Abstract
Liver lesions located adjacent to the middle hepatic vein (MHV) at the hepatocaval confluence are rare. Mini-mesohepatectomy (MMH) allows resection of these lesions with preservation of liver parenchymal volume thus reducing the risk of post-hepatectomy liver failure (PHLF). We evaluated our experience of MMH at our institution and assessed post-operative complications, disease free survival (DFS) and overall survival (OS). All patients undergoing MMH at our institution were included in the study. Intra-operative parameters, histopathological data, DFS and OS were evaluated. 11 patients with colorectal liver metastasis underwent MMH between Jan 2012 and Dec 2020. MMH resulted in R0 resection rate in all patients with no PHLF. There were 1 post-operative bile leaks but no mortality following MMH. Median DFS was 13.5 months with OS being 60 months. MMH offers safe oncological resection of lesions at the MHV at the hepatocaval confluence and should be considered in patients presenting with such lesions.
Abbreviations: EH, extended hepatectomy; IOUS, entra-operative ultrasound; MMH, mini-mesohepatectomy; MHV, middle hepatic vein; PHLP, post-hepatectomy liver failure; FLR, future liver remnant
Keywords: Colorectal liver metastasis, Liver resection, Middle hepatic vein, Mini-mesohepatectomy, Post-hepatectomy liver failure
Highlights
-
•
Mini-mesohepatectomy is a safe and oncological radical surgical approach for tumours at the hepatocaval confluence.
-
•
Utilising mini-mesohepatectomy as a parenchymal preserving liver resection technique leads to good long-term patient outcomes.
-
•
Mini-mesohepatectomy maybe adapted to other lesions/tumours at the original of the right and left hepatic veins.
1. Introduction
Liver lesions and/or tumours in contact with or invading the middle hepatic vein (MHV) at the hepatocaval confluence are rare and a number of surgical techniques can be employed to resect them [1], [2], [3], [4]. When planning surgery for any liver tumour attaining complete oncological resection (R0) and ensuring adequate future liver remnant (FLR) volume are the primary goals of surgery. Fig. 1a(i & ii) demonstrates a liver lesion that is located at the MHV at the hepatocaval confluence with patent right and left hepatic veins and no involvement of the hepatic hilum. Three surgical approaches can be used to resect such tumours; extended right or left liver hepatectomy, central liver resection (referred to hereafter as mesohepatectomy (MH) or mini-mesohepatectomy (MMH). Traditionally, tumours at the hepatocaval confluence would have been considered for extended right hepatectomy/right trisectionectomy [2], [3], [4]. Whilst these types of extended hepatectomy (EH) can increase R0 rates for such lesions the risk of post-hepatectomy liver failure (PHLF) is reported as 8 % with the associated risk of mortality [5]. However parenchymal preserving liver resection techniques such as partial hepatectomy/non-anatomical liver resection can be utilised to resect such lesion with the potential advantage of reducing the risk of PHLF whilst still attaining oncological resection. A recent meta-analysis comparing surgical outcomes between EH and parenchymal preserving liver surgery found that the latter approach was associated with lower operative time, less blood loss and less post-operative complications [6]. An alternative surgical technique to EH for such lesions is MH which involves the resection of parts of segments 4, 5 and 8 with concomitant resection of the MHV at the hepatocaval confluence and separation of the left and right liver aspects as first described by Hasegawa et al. [7]. A meta-analysis by Chan et al. demonstrated that mortality rates for MH ranged from 0 to 9 % with the most common cause of death being PHLF with morbidity ranging between 12 and 61 %, although these were still significantly lower than the complications observed after EH [8]. MMH is a variation of MH were the lesion at the hepatocaval confluence can be resected without dissection of the hepatic hilum and without complete separation of the liver, as described by Torzilli et al. [1]. MMH is a non-anatomical liver resectional technique that specifically involves partial resection of segment 4a and 8 with concomitant resection of the MHV (Fig. 2). MMH is associated with significantly lower frequencies of post-operative morbidity and mortality when compared to MH and EH [9] whilst also maximizing FLR and allowing oncological liver resection. In addition MMH can be used to achieve oncological resection for tumours at the hepatocaval confluence arising from but not limited to colorectal liver metastasis, hepatocellular carcinoma and metastases from ovarian and renal cell cancers.
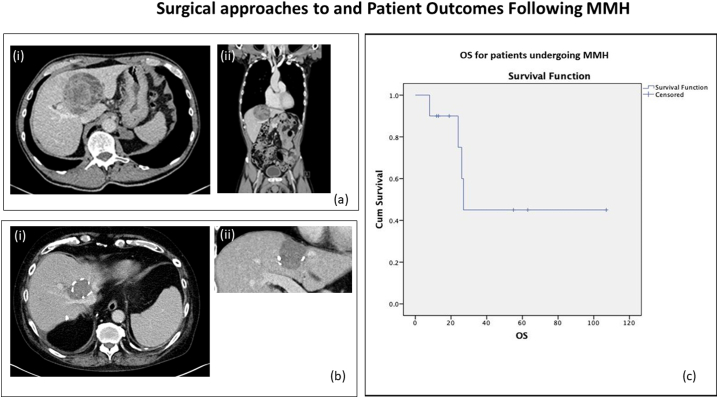
Fig. 1.
(a) Typical liver lesion/tumours amenable to MMH. The CT demonstrates a typical lesion that would be considered for MMH. The mass is within 4 cm of the MHV origin with no invasion of the RHV and LHV (ai). As demonstrated in (aii) there is no compromise to the hepatic inflow. (b) Post-operative cross-sectional imaging following MMH. The CT images demonstrate the post-operative appearances in a patient following MMH. The image on the left shows resection of the MHV at the IVC with preservation of the RHV and LHV, a preserved V7 branch to the RHV can be seen (bi). The transection plane is keep superior to the liver hilum (top right) and anterior to the RHV (bottom left) (bii). (c) OS for patients undergoing MMH.
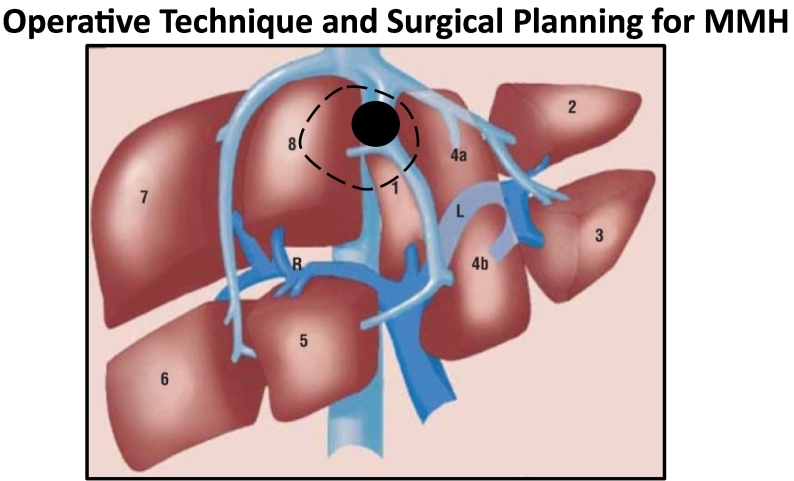
Fig. 2.
Demonstrates the surgical technique for MMH. Following mobilization of the liver by division of the right and left triangular ligaments the precise relationship between the tumour/lesion and hepatic veins is confirmed using intra-operative ultrasound. Principally it is ensured that the right and left hepatic veins and liver hilum are clear of the tumour/lesion. The transection line (dashed line) is then used to resect the lesion/tumour with partial resection of segment 4a and 8 and concomitant resection of the MHV at its origin hence obtaining oncologically clear margins with a non-anatomical rescetional approach.
2. Methods
Reviewing our departmental database between January 2012 and December 2020 inclusive demonstrated that 11 patients had undergone MMH. Our case series is reported in accordance with the PROCESS 2020 guidelines [10] and is registered study at our institution (GI_2223_001). Candidates for the MMH were patients with tumours with indefinite margins, or direct evidence of invasion of the MHV at the hepatocaval confluence, which was defined as the tract of the vein within 4 cm from the IVC (Fig. 2). The terminology for liver anatomy and resections used are as per Brisbane classification [11]. MMH was defined as the partial removal of segments 8 and 4a including the involved tract of MHV [1]. Pre-operative staging was with a chest, abdominal and pelvic computed tomography (CT) scan. All patients were discussed within a specialist hepatobiliary multidisciplinary team meeting prior to surgery. Magnetic Resonance Imaging (MRI) and CT-positron emission tomography (PET) were performed selectively in patients were indicated.
Surgery was carried out with a Makuuchi incision. After a partial mobilization of the liver, intraoperative ultrasound (IOUS) was performed to delineate the precise relationship between the tumour/lesion and MHV, V4/left superficial vein (LSV) and V8 as well as to assess for other liver lesions. In all patients IOUS with colour flow doppler was used to map the precise relationship between the tumour, right hepatic vein (RHV) and V8 and also the left hepatic vein (LHV) and LSV/V4 as to allow safe ligation of V8 and LSV/V4 during liver transection. Following this, the hepatocaval confluence was exposed, and the space between the RHV and MHV at the confluence into the IVC was dissected free. The LHV was then defined after the common truck of MHV and LHV was visualized. Following this, full mobilization of the right and left hemiliver was performed by dividing the triangular and coronary ligaments with preservation of all the short retrohepatic veins. The area of resection was marked on the liver surface under IOUS guidance, as described by Torzilli et al. [1]. Pringle was used at the operating surgeon's discretion and CUSA was used for hepatic transection in all cases. LSV/V4 and V8 were dissected and ligated under direct vision using clips. Abdominal drainage was achieved with Robinson drain. Fig. 1b(i & ii) demonstrates post-operative CT images of a patient following MMH. After surgery patients underwent clinical assessment and surveillance CT scans at regular intervals.
3. Results
Patients had a median age of 60 years (range 46–72 years). Table 1 demonstrates the demographics of the study cohort. In total 11 patients underwent MMH during the study period.
Table 1.
Demographics of patients undergoing MMH.
| Age: median (range) | 60 years (46–72 years) |
| Gender (M:F) | 4:7 |
| Performance status | |
| 0 | 9 |
| 1 | 2 |
| Type of tumour/lesion | |
| Synchronous CRLM | 1 |
| Rectal | 1 |
| Metachronous CRLM | 7 |
| Rectal | 3 |
| Sigmoid | 1 |
| Right colon | 2 |
| Hepatocellular carcinoma | 2 |
| Benign liver lesion | 1 |
| Number of lesions on pre-operative Imaging | |
| 1 | 8 |
| 2 | 2 |
| ≥3 | 1 |
| Tumour diameter: mean (range) | 43 mm (22-75 mm) |
| R0 (%) | 100 |
Of the 11 patients who underwent MMH, 8 patients had colorectal liver metastasis/es (CRLM). 1 patient with CRLM had a synchronous rectal tumour and received neoadjuvant FOLFIRI and cetuximab. 7 patients had metachronous CRLM. In these 7 patients the primary colorectal tumour was located in rectal (n = 4), 1 sigmoid (n = 1), right colon (n = 2). Of the 7 patients with metachronous disease 1 had not received adjuvant chemotherapy, 4 patients with rectal tumour had received FOLFIRI (n = 3) and FOLFIRI and cetuximab (n = 1), the patient with sigmoid primary tumour received FOLFOX and cetuximab and the 2 patients with right colon tumour received adjuvant CAPOX chemotherapy. Two patients had tumours that were radiologically consistent with primary liver tumours and 1 patient had a presumed colorectal liver metastasis that was a benign lesion on post-operative histology and has been excluded from the formal analysis below.
For MMH, following laparotomy and the exclusion of extrahepatic disease, intra-operative ultrasound (IOUS) was used to clarify and define the precise relationship between the tumour and MHV. In patients with additional metastasis IOUS was used to identify these lesions. Pringle manoeuvre was used in 4 patients and mean blood loss for the whole cohort being 827 mls (range 200-2000mls). Seven patients had a solitary liver lesion and 3 patients had multiple lesions (2 patients had 2 lesions and 1 patient had 4 lesions).
There was no incidence of PHLF and no 30-day or 90-day mortality in our series although there was 1 post-operative bile leak that was managed conservatively. R0 resection was achieved in all 10 patients (100 %) with a mean tumour diameter 43 mm (range 22-75 mm) noted on histopathology.
Median patient follow-up was 25 months (range 8–107 months). 1-year patient survival was 90 %. The median Disease-Free Survival (DFS) was 13.5 months (range 5–55 months). Six patients had not developed any recurrent disease by the time of their last follow-up. One patient developed liver only recurrence and underwent repeat hepatectomy. Three patients developed liver and lung metastases. Of these, 1 patient underwent radiofrequency ablation to the liver metastasis followed by FOLFOX and bevacizumab chemotherapy and 2 patients were treated with FOLFIRI. Local recurrence rate was 0 %, i.e., no patient developed recurrence at the resection margin. Overall Survival (OS) was 60 %, i.e., 6 patients were alive at the time of last follow-up (Fig. 1c). Four patients died (40 %) at a median time of 25 months (range 8–27 months).
4. Discussion
The use of MMH for tumours at the hepatocaval confluence merges the oncologic principles of cancer surgery with the concept of parenchymal preservation, in pursuit of the largest possible FLR volume [9], [12]. As reported by Torzilli et al., approaches such as MMH are associated with low mortality (1.8 %) and low morbidity (8 %) [1], [9]. Similarly, we reported no mortality in our series and no major morbidity. Moreover, utilising MMH as a parenchymal sparing approach/non-anatomical liver resectional technique, we report a R0 resection rate of 100 %, in spite of a relatively large tumour size (mean tumour diameter 43 mm). Previous studies have suggested that major hepatectomy, sometimes in the form of EH, may improve DFS over parenchyma-sparing surgical techniques such as MMH [13], [14]. Indeed a 10 % parenchymal-sparing surgery failure rate has been reported but it must be noted that this is similar to that reported with standard anatomical hepatectomy [15]. Importantly, parenchymal-sparing surgery has the significant advantage of allowing patients to undergo repeat hepatectomy in instances of recurrence as demonstrated by our series [15]. In addition, where oncologically feasible the parenchyma sparing policy has allowed surgery to be performed even in the setting of multiple bilobar colorectal liver metastases [9]. Indeed, 3 patients in our series underwent multiple non-anatomical liver resections in combination with MMH ensuring radical resection with reduced risks of PHLF. This approach renders otherwise potentially unfeasible surgical approaches to liver tumours feasible in a single procedure and can potentially be an option in 86 % patients with bilobar liver disease [9]. When MMH was initially described there was concern that the preservation of part of the right anterior section and of the left median section without the MHV represents a risk for venous congestion that might be a source of morbidity [1]. However, we have observed no evidence of this phenomenon in our patients and the only morbidity noted in our series was a post-operative bile leak that was successfully managed without intervention. In conclusion, MMH for resection of lesions in segment 8 and/or segment 4a that are in contact with or infiltrating the MHV is a feasible, safe and effective oncological operation, and we believe it should be part of the armamentarium of the liver surgeon. Application of MMH is versatile; it can be used in combination with other liver resections and offers excellent oncological resection with acceptable post-operative morbidity. The patient outcomes of MMH versus EH for tumours at the hepatocaval confluence can only be answered be conducted randomised controlled study and given the rarity of such of tumours this would be challenging to achieve.
Sources of funding
No sources of funding to declare.
Ethical approval
Ethical approval given by local ethics committee.
Approval of the research protocol
The Royal Marsden NHS Foundation Trust.
Informed consent
All patients had provided informed consent prior to surgery and no patient identifiable data has been used in the study.
Registry and registration No. of the study/trial
GI_2223_001.
Animal studies
Not applicable.
Guarantor
RH.B
CRediT authorship contribution statement
Conceptualization: RHB.
Data curation: PHP, JD, RHB.
Formal analysis: PHP, VKM, RHB.
Methodology: RHB.
Supervision: RHB.
Writing: VKM, SK, RHB.
Declaration of competing interest
The authors have no conflicts of interests to declare.
Acknowledgments
The work represents independent research supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at The Royal Marsden NHS Foundation Trust and the Institute for Cancer Research, London.
References
- 1.Torzilli G., Palmisano A., Procopio F., Cimino M., Botea F., Donadon M., et al. A new systematic small for size resection for liver tumors invading the middle hepatic vein at its caval confluence: mini-mesohepatectomy. Ann. Surg. 2010;251:33–39. doi: 10.1097/SLA.0b013e3181b61db9. PMID: 19858707. [DOI] [PubMed] [Google Scholar]
- 2.Wu C.C., Ho W.L., Chen J.T., Tang C.S., Yeh D.C., Liu T.J., et al. Mesohepatectomy for centrally located hepatocellular carcinoma: an appraisal of a rare procedure. J. Am. Coll. Surg. 1999;188:508–515. doi: 10.1016/s1072-7515(99)00026-5. PMID: 10235579. [DOI] [PubMed] [Google Scholar]
- 3.Hasegawa H., Makuuchi M., Yamazaki S., Gunvén P. Central bisegmentectomy of the liver: experience in 16 patients. World J. Surg. 1989;13:786–790. doi: 10.1007/BF01658437. PMID: 2560286. [DOI] [PubMed] [Google Scholar]
- 4.Stratopoulos C., Soonawalla Z., Brockmann J., Hoffmann K., Friend P.J. Central hepatectomy: the golden mean for treating central liver tumors? Surg. Oncol. 2007;16:99–106. doi: 10.1016/j.suronc.2007.05.002. PMID: 17583496. [DOI] [PubMed] [Google Scholar]
- 5.Kauffmann R., Fong Y. Post-hepatectomy liver failure. Hepatobiliary Surg. Nutr. 2014 Oct;3(5) doi: 10.3978/j.issn.2304-3881.2014.09.01. PMID: 2560286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng G., Li H., Jia G.Q., Fang D., Tang Y.Y., Xie J., et al. Parenchymal-sparing versus extended hepatectomy for colorectal liver metastases: a systematic review and meta-analysis. HPB (Oxford) 2019;8(14):6165–6175. doi: 10.1002/cam4.2515. PMID: 31464101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hasegawa H., Makuuchi M., Yamazaki S., Gunvén P. Central bisegmentectomy of the liver: experience in 16 patients. World J. Surg. 1989;13:786–790. doi: 10.1007/BF01658437. PMID: 2560286. [DOI] [PubMed] [Google Scholar]
- 8.Chan J., Perini M., Fink M., Nikfarjam M. The outcomes of central hepatectomy versus extended hepatectomy: a systematic review and meta-analysis. HPB (Oxford) 2018;20(6):487–496. doi: 10.1016/j.hpb.2017.12.008. PMID: 2560286. [DOI] [PubMed] [Google Scholar]
- 9.Torzilli G., Viganò L., Andrea Gatti G., Costa M., Cimino F., et al. Twelve-year experience of “radical but conservative” liver surgery for colorectal metastases: impact on surgical practice and oncologic efficacy. HPB(Oxford) 2017;19(9):775–784. doi: 10.1016/j.hpb.2017.05.006. PMID: 28625391. [DOI] [PubMed] [Google Scholar]
- 10.Agha R.A., Sohrabi C., Mathew G., Franchi T., Kerwan A., O'Neill N., for the PROCESS Group The PROCESS 2020 guideline: updating consensus Preferred Reporting Of CasE Series in Surgery (PROCESS) guidelines. Int. J. Surg. 2020;84:231–235. doi: 10.1016/j.ijsu.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Terminology Committee of the IHPBA: terminology of liver anatomy and resections. HPB Surg. 2000;2:333–339. doi: 10.1080/136518202760378489. PMID: 18332933. [DOI] [Google Scholar]
- 12.Adam R., DeGramont A., Figueras J., Guthrie A., Kokudo N., Kunstlinger F., et al. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist. 2012;17:1225–1239. doi: 10.1634/theoncologist.2012-0121. PMID: 2560286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kokudo N., Tada K., Seki M., Ohta H., Azekura K., Ueno M., et al. Anatomical major resection versus nonanatomical limited resection for liver metastases from colorectal carcinoma. Am. J. Surg. 2001;18:153–159. doi: 10.1016/s0002-9610(00)00560-2. PMID: 2560286. [DOI] [PubMed] [Google Scholar]
- 14.Makuuchi M., Hasegawa H., Yamazaki S., Takayasu K. Four new hepatectomy procedures for resection of the right hepatic vein and preservation of the inferior right hepatic vein. Surg. Gynecol. Obstet. 1987;164:68–72. PMID: 3026059. [PubMed] [Google Scholar]
- 15.Pawlik T.M., Scoggins C.R., Zorzi D., Abdalla E.K., Andres A., Eng C., et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann. Surg. 2005;241:715–722. doi: 10.1097/01.sla.000160703.75808.7d. PMID: 15849507. [DOI] [PMC free article] [PubMed] [Google Scholar]