Abstract
The effects of trichloroethylene (TCE) oxidation on toluene 2-monooxygenase activity, general respiratory activity, and cell culturability were examined in the toluene-oxidizing bacterium Burkholderia cepacia G4. Nonspecific damage outpaced inactivation of toluene 2-monooxygenase in B. cepacia G4 cells. Cells that had degraded approximately 0.5 μmol of TCE (mg of cells−1) lost 95% of their acetate-dependent O2 uptake activity (a measure of general respiratory activity), yet toluene-dependent O2 uptake activity decreased only 35%. Cell culturability also decreased upon TCE oxidation; however, the extent of loss varied greatly (up to 3 orders of magnitude) with the method of assessment. Addition of catalase or sodium pyruvate to the surfaces of agar plates increased enumeration of TCE-injured cells by as much as 100-fold, indicating that the TCE-injured cells were ultrasensitive to oxidative stress. Cell suspensions that had oxidized TCE recovered the ability to grow in liquid minimal medium containing lactate or phenol, but recovery was delayed substantially when TCE degradation approached 0.5 μmol (mg of cells−1) or 66% of the cells' transformation capacity for TCE at the cell density utilized. Furthermore, among B. cepacia G4 cells isolated on Luria-Bertani agar plates from cultures that had degraded approximately 0.5 μmol of TCE (mg of cells−1), up to 90% were Tol− variants, no longer capable of TCE degradation. These results indicate that a toxicity threshold for TCE oxidation exists in B. cepacia G4 and that once a cell suspension has exceeded this toxicity threshold, the likelihood of reestablishing an active, TCE-degrading biomass from the cells will decrease significantly.
Trichloroethylene (TCE), a suspected human carcinogen, is one of the most widespread groundwater contaminants. Although TCE can be reductively dehalogenated under anaerobic conditions, a common end product of this reaction is vinyl chloride, which is more water soluble than TCE and is a known human carcinogen (4, 21, 48). Removal of TCE from polluted sites or industrial discharge is therefore of great concern. Although TCE has not been shown to support microbial growth under aerobic conditions, a number of bacteria are capable of degrading this compound via aerobic cometabolism. In each case examined to date, a nonspecific oxygenase catalyzes TCE transformation. Bacterial strains that use oxygenases to grow on methane, butane, propene, ethylene, ammonia, toluene, phenol, isoprene, and dichlorophenoxyacetic acid have been characterized as TCE degraders (3, 8, 10, 11, 17, 18, 39, 45, 54). Although some of these bacteria exhibit high initial rates of TCE conversion, the process is invariably nonsustainable in the absence of a growth-supporting substrate (2, 19, 35, 49).
From the biological standpoint, aerobic cometabolism of TCE by microorganisms is largely dependent on two factors: (i) cellular energy requirements and (ii) the toxicity often associated with TCE oxidation. Energy problems can be overcome by carefully controlling the concentrations of the cometabolite and growth substrate (6, 9) or by selecting or designing microorganisms that are capable of expressing the TCE-degrading oxygenase while growing on a noncompetitive substrate (29, 32, 41). Perhaps more problematic is the toxicity that is often associated with TCE cometabolism.
Aerobic cometabolism of TCE is typically plagued by turnover-dependent loss of activity. Whole-cell studies with the ammonia-oxidizing bacterium Nitrosomonas europaea, the methanotroph Methylosinus trichosporium OB3b, and the toluene-oxidizing bacterium Pseudomonas putida F1 have shown that the rate of TCE degradation decreases rapidly with time (35, 37, 46, 49). The effects of TCE oxidation have also been examined with purified oxygenases from M. trichosporium OB3b (soluble methane monooxygenase), P. putida F1 (toluene dioxygenase), and Burkholderia cepacia G4 (toluene 2-monooxygenase). In each case, TCE turnover results in enzyme inactivation and is accompanied by covalent modification of each of the components of the oxygenase complex (14, 25, 34).
[14C]TCE labeling studies with N. europaea and P. putida F1 indicate that reactive intermediates of TCE oxidation can alkylate not only components of the transforming oxygenase but other cellular constituents as well, including DNA, RNA, lipids, proteins, and various small molecules (37, 50). Furthermore, it has been noted that general cellular damage can accompany TCE oxidation in a variety of bacteria (19, 20, 35, 50); however, the effect of TCE transformation on oxygenase activity has often been the focus of these studies, and characterization of the cytotoxic damage incurred during the reaction was not vigorously pursued. Two recent studies examining the effects of TCE oxidation in methanotrophs have been an exception. Van Hylckama Vlieg et al. reported that the predominant toxic effect of TCE degradation by M. trichosporium OB3b was not methane monooxygenase inactivation but rather general cellular damage resulting in an apparent decrease in cell viability (46). In that study, cell viability was determined by colony formation on Luria-Bertani (LB) agar plates, which decreased exponentially with the amount of TCE degraded. In another study, the respiratory activities (as measured by microscopic analysis of 5-cyano-2,3-ditolyl tetrazolium chloride-stained cells) of two methanotrophs, M. trichosporium OB3b and CAC-2, was analyzed following TCE degradation (7). The respiratory activity was found to decrease in a linear relationship with the amount of TCE degraded.
Because cellular toxicity can ultimately limit TCE oxidation, considerable effort has been directed towards identifying bacterial strains that can sustain high rates of TCE degradation. From these efforts, the toluene-oxidizing bacterium B. cepacia G4 has emerged as a promising agent for TCE bioremediation (32, 36, 43). Earlier studies had led to the assumption that this microorganism was relatively impervious to TCE-related toxicity. For example, stable rates of TCE consumption by B. cepacia G4 were observed during short-term resting-cell assays (13, 33). Furthermore, steady rates of TCE degradation were obtained in bioreactors containing phenol- and toluene-fed cultures of B. cepacia G4 (12, 23). However, other observations were made which suggest that B. cepacia G4 may indeed incur damage during TCE degradation (26, 27, 41). For example, when B. cepacia G4 cells were cultivated in a toluene-fed batch reactor and exposed to TCE under nongrowth conditions (the toluene feed was suspended), a fourfold increase in the maintenance energy requirements of the cells was observed (26). In addition, TCE oxidation by purified toluene 2-monooxygenase from this organism leads to inactivation of the enzyme (34). Because of the conflicting data surrounding TCE-related toxicity in B. cepacia G4 and the potential value of this organism in TCE bioremediation efforts, we decided to systematically examine the physiological consequences of TCE cometabolism in whole cells of the microorganism.
MATERIALS AND METHODS
Chemicals and reagents.
Toluene and TCE were obtained from Aldrich. Other reagents and their sources included bovine liver catalase (38,080 U/ml; catalog no. 100429; ICN Biomedicals Inc., Aurora, Ohio), N,N-dimethylformamide (Sigma, St. Louis, Mo.), ethylene (Airco, Murray Hill, N.J.), and 2-hexyne (Farchan Laboratories, Inc., Gainesville, Fla.).
Culture conditions.
B. cepacia G4 was maintained and grown essentially as described previously (53). Batch cultures were grown overnight at 30°C with shaking in sealed serum vials (160 ml) containing minimal medium (60 ml) with toluene (94 μmol; 1.0 mM aqueous phase concentration) or lactate (20 mM). Cultures grown on toluene in this manner attained an optical density at 600 nm (OD600) of approximately 0.25. Additional toluene (94 μmol) was added to toluene-grown cultures 4 to 5 h prior to harvesting them for experimental assays, enabling the cultures to obtain a final OD600 of 0.5 to 0.6. Lactate-grown cells were harvested from overnight cultures that had reached a final OD600 of 1.0 to 1.3. A dimensionless Henry's constant of 0.343 was used to calculate the aqueous phase concentration of toluene in two-phase systems at 30°C (53). To collect cells for experimental assays, the cultures were centrifuged, rinsed twice with 50 mM KH2PO4-K2HPO4 buffer, pH 7.0 (phosphate buffer), and resuspended in fresh phosphate buffer. The cell suspension was stored at room temperature for ≤2 h before use.
Determining Tcs.
Toluene-grown cells of B. cepacia G4 were added to serum vials (10 ml) that were sealed with Teflon-lined butyl rubber stoppers and contained either ethylene (3.4, 6.7, 8.9, or 13.4 μmol [40 to 157 μM initial aqueous concentration]) or TCE (1.3, 1.7, or 1.9 μmol [239 to 349 μM initial aqueous concentration]) in phosphate buffer. Additions were made to yield a final reaction mixture volume of 1 ml with either 0.42, 1.05, 2.1, or 4.2 mg of cells (dry weight) ml−1. The reaction vials were inverted and incubated at 30°C with shaking (150 rpm) for either 4 (TCE vials) or 8 (ethylene vials) h, at which time consumption of the compounds had ceased. To monitor substrate consumption, headspace samples were analyzed by gas chromatography immediately following the addition of cells and at the end of the incubation period. The transformation capacity (Tc) for each compound at each cell density was determined by dividing the total amount of substrate consumed (in micromoles) by the amount of cells (in milligrams [dry weight]) in the reaction vial. Control vials with heat-killed cells exhibited virtually no loss of ethylene or TCE when incubated under the conditions described above.
Because TCE concentrations above 400 to 500 μM were inhibitory to B. cepacia G4 (data not shown), the amount of TCE that could be added to a given reaction vial was limited (≤1.9 μmol [350 μM initial aqueous concentration]). Therefore, incubations with TCE at a cell density of either 2.1 or 4.2 mg of cells ml−1 required a second addition of TCE (1.7 μmol) after 2 h. The amount of TCE in the vials immediately prior to and after the second addition of TCE was determined by gas chromatography analysis of headspace samples.
TCE exposure for toxicity assays.
Aliphatic hydrocarbons (1.36 μmol of TCE [250 μM initial aqueous concentration] or 1.36 μmol of ethylene [16 μM initial aqueous concentration]) were added to phosphate buffer (≈700 μl) in glass serum vials (10 ml) that were sealed with Teflon-lined butyl rubber stoppers. The reaction vials were incubated at 30°C with shaking (150 rpm) for at least 15 min to allow equilibration of the volatile hydrocarbons between the gas and liquid phases. Reactions were initiated by the addition of toluene-grown B. cepacia G4 cells (final reaction volume, 1 ml), and the mixtures were incubated at 30°C with shaking. To monitor substrate consumption, headspace samples were analyzed by gas chromatography immediately following the addition of cells and at the end of the incubation period. After the desired incubation period, the reaction mixture was transferred to a 1.5-ml microcentrifuge tube and centrifuged at 12,000 rpm for 30 s. The supernatant was removed, and the pellet was washed with phosphate buffer (700 μl), recentrifuged, and finally resuspended in fresh phosphate buffer (100 μl). Samples of this concentrated cell suspension were then used to assay the cell viability, acetate-dependent O2 uptake, and toluene-dependent O2 uptake rates.
Assays for cell culturability and O2 uptake rates.
To measure cell viability following hydrocarbon exposures, a 10-fold dilution series of the concentrated cell suspension was prepared in sterile phosphate buffer, and aliquots (50 μl) from selected dilutions were plated in duplicate onto LB agar plates. Where indicated, catalase (210 U/plate; from a 38,080-U/ml sterile stock solution) or sodium pyruvate (60 mg/plate; from a 375-mg/ml filter-sterilized stock solution prepared in distilled H2O) was spread over the surfaces of LB agar plates and allowed to dry. Colony counts were performed after the plates had been incubated at 30°C for 3 days. Alternatively, the number of culturable cells in each concentrated cell suspension was determined by a most-probable-number (MPN) technique. Four identical 10-fold dilution series were prepared from each cell sample. The dilutions were carried out beyond extinction (10−12) in tubes containing sterile minimal medium with 20 mM lactate. The tubes were incubated for 6 days at 30°C and scored for growth. An MPN computer program was used to calculate the number of culturable cells per milliliter in each sample (52).
Cell viability was also assayed with the BacLight Live/Dead stain (Molecular Probes, Eugene, Oreg.). TCE-treated cells were diluted in phosphate buffer, and a sample (1 ml) was stained according to the manufacturer's directions. A portion of the stained cells (7 μl) was place on a slide under a coverslip and observed via epifluorescent microscopy. One hundred cells were examined from randomly chosen areas under the coverslip and scored as live or dead. Heat (70°C for 15 min) and alcohol (75% isopropanol) killed >95% of B. cepacia G4 cells as determined by this method (data not shown).
To examine toluene 2-monooxygenase activity following hydrocarbon exposure, the toluene-dependent O2 uptake rates of treated cells were determined with a Clark-style O2 electrode Yellow Springs Insurance Co., Yellow Springs, Ohio) mounted in a glass water-jacketed reaction vessel (1.6 ml) maintained at 30°C. The reaction vessel was filled with phosphate buffer, and cells were added to determine a basal rate of cellular respiration. The basal respiratory rate was subtracted from the toluene-stimulated (250 μM) O2 uptake rate to obtain the toluene-dependent O2 uptake rate. Acetate-dependent O2 uptake rates were determined similarly with the addition of 1 mM acetate.
Effect of TCE oxidation on surrounding cells.
B. cepacia TCS-100 is a Tn5 mutant of B. cepacia G4 that is resistant to tetracycline. This strain is phenotypically indistinguishable from wild-type B. cepacia G4 in terms of toluene oxidation activity, TCE degradation activity, and growth rates on lactate, toluene, and LB agar (C. M. Yeager, unpublished results). Toluene-grown cells of B. cepacia TCS-100 were mixed with toluene-grown wild-type B. cepacia G4 cells at a ratio of 1:9 in a sealed serum vial (10 ml) containing phosphate buffer and TCE (1.36 μmol [250 μM initial aqueous concentration]). The cell density in the TCE reaction vial (1-ml final volume) was 2.1 mg of cells (dry weight) ml−1.
Prior to cell mixing, toluene 2-monooxygenase was inactivated in select cell suspensions of B. cepacia TCS-100 and wild-type B. cepacia G4 by incubating the suspensions with 2-hexyne (5 μl from a 1:100 dilution of 2-hexyne in N,N-dimethylformamide) in sealed serum vials (10 ml) for 40 min at 30°C (53). After the 2-hexyne incubation, the cells were washed twice and resuspended in phosphate buffer. 2-Hexyne-treated cells did not degrade TCE (data not shown). Three combinations of TCS-100 and G4 cells were mixed and examined: (i) both strains treated with 2-hexyne; (ii) TCS-100 treated and G4 untreated strains; and (iii) neither strain treated.
The TCE reaction vials containing both strains were incubated for 30 min at 30°C with shaking. Following the incubation, the reaction mixtures were transferred to a 1.5-ml microcentrifuge tube and centrifuged at 12,000 rpm for 30 s. The supernatants were removed, and the cells were washed with phosphate buffer (700 μl), recentrifuged, and finally resuspended in fresh phosphate buffer (100 μl). The culturability of B. cepacia TCS-100 cells from the cell mixtures was then determined by plating LB agar plates with appropriate dilutions containing tetracycline (15 μg/ml) and counting the colonies after incubating the dilutions at 30°C for 3 days. Wild-type B. cepacia G4 did not grow on plates containing tetracycline at 15 μg/ml.
Recovery of growth by B. cepacia G4 cells exposed to TCE.
Toluene-grown B. cepacia G4 cells were incubated with TCE, as described in “TCE exposure for toxicity assays” above. At selected time points, samples (50 μl) were removed from the TCE reaction mixture and added to a sterile glass serum vial (160 ml) containing minimal medium (60 ml) with 20 mM lactate or 2.5 mM phenol. The inoculated vials were then incubated at 30°C with shaking, and 1-ml portions were removed periodically to monitor the OD600 of the culture.
Occurrence of Tol− variants upon TCE oxidation.
Toluene-grown B. cepacia G4 cells that had been exposed to TCE for 0, 15, 30, or 60 min were diluted and spread on LB agar plates. The percentage of TCE-treated cells able to grow on toluene was determined by streaking 100 colonies from the LB agar plates onto minimal-medium plates, which were then incubated in sealed, 1-gal polyethylene jars containing toluene vapors. Toluene vapors were supplied by adding neat toluene (150 μl) to a Durham tube, plugging the tube with cotton, and placing the tube in an empty petri dish at the bottom of the polyethylene jar. The plates were scored for growth after 3 days of incubation at 30°C. Ten B. cepacia G4 variants that were incapable of growth on toluene vapors were randomly chosen and analyzed for the presence of the TOM plasmid. Plasmid DNA was isolated as previously described (22) and visualized following separation on a 0.6% agarose gel.
Analytical and other methods.
Hydrocarbons were analyzed with a Shimadzu (Kyoto, Japan) GC-8A chromatograph equipped with a flame ionization detector and a stainless steel column (0.3 by 61 cm) packed with Porapak Q 80 to 100 mesh (Alltech, Deerfield, III.). To detect ethylene, a column temperature of 100°C was utilized, and for TCE the column temperature was 155°C. The injector and detector temperatures were set at 200°C for all analyses. Hydrocarbons were quantified by comparison of peak heights to standard curves constructed from known amounts of authentic compounds.
The aqueous concentration of TCE in two-phase systems at 30°C was calculated with a dimensionless Henry's constant of 0.494 (16). The concentration of ethylene in the aqueous phase at 30°C was calculated with a Henry's constant of 9.35 (derived from the data of Wilhelm et al. [51]).
The protein concentrations of cell suspensions were determined by measuring the OD600 of appropriate dilutions of the suspensions and converting them to protein concentrations (suspensions of B. cepacia G4 cells with an OD600 of 1.0 contained 0.2 mg of total cell protein ml−1). Protein concentrations were determined by the Biuret assay (15) following cell solubilization in 3 M NaOH for 30 min at 65°C. Bovine serum albumin was used as the standard. The dry weights of culture samples were determined by resuspending the cells in distilled H2O in preweighed Eppendorf tubes, drying them for 2 days at 55°C, and weighing the cell pellets. It was determined that 2.1 mg of B. cepacia G4 cells (dry weight) contains approximately 1.0 mg of protein.
RESULTS
Effects of TCE transformation on toluene 2-monooxygenase activity, general respiratory activity, and cell viability in B. cepacia G4.
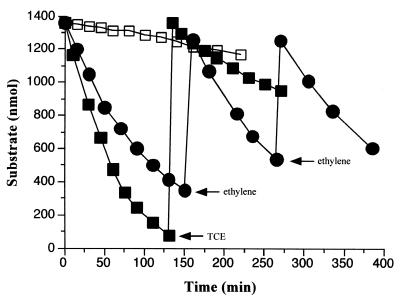
TCE degradation by toluene-grown B. cepacia G4 was examined in resting-cell assays (Fig. 1). The initial rate of TCE consumption at 30°C was 15 nmol min−1 mg of total cell protein−1. Folsom et al. previously reported a maximal TCE degradation rate of 8 nmol min−1 mg of protein−1 with phenol-grown B. cepacia G4 at 26°C, and Sun and Wood reported a rate of 9 nmol min−1 mg of protein−1 at 25°C (13, 44). After 4 h, the rate of TCE depletion had slowed to essentially that of the control vial containing heat-killed cells. In contrast, B. cepacia G4 cells consumed ethylene at high rates during the entire assay (up to 6 h). The initial aqueous phase concentrations of TCE and ethylene in the reaction mixtures were 250 and 16 μM, respectively. We previously determined a Ks for ethylene of 39.7 μM for B. cepacia G4 (53). Therefore, the nonlinear nature of ethylene consumption (Fig. 1) is likely due to concentration-dependent kinetics. Indeed, at higher ethylene concentrations (>200 μM), a constant rate of ethylene consumption was observed (data not shown).
FIG. 1.
Time course of TCE and ethylene consumption by B. cepacia G4. Toluene-grown cells (2.1 mg of cells) were harvested and incubated with 1.36 μmol of TCE (■) or ethylene (●). Additional TCE or ethylene was added where indicated (arrows) □, heat-killed cell control with TCE.
The Tcs of resting-cell suspensions of B. cepacia G4 for TCE and ethylene were determined. The Tc is defined as the maximum mass of a compound that can be degraded per mass of cells prior to inactivation (1). The Tc of B. cepacia G4 for ethylene was 10 to 18 times greater than that for TCE over a range of cell densities (Table 1). We have previously observed that B. cepacia G4 oxidizes ethylene to epoxyethane with no evidence of further breakdown of the product (53) and no evidence of toxicity (Fig. 1). Therefore, ethylene transformation should provide a measure of the effects of reductant drain on cells that should also be applicable during TCE transformation (unless extensive uncoupling of NADH utilization occurs during TCE oxidation relative to ethylene oxidation). Since the Tc of resting-cell suspensions of B. cepacia G4 for TCE was much lower than that of ethylene, it seemed likely that the time-dependent decrease in TCE degradation observed in Fig. 1 was largely due to toxicity rather than reductant depletion. The decrease in the Tc of B. cepacia G4 for TCE with increasing cell density is addressed below.
TABLE 1.
Tcs of resting cell suspensions of B. cepacia G4 for TCE and ethylene at different cell densities
| Cell density (mg of cells/ml) |
Tca
|
|
|---|---|---|
| Ethylene | TCE | |
| 0.42 | 9.0 ± 2.7 | 0.95 ± 0.14 |
| 1.05 | 10.4 ± 1.0 | 0.92 ± 0.10 |
| 2.1 | 11.0 ± 0.2 | 0.75 ± 0.07 |
| 4.2 | 10.6 ± 0.2 | 0.61 ± 0.07 |
Tc is expressed as micromoles of substrate transformed per milligram of cells (dry weight). All values are the means from three trials ± standard deviations.
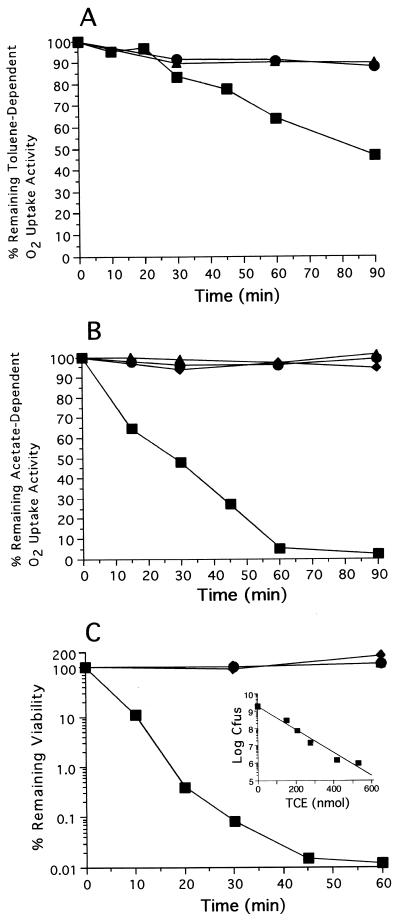
To determine if an irreversible loss of toluene 2-monooxygenase activity occured in B. cepacia G4 cells during TCE oxidation, rates of toluene-dependent O2 uptake were determined in cells exposed to TCE. The cells retained full toluene-dependent O2 uptake activity during the first 20 to 30 min of TCE degradation, after which a slow, linear decrease in activity was observed (Fig. 2A). The decrease in the toluene-dependent O2 uptake activity of cells during TCE oxidation could be attributed specifically to the loss of toluene 2-monooxygenase activity, since levels of 3-methylcatechol-dependent O2 uptake (3-methylcatechol is the ultimate product of toluene oxidation by toluene 2-monooxygenase in B. cepacia G4) remained constant in the TCE-treated cells (data not shown). However, our data were not sufficient to distinguish whether the loss of toluene 2-monooxygenase activity was a consequence of inactivation of a particular component of the oxygenase, decreased electron flow to the terminal oxygenase component, damage to the diiron center of the α subunit of the hydroxylase, or some other toxic effect.
FIG. 2.
Toluene-dependent O2 uptake activity (A), acetate-dependent O2 uptake activity (B), and cell viability (C) in B. cepacia G4 cells following exposure to alkenes. Resting suspensions (2.1 mg of cells ml−1) of toluene-grown cells were incubated with 1.36 μmol of TCE (■), ethylene (▴), or no substrate (●) for selected times, harvested, and assayed for toluene-dependent O2 uptake activity, acetate-dependent O2 uptake activity, and cell viability. Viability was measured by colony counts on LB agar plates. Toluene-grown cells were also pretreated with 2-hexyne to inactivate toluene 2-monooxygenase and then incubated with TCE (⧫). The inset in panel C depicts cell viability as a function of the amount of TCE degraded. For each graph (except the inset), values are the means of two experiments.
Acetate-dependent O2 uptake rates were used to examine the effects of TCE transformation on the general respiratory status of B. cepacia G4 cells for several reasons. First, toluene-grown cells of B. cepacia G4 exhibited robust, stable levels of acetate-dependent O2 consumption (acetate is an intermediate of toluene metabolism in this microorganism). Second, the majority of O2 consumed during acetate metabolism is associated with central metabolism, and therefore acetate-dependent O2 consumption is a good measure of the general respiratory status of the cell. Upon TCE exposure, the acetate-dependent O2 uptake activity of toluene-grown B. cepacia G4 cells decreased steadily until <5% of the original activity remained (Fig. 2B). TCE turnover was required to bring about the loss of acetate-dependent O2 uptake activity, because cells incubated with TCE and 2-hexyne (a mechanism-based inactivator of toluene 2-monooxygenase [53]) retained >94% of their original activity during the incubation period.
The effect of TCE oxidation on the culturability of B. cepacia G4 cells was examined by spreading TCE-treated cells on LB agar plates (Fig. 2C). TCE degradation resulted in a precipitous loss of cell culturability (CFU). The culturability of the cells was found to decrease exponentially with the amount of TCE transformed (Fig. 2C, inset). B. cepacia G4 cells incubated with ethylene or without substrate did not exhibit a loss of culturability. Apparently, utilization of reductant for the oxidation of ethylene (and by inference also TCE) had little affect on cell culturability. Cells pretreated with 2-hexyne and exposed to TCE remained culturable, again indicating that TCE transformation was required to elicit a toxic effect and that TCE itself was not the toxic agent. These results confirmed that TCE degradation in resting cell suspensions of B. cepacia G4 can cause extensive nonspecific cellular damage.
Further examination of the culturability of B. cepacia G4 cells following TCE transformation.
Because measures of cell culturability are often influenced by the methodology utilized, several different techniques were used to examine the culturability of B. cepacia G4 cells that had been exposed to TCE. Colony counts from minimal-medium plates with 20 mM lactate on which TCE-treated cells had been spread yielded results similar to those obtained with LB agar plates—cell culturability decreased in an exponential fashion upon TCE transformation (data not shown). However, strikingly different results were observed when culturability was assayed on LB agar plates containing catalase (Table 2). The number of CFU obtained from cell suspensions exposed to TCE were up to 2 orders of magnitude higher when catalase was added to the surfaces of the plates. Catalase addition did not affect the culturability of untreated cells. Furthermore, the addition of sodium pyruvate, a compound that degrades H2O2 (28, 30, 38), to the surfaces of LB agar plates also increased the number of CFU obtained from TCE-treated cells (data not shown).
TABLE 2.
Cell culturability of B. cepacia G4 cells exposed to TCE for selected times
| TCE exposure time (min) | Average amt of TCE degraded (nmol) | Culturable cells (109)a
|
||
|---|---|---|---|---|
| LB agar plates | LB agar plates with catalaseb | Liquid medium MPN | ||
| 0 | 0 | 1.69 ± 0.2 | 1.74 ± 1.0 | 6.24 ± 5.4 |
| 15 | 202 ± 64 | 0.48 ± 0.31 | 0.99 ± 0.14 | 8.44 ± 7.0 |
| 30 | 447 ± 58 | 0.0030 ± 0.0026 | 0.29 ± 0.11 | 2.06 ± 1.7 |
| 60 | 928 ± 232 | 0.000111 ± 0.00006 | 0.010 ± 0.004 | 0.12 ± 0.06 |
Toluene-grown cells (2.1 mg of cells [dry weight]) were exposed to TCE for the indicated times and assayed for culturability either with a liquid-medium MPN technique or via colony formation on LB agar plates. All values are the means for three to five trials ± standard deviations.
LB agar plates with catalase had 210 U of sterile bovine liver catalase spread on the agar surface.
Data from a liquid-medium MPN assay further showed that conditions on the surfaces of agar plates are not optimal for the growth of TCE-treated cells (Table 2). As measured by this technique, cell culturability was not affected significantly until larger quantities of TCE (>0.5 μmol) had been transformed, and even then, the loss of culturability was far less than that observed on LB agar plates. The results imply that TCE-treated cells were able to grow more readily in liquid broth than on the surfaces of agar plates. Of particular note, the terminal MPN tubes containing cells that had been exposed to TCE took longer to develop turbidity than those containing cells that had not been incubated with TCE. Furthermore, there was a positive correlation between the length of the delay before the appearance of turbidity and the amount of TCE transformed.
Data obtained with the BacLight Live/Dead stain showed that TCE-treated B. cepacia G4 cells remained impermeable to the nucleic acid stain propidium iodide (data not shown). Therefore, the toxicity associated with TCE oxidation in B. cepacia G4 does not appear to be a consequence of extensive structural damage to the cell membrane.
Recovery of growth of B. cepacia G4 cells following TCE transformation.
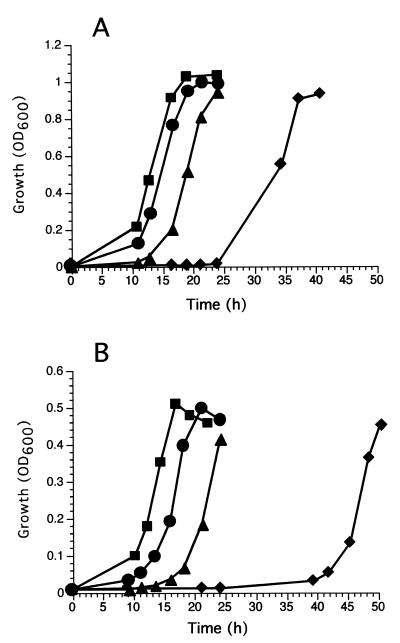
The aforementioned results suggested that cells of B. cepacia G4 could recover, while bathed in liquid medium, from the damage accrued during TCE transformation. To examine the recovery characteristics of B. cepacia G4 following TCE exposure, an aliquot of TCE-treated cells was resuspended in minimal medium containing lactate (20 mM) or phenol (2.5 mM initial aqueous concentration) and the ensuing growth was monitored by OD600 measurements. Toluene was not utilized in the recovery experiments because it was toxic to B. cepacia G4 at aqueous concentrations of >1.0 mM, therefore limiting the amount of growth that could be achieved in batch cultures with a single addition of substrate. Phenol was used instead, since it is less toxic than toluene yet still requires toluene 2-monooxygenase activity in order to be metabolized by B. cepacia G4.
Cells that had been exposed to TCE exhibited markedly longer lag periods before exponential growth was observed during the recovery experiment (Fig. 3). Once exponential growth was observed, there were no obvious differences in the growth rates among the TCE-treated and untreated cells. As a measure of recovery, we determined the time it took a culture to reach an OD600 of 0.3 following TCE exposure in three independent trials. Cells exposed to TCE for 0, 15, 30, and 60 min required 11.2 ± 0.9, 13.5 ± 0.5, 17.1 ± 0.9, and 30.5 ± 3.0 h, respectively, to reach an OD600 of 0.3 when lactate was used as the growth substrate for recovery. This method yielded results more consistent than those obtained with the liquid-medium MPN assay. Data from both experiments indicate that the culturability of cells within a TCE-degrading cell suspension decreases rapidly once the cells have degraded larger quantities of TCE (>400 to 500 nmol of TCE mg of cells−1). With phenol as the growth substrate, the recovery of TCE-treated cells was delayed even longer relative to the control cells. Cells exposed to TCE for 0, 15, 30, and 60 min required 14.8 ± 1.4, 18.4 ± 1.3, 24.0 ± 1.5, and 52.0 ± 3.4 h, respectively, to reach an OD600 of 0.3 with phenol as the growth substrate.
FIG. 3.
Time course for recovery of growth by B. cepacia G4 cells exposed to TCE. Cells harvested from cultures grown on toluene were incubated in phosphate buffer containing TCE (1.36 μmol) for 0 (■), 10 (●), 30 (▴) and 60 (⧫) min. TCE-treated cells were washed, and a set amount of the washed cells was added to vials containing minimal medium with 20 mM lactate (A) or 2.5 mM phenol (B). The cultures were then incubated at 30°C with shaking, and growth was monitored by OD600 readings.
Excessive TCE damage selects against toluene 2-monooxygenase activity in B. cepacia G4 populations.
Since TCE oxidation by B. cepacia G4 can result in cellular injury and death, it is possible that TCE degradation by cultures of this organism could select against those cells in the population that exhibit toluene 2-monooxygenase activity. To examine this possibility, B. cepacia G4 cells were exposed to TCE for selected times, diluted, and spread on LB agar plates. Colonies that grew on the LB agar plates were then streaked onto minimal-medium plates, which were incubated in the presence of toluene vapors. Among the colonies that grew on LB agar plates following TCE exposure, the percentage that were also capable of growth on toluene decreased with increasing TCE exposure times (Table 3). Additionally, none of the Tol− variants tested (n = 12) exhibited toluene 2-monooxygenase activity (assayed by toluene-dependent O2 uptake and toluene consumption) when grown on lactate and induced with toluene (data not shown).
TABLE 3.
Tol+ phenotype among B. cepacia G4 cells that survive TCE exposure
| TCE exposure time (min) |
% Colonies able to grow on toluenea |
|---|---|
| 0 | 99.7 ± 0.6 |
| 15 | 99.3 ± 1.2 |
| 30 | 62.7 ± 35.4 |
| 60 | 9.5 ± 11.6 |
Dilutions of cells that had been exposed to TCE for various times were spread on LB agar plates. The percentage of colonies that formed on the LB agar plates that were also capable of growing on toluene was determined by streaking 100 of the colonies onto minimal-medium plates, which were then incubated in the presence of toluene vapors. All values are the means of three to five trials ± standard deviations.
The genes required for toluene 2-monooxygenase activity have been localized to the TOM plasmid (approximately 108 kb) in B. cepacia G4 (40). To determine if the Tol− phenotype observed among strains recovered from TCE-treated cell suspensions was due to instability of the TOM plasmid, the plasmid profiles of 10 Tol− strains were compared to that of wild-type B. cepacia G4. The plasmid profile of each of the strains examined was identical to the pattern obtained from wild-type cells (data not shown). Furthermore, the plasmid profile of each strain was similar to that previously published for wild-type B. cepacia G4 (26, 40), with two bands clearly visible. Therefore, the Tol− phenotype observed among cells surviving TCE exposure was not typically due to plasmid loss. The genetic basis of the Tol− phenotype was not determined.
Nature of the toxic TCE intermediate(s).
Data from previous studies suggest that the toxic species associated with TCE oxidation by bacterial monooxygenases is a short-lived intermediate(s) of the reaction (14, 34, 35, 47, 50). With purified toluene 2-monooxygenase, the stable products of TCE oxidation are glyoxylate (10%), formate (21%), carbon monoxide (41%), and covalently modified oxygenase proteins (12%) (34). To test the possibility that the stable products of TCE oxidation are responsible for loss of cell viability and/or toluene 2-monooxygenase activity in B. cepacia G4, toluene-grown cell suspensions were incubated for 2 h with various amounts of glyoxylate, formate, and CO. None of the incubations altered the viabilities or toluene 2-monooxygenase activities of the B. cepacia G4 suspensions (data not shown). The incubations containing the highest levels of glyoxylate (1.5 μmol), formate (3.15 μmol), and CO (6.15 μmol) contained approximately 15 times the amount of products expected during one of the standard 60-min TCE degradation assays described here. Additionally, fresh toluene-grown B. cepacia G4 cells that were incubated with spent medium from a 90-min TCE degradation assay with other B. cepacia G4 cells retained full viability and toluene 2-monooxygenase activity (data not shown). These results indicate that the toxic intermediate(s) formed during TCE oxidation by B. cepacia G4 is a short-lived species.
B. cepacia TCS-100 has a Tn5-OT182 cassette integrated into its chromosome (Yeager, unpublished results). This mini-transposon cassette confers tetracycline resistance on the host (wild-type B. cepacia G4 is sensitive to tetracycline). Toluene-grown cells of this strain were mixed with toluene-grown wild-type B. cepacia G4 at a ratio of 1:9 and incubated in the presence of TCE. Prior to cell mixing, B. cepacia TCS-100, both B. cepacia TCS-100 and wild-type B. cepacia G4, or neither strain was treated with 2-hexyne to inactivate toluene 2-monooxygenase. Following TCE exposure, the viability of B. cepacia TCS-100 cells from the assay mixture was determined with LB agar plates containing tetracycline (15 μg/ml). The results are presented in Table 4. When both strains were pretreated with 2-hexyne, TCE exposure resulted in an 11% decrease in the viability of B. cepacia TCS-100 cells compared to that of the B. cepacia TCS-100 control cells (no TCE added). However, the viability of 2-hexyne-treated B. cepacia TCS-100 cells decreased 69% when incubated with fully active wild-type B. cepacia G4 cells in the presence of TCE. Although this loss of viability was relatively minor compared to that of B. cepacia TCS-100 cells containing active toluene 2-monooxygenase (99.7%), it indicates that TCE oxidation by a group of cells can have a negative impact on surrounding cells not transforming TCE. Similar results were obtained with a second mutant, B. cepacia TCS-101, which has the Tn5-OT182 cassette integrated into a different region of the chromosome than B. cepacia TCS-100 (data not shown). It has been previously documented that reactive TCE intermediate(s) can diffuse outside of the transforming cell (47); however, to our knowledge, this study provides the first evidence that TCE oxidation by a group of cells can adversely effect the health of surrounding cells not participating in TCE oxidation.
TABLE 4.
Diffusible nature of toxic TCE intermediate(s)
| 2-Hexyne pretreatmenta
|
% TCS-100 cells able to grow on LB- Tet plates following TCE exposureb | |
|---|---|---|
| Wild-type G4 | TCS-100 | |
| Yes | Yes | 89.0 ± 8.5 |
| No | No | 0.3 ± 0.25 |
| No | Yes | 31.5 ± 3.2 |
Cells pretreated with 2-hexyne lacked toluene 2-monooxygenase activity and were incapable of TCE transformation.
Toluene-grown cells of B. cepacia G4 and B. cepacia TCS-100 were or were not pretreated with 2-hexyne (as indicated). The cells were then mixed in a 9:1 ratio (G4–TCS-100) and exposed to TCE for 30 min. The culturability of the TCS-100 cells within the TCE-treated cell mixtures was determined by colony counts on LB agar plates containing tetracycline (15 μg/ml). All values are the means of three trials ± standard deviations.
Our observation of decreasing Tc with increasing cell density is consistent with production of a diffusible intermediate(s) that is more effectively toxic at high cell densities. Consistent with these observations, we have found that the Tc of B. cepacia G4 for TCE decreases with increasing cell density (above 1 mg of cells ml−1), while the Tc for ethylene of the same organism remains relatively constant over the range of cell densities tested (Table 1).
DISCUSSION
Previous studies have reported conflicting data on the susceptibility of B. cepacia G4 to the toxicity that is often associated with aerobic cometabolism of TCE (12, 13, 23, 26, 41). The metabolic diversity and robust culturability of B. cepacia G4 allowed us to critically examine the toluene 2-monooxygenase activity, general respiratory activity, and culturability of this microorganism following TCE oxidation. TCE oxidation was shown to have a detrimental effect on each of these properties. Interestingly, the general respiratory activity and culturability of cells were more prone to damage during TCE transformation than was toluene 2-monooxygenase activity. In resting-cell assays with B. cepacia G4, cells apparently retain the internal reductant pool and enzyme activities necessary to affect the oxidation of TCE, while general cell health as manifested by cell culturability or general respiratory activity can be severely compromised. These results underscore the idea that sustained rates of TCE oxidation by bacterial cells in short assays do not necessarily demonstrate a lack of toxicity associated with the reaction.
As reported with M. trichosporium OB3b (46), the culturability of B. cepacia G4 cells decreased exponentially upon TCE oxidation when determined by colony formation on LB agar plates. From studies performed primarily within the context of food microbiology and public health, it is known that catalase and pyruvate can increase the enumeration of physically or chemically injured bacteria on agar plates (5, 28, 30). It is thought that catalase and pyruvate act by preventing the accumulation of H2O2 in and/or around injured cells, which, in contrast to healthy cells, are apparently unable to tolerate even low levels of this reactive oxygen species (28, 38). In this study, we found that the addition of catalase or sodium pyruvate to the surfaces of the LB agar plates increased the culturability of TCE-treated cells of B. cepacia G4 by as much as 100-fold.
Viable cell count estimates performed in this study with a liquid-medium MPN assay also indicate that a dramatic decrease in cell culturability does not occur until a relatively large amount of TCE has been transformed (0.5 μmol mg of cells−1). From these results, it appears that the culturability of B. cepacia G4 does not necessarily decrease exponentially during TCE oxidation; however, cellular injuries that render the bacteria ultrasusceptible to oxidative stress do accumulate in an exponential fashion. Thus, conditions that support the formation of H2O2 either intracellularly or in the external environment (i.e., the surfaces of agar plates) may hinder the recovery of TCE-injured bacteria.
Our results indicate that there is a critical level of damage, or a toxicity threshold, that a population of B. cepacia G4 cells can accumulate during TCE oxidation, beyond which cell culturability drops considerably. In their work with the methanotrophs M. trichosporium OB3b and CAC-2, Chu and Alvarez-Cohen suggested that general cellular damage proceeds in a linear relationship with the amount of TCE degraded until a critical quantity of TCE is oxidized, at which point cells can no longer recover (7). The recovery of both M. trichosporium Ob3b and CAC-2 was severely limited once the general respiratory activity of the cells decreased to <5% of its original level. Chu and Alvarez-Cohen also suggest that the Tc for TCE provides a measure of the toxicity threshold exhibited by methanotrophs upon oxidation of this compound (7). When cells of M. trichosporium OB3b or CAC-2 had degraded an amount of TCE that approached their respective Tc values, respiratory activity decreased approximately 95% and cell recovery was severely limited. With B. cepacia G4 we determined a Tc of 0.75 μmol mg of cells−1 for TCE at the cell density (2.1 mg of cells ml−1) used for most assays performed in this study. Yet B. cepacia G4 cells that had degraded between 0.2 and 0.5 μmol of TCE mg of cells−1 exceeded their toxicity threshold as determined by decreased acetate-dependent O2 uptake rates and long recovery times. With B. cepacia G4, the toxicity threshold was clearly exceeded when the cells had degraded an amount of TCE that corresponded to approximately 66% of their Tc for this compound. While the Tc of B. cepacia G4 for TCE certainly describes the amount of TCE that can be degraded by nongrowing cells of the microorganism, it does not correspond to the toxicity threshold of the organism. The general applicability of relating Tc values to the resuscitation potential of TCE-injured bacterial cultures will require further research.
The negative impact of TCE oxidation on cellular recovery was clear and pronounced in B. cepacia G4 cells when phenol was used as the growth substrate for recovery. Since phenol has biocidal properties (42) and can inhibit the growth of B. cepacia G4 at high concentrations (13; our unpublished observations), it is possible that cells damaged extensively during TCE oxidation are unable to maintain the protective mechanisms or other physiological adaptations that are required for growth on phenol. Alternatively, TCE oxidation could selectively debilitate cells possessing toluene 2-monooxygenase activity, effectively enriching the number of Tol− variants within a population. If so, the amount of time required for a TCE-treated population of cells to recover (grow) on phenol would increase relative to the time required for the cells to recover with lactate as the growth substrate. Indeed, it was found in this study that among B. cepacia cells that had oxidized approximately 0.5 μmol of TCE (mg of cells−1), a disproportionate number of the survivors subsequently isolated on LB agar plates (up to 90%) lacked toluene 2-monooxygenase activity (Tol−).
The emergence of Tol− mutants from populations of B. cepacia cells originally containing toluene 2-monooxygenase activity has been documented previously. Mars et al. found that mutants of B. cepacia G4 which had lost the TOM plasmid took over a pure culture that was exposed to TCE and starved for carbon and energy over a period of days (26). Tol− variants have also been detected within toluene-grown batch cultures of another bacterium, P. putida 54G (24, 31). In one study, the percentage of Tol− variants within the toluene-grown population of P. putida 54G approached 10% under certain conditions (24). Furthermore, three types of Tol− variants were observed: one still harbored the 118-kb TOL-like plasmid (this 118-kb plasmid harbors the meta pathway for toluene catabolism in wild-type P. putida 54G), a second type harbored a TOL-like plasmid of reduced molecular weight, and the third was cured of the plasmid altogether. In the present study, the Tol− phenotype could not be attributed to the loss of the TOM plasmid. It seems plausible that the toluene-grown cell suspensions of B. cepacia G4 used in the TCE exposure assays contained a small percentage of Tol− members that nonetheless harbor the TOM plasmid and that the toxicity associated with TCE oxidation acted to enrich these Tol− members of the population by selectively debilitating cells that did display toluene 2-monooxygenase activity. Regardless of the mechanism, this selective pressure could certainly compromise the likelihood of resuscitating a TCE-degrading population of B. cepacia G4 in instances where the toxicity threshold of the cells had been exceeded.
In summary, results from this study demonstrate that B. cepacia G4 does incur damage during TCE oxidation, with injuries that impair general cellular processes, such as respiratory metabolism and cell culturability, outpacing inactivation of toluene 2-monooxygenase. Additionally, there appears to be a critical amount of damage that B. cepacia G4 cells can accumulate during TCE oxidation or a toxicity threshold beyond which cellular recovery is severely limited. These findings have practical implications for the development of sustainable bioremediation systems for TCE degradation.
ACKNOWLEDGMENTS
We thank Malcolm Shields for providing B. cepacia G4 and give special thanks to Miriam Sluis and Natsuko Hamamura for review and comments.
Funding for this study was provided by the office of Research and Development, U.S. Environmental Protection Agency, under Agreement PR-0345 through the Western Region Hazardous Substance Research Center.
REFERENCES
- 1.Alvarez-Cohen L, McCarty P L. A cometabolic biotransformation model for halogenated aliphatic compounds exhibiting product toxicity. Environ Sci Technol. 1991;25:1381–1386. [Google Scholar]
- 2.Alvarez-Cohen L, McCarty P L. Effects of toxicity, aeration, and reductant supply on trichloroethylene transformation by a mixed methanotrophic culture. Appl Environ Microbiol. 1991;57:228–235. doi: 10.1128/aem.57.1.228-235.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arciero D, Vannelli T, Logan M, Hooper A B. Degradation of trichloroethylene by the ammonia-oxidizing bacterium Nitrosomonas europaea. Biochem Biophys Res Commun. 1989;159:640–643. doi: 10.1016/0006-291x(89)90042-9. [DOI] [PubMed] [Google Scholar]
- 4.Bouwer E J, McCarty P L. Transformation of 1- and 2-carbon halogenated aliphatic organic compounds under methanogenic conditions. Appl Environ Microbiol. 1983;45:1286–1294. doi: 10.1128/aem.45.4.1286-1294.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calabrese J P, Bissonnette G K. Improved detection of acid mine water stressed coliform bacteria on media containing catalase and sodium pyruvate. Can J Microbiol. 1990;36:544–550. doi: 10.1139/m90-095. [DOI] [PubMed] [Google Scholar]
- 6.Chang H L, Alvarez-Cohen L. Model for the cometabolic biodegradation of chlorinated organics. Environ Sci Technol. 1995;29:2357–2367. doi: 10.1021/es00009a031. [DOI] [PubMed] [Google Scholar]
- 7.Chu K H, Alvarez-Cohen L. Evaluation of toxic effects of aeration and trichloroethylene oxidation on methanotrophic bacteria grown with different nitrogen sources. Appl Environ Microbiol. 1999;65:766–772. doi: 10.1128/aem.65.2.766-772.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiSpirito A A, Gulledge J, Shiemke A K, Murrel J C, Lidstrom M E, Krema C L. Trichloroethylene oxidation by the membrane-associated methane monooxygenase in Type I, Type II, and Type X methanotrophs. Biodegradation. 1992;2:151–164. [Google Scholar]
- 9.Ely R L, Hyman M R, Arp D J, Guenther R B, Williamson K J. A cometabolic kinetics model incorporating enzyme inhibition, inactivation, and recovery: II. Trichloroethylene degradation experiments. Biotechnol Bioeng. 1995;46:232–245. doi: 10.1002/bit.260460306. [DOI] [PubMed] [Google Scholar]
- 10.Ensign S A, Hyman M R, Arp D J. Cometabolic degradation of chlorinated alkenes by alkene monooxygenase in a propylene-grown Xanthobacter strain. Appl Environ Microbiol. 1992;58:3038–3046. doi: 10.1128/aem.58.9.3038-3046.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ewers J, Freier-Schroder D, Knackmuss H J. Selection of trichloroethene (TCE) degrading bacteria that resist inactivation by TCE. Arch Microbiol. 1990;154:410–413. doi: 10.1007/BF00276540. [DOI] [PubMed] [Google Scholar]
- 12.Folsom B R, Chapman P J. Performance characterization of a model bioreactor for the biodegradation of trichloroethylene by Pseudomonas cepacia G4. Appl Environ Microbiol. 1991;57:1602–1608. doi: 10.1128/aem.57.6.1602-1608.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folsom B R, Chapman P J, Pritchard P H. Phenol and trichloroethylene degradation by Pseudomonas cepacia G4: kinetics and interactions between substrates. Appl Environ Microbiol. 1990;56:1279–1285. doi: 10.1128/aem.56.5.1279-1285.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox B G, Borneman J G, Wackett L P, Lipscomb J D. Haloalkene oxidation by the soluble methane monooxygenase from Methylosinus trichosporium OB3b: mechanistic and environmental implications. Biochemistry. 1990;29:6419–6427. doi: 10.1021/bi00479a013. [DOI] [PubMed] [Google Scholar]
- 15.Gornall A G, Bardawill C J, David M M. Determination of serum proteins by means of the Biuret reaction. J Biol Chem. 1949;177:751–766. [PubMed] [Google Scholar]
- 16.Gossett J M. Measurement of Henry's law constants for C1 and C2 chlorinated hydrocarbons. Environ Sci Technol. 1987;21:202–208. [Google Scholar]
- 17.Hamamura N, Page C, Long T, Semprini L, Arp D J. Chloroform cometabolism by butane-grown CF8, Pseudomonas butanovora, and Mycobacterium vaccae JOB5 and methane-grown Methylosinus trichosporium OB3b. Appl Environ Microbiol. 1997;63:3607–3613. doi: 10.1128/aem.63.9.3607-3613.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harker A R, Kim Y. Trichloroethylene degradation by two independent aromatic-degrading pathways in Alcaligenes eutrophus JMP134. Appl Environ Microbiol. 1990;56:1179–1181. doi: 10.1128/aem.56.4.1179-1181.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heald S, Jenkins R O. Trichloroethylene removal and oxidation toxicity mediated by toluene dioxygenase of Pseudomonas putida. Appl Environ Microbiol. 1994;60:4634–4637. doi: 10.1128/aem.60.12.4634-4637.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hyman M R, Russell S A, Ely R L, Williamson K J, Arp D J. Inhibition, inactivation, and recovery of ammonia-oxidizing activity in cometabolism of trichloroethylene by Nitrosomonas europaea. Appl Environ Microbiol. 1995;61:1480–1487. doi: 10.1128/aem.61.4.1480-1487.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Infante P F, Tsongas T A. Mutagenic and oncogenic effects of chloromethanes, chloroethanes, and halogenated analogs of vinyl chloride. Environ Sci Res. 1987;25:301–327. [Google Scholar]
- 22.Kado C I, Liu S-T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981;145:1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landa A S, Sipkema E M, Weijma J, Beenackers A A, Dolfing J, Janssen D B. Cometabolic degradation of trichloroethylene by Pseudomonas cepacia G4 in a chemostat with toluene as the primary substrate. Appl Environ Microbiol. 1994;60:3368–3374. doi: 10.1128/aem.60.9.3368-3374.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leddy M B, Phipps D W, Ridgway H F. Catabolite-mediated mutations in alternate toluene degradative pathways in Pseudomonas putida. J Bacteriol. 1995;177:4713–4720. doi: 10.1128/jb.177.16.4713-4720.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li S, Wackett L P. Trichloroethylene oxidation by toluene dioxygenase. Biochem Biophys Res Commun. 1992;185:443–451. doi: 10.1016/s0006-291x(05)81005-8. [DOI] [PubMed] [Google Scholar]
- 26.Mars A E, Houwing J, Dolfing J, Janssen D B. Degradation of toluene and trichloroethylene by Burkholderia cepacia G4 in growth-limited fed-batch culture. Appl Environ Microbiol. 1996;62:886–891. doi: 10.1128/aem.62.3.886-891.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mars A E, Prins G T, Wietzes P, de Koning W, Janssen D B. Effect of trichloroethylene on the competitive behavior of toluene-degrading bacteria. Appl Environ Microbiol. 1998;64:208–215. doi: 10.1128/aem.64.1.208-215.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin S E, Flowers R S, Ordal Z J. Catalase: its effect on microbial enumeration. Appl Environ Microbiol. 1976;32:731–734. doi: 10.1128/aem.32.5.731-734.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McClay K, Streger S H, Steffan R J. Induction of toluene oxidation activity in Pseudomonas mendocina KR1 and Pseudomonas sp. strain ENVPC5 by chlorinated solvents and alkanes. Appl Environ Microbiol. 1995;61:3479–3481. doi: 10.1128/aem.61.9.3479-3481.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDonald L C, Hackney C R, Ray B. Enhanced recovery of injured Escherichia coli by compounds that degrade hydrogen peroxide or block its formation. Appl Environ Microbiol. 1983;45:360–365. doi: 10.1128/aem.45.2.360-365.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mirpuri R G, Jones W L, McFeters G A, Ridgway H F. Physiological stress in batch cultures of Pseudomonas putida 54G during toluene degradation. J Ind Microbiol Biotechnol. 1997;18:406–413. doi: 10.1038/sj.jim.2900407. [DOI] [PubMed] [Google Scholar]
- 32.Munakata-Marr J, McCarty P L, Shields M S, Reagin M, Francesconi S C. Enhancement of trichloroethylene degradation in aquifer microcosms bioaugmented with wild type and genetically altered Burkholderia (Pseudomonas) cepacia G4 and PR1. Environ Sci Technol. 1996;30:2045–2052. [Google Scholar]
- 33.Nelson M J, Montgomery S O, Mahaffey W R, Pritchard P H. Biodegradation of trichloroethylene and involvement of an aromatic biodegradative pathway. Appl Environ Microbiol. 1987;53:949–954. doi: 10.1128/aem.53.5.949-954.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newman L M, Wackett L P. Trichloroethylene oxidation by purified toluene 2-monooxygenase: products, kinetics, and turnover-dependent inactivation. J Bacteriol. 1997;179:90–96. doi: 10.1128/jb.179.1.90-96.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oldenhuis R, Oedzes J Y, van der Waarde J J, Janssen D B. Kinetics of chlorinated hydrocarbon degradation by Methylosinus trichosporium OB3b and toxicity of trichloroethylene. Appl Environ Microbiol. 1991;57:7–14. doi: 10.1128/aem.57.1.7-14.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radway J C, Santo Domingo J W, Hazen T C, Wilde E W. Evaluation of biodegradation potential of foam embedded Burkholderia cepacia G4. Biotechnol Lett. 1998;20:663–666. [Google Scholar]
- 37.Rasche M E, Hyman M R, Arp D J. Factors limiting aliphatic chlorocarbon degradation by Nitrosomonas europaea—cometabolic inactivation of ammonia monooxygenase and substrate specificity. Appl Environ Microbiol. 1991;57:2986–2994. doi: 10.1128/aem.57.10.2986-2994.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raymon M K, Aris B, El Derea H B. The effect of compounds which degrade hydrogen peroxide on the enumeration of heat-stressed cells of Salmonella senftenberg. Can J Microbiol. 1978;24:883–885. doi: 10.1139/m78-146. [DOI] [PubMed] [Google Scholar]
- 39.Shields M S, Montgomery S O, Cuskey S M, Chapman P J, Pritchard P H. Mutants of Pseudomonas cepacia G4 defective in catabolism of aromatic compounds and trichloroethylene. Appl Environ Microbiol. 1991;57:1935–1941. doi: 10.1128/aem.57.7.1935-1941.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shields M S, Reagin M J, Gerger R R, Campbell R, Somerville C. TOM, a new aromatic degradative plasmid from Burkholderia (Pseudomonas) cepacia G4. Appl Environ Microbiol. 1995;61:1352–1356. doi: 10.1128/aem.61.4.1352-1356.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shields M S, Reagin M J, Gerger R R, Somerville C, Schaubhut R, Campbell R, Hu-Primmer J. Constitutive degradation of trichloroethylene by an altered bacterium in a gas-phase bioreactor. In: Hinche R E, Leeson A, Semprini L, editors. Bioremediation of chlorinated and polycyclic aromatic hydrocarbon compounds. Boca Raton, Fla: Lewis; 1994. pp. 50–65. [Google Scholar]
- 42.Sikkema J, De Bont J A M, Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev. 1995;59:201–222. doi: 10.1128/mr.59.2.201-222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun A K, Hong J, Wood T K. Modeling trichloroethylene degradation by a recombinant Pseudomonad expressing toluene ortho-monooxygenase in a fixed-film bioreactor. Biotechnol Bioeng. 1998;59:40–51. doi: 10.1002/(sici)1097-0290(19980705)59:1<40::aid-bit6>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 44.Sun A K, Wood T K. Trichloroethylene degradation and mineralization by pseudomonads and Methylosinus trichosporium OB3b. Appl Microbiol Biotechnol. 1996;45:248–256. doi: 10.1007/s002530050679. [DOI] [PubMed] [Google Scholar]
- 45.Tsein H C, Brusseau G A, Hanson R S, Wackett L P. Biodegradation of trichloroethylene by Methylosinus trichosporium OB3b. Appl Environ Microbiol. 1989;55:3155–3161. doi: 10.1128/aem.55.12.3155-3161.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Hylckama Vlieg J E T, De Koning W, Janssen D B. Effect of chlorinated ethene conversion on viability and activity of Methylosinus trichosporium OB3b. Appl Environ Microbiol. 1997;63:4961–4964. doi: 10.1128/aem.63.12.4961-4964.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Hylckama Vlieg J E T, de Koning W, Janssen L P. Transformation kinetics of chlorinated ethenes by Methylosinus trichosporium OB3b and detection of unstable epoxides by on-line gas chromatography. Appl Environ Microbiol. 1996;62:3304–3312. doi: 10.1128/aem.62.9.3304-3312.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vogel T M, McCarty P L. Biotransformation of tetrachloroethylene to trichloroethylene, dichloroethylene, vinyl chloride, and carbon dioxide under methanogenic conditions. Appl Environ Microbiol. 1985;49:1053–1080. doi: 10.1128/aem.49.5.1080-1083.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wackett L P, Gibson D T. Degradation of trichloroethylene by toluene dioxygenase in whole-cell studies with Pseudomonas putida F1. Appl Environ Microbiol. 1988;54:1703–1708. doi: 10.1128/aem.54.7.1703-1708.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wackett L P, Householder S R. Toxicity of trichloroethylene to Pseudomonas putida F1 is mediated by toluene dioxygenase. Appl Environ Microbiol. 1989;55:2727–2725. doi: 10.1128/aem.55.10.2723-2725.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilhelm E, Battino R, Wilcock R J. Low-pressure solubility of gases in liquid water. Chem Rev. 1977;77:219–262. [Google Scholar]
- 52.Woomer P, Bennett J, Yost R. Overcoming the inflexibility of most probable number procedures. Agron J. 1990;82:349–353. [Google Scholar]
- 53.Yeager C M, Bottomley P J, Arp D J, Hyman M R. Inactivation of toluene 2-monooxygenase in Burkholderia cepacia G4 by alkynes. Appl Environ Microbiol. 1999;65:632–639. doi: 10.1128/aem.65.2.632-639.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zylstra G J, Wackett L P, Gibson D T. Trichloroethylene degradation by Escherichia coli containing the cloned Pseudomonas putida F1 toluene dioxygenase genes. Appl Environ Microbiol. 1989;55:3162–3166. doi: 10.1128/aem.55.12.3162-3166.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]