Abstract
Fungi often produce the phenoloxidase enzyme laccase during interactions with other organisms, an observation relevant to the development of biocontrols. By incorporating the laccase substrate 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) into agar, we analyzed laccase induction in the plant-pathogenic fungus Rhizoctonia solani when paired against isolates of the soil bacterium Pseudomonas fluorescens. Substantial induction of R. solani laccase was seen only in pairings with strains of P. fluorescens known to produce antifungal metabolites. To study laccase induction further, a range of chemical treatments was applied to R. solani liquid cultures. p-Anisidine, copper(II), manganese(II), calcium ionophore A23187, lithium chloride, calcium chloride, cyclic AMP (cAMP), caffeine, amphotericin B, paraquat, ethanol, and isopropanol were all found to induce laccase; however, the P. fluorescens metabolite viscosinamide did not do so at the concentrations tested. The stress caused by these treatments was assessed by measuring changes in lipid peroxidation levels and dry weight. The results indicated that the laccase induction seen in pairing plate experiments was most likely due to calcium or heat shock signaling in response to the effects of bacterial metabolites, but that heavy metal and cAMP-driven laccase induction was involved in sclerotization.
Rhizoctonia solani is a soil-living plant-pathogenic fungus which attacks a wide range of crop plants, including sugarbeet, potato, and rice. Seeds and seedlings are particularly susceptible to this fungus, which persists in soil in the form of resistant sclerotia. Fungicides can be used to control R. solani, but recently attention has focused on the development of biocontrol techniques. To this end, strains of the bacterium Pseudomonas fluorescens that are antagonistic to R. solani have been isolated and are currently being evaluated as biocontrol agents in our department. The most promising strain is P. fluorescens DR54, which produces the antifungal depsipeptide metabolite viscosinamide (18).
Inevitably, most fungi will encounter competitive or antagonistic organisms, whether bacterial, fungal or animal, during their life cycles. Fungi engaged in such competition frequently produce secondary metabolites, extracellular phenol-oxidizing enzymes, and differentiated structures in the zone of conflict (2, 4, 8). These responses may be critical in determining the outcome of a biocontrol treatment.
Our first aim was to study the induction pattern of the fungal phenol-oxidizing enzyme laccase in interactions between R. solani and P. fluorescens and how this related to the characteristics of different P. fluorescens strains. Laccase, ubiquitous in fungi and flexible in function (28), is often induced during antagonistic interactions, and R. solani has been shown to possess four laccase genes (29). Our second aim was to determine the pathways of laccase induction in R. solani and relate these to the effects of antagonistic bacteria. We believed that this work would aid the development of the biocontrol system and provide insights into the physiology and ecology of the two organisms.
MATERIALS AND METHODS
Media and chemicals.
Potato dextrose agar (PDA), glucose, and asparagine were obtained from Difco Laboratories. All other chemicals used were obtained from Sigma, apart from high-pressure liquid chromatography purified viscosinamide, which was provided in-house by Tommy H. Nielsen.
Organisms.
R. solani AG4 (strain 92009; Danisco Seed, Holeby, Denmark) was maintained on PDA. The growth conditions used were 25°C in darkness for both agar and liquid cultures. P. fluorescens strains were previously isolated from an experimental field of Danisco Seed and maintained in our departmental culture collection (18). The strains used were 96.578, DR1, DR2, DR3, DR4, DR12, DR17, DR20, DR34, DR41, DR46, DR48, DR50, DR52, DR54, DR56, PS1, PS3, PS7, PS8, PS12, PS16, and PS21. Several of these strains produce antifungal metabolites. DR54 produces the depsipeptide viscosinamide, similar in structure to the surfactant compound viscosin (14) but less polar. PS8 and PS16 produce 2,4-diacetylphloroglucinol (DAPG); PS7 and 96.578 produce, respectively, cyclic and noncyclic isoforms of a peptide similar to viscosinamide and tentatively named tensin (T. H. Nielsen, unpublished data). DR50 produces an uncharacterized antifungal metabolite, extractable by ethyl acetate (Nielsen, unpublished).
Pairings of R. solani and P. fluorescens.
Pairings were made between all P. fluorescens strains and R. solani. Agar plugs 4 mm in diameter were taken from an R. solani culture and transferred, approximately 2 cm off center, to PDA plates. Two parallel inoculation streaks of the P. fluorescens strain were then made facing the R. solani inoculation plug (see Fig 2A). To ensure reproducibility, inocula were positioned on the plates by reference to a drawn template. Three replicates were made of each pairing, and the experiment was repeated twice.
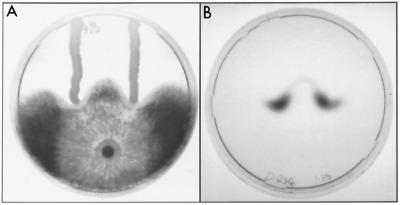
FIG. 2.
Induction of R. solani laccase by P. fluorescens DR54 on 9-cm-diameter PDA plates (3 days). R. solani shows appressed and inhibited mycelial growth near streaks of P. fluorescens DR54 (A). Laccase activity was visualized using an ABTS agar underlay. R. solani laccase activity was induced in the region of confrontation (B).
ABTS underlay visualization of laccase production.
The artificial laccase substrate 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) was used to visualize laccase distribution in agar cultures of R. solani. Indicator agar was made as follows. A mixture of 0.5487 g of 1 mM ABTS, 20 g of agar, and 50 ml of 20× mineral medium (per liter, 2 g each of K2HPO4, KCl, and MgSO4 · 7H2O 2.0 [pH 5.0]) was made to 1 liter with distilled water and autoclaved; 20-ml aliquots of indicator agar were pump dispensed into 9-cm-diameter plastic petri dishes. Laccase distribution in an agar culture was visualized by removing the colonized agar from its dish and laying it on top of a plate of indicator agar. Bubbles were excluded by gentle pressure from a gloved finger, and the plate was incubated for 120 min at room temperature. ABTS oxidized by laccase became visible as dark green regions on the indicator agar. The colonized agar was discarded, and the underlay agar then photographed on a light box using a 35-mm camera and Kodak Gold 200 print film. The contribution of peroxidase activity to color development was assessed by cutting agar from regions close to the interaction zone, extracting in Britton-Robinson buffer (0.1 M boric acid–0.1 M acetic acid–0.1 M phosphoric acid adjusted to pH 5.0 with 0.5 M NaOH), and measuring ABTS oxidation in the presence of catalase (1 mg/ml) (see description of laccase assay method below).
Image analysis procedure.
The photographic prints were digitized to produce 256-level greyscale images of approximately 1,800 pixels square. The Magic Wand function of Paint Shop Pro software (shareware version 4.12; JASC Inc.) was used to select regions within each plate that were at least 10 greyscale points darker than the background agar, which was deleted. The area, in pixels, of the dark regions was measured by using the Area function of Image-Pro Plus software (version 1.2; Media Cybernetics L.P.). The image of the petri dish was outlined using a circular selection tool, and its pixel area was measured. The area of visible ABTS oxidation was then expressed as a fraction of total petri dish area.
Liquid culture of R. solani.
R. solani was grown in a defined liquid medium modified from GAsnM (19). This contained (in grams per liter) glucose (9.0), asparagine (1.0), K2HPO4 (0.1), KCl (0.1), MgSO4 · 7H2O (0.1), and thiamine (10−3); 25 μl of a trace element solution was also added, consisting of (in grams per liter) FeEDTA (34.4), ZnSO4 · 7H20 (6.3), MnSO4 · H2O (15.4), CuSO4 · 5H2O (2.5), and NH4Mo7O2 · 4H2O (0.5). R. solani inoculum was prepared by plug inoculating a PDA plate covered with a cellophane membrane and incubating it at 25°C for 3 days. The mycelium was scraped from the plate using a sterile glass microscope slide, added to 10 ml of 0.3 M mannitol, and macerated for 1 min in a Sorval Omni-Mixer fitted with a 50-ml beaker. Conical flasks (300 ml) containing 40 ml of GAsnM medium were each inoculated with 400 μl of mycelial suspension. The flasks were incubated in the dark at 25°C without shaking.
Chemical treatment of R. solani cultures.
Chemical treatments were added to the liquid cultures of R. solani after 48 h of growth. Each experiment used the same batch of R. solani inoculum in two to four sets of five replicate flasks. A set of five control flasks was included in each experiment. The chemicals were chosen to treat the R. solani liquid cultures were either (i) known or suspected to induce fungal laccases as substrates or cofactors, (ii) likely to affect intracellular message pathways controlling gene regulation, or (iii) known to cause stress to the fungus by various means. The final concentration of each chemical used (see Fig. 3) was determined either by reference to available literature or preliminary experiments (indicated as “P”). The chemicals chosen were amphotericin B (P), p-anisidine (P), caffeine (23), CaCl2 (P), calcium ionophore A23187 (P), CuSO4 (P), cyclic AMP (cAMP) (23), ethanol (P), isopropanol (P), LiCl (13), MnSO4 (P), paraquat (methyl viologen) (P), and viscosinamide (C. Thrane, unpublished data). In cases where ethanol was used as a solvent (p-anisidine, calcium ionophore A23187, and viscosinamide), the same volume of ethanol was applied to control cultures. As the effects of CaCl2 and viscosinamide were small, these experiments were repeated three times.
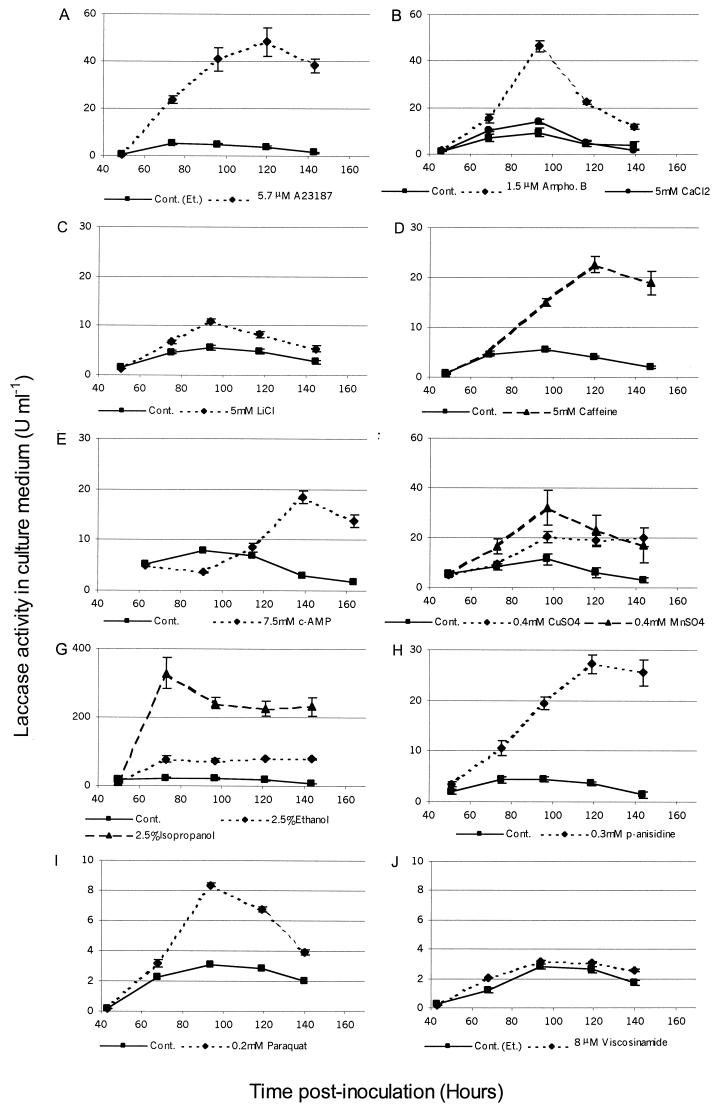
FIG. 3.
Profiles of laccase activity in liquid cultures of R. solani treated with various chemicals. One unit of activity is the amount of enzyme converting 1 μmol of substrate in 1 min. Each point is the mean of five replicates with SEM. Cont. (Et.), control (ethanol); Ampho. B, amphotericin B.
Assay of laccase activity in liquid cultures.
Five laccase assays of the culture medium were carried out at approximately 24-h intervals. The first assay was made immediately prior to treatment of the cultures. The assay buffer used was a mixture of 0.1 M boric acid, 0.1 M acetic acid, and 0.1 M phosphoric acid (Britton-Robinson buffer [29]) adjusted to pH 5.0 with NaOH. A 200-μl sample of culture medium was mixed with 750 μl of assay buffer in a 1.6-ml semimicrocuvette. To this was added 50 μl of a 20 mM aqueous solution of ABTS (extinction coefficient of 35 mM−1 cm−1 at 405 nm). The increase in absorbance at 405 nm was recorded over 1 min on a Shimadzu UV-160 UV-visible recording spectrophotometer.
LPO assay and dry weight measurement.
The Bioxytech LPO-586 lipid peroxidation (LPO) assay (Oxis International) was used to measure LPO products including 4-hydroxyalkenals and malonaldehyde. LPO and dry weight measurements were carried out approximately 200 h after inoculation. Mycelium from liquid cultures was harvested, blotted dry on paper towels, and then ground for 30 s in a mortar and pestle with 10 ml of distilled water; 1.5 ml of this sample was centrifuged at 10,000 × g for 15 min to remove debris; 200 μl of the supernatant was assayed for both malonaldehyde and 4-hydroxyalkenals according to the manufacturer's protocol. The dry weight of the mycelium was calculated by pouring the mycelial slurry into preweighed petri dishes and drying overnight at 55°C before reweighing.
RESULTS
Laccase induction in confrontations between R. solani and a panel of P. fluorescens strains.
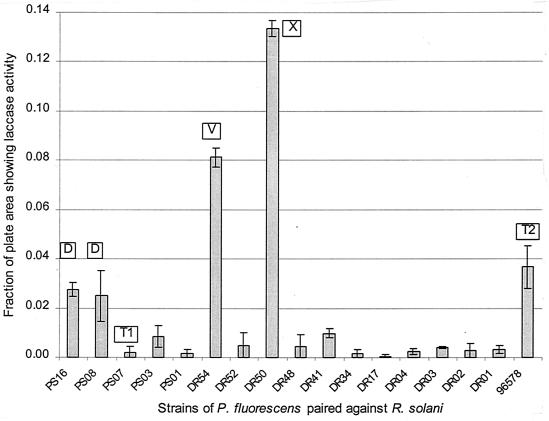
Twenty-three strains of P. fluorescens were tested for the ability to induce laccase activity in R. solani. These strains had previously been assessed for inhibitory effects on R. solani and screened for the production of pigments, endochitinases, and antifungal metabolites (18). None of the P. fluorescens strains were able to oxidize ABTS directly. The fractional area of the interaction plates where ABTS oxidation could be detected using image analysis is presented in Fig. 1. Of the 23 P. fluorescens strains tested, 17 induced the formation of measurable regions of laccase activity in interactions with R. solani. Of the remaining strains, three induced trace amounts of laccase and three caused no induction. The only P. fluorescens strains to induce laccase in more than 2% of the plate area were those known to produce antifungal agents. No positive correlation was seen between R. solani laccase induction and P. fluorescens strains expressing endochitinase or producing pigment. Assays of agar extracts did not show reduced ABTS oxidation in the presence of catalase, indicating that peroxidases were not involved.
FIG. 1.
Laccase response of R. solani to various P. fluorescens strains in agar cultures. The boxed letters refer to metabolites known to be produced by certain strains of P. fluorescens. D, DAPG; T1, tensin (cyclic); T2, tensin (linear); X, uncharacterized (ethyl acetate extractable); V, viscosinamide. The strains of P. fluorescens not inducing measurable areas of laccase activity were DR12, DR20, DR46, DR56, PS12, and PS21.
The pattern of ABTS oxidation seen in the R. solani-P. fluorescens DR54 interaction illustrated in Fig. 2 is typical of all cases where substantial laccase activity was observed, being localized to regions at the edge of the fungal colony where growth inhibition was evident. In some cases, small, sharply defined areas of low laccase activity appeared within the high-activity regions. Sclerotial formation was limited and did not correlate with regions of laccase activity. In interactions involving DAPG-producing strains of P. fluorescens, the region of ABTS oxidation (and no other regions) initially appeared orange, although further development of green coloration gradually masked this.
Induction of laccase in liquid cultures of R. solani by chemical treatments.
The liquid cultures of R. solani initially grew as a pale diffuse submerged mycelium, producing the first aerial growth after 36 to 48 hs and developing into a floating mycelial mat. Sclerotization was limited (<5% of mycelial surface) and commonly occurred in contact with the flask walls. In untreated cultures, laccase activity peaked at about 96 h, concomitant with the onset of yellow-brown pigmentation and the early stages of sclerotial formation. The mean peak laccase activity of control cultures (data pooled from all experiments) was 8.6 U ml−1, with a standard error of the mean of ±2.
The induction profiles of the liquid culture laccase assays are presented in Fig. 3, and the relative peak inductions are given in Table 1. Treatments with all of the chemicals except viscosinamide induced laccase to some extent. In most cases, the peak induction of laccase occurred at or after 48 h posttreatment. The exceptions were ethanol and isopropanol, with peak activity appearing by 24 h. cAMP inhibited laccase production at the first time point posttreatment (P < 0.001), with induction delayed until 72 h posttreatment. The most potent inducer of laccase was the calcium ionophore A23187, which induced laccase more than 13-fold at a concentration of 5.7 μM.
TABLE 1.
Effects of chemical treatments on laccase induction, final dry weight, and LPO levels in liquid cultures of R. solania
| Treatment | LPO | Dry wt | Relative laccase induction | Mycelial morphology |
|---|---|---|---|---|
| Compounds affecting calcium signaling | ||||
| Amphotericin B | 1.30** | 0.93 | 5.0** | Pale mycelium, many loose sclerotia |
| CaCl2 | 0.76** | 1.03 | 1.5* | No effect |
| Ionophore A23187 | 1.03 | 1.01 | 13.6** | No effect |
| LiCl | ND | 0.70** | 2.0** | Whole mycelium with brown pigmentation |
| Compounds affecting cAMP signaling | ||||
| Caffeine | 1.65** | 0.88* | 4.1** | Heavy sclerotization |
| cAMP | 0.57** | 0.79** | 2.3** | Heavy sclerotization with pale aerial mycelium |
| Heavy metal salts | ||||
| CuSO4 | 2.21** | 0.85* | 1.8* | Heavy sclerotization |
| MnSO4 | 1.03 | 0.97 | 2.9* | Heavy sclerotization with brown exudate |
| Simple organic compounds | ||||
| p-Anisidine | 1.02 | 0.99 | 6.1** | Whole mycelium purple-brown pigmentation |
| Ethanol | 2.18** | 0.96 | 3.5** | Pale mycelium, no sclerotia |
| Isopropanol | 3.75** | 0.44** | 14.1** | Pale mycelium, no sclerotia |
| Paraquat | 3.83* | 0.38** | 2.7** | Pale mycelium, no sclerotia |
| Purified P. fluorescens metabolite | ||||
| Viscosinamide | 1.08 | 0.97 | 1.1 | Aerial mycelium reduced at mat periphery |
Relative laccase induction was calculated by comparison of the mean peak laccase activity in control and induced cultures; values for LPO and final dry weight are relative to internal controls for each experiment. The mean LPO level of control cultures (pooled from all experiments) was equivalent to 0.257 mM malonaldehyde per g (dry weight) of mycelium (SEM < 4%). No LPO data are available for lithium chloride treatment, as this interfered with the assay. The mean dry weight of control cultures (pooled from all experiments) was 0.213 g (SEM < 1%). Significance was assessed by t test (**, P < 0.01; *, P < 0.05). ND, not determined.
Stress measurements and morphological changes in chemically treated cultures.
The LPO levels, dry weights, and morphological changes seen in chemically treated cultures are presented in Table 1. Correlated stress effects (LPO increase and dry weight decrease) were seen in caffeine, CuSO4, isopropanol, and paraquat treatments. Ethanol and amphotericin B increased LPO but did not reduce dry weight. Measurements of dry weight in older cultures indicated that R. solani could metabolize ethanol (data not shown). LiCl treatment reduced dry weight, but its effect on LPO could not be assessed because it interfered with the assay. cAMP reduced LPO and dry weight, and CaCl2 reduced LPO but left dry weight unaffected; both of these treatments may have antioxidant effects that limit LPO (see Discussion). Calcium ionophore A23187, p-anisidine, MnSO4, and viscosinamide did not cause significant effects.
Effects of the chemical treatments on the morphology of liquid cultures varied. Ethanol, isopropanol, and paraquat abolished pigmentation and sclerotial formation, whereas copper, manganese, caffeine, and cAMP caused heavy sclerotization with varying degrees of pigmentation. Amphotericin B-treated cultures developed a pale, felty mycelial mat containing many loose, pigmented spots resembling flattened sclerotia. Neither p-anisidine or LiCl altered the sclerotization of cultures, but both caused deep pigmentation of the mycelium and culture medium. Viscosinamide treatment inhibited aerial mycelium formation at the periphery of the mycelial mat, possibly due to its surfactant properties.
DISCUSSION
Laccase induction in pairing plates.
We found that several strains of P. fluorescens known to produce antifungal metabolites were able to induce substantial laccase production by R. solani. This was not linked to mycelial melanization or sclerotization, processes often associated with laccase. The pattern of induction suggested that the R. solani mycelium was reacting to agents diffusing from the bacteria within the agar medium. Both induction and inhibition of phenoloxidase activity have been reported in other fungai-bacterial interactions. Bacillus subtilis cells and extracts were found to induce laccase in the wood-decaying basidiomycete Hypholoma fasciculare (8), and Pseudomonas tolaasii enhanced tyrosinase activity in Agaricus bisporus fruit bodies by the action of a metabolite resembling viscosinamide (27). Exposure of the mycoparasite Phanaerochaete magnoliae and saprophyte Trichoderma viride to growth-inhibiting volatiles from soil bacteria inhibited laccase and induced or inhibited tyrosinase, depending on the bacterial strain (16). Our results show a great variability in laccase response to different strains of the same bacterial species, with the key inducing factor in this case being antifungal metabolite production. This implies scope for great complexity in the biochemical interplay between fungi and bacteria in the soil community.
Potential control pathways for R. solani laccases.
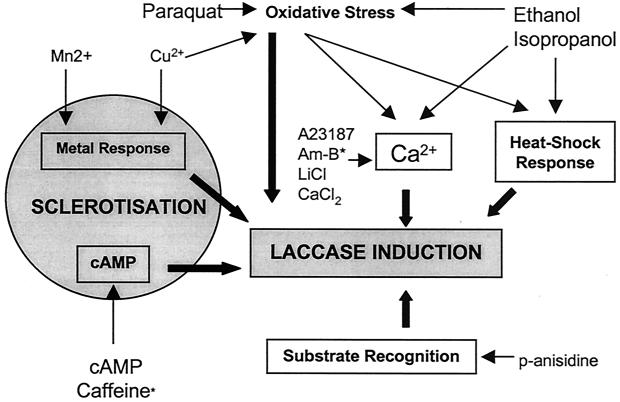
The results of the chemical treatment experiments shown in Table 1 and Fig. 3 indicate that several signaling pathways may be involved in laccase regulation in R. solani, and a hypothetical scheme for this is presented in Fig. 4.
FIG. 4.
Hypothetical scheme for laccase induction in R. solani. Thin arrows refer to the direct or indirect effects of different chemical treatments; heavy arrows indicate endogenous signaling pathways. Treatments marked * may have a minor oxidative stress effect. A23187 refers to the calcium ionophore; Am-B denote amphotericin B.
Calcium is a ubiquitous secondary messenger in fungi (6) and has been directly linked to laccase induction in the fungus Cryphonectria parasitica (13). Treatment of R. solani liquid cultures with calcium ionophore A23187, amphotericin B, or lithium chloride all induced laccase activity. All of these compounds mobilize calcium across membranes by various means (21, 25). The effect of the ionophore was particularly significant, as it was highly potent and not associated with stress effects or alterations in mycelial morphology. The lithium ion acts indirectly on calcium levels by stimulating the release of calcium from intracellular stores via inositol 1,4,5-trisphosphate-sensitive calcium channels (6, 13); the lithium ion-triggered induction of laccase seen here suggests that such a pathway functions in R. solani. Finally, although intracellular calcium levels are thought to be tightly controlled (6), addition of calcium chloride to the growth medium also led to a modest laccase induction.
cAMP is another important secondary messenger known to drive many processes including sclerotial formation in R. solani (26) and other fungi (6, 23). We found R. solani laccase activity and sclerotization to be strongly induced by both cAMP itself and caffeine, an antagonist of cAMP phosphodiesterase. These results point to the regulation of laccase by cAMP and the involvement of both in sclerotial morphogenesis (28). Laccase activity concomitant with sclerotization was also seen after both Cu2+ and Mn2+ treatments. Copper is both a cofactor (28) and a transcriptional inducer of fungal laccases (5), and the redox cycling of manganous ions is indirectly driven by laccase during ligninolysis (17). Our results imply that a metal-responsive induction pathway is involved in laccase induction and further strengthen the link between laccase and sclerotization.
The finding that ethanol and isopropanol are powerful laccase inducers is, to our knowledge, novel. This induction was rapid (<24 h) compared to all other treatments, and isopropanol induced the highest recorded laccase activity. Alcohols destabilize membrane and protein structures (10), and the cellular response to this is closely correlated with that of heat shock (20), which regulates laccase and peroxidase genes in some fungi (15, 24). Calcium influx and oxidative stress (due to membrane disruption and metabolic by-products, respectively) are also likely consequences of alcohol treatment. As isopropanol is more lipophilic than ethanol, it is correspondingly more disruptive, as reflected in its greater laccase-inducing potential. This intense, rapid inducing activity could provide a useful tool for further investigations of laccases in R. solani and other fungi.
The aromatic compound p-anisidine is known to induce laccase in Rhizoctonia species (29), and such specific induction may operate via receptor-mediated transcriptional activation (5). As expected, we found p-anisidine to be a powerful inducer of laccase in R. solani, and this induction was not linked to any stress effects at the concentration used.
It has been proposed that laccase induction (5, 23; M. Jaszek and A. Leonowicz, Abstr. Int. Conf. Plasma Membr. Redox Syst. Role Biol. Stress Dis., p. 49, 1998) and sclerotization (7) respond to oxidative stress. This may be directly sensed via a transcriptional activator or cause indirect activation of calcium and heat shock pathways due to membrane disruption and protein misfolding. Copper, paraquat, and alcohol treatments are known to cause oxidative stress via well-established mechanisms of free radical formation (9, 10), and we found that these treatments caused increased LPO and dry weight loss as well as laccase induction. Although copper and the alcohols have other, parallel effects (see above), a straightforward link between oxidative stress and laccase induction can be seen in the case of paraquat treatment. This link complicates the interpretation of some of the other chemical treatments (particularly caffeine and amphotericin B), as oxidative stress may be part of their laccase-inducing effect, a possibility overlooked in most previous studies. However, our evidence for the calcium, metal, and cAMP pathways includes, in each case, at least one treatment that does not cause oxidative stress as measured by LPO. In general, no clear correlation was evident between elevated LPO levels and enhanced sclerotization. The alcohol treatments increased LPO but actually inhibited sclerotization and pigmentation. Alcohols have a paradoxical effect; although their metabolic breakdown is a powerful source of free radicals, they also scavenge free radicals in solution, and this may interfere with melanogenesis (9).
Unexpectedly, calcium chloride and cAMP treatments were found to significantly decrease LPO. Calcium has been reported to protect yeast cells from Fe2+ and Cu2+ toxicity, probably by competing with these ions at the cell surface and thus limiting the production of hydroxyl radicals (9, 12). As well as reducing LPO, cAMP also caused an initial inhibition in laccase activity and may act as an antioxidant in addition to its direct intracellular signaling effect.
Viscosinamide was the only P. fluorescens metabolite purified in sufficient amounts for experimental use. The metabolite did not significantly induce laccase in liquid cultures, although it should be noted that the hydrophobicity of viscosinamide limited the concentration that could be applied without the excessive use of solvents. Viscosinamide's potency may be amplified on the pairing plates due to highly localized concentration effects and the environment (e.g., nutrient depletion) caused by the presence of live bacteria.
Significance of laccase induction to the biocontrol and virulence of R. solani.
We conclude from the evidence discussed above that the strong laccase induction seen in some R. solani- P. fluorescens interactions is most likely due to the triggering of calcium and/or heat shock signaling pathways by bacterial metabolites. Involvement of the substrate-, metal-, cAMP-responsive pathway seems improbable, as the known P. fluorescens metabolites are unlikely laccase substrates, Cu2+ and Mn2+ concentrations were low, and sclerotia (associated with cAMP elevation) were absent.
What is the role of laccase in the fungal-bacterial interaction? Calcium influx and heat shock pathway activation are both indicators that cell integrity is compromised, a potentially fatal stress. Reactions involving laccase-derived free radical products may restore homeostasis in two ways: by polymerizing and rendering cell walls less permeable (24) and/or by detoxifying antifungal compounds (1). Laccase may therefore be a determining factor in the efficacy of the bacterial biocontrol of R. solani. Moreover, it could play a similar role as a virulence factor in the host-fungus interaction, a role in which laccase has already been implicated (11, 13, 30). Further work is being undertaken to study which R. solani laccases are involved, their regulation, and how they act in soil or host systems.
ACKNOWLEDGMENTS
We are grateful to Tommy H. Nielsen for valuable information and help in investigating P. fluorescens metabolite production and to Mette Nielsen and Dorte Rasmussen for supplying and preparing P. fluorescens strains.
This research was supported by grant 9601035 (Functional Specialisation of the Mycelium) from the Danish Agricultural and Veterinary Research Council.
REFERENCES
- 1.Bollag J, Leonowicz A. Comparative studies of extracellular fungal laccases. Appl Environ Microbiol. 1984;48:849–854. doi: 10.1128/aem.48.4.849-854.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooke R C, Rayner A D M. Ecology of saprotrophic fungi. London, England: Longman; 1984. [Google Scholar]
- 3.Davidson J F, Whyte B, Bissinger P H, Schiestl R H. Oxidative stress is involved in heat-induced cell death in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1996;93:5116–5121. doi: 10.1073/pnas.93.10.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dyer H C, Boddy L, Preston-Meek C M. Effect of nematode Panagrellus redivivus on growth and enzyme production by Phanerochaete velutina and Stereum hirsutum. Mycol Res. 1992;96:1019–1028. [Google Scholar]
- 5.Fernández-Larrea J, Stahl U. Isolation and characterization of a laccase gene from Podospora anserina. Mol Gen Genet. 1996;252:539–551. doi: 10.1007/BF02172400. [DOI] [PubMed] [Google Scholar]
- 6.Gadd G M. Signal transduction in fungi. In: Gow N A R, Gadd G M, editors. The growing fungus. London, England: Chapman and Hall; 1994. pp. 183–210. [Google Scholar]
- 7.Georgiou C D. Lipid peroxidation in Sclerotium rolfsii: a new look into the mechanism of sclerotial biogenesis in fungi. Mycol Res. 1997;101:460–464. [Google Scholar]
- 8.Griffith G S, Rayner A D M R, Wildman H G. Interspecific interactions and mycelial morphogenesis of Hypholoma fasciculare (Agaricaceae) Nova Hedwig. 1994;59:47–75. [Google Scholar]
- 9.Halliwell B, Gutteridge J M C. Free radicals in biology and medicine. 2nd ed. Oxford, England: Clarendon Press; 1989. [Google Scholar]
- 10.Ingram L O, Buttke T M. Effects of alcohols on micro-organisms. Adv Microb Physiol. 1984;25:253–296. doi: 10.1016/s0065-2911(08)60294-5. [DOI] [PubMed] [Google Scholar]
- 11.Johansson M, Denekamp M, Asiegbu F O. Production and isozyme pattern of extracellular laccase in the S and P intersterility groups of the root pathogen Heterobasidion annosum. Mycol Res. 1999;103:365–371. [Google Scholar]
- 12.Karamushka V I, Sayer J A, Gadd G A. Inhibition of H+ efflux from Saccharomyces cerevisiae by insoluble metal phosphates and protection by calcium and magnesium: inhibitory effects a result of soluble metal cations? Mycol Res. 1996;100:707–713. [Google Scholar]
- 13.Larson T G, Choi G H, Nuss D L. Regulatory pathways governing modulation of fungal gene expression by a virulence-attenuating mycovirus. EMBO J. 1992;11:4539–4548. doi: 10.1002/j.1460-2075.1992.tb05555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laycock M V, Hildebrand P D, Thibault P, Walter J A, Wright J L C. Viscosin, a potent peptidolipid biosurfactant and phytopathogenic mediator produced by a pectolytic strain of Pseudomonas fluorescens. J Agric Food Chem. 1991;39:463–489. [Google Scholar]
- 15.Li D, Alic M, Brown J A, Gold M H. Regulation of manganese peroxidase gene transcription by hydrogen peroxide, chemical stress, and molecular oxygen. Appl Environ Microbiol. 1995;61:341–345. doi: 10.1128/aem.61.1.341-345.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackie A E, Wheatley R E. Effects and incidence of volatile organic compound interactions between soil bacterial and fungal isolates. Soil Biol Biochem. 1998;31:375–385. [Google Scholar]
- 17.Muñoz C, Guillén F, Martinez A T, Martinez M J. Laccase isozymes of Pleurotus eryngii: characterization, catalytic properties, and participation in activation of molecular oxygen and Mn2+ oxidation. Appl Environ Microbiol. 1997;63:2166–2174. doi: 10.1128/aem.63.6.2166-2174.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nielsen M, Sørenson J, Fels J, Pedersen H C. Secondary metabolite- and endochitinase-dependent antagonism toward plant-pathogenic microfungi of Pseudomonas fluorescens isolates from sugar beet rhizosphere. Appl Environ Microbiol. 1998;64:3563–3569. doi: 10.1128/aem.64.10.3563-3569.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olsson S. Mycelial density profiles of fungi on heterogeneous media and their interpretation in terms of nutrient reallocation patterns. Mycol Res. 1995;99:143–153. [Google Scholar]
- 20.Piper P W. The heat shock and ethanol stress responses of yeast exhibit extensive similarity and functional overlap. FEMS Microbiol Lett. 1995;134:121–127. doi: 10.1111/j.1574-6968.1995.tb07925.x. [DOI] [PubMed] [Google Scholar]
- 21.Ramos H, Attias-De-Murciano A, Cohen B E, Bolard J. The polyene antibiotic amphotericin B acts as a calcium ionophore in sterol-containing liposomes. Biochim Biophys Acta. 1989;982:303–306. doi: 10.1016/0005-2736(89)90069-2. [DOI] [PubMed] [Google Scholar]
- 22.Rayner A D M, Beeching J R, Crowe J D, Watkins Z R. Defining individual fungal boundaries. In: Worrall J J, editor. Structure and dynamics of fungal populations. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1999. pp. 19–41. [Google Scholar]
- 23.Rollins J A, Dickman M B. Increase in endogenous and exogenous cyclic AMP levels inhibits sclerotial development in Sclerotinia sclerotiorum. Appl Environ Microbiol. 1998;64:2539–2544. doi: 10.1128/aem.64.7.2539-2544.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saloheimo M, Niku-Paavola M L, Knowles J K C. Isolation and structural analysis of the laccase gene from the lignin-degrading fungus Phlebia radiata. J Gen Microbiol. 1991;137:1537–1544. doi: 10.1099/00221287-137-7-1537. [DOI] [PubMed] [Google Scholar]
- 25.Schmid J, Harold F M. Dual roles for calcium ions in apical growth of Neurospora crassa. J Gen Microbiol. 1988;134:2623–2631. doi: 10.1099/00221287-134-9-2623. [DOI] [PubMed] [Google Scholar]
- 26.Sharada K, Ikegami H, Hyakumachi M. 2,4-D induced, c-AMP mediated, sclerotial formation in Rhizoctonia solani. Mycol Res. 1992;96:863–866. [Google Scholar]
- 27.Soler-Rivas C, Arpin N, Olivier J M, Wichers H J. Activation of tyrosinase in Agaricus bisporus strains following infection by Pseudomonas tolaasii or treatment with a tolaasin-containing preparation. Mycol Res. 1997;101:375–382. [Google Scholar]
- 28.Thurston C F. The structure and function of fungal laccases. Microbiology. 1994;140:19–26. [Google Scholar]
- 29.Wahleithner J A, Xu F, Brown K M, Brown S H, Golightly E J, Halkier T, Kauppinen S, Pederson A, Schneider P. The identification and characterization of four laccases from the plant-pathogenic fungus Rhizoctonia solani. Curr Genet. 1996;29:395–403. doi: 10.1007/BF02208621. [DOI] [PubMed] [Google Scholar]
- 30.Williamson P R, Wakamatsu K, Ito S. Melanin biosynthesis in Cryptococcus neoformans. J Bacteriol. 1998;180:1570–1572. doi: 10.1128/jb.180.6.1570-1572.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]