., Yu H.-H., Huang T.-Y. Differentiation potential of SHEDs using biomimetic periosteum containing dexamethasone. Mater Sci Eng C. 2016;58:1036–1045. doi: 10.1016/j.msec.2015.09.077. [DOI] [PubMed] [Google Scholar]
- 72.Shi R., Gong M., Chi C., Huang Y., Li W., Li G., et al. Nano twin-fiber membrane with osteogenic and antibacterial dual functions as artificial periosteum for long bone repairing. J Biomed Nanotechnol. 2019;15:272–287. doi: 10.1166/jbn.2019.2687. [DOI] [PubMed] [Google Scholar]
- 73.Burdick J.A., Anseth K.S. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials. 2002;23:4315–4323. doi: 10.1016/s0142-9612(02)00176-x. [DOI] [PubMed] [Google Scholar]
- 74.Reyes M., Lund T., Lenvik T., Aguiar D., Koodie L., Verfaillie C.M. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood. 2001;98:2615–2625. doi: 10.1182/blood.v98.9.2615. [DOI] [PubMed] [Google Scholar]
- 75.Ma D., Yao H., Tian W., Chen F., Liu Y., Mao T., et al. Enhancing bone formation by transplantation of a scaffold-free tissue-engineered periosteum in a rabbit model. Clin Oral Implants Res. 2011;22:1193–1199. doi: 10.1111/j.1600-0501.2010.02091.x. [DOI] [PubMed] [Google Scholar]
- 76.Augello A., Kurth T.B., de Bari C. Mesenchymal stem cells: a perspective from in vitro cultures to in vivo migration and niches. Eur Cell Mater. 2010;20:e33. doi: 10.22203/ecm.v020a11. [DOI] [PubMed] [Google Scholar]
- 77.Kang Y., Ren L., Yang Y. Engineering vascularized bone grafts by integrating a biomimetic periosteum and β-TCP scaffold. ACS Appl Mater Interfaces. 2014;6:9622–9633. doi: 10.1021/am502056q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Doherty L., Yu J., Wang X., Hankenson K.D., Kalajzic I., Sanjay A. A PDGFRβ-PI3K signaling axis mediates periosteal cell activation during fracture healing. PLoS One. 2019;14 doi: 10.1371/journal.pone.0223846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ferretti C., Borsari V., Falconi M., Gigante A., Lazzarini R., Fini M., et al. Human periosteum-derived stem cells for tissue engineering applications: the role of VEGF. Stem Cell Rev Rep. 2012;8:882–890. doi: 10.1007/s12015-012-9374-7. [DOI] [PubMed] [Google Scholar]
- 80.Wu L., Gu Y., Liu L., Tang J., Mao J., Xi K., et al. Hierarchical micro/nanofibrous membranes of sustained releasing VEGF for periosteal regeneration. Biomaterials. 2020;227 doi: 10.1016/j.biomaterials.2019.119555. [DOI] [PubMed] [Google Scholar]
- 81.Stevens M.M., Marini R.P., Martin I., Langer R., Shastri V.P. FGF-2 enhances TGF-β1-induced periosteal chondrogenesis. J Orthop Res. 2004;22:1114–1119. doi: 10.1016/j.orthres.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 82.Du X., Xie Y., Xian C.J., Chen L. Role of FGFs/FGFRs in skeletal development and bone regeneration. J Cell Physiol. 2012;227:3731–3743. doi: 10.1002/jcp.24083. [DOI] [PubMed] [Google Scholar]
- 83.Romero R., Travers J.K., Asbury E., Pennybaker A., Chubb L., Rose R., et al. Combined delivery of FGF-2, TGF-β1, and adipose-derived stem cells from an engineered periosteum to a critical-sized mouse femur defect. J Biomed Mater Res. 2017;105:900–911. doi: 10.1002/jbm.a.35965. [DOI] [PubMed] [Google Scholar]
- 84.Ammann P., Rizzoli R., Muller K., Slosman D., Bonjour J.P. IGF-I and pamidronate increase bone mineral density in ovariectomized adult rats. Am J Physiol-Endoc M. 1993;265:E770–E776. doi: 10.1152/ajpendo.1993.265.5.E770. [DOI] [PubMed] [Google Scholar]
- 85.Srouji S., Blumenfeld I., Rachmiel A., Livne E. Bone defect repair in rat tibia by TGF-β1 and IGF-1 released from hydrogel scaffold. Cell Tissue Bank. 2004;5:223–230. doi: 10.1007/s10561-004-0503-7. [DOI] [PubMed] [Google Scholar]
- 86.Lei L., Wang S., Wu H., Ju W., Peng J., Qahtan A.S.A., et al. Optimization of release pattern of FGF-2 and BMP-2 for osteogenic differentiation of low-population density hMSCs. J Biomed Mater Res. 2015;103:252–261. doi: 10.1002/jbm.a.35168. [DOI] [PubMed] [Google Scholar]
- 87.Birek C., Pawson T., McCulloch C.A.G., Tenenbaum H.C. Dexamethasone Effects on induction of neoplastic transformation by fujinami sarcoma virus in an in vitro chick embryo periosteal model for osteosarcoma1. Cancer Res. 1988;48:7231–7236. [PubMed] [Google Scholar]
- 88.Tenenbaum H.C., Heersche J.N.M. Dexamethasone stimulates osteogenesis in chick periosteum in vitro. Endocrinology. 1985;117:2211–2217. doi: 10.1210/endo-117-5-2211. [DOI] [PubMed] [Google Scholar]
- 89.Tenenbaum H.C., Limeback H., McCulloch C.A.G., Mamujee H., Sukhu B., Torontali M. Osteogenic phase-specific co-regulation of collagen synthesis and mineralization by β-glycerophosphate in chick periosteal cultures. Bone. 1992;13:129–138. doi: 10.1016/8756-3282(92)90002-e. [DOI] [PubMed] [Google Scholar]
- 90.Kudva A.K., Luyten F.P., Patterson J. RGD-functionalized polyethylene glycol hydrogels support proliferation and in vitro chondrogenesis of human periosteum-derived cells. J Biomed Mater Res. 2018;106:33–42. doi: 10.1002/jbm.a.36208. [DOI] [PubMed] [Google Scholar]
- 91.Li Hong-ming1 Hu Xiao-xiong2 G.Y. Icariin promotes the proliferation of human periosteum cells and the underlying mechanism. Chinese J Tissue Eng Res. 2018;22:505–509. [Google Scholar]
- 92.Alblowi J., Kayal R.A., Siqueria M., McKenzie E., Krothapalli N., McLean J., et al. High levels of tumor necrosis factor-α contribute to accelerated loss of cartilage in diabetic fracture healing. Am J Pathol. 2009;175(4):1574–1585. doi: 10.2353/ajpath.2009.090148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen T.-H., Weber F.E., Malina-Altzinger J., Ghayor C. Epigenetic drugs as new therapy for tumor necrosis factor-α-compromised bone healing. Bone. 2019;127:49–58. doi: 10.1016/j.bone.2019.05.035. [DOI] [PubMed] [Google Scholar]
- 94.Dinarello C.A. The interleukin-1 family: 10 years of discovery1. Faseb J. 1994;8(15):1314–1325. [PubMed] [Google Scholar]
- 95.Sun K., Wang C., Xiao J., Brodt M.D., Yuan L., Yang T., et al. Fracture healing is delayed in the absence of gasdermin-interleukin-1 signaling. Elife. 2022;11 doi: 10.7554/eLife.75753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hwa S.Y., Burkhardt D., Little C., Ghosh P. The effects of orally administered diacerein on cartilage and subchondral bone in an ovine model of osteoarthritis. J Rheumatol. 2001;28(4):825–834. [PubMed] [Google Scholar]
- 97.Moss M., Kruger G.O., Reynolds D.C. The effect of chondroitin sulfate on bone healing. Oral Surg Oral Med Oral Pathol. 1965;20(6):795–801. doi: 10.1016/0030-4220(65)90143-x. [DOI] [PubMed] [Google Scholar]
- 98.Arm D.M., Tencer A.F., Bain S.D., Celino D. Effect of controlled release of platelet-derived growth factor from a porous hydroxyapatite implant on bone ingrowth. Biomaterials. 1996;17(7):703–709. doi: 10.1016/0142-9612(96)86740-8. [DOI] [PubMed] [Google Scholar]
- 99.Pierce G.F., Mustoe T.A., Senior R.M., Reed J., Griffin G.L., Thomason A., et al. In vivo incisional wound healing augmented by platelet-derived growth factor and recombinant c-sis gene homodimeric proteins. J Exp Med. 1988;167(3):974–987. doi: 10.1084/jem.167.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bonewald L., Mundy G.J. C.o., research r. Role of transforming growth factor-beta in bone remodeling. Clin Orthop Relat Res. 1990;(250):261–276. [PubMed] [Google Scholar]
- 101.Stevenson S., Emery S.E., Goldberg V.M. Factors Affecting bone graft incorporation. Clinical Clin Orthop Relat Res. 1996;324 doi: 10.1097/00003086-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 102.Zhu M., Ye H., Fang J., Zhong C., Yao J., Park J., et al. Engineering high-resolution micropatterns directly onto titanium with optimized contact guidance to promote osteogenic differentiation and bone regeneration. ACS Appl Mater Interfaces. 2019;11:43888–43901. doi: 10.1021/acsami.9b16050. [DOI] [PubMed] [Google Scholar]
- 103.Weston D.A. Investigating the specificity of periosteal reactions in pathology museum specimens. Am J Phys Anthropol. 2008;137(1):48–59. doi: 10.1002/ajpa.20839. [DOI] [PubMed] [Google Scholar]
- 104.Ragsdale B., Madewell J., Sweet D.J. Radiologic and pathologic analysis of solitary bone lesions. Part II: periosteal reactions. Radiol Clin. 1981;19(4):749–783. R. C. o. N. A. [PubMed] [Google Scholar]
- 105.Chen E.M., Masih S., Chow K., Matcuk G., Patel D. Periosteal reaction: review of various patterns associated with specific pathology. Contemp Diagn Radiol. 2012;35(17) [Google Scholar]
- 106.Shi X., Fujie T., Saito A., Takeoka S., Hou Y., Shu Y., et al. Periosteum-mimetic structures made from freestanding microgrooved nanosheets. Adv Mater. 2014;26:3290–3296. doi: 10.1002/adma.201305804. [DOI] [PubMed] [Google Scholar]
- 107.Yang Y., Xu T., Bei H.P., Zhao Y., Zhao X. Sculpting bio-inspired surface textures: an adhesive janus periosteum. Adv Funct Mater. 2021;31 [Google Scholar]
- 108.Shi X., Li L., Ostrovidov S., Shu Y., Khademhosseini A., Wu H. Stretchable and micropatterned membrane for osteogenic differentation of stem cells. ACS Appl Mater Interfaces. 2014;6:11915–11923. doi: 10.1021/am5029236. [DOI] [PubMed] [Google Scholar]
- 109.Yiannakopoulos C.K., Kanellopoulos A.D., Trovas G.P., Dontas I.A., Lyritis G.P. The biomechanical capacity of the periosteum in intact long bones. Arch Orthop Traum Su. 2008;128:117–120. doi: 10.1007/s00402-007-0433-5. [DOI] [PubMed] [Google Scholar]
- 110.Matsumoto T., Nakayama K., Kodama Y., Fuse H., Nakamura T., Fukumoto S. Effect of mechanical unloading and reloading on periosteal bone formation and gene expression in tail-suspended rapidly growing rats. Bone. 1998;22:89S–93S. doi: 10.1016/s8756-3282(98)00018-0. [DOI] [PubMed] [Google Scholar]
- 112.Turner C.H., Forwood M.R., Otter M.W. Mechanotransduction in bone: do bone cells act as sensors of fluid flow? Faseb J. 1994;8:875–878. doi: 10.1096/fasebj.8.11.8070637. [DOI] [PubMed] [Google Scholar]
- 113.Klein-Nulend J., Burger E.H., Semeins C.M., Raisz L.G., Pilbeam C.C. Pulsating fluid flow stimulates prostaglandin release and inducible prostaglandin g/h synthase mrna expression in primary mouse bone cells. J Bone Miner Res. 1997;12:45–51. doi: 10.1359/jbmr.1997.12.1.45. [DOI] [PubMed] [Google Scholar]
- 114.Kleinnulend J., Semeins C.M., Ajubi N.E., Nijweide P.J., Burger E.H. Pulsating fluid flow increases nitric oxide (no) synthesis by osteocytes but not periosteal fibroblasts - correlation with prostaglandin upregulation. Biochem Biophys Res Commun. 1995;217:640–648. doi: 10.1006/bbrc.1995.2822. [DOI] [PubMed] [Google Scholar]
- 115.Haddad J.B., Obolensky A.G., Shinnick P. The biologic effects and the therapeutic mechanism of action of electric and electromagnetic field stimulation on bone and cartilage: new findings and a review of earlier work. J Alternative Compl Med. 2007;13:485–490. doi: 10.1089/acm.2007.5270. [DOI] [PubMed] [Google Scholar]
- 116.Gittens R.A., Olivares-Navarrete R., Tannenbaum R., Boyan B.D., Schwartz Z. Electrical implications of corrosion for osseointegration of titanium implants. J Dent Res. 2011;90:1389–1397. doi: 10.1177/0022034511408428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rajabi A.H., Jaffe M., Arinzeh T.L. Piezoelectric materials for tissue regeneration: a review. Acta Biomater. 2015;24:12–23. doi: 10.1016/j.actbio.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 118.Zhang C., Liu W., Cao C., Zhang F., Tang Q., Ma S., et al. Modulating surface potential by controlling the β phase content in poly(vinylidene fluoridetrifluoroethylene) membranes enhances bone regeneration. Adv Healthcare Mater. 2018;7 doi: 10.1002/adhm.201701466. [DOI] [PubMed] [Google Scholar]
- 119.Grabowski G., Cornett C.A. Bone graft and bone graft substitutes in spine surgery: current concepts and controversies. J Am Acad Orthop Sur. 2013;21(1) doi: 10.5435/JAAOS-21-01-51. [DOI] [PubMed] [Google Scholar]
- 120.Xu, T., Yang, Y., Suo, D., Bei, H. P., Xu, X., Zhao, X., Electrosprayed regeneration-enhancer-element microspheres power osteogenesis and angiogenesis coupling. Small 2200314. [DOI] [PubMed]
- 121.Von Rechenberg B. Animal models in bone repair. Drug Discov Today Dis Model. 2014;13:23–27. [Google Scholar]
- 122.Li Y., Chen S.-K., Li L., Qin L., Wang X.-L., Lai Y.-X. Bone defect animal models for testing efficacy of bone substitute biomaterials. J Orthop Translat. 2015;3(3):95–104. doi: 10.1016/j.jot.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xin T., Mao J., Liu L., Tang J., Wu L., Yu X., et al. Programmed sustained release of recombinant human bone morphogenetic protein-2 and inorganic ion composite hydrogel as artificial periosteum. ACS Appl Mater Interfaces. 2020;12(6):6840–6851. doi: 10.1021/acsami.9b18496. [DOI] [PubMed] [Google Scholar]
- 124.Wang W., Yeung K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: a review. Bioact Mater. 2017;2(4):224–247. doi: 10.1016/j.bioactmat.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang M., Park S., Nam Y., Nielsen J., Low S.A., Srinivasarao M., et al. Bone-fracture-targeted dasatinib-oligoaspartic acid conjugate potently accelerates fracture repair. Bioconjugate Chem. 2018;29(11):3800–3809. doi: 10.1021/acs.bioconjchem.8b00660. [DOI] [PubMed] [Google Scholar]
- 126.Wakitani S., Yamamoto T. Response of the donor and recipient cells in mesenchymal cell transplantation to cartilage defect. Microsc Res Tech. 2002;58(1):14–18. doi: 10.1002/jemt.10111. [DOI] [PubMed] [Google Scholar]
- 127.Lee M.S., Lee D.H., Jeon J., Oh S.H., Yang H.S. Topographically defined, biodegradable nanopatterned patches to regulate cell fate and acceleration of bone regeneration. ACS Appl Mater Interfaces. 2018;10(45):38780–38790. doi: 10.1021/acsami.8b14745. [DOI] [PubMed] [Google Scholar]
- 128.McLaren J.S., Macri-Pellizzeri L., Hossain K.M.Z., Patel U., Grant D.M., Scammell B.E., et al. Porous phosphate-based glass microspheres show biocompatibility, tissue infiltration, and osteogenic onset in an ovine bone defect model. ACS Appl Mater Interfaces. 2019;11(17):15436–15446. doi: 10.1021/acsami.9b04603. [DOI] [PubMed] [Google Scholar]
- 129.Gupta S., Teotia A.K., Qayoom I., Shiekh P.A., Andrabi S.M., Kumar A. Periosteum-mimicking tissue-engineered composite for treating periosteum damage in critical-sized bone defects. Biomacromolecules. 2021;22(8):3237–3250. doi: 10.1021/acs.biomac.1c00319. [DOI] [PubMed] [Google Scholar]
- 130.Brey E.M., Cheng M.-H., Allori A., Satterfield W., Chang D.W., Patrick C., et al. Comparison of guided bone formation from periosteum and muscle fascia. Plast Reconstr Surg. 2007;119(4):1216–1222. doi: 10.1097/01.prs.0000254361.74614.bb. [DOI] [PubMed] [Google Scholar]
- 131.Raina D.B., Glencross A., Chaher N., Liu Y., Lidgren L., Isaksson H., et al. Synthesis and characterization of a biocomposite bone bandage for controlled delivery of bone-active drugs in fracture nonunions. ACS Biomater Sci Eng. 2020;6(5):2867–2878. doi: 10.1021/acsbiomaterials.9b01574. [DOI] [PubMed] [Google Scholar]
- 132.Polisel E.E.C., Beck W.R., Scariot P.P.M., Pejon T.M.M., Gobatto C.A., Manchado-Gobatto F.B. Effects of high-intensity interval training in more or less active mice on biomechanical, biophysical and biochemical bone parameters. Sci Rep. 2021;11(1):6414. doi: 10.1038/s41598-021-85585-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lu C., Miclau T., Hu D., Hansen E., Tsui K., Puttlitz C., et al. Cellular basis for age-related changes in fracture repair. J Orthop Res. 2005;23(6):1300–1307. doi: 10.1016/j.orthres.2005.04.003.1100230610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lu J.Y., Zhang X.X., Zhu Q.Y., Zhang F.R., Huang W.T., Ding X.Z., et al. Highly tunable and scalable fabrication of 3d flexible graphene micropatterns for directing cell alignment. ACS Appl Mater Interfaces. 2018;10:17704–17713. doi: 10.1021/acsami.8b04416. [DOI] [PubMed] [Google Scholar]
- 135.Metwally S., Stachewicz U. Surface potential and charges impact on cell responses on biomaterials interfaces for medical applications. Mater Sci Eng C. 2019;104 doi: 10.1016/j.msec.2019.109883. [DOI] [PubMed] [Google Scholar]
- 136.Dimitriou R., Tsiridis E., Giannoudis P.V. Current concepts of molecular aspects of bone healing. Injury. 2005;36(12):1392–1404. doi: 10.1016/j.injury.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 137.Kon T., Cho T.-J., Aizawa T., Yamazaki M., Nooh N., Graves D., et al. Expression of osteoprotegerin, receptor activator of nf-κb ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J Bone Miner Res. 2001;16(6):1004–1014. doi: 10.1359/jbmr.2001.16.6.1004. [DOI] [PubMed] [Google Scholar]
- 138.Gong M., Huang C., Huang Y., Li G., Chi C., Ye J., et al. Core-sheath micro/nano fiber membrane with antibacterial and osteogenic dual functions as biomimetic artificial periosteum for bone regeneration applications. Nanomed-Nanotechnol. 2019;17:124–136. doi: 10.1016/j.nano.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 139.Yin J., Qiu S., Shi B., Xu X., Zhao Y., Gao J., et al. Controlled release of FGF-2 and BMP-2 in tissue engineered periosteum promotes bone repair in rats. Biomed Mater. 2018;13 doi: 10.1088/1748-605X/aa93c0. [DOI] [PubMed] [Google Scholar]
- 140.Yilgor P., Hasirci N., Hasirci V. Sequential BMP-2/BMP-7 delivery from polyester nanocapsules. J Biomed Mater Res. 2010;93A:528–536. doi: 10.1002/jbm.a.32520. [DOI] [PubMed] [Google Scholar]
- 141.Jo J.Y., Jeong S.I., Shin Y.M., Kang S.S., Kim S.E., Jeong C.M., et al. Sequential delivery of BMP-2 and BMP-7 for bone regeneration using a heparinized collagen membrane. Int J Oral Maxillofac Surg. 2015;44:921–928. doi: 10.1016/j.ijom.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 142.Elgali I., Omar O., Dahlin C., Thomsen P. Guided bone regeneration: materials and biological mechanisms revisited. Eur J Oral Sci. 2017;125:315–337. doi: 10.1111/eos.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.D’Elía N.L., Rial Silva R., Sartuqui J., Ercoli D., Ruso J., Messina P., et al. Development and characterisation of bilayered periosteum-inspired composite membranes based on sodium alginate-hydroxyapatite nanoparticles. J Colloid Interface Sci. 2020;572:408–420. doi: 10.1016/j.jcis.2020.03.086. [DOI] [PubMed] [Google Scholar]
- 144.Hill E.L., Elde R. Distribution of CGRP-, VIP-, DβH-, SP-, and NPY-immunoreactive nerves in the periosteum of the rat. Cell Tissue Res. 1991;264(3):469–480. doi: 10.1007/BF00319037. [DOI] [PubMed] [Google Scholar]
- 145.Hohmann Elizabeth L., Elde Robert P., Rysavy Joseph A., Einzig S., Gebhard Roger L. Innervation of periosteum and bone by sympathetic vasoactive intestinal peptide-containing nerve fibers. Science. 1986;232:868–871. doi: 10.1126/science.3518059. [DOI] [PubMed] [Google Scholar]
- 146.Zhang Y., Xu J., Ruan Y.C., Yu M.K., O'Laughlin M., Wise H., et al. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat Med. 2016;22(10):1160–1169. doi: 10.1038/nm.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ye L., Xu J., Mi J., He X., Pan Q., Zheng L., et al. Biodegradable magnesium combined with distraction osteogenesis synergistically stimulates bone tissue regeneration via CGRP-FAK-VEGF signaling axis. Biomaterials. 2021;275 doi: 10.1016/j.biomaterials.2021.120984. [DOI] [PubMed] [Google Scholar]
- 148.Wang J., Xu J., Wang X., Sheng L., Zheng L., Song B., et al. Magnesium-pretreated periosteum for promoting bone-tendon healing after anterior cruciate ligament reconstruction. Biomaterials. 2021;268 doi: 10.1016/j.biomaterials.2020.120576. [DOI] [PubMed] [Google Scholar]
- 149.Il L.K., Ramya G., Merissa O., Yasunari I., Joe H., Jihye B., et al. Mohawk is a transcription factor that promotes meniscus cell phenotype and tissue repair and reduces osteoarthritis severity. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.aan7967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Debnath S., Yallowitz A.R., McCormick J., Lalani S., Zhang T., Xu R., et al. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature. 2018;562:133–139. doi: 10.1038/s41586-018-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Duchamp de Lageneste O., Julien A., Abou-Khalil R., Frangi G., Carvalho C., Cagnard N., et al. Periosteum contains skeletal stem cells with high bone regenerative potential controlled by Periostin. Nat Commun. 2018;9:773. doi: 10.1038/s41467-018-03124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tang D., Tare R.S., Yang L.-Y., Williams D.F., Ou K.-L., Oreffo R.O.C. Biofabrication of bone tissue: approaches, challenges and translation for bone regeneration. Biomaterials. 2016;83:363–382. doi: 10.1016/j.biomaterials.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 153.Zhang G., Li Q., Yuan Q., Zhang S. Spatial distributions, characteristics, and applications of craniofacial stem cells. Stem Cell Int. 2020;2020 doi: 10.1155/2020/8868593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kurenkova A.D., Medvedeva E v, Newton P.T., Chagin A.S. Niches for skeletal stem cells of mesenchymal origin. Front Cell Dev Biol. 2020;8 doi: 10.3389/fcell.2020.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Serowoky M.A., Arata C.E., Crump J.G., Mariani F v. Skeletal stem cells: insights into maintaining and regenerating the skeleton. Development. 2020;147:dev179325. doi: 10.1242/dev.179325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yang C., Ma H., Wang Z., Younis M.R., Liu C., Wu C., et al. 3D printed wesselsite nanosheets functionalized scaffold facilitates NIR-II photothermal therapy and vascularized bone regeneration. Adv Sci. 2021;8 doi: 10.1002/advs.202100894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Skoog T. The use of periosteum and surgicel® for bone restoration in congenital clefts of the maxilla: a clinical report and experimental investigation. Scand J Surg. 1967;1(2):113–130. doi: 10.3109/02844316709022841. [DOI] [PubMed] [Google Scholar]
- 158.Romana M.C., Masquelet A.C. Vascularized periosteum associated with cancellous bone graft: an experimental study. Plast Reconstr Surg. 1990;85(4):587–592. doi: 10.1097/00006534-199004000-00014. [DOI] [PubMed] [Google Scholar]
- 159.Khan S.N., Tomin E., Lane J.M. Clinical applications of bone graft substitutes. Orthop Clin N Am. 2000;31(3):389–398. doi: 10.1016/s0030-5898(05)70158-9. [DOI] [PubMed] [Google Scholar]