Abstract
Objective
We aimed to describe the persistence of symptoms in coronavirus disease 2019 (COVID-19) and quality of life (QoL) among patients 90 days after their discharge from the hospital for infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and to determine differences in QoL domains concerning the absence or presence of persistent symptoms.
Methods
To measure QoL, we used a validated Spanish version of the 36-item Short Form Health Survey (SF-36).
Results
We included 141 patients. Ninety days after discharge, COVID-19 symptoms persisted in 107 patients (75.9%), with fatigue (55.3%) and joint pain (46.8%) being the most frequent. According to the SF-36, the role-physical score was the dimension with the lowest values (median score, 25; interquartile range, 0–75). Patients with joint pain, fatigue, and dyspnea had lower scores than patients without those symptoms, with 10 of the 13 evaluated SF-36 scales showing lower levels.
Conclusion
Ninety days after hospital discharge from COVID-19 reference centers, most patients had persistent symptoms and had lower SF-36 scores than patients without symptoms. It is important to follow-up patients discharged from the hospital after SARS-CoV-2 infection, ideally through a post-COVID-19 health care clinic and rehabilitation program, to improve QoL in these patients.
Keywords: Quality of life, coronavirus disease 2019, severe acute respiratory syndrome coronavirus 2, 36-item Short Form Health Survey, persistent COVID-19 symptoms, long COVID
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has demonstrated high transmission and pathogenicity, claiming the lives of more than 5.5 million people around the world. 1 Survivors of COVID-19 number more than 166 million individuals, representing a challenge to health systems with increased patient demand for physical, respiratory, and mental health rehabilitation 2 –4 should they develop new or worsening symptoms and sequelae after acute infection.
There is some evidence that COVID-19 causes not only acute disease but also subsequent dysfunction, which experts have termed long COVID in reference to long-term effects of COVID-19 infection. 5 A recent study estimated that 80% of patients with a diagnosis of COVID-19 have at least one persistent symptom, occurring mainly among patients who experienced severe disease. The most common persistent symptoms are fatigue, headache, attention disorder, hair loss, and dyspnea. 5 Persistent symptoms and sequelae after hospital discharge owing to COVID-19 affect patients’ physical and psychological performance, 2,6,7 causing difficulties in performing daily activities among some patients, despite having survived severe SARS-CoV-2 infection. 8, 9 A recent report suggested that poor health and poor quality of life (QoL) post-COVID-19 could be attributed to an infection that might not be completely resolved, given the number of persistent symptoms 35 days after discharge. 8 Measurement of QoL after infection with COVID-19 is important to improve patient prognosis and alleviate disease burden with timely intervention. 3, 10
In Latin America, QoL has not been evaluated in patients who have been discharged after COVID-19. On the basis of what is known about COVID-19 infection and its impact on physical and mental health, 2 , 6 , 7 the disease can have a strong impact on QoL. 8, 11 Therefore, we aimed to describe the persistence of COVID-19 symptoms and QoL in individuals 90 days after their discharge from the hospital for SARS-CoV-2 infection and to determine differences in QoL domains with the absence or presence of persisting symptoms.
Methods
We conducted an observational, ambispective, longitudinal analytic study in two COVID-19 referral centers (Hospital Regional de Alta Especialidad del Bajío and Hospital Estatal de Atención COVID-19) in the state of Guanajuato, Mexico. This study was reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines, 12 Epidemiological and clinical data were obtained by reviewing the clinical records. The authors then interviewed patients and established a prospective database using a standard questionnaire to obtain the following variables: date at symptom onset, length of stay (LoS; the number of days elapsed between a patient’s hospital admittance and discharge), comorbidities, and persistence of symptoms after discharge (fatigue, joint pain, myalgia, dyspnea, anxiety, depression, headache, cough, chest pain, sore throat, vertigo, anosmia, diarrhea, memory loss, insomnia/poor sleep quality), complications, and sequelae attributed to COVID-19. Depression and anxiety were measured using a validated Spanish version of the nine-item Patient Health Questionnaire depression scale (PHQ-9) and seven-item Generalized Anxiety Disorder scale (GAD-7). 13 –15 To measure QoL, we used a validated version of the MOS/RAND 36-item Short Form Health Survey (SF-36) in Spanish for the Mexican population. 16 The characteristics of SF-36 measurement have been extensively studied to assess its reliability and validity. 17 –19 The questionnaire consists of 36 items that measure the following eight dimensions: physical functioning (PF, limitations in performing physical activities such as bathing or dressing), role-physical (RP, limitations in work and other daily activities as a consequence of physical health), bodily pain (BP, how severe and limiting pain is), general health (GH, how patients perceive their overall personal health), vitality (VT, feeling tired and worn out as opposed to feeling energetic), social functioning (SF, interference with usual social activities owing to physical or emotional problems), role-emotional (RE, limitations in work and other daily activities as a consequence of emotional problems), and mental health (MH, feeling nervous and depressed as opposed to peaceful, happy, and calm). Furthermore, we included the health change score (HC, change in overall health status since the previous year). Scores for each item ranged from 0 to 100, with higher scores indicating better health. Two additional scores were calculated with the orthogonal (uncorrelated) and oblique (correlated) method, the physical component summary (PCS) and the mental component summary (MCS). The PCS is derived from positive weighting for the PF, RP, BP, GH, and VT scales and negative weighting for the SF, RE, and MH scales. The scoring algorithm for MCS includes positive weighting for the VT, SF, RE, and MH scales and negative weighting for the PF, RP, BP, and GH scales. The PCS and MCS are presented as T-scores; these scores were calculated following the methodology proposed by Ware and Hays, creators of these scores. 20 –22
We included men and women older than age 18 years, with a confirmed diagnosis of COVID-19 who had been hospitalized for their illness. We excluded patients who did not complete at least 80% of the SF-36 questionnaire. SARS-CoV-2 infection was confirmed using real-time reverse transcription polymerase chain reaction (RT-PCR) during hospitalization.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Ethics and Research Committee of the Hospital Regional de Alta Especialidad del Bajío (approval number CEI-25-2021). All participants provided their verbal informed consent.
Statistical analysis
Quantitative variables are described using median with interquartile range (IQR) or mean ± standard deviation. Categorical data are described using absolute and relative frequencies. Normal distribution was assessed for continuous variables using the Kolmogorov–Smirnov test. In comparative analysis among SF-36 scores with respect to the persistence or absence of symptoms or sequelae attributable to COVID-19, the data were compared using the Mann–Whitney U test. Statistical differences between study groups (patients with and without persistent symptoms) were calculated using the χ2 test for the comparison of incidence rates (%). The odds ratio (OR) was calculated for qualitative dichotomous variables. Statistical analysis was performed using VassarStats. 23 A p-value of <0.01 was considered significant.
Results
A total of 141 patients meeting the respective hospitals' discharge criteria were investigated 90 days after hospitalization owing to COVID-19. The sex ratio was 1.47:1. Mean patient age at the time of the survey was 52.24 ± 14.76 years. At the time of the study, 112 (79.4%) patients had associated chronic comorbidities; the most frequent were hypertension (46.1%), diabetes (33.3%), and overweight (31.9%). Other comorbidities were observed in 13 (9.2%) individuals, including malnutrition, hypothyroidism, panhypopituitarism, latent tuberculosis, arterial insufficiency, and bipolar disorder (Table 1).
Table 1.
General characteristics of the study population (N = 141).
| Characteristics | Value |
|---|---|
| Age (years), mean ( ± SD) | 52.24 (±14.76) |
| Male sex, n (%) | 84 (59.6%) |
| Female sex, n (%) | 57 (40.4%) |
| Smoking, n (%) | 38 (27%) |
| Comorbidities, n (%) | 112 (79.4%) |
| Diabetes, n (%) | 47 (33.3%) |
| Hypertension, n (%) | 65 (46.1%) |
| Overweight, n (%) | 45 (31.9%) |
| Chronic kidney disease, n (%) | 12 (8.5%) |
| COPD, n (%) | 4 (2.8%) |
| Cardiopathy, n (%) | 7 (4.96%) |
| Dyslipidemia, n (%) | 10 (7.1%) |
| Depression, n (%) | 2 (1.4%) |
| Asthma, n (%) | 4 (2.8%) |
| Cancer, n (%) | 5 (3.55%) |
| Other comorbidities, n (%) | 13 (9.2%) |
| Days between the onset of symptoms and hospitalization, median (IQR) | 7 (5–10) |
| Days since symptoms onset, median (IQR) | 98 (95–109.5) |
| Length of hospital stay in days, median (IQR) | 8 (5–19.5) |
| ICU/mechanical ventilation, n (%) | 45 (31.9%) |
| Number of days with mechanical ventilation, mean (±SD) | 12.2 (±9.98) |
| Nosocomial infection, n (%) | 12 (8.5%) |
| Complications/sequelae owing to COVID-19 at discharge, n (%) | 133 (94.3%) |
| Home supplemental oxygen, n (%) | 133 (94.3%) |
| Critical illness neuropathy/myopathy, n (%) | 28 (19.9%) |
| Myocardial infarction, n (%) | 1 (0.7%) |
| Other complications/sequelae owing to COVID-19, n (%) | 3 (2.1%) |
COVID-19, coronavirus disease 2019; ICU, intensive care unit; COPD, chronic obstructive pulmonary disease; IQR, interquartile range; SD, standard deviation.
The median LoS was 8 (IQR 5–19) days. During hospitalization, 45 (31.9%) patients required treatment in the intensive care unit (ICU) or mechanical ventilation, with a mean duration of 12.2 ± 9.98 days. Complications and sequelae at discharge attributed to COVID-19 were present in 133 patients (94.3%); a requirement for home supplemental oxygen (94.3%) and critical illness neuromyopathy (19.9%) were the most relevant (Table 1).
Hospital medication management included macrolides in 84 patients (59.6%), ivermectin in 37 (26.2%), hydroxychloroquine in 4 (2.8%), and convalescent plasma in 2 (1.4%).
Concerning persistence of sequelae, 90 days after discharge, some patients (4.9%) still reported needing home supplemental oxygen and 8.5% were unable to walk.
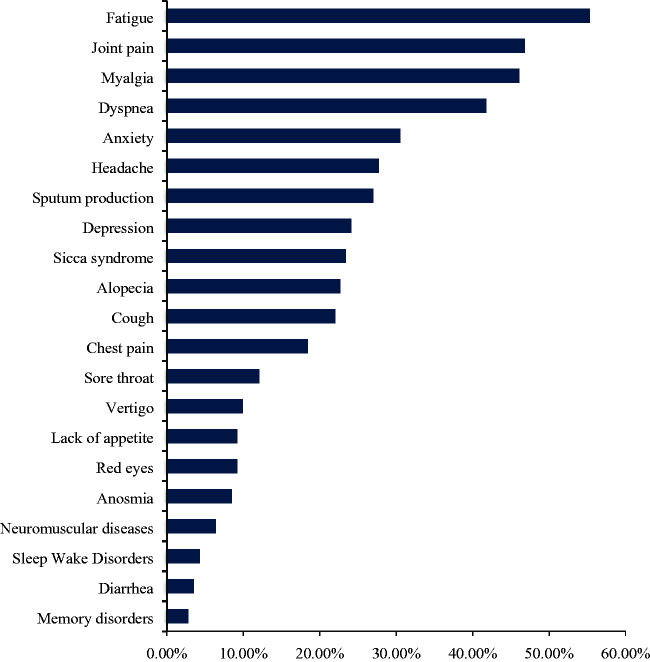
Persistence of at least one symptom 90 days after hospital discharge was present in 107 (75.9%) patients; only 34 (24.1%) were completely free of any COVID-19-related symptoms. Among those with persistent symptoms, 9 (6.38%) patients had one symptom, 13 (9.22%) had two symptoms, and 85 (60.28%) had three or more. The most frequent symptoms were fatigue (55.3%, 95% confidence interval [CI] 47.0–63.0), joint pain (46.8%, 95% CI 38.4–55.4), myalgia (46.1%, 95% CI 37.7–54.7), and dyspnea (41.8%, 95% CI 34–50). Other reported symptoms were anxiety (30.5%, 95% CI 22.7–30.8), depression (24.1%, 95% CI 17.7–31.2), sputum production (27%, 95% CI 19.9–34), headache (27.7%, 95% CI 20.6–34.8), sicca syndrome (23.4%, 95% CI 16.3–30.5), alopecia (22.7%, 95% CI 15.6–29.8), memory loss (2.8%, 95% CI 0.7–5.7), and insomnia or poor sleep quality (4.3%, 95% CI 1.4–7.8) (Figure 1).
Figure 1.
Persistent symptoms 90 days after hospital discharge owing to coronavirus disease 2019 (COVID-19).
To assess the influence of comorbidities and ICU/mechanical ventilation on the persistence of symptoms, we performed a comparison using the χ2 test. Although patients with hypertension and overweight showed a higher proportion of persistent symptoms, statistical significance was found only for patients who required ICU management and mechanical ventilation as opposed to those who did not (39.3% vs. 8.8%, p = 0.001), with an odds ratio (OR) of 6.68 (95% CI 1.92–23.23), shown in Table 2.
Table 2.
Influence of sex, comorbidities, and intensive care unit/mechanical ventilation management in the persistence of symptoms 90 days after hospital discharge owing to COVID-19.
| Study population,N = 141 |
||||
|---|---|---|---|---|
| With persistentsymptoms (n = 107) | Without persistentsymptoms(n = 34) | p-value* | OR(95% CI) | |
| Male sex, n (%) | 63 (58.9) | 21 (61.8) | 0.765 | 0.89 (0.40–1.96) |
| Hypertension, n (%) | 54 (50.5) | 11 (32.4) | 0.065 | 2.13 (0.95–4.8) |
| Overweight, n (%) | 37 (34.6) | 8 (23.5) | 0.229 | 1.72 (0.71–4.17) |
| Diabetes, n (%) | 33 (30.8) | 14 (41.2) | 0.265 | 0.64 (0.29–1.41) |
| ICU/mechanical ventilation, n (%) | 42 (39.3) | 3 (8.8) | 0.001 | 6.68 (1.92–23.23) |
| Macrolide treatment | 61 (57) | 23 (67.6) | 0.271 | 0.63 (0.29–1.43) |
| Ivermectin treatment | 29 (27.1) | 8 (23.5) | 0.680 | 1.2 (0.49–2.97) |
*Statistical significance calculated using χ2 test.
COVID-19, coronavirus disease 2019; OR, odds ratio; ICU, intensive care unit; CI, confidence interval.
The SF-36 questionnaire was administered to the entire study group to assess QoL 3 months after hospitalization owing to COVID-19 infection. Data analysis showed that RP scores were the lowest (median score 25; IQR 0–75), followed by HC scores (median score 50; IQR 25–75). Median orthogonal and oblique PCS scores were 45.2 and 45.3, respectively, which were lower than the median orthogonal and oblique MCS scores of 52.3 and 49.2, respectively (Table 3).
Table 3.
SF-36 total scores 90 days after hospital discharge owing to COVID-19.
| Study population, N = 141 | Score, median (IQR) |
|---|---|
| Physical function | 80 (55–90) |
| Role physical function | 25 (0–75) |
| Body pain | 80 (57.5–100) |
| General health | 65 (42.5–80) |
| Mental health | 76 (64–88) |
| Role emotional | 100 (33–100) |
| Vitality | 70 (55–85) |
| Social function | 75 (50–100) |
| Health change | 50 (25–75) |
| Uncorrelated physical health component | 45.2 (36.1–50.9) |
| Correlated physical health component | 45.3 (38.3–50.6) |
| Uncorrelated mental health component | 52.3 (42.9–56.7) |
| Correlated mental health component | 49.2 (43.6–55.3) |
COVID-19, coronavirus disease 2019; SF-36; 36-item Short Form Health Survey; IQR, interquartile range.
SF-36 scores were compared among patients with and without complications and persistent symptoms 3 months after hospital discharge. Concerning the presence or absence of persistent symptoms after SARS-CoV-2 infection, we performed a comparative analysis according to the predominant scores. Patients with joint pain, fatigue, and dyspnea showed lower scores than patients who did not have those symptoms in 10 of the 13 evaluated SF-36 scales (p < 0.01) (Table 4). Additionally, patients with symptoms suggestive of depression and those with myalgia had lower scores in 8 of the 13 evaluated SF-36 parameters (p < 0.01). Patients with cough, sore throat, and chest pain had lower scores in 7 of the 13 scales when compared with patients who did not have those symptoms (Table 4). However, patients with vertigo had lower scores in 6 of the 13 evaluated SF-36 parameters, and patients with symptoms suggestive of anxiety and sicca syndrome presented lower scores in 5 of the 13 scales compared with their counterparts who did not have those symptoms (Table 4).
Table 4.
SF-36 scores 90 days after hospital discharge regarding persistence of symptoms after COVID-19 infection.
|
Dyspnea, median (IQR) |
Joint pain, median (IQR) |
|||||
|
|
Positive, n = 59 |
Negative, n = 82 |
p-value |
Positive, n = 66 |
Negative, n = 75 |
p-value |
| PF | 60 (40–85) | 85 (75–95) | <0.001 | 65 (40–85) | 85 (70–95) | <0.001 |
| RP | 0 (0–50) | 50 (25–100) | <0.001 | 25 (0–50) | 75 (0–100) | 0.001 |
| RE | 67 (0–100) | 100 (66.7–100) | 0.058 | 100 (24.7–100) | 100 (33–100) | 0.724 |
| VT | 60 (50–75) | 80 (63.7–90) | <0.001 | 65 (53.7–75) | 80 (60–90) | <0.001 |
| MH | 72 (56–80) | 80 (68–92) | 0.001 | 72 (63–80) | 80 (68–92) | 0.001 |
| SF | 75 (50–87.5) | 75 (50–100) | 0.525 | 62.5 (50–75) | 75 (62.5–100) | 0.001 |
| BP | 67.5 (45–90) | 90 (76.8–100) | <0.001 | 61.5 (45–90) | 90 (78–100) | <0.001 |
| GH | 55 (35–70) | 70 (53.7–81.2) | 0.001 | 60 (38.7–71.2) | 70 (50–80) | 0.022 |
| HC | 25 (25–50) | 50 (50–75) | <0.001 | 25 (25–50) | 50 (25–75) | 0.006 |
| PCSuc | 38.6 (31.8–45.3) | 48.2 (43.8–52.5) | <0.001 | 39.8 (32.4–45.8) | 48.7 (42.5–51.9) | <0.001 |
| MCSuc | 51.1 (38.6–55.5) | 52.5 (46.5–57.4) | 0.107 | 51.3 (40.2–56.7) | 52.7 (47.2–56.9) | 0.226 |
| PCSc | 39.7 (31.2–45) | 48.9 (42.8–52.9) | <0.001 | 40.5 (31.3–48.3) | 48.9 (42.7–53.6) | <0.001 |
|
MCSc |
47.5 (37–50.8) |
52.2 (47.2–56.2) |
<0.001 |
47.8 (37.2–50.9) |
52.7 (46.6–56.7) |
<0.001 |
|
Fatigue, median (IQR) |
Myalgia, median (IQR) |
|||||
|
|
Positive, n = 78 |
Negative, n = 63 |
p–value |
Positive, n = 65 |
Negative, n = 76 |
p–value |
| PF | 65 (40–85) | 85 (75–95) | <0.001 | 65 (42.5–85) | 85 (75–95) | <0.001 |
| RP | 25 (.00–50) | 75 (25–100) | <0.001 | 25 (0–75) | 50 (0–100) | 0.030 |
| RE | 100 (24.75–100) | 100 (33.3–100) | 0.751 | 100 (0–100) | 100 (66.7–100) | 0.199 |
| VT | 60 (50–75) | 80 (65–95) | <0.001 | 60 (50–75) | 80 (65–90) | <0.001 |
| MH | 72 (64–84) | 80 (64–92) | 0.008 | 72 (58–80) | 80 (68–92) | 0.001 |
| SF | 75 (50–87.5) | 75 (50–100) | 0.227 | 63 (50–94) | 75 (50–100) | 0.205 |
| BP | 67.5 (45–90) | 100 (78–100) | <0.001 | 67.5 (45–90) | 100 (78–100) | <0.001 |
| GH | 60 (35–70) | 70 (55–85) | <0.001 | 65 (35–70) | 70 (50–83.7) | 0.007 |
| HC | 25 (25–75) | 50 (50–75) | 0.009 | 50 (25–75) | 50 (25–75) | 0.131 |
| PCSuc | 40.2 (32.4–45.6) | 50.1 (45.2–53) | <0.001 | 38.9 (32.7–45.5) | 48.5 (43.2–51.9) | <0.001 |
| MCSuc | 51.5 (40.8–56.3) | 52.9 (46.3–57.1) | 0.341 | 50.7 (38.9–56.7) | 52.9 (47.6–56.8) | 0.061 |
| PCSc | 41.1 (31.7–46.2) | 50.3 (45.1–54.2) | <0.001 | 40.5 (31.7–47.2) | 49.2 (42.2–53.2) | <0.001 |
|
MCSc |
48 (39–52) |
53.5 (47.5–57) |
<0.001 |
46.9 (37–51.9) |
52.4 (47.8–56.6) |
<0.001 |
|
Depression, median (IQR) |
Cough, median (IQR) |
|||||
|
|
Positive, n = 34 |
Negative, n = 107 |
p–value |
Positive, n = 31 |
Negative, n = 110 |
p–value |
| PF | 65 (38.7–80) | 80 (60–90) | <0.001 | 65 (35–80) | 80 (60–90) | 0.003 |
| RP | 0 (0–75) | 50 (0–100) | 0.013 | 25 (0–50) | 50 (0–100) | 0.158 |
| RE | 33 (0–100) | 100 (66.7–100) | <0.001 | 100 (0–100) | 100 (33–100) | 0.665 |
| VT | 55 (28.7–81.2) | 70 (60–85) | <0.001 | 60 (50–80) | 70 (60–85) | 0.013 |
| MH | 60 (39–76) | 80 (68–88) | <0.001 | 72 (56–76) | 80 (68–88) | 0.001 |
| SF | 62.5 (50–90.6) | 75 (62.5–100) | 0.047 | 62.5 (50–75) | 75 (62.5–100) | <0.001 |
| BP | 67.5 (41.8–90) | 90 (67.5–100) | 0.002 | 67.5 (45–100) | 89 (66.8–100) | 0.034 |
| GH | 62.5 (30–72.5) | 70 (50–80) | 0.040 | 55 (30–70) | 67.5 (45–80) | 0.035 |
| HC | 25 (25–56.2) | 50 (25–75) | 0.013 | 25 (25–50) | 50 (25–75) | 0.008 |
| PCSuc | 40.3 (33–47.1) | 46.5 (38.6–51.3) | 0.026 | 37.5 (32.5–48.3) | 46 (39.2–51.6) | 0.005 |
| MCSuc | 39.9 (34.1–52.7) | 53.2 (47.9–57.7) | <0.001 | 49.6 (38.6–54.2) | 52.9 (44.9–57.1) | 0.027 |
| PCSc | 39.2 (30.7–45) | 47.2 (40.9–52) | <0.001 | 40.5 (31.4–49.1) | 46.7 (38.7–52.1) | 0.001 |
|
MCSc |
38.2 (31.9–49.8) |
50.6 (47.3–55.9) |
<0.001 |
47.2 (37.2–50.6) |
50.6 (45.9–56.1) |
0.002 |
|
Sore throat, median (IQR) |
Chest pain, median (IQR) |
|||||
|
|
Positive, n = 38 |
Negative, n = 103 |
p–value |
Positive, n = 26 |
Negative, n = 115 |
p–value |
| PF | 67.5 (38.7–85) | 80 (60–90) | 0.003 | 60 (40–85) | 80 (60–90) | 0.014 |
| RP | 25 (0–75) | 50 (0–100) | 0.233 | 25 (0–56.2) | 50 (0–100) | 0.112 |
| RE | 100 (0–100) | 100 (33–100) | 0.375 | 83.3 (33–100) | 100 (33–100) | 0.470 |
| VT | 65 (42.5–75) | 70 (60–90) | 0.006 | 60 (33.7–75) | 70 (60–85) | 0.002 |
| MH | 72 (54–81) | 76 (68–88) | 0.008 | 72 (59–78) | 76 (64–88) | 0.028 |
| SF | 62.5 (47–75) | 75 (62.5–100) | 0.009 | 63 (50–75) | 75 (50–100) | 0.181 |
| BP | 70 (45–100) | 90 (65–100) | 0.036 | 58 (45–78) | 90 (67.5–100) | <0.001 |
| GH | 57.5 (30–70) | 70 (45–80) | 0.044 | 60 (35–70) | 65 (45–80) | 0.135 |
| HC | 25 (25–50) | 50 (25–75) | 0.003 | 25 (25–50) | 50 (25–75) | <0.001 |
| PCSuc | 37.7 (33–48.7) | 46.1 (39.8–51.3) | 0.015 | 34.9 (32–43.5) | 46.5 (38.9–51.3) | 0.002 |
| MCSuc | 48.7 (38.2–54.8) | 52.9 (46.3–57.1) | 0.018 | 47 (39.6–55.4) | 52.7 (45.3–56.9) | 0.131 |
| PCSc | 40.1 (31.1–49.5) | 46.8 (40.9–51.6) | 0.004 | 38.9 (31.5–43.9) | 46.8 (40.6–51.8) | 0.001 |
|
MCSc |
47.1 (35.7–52.7) |
50.6 (46.2–55.9) |
0.005 |
46.7 (35.6–49.9) |
50.6 (45.1–56.1) |
0.002 |
|
Vertigo, median (IQR) |
Anxiety, median (IQR) |
|||||
|
|
Positive, n = 14 |
Negative, n = 127 |
p–value |
Positive, n = 43 |
Negative, n = 098 |
p–value |
| PF | 57.5 (33.7–77.5) | 80 (60–90) | 0.012 | 75 (55–90) | 80 (55–90) | 0.678 |
| RP | 0 (0–75) | 50 (0–100) | 0.024 | 50 (0–100) | 25 (0–75) | 0.567 |
| RE | 0 (0–75.2) | 100 (33.3–100) | 0.001 | 66.7 (0–100) | 100 (66.7–100) | 0.008 |
| VT | 47.5 (23.7–71.2) | 70 (60–85) | 0.012 | 70 (50–80) | 70 (60–85) | 0.112 |
| MH | 62 (39–73) | 76 (68–88) | 0.002 | 68 (52–80) | 80 (68–88) | <0.001 |
| SF | 50 (47–78.1) | 75 (50–100) | 0.061 | 63 (50–75) | 75 (59.3–100) | 0.079 |
| BF | 51.5 (30.6–75) | 88 (65–100) | 0.002 | 65 (48–90) | 90 (67.5–100) | 0.009 |
| GH | 62.5 (37.5–72.5) | 65 (45–80) | 0.306 | 65 (40–80) | 65 (45–75) | 0.684 |
| HC | 25 (25–31.2) | 50 (25–75) | 0.016 | 25 (25–50) | 50 (25–75) | 0.037 |
| PCSuc | 36.1 (32.1–44.4) | 45.6 (37.3–51.3) | 0.035 | 44.9 (34.3–53) | 45.3 (36.1–49.7) | 0.431 |
| MCSuc | 38.9 (35.1–48.4) | 52.7 (46.3–56.9) | 0.001 | 45.1 (37.9–53.2) | 53.6 (48.4–57.7) | <0.001 |
| PCSc | 33.1 (30.7–41.4) | 46.2 (39.3–50.8) | 0.002 | 44.4 (37.9–50.3) | 45.8 (38.4–51.8) | 0.407 |
|
MCSc |
37.1 (31.9–49) |
50.4 (46.2–55.5) |
0.001 |
47.2 (36.9–52.1) |
50.7 (45.1–55.8) |
0.003 |
|
Sicca syndrome, median (IQR) |
Headache, median (IQR) |
|||||
|
|
Positive, n = 33 |
Negative, n = 108 |
p–value |
Positive, n = 39 |
Negative, n = 102 |
p–value |
| PF | 60 (40–85) | 80 (61.2–90) | 0.006 | 75 (55–85) | 80 (55–90) | 0.239 |
| RP | 25 (0–75) | 50 (0–100) | 0.041 | 25 (0–100) | 50 (0–75) | 0.600 |
| RE | 67 (0–100) | 100 (33.1–100) | 0.167 | 100 (0–100) | 100 (33–100) | 0.484 |
| VT | 65 (40–80) | 70 (60–85) | 0.080 | 65 (55–70) | 75 (60–90) | 0.004 |
| MH | 72 (56–92) | 76 (64–84) | 0.505 | 72 (60–76) | 80 (67–88) | 0.018 |
| SF | 62.5 (50–100) | 75 (53.1–97) | 0.317 | 62.5 (50–75) | 75 (59.3–100) | 0.020 |
| BP | 70 (45–90) | 90 (67.5–100) | 0.005 | 70 (48–90) | 90 (66.8–100) | 0.019 |
| GH | 65 (37.5–75) | 65 (46.2–80) | 0.195 | 55 (40–80) | 67.5 (48.7–80) | 0.335 |
| HC | 25 (25–50) | 50 (25–75) | 0.004 | 25 (25–50) | 50 (25–75) | 0.069 |
| PCSuc | 39.3 (32.3–45.8) | 46.5 (38.6–51.4) | 0.002 | 44.5 (33.1–51.4) | 45.5 (37.1–50.9) | 0.330 |
| MCSuc | 51.2 (38.3–57.4) | 52.3 (44.6–56.5) | 0.665 | 51.1 (39.2–54.8) | 52.7 (46.1–57.7) | 0.038 |
| PCSc | 41.3 (30.6–48.2) | 46.5 (39.6–51.9) | 0.006 | 44.3 (31.8–50.3) | 45.8 (38.7–51.7) | 0.125 |
| MCSc | 46.6 (37.3–56.2) | 50 (46.4–55.2) | 0.129 | 48.1 (37–52) | 50.6 (45.1–56.1) | 0.019 |
*Statistical significance calculated using Mann–Whitney U test.
SF-36; 36-item Short Form Health Survey; COVID-19, coronavirus disease 2019; PF, physical function; RP, role-physical; BP, body pain; GH, general health; VT, vitality; SF, social function; RE, role-emotional; MH, mental health; HC, health change; PCSuc, uncorrelated physical health component; MCSuc, uncorrelated mental health component; PCSc, correlated physical health component; MCSc, correlated mental health component; IQR, interquartile range.
Regarding complications or sequelae, when comparing patients with or without the inability to walk, lower scores were observed in 2 of the 13 SF-36 parameters among patients with this complication (p < 0.01). Median PF and RE scores were lower (median PF score 40, IQR 11.2–66.2; median RE score 0, IQR 0–83.3) among patients with an inability to walk in comparison with patients who did not have this complication (median PF score 80, IQR 60.0–90.0; median RE score 100, IQR 33.3–100.0). However, when comparing patients with or without the need for supplemental home oxygen 3 months after discharge, no significant difference was found for the 13 parameters evaluated in the SF-36 (Table 5).
Table 5.
SF-36 scores 90 days after hospital discharge for inability to walk after COVID-19 infection and ICU/mechanical ventilation management during hospitalization.
| Inability to walk, median (IQR) |
ICU/ mechanical ventilation, median (IQR) |
|||||
|---|---|---|---|---|---|---|
| Positive n = 12 | Negative n = 129 | p-value | Positive n = 45 | Negative n = 96 | p-value | |
| PF | 40 (11.2–66.2) | 80 (60–90) | 0.001 | 70 (45–85) | 80 (60–90) | 0.300 |
| RP | 0 (0–75) | 50 (0–75) | 0.128 | 0 (0–50) | 50 (0–100) | <0.001 |
| RE | 0 (0–83.3) | 100 (33.3–100) | 0.002 | 100 (0–100) | 100 (33–100) | 0.438 |
| VT | 57.5 (38.7–95) | 70 (60–85) | 0.463 | 65 (60–80) | 70 (55–85) | 0.437 |
| MH | 66 (49–87) | 76 (64–88) | 0.273 | 72 (64–84) | 78 (64–88) | 0.209 |
| SF | 50 (40.6–81.3) | 75 (50–100) | 0.049 | 62.5 (50–81.25) | 75 (50–100) | 0.048 |
| BP | 70 (43.1–100) | 80 (58–100) | 0.513 | 77.5 (51.5–100) | 84 (58–100) | 0.568 |
| GH | 57.5 (41.2–65) | 65 (42.5–80) | 0.220 | 60 (40–70) | 70 (46.25–80) | 0.062 |
| HC | 25 (25–50) | 50 (25–75) | 0.054 | 50 (25–75) | 50 (25–75) | 0.728 |
| PCSuc | 38.6 (29.8–46.4) | 45.4 (36.9–51.3) | 0.044 | 41.1 (31.2–47.1) | 46.7 (38.3–51.9) | 0.001 |
| MCSuc | 43 (37.9–50.1) | 52.7 (44.1–56.8) | 0.035 | 51.49(43.0–57.3) | 52.8 (42.9–56.7) | 0.818 |
| PCSc | 36.1 (29.1–44.5) | 46 (38.5–50.7) | 0.028 | 41.6 (34.6–45.5) | 48.3 (38.6–51.9) | 0.001 |
| MCSc | 43.1 (34.5–50.4) | 49.7 (45.1–55.5) | 0.048 | 58.1 (41.3–53.35) | 50.5 (45.4–55.9) | 0.110 |
COVID-19, coronavirus disease 2019; PF, physical function; RP, role-physical; BP, body pain; GH, general health; VT, vitality; SF, social function; RE, role-emotional; MH, mental health; HC, health change; PCSuc, uncorrelated physical component; MCSuc, uncorrelated mental component; PCSc, correlated physical component; MCSc, correlated mental component; ICU, intensive care unit; SF-36, 36-item Short Form Health Survey; IQR, interquartile range.
*Statistical significance was calculated using Mann–Whitney U test.
SF-36 scores were also compared among patients with and without comorbidities (hypertension, diabetes, overweight), and the need for ICU management or mechanical ventilation. A significant difference (p < 0.01) was found only when comparing patients who required ICU management or mechanical ventilation against those who did not. Patients who required ICU management or mechanical ventilation showed lower SF-36 scores in the RP (median score 0; IQR 0.0–50.0), orthogonal PCS (median score 41.1; IQR 31.2–47.1), and oblique PCS (median score 41.6; IQR 34.6–45.5) scales. In patients who did not require ICU management or mechanical ventilation, the observed scores were higher for RP (median score 50; IQR 50.0–100.0), orthogonal PCS (median score 46.7; IQR 38.3–51.9), and oblique PCS (median score 48.3; IQR 38.6–51.9) (Table 5).
Discussion
We examined 141 patients 90 days after hospital discharge for SARS-CoV-2 infection and described the persistence of COVID-19 symptoms and QoL and determined differences in QoL domains with respect to the absence or presence of persisting symptoms. We obtained epidemiological and relevant clinical data and observed that a greater proportion of male patients experienced severe illness and were more frequently hospitalized for COVID-19 in comparison with female patients, as previously reported internationally. 24, 25 Advanced age is another risk factor that predisposes individuals to severe illness; 26, 27 however, the mean age of patients in our study (52.24 years) was lower than that reported previously (mean ages of 57 and 62 years, respectively). 6, 7 Hypertension, diabetes, and overweight were the most prevalent comorbidities, which was consistent with several studies conducted worldwide among hospitalized patients with COVID-19 infection. 26, 28, 29
In our study, the median length of stay (LoS) in the hospital was 8 days, which was similar to that reported by Halpin et al., 2 Chopra et al., 7 and Jacobs et al., 8 who observed a median LoS of 6.5 days, 5 days, and 7 days, respectively. In contrast, Huang et al. reported a longer LoS, with a median 14 days. 6 We found a high prevalence of complications reported by patients with COVID-19 just after hospital discharge. For instance, respiratory illness with consequent supplemental oxygen requirement at discharge was reported in 94.3% of patients, which decreased to 4.9% by the time of the survey. This proportion was higher than the 3% reported by Daher et al. in German patients, even with follow-up conducted sooner, at 6 weeks; however, those authors only included patients who did not require mechanical ventilation, which could partly explain the lower proportion observed. 30
Critical illness neuropathy/myopathy is another important sequela during hospitalization, found in 19.9% of our study participants. This rate was higher than that reported among Swedish patients, with an incidence of 10%. 31 The difference may be because our patients were only clinically diagnosed with neuromyopathy without a complementary electrophysiology study.
At the time of the survey at 90 days post-discharge, 8.5% of our patients were unable to walk, an important sequela that has been poorly documented in international COVID-19 studies. A study in Bangladesh found that of 734 patients who had recovered from COVID-19, 1.8% remained confined to their bed after 4 weeks. 32 The fact that this complication was observed in a high proportion of our patients represents an opportunity to improve medical care during hospitalization to minimize this condition. Additionally, subsequent monitoring of patients and their contacts should be provided, as well as physical rehabilitation and psychotherapy. Telerehabilitation using digital communications technology can serve as an alternative approach that permits safe and efficient assessment and remote monitoring of patients during rehabilitation. 33 There is some evidence that telerehabilitation can be helpful in reducing rates of rehospitalization and in improving health status as well as QoL. Telerehabilitation does not replace rehabilitation services in the hospital; however, implementing a telerehabilitation program for patients who have recovered from COVID-19 can positively impact their QoL. Marotta et al. described that among patients with a confirmed diagnosis of multiple sclerosis, Parkinson disease, or stroke who participated in telerehabilitation programs, their QoL continued to improve despite not receiving in-person rehabilitation therapy. 34
Symptom persistence is a common outcome in many patients after infection with SARS-CoV-2, and 75.9% of the patients in our survey reported the persistence of at least one symptom. This proportion was lower than the 87.4% observed in a study from Italy, but higher than rates in the United States and Spain, with reported frequencies of 32.6% and 62.5%, respectively. 35 –37 The reason for differences in the observed proportions of symptom persistence among studies remains unclear but this could be owing to several factors like age, access to health care, hospital LoS, and timing of the survey after discharge. Despite the varying proportions, the persistence of symptoms after discharge remains a constant in numerous studies, reaffirming the need for long-term follow-up of patients who have recovered from COVID-19 infection.
Whereas we did not control for co-occurring symptoms, our results showed that 55.3% of the current sample reported fatigue 3 months after infection. This proportion was similar to that reported in other studies where fatigue was the most frequent symptom remaining after COVID-19. For example, among studies in Germany, Bangladesh, and France, the persistence of fatigue was identified in 45%, 33%, and 55% of patients, respectively. 11, 30, 38
Joint pain was the second most commonly reported symptom among our patients (46.8%) 3 months after hospital discharge, which was higher than that reported in Italian (22.7%) and Egyptian (31.4%) populations. 39, 40 Myalgia is an infrequently remaining symptom according to global reports, accounting for less than 10% of patients in a study in Italy, 15% in a German study, and 1.2% of patients in a report from Bangladesh. 30, 37, 38 It is intriguing that 46.10% of our patients had persistent myalgia 3 months after hospital discharge. Although we do not know the reason for this finding, its effect on daily life is evident. Dyspnea is the second leading persistent symptom in most reports, such as in those by Carfi et al. and Garrigues et al., with 43.4% and 42%, respectively. 11, 37 In our study, dyspnea ranked fourth, but with a similar frequency (41.80%).
The ongoing COVID-19 pandemic has caused multiple psychological and neurological problems such as anxiety, depression, memory loss, and insomnia. 5 In our study, patients reported symptoms suggestive of anxiety and depression at frequencies of 30.5% and 24.1%, respectively, similar to those reported by Kamal et al. of 38% and 28.6%, respectively. 40
In our survey, memory loss and insomnia or poor sleep quality were uncommonly reported persistent symptoms, with a frequency of 2.8% and 4.3%, respectively. These symptoms seem to be reported more frequently in other studies. For example, memory loss had a frequency of 34.2% and 28.33% in studies conducted among French and Chinese patients, respectively, 11, 41 whereas insomnia or poor sleep quality was reported at rates of 19.7% and 17.7% in Bangladesh and China, respectively. 32,42
Huang et al. and Xiong et al. 6, 42 identified alopecia as a complication after COVID-19 infection in 22% and 28.6% of patients, respectively, which is close to the frequency of 22.7% observed in the present study. Although this complication has been explained as an androgenic influence or the result of pharmacological interventions to treat COVID-19, the reason for its occurrence remains unknown. 38, 43 Further studies are necessary to clarify the specific etiology of alopecia and the degree of recovery after COVID-19.
Concerning the SF-36 QoL questionnaire, we observed that the PR domain was the most impaired, with a median score of 25 (IQR 0–75). This is comparable to a previous report from Italy, where the PR score measured 1 month after COVID-19 infection was the lowest, with a mean of 28.33 ± 41.04. 44 Interestingly, similar PR scores have been shown in other lung diseases, according to the SF-36. For instance, in studies among patients with idiopathic interstitial pneumonia carried out in Poland and China, the PR domain was severely affected, with mean scores of 16.9 ± 25.1 and 25 (IQR 0–81.2), respectively. 45, 46 Likewise, Lutogniewska et al. reported a mean PR score of 20.7 ± 23.2 among patients with idiopathic pulmonary fibrosis prior to lung transplantation. 46
Although we observed serious psychological effects in our patients after infection COVID-19, poor physical condition imposed a greater burden on their QoL. Orthogonal and oblique PCS had lower median scores (45.2 and 45.3, respectively) than median scores for orthogonal and oblique MCS (52.3 and 49.2, respectively). This is consistent with reports from Spain, where the SF-12 QoL questionnaire was used among patients after COVID-19, in whom the mean PCS score was lower than the mean MCS score (42.5 ± 11.2 and 45.5 ± 11.5, respectively). 47 Conversely, researchers from China and Italy administered the SF-36 after SARS-CoV-2 infection and found that the mean score for PCS was higher than that for MCS (China: PCS = 55.96 ± 7.24 and MCS = 48.92 ± 10.81; Italy: PCS = 56.25 ± 23.15 and MCS = 53.63 ± 28.11). 44, 48 The differences observed in PCS and MCS among these studies could be influenced, in part, by the methods used to calculate these measures. It is recommended to use the methodology proposed by the developers of these scores, as in the present study.
We found that patients who received ICU management or mechanical ventilation had a higher proportion of persistent symptoms than patients who did not have persistent symptoms (39.3% vs. 8.8%, p = 0.001; OR = 6.68). Moreover, patients who required ICU management or mechanical ventilation showed diminished SF-36 scores in orthogonal PCS (median score 41.1, IQR 31.9–47.1) and oblique PCS (median score 41.6, IQR 34.7–45.5) compared with their counterparts who did not require such management. These findings are consistent with other reports where mechanical ventilation or ICU management affected the persistence of symptoms and the patient’s QoL. For example, Vlake et al. reported a median PCS of 40 (IQR 19–58) among patients with COVID-19 in the ICU who were treated with mechanical ventilation, which is similar to the median PCS observed in our study. It should be noted, however, that those authors did not detail whether their PCS analysis was orthogonal or oblique. 49
To our knowledge, our study is one of the first to show a difference among QoL scales between patients with persistent symptoms or sequelae after COVID-19 infection and those without persistent symptoms. Patients with joint pain, fatigue, and dyspnea had lower QoL scores than patients who did not have these symptoms in 10 of the 13 SF-36 scales (p < 0.01); this was followed by patients with depression-related symptoms and myalgia, who presented lower scores in 8 of the 13 evaluated scales (p < 0.01).
In our study, patients with symptoms suggestive of depression had lower scores than those without depression-related symptoms in 8 of the 13 SF-36- scales. As mentioned by Rogers et al., 50 although depression and other common psychiatric disorders, such as anxiety and fatigue, have been associated with severe coronavirus infections like SARS-CoV or MERS-CoV, the apparently high prevalence of such symptoms may be unrelated to coronavirus infection and could instead by a consequence of selection bias. This is a limitation in our study. Future investigation among a prospective cohort of patients with SARS-CoV-2 should measure other possible confounding factors and assess mental health status prior to infection using standardized questionnaires or scales.
Because we did not control for or examine co-occurring symptoms, it is important to acknowledge that the observed differences in QoL domains are unlikely to be solely related to a single symptom. Future research could examine whether differences in QoL domains are affected by the presence or absence of multiple simultaneously occurring symptoms versus a single persisting symptom. This study has several other limitations including the sample size, which could decrease the power of the study. Also, a longer follow-up would be desirable; 90 days may not be sufficient to assess the duration of persistent symptoms and the impact on QoL. To minimize the probability of a type I error, a p-value <0.01 was chosen to assess statistical significance; however, further studies using different approaches are needed to corroborate our findings.
In conclusion, we found that 90 days after hospital discharge, most patients with COVID-19 infection (75.9% in our study) reported the persistence of symptoms, particularly fatigue and joint pain, and most had lower SF-36 QoL scores in comparison with patients who did not have these symptoms. It is important to ensure follow-up of patients after illness, ideally in a post-COVID-19 health care clinic, to minimize the burden of disease on patients and their families and to consequently improve their QoL.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Eduardo Guaní-Guerra https://orcid.org/0000-0001-6494-5511
References
- 1.World Health Organization. COVID-19 Weekly Epidemiological Update. August 29, 2021.
- 2.Halpin SJ, McIvor C, Whyatt G, et al. Post-discharge symptoms and rehabilitation needs in survivors of COVID‐19 infection: A cross‐sectional evaluation. J Med Virol 2021; 93: 1013–1022. [DOI] [PubMed] [Google Scholar]
- 3.Demeco A, Marotta N, Barletta M, et al. Rehabilitation of patients post-COVID-19 infection: a literature review. J Int Med Res 2020; 48: 0300060520948382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vlake JH, Wesselius S, Van Genderen ME, et al. Psychological distress and health-related quality of life in patients after hospitalization during the COVID-19 pandemic: A single-center, observational study. PLoS One 2021; 16: e0255774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep 2021; 11: 16144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021; 397: 220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chopra V, Flanders SA, O’Malley M, et al. Sixty-Day Outcomes Among Patients Hospitalized With COVID-19. Ann Intern Med 2021; 174: 576–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs LG, Gourna Paleoudis E, Lesky-Di Bari D, et al. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID-19 infection. PLoS One 2020; 15: e0243882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang F, Kream RM, Stefano GB. Long-Term Respiratory and Neurological Sequelae of COVID-19. Med Sci Monit 2020; 26: e928996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu K, Zhang W, Yang Y, et al. Respiratory rehabilitation in elderly patients with COVID-19: A randomized controlled study. Complement Ther Clin Pract 2020; 39: 101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garrigues E, Janvier P, Kherabi Y, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect 2020; 81: e4–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 13.Gaitán-Rossi P, Pérez-Hernández V, Vilar-Compte M, et al. Prevalencia mensual de trastorno de ansiedad generalizada durante la pandemia por Covid-19 en México. Salud Publica Mex 2021; 63: 478–485. [DOI] [PubMed] [Google Scholar]
- 14.Baader MT, Molina F JL, Venezian BS, et al. Validación y utilidad de la encuesta PHQ-9 (Patient Health Questionnaire) en el diagnóstico de depresión en pacientes usuarios de atención primaria en Chile. Revista Chilena de Neuro-Psiquiatría 2012; 50: 10–22. [Google Scholar]
- 15.Tadeo-Álvarez MA, Munguía-Ortíz CD, Benítez-López V, et al. Presence of depressive symptoms in medical students in a Mexican public university. Salud Mental 2019; 42: 131–136. [Google Scholar]
- 16.Zúñiga M, Carrillo-Jiménez G, Fos P, et al. Evaluación del estado de salud con la Encuesta Evaluación del estado de salud con la Encuesta SF-36: resultados preliminares en México. Salud Publica Mex 1999; 41: 110–118. [PubMed] [Google Scholar]
- 17.Ware JE, Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF-36): I. Conceptual Framework and Item Selection. Med Care 1992; 30: 473–483. [PubMed] [Google Scholar]
- 18.Barile JP, Horner-Johnson W, Krahn G, et al. Measurement characteristics for two health-related quality of life measures in older adults: The SF-36 and the CDC Healthy Days items. Disabil Health J 2016; 9: 567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Del Core MA, Ahn J, Wukich DK, et al. Gender Differences on SF-36 Patient-Reported Outcomes of Diabetic Foot Disease. Int J Low Extrem Wounds 2018; 17: 87–93. [DOI] [PubMed] [Google Scholar]
- 20.Ware J, Kosinski M, Keller S. SF-36 Physical and Mental health summary scales: a user´s manual. 5th ed. Boston: Health Assessment Lab, 1994. [Google Scholar]
- 21.Farivar SS, Cunningham WE, Hays RD. Correlated physical and mental health summary scores for the SF-36 and SF-12 Health Survey, V.1. Health Qual Life Outcomes 2007; 5: 54. DOI: 10.1186/1477-7525-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Ann Med 2001; 33: 350–357. [DOI] [PubMed] [Google Scholar]
- 23.VassarStats. Website for Statistical Computation. http://vassarstats.net/ (accessed May 9, 2021).
- 24.Parohan M, Yaghoubi S, Seraji A, et al. Risk factors for mortality in patients with Coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of observational studies. The Aging Male 2020; 23: 1416–1424. [DOI] [PubMed] [Google Scholar]
- 25.Peckham H, De Gruijter NM, Raine C, et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun 2020; 11: 6317. DOI: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao Y, Ding M, Dong X, et al. Risk factors for severe and critically ill COVID‐19 patients: A review. Allergy 2021; 76: 428–455. [DOI] [PubMed] [Google Scholar]
- 27.Rashedi J, Mahdavi Poor B, Asgharzadeh V, et al. Risk Factors for COVID-19. Infez Med 2020; 4: 469–474. [PubMed] [Google Scholar]
- 28.Tadic M, Cuspidi C, Sala C. COVID‐19 and diabetes: Is there enough evidence? J Clin Hypertens (Greenwich) 2020; 22: 943–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salazar M, Barochiner J, Espeche W, et al. COVID-19, hipertensión y enfermedad cardiovascular. Hipertens Riesgo Vasc 2020; 37: 176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daher A, Balfanz P, Cornelissen C, et al. Follow up of patients with severe coronavirus disease 2019 (COVID-19): Pulmonary and extrapulmonary disease sequelae. Respir Med 2020; 174: 106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frithiof R, Rostami E, Kumlien E, et al. Critical illness polyneuropathy, myopathy and neuronal biomarkers in COVID-19 patients: A prospective study. Clin Neurophysiol 2021; 132: 1733–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akter F, Mannan A, Mehedi HMH, et al. Clinical characteristics and short-term outcomes after recovery from COVID-19 in patients with and without diabetes in Bangladesh. Diabetes Metab Syndr 2020; 14: 2031–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carda S, Invernizzi M, Bavikatte G, et al. COVID-19 pandemic. What should physical and rehabilitation medicine specialists do? A clinician’s perspective. Eur J Phys Rehabil Med 2020; 56: 515–524. DOI: 10.23736/S1973-9087.20.06317-0. [DOI] [PubMed] [Google Scholar]
- 34.Marotta N, Demeco A, Moggio L, et al. Why is telerehabilitation necessary? A pre-post COVID-19 comparative study of ICF activity and participation. J Enabling Technologies 2021; 15: 117–121. [Google Scholar]
- 35.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nature Medicine 2021; 27: 601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosales-Castillo A, García de los Ríos C, Mediavilla García JD. Persistent symptoms after acute COVID-19 infection: importance of follow-up. Med Clin (Barc) 2021; 156: 35–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carfì A, Bernabei R, Landi F. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020; 324: 603–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahmud R, Rahman MM, Rassel MA, et al. Post-COVID-19 syndrome among symptomatic COVID-19 patients: A prospective cohort study in a tertiary care center of Bangladesh. PLoS One 2021; 16: e0249644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Landi F, Carfì A, Benvenuto F, et al. Predictive Factors for a New Positive Nasopharyngeal Swab Among Patients Recovered From COVID-19. Am J Prev Med 2021; 60: 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kamal M, Abo Omirah M, Hussein A, et al. Assessment and characterisation of post‐COVID‐19 manifestations. Int J Clin Pract 2021; 75: e13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu Y, Li X, Geng D, et al. Cerebral Micro-Structural Changes in COVID-19 Patients – An MRI-based 3-month Follow-up Study. EClinicalMedicine 2020; 25: 100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiong Q, Xu M, Li J, et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect 2021; 27: 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moradi F, Enjezab B, Ghadiri-Anari A. The role of androgens in COVID-19. Diabetes Metab Syndr 2020; 14: 2003–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Temperoni C, Grieco S, Pasquini Z, et al. Clinical characteristics, management and health related quality of life in young to middle age adults with COVID-19. BMC Infect Dis 2021; 21: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuan X-Y, Zhang H, Huang LR, et al. Evaluation of health-related quality of life and the related factors in a group of Chinese patients with interstitial lung diseases. PLoS One 2020; 15: e0236346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lutogniewska W, Jastrzebski D, Wyrwol J, et al. Dyspnea and quality of life in patients referred for lung transplantation. Eur J Med Res 2010; 15: 76–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Méndez R, Balanzá‐Martínez V, Luperdi SC, et al. Short‐term neuropsychiatric outcomes and quality of life in COVID‐19 survivors. J Intern Med 2021; 621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen KY, Li T, Gong FH, et al. Predictors of Health-Related Quality of Life and Influencing Factors for COVID-19 Patients, a Follow-Up at One Month. Front Psychiatry 2020; 11: 668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vlake JH, Wesselius S, Van Genderen ME, et al. Psychological distress and health-related quality of life in patients after hospitalization during the COVID-19 pandemic: A single-center, observational study. PLoS One 2021; 16: e0255774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020; 7: 611–627. [DOI] [PMC free article] [PubMed] [Google Scholar]