Abstract
Background
Glenohumeral joint instability is associated with structural deficits and/or alterations in sensory and motor processing; however, a proportion of patients with glenohumeral joint instability fail to respond to surgical and rehabilitative measures. This systematic review aimed to establish if functional cortical changes occur in patients with glenohumeral joint instability.
Methods
AMED, CINAHL, Cochrane Central Register of Controlled Trials, Embase, Medline, PEDro, Pubmed, PsychINFO and Scopus were searched from inception to 17 March 2021. Randomised controlled trials and non-randomised trials were included and quality was appraised using the Downs and Black tool.
Results
One thousand two hundred seventy-nine records were identified of which five were included in the review. All studies showed altered cortical function when comparing instability patients with healthy controls and included areas associated with higher cortical functions.
Discussion
The findings of this systematic review offer some insight as to why interventions addressing peripheral pathoanatomical factors in patients with glenohumeral joint instability may fail in some cases due to functional cortical changes. However, data are of moderate to high risk of bias. Further high-quality research is required to ascertain the degree of functional cortical changes associated with the type and duration of glenohumeral joint instability.
Keywords: Glenohumeral joint instability, cortical reorganisation
Introduction
The glenohumeral joint (GHJ) is the least stable joint in the body. 1 Whilst instability is most commonly caused by trauma, a group of patients experience instability in the absence of trauma. 2 A common clinical sign of GHJ instability is apprehension,3–5 but what causes this apprehension is not certain. Whether it is structural, due to a mismatch in sensory or motor processing 6 or due to cerebral patterning whereby patterns of functional connectivity in the brain arouse memories of unpleasant sensations and induce motor resistance and anxiety 7 remains unclear.
Surgery to rectify structural deficits in GHJ instability and rehabilitation to correct altered motor patterns and proprioceptive deficits can have good results; however, there are a subset of patients who fail to improve with such measures.2,8 What was once considered a purely peripheral pathology is now thought to be associated with neural alterations including aberrant cortical activity levels.4,5
Representation of the physical body within the cortex has been found to be altered in persistent pain states 9 as measured by activity within specific areas of the cortex.
Tactile acuity is suggested to be reflective of cortical activity and cortical representation9,10 and changes in tactile acuity are thought to be due to neuroplastic reorganisation of the higher brain centres. 11 Tactile acuity has been found to be altered in multiple persistent pain states 10 such as chronic regional pain syndrome (CRPS),12,13 phantom limb pain, 14 chronic neck pain 15 and low back pain (LBP). 14 Changes in cortical function have also been found in patients with GHJ instability.4,7,16–19 Whether these changes are the cause or the result of GHJ instability is yet to be established.
Altered motor imagery as an indirect measure of functional cortical activity has been demonstrated in patients with chronic musculoskeletal pain using left/right judgement tasks (LRJT). 20 Recent evidence has identified altered reaction times in patients with shoulder pain 21 and frozen shoulder22,23 in LRJT. However, Breckenridge et al. found the accuracy in LRJT was not statistically significantly different in frozen shoulders 22 and shoulder pain 21 when compared to the unaffected shoulder or control participants respectively. Conversely, Mena-del Horno et al. 23 found that accuracy was reduced in those with frozen shoulders when compared to control participants. Furthermore, two-point discrimination was altered in patients with frozen shoulders when compared to controls 23 or the unaffected shoulder. 22 These studies indicate altered cortical function within certain shoulder pathologies. Therefore, it could be suggested that this may also be possible in patients with GHJ instability.
The identification of functional cortical changes has provided potential targets for treatments in some certain persistent pain states with the aim of normalising the cortical remapping.24,25 Therefore, there is potential to incorporate such treatment approaches into the management of GHJ instability which may provide the key to improved outcomes in certain subgroups of patients. To the authors’ knowledge, this is the first systematic review to date evaluating the current literature regarding functional cortical changes in GHJ instability. This systematic review therefore aims to provide an overview of the current available research on cortical changes in patients with GHJ instability when compared to controls. Increasing knowledge in this area may enhance our understanding of why current treatment approaches may sometimes fail and highlight potential areas for research.
Methods
This systematic review was undertaken in accordance with the Cochrane Handbook for Systematic Reviews of interventions 26 using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.27,28 The protocol for this systematic review was devised and registered with PROSPERO (ID number CRD42019132074). https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=132074
Search strategy
AMED, CINAHL, Cochrane Central Register of Controlled Trials (CENRAL), Embase, Medline, PEDro, Pubmed, PsychINFO and Scopus were searched from inception to 17 March 2021. Grey literature (open grey) and trial registers (clinicaltrails.gov) were searched for work that was not published before 17 March 2021. Hand searching of the reference lists of included articles for relevant trials was also undertaken. See Supplementary Appendix 1 for example of search strategy used. This search strategy was reproduced as closely as possible with all other databases. The electronic searches of all databases were carried out by the main author (ML). Two authors (ML and HP) independently screened titles, abstracts and full text articles for eligibility. Disagreements were resolved by discussion and consensus. If not achieved, a third reviewer (LD) was approached to decide if the publications were to be included in the review.
Eligibility
Studies were eligible for inclusion if they were case-controlled, cross-sectional, randomised controlled trials (RCTs) or non-randomised controlled trials (NRCTs). RCTs and NRCTs were included if they measured cortical activity prior to the experimental intervention and only baseline results were included in the review.
Trials were eligible if participants were diagnosed with traumatic or atraumatic GHJ instability. This required positive tests for apprehension (specifically the apprehension and/or apprehension relocation test), a history of traumatic dislocation or diagnosis through clinical assessment by a specialist clinician. No restrictions were placed on language or the classification of type or direction of instability. Trials which assessed functional cortical activity either directly through Functional Magnetic Resonance Imaging (fMRI) or indirectly through measures of tactile acuity/discrimination (e.g. two-point discrimination, tactile localisation or graphaesthesia) were included. Indirect measures of cortical function considered for inclusion were any which assessed tactile acuity which is the precision of the sense of touch. 10 Changes of the response profile of neurones to tactile stimulus are associated with cortical reorganisation within the brain. 29 These two methods of functional cortical activity were chosen in order to maximise the data on cortical function within this niche area.
Control groups could be healthy controls defined as those without signs or symptoms of shoulder pain, dysfunction or hyperlaxity or the contralateral asymptomatic side of individual participants. Exclusion criteria were any trials that included patients with neurological disorders such as a cerebrovascular accident or head injuries. A screening tool based on the PICOS criteria for this systematic review was devised to facilitate the inclusion and exclusion of trials. The tool was piloted by both reviewers using the first 50 citations brought up on EMBASE.
Risk of bias
All included studies were appraised for risk of bias by two reviewers (ML and HP) and disagreements resolved by arbitration of the third reviewer (LD). Risk of bias was assessed using the Downs and Black (DaB) tool. 30 Using only one tool enabled direct comparison across studies.
The checklist was modified in line with other studies whereby item 27 was changed to a score of 0 (no/unable to determine) or 1 (yes) for the presence or absence of power calculations.31–33 Items 4, 8, 9, 14, 17, 19, 21, 23, 24 and 26 were excluded as they were originally included to assess interventions/treatments in RCTs and item 10 was excluded as inferential statistics were not relevant in cross-sectional studies. 34 From the scoring of each study, a Quality Index (QI) score was calculated as a normalised proportion of the points assigned to a study using the following formula: QI% = (sum of scores × 100)/number of items used. As per Adamczyk et al., 34 a score of >75% was deemed high quality (low risk of bias), 50-75% moderate quality/risk of bias and <50% poor quality (high risk of bias).
Data extraction
The two reviewers (ML and HP) independently extracted information using a standardised data extraction form. Disagreements were resolved by consensus and in the event this was not possible, a third reviewer (LD) was used for a majority decision.
Synthesis of results
Due to the heterogeneity of included trials, it was decided that data be analysed using narrative synthesis as recommended by The Cochrane Handbook for Systematic Reviews of Interventions. 35
The primary outcomes were the results of the measure of cortical activity of participants compared to controls, whether that be the identified areas of the cortex as through fMRI or the distances or errors associated with more indirect measures such as two-point discrimination. Risk of bias is presented in a table order to compare individual studies and their component parts using the DaB tool.
Results
Search
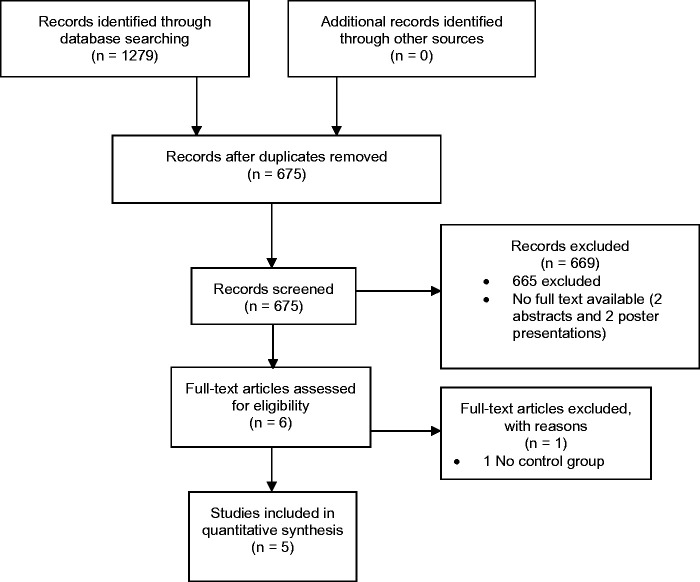
Database searches yielded 1279 citations; 675 duplicates were removed and a further 669 removed after screening of all titles and abstracts. Six full text articles were then screened for eligibility with one more being excluded leaving five articles for inclusion. Figure 1 presents a PRISMA flow diagram with reasons for exclusion. No further articles were found during grey literature or hand searching. All included studies were published after 2013. Three of the five studies were from Switzerland with the remaining two from the United Kingdom and Japan.
Figure 1.
PRISMA flow chart of search.
Study characteristics
All studies appeared to be prospective case-controlled designs, however only Cunningham et al. 18 explicitly stated their study design.
Participants
Participant numbers of included studies varied from 7 to 28. The mean age of participants ranged from 26.8 years (±1.2) 18 to 28.2 (±8.6) years 17 with a mean across all studies of 26.8 (±1.25) years. Three out of the five studies studied only male participants whilst the other two included males and females.
Two out of the five studies included only right-handed participants,17,18 another only had right-handed individuals although it was not clear if this was part of the inclusion criteria. 17 The other two studies used left- and right-handed individuals.4,36 Howard et al. 36 flipped the images of left-handed individuals in order to ensure cortical activation contralateral to the affected side was matched in all individuals. A summary of the demographic data of included studies is presented in Table 1.
Table 1.
Summary of study demographics.
| Reference | Sample size (females) R handed: L
handed |
Age (mean ± SD) (years) |
Type of GHJ instability | Side of GHJ instability R:L | Assessment of cortical function | ||
|---|---|---|---|---|---|---|---|
| Patients | Controls | Patients | Controls | ||||
| Cunningham et al. 18 | 28 (0) 28:0 | 10 (0) 10:0 | 26.8 ± 1.2 | 29.6 ± 1.3 | Not defined | 18:10 | fMRI |
| Haller et al. 7 | 15 (0) 15:0 | 10(0) 10:0 | 27.5 ± 6.4 | 29.0 ± 4.7 | Not defined | 9:6 | fMRI |
| Howard et al. 36 | 16 (15) 12:4 | 16 (15) 12:4 | 24.2 ± 6.0 | 23.8 ± 5.1 | Stanmore Classification Type II/III | 12:4 | fMRI |
| Shitara et al. 17 | 14 (3) 14:0 | 12 (4) 12:0 | 28.2 ± 8.6 | 23.2 ± 3.2 | Not defined | 14:0 | fMRI |
| Zanchi et al. 4 | 14 (0) 10:4 | 10 (0) Not given | 27.3 ± 2.0 | 29.6 ± 1.3 | Not defined | 10:4 | fMRI |
MRI: functional magnetic resonance imaging.
Stanmore Classification of GHJ instability III.
All but one study did not classify the type of GHJ instability included however inferred anterior instability through the use of a positive apprehension test for diagnosis. Whilst Zanchi included patients with traumatic anterior instability, 4 Howard et al. 36 was the only study to classify the type of instability and recruited Stanmore Triangle Classification type II/III instability participants. 3 The other studies appeared to exclude this patient group including only participants with a history of previous traumatic dislocations or excluding multi-directional instability.4,17 Whilst three of the studies specified anterior instability within their inclusion criteria,4,17,18 one did not specify any direction7,36 whilst the other alluded to anterior instability through the inclusion of patients with a positive apprehension test. 7 The diagnosis of instability varied between studies and is demonstrated in Table 2 along with other key study design characteristics of the included studies.
Table 2.
Key study design characteristics of included studies.
| Reference | Method of clinical diagnosis | Experimental task | fMRI technique | Analysis |
|---|---|---|---|---|
| Cunningham et al. 18 | Apprehension test | Apprehension and control videos | ICA VBM GLM DTI and TBSS | GLM ICA |
| Haller et al. 7 | Apprehension test | Apprehension and control videos | ICA VBM DTI and TBSS GLM | GLM ICA |
| Howard et al. 36 | History, physical examination and arthroscopy. | Active flexion, abduction or rest | Parametric mapping using MNI | Cluster level correct |
| Shitara et al. 17 | History of >1 traumatic instability, positive apprehension and relocation test and Bankart lesion identified by MR arthrogram or arthroscopy | Motor imagery using pictures, passive shoulder motion task | MNI GLM | GLM |
| Zanchi et al. 4 | History of traumatic anterior glenohumeral instability, positive apprehension test and radiological evidence on MR arthrogram or CT | Apprehension and control videos | DTI and TBSS ICA GLM | ICA |
ICA: independent component analysis; VBM: voxel based morphometry; GLM: general linear model; DTI: diffusion tensor imaging; TBSS: tract based spatial statistics; MNI: Montreal neurological imaging.
Intervention characteristics
All studies utilised a task-based fMRI study design using cue stimulation during scanning to induce brain activity. Three studies asked the participants to watch self-made videos intended to induce states of both rest/non-stimulating (control) and apprehension.4,7,18 Shitara et al. 17 used pictures/photographs of a person in three different movement conditions (control, lifting a kettle into abduction and forced passive GHJ abduction and external rotation (ABER) and asked the participants to imagine completing these movements themselves. Additionally, they used a passive shoulder motion task where the shoulder was moved into internal and external rotation in 90° abduction. Finally, Howard et al. 36 used blocks of purely active shoulder movement (flexion, abduction or rest) during fMRI assessment.
Assessment of cortical function
Despite the search criteria being open to both direct and indirect measures of cortical function, no studies were found which investigated tactile acuity, graphaesthesia or any other clinical indirect measure of cortical function. All included studies used fMRI as an assessment of cortical activity.
Risk of bias
The results of the risk of bias assessment are presented in Table 3. Four out of the five articles had a Quality Index score over 50% indicating a moderate risk of bias.7,17,18,36 Cunningham et al. 18 and Howard et al. 36 scored the highest with 64.70% whilst Zanchi et al. 4 scored lowest with 47.06% and was the only study to be deemed of poor quality and therefore at high risk of bias. The mean QI% for all studies was 57.64% (±6.86).
Table 3.
Risk of bias of included studies as assessed by DaB tool.
| Reference | Reporting (n = 7) | External validity (n = 3) | Internal validity – bias (n = 4) | Internal validity – cofounding (n = 2) | Power (n = 1) | Total score | QI score % |
|---|---|---|---|---|---|---|---|
| Cunningham et al. 18 | 7 | 0 | 3 | 1 | 0 | 11 | 64.70 |
| Haller et al. 7 | 6 | 0 | 3 | 0 | 0 | 9 | 52.94 |
| Howard et al. 36 | 7 | 0 | 3 | 1 | 0 | 11 | 64.70 |
| Shitara et al. 17 | 7 | 0 | 3 | 0 | 0 | 10 | 58.82 |
| Zanchi et al. 4 | 5 | 0 | 3 | 0 | 0 | 8 | 47.06 |
n: number of constituent items within each section; QI %: Quality Index %.
All included studies scored poorly for external validity due to participants not being truly representative of the normal instability population (variability in gender, hand dominance and side of instability). The lack of adjustment for these cofounders, lack of randomisation (which would not be possible in case-controlled trials) and the lack of information on the time of recruitment also led to low scores for internal bias for most studies. However, all studies scored at least 3/6 for internal validity indicating low systematic bias.
None of the included studies included a power calculation thus all failed to obtain a score in this domain. Howard et al. 36 did discuss their sample size but referred to a sample size used in a previous study and did not undertake their own calculation.
Study findings
Table 4 summarises the key study findings from all included studies.
Table 4.
Summary of key findings from all included studies grouped by experimental task.
| Reference | Experimental task | Cortical activity identified in patients (p) | Cortical activity identified in controls (p) |
|---|---|---|---|
| Videos | |||
| Cunningham et al. 36 | Apprehension and control videos | Pain VAS/Rowe/WOSI: increased activity in bilateral anterior insula, anterior cingulate cortex, posterior cingulate cortex, bilateral dorsomedial prefrontal cortex, somatosensory area and somatosensory cortex. Rowe score additionally associated with midcingulate cortex and visual and attention areas (p < 0.05) Significant differences between brain networks of patients and controls (p < 0.01) Post hoc correlation: Rowe score negatively correlated with activity in bilateral frontal pole and posterior division of the left inferior temporal gyrus SSV correlated with activity in the bilateral precentral gyrus, bilateral postcentral gyrus and bilateral superior parietal lobe No statistical difference in grey and white matter density | None given |
| Haler et al. 7 | Apprehension and control videos | Higher functional connectivity in bilateral primary sensory-motor area, dlPFC, anterior insula, and dACC (p < 0.05). Reduced functional connectivity in a bilateral higher-level visual network including the parietal region (p < 0.05) Increased functional connectivity activation strength in task-positive networks with increasing unpleasantness (p < 0.022) GLM: Activation of left primary sensory-motor area and dlPFC. Contralateral regions showed clear non-significant trend No statistical difference in white and grey matter. | None given |
| Zanchi et al. 4 | Apprehension and control videos | Hypoactivation in the ventral ACC, posterior cinculate and precuneus Hyperactivation of anterior insula, motor and somatosensory cortex Increased FA in left internal capsule and thalamus (p < 0.05) | Non-given |
| Movement stimulus | |||
| Howard et al. 36 | Movement block of flexion, abduction or rest | Hyperactivation in left primary motor cortex (BA4) and supramarginal gyrus (BA40), left inferior frontal gyrus (BA44), left precentral gyrus (BA6) and left middle frontal gyrus (BA6) (p < 0.05) Voxel wise correction: 1 voxel in primary motor cortex (BA4) where activation was greater (p < 0.05) | Greater activation in right parahippocampal gyrus (BA27) and perirhinal cortex (BA36) (p < 0.05). |
| Shitara et al. 17 | Passive shoulder motion task | No increase in activity in those with instability vs. controls. Covariate analysis with apprehension and activity: Hyperactivation of left middle temporal gyrus, left superior orbital gyrus, left middle frontal gyrus and left inferior parietal lobule. | Increased activity in right pre- and postcentral gyrus, right superior frontal gyrus, right medial temporal pole, left middle and inferior temporal gyri and left angular gyrus (p < 0.001). |
| Motor imagery | |||
| Shitara et al. 17 | Motor imagery task using picture | Greater activity in right hippocampus and amygdala, right precentral gyrus and left hippocampus (p < 0.001). Covariate analysis with apprehension and activity: ABER: Increased activity in anterior cingulate cortex (ACC), right S1, hippocampus, parahippocampal gyrus, middle occipital gyrus, superior parietal lobule, linguinal gyrus, bilateral cerebellum, left thalamus, fusiform gyrus, precuneus and calcarine gyrus in motor imagery. Kettle: Hyperactivation in left amygdala, thalamus, inferior frontal gyrus, right rolandic operculum and middle occipital, linguinal and rectal gyri during motor imagery. | Kettle condition: Increased activity in left precentral gyrus, left postcentral gyrus, left posterior cingulate cortex, right rectal gyrus, right superior occipital gyrus, right inferior parietal lobule, right superior parietal lobule, right rectal gyrus, right middle orbital gyrus, left paracentral lobule (kettle) (p < 0.001). ABER: Increased activity in left precentral gyrus, left postcentral gyrus, right inferior parietal lobule, left paracentral lobule, right inferior temporal gyrus, right precentral gyrus, left inferior parietal lobule, left thalamus, left fusiform gyrus, left middle frontal gyrus and right fusiform gyrus (p < 0.001). |
VAS: Visual Analogue Scale; Western Ontario Shoulder Instability Index; SSV: subjective shoulder value; dACC: dorsal anterior cingulate cortex; GLM: general linear model; dlPFC: dorsolateral prefrontal cortex; FA: functional anisotropy; BA: Brodmann area; ACC: anterior cingulate cortex; ABER: abduction external rotation condition.
Video apprehension cues
Three studies investigated cortical function using self-made videos to assess changes in cortical function.4,7,18 and all used ICA and TBSS for their analysis. Three studies showed increased activity in the anterior insula, primary somatosensory cortex and primary motor cortex4,7,18 in participants with GHJ instability compared to control participants. Two studies demonstrated increased activity in the anterior cingulate cortex (ACC) and posterior cingulate cortex (PCC)4,18 in those with GHJ instability. Further activity was found within the prefrontal cortex including the dorsomedial prefrontal cortex (dmPFC).7,18 frontal pole and dorsolateral prefrontal cortex (dlPFC).7,18 Additionally, increased activity was also noted within the occipital lobe, inferior temporal gyrus 18 and the superior parietal lobe.4,18
Motor imagery
Shitara et al. 17 was the only study to use motor imagery as a stimulus in the form of photos depicting a control position of the shoulder, a movement condition (kettle) and an apprehension condition (ABER). The results identified increased activity (p < 0.001) in participants with GHJ instability in the kettle (ipsilateral hippocampus and amygdala) and in the ABER condition (contralateral hippocampus and ipsilateral precentral gyrus; p < 0.001). In contrast, control participants showed increased activity in the superior parietal lobule and motor network on the ipsilateral side to the ‘affected’ shoulder and contralateral premotor cortex, primary motor and sensory cortices and the thalamus (p < 0.001). Using covariate analysis, additional areas of activity were noted in participants with instability as shown in Table 4.
Movement stimulus
Two studies looked at the effect of movement on cortical activity.17,36 Shitara et al., 17 in addition to motor imagery, investigated the effect of passive movement on cortical activity. Participants’ shoulders were positioned into approximately 90° abduction and then internally and externally rotated. The authors found no significantly different brain activity in instability participants compared to controls with passive movement. However, the control participants showed increased activity in the pre- and postcentral gyrus, superior frontal gyrus and medial temporal pole ipsilateral to the side of instability and the middle and inferior temporal gyri and angular gyrus contralateral to the affected shoulder.
In contrast, Howard et al. 36 used the active movements of flexion and abduction to investigate cortical activity. They found significantly increased activity in the contralateral primary motor cortex, supramarginal gyrus, frontal gyrus, precentral gyrus and middle frontal gyrus in participants with GHJ instability. Further voxel wise correction showed a specific voxel in Brodmann area 4, the primary motor cortex, which was greater in all instability participants except one who had normal shoulder related outcome measures. In contrast, control participants showed significantly greater activity in the ipsilateral parahippocampal gyrus and the perirhinal cortex.
One publication excluded from the review as it did not meet the inclusion criteria does however merit discussion. Ladermann et al. 19 produced a poster presentation and the authors were contacted; however, this study was not published for unknown reasons. The study used video cues as a stimulus with fMRI and utilised independent component analysis (ICA). No specific anatomical brain regions were identified in the results; however, their results showed increased functional connectivity in ipsilateral motor areas and the default mode network, an area of the brain which includes the dmPFC, PCC and the inferior parietal lobule. 37
Discussion
The aim of this article was to use a systematic review to investigate if GHJ instability was associated with functional cortical changes within the brain. It provides an accessible synthesis of the current knowledge base within this area and provides a narrative synthesis to assist both clinicians and researchers. All studies demonstrated a difference in cortical function when comparing participants with GHJ instability to healthy controls. The included studies also showed that the area of cortical function differed depending on the stimulus used to induce cortical activity.
The findings of this review were not unexpected as functional cortical changes have been observed in musculoskeletal conditions such as LBP,38,39 Medial patellofemoral ligament instability (MPFL), 40 anterior cruciate ligament instability (ACL)41,42 and CRPS.43,44 What was unexpected is that the areas activated in GHJ instability patients are not limited to the motor or sensory cortex but are much more complex in nature. The cortical changes observed in patients with GHJ instability involve many areas of the brain including the prefrontal cortices, insula, perirhinal and hippocampal cortex, hippocampus, cerebellum and the amygdala. These areas are associated with higher cortical functions including anxiety, pain, fear, fearful memories, cognitive control of motor behaviour and anticipation.7,17,18,45
Apprehension and functional changes in cortical activity
The results suggest that people with GHJ instability appear to be working harder to achieve stability, 36 have higher levels of motor resistance 7 and pay greater attention to external stimuli of threat. 17 Noxious stimuli is associated with increased functional activity within the ‘pain matrix’, a brain network including the cingulate cortex, insula, limbic system, primary and secondary motor cortices and the frontal and parietal lobes. 46 These are the areas of the brain noted to have altered functional activity in patients with GHJ instability included within this systematic review (dlPFC, dACC, somatosensory area, anterior insula and the dmPFC). They are collectively associated with pain modulation, expectancy of pain, anxiety, 18 the cognitive control of motor behaviour and appraisal of negative input, 7 emotional regulation, 7 the processing of tactile information and motor resistance, preparation and readiness. 7
Recent work has identified that the pain-matrix is not exclusive to pain and is activated by other non-painful stimuli. 37 For example, areas of the pain-matrix are also involved in sensorimotor control in patients with LBP which is suggested to indicate altered sensorimotor and pain processing which may act as a warning system serving to respond to an aparrent threat. 38 Therefore, one interpretation is that the participants in the studies included within the review were interpreting the experimental stimuli as threatening. Goossens et al. 38 suggest that LBP participants were over-attentive or over-reactive to potential threatening stimuli resulting in overgeneralised motor responses to protect the spine which could correlate with the increased motor resistance noted in participants with GHJ instability.7,17
Movement and function changes in cortical activity
Two studies examined the impact of movement upon cortical activity.17,36 Howard et al. 36 used active movements and highlighted a single voxel found in participants with GHJ instability overlapping both motor and sensory cortices emphasising the close association between sensory processing and movement production. This single voxel corresponds to an area reported to have an inhibitory effect on distant sites within the motor cortex and is associated with impaired motor function. These findings were also found in chronic stroke patients 47 indicating that centrally driven inhibition may have a potential role in chronic GHJ instability. 36
Similar to patients with GHJ instability, Kadowaki et al. 40 suggested that apprehension regarding patella dislocation in patients with MPFL deficiency evokes a fear memory as a result of previous or recurrent dislocation. Feelings of apprehension and instability may continue even after surgery which has been found in GHJ instability. 48 Similar to some of the studies within the review, increased activity in the visual cortex has been found in patients with MPFL instability 40 and ACL injuries 41 and is proposed to be due to restricted proprioceptive feedback from injured ligaments and a reliance on visual feedback. Howard et al. 36 found increased activity within areas associated with the higher level of processing of sensory information that is thought to contribute to GHJ stability. Therefore patients with GHJ instability could have an increased reliance on visual feedback to compensate for a lack of proprioceptive feedback as visual and proprioceptive feedback combine to produce accurate limb movement. 49
Study heterogeneity and bias
There was a high level of heterogeneity among all studies including the study populations, fMRI parameters and the methods used to stimulate cortical activity. Shitara et al. 17 found the areas of the brain activated with motor imagery and passive range testing were completely different, indicating that different protocols may impact results therefore limiting comparison with one another. Of note is that the included studies all used different fMRI parameters which can impact upon image resolution and detail. Additionally, different analysis techniques were used including the General Linear Model,7,17,18 ICA4,7,18 and cluster level corrections 36 all of which limited comparisons between studies.
Most significant was the heterogeneity in the selected participants, namely the absence of explicit verification of the type of instability included. Howard et al. 36 and Zanchi 4 were the only authors to explicitly state the types of instability included; Stanmore type II/III and anterior instability respectively. Such heterogeneity limits the ability to infer any findings to the general GHJ instability population and limits the ability to draw definitive conclusions from the review.
All studies had a moderate to high risk of bias and all scored low on external validity as the study populations and the environment they were conducted in were not representative of normal clinical practice. Internal validity scores for cofounding variables were also low due to variations in population demographics that was not commonly accounted for within the analyses. However, in small-scale studies such diversity would add significant confounders which could skew the results. For example, including both left and right handed or male and female participants would require data analysis of these groups individually to ascertain for any differences. However, due to the small number of participants within the studies, these calculations would be significantly underpowered and may lead to type II error. Whilst a sample size of at least 20 is suggested to result in adequate reliability for investigations using fMRI. 50 However, smaller sample sizes are not uncommon in fMRI studies due to the financial implications but there is a risk of publication of underpowered studies 51 and where no power calculations were conducted in any of the included studies. The implications of underpowered studies are that any differences in cortical function between controls and instability patients may not be detected. Additionally, there may be higher variance in the results with large numbers of cortical areas identified as having altered levels of activity. The studies in this review achieved a moderate to high risk of bias indicating moderate to low quality so meaningful conclusions cannot be made confidently from the results of this systematic review but they provide a good starting point for further research.
Strengths and limitations
Strengths of this review include the use of a strict protocol, including non-English articles and using a risk of bias tool and using the PRISMA guidelines. 28 The limited amount of research available within this area, the heterogeneity of data and the differing study protocols making undertaking a meta-analysis unfeasible were the limitations. Generalisability of the results of this review is limited due to the specific populations of studies such as variations in the type of instability investigated and the gender bias towards men.
Clinical implications
A subset of patients with GHJ instability have failed conservative and surgical management which may be due to altered cortical function in these patients. Ascertaining possible differences in cortical activity could help identify new treatment options resulting in the more successful management of this patient group.
The findings do not support a need for a change in current practice. The British Elbow and Shoulder Society recommend the management of Traumatic and Atraumatic GHJ instability patients in line with their guidelines. All but one of the included studies appeared to only assess patients with anterior instability and predominantly traumatic dislocations and therefore are not representative of the entire GHJ instability population and more specifically, of the subset who fail to improve with treatment. This review highlights the need for high quality studies into the functional cortical changes associated with different classifications of GHJ instability. This could enable more specific management approaches tailored specifically towards the type of instability. Furthermore, research to explore if cortical changes are seen in those who have been successfully treated for instability and no longer show symptoms could provide further insight into the cause or effect of functional cortical changes in shoulder instability. Additionally, studies should focus on techniques to assess these changes within the clinical setting which would reduce costs and allow appropriately powered studies. The results indicate that there is an association between cortical activity in areas associated with cognition and emotion in patients with GHJ instability. This highlights the potential for exploration of therapeutic interventions which may induce changes in functional cortical activity such as two-point discrimination and graphaesthesia training in addition to psychological therapies which may impact prefrontal cortex activity. Results suggest that there may be cortical changes involved in the presentation of shoulder instability which warrants further research. Therefore, treatment may need to move away from local musculoskeletal exercise-based rehabilitation and consider treatments which can impact cortical activity.
Conclusion
This systematic review found evidence to suggest that shoulder instability is associated with functional cortical changes within the brain; however, the evidence was at moderate to high risk of bias. The differing methodologies, small sample sizes and variability in patient demographics, including the instability classification, overall study heterogeneity and bias make definitive conclusions difficult. Areas of increased activity and functional connectivity included areas associated with the sensory, cognitive and emotional processing of pain. This could be a reason why focusing primarily on peripheral pathoanatomical assessment and treatment fails in some patient groups. Identifying and targeting higher cortical areas associated with GHJ instability with the appropriate interventions could facilitate better management of this subgroup of patients.
Supplemental Material
Supplemental material, sj-pdf-1-sel-10.1177_17585732211019016 for Functional cortical changes associated with shoulder instability – a systematic review by Morissa F Livett, Deborah Williams, Hayley Potter and Melinda Cairns in Shoulder & Elbow
Acknowledgements
We would like to thank Katy Oak, Ben Hughes and Lucy Dove for their support and guidance throughout this research.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Contributorship: DW and BH conducted the initial research scoping and conceived the research questions. ML devised the formal question and protocol and conducted the formal systematic review. MC was the supervisor of the research project. ML wrote the first draft of the manuscript and all authors reviewed and edited the manuscript and approved the final version.
Guarantor: ML.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Morissa F Livett https://orcid.org/0000-0001-5087-0053
Deborah Williams https://orcid.org/0000-0003-2935-0621
References
- 1.Brogan K, Baxter JA, Tennent D. Managing patients with shoulder instability. Orthop Trauma 2018; 32: 153–158. [Google Scholar]
- 2.Bateman M, Jaiswal A, Tambe AA. Diagnosis and management of atraumatic shoulder instability. J Arthrosc Jt Surg 2018; 5: 79–85. [Google Scholar]
- 3.Lewis A, Kitamura T, Bayley JIL. The classification of shoulder instability: new light through old windows!. Curr Orthop 2004; 18: 97–108. [Google Scholar]
- 4.Zanchi D, Cunningham G, Ladermann A, et al. Structural white matter and functional connectivity alterations in patients with shoulder apprehension. Sci Rep 2017; 7: 42327–42327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zanchi D, Cunningham G, Ladermann A, et al. Brain activity in the right-frontal pole and lateral occipital cortex predicts successful post-operatory outcome after surgery for anterior glenoumeral instability. Sci Rep 2017; 7: 498–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrett C. The clinical physiotherapy assessment of non-traumatic shoulder instability. Shoulder Elbow 2015; 7: 60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haller S, Cunningham G, Laedermann A, et al. Shoulder apprehension impacts large-scale functional brain networks. Am J Neuroradiol 2014; 35: 691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noorani A, Goldring M, Jaggi A, et al. BESS/BOA patient care pathways: atraumatic shoulder instability. Shoulder Elbow 2019; 11: 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lotze M, Moseley GL. Role of distorted body image in pain. Curr Rheumatol Rep 2007; 9: 488–496. [DOI] [PubMed] [Google Scholar]
- 10.Catley MJ, O'Connell NE, Berryman C, et al. Is tactile acuity altered in people with chronic pain? a systematic review and meta-analysis. J Pain 2014; 15: 985–1000. [DOI] [PubMed] [Google Scholar]
- 11.Baliki MN, Schnitzer TJ, Bauer WR, et al. Brain morphological signatures for chronic pain. PLoS One 2011; 6: e26010–e26010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juottonen K, Gockel M, Silen T, et al. Altered central sensorimotor processing in patients with complex regional pain syndrome. Pain 2002; 98: 315–323. [DOI] [PubMed] [Google Scholar]
- 13.Vartiainen NV, Kirveskari E, Forss N. Central processing of tactile and nociceptive stimuli in complex regional pain syndrome. Clin Neurophysiol 2008; 119: 2380–2388. [DOI] [PubMed] [Google Scholar]
- 14.Flor H, Braun C, Elbert T, et al. Extensive reorganization of primary somatosensory cortex in chronic back pain patients. Neurosci Lett 1997; 224: 5–8. [DOI] [PubMed] [Google Scholar]
- 15.Harvie DS, Edmond-Hank G, Smith AD. Tactile acuity is reduced in people with chronic neck pain. Musculoskelet Sci Pract 2018; 33: 61–66. [DOI] [PubMed] [Google Scholar]
- 16.Frostick SP, Howard AJ, Kemp G. Does cortical activation hold the key to shoulder instability? J Shoulder Elbow Surg 2018; 27: e135–e136. [Google Scholar]
- 17.Shitara H, Shimoyama D, Sasaki T, et al. The neural correlates of shoulder apprehension: a functional MRI study. PloS one 2015; 10: n.p–n.p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cunningham G, Zanchi D, Emmert K, et al. Neural correlates of clinical scores in patients with anterior shoulder apprehension. Med Sci Sports Exerc 2015; 47: 2612–2620. [DOI] [PubMed] [Google Scholar]
- 19.Ladermann A, Haller S, Cunningham G, et al. Cerebral activation related to shoulder apprehension in patients with glenohumeral instability and in healthy volunteers. Swiss Med Wkly 2013; 198: 7S–7S. Conference abstract. [Google Scholar]
- 20.Breckenridge JD, Ginn KA, Wallwork SB, et al. Do people with chronic musculoskeletal pain have impaired motor imagery? A meta-analytical systematic review of the left/right judgment task. J Pain 2019; 20: 119–132. [DOI] [PubMed] [Google Scholar]
- 21.Breckenridge JD, McAuley JH, Moseley GL, et al. Is implicit motor imagery altered in people with shoulder pain? The shoulder left/right judgement task. Musculoskelet Sci Pract 2020; 48: 102159–102159. [DOI] [PubMed] [Google Scholar]
- 22.Breckenridge JD, McAuley JH, Ginn KA. Motor imagery performance and tactile spatial acuity: are they altered in people with frozen shoulder? Int J Environ Res Public Health 2020; 17: 7464–7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mena-del Horno S, Balasch-Bernat M, Dueñas L, et al. Laterality judgement and tactile acuity in patients with frozen shoulder: a cross-sectional study. Musculoskelet Sci Pract 2020; 47: 102136–102136. [DOI] [PubMed] [Google Scholar]
- 24.Daffada PJ, Walsh N, McCabe CS, et al. The impact of cortical remapping interventions on pain and disability in chronic low back pain: a systematic review. Physiotherapy 2015; 101: 25–33. [DOI] [PubMed] [Google Scholar]
- 25.Ryan C, Harland N, Drew BT, et al. Tactile acuity training for patients with chronic low back pain: a pilot randomised controlled trial. BMC Musculoskelet Disord 2014; 15: 59–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins JPT and Green S. Cochrane handbook for systematic reviews of interventions, 2008.
- 27.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6: e1000100–e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097–e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moseley LG, Zalucki NM, Wiech K. Tactile discrimination, but not tactile stimulation alone, reduces chronic limb pain. Pain 2008; 137: 600–608. [DOI] [PubMed] [Google Scholar]
- 30.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998; 52: 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morton S, Barton CJ, Rice S, et al. Risk factors and successful interventions for cricket-related low back pain: a systematic review. Br J Sports Med 2014; 48: 685–685. [DOI] [PubMed] [Google Scholar]
- 32.Korakakis V, Whiteley R, Tzavara A, et al. The effectiveness of extracorporeal shockwave therapy in common lower limb conditions: a systematic review including quantification of patient-rated pain reduction. Br J Sports Med 2018; 52: 387–407. [DOI] [PubMed] [Google Scholar]
- 33.Trac MH, McArthur E, Jandoc R, et al. Macrolide antibiotics and the risk of ventricular arrhythmia in older adults. CMAJ 2016; 188: e120–e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adamczyk W, Luedtke K, Saulicz E. Lumbar tactile acuity in patients with low back pain and healthy controls: systematic review and meta-analysis. Clin J Pain 2018; 34: 82–94. [DOI] [PubMed] [Google Scholar]
- 35.Reeves BC, Deeks JJ, Higgins JPT, et al. Chapter 13: Including non-randomized studies. In: Higgins Julian PT and Green S (eds) Cochrane handbook for systematic reviews of interventions version 510 (updated March 2011). Cochrane, 2011, www.cochrane-handbook.org.
- 36.Howard A, Powell JL, Gibson J, et al. A functional magnetic resonance imaging study of patients with polar type II/III complex shoulder instability. Sci Rep 2019; 9: 6271–6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ng SK, Urquhart DM, Fitzgerald PB, et al. The relationship between structural and functional brain changes and altered emotion and cognition in chronic low back pain brain changes: a systematic review of MRI and fMRI studies. Clin J Pain 2018; 34: 237–261. [DOI] [PubMed] [Google Scholar]
- 38.Goossens N, Rummens S, Janssens L, et al. Association between sensorimotor impairments and functional brain changes in patients with low back pain: a critical review. Am J Phys Med Rehabil 2018; 97: 200–211. [DOI] [PubMed] [Google Scholar]
- 39.Ng C, Bialocerkowski A, Hinman R. Effectiveness of arthroscopic versus open surgical stabilisation for the management of traumatic anterior glenohumeral instability. Int J Evid Based Healthc 2007; 5: 182–207. [DOI] [PubMed] [Google Scholar]
- 40.Kadowaki M, Tadenuma T, Kumahashi N, et al. Brain activity changes in somatosensory and emotion-related areas with medial patellofemoral ligament deficiency. Clin Orthop Relat Res 2017; 475: 2675–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kapreli E, Athanasopoulos S, Gliatis J, et al. Anterior cruciate ligament deficiency causes brain plasticity: a functional MRI study. Am J Sports Med 2009; 37: 2419–2426. [DOI] [PubMed] [Google Scholar]
- 42.Grooms DR, Page SJ, Nichols-Larsen DS, et al. Neuroplasticity associated with anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther 2017; 47: 180–189. [DOI] [PubMed] [Google Scholar]
- 43.Ushida T, Ikemoto T, Taniguchi S, et al. Virtual pain stimulation of allodynia patients activates cortical representation of pain and emotions: a functional MRI study. Brain Topogr 2005; 18: 27–35. [DOI] [PubMed] [Google Scholar]
- 44.Pleger B, Ragert P, Schwenkreis P, et al. Patterns of cortical reorganization parallel impaired tactile discrimination and pain intensity in complex regional pain syndrome. Neuroimage 2006; 32: 503–510. [DOI] [PubMed] [Google Scholar]
- 45.Nijs J, Clark J, Malfliet A, et al. In the spine or in the brain? Recent advances in pain neuroscience applied in the intervention for low back pain. Clin Exp Rheumatol 2017; 35: 108–115. [PubMed] [Google Scholar]
- 46.Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 2013; 14: 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grefkes C, Nowak DA, Wang LE, et al. Modulating cortical connectivity in stroke patients by rTMS assessed with fMRI and dynamic causal modeling. Neuroimage 2010; 50: 233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lädermann A, Lubbeke A, Stern R, et al. Risk factors for dislocation arthropathy after Latarjet procedure: a long-term study. Int Orthop 2013; 37: 1093–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scheidt RA, Conditt MA, Secco EL, et al. Interaction of visual and proprioceptive feedback during adaptation of human reaching movements. J Neurophysiol 2005; 93: 3200–3213. [DOI] [PubMed] [Google Scholar]
- 50.Thirion B, Pinel P, Meriaux S, et al. Analysis of a large fMRI cohort: statistical and methodological issues for group analyses. Neuroimage 2007; 35: 105–120. [DOI] [PubMed] [Google Scholar]
- 51.Moayedi M, Salomons TV, Atlas LY. Pain neuroimaging in humans: a primer for beginners and non-imagers. J Pain 2018; 19: 961.e961–961.e921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-sel-10.1177_17585732211019016 for Functional cortical changes associated with shoulder instability – a systematic review by Morissa F Livett, Deborah Williams, Hayley Potter and Melinda Cairns in Shoulder & Elbow