Abstract
The bacteria colonizing geologic core sections (attached) were contrasted with those found suspended in the groundwater (unattached) by examining the microbiology of 16 depth-paired core and groundwater samples using a suite of culture-independent and culture-dependent analyses. One hundred twenty-two meters was continuously cored from a buried chalcopyrite ore hosted in a biotite-quartz-monzonite porphyry at the Mineral Park Mine near Kingman, Ariz. Every fourth 1.5-m core was acquired using microbiologically defensible methods, and these core sections were aseptically processed for characterization of the attached bacteria. Groundwater samples containing unattached bacteria were collected from the uncased corehole at depth intervals corresponding to the individual cores using an inflatable straddle packer sampler. The groundwater was acidic (pH 2.8 to 5.0), with low levels of dissolved oxygen and high concentrations of sulfate and metals, including ferrous iron. Total numbers of attached cells were less than 105 cells g of core material−1 while unattached cells numbered about 105 cells ml of groundwater−1. Attached and unattached acidophilic heterotrophs were observed throughout the depth profile. In contrast, acidophilic chemolithotrophs were not found attached to the rock but were commonly observed in the groundwater. Attached communities were composed of low numbers (<40 CFU g−1) of neutrophilic heterotrophs that exhibited a high degree of morphologic diversity, while unattached communities contained higher numbers (ca. 103 CFU ml−1) of neutrophilic heterotrophs of limited diversity. Sulfate-reducing bacteria were restricted to the deepest samples of both core and groundwater. 16S ribosomal DNA sequence analysis of attached, acidophilic isolates indicated that organisms closely related to heterotrophic, acidophilic mesophiles such as Acidiphilium organovorum and, surprisingly, to the moderately thermophilic Alicyclobacillus acidocaldarius were present. The results indicate that viable (but possibly inactive) microorganisms were present in the buried ore and that there was substantial distinction in biomass and physiological capabilities between attached and unattached populations.
Compositional differences between attached and unattached (suspended in associated aqueous phase) microbial communities may develop due to selective advantages conferred on some populations by attachment to environmental surfaces (28). Attachment of individual bacterial cells (of a single population) has frequently been associated with substantial changes in cell physiology, although the direction of change for a given physiological parameter is not predictable and the causal factors of these changes are unclear (42). Accordingly, several recent publications have described both structural and functional differences between attached and unattached bacterial communities in marine and freshwater aquatic environments (7, 12, 37). Although similar distributions of microbial biomass and activities in geologic media and groundwater may impact solute transport in aquifers (19, 33, 43), few studies have systematically compared the microbial communities in paired core and groundwater samples.
Studies examining the microbiology of core and groundwater samples from unconsolidated sedimentary aquifers generally have indicated a predominance of attached biomass (5, 13, 16, 17, 25, 40). Therefore, it has been suggested that core is more representative of the subsurface microflora than groundwater (1, 14, 17, 32). However, numerical dominance of unattached organisms has been observed in some of these reports (5, 40), and differences in composition between attached and unattached communities are common to all of these studies (5, 13, 16, 17, 25, 40). The studies cited above are not all directly comparable because there are differences in formation lithology, degree of nutrient enrichment, factors related to sample pairing (e.g., same depth and same corehole), methods used to acquire samples, the type of analyses performed, and the units of the reported results. Given the constraints for interpreting current data regarding attached and unattached subsurface bacteria, it seems premature to limit the study of subsurface bacteria to core or groundwater alone. Under a stable set of conditions, it is probable that a dichotomy between attached and unattached organisms will be established (18) which may be reflected in significant differences in the functional capabilities of attached and unattached populations. There is a particular lack of data from paired rock and groundwater samples from crystalline, fractured rock aquifers.
In order to evaluate potential differences between attached and unattached subsurface bacterial communities, we applied a variety of culture-independent and -dependent techniques to characterize core and groundwater samples that were closely paired in depth of origin from a single corehole in a biotite-quartz-monzonite porphyritic intrusive. This igneous rock hosts a low-grade sulfidic ore that was under study by the U.S. Bureau of Mines for the development of advanced in situ leach mining techniques prior to the elimination of the Bureau of Mines in 1996 (R. D. Schmidt and D. Earley, U.S. Bureau of Reclamation open file report, 1998). Deeply buried, low-grade sulfidic ores comprise most of world's remaining copper reserves (8), and the microbial ecology of these intact, crystalline ore bodies is largely unknown. The microbiological assays were aimed at populations that were expected to exist in this acidic environment and are involved in sulfide mineral oxidation (i.e., acidophilic chemolithotrophs and heterotrophs) and those that have been widely detected in subsurface environments (i.e., aerobic chemoheterotrophs).
MATERIALS AND METHODS
Site description.
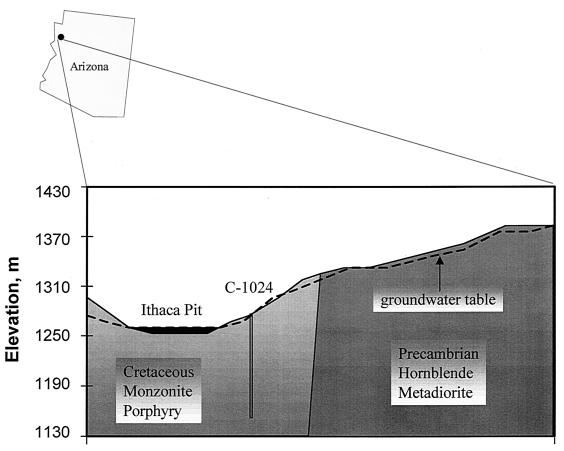
The Mineral Park mine (operated by Cyprus Mineral Park, a subsidiary of Cyprus Mining Corporation) is located in the central part of the Wallapai mining district of the Cerbat Mountains, 26 km north of Kingman, Ariz., and 6.5 km east of Highway 95 (3,916,900 m northing, 759,600 m easting; Universal Transverse Mercator grid, zone 11; North American Datum 1927) (Fig. 1). The 122-m-deep corehole was sited slightly north of Turquoise Mountain, midway down into Ithaca Pit (formerly Ithaca Peak). The geology of the corehole (C-1024) site is characterized by a thin layer of avalanche and mudslide debris (1 m thick) overlying the Ithaca Peak stock, which is a biotite-quartz-monzonite porphyry of the late Cretaceous period (ca. 71.5 million years ago) that intruded into the surrounding Precambrian hornblende metadiorite. The igneous host rock was heavily fractured with hydrothermal mineralization, creating numerous cross-cutting veins filled with quartz, pyrite, chalcopyrite, and molybdenite; the average grade for the ore is 0.067% Cu and 0.049% Mo (46). A stratigraphic profile of the corehole based on the geologic field log of the core includes a description of the rock type and dominant alteration features (Fig. 2). Bulk formation permeabilities are on the order of millidarcies (Schmidt and Earley, U.S. Bureau of Reclamation open file report). The elevation at the wellhead is 1,274 m above sea level. The local water table at this location is about 6.4 m below land surface, which was the approximate depth of the surface casing. The hydrologic computer program MINEFLO has been used to construct a hydrologic model for the Mineral Park mine (R. D. Schmidt, L. J. Dahl, K. Kim, F. Paillet, and D. Earley, presented at the SME-AIME Annual Meeting, Denver, Colo., 1995). According to the MINEFLO model, the groundwater table varies from 15.2 m below the surface to 3.1 m above the surface along the cross section shown in Fig. 1. The locations of artesian head conditions predicted by the model correlate with natural springs, which flow almost year round, observed on the east highwall of the pit.
FIG. 1.
One-kilometer cross-section (W-E) locating the corehole (C-1024) at the Mineral Park Mine north of Kingman, Ariz., and its relationship with local geology, topography, and groundwater table.
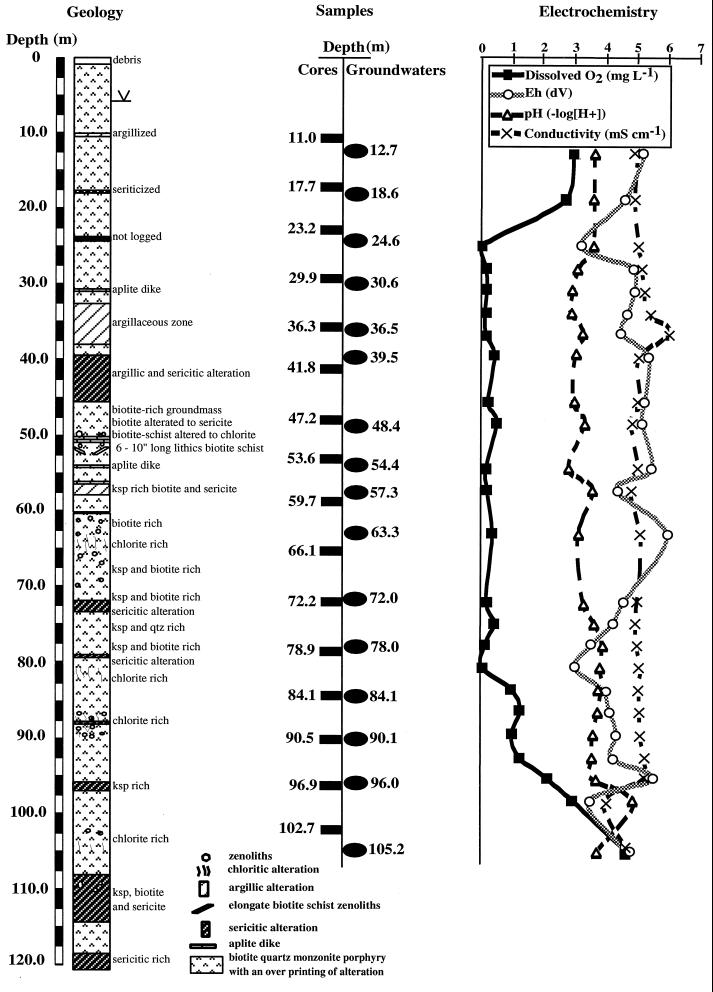
FIG. 2.
Lithological profile of borehole derived from field log observations, vertical profile of electrochemistry results, and depths from which cores and groundwater samples were retrieved. The depth indicated for groundwaters is the midpoint of the 3-m packed-off zone. Eleven cores were taken within the 3-m span of their corresponding groundwater sample; five cores (CM102.7, CM66.1, CM59.7, CM41.8, and CM11.0) were taken just outside the 3-m packed-off interval of their corresponding groundwater sample.
Core sample acquisition and processing.
One hundred twenty-two continuous meters of rock was cored with conventional rotary methods over a 2-week period in February 1995. Cores (1.5 m long, 6.11 cm in diameter) were retrieved by wireline. Groundwater pumped from the downgradient Sacramento Valley regional aquifer was amended with sodium hypochlorite to a concentration of 60 mg liter−1 and used as drilling fluid. Drilling fluid was continually discharged (not recirculated) during coring. Nineteen of the seventy-six cores required to advance the hole past the surface casing to total depth were processed for microbiological analyses (every fourth core). Sixteen of these cores could be closely matched in depth with groundwater samples obtained by straddle packer sampling (described below) (Fig. 2). No groundwater samples could be taken to compare with three cores taken between 102.7 and 122.0 m because of the length of the straddle packer sampler and accumulated debris in the corehole. Control and assessment of contamination introduced during coring were consistent with procedures reviewed by Fredrickson and Phelps (11) and Griffin et al. (15), including the use of carboxylated, fluorescent microspheres (0.9-μm diameter; Polysciences, Inc., Warrington, Pa.) (38) and soluble perfluorocarbon tracers (30) to assess drilling fluid intrusion. New drilling rods were purchased and dedicated to this project. All drill steel was steam cleaned prior to insertion into the corehole, and the inner core barrel was similarly cleaned prior to each trip downhole. The inner barrel was handled only with clean cotton gloves by researchers and drillers, and no lubricant was used on the pipe threads. For microbiological cores, a Lexan liner that had been cleaned with 10% bleach solution, rinsed with distilled water, and air dried was placed inside the inner core barrel.
Extruded cores (in liners) were immediately capped and transported to an on-site laboratory trailer for aseptic processing in a glovebag under an argon atmosphere (11). From each 1.5-m section of core, an intact 0.3-m section was chosen as a “common interval” which was pared, homogenized, and distributed into sterile bags which were sealed in mason jars under argon and held in a refrigerator (4°C) for cultural analyses or in a freezer (−20°C) for noncultural analyses. Core samples are referred to in the text by the depth in meters from which they were retrieved preceded by the project designation, CM (e.g., CM11.0). Results of microbiological analyses of core samples were considered to represent attached portions of the subsurface community. Subsamples of pared core and parings were placed in methanol in tared volatile organic carbon bottles for perfluorocarbon tracer analysis. Outer parings from the common interval were bagged for microsphere analyses and petrologic and geochemical characterization. A “blank” core consisting of Berea sandstone fired repeatedly at 550°C was processed and analyzed in the same manner as other cores to estimate the contamination due to sample handling. Samples from potential sources of contamination included groundwater used as drilling fluid (MW1 [start of coring] and MW2 [end of coring]), surface rubble, and water accumulated in the adjacent Ithaca Pit; these samples were collected aseptically and analyzed like the core samples. All samples were mailed in insulated boxes to the analysis laboratory by overnight express mail on ice or dry ice (depending on the analyses).
Groundwater sampling and geochemistry.
Groundwater sampling occurred 4 to 6 months following core sampling to allow subsidence of the disturbance to ambient groundwater composition introduced during coring activities (18). Groundwater samples were collected from discrete 3-m intervals in the open (uncased) corehole by sealing off portions of the corehole above and below the sampling interval with an inflatable straddle packer (Schmidt and Earley, U.S. Bureau of Reclamation open file report). The straddle packer consisted of two inflatable bladders connected to a riser pipe that was screened in the interval between the two bladders (39). The straddle packer was lowered to the desired sampling point, and the bladders were inflated to isolate the sampling interval. A pump was lowered through the riser pipe to pump water from this screened interval to the surface. In the intervals where recharge rates allowed, three interval bore volumes were purged from the isolated zone before sampling. In all cases, the interval was purged prior to sampling until the electrochemistry (e.g., pH, temperature, dissolved oxygen, etc.) of the groundwater had stabilized. The isolation of the sampling interval was confirmed by monitoring pressure (head) in pressure transducers placed above and below the packed-off interval. A second indicator of successful isolation was the speed of recovery within the sampling interval after pumping was discontinued. If the head between the packers (monitored by a transducer) recovered more quickly than expected given the aquifer properties, then the packer was reseated. Two methods were used to collect the samples following isolation and purging of the sample interval. In the first method, groundwater was pumped from the isolated interval directly to a sampling port (flowthrough method). In the second method, samples were collected with a bailer after the pump was pulled (pump-and-retreat method).
A total of 32 groundwater samples were collected from 3-m intervals isolated by the straddle packer; 16 of these interval depths corresponded to the depths of cores. Interval groundwaters are referred to by the designation SP followed by the depth in meters of the midpoint of the 3-m packed-off interval. Groundwater collected from packed-off intervals for microbiological analyses was pumped or collected from the bailer into sterile, polypropylene containers and held on ice prior to shipment as was done for the core samples. Results of microbiological analyses of groundwater samples were considered to represent unattached portions of the subsurface community. Geochemistry samples were filtered through a 0.2-μm-pore-size filter, acidified with 0.5 ml of 1:1 HCl, and placed on ice. The concentration of Fe2+ was determined within 12 h by titration with KMnO4. Comprehensive geochemical analysis of the samples was performed at the USBM Twin Cities Research Center laboratory using inductively coupled plasma atomic emission spectrometry for total sulfur and all cations except K, Pb, Se, As, and Hg. Potassium was determined using flame atomic absorption spectrometry, and the remaining cations were determined using graphite furnace atomic absorption spectrometry. Ion chromatography was used for anion concentration determinations.
The pH, temperature, Eh, dissolved oxygen content, and conductivity of the interval groundwater samples were determined for each interval sample with a calibrated H20G multiprobe (Hydrolab Corp., Austin, Tex.). In the flowthrough mode the water was pumped directly through an acrylic chamber and past the sensors of the Hydrolab multiprobe. With the bailer sampling method, the multiprobe was lowered into the well until it was less than 1 m above the pipe-packer fitting following sample collection with the bailer. The electrodes were allowed to equilibrate with the groundwater for 15 to 20 min before the reading was taken. A low-flow membrane (developed by Hydrolab) was used on the dissolved-oxygen sensor in order to facilitate readings in standing water prior to the collection of bailer samples. The standard membrane was used when the instrument was used in the flowthrough mode. In addition to the electrochemistry performed on interval samples, a calibrated multiprobe (Hydrolab H20G) was used to profile the entire column of groundwater in the uncased well prior to straddle packer pump sample collection in August and September 1995. The temperature, dissolved oxygen content, conductivity, pH, and Eh were recorded at approximately 1-m intervals.
Microbiological analyses. (i) Sample preparation
Rock fragments from the pared core and surface rubble samples were reduced in particle size to a fine dust by a Spex mill (model 8500; Spex Industries, Inc., Edison, N.J.) in the laboratory. The crushing was performed aseptically, with the critical components being wiped with 1% bleach solution and dried under vacuum between samples. Pilot studies using samples spiked with Pseudomonas chlororaphis and Acidithiobacillus ferrooxidans indicated no biological carryover between samples and no ill effect of the crushing process or disinfection on the viability of these organisms (data not shown). Dilutions of crushed rock and groundwaters were made in phosphate-buffered saline (1.18 g of Na2HPO4, 0.223 g of NaHPO4·H20, and 8.5 g of NaCl per liter; pH 7.0) for neutrophilic organisms and in a pH 3 salts solution [1.25 g of (NH4)2SO4, 0.5 g of MgSO4 · 7H2O, and 0.25 g of tryptone soy broth per liter] for acidophilic organisms.
(ii) Total cell counts and PLFA analysis.
Acridine orange direct cell counts were performed on all core and groundwater samples to estimate the total number of bacterial cells per gram of rock or milliliter of water by the method of Kieft et al. (24). Phospholipid fatty acid (PLFA) analyses were performed on rock and groundwater samples using standardized methods by Microbial Insights (Knoxville, Tenn.) (45). Biomass was estimated by measurement of the quantity of ester-linked phospholipid fatty acids in the samples, and a structural community profile was generated for each sample by community-level PLFA analyses (45). The molar percentages of the different classes of PLFA were compared by multivariate techniques, and sample profiles were examined for signature markers for specific taxonomic groups of microorganisms.
(iii) Enumeration and enrichment of culturable heterotrophs—aerobic, anaerobic, iron-reducing, and sulfate-reducing.
Standard spread plate count methods for aerobic heterotrophic organisms at circumneutral pH were performed with sample dilutions on triplicate plates of 10% TSA (29) and R2A (36) media incubated at room temperature for 2 weeks. Because of the low neutrophilic, heterotrophic biomass anticipated in the rock samples, supplementary direct contact plates were created by sprinkling solid material across the agar surface. Fermenters and facultative anaerobic heterotrophs were assayed by incubation of triplicate spread plates of R2A under a 95% N2–5% CO2 atmosphere at room temperature for 4 weeks prior to counting. Aerobic and anaerobic heterotrophic plate counts on interval groundwaters were performed using 10% TSA only. Estimates of culturable heterotrophic diversity were obtained by isolating morphologically distinct colonies (using size, color, edge, elevation, and consistency as parameters) from each group of samples (i.e., core or groundwater). The presence of dissimilatory-iron-reducing bacteria was determined by enrichment of 1 g or 1 ml of sample in iron oxyhydroxide media in anoxic serum vials at pH 7.0 (27). The presence of sulfate-reducing bacteria was determined by enrichment of 1 g or 1 ml of sample in lactate media (35) in anoxic serum vials at pH 7.0. Primary enrichments of iron- or sulfate-reducing bacteria that were positive by presumptive evidence (i.e., precipitates) were transferred to fresh medium. Secondary enrichments that were positive by presumptive evidence were examined for the presence of high numbers of cells by direct observation of acridine orange-stained smear preparations. Secondary enrichments with confirmed presence of high numbers of cells were recorded as positive.
(iv) Acidophiles cultured on solid media.
Acidophilic heterotrophs and acidophilic chemolithotrophs were enumerated from crushed cores and groundwater by spread plating on glycerol (0.1%)- and yeast extract (0.02%)-amended basal salts–0.025% tryptone soy broth medium (solidified with 0.5% agarose) (22) and tetrathionate-ferrous iron-amended overlay medium (20), respectively. Media were prepared at pH 3.0. Crushed rock samples were prediluted with basal salts medium, mixed, and shaken for several hours prior to plating. Enumerations were performed on both media after 2 weeks and reported as acidophilic heterotrophs and chemolithotrophs, respectively. Colony morphology was used to distinguish different physiologies growing on the sulfur-ferrous iron-amended plates, i.e., iron-oxidizing chemotrophs versus sulfur-oxidizing chemotrophs versus iron-oxidizing heterotrophs (20). Representatives of distinct colony morphologies of heterotrophic and iron-oxidizing acidophilic bacteria were isolated and purified for genetic analysis. Genomic DNA was obtained from the isolates after lysis with lysozyme-proteinase K (4), and 16S ribosomal DNA (rDNA) was amplified by PCR performed using Tfl DNA polymerase (Thermus flavus; Epicentre Technologies, Madison, Wis.), a Thermolyne PTC-100 thermocycling apparatus, and eubacterial 8F and 1492R primers (44). PCR products were cloned into plasmid pUC18 using the CloneAMP pUC18 system (GIBCO BRL, Bethesda, Md.) and transformed into Escherichia coli DH5α. Plasmid DNA was isolated from transformed colonies and purified by CsCl density gradient centrifugation. The resulting DNA was sequenced (4000L automated DNA sequencer; LiCor, Inc., Lincoln, Nebr.), manually aligned, and compared with 16S rRNA sequences deposited in the Ribosomal Database Project. Sequence analysis and determination of phylogenetic position were performed by the method of De Soete (9), and the sequences are presented relative to their nearest neighbors as indicated by BLAST analysis (2).
(v) Autotrophic acidophilic liquid enrichments.
The presence or absence of acidophilic chemolithotrophs using specific electron donors was determined by inoculating 1 g or 1 ml of samples into five different types of basal salts liquid enrichment medium [1.5 g of (NH4)2SO4, 0.5 g of KCl, 5.0 g of MgSO4 · 7H2O, and 0.1 g of Ca(NO3)2 per liter] containing a trace element solution (23) and either 20 mM ferrous sulfate, 0.1% (wt/vol) pyrite, 0.1% (wt/vol) elemental sulfur, 5 mM tetrathionate, or 5 mM tetrathionate plus 20 mM thiosulfate as a donor, with the medium pH ranging from 1.8 to 2.5. Liquid acidophilic enrichments were incubated at room temperature in the dark with shaking. Primary enrichments that were positive by presumptive evidence (i.e., precipitates or turbidity) were transferred to fresh medium. Secondary enrichments that were positive by presumptive evidence were examined for the presence of high numbers of cells by direct observation of acridine orange-stained smear preparations. Secondary enrichments with confirmed presence of high numbers of cells were recorded as positive. For all culture assays, uninoculated media and solid media spread with sterile dilution buffer were maintained under incubation conditions as negative controls; positive controls consisted of media inoculated with a culture of the target physiological type and maintained under similar conditions.
(vi) Direct extraction of bulk DNA.
Direct extraction of DNA from core materials was attempted using a variation of the method of Tsai and Olson (41). Five grams of pulverized core material was mixed with 10 ml of 120 mM sodium phosphate buffer, pH 8.0. The slurry was shaken for 15 min at room temperature, after which the mixture was centrifuged at 6,000 × g for 10 min, and the resulting pellet was resuspended in 10 ml of lysis solution (0.15 M NaCl, 0.1 M Na2EDTA [pH 8.0], 5 μg of proteinase K ml−1). After incubation for 2 h with agitation at 30-min intervals, 10 ml 0.1 M NaCl–0.5 M Tris HCl (pH 8.0)–10% sodium dodecyl sulfate was added. The resulting suspension was subjected to three cycles of freeze-thawing, freezing in a dry ice-ethanol bath, and thawing in a 65°C water bath. The lysed suspension was centrifuged for an additional 10 min at 6,000 × g, after which sodium dodecyl sulfate was removed from solution using detergent absorber beads (Boehringer Mannheim) according to manufacturer's instructions. Nucleic acids were precipitated with the addition of 0.6 volume of isopropanol and overnight incubation of the solution at −20°C, followed by centrifugation at 10,000 × g for 20 min at 4°C. The nucleic acid-containing pellet was dried briefly under vacuum and resuspended in sterile, 0.2-μm filtered TE buffer (10 mM Tris HCl, 1 mM EDTA [pH 8.0]). 16S rDNA amplification was subsequently performed as described above, and gel electrophoresis was used to screen for products.
Nucleotide sequence accession numbers.
Partial 16S rDNA sequences (ca. 1,250 bases) for the following isolates have been deposited in the GenBank nucleotide sequence database (accession numbers in parentheses): MPH2 (AF352793), MPH3 (AF352794), MPH5 (AF352792), and MPH6 (AF352791).
RESULTS
Geochemistry.
The groundwater electrochemical profiles taken from the uncased, unpacked corehole showed sharp fluctuations in parameters as a function of depth, most likely a result of fracture-controlled flow (Fig. 2). All groundwater samples were acidic (pH ∼2.8 to 5.0) and contained high concentrations of dissolved solids with respect to groundwater compositions upgradient from the mine pit in undisturbed areas (data not shown). However, the groundwater samples are dilute in ionic strength compared with the surface water found in the mine pits, which is constantly being recirculated through copper-bearing dumps and drill and blast areas (data not shown). Through most of the profile the dissolved oxygen levels were below detection (<0.5 mg liter−1) except at the top and at the bottom of the well. The high dissolved oxygen levels in the bottom of the well may be attributed to high flow from a large fracture detected at 100 m. The deeper intervals also showed anomalous pH, Eh, and conductivity values that are similar to those for upgradient groundwater that has not been affected by mining activity. Temperatures recorded in the undisturbed well before interval pumping climbed steadily from 18°C at the top of the well to 23°C at the bottom. The temperatures at the bottom of the unpacked corehole are consistent with measurements made with the downhole multiprobe using the pump-and-retreat method for interval groundwater sampling. Temperature measurements made in the flow cell at the surface were unreliable due to solar heating of the pump hose.
The two sampling methods (flowthrough and pump-and-retreat) yielded equivalent concentrations of groundwater constituents and enumerations of acidophilic and neutrophilic bacteria on the same interval sampled consecutively (SP52.8; data not shown). To control for temporal effects on the two episodes of interval groundwater sampling (August and September 1995), a single interval (SP75.0/75.8) was sampled on both occasions, and concentrations of groundwater constituents and enumerations of acidophilic and neutrophilic bacteria were consistent (data not shown). The geochemistry of groundwater samples from several representative intervals spanning a range of compositions encountered is provided in Table 1. The geochemical speciation model, EQ3 (UCRL-MA-110662 PT1; Lawrence Livermore National Laboratory, Livermore, Calif.), indicated a high quality of the analyses and complete coverage of the major analytes as the respective maximum and minimum charge imbalances were 1% and −5% of the total charge. The majority of the calculated charge imbalances were within ±1% of the total charge. Ferrous iron represented over 95% of the total dissolved iron (ca. 200 to 300 mg liter−1) found in the interval groundwaters. EQ3 speciation of the waters showed that approximately 64% of the ferrous iron was “free” (unassociated with ligands) and 36% was associated with sulfate as FeSO4(aq). The activities of other ferrous iron species were negligible. The EQ3 speciation results showed that the potential of the ferrous-ferric iron couple was 0.5 to 1.9 V greater than the electrode potential (Eh). Iron-oxidizing bacteria could be responsible for maintaining this disequilibrium. The cations Si, Al, Mn, Na, Cu, and Zn were present at concentrations in the tens to hundreds of milligrams per liter. The predominant anion was sulfate, which ranged from approximately 3,000 to 4,000 mg liter−1. This result along with EQ3 speciation results showed that other forms of dissolved sulfur occurred only in minute amounts. Chloride and fluoride levels ranged from 10 to 80 mg liter−1, while nitrate and phosphate levels were below detection (<6 mg liter−1).
TABLE 1.
Representative interval groundwater compositions from well C-1024
| Depth (m) | Concn (mg liter−1) ofa:
|
Condc (mS cm−1) | pH | Eh (dV) | Temp (°C) | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al | Mn | Total Fe | Fe2+ (%) | Mg | Ca | Na | K | Cr | Ni | Pb | As | Cu | Zn | Cd | SO4 | Total S | Cl | F | NO3 | PO4 | O2b | |||||
| 18.6 | 97.0 | 152.9 | 57.7 | 216.8 | 208 (96) | 354.0 | 512.5 | 68.2 | 2.3 | 0.10 | 0.81 | 0.03 | <0.010 | 106.2 | 81.8 | 0.22 | 4,202.1 | 1,429.1 | 56.4 | 37.7 | <6.0 | <7.0 | 2.8 | 5.00 | 3.68 | 4.7 | 36.5 |
| 24.6 | 99.8 | 155.1 | 58.0 | 218.5 | 208 (95) | 348.4 | 515.8 | 68.9 | 2.7 | 0.08 | 0.79 | 0.06 | <0.010 | 105.7 | 80.4 | 0.21 | 4,200.0 | 1,453.2 | 56.2 | 37.5 | <6.0 | <7.0 | 0.0 | 5.13 | 3.65 | 3.3 | 28.3 |
| 36.5 | 73.1 | 225.8 | 79.1 | 379.0 | 380 (100) | 486.0 | 483.5 | 66.1 | 8.7 | 0.07 | 0.92 | 0.18 | <0.010 | 58.4 | 76.3 | 0.24 | 5,226.9 | 1,785.7 | 61.6 | 81.2 | <6.0 | <7.0 | 0.2 | 6.20 | 3.34 | 4.6 | 26.8 |
| 54.4 | 100.3 | 148.4 | 55.2 | 202.9 | 195 (96) | 332.4 | 511.2 | 67.5 | 4.6 | 0.09 | 0.72 | 0.13 | <0.010 | 86.1 | 54.7 | 0.20 | 4,087.0 | 1,356.2 | 69.6 | 40.2 | <6.0 | <7.0 | 0.2 | 5.10 | 2.86 | 5.6 | 24.1 |
| 63.3 | 96.7 | 144.8 | 55.4 | 189.8 | 182 (96) | 342.8 | 535.7 | 69.2 | 5.2 | 0.09 | 0.69 | 0.09 | <0.010 | 106.6 | 61.6 | 0.22 | 4,076.3 | 1,424.8 | 63.5 | 38.4 | <6.0 | <7.0 | 0.4 | 5.18 | 3.16 | 6.1 | 25.9 |
| 84.1 | 88.7 | 128.6 | 53.3 | 190.6 | 191 (100) | 338.8 | 523.7 | 68.7 | 6.7 | 0.07 | 0.74 | 0.05 | <0.010 | 104.0 | 53.3 | 0.19 | 4,006.1 | 1,334.6 | 59.8 | 37.0 | <6.0 | <7.0 | 1.0 | 5.15 | 3.92 | 4.0 | 27.6 |
| 90.1 | 89.3 | 137.2 | 52.7 | 177.3 | 177 (100) | 330.6 | 539.8 | 69.0 | 6.8 | 0.07 | 0.64 | 0.05 | <0.010 | 93.0 | 52.7 | 0.20 | 3,962.0 | 1,322.4 | 54.9 | 36.6 | <6.0 | <7.0 | 1.0 | 5.18 | 3.64 | 4.4 | 26.3 |
| 96.0 | 94.1 | 149.4 | 53.8 | 162.3 | 162 (100) | 347.1 | 525.3 | 70.0 | 5.8 | 0.06 | 0.69 | 0.04 | <0.010 | 96.1 | 53.8 | 0.18 | 3,990.5 | 1,360.0 | 59.9 | 36.9 | <6.0 | <7.0 | 2.2 | 5.29 | 3.62 | 5.6 | 25.4 |
| 105.2 | 91.1 | 126.1 | 54.1 | 183.1 | 179 (98) | 322.5 | 532.6 | 73.3 | 7.3 | 0.06 | 0.59 | 0.05 | 0.01 | 78.0 | 43.7 | 0.19 | 3,970.8 | ND | 69.9 | 27.3 | <12 | <14 | 4.6 | 4.70 | 3.90 | 4.6 | 23.5 |
At all depths, values for Ti were <0.005 mg liter−1, values for Se were <0.010 mg liter−1, and values for Hg and Ag were <0.05 mg liter−1. ND, no data.
Dissolved O2 readings below 0.5 mg liter−1 are considered to be below detection, but absolute instrument readings are reported.
Cond, conductivity.
Exogenous tracer analyses.
A reduction of one or more orders of magnitude in microsphere counts was typically observed in the subcores relative to the core parings (Table 2). Residual microsphere counts in the subcores and the combusted sandstone core were generally less than 2,550 spheres g (wet weight)−1, which compares to values of ca. 105 spheres g−1 reported during similar deployment of microspheres in another fractured rock aquifer (6). Core CM41.8, noted to have the highest abundance of veins in the geologic log, and core CM72.2 had high residual microsphere counts. Since several cross sections from a single common interval were pared in the same sterilized pan, there was a possibility of smearing of microspheres from parings onto subsequent subcore sections. Microspheres appearing in the subcore via this mechanism do not necessarily represent infiltration of contaminant organisms; this effect has been noted in other subsurface studies (J. K. Fredrickson, personal communication). It is unclear if the methods for biological disinfection of processing pans between samples (washing with bleach, rinsing with distilled water, and flaming with a propane torch) effectively remove all microspheres. Perfluorocarbon analyses for all drilling fluid, subcore, and paring samples were below method detection limits (ca. 30 ng ml−1 or g−1). The failure to detect this analyte may have been due to the extended holding time (3 to 4 mo) prior to analyses.
TABLE 2.
Core quality assessed by microsphere analysis
| Core depth (m) | Microsphere count (per g)a
|
Reduction factorb | |
|---|---|---|---|
| Subcore | Parings | ||
| 11.0 | 1,577 | NDd | ND |
| 17.7 | 531 | 115,000 | 217 |
| 23.2 | 521 | ND | ND |
| 29.9 | BDc | 291,000 | —e |
| 36.3 | 400 | 1,130 | 3 |
| 41.8 | 70,200 | 84,500 | 1 |
| 47.2 | 467 | 2,800 | 6 |
| 53.6 | 200 | 597 | 3 |
| 59.7 | 196 | 9,150 | 47 |
| 66.1 | 130 | 7,680 | 59 |
| 72.2 | 8,334 | 130,000 | 16 |
| 78.9 | 2,067 | 534 | 0 |
| 84.1 | 1,460 | 53,300 | 37 |
| 90.5 | 2,546 | 12,300 | 5 |
| 96.9 | 1,183 | 1,260 | 1 |
| 102.7 | 398 | 107,000 | 269 |
| Avg (all) | 5,638 | 58,304 | 51 |
| SD (all) | 17,334 | 82,719 | 88 |
Average of three independent preparations.
Count of parings divided by count of subcore.
BD, below detection limit (65 microspheres g [wet weight]−1).
ND, no data.
Infinity.
Microbiology. (i) Total cells.
Total cell counts for attached bacteria in all core samples, the combusted blank core and the surface rubble were at or below the detection limit (ca. 105 cells g [wet weight]−1) for this method. For the unattached bacteria present in the groundwaters, direct counts were all around 105 cells ml−1, with relatively little variability (mean ± standard deviation [SD], 3.14 × 105 ± 1.56 × 105; n = 16). Total cell counts were 6.6 × 105 cells ml−1 and 6.4 × 105 cells ml−1 for MW1 and MW2, respectively. The water in the pit had 2.7 × 105 cells ml−1 and contained filamentous microalga species not observed in other samples.
(ii) PLFA.
The amount of attached and unattached biomass as measured by total PLFA was at or below the detection limits for the assay as performed on these core and groundwater samples (≤4 pmol g−1 or ml−1). The greatest overall biomass was observed in the surface sample of weathered outcrop (90 pmol of PFLA g−1). Based on prior studies, this amount of biomass can be converted to roughly 5 × 106 viable cells (45). The drilling fluid makeup groundwaters (MW1 and MW2) contained 4.5 pmol of PLFA ml−1 (3 × 105 cells ml−1), and the pit water contained 11 pmol of PLFA ml−1 (7 × 105 cells ml−1). The molar percentages of PLFA in the drilling fluid makeup groundwaters indicated a community composed of gram-negative bacteria, possibly Pseudomonas spp., that contain high proportions of monoenoic fatty acids such as 18:1ω9c and 18:1:ω7c (D. Ringelberg, personal communication). In the weathered surface rubble, the PLFA signature suggested that Actinomycetes might be the dominant organisms due to the abundance of mid-chain-branched saturates, while in the Ithaca Pit surface water samples, the high percentages of 18:1ω7c and 17:0 suggested gram-negative organisms (like A. ferrooxidans) (D. Ringelberg, personal communication).
(iii) Neutrophilic heterotrophs.
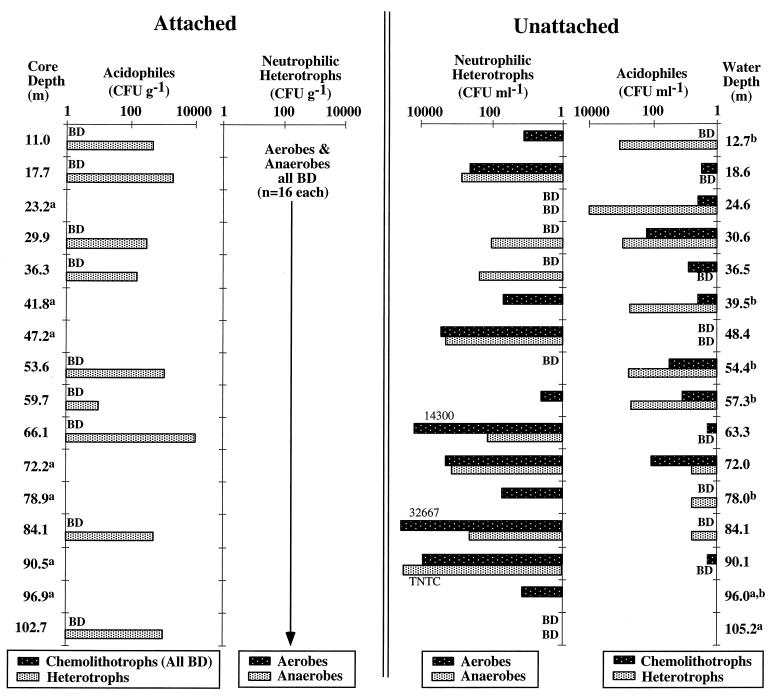
The numbers of attached aerobic and anaerobic neutrophilic heterotrophs were below method detection limits (ca. 40 CFU g [wet weight]−1) for all 16 core samples and the combusted blank core which were spread on 10% TSA and R2A media. However, occasional colonies resulting from inoculation of core samples onto the spread and sprinkle plates (both 10% TSA and R2A) were isolated and morphologically characterized. By streaking out distinct colony types for each core sample, a total of 40 morphologically distinct aerobic neutrophilic heterotrophs were isolated from the sum of all plates from all 16 cores. A maximum of five morphologically distinct colonies were found in a single core; not all cores were represented. Similarly, four anaerobic heterotrophic isolates were obtained from the sum of all plates from all 16 cores spread on R2A media.
The numbers of unattached aerobic and anaerobic neutrophilic heterotrophs ranged from below detection (<10 CFU ml−1) to more than 104 CFU ml−1, depending on the groundwater interval sampled (Fig. 3). The mean numbers of unattached aerobic and anaerobic heterotrophs were 3.80 × 103 CFU ml−1 (1 SD, 8.67 × 103; n = 16) and 9.32 × 102 CFU ml−1 (1 SD, 1.14 × 103; n = 9), respectively (Fig. 3). There was no significant difference between the mean numbers of aerobes and anaerobes for the groundwater samples possessing both enumerations (P = 0.199, Student's two-tailed paired t test; n = 9). A total of eight morphologically distinct aerobic heterotrophs were isolated from the sum of all plates for all 16 interval groundwaters spread on 10% TSA media; no more than one isolate type was observed per sample. Four anaerobic chemoheterotrophic isolates were recovered from 10% TSA from the sum of all plates from nine interval groundwaters.
FIG. 3.
Results of microbiological analyses using solid media plating. Populations cultured from the core (“attached”) are juxtaposed with populations cultured from the groundwater (“unattached”). Depth is expressed in meters (m) below land surface. BD, below detection limits; TNTC, too numerous to count. a, neither acidophilic enumeration was performed on this sample; b, no enumeration of neutrophilic anaerobes was done for this sample.
Aerobic neutrophilic heterotrophic plate counts for the makeup waters taken at the start (MW1) and end (MW2) of the 2-week sampling period were both 2.3 × 105 CFU ml−1. No colonies were apparent on makeup water samples after sodium hypochlorite was added, indicating effectiveness of the drilling fluid pretreatment. Weathered surface rubble from the drilling site contained 7.7 × 104 CFU g (wet weight)−1 and acidic pit waters (pH 2.5) contained 3.0 × 102 CFU of aerobic neutrophilic heterotrophs ml−1. Only two morphologically distinct aerobic neutrophilic heterotrophic isolates were cultured from the groundwater samples, MW1 and MW2 (same two isolates at both sampling times). These two aerobic chemoheterotrophs cultured from the drilling makeup water (prior to disinfection) were never cultured from any cores. Four morphologically distinct isolates were cultured from both the surface rubble and the pit water. No colonies were formed from spread plate inoculations of the combusted core.
(iv) Iron and sulfate reducers.
Enrichments for heterotrophic iron-reducing bacteria were negative for all cores and groundwater samples and positive only for Ithaca Pit surface water. Enrichments for sulfate-reducing bacteria were successful only for samples MW1, CM72.2, CM96.9, CM102.7, and SP105.2.
(v) Culturable acidophiles on solid media.
Attached acidophilic heterotrophs (i.e., Acidiphilium spp.) were commonly recovered through out the depth profile, with CFU ranging from 102 to 104 g (wet weight)−1. In contrast, attached iron- or sulfur-oxidizing chemolithotrophs were never recovered (detection limit, 10 CFU g [wet weight]−1) (Fig. 3). While no strictly chemolithotrophic iron oxidizers were recovered from any of the core samples, there was evidence of iron-oxidizing heterotrophs which grew sparingly on iron media alone, presumably utilizing contaminants in the agarose-gelled media as a carbon source. These organisms were grown much more efficiently after transfer to heterotrophic acidophile media. In the groundwaters, however, unattached chemolithotrophs were present in numbers from 10 to 102 CFU ml−1 in addition to acidophilic heterotrophs numbering 10 to 104 CFU ml−1. Based on characteristic colony morphologies, at least three different iron-oxidizing types were identified, including A. ferrooxidans, iron-oxidizing heterotrophs of the T21 type, i.e., “Ferromicrobium acidophilus ” (as described by Johnson et al. [21]), and novel iron oxidizers producing irregular colony margins, often with heavy zones of oxidized iron precipitation surrounded by areas of more uniform ferric iron deposition. Another unusual morphology observed was a colony type in which white sulfur compounds crystallized in a background of regular ferric precipitate. The sulfur-oxidizing A. thiooxidans was also identified at several groundwater interval depths by an absence of iron precipitates accumulating around colonies.
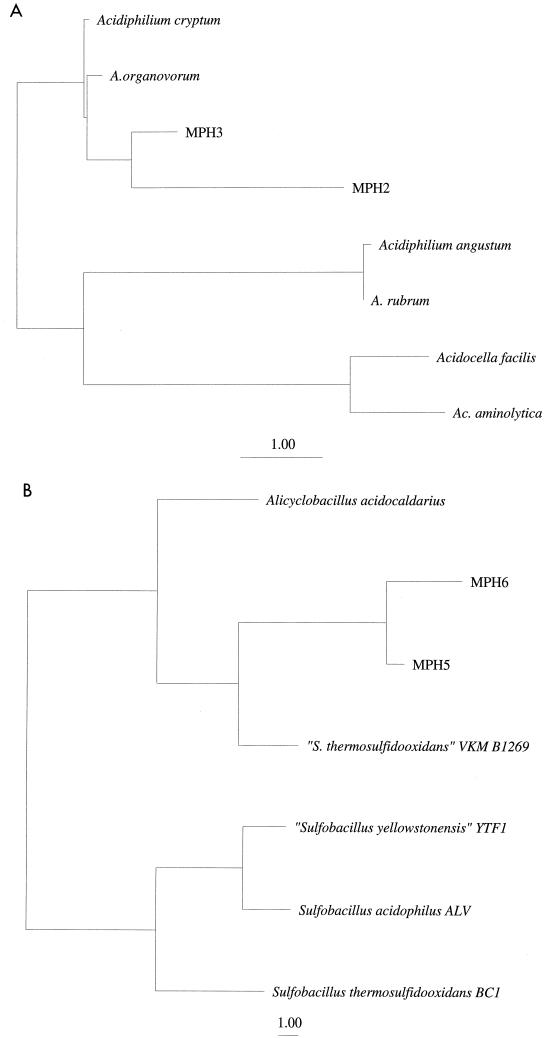
In an effort to definitively identify the various bacteria which have been provisionally identified based on behavior on the various acidophilic media utilized in this study, single colonies with unique morphologies, as well as those with similar morphologies from different depths, were grown to allow large-scale isolation of genomic DNA for subsequent amplification of 16S rDNA sequences and phylogenetic analysis. As expected, several of the acidophilic heterotrophs (MPH2 and MPH3) cultivated from core samples (non-iron-oxidizing isolates) appear to be closely related to previously described members of the genus Acidiphilium (Fig. 4A). However, other acidophilic heterotrophs (MPH5 and MPH6) yielded 16S rDNA sequences most similar to those of gram-positive, moderately thermophilic Alicyclobacillus acidocaldarius (Fig. 4B). These isolates have been grown at elevated temperatures (47°C).
FIG. 4.
(A) Phylogenetic positions of Mineral Park mesophilic heterotrophic acidophiles, MPH2 and MPH3, relative to Acidiphilium and Acidocella species of the alpha subclass of Proteobacteria (unrooted). Scale bar, 1% sequence divergence. (B) Phylogenetic position of Mineral Park moderately thermophilic acidophiles, MPH5 and MPH6, relative to other sulfobacilli of the high-G+C DNA content gram-positive bacteria (unrooted). From the positions of MPH5 and MPH6, they would be predicted to be non-iron-oxidizing sulfobacilli. Quotation marks indicate nonauthoritative nomenclature.
(vi) Liquid acidophilic, autotrophic enrichments.
For attached bacteria, liquid enrichments with ferrous sulfate were positive from the lower portion of the corehole, and two cores, CM36.3 and CM78.9, were positive with elemental sulfur as an energy source (Table 3). In contrast, liquid enrichments for unattached acidophilic chemoautotrophic bacteria were widely successful with elemental sulfur, tetrathionate, tetrathionate-thiosulfate, and, to a lesser extent, ferrous sulfate. No enrichments with pyrite as the electron donor were positive for either attached or unattached bacteria, although organisms closely associated with the pyrite surface may have been overlooked in examination of the liquid. Liquid enrichments were more favorable for the initial cultivation of sulfur oxidizers than the solid overlay media. Once grown in the liquid enrichment media, sulfur-oxidizing organisms could be transferred successfully to solid overlay media amended with ferrous iron and tetrathionate (data not shown).
TABLE 3.
Results of acidic liquid media enrichments with different electron donors for paired core and groundwater samples
| Sample type and depth BLS (m)a | Resultb with electron donor
|
Sample type and depth BLS (m)c | Resultb with electron donor
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FeSO4 | (S4O6)2− | (S2O3)2−/(S4O6)2− | S0 | FeSO4 | (S4O6)2− | (S2O3)2−/(S4O6)2− | S0 | |||
| Core (attached) | Groundwater (unattached) | |||||||||
| 11.0 | − | − | − | − | 12.7 | − | + | + | + | |
| 17.7 | − | − | − | − | 18.6 | − | + | + | + | |
| 23.2 | − | − | − | − | 24.6 | − | + | + | + | |
| 29.9 | − | − | − | − | 30.6 | − | + | + | ND | |
| 36.3 | − | − | − | + | 36.5 | + | + | + | + | |
| 41.8 | − | − | − | − | 39.5 | − | + | + | ND | |
| 47.2 | − | − | − | − | 48.4 | − | + | + | + | |
| 53.6 | + | − | − | − | 54.4 | + | + | + | + | |
| 59.7 | − | − | − | − | 57.3 | + | + | + | + | |
| 66.1 | + | − | − | − | 63.3 | + | + | + | + | |
| 72.2 | + | − | − | − | 72.0 | + | + | + | + | |
| 78.9 | + | − | − | + | 78.0 | − | + | + | + | |
| 84.1 | + | − | − | − | 84.1 | − | + | + | + | |
| 90.5 | + | − | − | − | 90.1 | − | + | + | ND | |
| 96.9 | + | − | − | − | 96.0 | + | + | + | + | |
| 102.7 | + | − | − | − | 105.2 | − | + | + | + | |
Depth below land surface (BLS) indicates the depth of the 0.3-m core segment (of a 1.5-m core) that was processed.
Positive (+) and negative (−) enrichments are indicated. ND, no data.
Depth below land surface (BLS) was measured from the midpoint of the 3-m packed-off interval that was sampled.
(vii) Direct extraction of bulk DNA.
In an effort to reconstruct the in situ microbial community structure using phylogenetically informative sequences (i.e., 16S rDNA gene sequences), DNA was extracted directly from core samples, CM17.7 and CM41.8, and amplification of 16S rDNA sequences was attempted. Estimates of the sensitivity of this technique suggest that as little as 1 pg of target DNA can serve as an efficient template for the technique (10, 41). Due to interfering materials (possibly high concentrations of cations), our limit of detection was 100 pg. Based on an estimate of the DNA content of a “typical” bacterial cell (E. coli) of about 1 × 10−14 g of DNA cell−1 (31), our limit of detection would be on the order of 2 × 103 cells g−1, given that the isolation procedure was scaled to handle 5 g of core material. In no case were we able to detect amplified 16S rDNA fragments from the two samples tested, even after a second round of amplification, except in positive controls spiked with control bacterial genomic DNA. This result is consistent with data from PLFA analyses and acridine orange direct counts also performed on core materials, which suggests that cell numbers were below the limits of detection of those techniques as well (again on the order of 104 cells g−1). DNA extraction on groundwater samples was not attempted due to the limited volume retrieved, the low biomass, and the high concentrations of cations.
DISCUSSION
In this study of 16 paired core and groundwater samples from a single corehole in a crystalline, fractured rock aquifer, the majority of organisms were suspended in the groundwater and not attached to rock surfaces. There were decided compositional differences between attached and unattached communities with respect to acidophilic and neutrophilic organisms. Acidophilic chemolithotrophs were absent in attached communities and commonly present in unattached communities. Since over 95% of the dissolved iron in the groundwater was in the ferrous state and core surfaces were largely unoxidized, it is likely that the activity of these unattached chemolithotrophs was limited, probably by available oxygen, nitrogen, or CO2. There were quantitative differences between the numbers of attached and unattached neutrophilic chemoheterotrophs, and qualitative differences in these populations were suggested by the greater morphologic diversity of colonies cultured from cores than of those from groundwater. The in situ activity (at low pH) of the neutrophilic chemoheterotrophs is uncertain. The isolation of moderately thermophilic organisms related to Alicyclobacillus acidocaldarius raises the interesting question of how these organism have come to reside in subsurface mineral deposits and what role they may play in the microbial ecology of such an environment.
Since the report of Harvey et al. (16), researchers have generally concluded that attached bacteria dominate subsurface environments in biomass and activity and that planktonic cells are inactive subsets of the attached organisms or transients (17, 34). Our finding of higher numbers and diversity of unattached bacteria than of attached bacteria appears to be at odds with this general conclusion. However, there are only limited microbiological data on multiple observations of authentic, defensible, depth-paired core and water from the same corehole to support this generalization. A review of the studies that fulfill these criteria yields the following observations. Koebel-Boelke et al. (25) found more attached biomass in a sandy aquifer with some differences between culturable organisms in the groundwater and those colonizing the sediment. Godsy et al. (13) reported more biomass attached to sandy sediments than in the comparative groundwater, although some physiological groups (methanogens) were found mostly in the groundwater. Bekins et al. (5) found a bimodal distribution of the relative abundance of planktonic bacteria (the modes were 15 and 100% of the total cells) in a set of cores and groundwater from a sedimentary aquifer. Those authors also reported that methanogens were recovered more often in the groundwater than from the sediments. Therefore, when comparisons are carefully controlled, it does seem that there are organisms that are unique to the groundwater and are probably not transients (i.e., methanogens) and that unattached biomass may predominate over attached biomass under some circumstances.
The three studies cited above were all conducted in unconsolidated sedimentary aquifers which have been the sites for other studies that have concluded that the majority of subsurface biomass is attached (16, 17, 40). In contrast, our data were taken from a crystalline, fractured rock aquifer where groundwater flow is not through true porous media but largely confined to fractures in the low-permeability matrix. Because organisms might be expected to concentrate on surfaces of fracture faces, aseptic dissection of fracture surfaces for independent characterization was attempted, but it was not technically feasible, and the attached biomass was expressed per unit of mass of bulk rock. It may be appropriate to express attached biomass in fractured rock aquifers in other units (i.e., surface area or unit of volume of aquifer), as suggested by Pederson and Ekendahl (32, 34), who used colonized artificial substrata to assess attached microbes in a crystalline rock aquifer. A limited number of samples (one groundwater and three rock samples) were compared in the only (to our knowledge) study that compared attached and unattached bacteria in actual samples taken from a crystalline, fractured rock formation (3). Those authors found about equal numbers of attached (per gram) and unattached (per milliliter) heterotrophs in ashfall tuff and the corresponding groundwater accessed from tunnels at the Nevada Test Site but found that the identities of these groups of isolates were very different. Colwell and Lehman (unpublished data) have observed little biomass and activity associated with basalt cores compared to groundwater taken from the same depth in a single corehole. While there is difficulty in equitably comparing attached and unattached biomass in crystalline, fractured rock aquifers, the existing data support compositional differences between attached and unattached communities in these settings.
Another factor which may differentiate shallow, unconsolidated sedimentary aquifers and deeper, crystalline, fractured rock aquifers is the amount of organic matter that is present. Ghiorse and Wilson (12a) discussed the paradigm that organisms should be attached in proximity to large amounts of surface area in porous media. This expectation is generated by the knowledge that surfaces also tend to concentrate metabolizable organic matter. The studies that have reported the dominance of attached biomass have been conducted in sedimentary aquifers which contain particulate organic carbon deposited in the geologic media, as opposed to the quartz-monzonite porphyry investigated in this study. Further it might be expected that shallow groundwaters passing through porous media may contain greater amounts of dissolved carbon than are present in deeper, crystalline rock aquifers such as the one in the present study (groundwater total organic carbon, ca. 2 to 3 mg liter−1). However, the effect of carbon and nutrient enrichment on the distribution of aquifer organisms between attached and unattached states is controversial. Nutrient enrichment of groundwaters is thought by some researchers to favor increased planktonic biomass (16, 17), while the opposite finding has been reported in one case (5) and suggested by literature on ultramicrobacteria (26). Therefore, it is difficult to conclude if the relatively small amount of organic carbon in the aquifer studied contributed to the lower numbers and diversity of attached organisms.
The partitioning of populations and physiologies between attached and unattached communities underscores the need to sample both core and groundwater to achieve comprehensive microbiological characterization of aquifers. The segregation of functional potential between attached and unattached communities and the known changes in cell enzyme expression associated with surface attachment indicate a probable impact of microbial partitioning on solute transport in aquifers. The partitioning of in situ function between attached and unattached microorganisms in this and other aquifers remains unknown and represents the critical information for applications. Plans for in situ manipulation of saturated subsurface environments for mining, fossil fuel extraction, bioremediation, or waste repository purposes should reflect knowledge of the location and mobility of indigenous organisms, attached or unattached, and their activities.
ACKNOWLEDGMENTS
This research was supported by funding provided by the former U.S. Bureau of Mines to the Idaho National Engineering and Environmental Laboratory (INEEL), operated at that time by Lockheed Martin Idaho Technologies Co. under contract DE-AC07-94ID13223, and by funding provided by the Department of Energy, Office of Environmental Management to the INEEL operated by Bechtel BWXT, LLC., under contract DE-AC07-99ID13727.
REFERENCES
- 1.Alfreider A, Krossbacher M, Psenner R. Groundwater samples do not reflect bacterial densities and activity in subsurface systems. Water Res. 1997;31:832–840. [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Amy P S, Haldeman D L, Ringelberg D, Hall D H, Russell C. Comparison of identification systems for classification of bacteria isolated from water and endolithic habitats within the deep subsurface. Appl Environ Microbiol. 1992;58:3367–3373. doi: 10.1128/aem.58.10.3367-3373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ausubel F M, Brent R, Kingston R E, Moore D D, Seldman J G, Smith J A, Sturtert R, editors. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons, Inc.; 1999. [Google Scholar]
- 5.Bekins B A, Godsy E M, Warren E. Distribution of microbial physiologic types in an aquifer contaminated by crude oil. Microb Ecol. 1999;37:263–275. doi: 10.1007/s002489900149. [DOI] [PubMed] [Google Scholar]
- 6.Colwell F S, Stormberg G J, Phelps T J, Birnhaum S A, McKinley J P, Rawson S A, Veverka C, Goodwin S, Long P E, Russell B F, Garland T, Thompson D, Skinner P, Grover S. Innovative techniques for collection of saturated and unsaturated subsurface basalts and sediments for microbiological characterization. J Microbiol Methods. 1992;15:279–292. [Google Scholar]
- 7.Crump B C, Armbrust E V, Baross J A. Phylogenetic analysis of particle-attached and free-living bacterial communities in the Columbia River, its estuary, and the adjacent coastal ocean. Appl Environ Microbiol. 1999;65:3192–3204. doi: 10.1128/aem.65.7.3192-3204.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Debus K H. Identifying the biohydrometallurgical processes with the greatest probability of commercial adoption. In: Salley J, McCready R G L, Wichlacz P, editors. Biohydrometallurgy '89. Ottawa, Canada: Canmet; 1989. pp. 487–498. [Google Scholar]
- 9.De Soete G. A least squares algorithm for fitting additive trees to proximity data. Psychometrika. 1983;48:621–626. [Google Scholar]
- 10.Erlich H A. PCR technology: principles and applications for DNA amplification. New York, N.Y: Stockton Press; 1989. [Google Scholar]
- 11.Fredrickson J K, Phelps T J. Subsurface drilling and sampling. In: Hurst C J, Knudsen G R, McInerney M J, Stetzenbach L D, Walter M V, editors. Manual of environmental microbiology. Washington, D.C.: ASM Press; 1997. pp. 526–540. [Google Scholar]
- 12.Friedrich U, Schallenberg M, Holliger C. Pelagic bacteria-particle interactions and community-specific growth rates in four lakes along a trophic gradient. Microb Ecol. 1999;37:49–61. doi: 10.1007/s002489900129. [DOI] [PubMed] [Google Scholar]
- 12a.Ghiorse W C, Wilson J T. Microbial ecology of the terrestrial subsurface. Adv Appl Microbiol. 1988;33:107–172. doi: 10.1016/s0065-2164(08)70206-5. [DOI] [PubMed] [Google Scholar]
- 13.Godsy E M, Goerlitz D F, Grbic Galic D. Methanogenic biodegradation of creosote contaminants in natural and simulated groundwater ecosystems. Ground Water. 1992;30:232–242. [Google Scholar]
- 14.Gounot A M. Microbial ecology of groundwaters. In: Gibert J, Danielopol D L, Stanford J A, editors. Groundwater ecology. San Diego, Calif: Academic Press; 1994. pp. 189–504. [Google Scholar]
- 15.Griffin W T, Phelps T J, Colwell F S, Long P E. Methods for obtaining deep subsurface microbiological samples by drilling. In: Amy P S, Haldeman D L, editors. The microbiology of the terrestrial deep subsurface. Boca Raton, Fla: CRC Press; 1997. pp. 23–44. [Google Scholar]
- 16.Harvey R W, Smith R L, George L. Effect of organic contamination upon microbial distributions and heterotrophic uptake in a Cape Cod, Mass., aquifer. Appl Environ Microbiol. 1984;48:1197–1202. doi: 10.1128/aem.48.6.1197-1202.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hazen T C, Jimenez L, de Victoria G L, Fliermans C B. Comparison of bacteria from deep subsurface sediment and adjacent groundwater. Microb Ecol. 1991;22:293–304. doi: 10.1007/BF02540231. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch P, Rades-Rohkohl E. Some special problems in the determination of viable counts of groundwater microorganisms. Microb Ecol. 1988;16:99–113. doi: 10.1007/BF02097408. [DOI] [PubMed] [Google Scholar]
- 19.Holm P E, Nielsen P H, Albrechtsen H-J, Christensen T H. Importance of unattached bacteria and bacteria attached to sediment in determining potentials for degradation of xenobiotic organic contaminants in an aerobic aquifer. Appl Environ Microbiol. 1992;58:3020–3026. doi: 10.1128/aem.58.9.3020-3026.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson D B. Selective solid media for isolating and enumerating acidophilic bacteria. J Microbiol Methods. 1995;23:205–218. [Google Scholar]
- 21.Johnson D B, Bacelar-Nicolau P, Bruhn D F, Roberto F F. Iron-oxidizing heterotrophic acidophiles: ubiquitous novel bacteria in leaching environments. In: Vargas T, Jerez C A, Wiertz J V, Toledo H, editors. Biohydrometallurgical processing '95. Vol. 1. Santiago, Chile: University of Chile; 1995. pp. 47–56. [Google Scholar]
- 22.Johnson D B, Macvicar J H M, Rolfe S. A new solid medium for the isolation and enumeration of Thiobacillus ferrooxidans and acidophilic heterotrophic bacteria. J Microbiol Methods. 1987;7:9–18. [Google Scholar]
- 23.Johnson D B, McGinness S. A highly efficient and universal solid medium for growing mesophilic and moderately thermophilic, iron-oxidizing, acidophilic bacteria. J Microbiol Methods. 1991;13:113–122. [Google Scholar]
- 24.Kieft T L, Amy P S, Brockman F J, Fredrickson J K, Bjornstad B N, Rosacker L L. Microbial abundance and activities in relation to water potential in the vadose zones of arid and semiarid site. Microb Ecol. 1993;26:59–78. doi: 10.1007/BF00166030. [DOI] [PubMed] [Google Scholar]
- 25.Kolbel-Boelke J, Anders E-M, Nehrkorn A. Microbial communities in the saturated groundwater environment. II. Diversity of bacterial communities in a Pleistocene sand aquifer and their in vitro activities. Microb Ecol. 1988;16:31–48. doi: 10.1007/BF02097403. [DOI] [PubMed] [Google Scholar]
- 26.Lappin-Scott H M, Costerton J W. Starvation and penetration of bacteria in soils and rocks. Experientia. 1990;46:807–812. [Google Scholar]
- 27.Lovley D R, Phillips E J P. Organic-matter mineralization with reduction of ferric iron in anaerobic sediments. Appl Environ Microbiol. 1986;51:683–689. doi: 10.1128/aem.51.4.683-689.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall K C. Planktonic versus sessile life of prokaryotes. In: Balows A, Truper H G, Dworkin M, Harder W, Schleifer K-H, editors. The Prokaryotes: a handbook on the biology of bacteria: ecophysiology, isolation, identification, applications. 2nd ed. New York, N.Y: Springer-Verlag; 1992. pp. 262–275. [Google Scholar]
- 29.Martin J K. Comparison of agar media for counts of viable soil bacteria. Soil Biol Biochem. 1975;7:401–402. [Google Scholar]
- 30.McKinley J P, Colwell F S. Application of perfluorocarbon tracers to microbial sampling in subsurface environments using mud-rotary and air-rotary drilling techniques. J Microbiol Methods. 1996;26:1–9. [Google Scholar]
- 31.Neidhardt F C, Ingraham J C, Schaechter M. Physiology of the bacterial cell: a molecular approach. Sunderland, Mass: Sinauer Associates; 1990. [Google Scholar]
- 32.Pedersen K. The deep subterranean biosphere. Earth-Sci Rev. 1993;34:243–260. [Google Scholar]
- 33.Pedersen K. Investigations of subterranean bacteria in deep crystalline bedrock and their importance for the disposal of nuclear waste. Can J Microbiol. 1996;42:382–391. [Google Scholar]
- 34.Pedersen K, Ekendahl S. Distribution and activity of bacteria in deep granitic groundwaters of southeastern Sweden. Microb Ecol. 1990;20:37–52. doi: 10.1007/BF02543865. [DOI] [PubMed] [Google Scholar]
- 35.Postgate J R. The sulphate-reducing bacteria. Cambridge, United Kingdom: Cambridge University Press; 1979. [Google Scholar]
- 36.Reasoner D J, Geldreich E E. A new medium for the enumeration and subculture of bacteria from potable water. Appl Environ Microbiol. 1985;49:1–7. doi: 10.1128/aem.49.1.1-7.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riemann L, Steward G F, Azam F. Dynamics of bacterial community composition and activity during a mesocosm diatom bloom. Appl Environ Microbiol. 2000;66:578–587. doi: 10.1128/aem.66.2.578-587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russell B F, Phelps T J, Griffin W T, Sargent K A. Procedures for sampling deep subsurface microbial communities in unconsolidated sediments. Ground Water Monit Rev. 1992;12:96–104. [Google Scholar]
- 39.Shuter E, Pemberton R R. Inflatable straddle packers and associated equipment for hydraulic fracturing and hydraulic testing USGS Water-Investigations 78–55. 1978. [Google Scholar]
- 40.Thomas J M, Lee M D, Ward C H. Use of ground water in assessment of biodegradation potential in the subsurface. Environ Toxicol Chem. 1987;6:607–614. [Google Scholar]
- 41.Tsai Y-L, Olson B H. Rapid method for direct extraction of DNA from soils and sediments. Appl Environ Microbiol. 1991;57:1070–1074. doi: 10.1128/aem.57.4.1070-1074.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Loosdrecht C M M, Lyklema J, Norde W, Zehnder A J B. Influence of interfaces on microbial activity. Microbiol Rev. 1990;54:75–87. doi: 10.1128/mr.54.1.75-87.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Schie P M, Fletcher M. Adhesion of biodegradative anaerobic bacteria to solid surfaces. Appl Environ Microbiol. 1999;65:5082–5088. doi: 10.1128/aem.65.11.5082-5088.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weisburg W G, Barns S M, Pelletier D A, Lane D J. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White D C. Lipid analysis of sediments for microbial biomass and community structure. In: Litchfield C D, Seyfreid P L, editors. Methodology for biomass determinations and microbial activities in sediments. ASTM STP 673. Philadelphia, Pa: American Society for Testing and Materials; 1979. pp. 87–103. [Google Scholar]
- 46.Wilkinson W H, Vega L A, Titley S R. Geology and ore deposits at Mineral Park, Mohave County, Arizona. In: Titley S R, editor. Advances in geology of the porphyry copper deposits, southwestern North America. Tucson: University of Arizona Press; 1982. pp. 523–542. [Google Scholar]