Abstract
Context:
Cymbidium mosaic virus (CymMV) is one of the most devastating viruses causing losses in the orchid industry, affecting economies worth millions of US dollars. CymMV significantly affects the orchid population and could be controlled through an integrated management strategy consisting of virus detection, good sanitation care of gardeners and their tools, and maintaining virus-free explants.
Evidence acquisition:
This review was written based on research publications relevant to the CymMV infection in orchids. The literature cited were obtained from online literature databases such as web of Science, Scopus, and Google Scholar. The searched term used was “Cymbidium mosaic virus”. Related publications to the initial search were also examined.
Results & Conclusions:
This review describes the threat of CymMV to the orchid population by examining its history, genome organization, symptoms on individual orchids, detection, and management. Current research has been focusing on the prospect of transgenic orchids with viral resistance. This review also highlights the potential role of the symbiotic relationship between orchids and arbuscular mycorrhiza fungi that could be useful to improve the protection of orchids against virus infection. Overall, this review provides information on how CymMV infection impacts the orchid population.
Keywords: Orchid virus, Orchid family, Plant viruses
1. Background
Orchids are monocotyledonous ornamentals that belong to the Orchidaceae family with global distribution and are considered as one of the most popular garden plants. Orchidaceae is one of the biggest plant families consists of a large number of species – around 500 new species described every year since 2003 – with wide varieties of colours, shapes, and scents ( 1 , 2 ). Many wild orchids were identified and recorded in addition to thousands of hybrids that were described and cultivated ( 1 ). Most cultivated orchids are economically valuable for their aesthetics, therapeutic, fragrant potential, as well as cosmetics values ( 1 - 3 ). Orchids play a major role in the global floriculture industry and many countries are involved in its import-export trade with an average value of at least US$500 million in the last decade ( 4 ). Like other plants, orchids are susceptible to viral infections. Records show that viral infection in wild orchids is relatively unknown and almost unnoticed compared to the cultivated ones ( 5 ). Besides, the infection in cultivated orchids has been the subject of research since they are significantly affecting the cut flower industry ( 6 , 7 ). There are at least 50 viruses that have been found to infect orchids, where the most prevalent ones are the Cymbidium mosaic virus (CymMV) and Odontoglossum ringspot virus (ORSV) (Table 1) ( 6 , 8 ). CymMV was first found in Cymbidium orchids ( 9 ) while ORSV was first found in tiger orchids, Odontoglossum grande (another name: Rossioglossum grande) ( 10 ). Several studies have found the ability of CymMV and ORSV to co-infect the same host ( 11 - 16 ). Co-infection can be associated with synergism between viruses and it usually produces more conspicuous disease symptoms compared to a single infection ( 8 , 12 , 16 - 19 ).
Table 1.
List of several viruses found infecting orchids and the geographical records. The information from this table is taken from ( 6 ).
| Virus name | Geographical location |
|---|---|
| Bean yellow mosaic virus (BYMV) | Japan, India |
| Calanthe mild mosaic virus (CalMMV) | Japan, India |
| Calanthe mosaic virus (CalMV) | Japan |
| Ceratobium mosaic virus (CerMV) | Australia |
| Clover yellow vein virus (ClYVV) | United Kingdom |
| Colombian datura virus (CDV) | Europe, Japan, Canada, United States |
| Dasheen mosaic virus (DsMV) | French Polynesia, India |
| Dendrobium mosaic virus (DeMV) | Hawaii |
| Diurus virus Y | Australia |
| Habeneria mosaic virus (HaMV) | Japan |
| Pecteilis mosaic virus (PcMV) | Japan |
| Phalaenopsis chlorotic spot virus (PhCSV) | Taiwan |
| Sarcochilus virus Y (SVY) | Australia |
| Spiranthes mosaic virus 2 (SpiMV2) | United States |
| Spiranthes mosaic virus 3 (SpiMV3) | United States |
| Turnip mosaic virus (TuMV) | Australia, United Kingdom |
| Vanilla mosaic virus (VanMV) | French Polynesia, India |
| Vanilla necrosis virus (VNV) | Fiji, Tonga, India |
| Cymbidium mosaic virus (CymMV) | Singapore, China, India, Taiwan, Malaysia, United States, Japan, Korea |
| Phaius virus X (PhaVX) | Japan |
| Carnation mottle virus (CarMV) | Taiwan |
| Cymbidium ringspot virus (CyRSV) | England, India |
| Cucumber mosaic virus (CMV) | French Polynesia, India |
| Dendrobium vein necrosis virus (DVNV) | Europe, United States |
| Orchid fleck virus (OFV) | Australia, Brazil, Japan, India |
| Tomato ringspot virus (ToRSV) | Puerto Rico |
| Capsicum chlorosis virus (CaCV) | Australia, Taiwan, Thailand |
| Impatiens necrotic spot virus (INSV) | Europe, United States |
| Tomato spotted wilt virus (TSWV) | Hawaii |
| Odontoglossum ringspot virus (ORSV) | United States, Thailand, Lithuania, Singapore, India |
| Tobacco rattle virus (TRV) | Japan, China |
| Groundnut bud necrosis virus (GBNV) | India |
Infections on orchids negatively affect the yield and the quality of orchids, significantly decreasing the number of total orchids viable for the cut flower trading in the orchid industry ( 6 , 8 , 12 ).
Consistently, CymMV is more common in the orchid population compared to ORSV ( 8 , 15 , 20 ). Similar to other important viruses, this review will be focusing on CymMV and its prevalence in the orchid population, emphasising the characteristics and comparison of the virus and relevant diagnostic tools, as well as current and future approaches in controlling the virus ( 21 - 23 ).
2. Virus Discovery and Symptomatology
CymMV was first found to infect Cymbidium orchids in California, USA in 1950 ( 9 ). Since then, CymMV has been found infecting various orchids globally ( 8 , 13 , 15 , 20 , 24 ). CymMV genome is accessible at the National Center for Biotechnology Information (NCBI) online database (Table 2). The first complete sequence of CymMV genome came from Singapore ( 6 , 8 ). So far, the virus has been found in various orchid species and two non-orchid hosts; Nicotiana benthamiana and Datura stramonium ( 6 , 8 , 24 ). The wide host-range of CymMV is supported by records showing positive CymMV infection, mainly in orchids; Dendrobium sp., Epidendrum sp., Laelia sp., Oncidium sp., Phalaenopsis sp., Vanda sp., Zygopetalum sp., Phaius tankervilliae, Vanilla planifolia, and in one non-orchid host – Cephalotaxus hainanensis (a species of conifer) found in nature (Table 2) ( 8 , 24 ).
Table 2.
List of current NCBI entries containing the information for Cymbidium mosaic virus (CymMV) isolates with complete genome sequences.
| CymMV Isolate (nt) | Host | Geographical location | Accession number | Reference |
|---|---|---|---|---|
| CymMV (6,227) | Cattleya sp. | Singapore | U62963 | ( 27 ) |
| China (6,203) | Cephalotaxus hainanensis | China | KR185347 | (unpublished) |
| HNXL (6,224) | Vanilla planifolia | Hainan, China | HQ681906 | ( 20 ) |
| NJ-1 (6,225) | Phalaenopsis sp. | Nanjing, China | JQ860108 | (unpublished) |
| Korean (6,227) | Cattleya sp. | Korea | AF016914 | ( 53 ) |
| 18-29 (6,218) | Dendrobium sp. | Hawaii, USA | EF125180 | ( 6 ) |
| 18-30 (6,218) | Dendrobium sp. | Hawaii, USA | EF125179 | ( 6 ) |
| 18-1 (6,218) | Dendrobium sp. | Hawaii, USA | EF125178 | ( 6 ) |
| plm1 (6,227) | Phaius tankervilliae | Palampur, India | AM055720 | ( 6 ) |
| M2 (6,226) | N/A | Taiwan | EU314803 | (unpublished) |
| Taiwan (6,227) | N/A | Taiwan | AY571289 | (unpublished) |
| CymMV (6,226) | N/A | Japan | AB197937 | (unpublished) |
| Cat (6,226) | Cattleya sp. | Japan | LC125633 | ( 54 ) |
| Malaysia (6,196) | Orchids | Malaysia | MK816927 | (unpublished) |
| SMi2 (6,225) | N/A | Yunnan, China | AM055640 | (unpublished) |
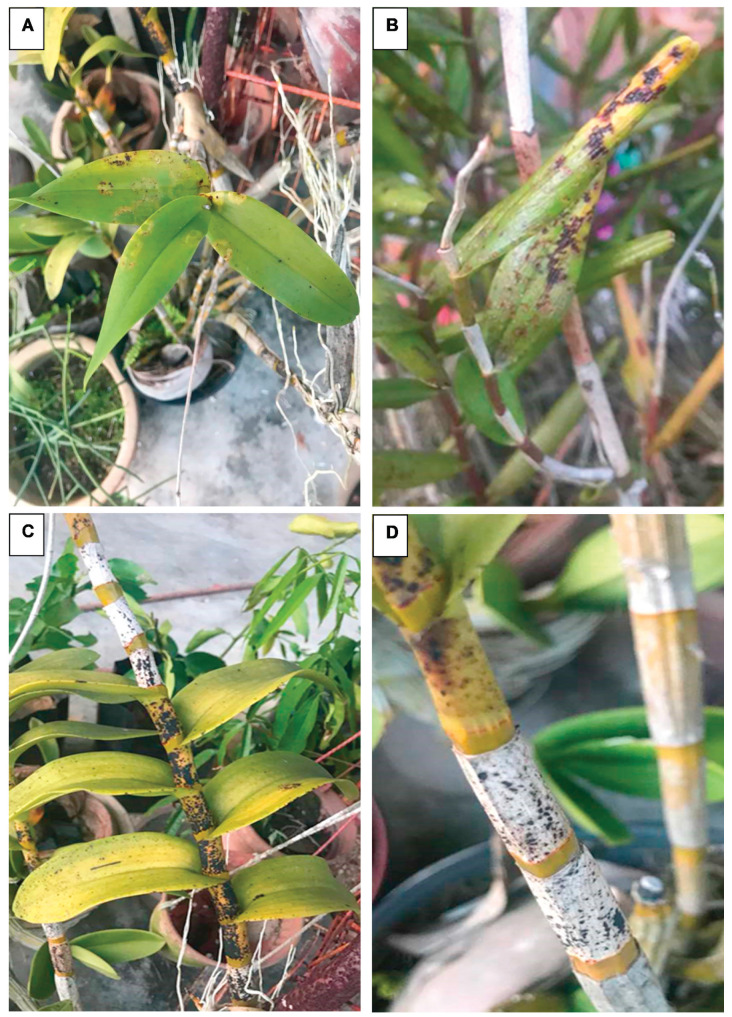
CymMV infection leads to asymptomatic or symptomatic orchids. So far, the symptoms are similar in different orchids regardless of their geographical location. CymMV infection in orchids can be detected from the following symptoms such as mosaic patterns, flowers with deformities and colour breaking, sunken chlorotic or necrotic patches on leaves and stems (Fig. 1) ( 8 , 12 , 24 ). Additionally, CymMV can also negatively affect the metabolic pathways and growth of infected orchids, i.e., dwarfing ( 6 , 25 ).
Figure 1.
Cymbidium mosaic virus infecting Dendrobium sp. systemically, causing observable symptoms. A) Leaves showing mosaic patterns B) Leaves showing yellowish colouration and dark necrotic spots.C) D) Stem showing dark necrotic spots. Photos have been taken by the authors for this manuscript.
3. Viral Genome Structure & Diversity
CymMV belongs to the Alphaflexiviridae family (genus: Potexvirus) and the virions have flexuous rod-shaped particles of approximately 500 nm (length) and 15 nm (width) ( 6 , 10 , 12 ). The genome consists of a positive, single-stranded monopartite RNA with around 6200 nucleotides containing five open reading frames (ORFs). A typical potexvirus, the ORFs of CymMV’s genome are flanked by the untranslated 5’- and 3’- ends and a poly-A tail at the 3’ end (Fig. 2) ( 6 ). ORF1 encodes for a 160-kDa putative RNA-dependent RNA polymerase (RdRp) protein. RdRp contains three conserved domains: (i) methyltransferase at the N-terminal region, (ii) RNA helicase and (iii) core binding domain – responsible for the RNA polymerase activities – both at the C-terminal region ( 6 , 8 ). However, the knowledge of the specific structure of the domains is quite limited ( 26 ).
Figure 2.
Schematic representation of Cymbidium mosaic virus genome organisation. The 5’ to 3’ end of the non-coding regions are represented as a single line. The open boxes represent the open reading frames (ORFs); ORF 1 encodes an RNA-dependent RNA polymerase (RdRp). ORF 2, 3, 4 encode triple gene block (TGB) proteins 1, 2, and 3, respectively. ORF 5 encodes the coat protein (CP). (A)n represents the poly-A tail. The numbers represent nucleotide positions. Figure adapted from ( 30 ).
ORF 2, 3, and 4 are overlapped and referred to as the triple gene block (TGB) which encode three proteins, namely, TGB1 (26-kDa), TGB2 (13-kDa), and TGB3 (10-kDa), respectively. TGB1 plays a role in cell-to-cell movement and the suppression of RNA silencing ( 6 ). ORF 5 encodes for the viral coat protein (CP) of 24 kDa, which also involves cell-to-cell movement and viral encapsulation ( 12 ).
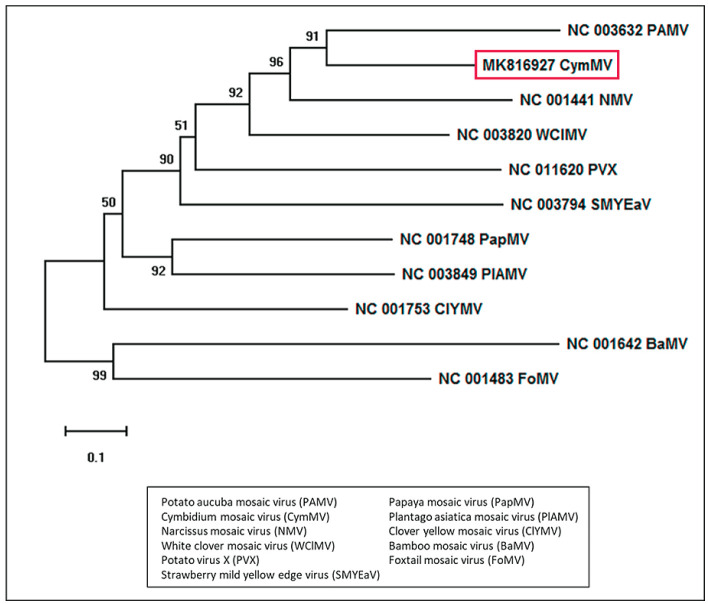
Various studies were conducted to examine the genome sequence variability of CymMV isolates. The phylogenetic comparison of the RdRp and CP shows the relationship of CymMV to other potexviruses, including but not limited to Clover yellow mosaic virus (ClYMV), White clover mosaic virus (WClMV), Narcissus mosaic virus (NMV), Potato aucuba mosaic virus (PAMV), and Strawberry mild yellow edge virus (SMYEaV) (Fig. 3) ( 5 , 6 , 8 , 27 ). The RdRp gene of CymMV contains the conserved motifs of the methyltransferase – It1 (nt 348-381), IIt1 (nt 502-523), IIt2 (nt 552-612), and IIt3 (nt 622-663) – among potexviruses ( 27 ).
Figure 3.
Phylogenetic tree showing the comparative analysis of coat protein nucleotide sequences of Cymbidium mosaic virus isolate of Malaysia (red box) with different potexviruses. The figure shows each isolate with its respective accession number and the potexvirus name abbreviation. The tree was generated using MEGA X software. The neighbor-Joining method based on the Kimura 2-parameter model was utilized in the phylogeny construction. Numbers at the branching points indicate the bootstrap support calculated for 1000 replicates, with values less than 50% not shown.
The RdRp gene among different CymMV isolates showed high sequence similarity (around 97-98%) which proves that the RdRp in CymMV is a conserved region ( 5 , 27 ). Meanwhile, the CP can also be considered a conserved region of CymMV due to the high sequence similarity (around 85-100%) across different isolates ( 5 , 6 , 8 , 27 ). Additionally, the amino acid sequence in the C-terminal of the CymMV CP varies significantly as compared to the N-terminal region ( 12 , 28 ).
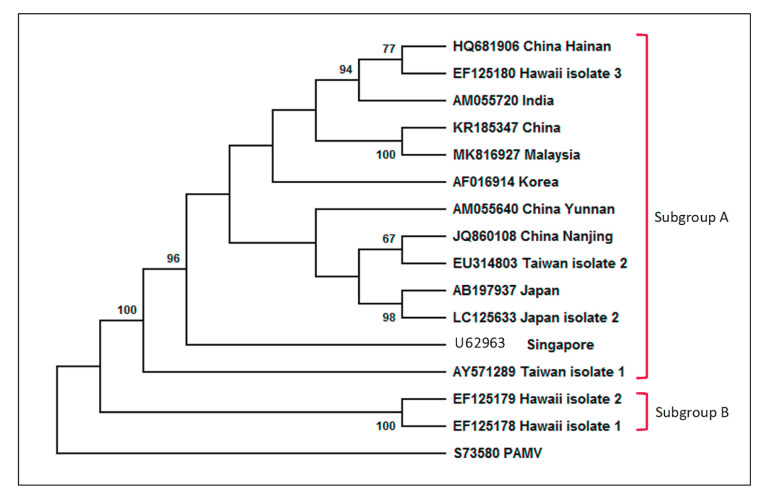
Sequence comparison of CP and RdRp in CymMV populations from different geographical origins revealed sequence similarity of more than 90% at both nucleotide and amino acid levels ( 5 , 6 , 28 ). Even though the phylogenetic analyses have shown that CymMV isolates can be classified into two subgroups, however, the variation between the subgroups is not significant enough to explain their geographical differences ( 5 , 6 , 8 , 11 , 29 ) and the phylogenetic analysis of CymMV isolates from Table 2 supports that the genomic variability is not influenced by their geographical locations (Fig. 4). Hence there is no clear correlation between the geographical origin of CymMVs and their sequence variation. However, several studies suggested that the extensive movement and exchanges of orchid varieties have not only caused the global virus transmission but has potentially muddled the true geographical origin of the isolates ( 4 - 6 ). The virus can be transmitted through sap inoculation for laboratory studies, but there are only mechanical means of transmission found in nature ( 6 ). So far, CymMV is not considered to be transmitted through seed or insect vectors, although there is a study suggesting that prolonged exposure of an insect towards infected plants could eventually lead to the insect propagating the virus to other healthy plants ( 8 , 30 ).
Figure 4.
Phylogenetic tree of the complete genome nucleotide sequences of published Cymbidium mosaic virus isolates as listed in Table 1 was generated using MEGA X software. The figure shows each isolate with its respective accession number and geographic origin. Maximum Likelihood method based on the Tamura 3-parameter model was utilized in the phylogeny construction, forming two major subgroups; subgroup A (isolates four China, two Taiwan, two Japan, Hawaii 3, India, Malaysia, Korea and Singapore) and subgroup B (isolates Hawaii 1 and Hawaii 2). The positing of CymMV-Malaysia in subgroup A was supported by a bootstrap value of 100%. The complete genome sequence of the Potato aucuba mosaic virus (PAMV) (GenBank accession no. S73580) was included as an outgroup. Numbers at the branching points indicate the bootstrap support calculated for 1000 replicates, with values less than 50% not shown.
There is a consensus variation in the amino acid sequences among potexviruses from the alignments of the TGB proteins of CymMV, in which that TGB1, TGB2, and TGB3 of CymMV showed the highest amino acid similarity to PAMV, WClMV, and Bamboo mosaic virus (BaMV), respectively (ranging from 55.4% - 60%) ( 27 ). Interestingly, TGB1 of CymMV was found to encode for an NTP-binding helicase motif, which is also found in the ORF1, suggesting a co-requirement for successful infection ( 6 , 8 , 27 ).
4. Detection Methods for CymMV in Orchids
Accurate viral diagnosis and identification are essential in the orchid industry as precise and reliable diagnostic procedures are required to manage and control CymMV. To make things worse, asymptomatic infected individuals also allow the virus to spread into the population ( 12 ). An enzyme-linked immunosorbent assay (ELISA) was developed for rapid and reliable detection of CymMV infection in large sample sets of orchids ( 6 , 8 , 14 ), followed by the development of immunocapture-RT-PCR (IC-RT-PCR) that can detect both viral nucleic acid and its capsid protein at optimum annealing temperature (55 °C) ( 31 ). Additionally, the virus detection by IC-RT-PCR can be achieved from dilution equivalent to 100 ng of orchid material, hence these abilities showed that IC-RT-PCR is more sensitive than ELISA by 10 to 100-fold ( 31 ). Using both ELISA and IC-RT-PCR allows for a more efficient virus screening ( 31 ). Since then, multiple detection methods that target the nucleic acid and/or the protein of the virus in orchids have been developed. These methods such as digoxigenin (DIG)-labelled cRNA probes, electron microscopy, mass spectrometry, molecular beacon hybridisation, and quartz crystal microbalance (QCM) immunosensors are used individually or ‘mix-and-match’ to confirm the occurrence of CymMV infection in the host ( 8 , 24 , 32 , 33 ). Table 3 provides a summary of available CymMV detection methods that are viable for quarantine authentication, germplasm collection, and selection for resistant plants. These diagnostic procedures allow for a range of methods to be carried out by countries and laboratories that are involved in the research and business involving orchids. Many research laboratories use RT-PCR is widely used as a part of their diagnostic tests in many research labs because it is a reliable, cost-effective, and straightforward procedure ( 24 , 33 ). The efficiency of CymMV screening in plants is most sought after in the industry and is constantly being improved and refined for a more accurate and precise rapid screening of large samples without compromising sensitivity and specificity ( 12 ).
Table 3.
List of the detection methods for Cymbidium mosaic virus alongside their respective descriptions and references.
| Detection Method | Description | Reference(s) |
|---|---|---|
| Enzyme-linked immunosorbent assay (ELISA) | Practical for routine detection of virus in a large number of samples. Downsides include a limited supply of viral antiserum and potential interference due to the high viscosity of the plant sap. | ( 55 ) |
| Immunocapture-polymerase chain reaction (IC-PCR) | Includes the use of polyclonal antibodies for increased sensitivity. Used in conjunction with ELISA, this method is suitable for the certification of virus-free orchid materials. | ( 31 ) |
| Reverse transcription-polymerase chain reaction (RT-PCR) | A direct, simple, and sensitive method to detect CymMV. Only requires a small amount of template nucleic acid. Requires no antibodies. | ( 24 , 33 ) |
| Touch-down polymerase chain reaction (TD-PCR) | The annealing temperature starts higher and gradually lowered in subsequent cycles, ensuring higher yield by reducing chances of mispriming. Also, it can be used to simultaneously detect multiple viruses with the same primer pairs. | ( 6 , 8 ) |
| TaqMan® real-time RT-PCR | A detection method combining quantitative RT-PCR and fluorescent detection. Probes can be designed to target RdRp and CP genes. | ( 6 , 8 ) |
| Reverse transcription loop-mediated isothermal amplification (RT-LAMP) assay | This assay involves only one step, making it a rapid and easy detection method. However, it is yet to be fully optimised – examination of parameters, buffer, temperature, and concentration. | ( 56 - 58 ) |
| Multiplex RT-PCR | Conducted using two separate primer pairs that are specific to two separate viruses, allowing simultaneous detection of the presence of two different viruses in the same sample. This can be a rapid, simple, relatively cheaper, and precise tool across a wide range of plant species. | ( 6 , 8, 53 ) |
| Electron microscopy | A direct method to observe the presence of potexvirus particles in a sample. This method is usually used as an additional confirmation. | ( 24 ) |
| Digoxigenin (DIG)-labelled cRNA probe | Increased localisation study means that this method can be used to examine the viral distribution patterns in plants’ leaves and stems. | ( 6 , 8 ) |
| Immuno-Capillary Zone Electrophoresis (I-CZE) | Suitable for routine detection when dealing with many samples. This method analyses antibody-antigen reaction in real-time, thus, a shorter time is taken compared to other antibody-based assays. | ( 6 , 8 ) |
| Mass spectrometry: - | Shorter time for sample preparation, resulting in rapid and accurate detection of CymMV. However, other confirmation tests e.g., electron microscopy must be done. | |
| -Liquid chromatography (LC/MS) | ( 6 , 8 ) | |
| -matrix-assisted laser desorption-ionization (MALDI) | ||
| Molecular beacon | Very specific and can be used for simultaneous detection of different viruses in the same sample. Furthermore, the analysis can be done in real-time, with relatively high throughput. Disadvantages of this method include high costs. | ( 6 , 8 ) |
| Quartz crystal microbalance (QCM) | Rapid detection method using plant crude saps. Can also be optimised for different viruses in different hosts. In the long run, there is potential to be more cost-effective for rapid screening. | ( 59 ) |
| Fibre optic particle plasmon resonance immunosensor | A label-free detection tool using gold nanorods as the sensor. The nanorods are functioning through antibodies that can detect corresponding viruses in a sample. The potential includes faster multiplex analysis, better reproducibility, portability, and quality control in the orchid industry. | ( 60 ) |
5. Current Management & Prospects for the Orchid Industry
Orchids are classified in appendix II of the Convention on International Trade in Endangered Species (CITES), and it has impacted the movement of orchids across international borders. Alongside the local phytosanitary protocols, these regulations aim to limit the transmission of orchid viruses by limiting the host movement ( 5 , 21 ). In addition to the border control enforcement, the management of plant viral diseases does integrate other approaches such as the sanitation of the gardeners and their tools, development of virus-free seedlings and plantlets, and virus screening for exported and imported orchids ( 6 ). The examples include proper handwashing with soap, the usage of disposable gloves, pots sterilisation, and handling the younger plants first before attending to the older ones, amongst others. Furthermore, regular disinfection of tools should be carried out by using chemical disinfectants such as phosphate, bleach solution, and even bacterial culture filtrate ( 5 , 6 ). The development of virus-free orchids was proposed through plant meristem tissue culture and intense management to control virus transmission across generations and maintaining healthy plant stocks ( 7 , 34 , 35 ). CymMV-free plants have been grown successfully through tissue culture using various types of antiviral compounds, such as ribavirin (virazole), dithiouracil, and also colchicine treatment ( 6 ). Generally, the meristem culture is established through several steps: sample collection and sterilisation, generation of aseptic culture in a media, growing the explants, and analysis of their stability ( 6 , 7 , 36 ). This approach is widely used commercially to maintain a steady supply of healthy, virus-free explants for various plant species ( 6 , 36 ). Additionally, meristem culture could be supplanted with thermo-, cryo-, and chemo- therapies to better eliminate pathogens from the plants ( 37 ). The above-mentioned diagnostic methods could be used to screen plants crossing the border ( 33 ). The implementation of the integrated management strategy does incur additional time and could affect the quality and quantity of orchids being transported globally; however, it is the best way to control viral transmission especially for those plants that are not native. Hence an easier way to control viral transmission is to grow the orchids locally, although it remains to be proven to better protect this lucrative floriculture industry and also the wild and native orchid population ( 5 ).
A recent strategy to control virus transmission is by producing transgenic orchids that can naturally induce virus resistance. This approach is feasible by using several approaches such as RNA silencing, gene stacking, host-derived resistance genes, and host defence factors ( 12 , 38 , 39 ). Various research has identified at least 30 resistance genes and transcription factors that can potentially act as a viral resistance in the transgenic lines ( 6 , 38 ). The transgenic lines of N. benthamiana, Dendrobium sp., and Phalaenopsis sp. with expressed CymMV-CP have shown possible protection against CymMV ( 18 , 40 ). Besides, the CP-mediated transgenic lines of Nicotiana sp. and Dendrobium sp. could protect against dual infection by CymMV and ORSV ( 18 , 40 ). Recently, a high degree of protection against dual infection of CymMV and ORSV was found in the transgenic lines of N. benthamiana through RNA silencing ( 41 ). Another overexpression study shows that the Argonaute proteins (AGOs) play a central role in the antiviral defence mechanism in plants ( 42 ). This current progress, combined with the increasing interest in using genome editing tools such as CRISPR/Cas, shows a potential, feasible and effective approach to generate resistant orchids that can highly benefit the industry ( 42 - 45 ).
Another interesting prospect to consider is the relationship between orchids and arbuscular mycorrhiza (AM) symbiont fungi. AM fungi play an essential role in plant growth and development. Recently, the molecular mechanisms behind this relationship are explored through whole genome sequencing. Transcriptomic studies have been used to identify the molecular processes involved in seed germination and protocorm development during the symbiosis of several orchids’ species with their respective AM fungus ( 46 ). Phylogenetic analyses on the RNA datasets obtained from orchids and their associated AM fungi suggested a specific evolutionary advantage for both host and fungi during symbiosis ( 47 , 48 ). Besides being actively involved in nutrient exchanges, the AM fungi also act as bioprotectant against phytopathogens ( 49 , 50 ). Hao et al suggested the possibility that symbiosis protects the plants against virus via three main mechanisms; (i) modulated plant tolerance, (ii) manipulation of induced systemic resistance, and (iii) pressuring the vector, e.g., affecting the insect reproduction and restricting the development of nematodes ( 50 ). However, some argue that AM fungi could also cause exacerbation of viral infection in host plants, rather than symptom attenuation, as observed during the introduction of an AM fungus into potato plant that led to the attenuated and exacerbated conditions after the plant is infected by Potato virus Y ( 49 - 52 ). Until now, it is yet to be explored if the symbiotic relationship could protect orchids against CymMV or otherwise and these studies shall provide the foundation to further understand the tripartite association of plant-fungi-virus from the orchid cultivation industry perspective.
6. Conclusion
This review outlines the characteristics and importance of CymMV. The combination of multiple diagnostic tests could fully utilise the advantages and limiting their respective disadvantages, which would result in the best screening strategy for the orchids and other plants, too. Orchids in the floriculture industry must be virus-free, and this can be achieved via the implementation of an integrated management approach that incorporates advanced analyses and strategies. Current progress in transgenic orchids research and development has a truly promising prospect to help protect the orchid lines against virus infection, leading to the improvement of the economic standing in the orchid industry. Realising the potential of research in orchid virus management would greatly benefit the industry.
Conflicts of Interests
The authors declare that they have no conflict of interest in the publication.
Acknowledgements
Research was funded under the grant Universiti Kebangsaan Malaysia DPP-2018-010 and GUP-2017-035. The authors would like to acknowledge a colleague, Maathavi Kannan, for advice and aid in the construction of phylogenetic trees.
References
- 1.Chase MW, Cameron KM, Freudenstein J V, Pridgeon AM, Salazar G, van den Berg C, et al. An updated classification of Orchidaceae. Bot J Linn Soc. 2015;177(2):151–174. doi: 10.1111/boj.12234. [DOI] [Google Scholar]
- 2.Christenhusz MJM, Byng JW. The Number of Known Plants Species in the World and its Annual Increase. Phytotaxa. 2016;261(May):201–217. doi: 10.11646/phytotaxa.261.3.1. [DOI] [Google Scholar]
- 3.Julsrigival J, Songsak T, Kirdmanee C, Chansakaow S. Determination of volatile constituents of Thai fragrant orchids by gas chromatography-mass spectrometry with solid-phase microextraction. Chiang Mai Univ J Nat Sci. 2013;12(1):43–58. doi: 10.12982/CMUJNS.2013.0005. [DOI] [Google Scholar]
- 4.De LC, Pathak P, Rao AN, Rajeevan PK. Global Orchid Industry. In: Commercial Orchids. Berlin, Boston: De Gruyter; 2014. pp. 13–9. [Google Scholar]
- 5.Fogell DJ, Kundu S, Roberts DL. Genetic homogenisation of two major orchid viruses through global trade- based dispersal of their hosts. Plants People Planet. 2019;1(4):356–362. doi: 10.1002/ppp3.46. [DOI] [Google Scholar]
- 6.Pant RP, Rashmi ER, Manjunath N, Baranwal VK. Status of orchid viruses in India and management strategies for them.In: Applied Plant Virology. Elsevier Inc: 2020. pp. 725–745. [DOI] [Google Scholar]
- 7.Pradhan S, Regmi T, Ranjit M, Pant B. Production of virus-free orchid Cymbidium aloifolium (L.) Sw. by various tissue culture techniques. Heliyon. 2016;2(10) doi: 10.1016/j.heliyon.2016.e00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ajjikuttira P, Wong SM. Molecular biology of two orchid infecting viruses: Cymbidium mosaic potexvirus and odontoglossum ringspot tobamovirus. Orchid Biol Rev Perspect X. 2009:251–277. doi: 10.1007/978-1-4020-8802-5-8. [DOI] [Google Scholar]
- 9.Jensen DD. Mosaic of Cymbidium Orchids. Phytopatholog. 1950;40:966–967. [Google Scholar]
- 10.Jensen DD, Gold AH. A virus ring spot of Odontoglossum Orchid: symptoms, transmission, and electron microscopy. Phytopatholog. 1951;41(7):648–653. [Google Scholar]
- 11.Rao X, Li Y, Sun J, Li X, Li M, Xiang M. Genetic diversities of cymbidium mosaic virus and odontoglossum ringspot virus isolates based on the coat protein genes from orchids in guangdong province. China. J Phytopathol. 2015;163(4):324–329. doi: 10.1111/jph.12285. [DOI] [Google Scholar]
- 12.Koh KW, Lu HC, Chan MT. Virus resistance in orchids. Plant Sci. 2014;228:26–38. doi: 10.1016/j.plantsci.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Soto-Valladares AG, De La Torre- Almaraz R, Xoconostle-Cazares B, Ruiz-Medrano R. First Report of Cymbidium mosaic virus and Odontoglossum ringspot virus in Orchids in Mexico. Plant Dis. 2011;96(1):464. doi: 10.1094/PDIS-08-11-0655. [DOI] [PubMed] [Google Scholar]
- 14.Rani P, Pant RP, Jain RK. Serological detection of Cymbidium mosaic and Odontoglossum ringspot viruses in orchids with polyclonal antibodies produced against their recombinant coat proteins. J Phytopathol. 2010;158(7-8):542–545. doi: 10.1111/j.1439-0434.2009.01668.x. [DOI] [Google Scholar]
- 15.Liu FX, Han YC, Li WD, Shi XQ, Xu W, Lin MG. Incidence of Cymbidium mosaic virus and Odontoglossum ringspot virus affecting Oncidium orchids in Hainan Island, China. Crop Prot. 2013;54:176–80. doi: 10.1016/j.cropro.2013.08.013. [DOI] [Google Scholar]
- 16.Hu WW, Wong SM, Loh CS, Goh CJ. Synergism in replication of cymbidium mosaic potexvirus(CymMV) and odontoglossum ringspot tobamovirus (ORSV) RNA in orchid protoplasts. Arch Virol. 1998;143(7):1265–1275. doi: 10.1080/02508281.2010.11081633. [DOI] [PubMed] [Google Scholar]
- 17.Wong SM. Studies on synergism and complementation of long distance movement of cymbidium mosaic virus RNA by odontoglossum ringspot virus coat protein. Acta Hortic. 2010; 878:467–72. doi: 10.1099/vir.0.80772-0. [DOI] [PubMed] [Google Scholar]
- 18.Petchthai U, Xie Z, Wong SM. Transgenic nicotiana benthamiana resistance to synergistic infection of two orchid viruses CymMV and ORSV. Acta Hortic. 2018;1193:69–76. doi: 10.17660/ActaHortic.2018.1193.10. [DOI] [Google Scholar]
- 19.Pai H, Jean WH, Lee YS, Chang YCA, Lin NS. Genome-wide analysis of small RNAs from Odontoglossum ringspot virus and Cymbidium mosaic virus synergistically infecting Phalaenopsis. Mol Plant Pathol. 2019;21(2):188–205. doi: 10.1111/mpp.12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He Z, Jiang D, Liu A, Sang L, Li W, Li S. The complete sequence of Cymbidium mosaic virus from Vanilla fragrans in Hainan. China. Virus Genes. 2011;42(3):440–443. doi: 10.1007/s11262-011-0592-x. [DOI] [PubMed] [Google Scholar]
- 21.Yusop MSM, Saad MFM, Talip N, Baharum SN, Bunawan H. A Review on Viruses Infecting Taro (Colocasia esculenta (L.) Schott) Pathogens. 2019;8(2):1–13. doi: 10.3390/pathogens8020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kannan M, Ismail I, Bunawan H. Maize dwarf mosaic virus: From genome to disease management. Viruses. 2018;10(9):1–23. doi: 10.3390/v10090492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kannan M, Saad MM, Zainal Z, Kassim H, Ismail I, Talip N, et al. Sequence and phylogenetic analysis of the first complete genome of rice tungro spherical virus in Malaysia. Iran J Biotechnol. 2020;18(4):9–17. doi: 10.30498/IJB.2020.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cánovas SE, Ballari MC, Nome CF. First report of Cymbidium mosaic virus and Odontoglossum ring spot virus in Argentina. Australas Plant Dis Notes. 2016;11(1):1–3. doi: 10.1007/s13314-015-0189-7. [DOI] [Google Scholar]
- 25.Gourdel S, Leclercq-Le Quillec F. Coat protein gene of Cymbidium mosaic virus from Vanilla fragrans in Reunion Island. J Phytopathol. 2001;149(9):561–564. doi: 10.1046/j.1439-0434.2001.00633.x. [DOI] [Google Scholar]
- 26.Jia H, Gong P. A structure-function diversity survey of the rna-dependent rna polymerases from the positive-strand rna viruses. Front Microbiol. 2019;10(AUG):1–11. doi: 10.3389/fmicb.2019.01945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong SM, Mahtani PH, Lee KC, Yu HH, Tan Y, Neo KK, et al. Cymbidium mosaic potexvirus RNA: Complete nucleotide sequence and phylogenetic analysis. Arch Virol. 1997;142(2):383–391. doi: 10.1007/s007050050084. [DOI] [PubMed] [Google Scholar]
- 28.Moraes LA, Krause-Sakate R, Pavan MA. Incidence and characterization of viruses infecting orchids in São Paulo state, Brazil. Trop Plant Pathol. 2017;42(2):126–131. doi: 10.1007/s40858-016-0126-0. [DOI] [Google Scholar]
- 29.Yoon JY, Chung BN, Choi GS, Choi SK. Genetic variability in the coat protein genes of Cymbidium mosaic virus isolates from orchids. Virus Genes. 2012;44(2):323–8. doi: 10.1007/s11262-011-0683-8. [DOI] [PubMed] [Google Scholar]
- 30.Allen C. Virus transmission in orchids through the feeding damage of australian cockroach, periplaneta australasiae. Acta Hortic. 2010;878:375–380. doi: 10.17660/actahortic.2010.878.47. [DOI] [Google Scholar]
- 31.Barry K, Hu JS, Kuehnle AR, Sughii N. Sequence analysis and detection using immunocapture-PCR of cymbidium mosaic virus and odontoglossum ringspot virus in Hawaiian orchids. J Phytopathol. 1996;144(4):179–186. doi: 10.1111/j.1439-0434.1996.tb01511.x. [DOI] [Google Scholar]
- 32.Varma A, Singh MK. Appl Plant Virolog. INC: 2020. pp. 79–92. [Google Scholar]
- 33.Ali RN, Dann AL, Cross PA, Wilson CR. Multiplex RT-PCR detection of three common viruses infecting orchids. Arch Virol. 2014;159(11):3095–3099. doi: 10.1007/s00705-014-2161-9. [DOI] [PubMed] [Google Scholar]
- 34.Retheesh ST, Bhat AI. Simultaneous elimination of Cucumber mosaic virus and Cymbidium mosaic virus infecting Vanilla planifolia through meristem culture. Crop Prot. 2010;29(10):1214–1217. doi: 10.1016/j.cropro.2010.05.017. [DOI] [Google Scholar]
- 35.Chien KW, Agrawal DC, Tsay HS, Chang CA. Elimination of mixed “Odontoglossum ringspot” and “Cymbidium mosaic” viruses from Phalaenopsis hybrid “V3” through shoot-tip culture and protocorm-like body selection. Opt Laser Technol. 2015;67:1–6. doi: 10.1016/j.cropro.2014.09.008. [DOI] [Google Scholar]
- 36.Pe PPW, Naing AH, Soe MT, Kang H, Park K Il, Kim CK. Establishment of meristem culture for virus-free and genetically stable production of the endangered plant Hosta capitata. Sci Hortic (Amsterdam) 2020;272:109591. doi: 10.1016/j.scienta.2020.109591. [DOI] [Google Scholar]
- 37.Varveri C, Maliogka VI, Kapari-Isaia T. Principles for supplying virus-tested material. In: Advances in Virus Research. 1st ed. Elsevier Inc: 2015. pp. 1–32. [DOI] [PubMed] [Google Scholar]
- 38.Cillo F, Palukaitis P. Transgenic Resistance. In: Advances in Virus Research. Elsevier B.V: 2014. pp. 35–146. [DOI] [PubMed] [Google Scholar]
- 39.Ren R, Wei Y, Ahmad S, Jin J, Gao J, Lu C, et al. Identification and characterization of NPR1 and PR1 homologs in Cymbidium orchids in response to multiple hormones, salinity and viral stresses. Int J Mol Sci. 2020;21(6):1–20. doi: 10.3390/ijms21061977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petchthai U, Yee CS Le, Wong SM. Resistance to CymMV and ORSV in artificial microRNA transgenic Nicotiana benthamiana plants. Sci Rep. 2018;8(1):1–8. doi: 10.1038/s41598-018-28388-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen T-Y, Pai H, Hou L-Y, Lee S-C, Lin T-T, Chang C-H, et al. Dual resistance of transgenic plants against Cymbidium mosaic virus and Odontoglossum ringspot virus. Sci Rep. 2019;9(1):10230. doi: 10.1038/s41598-019-46695-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuo SY, Hu CC, Huang YW, Lee CW, Luo MJ, Tu CW, et al. Argonaute 5 family proteins play crucial roles in the defence against Cymbidium mosaic virus and Odontoglossum ringspot virus in Phalaenopsis aphrodite subsp. formosana. Mol Plant Pathol. 2021:1–17. doi: 10.1111/mpp.13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsai W-C, Dievart A, Hsu C-C, Hsiao Y-Y, Chiou S-Y, Huang H, et al. Post genomics era for orchid research. Bot Stud. 2017;58(61):1–22. doi: 10.1186/s40529-017-0213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kui L, Chen H, Zhang W, He S, Xiong Z, Zhang Y, et al. Building a Genetic Manipulation Tool Box for Orchid Biology: Identification of Constitutive Promoters and Application of CRISPR/Cas9 in the Orchid, Dendrobium officinale. Front Plant Sci. 2016;7:2036. doi: 10.3389/fpls.2016.02036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boutigny AL, Dohin N, Pornin D, Rolland M. Overview and detectability of the genetic modifications in ornamental plants. Hortic Res. 2020;7(1) doi: 10.1038/s41438-019-0232-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yeh C-M, Chung K, Liang C-K, Tsai W-C. New Insights into the Symbiotic Relationship between Orchids and Fungi. Appl Sci. 2019;9(3):585. doi: 10.3390/app9030585. [DOI] [Google Scholar]
- 47.Shimura H, Masuta C, Koda Y. Metagenomic Analyses of the Viruses Detected in Mycorrhizal Fungi and Their Host Orchid. Methods Mol Biol. 2018;1746:161–172. doi: 10.1007/978-1-4939-7683-6_12. [DOI] [PubMed] [Google Scholar]
- 48.Ikeda Y, Shimura H, Kitahara R, Masuta C, Ezawa T. A Novel Virus-Like Double-Stranded RNA in an Obligate Biotroph Arbuscular Mycorrhizal Fungus: A Hidden Player in Mycorrhizal Symbiosis. Mol Plant-Microbe Interact. 2012;25(7):1005–1012. doi: 10.1094/mpmi-11-11-0288. [DOI] [PubMed] [Google Scholar]
- 49.Deja-Sikora E, Mercy L, Baum C, Hrynkiewicz K. The Contribution of Endomycorrhiza to the Performance of Potato Virus Y-Infected Solanaceous Plants: Disease Alleviation or Exacerbation? Front Microbiol. 2019;10:516. doi: 10.3389/fmicb.2019.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hao Z, Xie W, Chen B, Hao Z, Xie W, Chen B. Arbuscular Mycorrhizal Symbiosis Affects Plant Immunity to Viral Infection and Accumulation. Viruses. 2019;11(6):534. doi: 10.3390/v11060534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu P, Huang D, Chang D. Mycorrhizal symbiosis enhances Phalaenopsis orchid’s growth and resistance to Erwinia chrysanthemi. African J Biotechnol. 2011;10(50):10095–10100. doi: 10.5897/AJB11.1310. [DOI] [Google Scholar]
- 52.Miura C, Yamaguchi K, Miyahara R, Yamamoto T, Fuji M, Yagame T, et al. The Mycoheterotrophic Symbiosis Between Orchids and Mycorrhizal Fungi Possesses Major Components Shared with Mutualistic Plant-Mycorrhizal Symbioses. Mol Plant Microbe Interact. 2018;31(10):1032–1047. doi: 10.1094/MPMI-01-18-0029-R. [DOI] [PubMed] [Google Scholar]
- 53.Kim SM, Choi SH. Simultaneous detection of Cymbidium mosaic virus and Odontoglossum ringspot virus in orchids using multiplex RT-PCR. Virus Genes. 2015;51(3):417–422. doi: 10.1007/s11262-015-1258-x. [DOI] [PubMed] [Google Scholar]
- 54.Keima T, Hagiwara-Komoda Y, Hashimoto M, Neriya Y, Koinuma H, Iwabuchi N, et al. Deficiency of the eIF4E isoform nCBP limits the cell-to-cell movement of a plant virus encoding triple-gene-block proteins in Arabidopsis thaliana. Sci Rep. 2017;7:1–13. doi: 10.1038/srep39678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sherpa AR, Hallan V, Zaidi AA. In vitro expression and production of antibody against cymbidium mosaic virus coat protein. Indian J Virol. 2012;23(1):46–49. doi: 10.1007/s13337-012-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee M-S, Yang M-J, Hseu Y-C, Lai G-H, Chang W-T, Hsu Y-H, et al. One-step reverse transcription loop-mediated isothermal amplification assay for rapid detection of Cymbidium mosaic virus. J Virol Methods. 2011;173:43–48. doi: 10.1007/s10658-015-0821-6. [DOI] [PubMed] [Google Scholar]
- 57.Chang WH, Yang SY, Lin CL, Wang CH, Li PC, Chen TY, et al. Detection of viruses directly from the fresh leaves of a Phalaenopsis orchid using a microfluidic system. Nanomedicine Nanotechnology. Biol Med. 2013;9(8):1274–1282. doi: 10.1016/j.nano.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 58.Chang WH, Yang SY, Wang CH, Lee G Bin, Chen TY, Li PC, et al. Pathogen detection from phalaenopsis orchids by using an integrated microfluidic system. Ieee Int Conf Nano/Mol Med Eng Nanomed. 2012:6–10. doi: 10.1109/NANOMED.2012.6509132. [DOI] [Google Scholar]
- 59.Chang CA, Chen HR, Chiu CH. Disinfection of Odontoglossum ringspot virus and Cymbidium mosaic virus from tools used during orchid cultivation. In Ist Int’ernational Orchid Symposium. 2010. pp. 381–388. [Google Scholar]
- 60.Lin HY, Huang CH, Lu SH, Kuo IT, Chau LK. Direct detection of orchid viruses using nanorod-based fiber optic particle plasmon resonance immunosensor. Biosens Bioelectron. 2014;51:371–378. doi: 10.1016/j.bios.2013.08.009. [DOI] [PubMed] [Google Scholar]