Abstract
Background
Accidental awareness during general anaesthesia (AAGA) is when a patient unintentionally becomes conscious during a procedure performed with general anaesthesia and subsequently has explicit recall of this event. Incidence estimates for AAGA vary, with the most common estimate being one to two cases per 1000 general anaesthetics. Evidence linking nitrous oxide use and an increased risk of AAGA has come from observational studies data but the literature is contradictory, with some studies finding a protective effect of nitrous oxide.
Objectives
To assess the effect of general anaesthesia including nitrous oxide on the risk of AAGA in patients aged five years and over.
Search methods
We searched the following databases: Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and trial registers ((www.clinicaltrials.gov), the WHO International Clinical Trials Registry Platform (www.who.int/ictrp/network/en/) and Current Controlled Trials (www.isrctn.com/)) for eligible studies on December 9 2015. In addition, we conducted forward and backward citation searching using key identified papers.
Selection criteria
We considered all randomized controlled trials (RCTs), including quasi‐randomized studies and cluster‐randomized studies, of participants aged five years or older receiving general anaesthesia for any type of surgery.
We included trials in which participants receiving general anaesthesia that included nitrous oxide for maintenance at a concentration of at least 30% were compared with participants receiving no nitrous oxide during general anaesthesia. The intervention group must have received nitrous oxide in conjunction with an additional anaesthetic. We excluded studies where the depth of anaesthesia differed between the study arms. For inclusion in the review, studies needed to state in their methods that they planned to assess AAGA. We defined this as when a patient becomes conscious during a procedure performed with general anaesthesia and subsequently has explicit recall of this event.
Data collection and analysis
We used standard methodological procedures expected by Cochrane to identify studies. We extracted data and conducted 'Risk of bias' assessment using the Covidence database.
Main results
We included 15 studies. The total number of participants included in the analyses was 3520. Most studies were small with fewer than 120 participants, although two larger studies with 2012 and 671 participants were included. There was considerable variation in many of the study characteristics, including the anaesthetics used. The concentrations of nitrous oxide varied between 50% and 70%, and half of the studies used clinical signs and haemodynamic changes to monitor depth of anaesthesia.
As it was not possible to blind the anaesthetist to the anaesthetic used, we rated all studies at high risk of performance bias and we therefore downgraded the quality of evidence by one level for risk of bias using the GRADE approach. Other types of bias were generally low, or were rated unclear due to missing information.
No studies were designed to measure AAGA as the primary outcome, and were therefore statistically underpowered to answer this review question. Despite the inclusion of 3520 participants, only three awareness events were reported by two studies. In one study the event was due to technical failure. Due to the rarity of the events, we did not consider it appropriate to pool the data, and we therefore downgraded the quality of evidence by a further level for imprecision using GRADE.
Authors' conclusions
It is not possible to draw any conclusions from this review. The included studies were mainly small (fewer than 120 participants) and there were limited estimates of effect, with only two studies reporting any events. We cannot therefore determine whether the use of nitrous oxide in general anaesthesia increases, decreases or has no effect on the risk of accidental awareness.
Keywords: Adult; Child; Humans; Middle Aged; Anesthesia, General; Anesthetics, Inhalation; Intraoperative Awareness; Mental Recall; Nitrous Oxide; Surgical Procedures, Operative; Randomized Controlled Trials as Topic
Plain language summary
Nitrous oxide‐based versus nitrous oxide‐free general anaesthesia and accidental awareness during general anaesthesia in surgical patients
Review question
We reviewed the evidence about the effect of nitrous oxide used as part of a general anaesthesia on the risk of accidental awareness during anaesthesia in people over the age of five years undergoing surgery.
Background
Accidental awareness during general anaesthetic is when a person accidentally becomes conscious during surgery, performed with general anaesthesia, and can remember the event once they have woken up. Accidental awareness is an uncommon event, but it can be extremely distressing for the patient and the doctors. There is some evidence suggesting that use of nitrous oxide may increase the risk of accidental awareness. However, the literature is contradictory, with some studies finding no difference and others a decreased risk of awareness in persons anaesthetized with nitrous oxide.
Search date
We searched for studies in December 2015.
Study characteristics and key results
We included 15 studies. The studies covered 3520 people. Although most studies were small with fewer than 120 participants, there were two larger studies with 2012 and 671 participants included. There was a great variation in many of the important elements among the studies, including the type of anaesthetics used and the levels of nitrous oxide used.
No study was designed to measure accidental awareness, but rather they measured it as a secondary outcome. Although there were 3520 participants included in the studies, there were only three reports of a participant becoming aware. These were reported in two studies, and one was thought to be due to an error in the anaesthetic procedure.
Nine studies reported where the funds for the research were obtained. Two were funded by pharmaceutical companies, suggesting a potential bias, whereas five were funded through Universities or Government health research grants or a charity, limiting the risk of bias. The remaining two studies reported that there was no conflict of interest, also reducing the risk of bias in these studies.
Quality of the evidence
Due to safety issues, all of the anaesthetists had to know what anaesthesia was being used. However, this means that the study results may have been biased. Other indicators suggested a low risk of bias, or an unclear risk because of missing information. The quality of the evidence is also low due to the lack of reports of a participant becoming aware.
Conclusions
It is not possible to draw any conclusions from this review. The included studies were mainly too small, and only two studies reported any events. The review question is inadequately supported by the lack of strong evidence. The effect of nitrous oxide is hardly observed due to the small sample size.
Summary of findings
Summary of findings for the main comparison. What is the effect of general anaesthesia including nitrous oxide compared to without nitrous oxide on the risk of accidental awareness during general anaesthesia in patients aged five years and over?
| What is the effect of general anaesthesia including nitrous oxide compared to without nitrous oxide on the risk of accidental awareness during general anaesthesia in patients aged five years and over? | ||||||
|
Patient or population: surgical patients Setting: Seven studies in Europe, three in North America, two in Japan, one in India, one in Hong Kong and one international multi centred Intervention: Nitrous oxide‐based Comparison: Nitrous oxide ‐free | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk without Nitrous oxide | Risk with Nitrous oxide | |||||
| Accidental awareness ‐ Overall (AAGA) assessed with: Any | Study population | not estimable | 3439 (14 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | Results not pooled due to rarity of events | |
| not pooled | not pooled | |||||
| Accidental awareness ‐ In recovery (AAGA) assessed with: Any | Study population | not estimable | 263 (5 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | Results not pooled due to rarity of events | |
| not pooled | not pooled | |||||
| Accidental awareness ‐ 24 hours (AAGA) assessed with: Any follow‐up: 1 days | Study population | not estimable | 556 (7 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | Results not pooled due to rarity of events | |
| not pooled | not pooled | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1The anaesthetist delivering the anaesthetic was aware of the allocation in all studies, as this is essential for patient safety, so we rated all studies at high risk of performance bias.
2Due to the rarity of the events no pooling was appropriate.
Background
Description of the condition
Accidental awareness during general anaesthetic (AAGA) is when a patient unintentionally becomes conscious during a procedure performed with general anaesthesia and subsequently has explicit recall of this event. The Michigan awareness classification instrument (Mashour 2010) describes the features of awareness, which range from no awareness through isolated auditory perceptions to the experience of paralysis and pain. Awareness may be an extremely unpleasant experience and can have serious long‐term consequences for patients.
Estimates of the incidence of long‐term psychological problems in patients who have experienced AAGA are variable (Lennmarken 2002; Samuelsson 2007) but have been as high as 71% (Leslie 2010). Explicit awareness requires consciousness (involving both arousal and experience) and recall. Our limited understanding of the underlying mechanisms of awareness and its risk factors reflect current uncertainties in models for consciousness and for memory.
Incidence estimates for AAGA vary with the method of ascertainment. The most widely used method, the Brice protocol (Brice 1970), involves asking the patient directly about dreams, recall or other experiences between going to sleep and waking up. The patients are asked these questions on three separate occasions over a period postoperatively of up to 30 days. Most studies using this method or a variation have found that possible or definite awareness occurred in one to two cases per 1000 general anaesthetics (Avidan 2008; Avidan 2011; Mashour 2012; Myles 2004; Sandin 2000; Sebel 2004; Wennervirta 2002). These estimates are stable despite differences in study design (data from both randomized controlled trials (RCTs) (Avidan 2008; Avidan 2011; Mashour 2012; Myles 2004) and observational studies (Sandin 2000; Sebel 2004; Wennervirta 2002)); changes in number and timing of interviews (interviews performed three times (Avidan 2008; Myles 2004; Sandin 2000), twice (Avidan 2011; Sebel 2004) or only once (Wennervirta 2002)); and whether the study population was unselected (Mashour 2012; Sandin 2000; Sebel 2004; Wennervirta 2002) or considered at high risk of awareness (Avidan 2008; Avidan 2011; Myles 2004). Pooled data from five studies in children reported an incidence of 7.4/1000 (Davidson 2011). Pollard 2007 reported a much lower incidence of 1/14,500 cases in adults. This study took place as part of quality assurance programme and used a modified Brice questionnaire. Incidence estimates, based on surveys of anaesthetic staff reporting awareness events that had been voluntarily reported by patients rather than through a proactive questionnaire, are also much lower, 1/15,000 (Pandit 2013). The 5th National Audit Project (NAP5) was conducted in the UK and Ireland during 2012 to 2013, and included 141 certain, probable or possible reports of AAGA arising from an estimated approximately 3 million general anaesthetics. This study relied on spontaneous reports rather than direct questioning, and estimated the incidence of certain/probable/possible awareness in the UK as 1/19,600 (95% confidence interval (CI) 1/16,700 to 1/23,450) anaesthetics (Pandit 2014a; Pandit 2014b).
Risk factors for awareness are not well understood, but are likely to be associated with an inadequate dose of the anaesthetic agent. This may be intentional (due to clinical circumstance) or unintentional (due to clinical error or unpredicted variation in patient requirement). The incidence may be higher in women having a caesarean section, with a recent estimate, based on only two cases, of 2.6/1000 (Paech 2008). This increased risk may be due to a lighter anaesthesia being given to reduce harm to the baby. There is also evidence that cardiac cases are at higher risk of awareness (Ghoneim 2009; Sebel 2004). Other risk factors may include female sex and younger age (Ghoneim 2009) and American Society of Anesthesiologists (ASA) class III or IV (Sebel 2004), but data are sparse and contradictory. Increased metabolism of the anaesthetic agent, due to genetic variation or induction of enzymes systems such as cytochrome P450 by alcohol or other drugs, may also increase the risk of awareness (Mashour 2011). In NAP5 which collected the largest ever cohort of cases of AAGA reported in one study, factors increasing the risk of reports of accidental awareness included female sex, age (younger adults but not children), obesity, anaesthetist seniority (junior trainees), previous awareness, out‐of‐hours operating, emergencies, type of surgery (obstetric, cardiac, thoracic) and the use of neuromuscular blockade. ASA physical status, race and use or omission of nitrous oxide were not risk factors for reporting accidental awareness (Pandit 2014a; Pandit 2014b)
Studies of prevention of accidental awareness during general anaesthesia (AAGA)
Some trials investigating awareness have focused on the use of interventions which may help to prevent AAGA. These interventions include monitoring of brain activity and monitoring of anaesthetic concentrations or clinical signs that allow the patient's level of consciousness to be tracked. Monitoring of brain activity may be based on spontaneous electroencephalogram (EEG) activity or evoked brain electrical activity, often auditory evoked potentials (AEP) (ASA 2006). Bispectral index (BIS) monitors are used to assess spontaneous EEG activity but trials of their use to prevent awareness, compared to either routine care (Myles 2004; Zhang 2011) or anaesthetic concentration monitoring (Avidan 2008; Mashour 2012), have given mixed results (Pandit 2013).
A Cochrane review (Punjasawadwong 2014), which was last updated in 2014, reported a significant effect of BIS‐guided anaesthesia in reducing the risk of awareness among surgical patients considered at high risk of awareness, compared to using clinical signs in the control groups (2493 participants; odds ratio (OR) 0.24, 95% CI 0.08 to 0.69). This effect was not demonstrated in studies using anaesthetic concentration monitoring as the control group (1981 participants; OR 1.01, 95% CI 0.14 to 7.16). Another meta‐analysis (Shepherd 2013) demonstrated a significant reduction in awareness associated with BIS use (OR 0.45, 95% CI 0.25 to 0.81), but highlighted the high heterogeneity between studies. This meta‐analysis did not include Mashour 2012, which found no effect for BIS monitoring in a study population with no increased risk of awareness where a protocol was used, but a reduction in awareness compared to ‘routine care’ without a protocol to manage depth of anaesthesia. Guidance from the National Institute for Health and Clinical Excellence in the UK (NICE 2012) recommended the use of BIS monitors as an option in patients at high risk of awareness. It also concluded that although there was greater uncertainty about the clinical benefit of other models of EEG monitors, such as Narcotrend or Entropy, they should also be considered as an option in patients at high risk of awareness.
Challenges when studying accidental awareness during general anaesthesia
As AAGA is an uncommon event, RCTs are rarely large enough to achieve statistical power. One RCT with 21,601 participants enrolled was terminated due to inability to detect a difference in the incidence of awareness between different anaesthetic protocols (Mashour 2012). The use of non‐randomized designs such as case‐control studies or analysis of routine data may offer the potential for increasing power, but there are concerns about differences other than nitrous oxide use between the intervention and comparison groups which might bias the results of these studies. These potential confounders include depth of anaesthesia and other risk factors for awareness, such as type of surgery and other anaesthetic agents used. Meta‐analysis can be useful in aggregating results across RCTs but it is important that the methods for assessing awareness are comparable across studies and that the intervention and comparison groups are equivalent for other risk factors for AAGA. Studies which randomize participants to different anaesthetic techniques, such as intravenous versus inhalational and which include nitrous oxide in one arm only, are not suitable for assessing the impact of nitrous oxide on AAGA.
Description of the intervention
Nitrous oxide gas has been used in general anaesthesia since its early pioneering days. It is now commonly used with oxygen for the maintenance of anaesthesia (Sury 2014). Such use has been questioned due to the recognised side effects of nitrous oxide, including the oxidation of vitamin B12 which results in the inhibition of methionine synthesis and an increase in plasma homocysteine levels for several days after surgery. This increase in homocysteine affects endothelial function, which has the potential to destabilize atherosclerotic plaques (Leslie 2011). Nitrous oxide is a weak anaesthetic and is insufficient to provide anaesthesia as a single agent. It is typically used in the range of 50% to 70% nitrous with oxygen 30% to 50% and either an additional volatile anaesthetic agent or an intravenous infusion of anaesthetic. Its use precludes very high inspired oxygen concentrations. Some studies have found that high oxygen levels may improve wound healing and reduce nausea and vomiting (Myles 2007). Evidence from previous RCTs suggest short‐ and long‐term adverse effects of nitrous oxide use during anaesthesia, including increased risk of postoperative nausea and vomiting, major complications within 30 days (Myles 2007), and cardiovascular events up to five years after the anaesthetic (Leslie 2011). However, two recent observational studies failed to find any association between nitrous oxide anaesthesia and increased rates of mortality, one using routine data in an unselected group (Turan 2013) and the second a post hoc analysis of a trial of beta‐blockers in participants at increased risk of cardiovascular complications (Leslie 2013b). A recent large RCT designed to explore the risks of nitrous oxide in major surgery found no impact on mortality or cardiovascular morbidity but did find an increase in severe nausea and vomiting (Myles 2014a). Two systematic reviews on the effects of nitrous oxide have recently been published (Imberger 2014; Sun 2015). The Cochrane review (Sun 2015) investigated the differences in outcomes between nitrous oxide‐based and nitrous oxide‐free general anaesthesia in adults undergoing surgery. It concluded that "the avoidance of nitrous oxide may be reasonable in participants with pre‐existing poor pulmonary function or at high risk of postoperative nausea and vomiting". The non‐Cochrane review (Imberger 2014) looked at the cardiovascular effects of nitrous oxide and concluded that there was insufficient robust evidence to determine the effects.
How the intervention might work
Evidence linking nitrous oxide use with an increased risk of explicit awareness has come from observational studies data in both adults (Errando 2008) and children (Davidson 2011), but the literature is contradictory, with some studies finding a protective effect of nitrous oxide (Cook 2008; Rungreungvanich 2007). A meta‐analysis from 1996 of seven RCTs (Tramer 1996) reported a decreased risk of awareness in participants anaesthetized with nitrous oxide (OR 4.5, 95% CI 1.1 to 18). The results of the Tramer 1996 review in respect of AAGA should be treated with caution, as the review was designed to investigate the association between nitrous oxide use and postoperative nausea and vomiting rather than AAGA. This means that the literature search was restricted to studies reporting on nausea and vomiting and other studies reporting an AAGA outcome may have been missed. In addition the studies included in the Tramer 1996 were not all designed to identify awareness (Lampe 1990; Sengupta 1988) and some compared different anaesthetic techniques (Wrigley 1991).
Nitrous oxide acts predominately as a N‐methyl‐D‐aspartate (NMDA) antagonist whereas the majority of conventional anaesthetic agents are gamma‐aminobutyric acid (GABA) agonists (De Vasconcellos 2013). It is not clear why nitrous oxide use should affect the risk of awareness. Hopkins 2005 suggests that nitrous oxide may decrease the risk of AAGA compared to other anaesthetic agents due to more stable pharmacokinetics, so that clinicians are able to better predict the actual dose received by the patient, and because nitrous oxide is more potent at suppressing the memory of a noxious stimulus than other inhalational anaesthetic agents, but this observation was from animal studies (Alkire 2004). Nitrous oxide is now always used with other anaesthetic agents and the combination effects are thought to be additive (although the ENIGMA II trial did not find this; Myles 2014a). In practice this may be difficult to 'titrate' and there may be antagonism between anaesthetic agents which act on GABA receptors and the anti‐nociceptive effects of nitrous oxide, which may increase the risk of awareness (Sanders 2010). At present we do not know whether nitrous oxide affects awareness due to a direct action on the brain, alters the action of other anaesthetic agents to affect awareness, or has no effect on awareness. The use of brain activity monitors in patients anaesthetized using nitrous oxide is complex, since NMDA antagonists suppress cortical EEG less than GABA‐ergic agents. It has been shown that BIS values do not change during nitrous oxide sedation (Isik 2007). Using brain monitors to titrate nitrous oxide‐based anaesthesia may therefore lead to an increase in dose and inappropriately deep anaesthesia (De Vasconcellos 2013).
Why it is important to do this review
Unintentional explicit awareness during surgery is extremely unpleasant and may have long‐term consequences for the patient. Another Cochrane review is considering anaesthetic interventions for the prevention of awareness (Messina 2008), but this review does not specifically evaluate nitrous oxide. The existing meta‐analysis of the association between nitrous oxide use and AAGA (Tramer 1996) has limitations and should be updated.
There has been recent concern about the possible adverse consequences of using nitrous oxide as an anaesthetic agent. As part of the ongoing debate about its future use, it is important to clarify the relationship between nitrous oxide and awareness and whether its use increases or decreases the risk of unintentional awareness.
Objectives
To assess the effect of general anaesthesia including nitrous oxide on the risk of AAGA in patients aged five years and over.
Methods
Criteria for considering studies for this review
Types of studies
We considered all randomized controlled trials (RCTs) including quasi‐randomized studies and cluster‐randomized studies.
Types of participants
We included trials of participants aged five years or more, receiving general anaesthesia for any type of surgery.
Types of interventions
We included trials in which participants receiving general anaesthesia that included nitrous oxide for maintenance at a concentration of at least 30% were compared with participants receiving no nitrous oxide during general anaesthesia. The intervention group must have received nitrous oxide in conjunction with an additional anaesthetic. This could have been another inhalation anaesthetic (such as sevoflurane, enflurane or isoflurane) or intravenous anaesthetic (such as propofol). We excluded studies where nitrous oxide was used as the sole maintenance anaesthetic, which was an old technique that is no longer clinically relevant. We excluded studies where participants were randomized to different anaesthetic techniques apart from the administration of nitrous oxide, for example inhalation versus intravenous anaesthetic.
Since depth of anaesthesia will affect the likelihood of accidental awareness, we excluded studies where the two arms had different depths of anaesthetic. In order to assess these we used the reported minimum alveolar concentration (MAC) in the published reports. MAC is the concentration of the vapour in the lungs that is needed to prevent movement in 50% of people in response to surgical stimulus. MAC is used to compare the strengths, or potency, of anaesthetic agents. We assumed that, broadly, MACs are additive, and used this to determine whether the two intervention arms had 'similar depth anaesthetics'.
In some studies of nitrous oxide (for example Myles 2004) the other anaesthesia protocol was not specified but left to the discretion of the anaesthetist. In these studies the intention of randomization was nitrous oxide or not, but both the control and intervention groups could contain a mixture of different techniques and agents and presumably depth of anaesthesia. We included studies of this design, as randomization should even out these differences.
The main analyses amalgamated all types of additional anaesthetic agent. If we had had sufficient studies with outcome events we would have undertaken subgroup analyses for different additional anaesthetics, for example nitrous oxide in conjunction with other volatile inhalation anaesthetic agents versus the volatile inhalation agents alone, or nitrous oxide in conjunction with propofol versus propofol alone. These two analytic strategies would have allowed us to examine whether nitrous oxide affects the risk of explicit awareness regardless of the additional anaesthetic used or whether any effect is due to interaction with a particular class of anaesthetic agent.
Types of outcome measures
Primary outcomes
Accidental awareness during general anaesthesia (AAGA): defined as when a patient becomes conscious during a procedure performed with general anaesthesia and subsequently has explicit recall of this event. The qualitative aspects of awareness may be reported on a scale such as the Michigan awareness classification instrument (Mashour 2010), and we included recall of any type of event (auditory or tactile with or without distress, i.e. class 1 and above on the Michigan awareness instrument). Study investigators may also classify any reports of awareness as definite, probable or possible. Precise definitions vary between studies (Mashour 2009; Sandin 2000) but definite events are often those confirmed by attending personnel; probable events are those that the investigators were convinced were real, but for which no confirmation could be obtained; and possible cases occur in patients who were unable to recall any event definitely that would have been indicative of true awareness. For studies which divide awareness in this way, we included probable and definite awareness events only. We classified reported events with a high probability of occurring before or after anaesthesia as no awareness.
We included studies which used the Brice protocol (Brice 1970), questions for ascertainment or those with other direct questioning methods over a shorter period. We did not include studies which relied on unsolicited self reports of awareness.
Secondary outcomes
There were no secondary outcomes for this review.
Search methods for identification of studies
Electronic searches
We searched for eligible trials in the following databases: Cochrane Central Register of Controlled Trials (CENTRAL; 2015, issue 12) , MEDLINE (via Ovid) (from 1946 to the 8th December 2015) and EMBASE (via Ovid) (from 1974 to 8th December 2015). We applied the Cochrane highly sensitive filter for RCTs in MEDLINE and EMBASE (Higgins 2011b).
We also searched the following trial registers: www.clinicaltrials.gov, the WHO International Clinical Trials Registry Platform (www.who.int/ictrp/network/en/) and Current Controlled Trials (www.isrctn.com/) for ongoing trials on 9th December 2015. Our search strategies are presented in Appendix 1.
We included any publication that reported study data, including abstracts, letters and articles. We did not place any restriction on language of publication.
Searching other resources
We undertook forward citation on the key review article (Tramer 1996) and backward citation on Tramer 1996 and Schallner 2013 identified from the electronic resources using Scopus and Web of Science on the 9th December 2015.
Data collection and analysis
Selection of studies
We collated the results of the searches and removed duplicates. The selection of eligible articles took place in two stages.
Two out of three authors (JH, JG and AN) screened all titles and abstracts to remove studies that were very unlikely to be eligible. We piloted 100 titles before reviewing all titles in order to clarify criteria for discarding articles at this stage. If no abstract was available but the title was possibly relevant, we obtained the full text of the article. Because many trials of nitrous oxide in general anaesthesia are conducted with the purpose of assessing cardiovascular and other outcomes, we could not discard them at the title/abstract stage, as it was possible that awareness had been included as a secondary outcome but not included in the abstract. We therefore reviewed all trials with eligible design, population, intervention and comparison groups in full text.
When we had screened all titles and abstracts, two of the same three authors reviewed the full texts of potentially relevant titles. We used Covidence for this stage of the review, and recorded the reasons for exclusion in Covidence. We piloted 10 papers, after which the authors met to compare results and to standardize their procedure and decision making as required. We then read all potentially relevant papers. The Covidence programme compared results and the authors met to discuss discrepancies. We referred any differences that we could not resolve to TC or AS. We recorded the numbers of papers retrieved and exclusions at each stage, with reasons for those reviewed in full text, in a PRISMA flowchart (Figure 1). We summarize the details of ineligible papers which we reviewed in full text in the 'Characteristics of excluded studies' table.
Data extraction and management
Two authors (JH and AN) extracted data from eligible studies using Covidence, with the form template adapted as required (Appendix 2; Appendix 3). We reviewed the template after data from the first three papers had been entered, and modified it as required. If there were duplicate publications from the same study, we created a composite dataset from all the eligible publications.
We resolved disagreements by discussion and, if necessary, consultation with TC or AS.
Assessment of risk of bias in included studies
We used the Cochrane 'Risk of bias' tool to assess the quality of study design and extent of potential bias (Higgins 2011a). We considered the following domains.
Random sequence generation
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data
Selective reporting
Other potential sources of bias
Random sequence generation
We assessed studies as having a low risk of bias if the methods of sequence generation were clearly stated and were truly random. Where the information was unclear we assessed studies as being at unclear risk of bias and where studies were not truly random, e.g. alternating allocation, we assessed studies as being at high risk of bias.
Allocation concealment
We assessed studies as having a low risk of bias if the methods of allocation concealment were clearly stated and allocation was truly concealed, e.g. sealed opaque envelopes. Where the information was unclear, we assessed studies as being at unclear risk of bias and where allocation was not concealed we assessed studies as being at high risk of bias.
Blinding of participants and personnel (performance bias)
It was unlikely that any study would blind the anaesthetist to the intervention, as this is essential for participant safety. We therefore assessed all studies as being at high risk of performance bias.
Blinding of outcome assessment (detection bias)
We assessed detection bias as low risk if the paper stated that assessors or participants, or both, were blinded; unclear risk of bias if it was not possible to determine if participants and assessors were blinded; and high risk of bias if the assessors or participants, or both, were not blinded.
Incomplete outcome data (attrition bias)
We assessed studies as being at low risk of bias if there was low (< 20%) attrition equivalent across groups and the reasons were unlikely to be related to AAGA. We assessed studies as being at unclear risk of bias if it was not possible to assess the level of attrition across groups. We assessed groups with greater than 20% attrition, and either differences between groups or reasons that were related to AAGA, or both, as having a high risk of bias.
Selective reporting (reporting bias)
As this review only included studies that prespecified the outcome in the Methods section of the paper, this bias was not relevant for included studies.
Other sources of bias
Cluster designs may be used in this topic, with anaesthetist, operating theatre or hospital being the unit of randomization. For any cluster‐randomized trials that we included, we would have paid particular attention to baseline characteristics of the participants and the expertise of the anaesthetist. However we identified no cluster‐randomized trials.
We completed a 'Risk of bias' table for each included study within Covidence. For each outcome, we summarized the risk of bias assessments for each domain in 'Risk of bias' graphs and figures.
We then imported data entered into Covidence into Review Manager 5 (RevMan 5.3) and two authors (AN and JH) checked them.
Measures of treatment effect
The single outcome in this review is a dichotomous outcome (occurrence of accidental awareness). For this dichotomous outcome we entered total numbers and numbers of definite awareness events within each randomization group into RevMan 5.3 and calculated odds ratios (ORs) with 95% confidence intervals (CIs). We aimed to use Peto ORs as AAGA is a rare event and we anticipated that event data would be sparse. We would have entered data as odds or risk ratios (and used a general inverse variance model) if we had been unable to extract or obtain the raw data of numbers of definite awareness and total numbers from the study.
We used the Peto odds ratio for meta‐analysis of dichotomous outcomes as this method performs well when events are rare. The Peto method uses a fixed‐effect model but our final choice of a fixed‐effect or random‐effects statistical model for any meta‐analysis would have been influenced by the study characteristics such as control anaesthetic agent and method of ascertainment
Unit of analysis issues
For any cluster‐randomized trials included in the review, we planned to extract data directly from the publication only if the analysis used accounted for the cluster design with a method such as multi‐level modelling or generalized estimating equations. If these adjustments were not made within the report, we planned to undertake approximate analyses by recalculating standard errors or sample sizes based on the design effect. We would have analysed the resulting effect estimates and their standard errors using the generic inverse variance method in RevMan. However we identified no cluster‐randomized trials.
Four studies included more than two arms, with two different anaesthetics combined with nitrous oxide. Where studies made more than one comparison we included the study multiple times if all arms included separate groups. Where comparisons were made with the same control group we split the control group and entered both comparisons.
Dealing with missing data
We planned to perform sensitivity analyses to compare the effect of complete‐case analysis, the worst‐case scenario, and last observation carried forward options on the results of individual studies and any meta‐analyses.
Assessment of heterogeneity
We expected that the findings may differ between studies included in the review. This heterogeneity may be due to:
method of ascertainment of awareness
concentration of nitrous oxide used
class of additional anaesthetics given, such as inhalational or intravenous
co‐interventions such as premedications given
age group
type of surgery and other factors affecting underlying risk of awareness
We would have assessed the degree of heterogeneity by visual inspection of forest plots and by examining the Chi² test for heterogeneity. Heterogeneity would have been quantified using the I² statistic. We would have considered an I² statistical value of 50% or more to represent substantial levels of heterogeneity, but this value would have been interpreted in light of the size and direction of effects and the strength of the evidence for heterogeneity, based on the P value from the Chi² test (Higgins 2011b). If we had detected substantial clinical, methodological or statistical heterogeneity across included studies we would not have reported pooled results from meta‐analysis, but instead used a narrative approach to data synthesis. Due to lack of studies reporting any AAGA event, we were unable to pool any data.
Assessment of reporting biases
We planned to examine funnel plots to assess the potential for publication bias if we identified 10 or more studies reporting events for awareness. We would have used visual assessment supplemented by Egger’s test for asymmetry(Egger 1997). Heterogeneity between studies may lead to asymmetry and we would have considered this possibility when reviewing the results.
Data synthesis
We planned meta‐analysis if we had comparable effect measures from more than one study and where measures of heterogeneity indicated that pooling of results was appropriate. Initial analyses would have included all studies and results would have been pooled across all types of additional anaesthetic agent. An I² statistical value of more than 80% would argue against an overall pooled estimate being presented. If we had found this degree of heterogeneity we would have investigated the causes using subgroup analyses as described above (Assessment of heterogeneity). .
Subgroup analysis and investigation of heterogeneity
If we had had sufficient studies with outcome events we would have attempted subgroup analyses to investigate the potential sources of heterogeneity described above (Assessment of heterogeneity).
Method of ascertainment of awareness such as questionnaire, differences in classification of definite or probable events
Class of additional anaesthetics given, such as nitrous oxide in conjunction with other volatile inhalation anaesthetic agents versus volatile inhalation agents alone, or nitrous oxide in conjunction with propofol versus propofol alone
Co‐interventions such as premedications given
Age group
Type of surgery and underlying risk of awareness: high risk population or unselected population
We would have used the I² statistic to assess the reduction in heterogeneity when introducing subgroups.
Sensitivity analysis
We planned to undertake sensitivity analyses to explore the potential impact of missing data as described in the section Dealing with missing data. We would have carried out analyses stratified by risk of bias, and explored the impact of model choice on the results of any meta‐analyses.
Summary of findings
We used the principles of the GRADE system to give an overall assessment of the evidence relating to AAGA (Guyatt 2008).
The GRADE approach incorporates risk of bias, directness of evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias to give an overall measure of how confident we can be that our estimate of effect is correct. JH and JG independently used the GRADEpro software to create a 'Summary of findings' table for the outcome of AAGA. We discussed any discrepancies and if necessary would have referred them to SL for a final decision.
Results
Description of studies
Results of the search
Figure 1 summarizes the results of the searches. We retrieved 8976 records from the electronic databases and 226 from citation and trial searches. After duplicates were removed using Endnote X7, we screened 4539 records for inclusion. Of these, we selected 225 for full‐text review. This identified 22 publications covering 15 studies for inclusion in the review. Of those excluded at full‐text review most met all the inclusion criteria but were not designed to measure AAGA as a primary or secondary outcome.
1.

Study flow diagram
Included studies
We included 15 studies reported in 22 publications: Aceto 2002; Albertin 2005; Arellano 2000; Crawford 1998; Dedola 2008; ENIGMA; Girardi 1994; Handa 2010; Handa Tsutsui 2007; Heath 1996; Lindekaer 1995; Ngan Kee 2002; Singh 2011; Sukhani 1994; Vanacker 1999. The total number of participants included in the analyses was 3520. Details of the studies are reported in the 'Characteristics of included studies' table.
Only one study (Heath 1996) was conducted in the UK, although one further multicentre study did include a UK site (ENIGMA). Six studies were conducted in Europe (Aceto 2002; Albertin 2005; Dedola 2008; Girardi 1994; Lindekaer 1995; Vanacker 1999), four of which were conducted in Italy (Aceto 2002; Albertin 2005; Dedola 2008; Girardi 1994). Three studies were conducted in North America; two in Canada (Arellano 2000; Crawford 1998) and one in the USA (Sukhani 1994). The remaining studies were conducted in Japan (Handa 2010; Handa Tsutsui 2007), India (Singh 2011) and Hong Kong (Ngan Kee 2002).
Six studies did not report how the study was funded (Aceto 2002; Girardi 1994; Handa 2010; Heath 1996; Sukhani 1994; Vanacker 1999); two reported there were no conflicts of interest (Handa Tsutsui 2007; Singh 2011) and four were funded by Universities or Government health research grants (Albertin 2005; Dedola 2008; ENIGMA; Ngan Kee 2002). Two studies were funded by pharmaceutical companies (Crawford 1998; Lindekaer 1995) and one by a charity (Arellano 2000).
All but two studies included fewer than 120 participants in their final analyses, with numbers ranging from 35 to 118. The remaining two studies were much larger with Arellano 2000 including 617 participants and the ENIGMA study including 2012 participants in their final analyses.
Population
All but three studies (Arellano 2000; Handa 2010; Heath 1996) reported the mean age of participants, with all but one reporting similar mean ages ranging from 30 to 56. The exception was Crawford 1998, which included children aged three to 12 years and reported a mean age of six years.
The proportion of male participants included in the studies varied, with eight studies (Albertin 2005; Arellano 2000; Girardi 1994; Handa Tsutsui 2007; Heath 1996; Ngan Kee 2002; Sukhani 1994; Vanacker 1999) only including female participants (due to the gynaecological nature of the types of surgeries being carried out), although Dedola 2008 did include male participants but 88% of the study population was female. In comparison Lindekaer 1995 included 93% male participants.
Intervention and comparison
Eleven studies included a single comparison of an arm with nitrous oxide and an arm without nitrous oxide (Arellano 2000; Crawford 1998; ENIGMA; Girardi 1994; Handa 2010; Handa Tsutsui 2007; Heath 1996; Lindekaer 1995; Singh 2011; Sukhani 1994; Vanacker 1999) and four studies included more than one comparison (Aceto 2002; Albertin 2005; Dedola 2008; Ngan Kee 2002).
Aceto 2002 included four arms: anaesthesia was maintained either with sevoflurane plus or minus nitrous oxide (comparison one) or with Isoflurane plus or minus nitrous oxide (comparison two). As all arms were distinct groups, we included them as separate comparisons in the analyses.
Albertin 2005 also included four arms, with the dose of remifentanil differing between arms; sevoflurane plus remifentanil 3ng.ml‐1 with or without nitrous oxide and sevoflurane plus remifentanil 1ng.ml‐1 with or without nitrous oxide. As all arms were distinct groups, we included both of them as separate comparisons in the analyses.
Likewise, Dedola 2008 included four arms with the dose of remifentanil differing between arms. Desflurane was used: desflurane plus remifentanil 3 ng.ml‐1 with or without nitrous oxide and desflurane plus remifentanil 1 ng.ml‐1 with or without nitrous oxide. As all arms were distinct groups, we included both of them as separate comparisons in the analyses.
Finally, Ngan Kee 2002 included three arms. All arms received sevoflurane, but one arm received fraction‐inspired oxygen (FiO₂) at 0.5, one arm FiO₂ 0.7 and one arm no nitrous oxide. As there was only one nitrous oxide‐free arm the results for the nitrous oxide‐free arm were split into two groups between the two comparisons and added to the analyses.
The anaesthetics used in the studies varied, with seven using propofol (Arellano 2000; Crawford 1998; Handa 2010; Handa Tsutsui 2007; Heath 1996; Lindekaer 1995; Sukhani 1994), three using sevoflurane (Aceto 2002; Albertin 2005; Ngan Kee 2002), three using isoflurane (Aceto 2002; Girardi 1994; Singh 2011) and two using desflurane (Dedola 2008; Vanacker 1999). One study left other anaesthetics to the discretion of anaesthetists (ENIGMA).
Concentrations of nitrous oxide used in the included studies varied, with two studies using 50% (Handa Tsutsui 2007; Ngan Kee 2002), four studies 60% (Albertin 2005; Dedola 2008; Girardi 1994; Singh 2011), one study 65% (Arellano 2000), one study 66% (Heath 1996), two studies 67% (Handa 2010; Vanacker 1999) and five studies 70% (Crawford 1998; ENIGMA; Lindekaer 1995; Ngan Kee 2002; Sukhani 1994).
The method of establishing depth of anaesthesia and/or equivalence between the two groups (i.e. the monitoring method used) also varied between the studies. Eight studies used clinical signs/haemodynamic changes (Arellano 2000; Crawford 1998; ENIGMA; Girardi 1994; Handa 2010; Lindekaer 1995; Sukhani 1994; Vanacker 1999). Four studies delivered fixed concentrations (Albertin 2005; Dedola 2008; Handa Tsutsui 2007; Ngan Kee 2002), two studies used clinical experience but with no criteria stated (Heath 1996; Singh 2011), and three studies used EEG‐based monitoring, one as the sole monitor of anaesthetic depth (Aceto 2002) and two in addition to other forms of monitoring (ENIGMA; Girardi 1994).
Method of outcome assessment
To be eligible, studies had to outline in the Methods section that they were going to measure AAGA. All studies reported results for AAGA, although no studies had AAGA as a primary outcome. One study (ENIGMA) used a recognized instrument (Brice 1970), and two studies used a modified Brice protocol (Aceto 2002; Singh 2011). A further two studies used a structured questionnaire (Girardi 1994; Handa 2010) and it was unclear what method Sukhani 1994 used. All other studies "asked" or questioned participants.
The timing of assessment varied, with seven studies assessing AAGA whilst participants were in recovery (Arellano 2000; Crawford 1998; Girardi 1994; Handa Tsutsui 2007; Heath 1996; Sukhani 1994; Vanacker 1999) and 10 studies 24 hours later (Aceto 2002; Albertin 2005; Arellano 2000; Dedola 2008; ENIGMA; Girardi 1994; Handa 2010; Heath 1996; Ngan Kee 2002; Singh 2011). Lindekaer 1995 reported in the Methods that AAGA was assessed at discharge and in the Results section reported the results for AAGA at two hours after anaesthesia.
Excluded studies
We excluded 188 papers after full‐text review. Twenty were not of the required design, nine had participants under five years of age, 28 had an ineligible intervention or comparison e.g. not general anaesthetic or comparing different anaesthetics, one paper was a conference proceeding (all abstracts were checked). The main reason for studies being excluded (130 instances) was that although they met all other inclusion criteria they did not state that AAGA was a predetermined outcome.
After full‐text review, we identified a further 15 papers, reporting on 12 studies, as not meeting the inclusion criteria. Details of these 12 excluded studies (Chowdhury 2014; Goto 1997; Goto 1997a; Inada 1999; Kang 2013; ENIGMA‐II; Liu 2014; Luginbuhl 2005; Nakata 1999; Ochiai 1999; Rocca 2000; Ropcke 2001) are shown in Characteristics of excluded studies. Of these 12 studies, six did not include an intervention or comparator relevant to this review (Goto 1997; Goto 1997a; Luginbuhl 2005; Nakata 1999; Ochiai 1999; Ropcke 2001), for five studies the depth of anaesthesia differed between study arms (Chowdhury 2014; Inada 1999; Kang 2013; Liu 2014; Rocca 2000) and two papers reported on the ENIGMA II study (ENIGMA‐II), which did not measure AAGA.
Studies awaiting classification
There are no studies awaiting classification.
Ongoing studies
There are no ongoing studies
Risk of bias in included studies
The results of the 'Risk of bias' assessments are shown in Figure 2; Figure 3 and described below.
2.
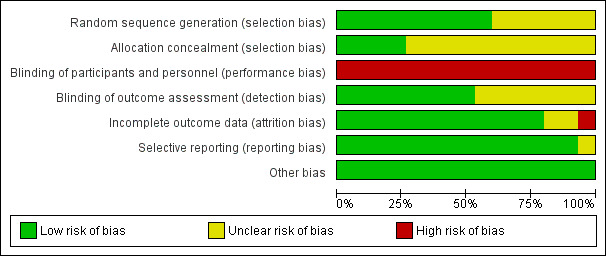
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
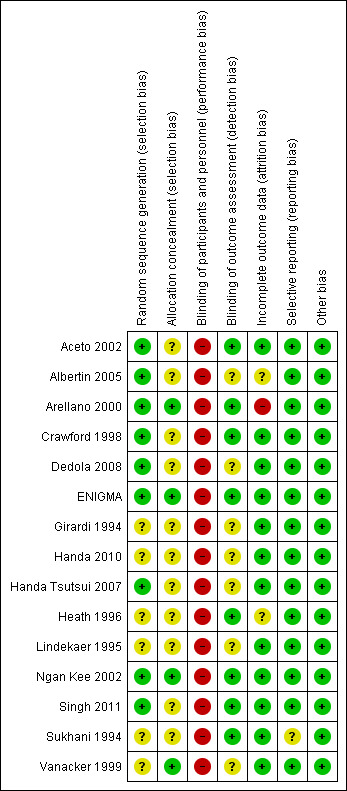
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The methods of random sequence generation and allocation concealment were generally poorly reported, with six studies failing to report methods of randomization thoroughly enough to determine whether the sequence generation was truly random (Girardi 1994; Handa 2010; Heath 1996; Lindekaer 1995; Sukhani 1994; Vanacker 1999). Where adequate information was reported we rated all studies at low risk of bias (Aceto 2002; Albertin 2005; Arellano 2000; Crawford 1998; Dedola 2008; ENIGMA; Handa Tsutsui 2007; Ngan Kee 2002; Singh 2011). Allocation concealment was adequately described in only four studies (Arellano 2000; ENIGMA; Ngan Kee 2002; Vanacker 1999), and was unclear due to lack of information in the remaining studies (Aceto 2002; Albertin 2005; Crawford 1998; Dedola 2008; Girardi 1994; Handa 2010; Handa Tsutsui 2007; Heath 1996; Lindekaer 1995; Singh 2011; Sukhani 1994).
Blinding
Performance bias
The anaesthetist delivering the anaesthetic was aware of the allocation in all studies, as this was essential for participant safety and so we rated all studies at high risk of bias.
Detection bias
Seven studies (Aceto 2002; Arellano 2000; Crawford 1998; ENIGMA; Heath 1996; Singh 2011; Sukhani 1994) stated that the investigator asking about awareness was blinded to participant allocation, but in the other eight studies this was unclear. Since many studies did not use a standardized instrument to ask about accidental awareness this was a potential source of bias. Furthermore as this was a self‐reported outcome if the participants were aware of their allocation this could be a source of detection bias. However only four studies stated that the participant was blinded to allocation, and for the other 11 studies this was unclear (Aceto 2002; ENIGMA; Ngan Kee 2002; Singh 2011). For studies where the investigator was blind to group allocation we assessed studies to be at a low risk of detection bias, and where it was unclear we assessed studies to be at unclear risk. No studies stated that personnel or participants were not blinded, so we rated none of them at high risk of bias.
Incomplete outcome data
Eight of the studies reported no participant attrition (Aceto 2002; Girardi 1994; Handa 2010; Handa Tsutsui 2007; Lindekaer 1995; Ngan Kee 2002; Sukhani 1994; Vanacker 1999), and three low levels of attrition, i.e. less than 20% (Crawford 1998; Dedola 2008; ENIGMA). Two studies reported levels of attrition higher than 20%. Arellano 2000 reported 59% attrition without any reasons stated and was therefore assessed as high risk of bias. Singh 2011 reported 23.3% and 26.8% attrition for the nitrous oxide‐free and ‐based groups respectively. As these levels were similar across groups and the reason for the missing data unrelated to AAGA, we rated this study at low risk. We judged a further two studies to be at unclear risk of bias, as it was not clear which groups, if any, had missing data (Albertin 2005; Heath 1996).
Selective reporting
As this review only included studies that prespecified the outcome in the Methods section of the paper, this bias was not relevant for included studies.
Other potential sources of bias
We identified no other potential sources of bias.
Effects of interventions
See: Table 1
See: Table 1.
Primary outcome one: Accidental awareness during general anaesthesia (AAGA): defined as when a patient becomes conscious during a procedure performed with general anaesthesia and subsequently has explicit recall of this event.
Of the 15 included studies, one (Heath 1996) could not be included in the analyses as the number of participants in each group was not reported. The AAGA data for the remaining 14 studies are shown in Analysis 1.1. As discussed in the section Included studies/interventions and comparisons, four studies included more than one comparison and are therefore included in the analysis more than once i.e. for each comparison (Aceto 2002; Albertin 2005; Dedola 2008; Ngan Kee 2002).
1.1. Analysis.
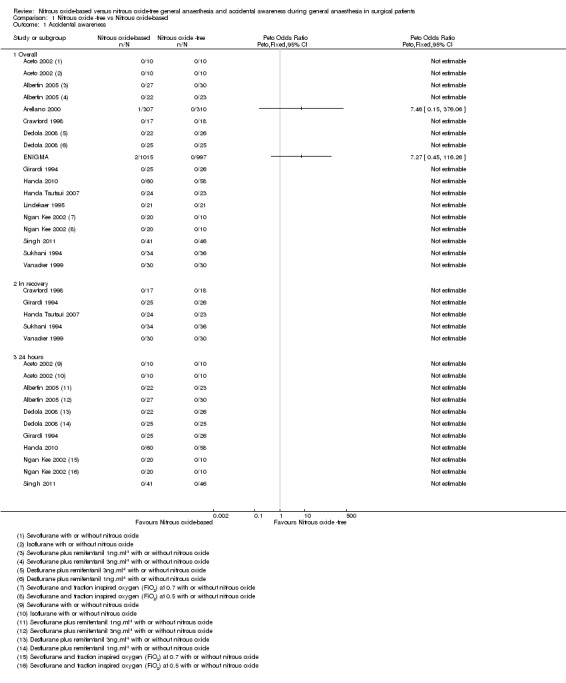
Comparison 1 Nitrous oxide ‐free vs Nitrous oxide‐based, Outcome 1 Accidental awareness.
Firstly, we consider the overall data, regardless of timing of the assessment of AAGA.
AAGA assessed at any time point
The results of 18 comparisons from the 14 studies (Aceto 2002; Albertin 2005; Arellano 2000; Crawford 1998; Dedola 2008; ENIGMA; Girardi 1994; Handa 2010; Handa Tsutsui 2007; Lindekaer 1995; Ngan Kee 2002; Singh 2011; Sukhani 1994; Vanacker 1999), involving 3439 participants, are shown in Analysis 1.1.1. There were three AAGA events reported in the included studies, all in the nitrous oxide‐based group. There were two events in the large ENIGMA study, resulting in an odds ratio of 7.27 with wide 95% confidence intervals (95% CI 0.45 to 116.26). It was not possible to determine from the published paper when the AAGA was assessed, i.e. in recovery or at 24 hours postoperatively. The one event in Arellano 2000 was due to a technical anaesthesia failure rather than a pharmacological effect, and if it had been appropriate to conduct sensitivity analyses we would have done so excluding this study. However with only two studies reporting any events, one of which was due to technical failure (Arellano 2000), no pooling or meta‐analysis was appropriate.
AAGA assessed in recovery.
Analysis 1.1.2 reports the result for AAGA assessed in recovery. For the seven studies stating that AAGA was measured in recovery (Arellano 2000; Crawford 1998; Girardi 1994; Handa Tsutsui 2007; Heath 1996; Sukhani 1994; Vanacker 1999), it was not possible to determine the time point of the results reported by Arellano 2000, and Heath 1996 could not be included in the analysis as the total numbers in each group were not reported. The remaining five studies (Crawford 1998; Girardi 1994; Handa Tsutsui 2007; Sukhani 1994; Vanacker 1999), included 263 participants and are shown in the analysis. No events of AAGA were reported in either group.
AAGA assessed 24 hours after the anaesthetic
Analysis 1.1.3 reports the results for AAGA assessed 24 hours after the anaesthetic. Of the 10 studies (Aceto 2002; Albertin 2005; Arellano 2000; Dedola 2008; ENIGMA; Girardi 1994; Handa 2010; Heath 1996; Ngan Kee 2002; Singh 2011) stating that AAGA was measured at 24 hours after anaesthesia, seven studies reporting on 11 comparisons are included in the analyses (Aceto 2002; Albertin 2005; Dedola 2008; Girardi 1994; Handa 2010; Ngan Kee 2002; Singh 2011). Again, Arellano 2000 and Heath 1996 could not be included. It was also not possible to include the ENIGMA study, as the timing of the reported outcomes was unclear. None of the seven studies/11 comparisons included in the analysis reported AAGA events (Aceto 2002; Albertin 2005; Dedola 2008; Girardi 1994; Handa 2010; Ngan Kee 2002; Singh 2011).
As no pooling or meta‐analysis was possible, we could not carry out any of the planned subgroup analyses, investigation of heterogeneity, sensitivity analyses or investigation of publication bias.
Using GRADE, we downgraded the quality of the evidence by one level (poor), due to concerns about the risk of bias, and by two levels (very poor) due to concerns around imprecision.
Discussion
Summary of main results
We included 15 studies, which had randomized participants to receive nitrous oxide or not as part of a general anaesthetic (Aceto 2002; Albertin 2005; Arellano 2000; Crawford 1998; Dedola 2008; ENIGMA; Girardi 1994; Handa 2010; Handa Tsutsui 2007; Heath 1996; Lindekaer 1995; Ngan Kee 2002; Singh 2011; Sukhani 1994; Vanacker 1999). None of the included trials was designed to measure awareness as a primary outcome, and all were underpowered to study this outcome. Despite a total number of 3520 participants, only three awareness events were reported by two studies (Arellano 2000; ENIGMA), and we considered pooling of data to be inappropriate.
Overall completeness and applicability of evidence
It is not possible to draw any conclusions from this review. The included studies were mainly small (the majority with fewer than 120 participants) and there were limited estimates of effect, since only two studies reported any events with one of these due to technical failure.
Quality of the evidence
Due to safety concerns, no anaesthetists were blinded to the use of nitrous oxide, so all studies had high risk of performance bias, resulting in us downgrading the quality of the evidence by one level in the Table 1. Studies were not designed to measure awareness and were therefore underpowered, resulting in us downgrading the quality of the evidence by a further level for imprecision. Overall we judged the evidence to be of very low quality. See Table 1 for details. Furthermore, the variety of the methods used for assessment of recall in the included studies (Brice, modified Brice, ‘questioning’, etc.) as well as the timing of assessment might have also added to the potential risk of bias.
Potential biases in the review process
We carried out a thorough search to identify all randomized studies of the use of nitrous oxide in general anaesthesia since 1994. Our inclusion criteria required that the accidental awareness outcome was listed in the Methods section of the paper and that participants were asked directly about awareness. We excluded 132 additional studies, which met the inclusion criteria for population, intervention and comparison group, as awareness was not reported as a predefined outcome. Some of these studies may have included spontaneous reports of AAGA and could have potentially contributed to the review. However, the inherent bias of relying on spontaneous reports of an outcome and the very different means of ascertainment of AAGA mean that these studies were not eligible for inclusion in the review.
We excluded studies where it was possible to determine that the study arms had different depths of anaesthesia (Chowdhury 2014; Inada 1999; Kang 2013; Kang 2014; Liu 2014; Rocca 2000). However, some studies did not report sufficient details to determine whether the depths of anaesthesia were equivalent, and we have not excluded these studies. This is a potential source of bias, in that more poorly‐reported trials are more likely to be included. However, none of the trials excluded for differing depths of anaesthesia reported any events of AAGA, so including or excluding them would not have changed the findings of the review. In assessing whether depths of anaesthesia differed, we assumed that MACs are broadly additive.
Agreements and disagreements with other studies or reviews
As our review failed to find sufficient evidence to draw any conclusions, it is not possible to agree or disagree with previous reviews.
The Tramer 1996 review, which is the only existing meta‐analysis of trials of the association between the use of nitrous oxide and accidental awareness, reported an increased risk of awareness in participants who did not receive nitrous oxide, with a calculated number needed to treat to prevent one additional instance of accidental awareness of 46. However the Tramer 1996 review was not designed to answer this question, but was rather designed to investigate the association between nitrous oxide use and postoperative nausea and vomiting. The search was restricted to studies reporting on nausea and vomiting, and there was no restriction by type of anaesthetic (Tramer 1996). Other narrative reviews (De Vasconcellos 2013; Ghoneim 2009; Hopkins 2005), have incorporated the findings of the Tramer review.
We are not aware of any other systematic reviews of accidental awareness and nitrous oxide. However, a recent large observational study (5th National Audit Project) did not find an association between the use of nitrous oxide and accidental awareness (Pandit 2014a; Pandit 2014b).
Authors' conclusions
Implications for practice.
We are unable to draw any implications for clinical practice from the results of this review. We have not been able to confirm the findings from the previous review (Tramer 1996), using more recent trial data and a more inclusive search. We cannot determine whether the use of nitrous oxide in general anaesthesia increases, decreases or has no effect on the risk of AAGA.
Implications for research.
The findings of this review have wider implications for the study of rare outcomes and adverse events. Although meta‐analysis has the potential to increase power by amalgamating results, this is not feasible if there are few or no events reported in the included trials. Assuming an incidence of 1/500 patients receiving general anaesthesia and that nitrous oxide leads to a 50% increase in awareness, a trial would need to have over 11,737 participants in each arm to have 80% power to detect the increase at 5% significance level. This would increase to 23,511 participants in each arm if the incidence was assumed to be 1/1000. An RCT with almost 11,000 in each arm investigating the use of bispectral index (BIS) was recently terminated due to futility, and no significant difference in incidence between the arms could be detected (Mashour 2012).
If such studies were to be done, it may be advisable to focus on higher‐risk groups such as those undergoing lower segment Caesarean section, cardiac surgery, or receiving neuromuscular blocking drugs or total intravenous anaesthesia (TIVA). However, it is not clear that the costs involved would be justified. Without such studies it is unlikely that any future meta‐analyses would be able to draw any implications for clinical practice.
Observational studies have the potential to address this issue and the recently‐published NAP5, which examined new reports of AAGA arising from approximately three million general anaesthetics (Pandit 2014a; Pandit 2014b), found no association between awareness and nitrous oxide use. The lower overall incidence of reports of awareness (1/19,600) reflects that ascertainment relied on spontaneous report of accidental awareness but there is no reason to assume that any association with nitrous oxide would be distorted by under‐reporting. The routine use of direct questioning in conjunction with routine data has the potential to provide further data.
Acknowledgements
We would like to thank Rodrigo Cavallazzi (content editor), Cathal Walsh (statistical editor), Kate Leslie, Richard P Dutton, James Palmer (peer reviewers), Patricia Tong (consumer referee) for their help and editorial advice during the preparation of this systematic review.
We would also like to thank Rodrigo Cavallazzi (content editor), Cathal Walsh (statistical editor), George A Mashour and Kate Leslie (peer reviewers) for their help and editorial advice during the preparation of the protocol (Nicholson 2014) for the systematic review.
We would also like to thank Eleanor Kotas for her assistance in merging search results and undertaking searches of trial registries and citation tracking. We would like to thank Gareth Jones for assistance with finding the full texts of publications and uploading them to Covidence.
Appendices
Appendix 1. Search strategies
Ovid MEDLINE(R) 1946 to July Week 3 2014, Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations July 29, 2014, Ovid MEDLINE(R) Daily Update July 29, 2014 1 ((randomized controlled trial or controlled clinical trial).pt. or randomi$ed.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh. 2 exp Intraoperative Complications/ 3 exp surgical procedures, operative/ 4 (surger* or surgical or intraoper* or postoper* or operat*).mp. 5 or/2‐4 6 nitrous oxide.mp. or exp Nitrous Oxide/ or entonox.mp. 7 6 and 5 and 1 8 6 and 1 9 (anaes* or anes*).mp. 10 exp Anesthesia/ 11 or/5,9‐10 12 6 and 11 and 1 13 6 and (9 or 10) and 1 Cochrane Search Search Name:nitrous oxide June 14 Date Run:30/07/14 13:10:51.810 Description: IDSearchHits #1MeSH descriptor: [Nitrous Oxide] explode all trees #2nitrous oxide or entonox #3#1 or #2 #4surger* or surgical or intraoper* or postoper* or operat* or anes* or anes* #5MeSH descriptor: [Anesthesia] explode all trees #6MeSH descriptor: [Intraoperative Complications] explode all trees #7MeSH descriptor: [Surgical Procedures, Operative] explode all trees #8{or #4‐#7} Database: Embase <1988 to 2014 Week 32> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp peroperative complication/ (19525) 2 exp surgery/ (2788966) 3 (surger* or surgical or intraoper* or postoper* or operat*).mp. (2388900) 4 or/1‐3 (3544644) 5 (nitrous oxide or entonox).mp. or exp nitrous oxide/ (21319) 6 randomized controlled trial/ or exp "randomized controlled trial (topic)"/ or randomi$ed.ab. or placebo.ab. or randomly.ab. or trial.ab. or groups.ab. (2181006) 7 and/4‐6 (3958) 8 limit 7 to (human and yr="1994 ‐Current") (3206) ***************************
Appendix 2. Data extraction form from Covidence
| Study ID | ||||
| Identification | ||||
| Field | Value | |||
| Sponsorship source | ||||
| Country | ||||
| Setting | ||||
| Comment | ||||
| Author's name | ||||
| Institution | ||||
| Address | ||||
| Study design | ||||
| Field | Value | |||
| Study Design | ||||
| Group | ||||
| Participants | ||||
| Field | Value | |||
| Inclusion Criteria | ||||
| Exclusion Criteria | ||||
| Baseline characteristics | ||||
| Characteristics | Nitrous oxide ‐ free ‐ A | Nitrous oxide ‐ based ‐ A | Nitrous oxide ‐ free ‐ B | Nitrous oxide‐based ‐ B |
| Number randomized | ||||
| Number analysed | ||||
| Age (mean) | ||||
| % male | ||||
| Type of surgery | ||||
| Other information | ||||
| Intervention characteristics | ||||
| Characteristics | Nitrous oxide ‐ free ‐ A | Nitrous oxide ‐based | ||
| Induction | ||||
| Maintenance | ||||
| Recovery | ||||
| Other drugs used | ||||
| Premedication | ||||
| Name | ||||
| Pre‐treatment | ||||
| Field | Value | |||
| Group Differences | ||||
Appendix 3. Data extraction outcome form from Covidence
| Outcomes: Dichotomous | ||||||||
| Treatment or comparator: Nitrous oxide ‐ free ‐ A | ||||||||
| Outcome Measure | After 48 hours | In recovery | 24 hours | Overall | ||||
| n | N | n | N | n | N | n | N | |
| Accidental awareness | ||||||||
| Treatment or comparator: Nitrous oxide‐based ‐ A | ||||||||
| Outcome Measure | After 48 hours | In recovery | 24 hours | Overall | ||||
| n | N | n | N | n | N | n | N | |
| Accidental awareness | ||||||||
| Treatment or comparator: Nitrous oxide ‐free ‐ B | ||||||||
| Outcome Measure | After 48 hours | In recovery | 24 hours | Overall | ||||
| n | N | n | N | n | N | n | N | |
| Accidental awareness | ||||||||
| Treatment or comparator: Nitrous oxide‐based ‐ B | ||||||||
| Outcome Measure | After 48 hours | In recovery | 24 hours | Overall | ||||
| n | N | n | N | n | N | n | N | |
| Accidental awareness | ||||||||
Data and analyses
Comparison 1. Nitrous oxide ‐free vs Nitrous oxide‐based.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Accidental awareness | 14 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 1.1 Overall | 14 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 In recovery | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 24 hours | 7 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aceto 2002.
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group |
|
| Participants |
Baseline Characteristics Nitrous oxide‐free ‐ A Number randomized: 10 Number analysed: 10 Age (mean): 54 (50 ± 58) % male: 50 Type of surgery: Laparoscopic cholecystectomy Nitrous oxide‐based ‐ A Number randomized: 10 Number analysed: 10 Age (mean): 49 (39 ± 59) % male: 40 Type of surgery: Laparoscopic cholecystectomy Nitrous oxide‐free ‐ B Number randomized: 10 Number analysed: 10 Age (mean): 52 (47 ± 57) % male: 40 Type of surgery: Laparoscopic cholecystectomy Nitrous oxide‐based ‐ B Number randomized: 10 Number analysed: 10 Age (mean): 50 (43 ± 57) % male: 60 Type of surgery: Laparoscopic cholecystectomy Included criteria: ASA I ‐ II undergoing elective laparoscopic cholecystectomy, aged 18 ‐ 70 yrs, Christians Excluded criteria: History of neurological or mental disease and hearing impairment. Patients having major haemodynamic changes (mean arterial pressure and heart rate) greater than 15% compared with baseline values), and blood loss with acute anaemia as a result of intraoperative surgical complications were also excluded |
|
| Interventions |
Intervention Characteristics Nitrous oxide‐free ‐ A Name: sevoflurane + air (FiO₂ 40%) Induction: Thiopental sodium (5 mg/kg), fentanyl (5 mcg/kg) and vecuronium bromide (0.08 mg/kg) Maintenance: sevoflurane + air (FiO₂ 40%) Recovery: NR Other drugs used: Boluses of fentanyl (2 mcg/kg) and additional vecuronium according to clinical necessity Premedication: None Duration of anaesthesia (min): 91 (70 ± 112) Nitrous oxide‐based ‐ A Name: Sevoflurane + N₂O (60%) in air (FiO₂ 40%) Induction: Thiopental sodium (5 mg/kg), fentanyl (5 mcg/kg) and vecuronium bromide (0.08 mg/kg) Maintenance: Sevoflurane + N₂O (60%) in air (FiO₂ 40%) Recovery: NR Other drugs used: Boluses of fentanyl (2 mcg/kg) and additional vecuronium according to clinical necessity Premedication: None Duration of anaesthesia (min): 107 (89 ± 125) Nitrous oxide‐free ‐ B Name: isoflurane + air (FiO₂ 40%) Induction: Thiopental sodium (5 mg/kg), fentanyl (5 mcg/kg) and vecuronium bromide (0.08 mg/kg) Maintenance: Isoflurane + air (FiO₂ 40%) Recovery: NR Other drugs used: Boluses of fentanyl (2 mcg/kg) and additional vecuronium according to clinical necessity Premedication: None Duration of anaesthesia (min): 90 (65 ± 115) Nitrous oxide‐based ‐ B Name: Isoflurane + N₂O (60%) in air (FiO₂ 40%) Induction: Thiopental sodium (5 mg/kg), fentanyl (5 mcg/kg) and vecuronium bromide (0.08 mg/kg) Maintenance: isoflurane + N₂O (60%) in air (FiO₂ 40%) Recovery: NR Other drugs used: Boluses of fentanyl (2 mcg/kg) and additional vecuronium according to clinical necessity Premedication: None Duration of anaesthesia (min): 97 (78 ± 116) Monitoring: (ML‐AERs) recorded before anaesthesia, at 1 MAC and 30 mins after awakening. The concentration of anaesthetic, monitored with an anaesthetic‐respiratory gas analyser, was maintained at 1 MAC for at least 20 mins before the intraoperative recording of MLAERs, 5 mins after surgical incision |
|
| Outcomes | Accidental awareness 24 hrs after awakening participants were assessed for explicit and implicit memory. Explicit memory was assessed with a recall test. Participants were asked about the last thing they remembered before going to sleep; the first thing they remembered when they woke up; and anything which happened in between, including sounds, dreams, and imagination. (i.e. modified Brice questionnaire) Other stimulation during surgery One of 4 audiotapes was played immediately after completion of MLAER recording. Each audiotape contained 1 of the following stories: (i) The fox and the grapes; (ii) Jesus's birth; (iii) The prodigal son; and (iv) The miracle of the loaves and fishes. At the end of each of the stories, 4 key words had been recorded. Recall of relevant words in these stories used to detect implicit recall |
|
| Identification |
Country: Italy Setting: Department of Anaesthesiology and Intensive Care Authors name: P Aceto Institution: Catholic University of the Sacred Heart Email: gdecosmo@rm.unicatt.it Address: Department of Anaesthesiology and Intensive Care, Policlinico A. Gemelli, L.go A. Gemelli 8,I‐00168 Rome, Italy |
|
| Aim of study | The aim of this study was to investigate the presence of subconscious awareness during anaesthesia and to examine its relationship to the ML‐AERs | |
| Notes | Sponsorship source: No details given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using randomization tables" |
| Allocation concealment (selection bias) | Unclear risk | Comment: No details reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The patients were blinded to the method of anaesthesia used and to the contents of the tape (they were not told that there would be a story on the tape)." Comment: Participants were blinded, as was the anaesthetist playing the tapes. No mention of the anaesthetist giving the anaesthesia, presumably not |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The anaesthesia resident that conducted the post‐ operative interview did not know which anaesthetic had been used or which story had been played." Comment: Outcome assessor and participants were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No losses to follow‐up reported |
| Selective reporting (reporting bias) | Low risk | Comment: All relevant outcomes specified in Methods reported |
| Other bias | Low risk | Comment: None identified |
Albertin 2005.
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group |
|
| Participants |
Baseline Characteristics Nitrous oxide‐free ‐A Number randomized: NR Number analysed: 23 Age (mean): 40 (± 8) % male: 0 Type of surgery: Elective abdominal surgery requiring skin incision Nitrous oxide‐free ‐B Number randomized: NR Number analysed: 30 Age (mean): 39 (± 7) % male: 0 Type of surgery: Elective abdominal surgery requiring skin incision Nitrous oxide‐based ‐B Number randomized: NR Number analysed: 27 Age (mean): 38 (± 7) % male: 0 Type of surgery: Elective abdominal surgery requiring skin incision Nitrous oxide‐based ‐A Number randomized: NR Number analysed: 22 Age (mean): 36 (± 8) % male: 0 Type of surgery: Elective abdominal surgery requiring skin incision Included criteria: Women, aged 20 – 50 yrs, ASA I, scheduled for elective abdominal surgery requiring skin incision Excluded criteria: Patients undergoing laparoscopic procedures, obesity (BMI 30 kg/m²), history of cardiac, pulmonary or renal diseases, drug or alcohol abuse, or current use of any medications affecting the cardiovascular system or blocking the adrenergic responses to surgical incision |
|
| Interventions |
Intervention Characteristics Nitrous oxide‐free ‐A Name: Nitrous oxide‐free remifentanil 3 ng/ml Induction: IV propofol (2 mg/kg) and TCI remifentanil 4 ng/ml for intubation Maintenance: Sevoflurane combined with oxygen and air FiO₂ 40%, TCI remifentanil 3 ng/ml Recovery: NR Other drugs used: NR Premedication: None used Duration of anaesthesia (min): NR Nitrous oxide‐free ‐B Name: Nitrous oxide‐free remifentanil 1 ng/ml Induction: IV propofol (2 mg/kg) and TCI remifentanil 4 ng/ml for intubation Maintenance: Sevoflurane combined with oxygen and air FiO₂ 40% TCI remifentanil 3 ng/ml Recovery: NR Other drugs used: NR Premedication: None used Duration of anaesthesia (min): NR Nitrous oxide‐based ‐B Name: Nitrous oxide‐based remifentanil 1 ng/ml Induction: IV propofol (2 mg/kg) and TCI remifentanil 4 ng/ml for intubation Maintenance: Sevoflurane combined with 60% N₂O in oxygen, TCI remifentanil 1 ng/ml Recovery: NR Other drugs used: NR Premedication: None used Duration of anaesthesia (min): NR Nitrous oxide ‐based ‐A Name: Nitrous oxide‐based remifentanil 3 ng/ml Induction: IV propofol (2mg/kg) and TCI remifentanil 4ng/ml for intubation Maintenance: Sevoflurane combined with 60% N2O in oxygen, TCI remifentanil 3ng/ml Recovery: NR Other drugs used: NR Premedication: None used Duration of anaesthesia (min): NR Monitoring The remifentanil infusion was set at the desired concentration ensuring an adequate equilibration time between plasma and effect site (based upon the very short equilibration time between plasma and effect site (KeO)). An up/down technique was then used to determine the MAC of sevoflurane. The first participant assigned to all groups received 1.5 MAC of sevoflurane adjusted for age (3%) . The subsequent participant then received a variable dose of sevoflurane (decreased or increased by 0.25 MAC (0.5%)) according to whether the preceding participant had responded (increase in heart rate of blood pressure by 15% or not after surgical incision). After 3 sequential negative deflections the change in MAC for each up/down response was reduced to 0.1 MAC (0.2%) |
|
| Outcomes |
On the first postoperative day visit participants were questioned about any recall of intraoperative events. |
|
| Identification |
Country: Italy Setting: NR Authors name: Andrea Albertin Institution: Vita‐Salute University of Milano Email: albertin.andrea@hsr.it Address: Department of Anesthesiology, IRCCS H, San Raffaele, Vita‐Salute University of Milano, Via Olgettina 60, 20132 Milan, Italy |
|
| Aim of study | To determine the effects of adding nitrous oxide on sevoflurane requirement for blunting sympathetic responses after surgical incision combined with 2 different target‐controlled concentrations of remifentanil (1 and 3 ng/ml) in women. | |
| Notes | Depth of anaesthesia would not be equivalent in nitrous vs nitrous‐free ‐ at induction (before MAC monitoring) Lack of clarity on numbers studied: "102 female patients..... were prospectively enrolled" but "A total of 102 female patients completed the study" and "Three patients in Group N3 and one patient in Group A1 were withdrawn from the study for hypotension requiring vasoactive agents." Sponsorship source: This study was supported by the Vita‐Salute University of Milano |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated sequence of numbers," Comment: No further details |
| Allocation concealment (selection bias) | Unclear risk | Comment: No details given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "anaesthesiologist recording cardiovascular parameters and determining the positive–negative response to surgical incision was blinded to patient grouping." Comment: Anaesthetist giving anaesthetic not blinded. Study described as double‐blind but no details of participant blinding. Anaesthetist delivering anaesthetic presumably not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Not reported as to whether the outcome assessor for awareness was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Unclear as to whether 102 participants were randomized or analysed; 3 withdrawals stated but whether they were included in the final analysis is unclear |
| Selective reporting (reporting bias) | Low risk | Comment: All relevant outcomes described in Methods were reported |
| Other bias | Low risk | Comment: None identified |
Arellano 2000.
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group |
|
| Participants |
Baseline Characteristics Nitrous oxide‐free – A Number randomized: 740 Number analysed: 310 Age (mean): NR for subsample with outcome % male: 0 Type of surgery: Outcome data ‐ 231 terminations of pregnancy: 79 laparoscopy Nitrous oxide‐based – A Number randomized: 750 Number analysed: 307 Age (mean): NR for subsample with outcome % male: 0 Type of surgery: Outcome data ‐ 235 terminations of pregnancy: 72 laparoscopy Included criteria: Women undergoing termination of pregnancy (TOP) or ambulatory gynaecologic laparoscopy (LAP). ASA status I or II, between 18 and 55 yrs of age, all day‐surgery patients Excluded criteria: Patients undergoing other ambulatory gynaecologic procedures were not studied, to reduce heterogeneity in study population; history of psychiatric disease, narcotic/sedative use, drug abuse, or morbid obesity (30% above ideal body weight) |
|
| Interventions |
Intervention Characteristics Nitrous oxide‐free – A Name: Propofol + 100% O₂ Induction: TOP: IV fentanyl 0.7 mcg/kg. 20 mg lidocaine and 2.0 mg/kg propofol IV over 40 secs, further propofol titrated to loss of lid reflex LAP: Fentanyl 1.5 mcg/kg and d‐tubocurare 3 mg IV. 20 mg lidocaine and 2.0 mg/kg propofol IV over 40 secs, further propofol titrated to loss of lid reflex. Succinylcholine 1.5 mg/kg IV, oral intubation. After induction, 0.075 – 0.1 mg/kg vecuronium IV Maintenance: TOP: 100% O₂. Intermittent bolus doses of 20 mg propofol in response to clinical signs of light anaesthesia (movement, laccrymation, or phonation in response to surgical stimuli, or increases in blood pressure, pulse rate, or respiratory rate of ≥ 20%) LAP: 100% O₂. Infusion of propofol 100 – 200 mcg/kg/min supplemented by intermittent bolus doses of 20 mg propofol in response to clinical signs of light anaesthesia (movement, laccrymation in response to surgical stimuli or increases in blood pressure, or pulse rate of ≥20%) Recovery: At the end of surgery, neuromuscular blockade was reversed with atropine 0.02 mg/kg and neostigmine 0.04 mg/kg. In all participants, propofol and N₂O were discontinued when the dressing was applied at the end of surgery Other drugs used: NR Premedication: No premedication was given Duration of anaesthesia (min): NR Nitrous oxide‐based – A Name: Propofol + 65% N₂O Induction: TOP: Fentanyl 0.7 mcg/kg IV. 20 mg lidocaine and 2.0 mg/kg propofol IV over 40 secs, further increments of propofol titrated to loss of lid reflex LAP: Fentanyl 1.5 mcg/kg and d‐tubocurare 3 mg IV. 20 mg lidocaine and 2.0 mg/kg propofol IV over 40 secs, further increments of propofol titrated to loss of lid reflex. Succinylcholine 1.5 mg/kg IV and oral intubation. After induction, 0.075 – 0.1 mg/kg vecuronium IV Maintenance: TOP: N₂O and O₂ FiO₂ 35% administered by mask. Intermittent bolus doses of 20 mg propofol in response to clinical signs of light anaesthesia (movement, laccrymation, or phonation in response to surgical stimuli, or increases in blood pressure, pulse rate, or respiratory rate of ≥ 20%) LAP: N₂O and O₂ FiO₂ 35%. Anaesthesia maintained with an infusion of propofol 100 – 200 mcg/kg/min supplemented by intermittent bolus doses of 20 mg propofol in response to clinical signs of light anaesthesia (movement or laccrymation in response to surgical stimuli or increases in blood pressure, or pulse rate of ≥ 20%) Recovery: At the end of surgery, neuromuscular blockade was reversed with atropine 0.02 mg/kg and neostigmine 0.04 mg/kg. In all participants, propofol and N₂O were discontinued when the dressing was applied at the end of surgery Other drugs used: NR Premedication: No premedication was given Duration of anaesthesia (min): NR Monitoring: Clinical signs as described above |
|
| Outcomes | Accidental awareness The incidence of perioperative dreaming and awareness during anaesthesia was assessed in 649 participants 1 hr and 24 hrs after surgery, face‐to‐face or telephone interview using a questionnaire. No reason given why only in subsample and not clear whether based on 1‐ or 24‐hr interview. Only 1 participant in this study reported intraoperative awareness (laparoscopy, N₂O group). The attending anaesthesiologist noted that "this event was likely caused by a kinked IV line that interrupted the flow of propofol for a short period" |
|
| Identification |
Country: Canada Setting: 4 hospitals in Ontario Authors name: Ramiro J. Arellano Institution: Department of Anesthesia, University of Toronto Email: arellano@is.dal.ca Address: Department of Anesthesia,Queen Elizabeth II Health Sciences Center, Halifax Infirmary, 1796 Summer Street, Halifax, Nova Scotia, Canada, B3H 3A7 |
|
| Aim of study | This study in women undergoing ambulatory gynaecologic surgery compares outcomes in participants administered total intravenous anaesthesia with propofol versus the propofol plus N₂O. The primary outcome was the time to home readiness. Secondary outcomes included the incidence of postanaesthetic adverse events | |
| Notes | "Six hundred forty‐nine patients were questioned postoperatively about perioperative dreams." Numbers in relevant table (table 5 of paper) do not add up to 649 for either 1‐hr or 24‐hr column. We have used the 24‐hr column numbers. Numbers randomized: propofol: 497 TOP: 243 lap; propofol +N₂O 503 TOP: 247 lap Sponsorship source: Supported by a grant from Physicians Services Incorporated Foundation, Toronto, Ontario, Canada |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly allocated by computer‐generated random numbers in blocks of four to receive either total intravenous anesthesia with propofol (TIVA group) or propofol and N₂O (N₂O group). Stratification by hospital site and surgical procedure ensured that roughly equal numbers of subjects within both treatment groups were enrolled at each site" |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were allocated to either the TIVA or N₂O group when the anesthesiologist opened the sealed opaque envelopes at induction of anesthesia" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "anesthesiologists were not blinded to treatment allocation to ensure safe anesthetic care. Biased administration of the anesthetics and unblinding of the research assistants were prevented by the following: (1) pre‐enrollment training of anesthesiologists to standardize anesthetic administration; (2) random visits by the principal investigator to discuss the anesthetic protocol with the anesthesiologists; (3) ongoing review of the anesthetic study sheets by the principal investigator; (4) restricting the research assistants from access to the operating rooms or patients’ charts" Comment: No mention of participant blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Four research assistants blinded to treatment allocation enrolled patients into the study, obtained demographic and baseline information, and collected postoperative data" Comment: No mention of participant blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Six hundred forty‐nine patients were questioned post‐ operatively about perioperative dreams" Comment: Only 617/1490 participants had outcome data. No reason given |
| Selective reporting (reporting bias) | Low risk | Comment: All relevant outcomes described in Methods reported |
| Other bias | Low risk | Comment: None identified |
Crawford 1998.
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group |
|
| Participants |
Baseline Characteristics Nitrous oxide‐free – A Number randomized: NR Number analysed: 18 Age (mean): 6.7 (± 2.5) % male: NR Type of surgery: Minor orthopaedic, urological and general surgical Nitrous oxide‐based – A Number randomized: NR Number analysed: 17 Age (mean): 6.2 (± 2.2) % male: NR Type of surgery: Minor orthopaedic, urological and general surgical Included criteria: Age 3 ‐ 12 ys, outpatient surgery lasting approximately 1 hr Excluded criteria: Children excluded if they had a history of cardiorespiratory, gastrointestinal or CNS disease or if they requested premedication or inhalational induction |
|
| Interventions |
Intervention Characteristics Nitrous oxide‐free – A Name: Propofol Induction: Lignocaine 0.1 mg/kg vecuronium 0.15 mg/kg propofol 3 mg/kg Maintenance: Propofol infusion with oxygen 30% in air. Initial infusion rate of propofol was 300 mcg/kg. Titrated to keep HR and BP within 20% of baseline values. Maintained above min of 50 mcg/kg with bolus of 25% of induction dose and infusion increased by 25% if tachycardia or BP increased. Max infusion rate 300 mcg/kg. If signs of light anaesthesia persisted ‐ fentanyl 2 mcg/kg. Mean infusion rate 220 (± 37) and median 1 bolus (range 0 ‐ 2) Recovery: Neostigmine 50 mcg/kg and atropine 25 mcg/kg. All anaesthetic drugs discontinued. Other drugs used: NR Premedication: None Duration of surg/anaes (mins): 55 (± 21) /83(±33) Nitrous oxide‐based – A Name: Propofol plus N₂O Induction: Lignocaine 0.1 mg/kg vecuronium 0.15 mg/kg propofol 3 mg/kg Maintenance: Propofol infusion with N₂O 70% in oxygen. Initial infusion rate 00 mcg/kg. Titrated to keep HR and BP within 20% of baseline values. Maintained above min of 50 mcg/kg with bolus of 25% of induction dose and infusion increased by 25% if tachycardia or BP increased. Max infusion rate 300 mcg/kg. If signs of light anaesthesia persisted fentanyl 2 mcg/kg. Mean infusion rate 180 (± 39) and median 0 bolus Recovery: Neostigmine 50 mcg/kg and atropine 25 mcg/kg. All anaesthetic drugs discontinued. Other drugs used: NR Premedication: None Duration of surg/anaes (mins): 47 (± 35)/69 (± 40) Monitoring: Infusion rate of propofol was titrated to maintain heart rate and systolic arterial pressure to within 20% of baseline values |
|
| Outcomes | Accidental awareness Before discharge from PACU children asked if they had any recall of intraoperative events |
|
| Identification |
Country: Canada Setting: Hospital for Sick Children, University of Toronto Authors name: Jerrold Lerman Institution: Hospital for Sick Children, University of Toronto Email: NR Address: J. Lerman, Dept of Anaesthesia, Hospital for Sick Children, 555 University Ave, Toronto |
|
| Aim of study | The present study examined the effect of nitrous oxide on the recovery characteristics of propofol anaesthesia, and compared these data with those for halothane/nitrous oxide anaesthesia. | |
| Notes | A further group of 19 participants received halathone plus N₂O 60 children randomized; 6 were excluded when converted to regional anaesthesia after induction. Not reported from which group Sponsorship source: Study supported in part by a grant from ICI Pharma Ltd |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "assigned using a table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Comment: No details given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Anaesthetist giving anaesthetic not blinded. Clear criteria for adjusting the depth of anaesthetic. No mention of participant blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Postoperative data were gathered by an investigator who was unaware of the anaesthetic regimen administered" Comment: No mention of participant blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 10% excluded due to regional anaesthesia used ‐ but after induction |
| Selective reporting (reporting bias) | Low risk | Comment: All relevant outcomes described in Methods reported. |
| Other bias | Low risk | Comment: None identified |
Dedola 2008.
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group |
|
| Participants |
Baseline Characteristics Nitrous oxide‐based – A Number randomized: 24 Number analysed: 22 Age (mean): 43 (± 7) % male: 9 Type of surgery: Elective abdominal surgery (laparotomic gynaecological surgery, laparotomic correction of laparocele, pancreatoduodenectomy, hepatectomy) Nitrous oxide‐based – B Number randomized: 26 Number analysed: 25 Age (mean): 40 (± 6) % male: 8 Type of surgery: Elective abdominal surgery (laparotomic gynaecological surgery, laparotomic correction of laparocele, pancreatoduodenectomy, hepatectomy) Nitrous oxide‐free – A Number randomized: 26 Number analysed: 26 Age (mean): 39(± 8) % male: 8 Type of surgery: Elective abdominal surgery (laparotomic gynaecological surgery, laparotomic correction of laparocele, pancreatoduodenectomy, hepatectomy) Nitrous oxide‐free – B Number randomized: 27 Number analysed: 25 Age (mean): 44(± 9) % male: 24 Type of surgery: Elective abdominal surgery (laparotomic gynaecological surgery, laparotomic correction of laparocele, pancreatoduodenectomy, hepatectomy) Included criteria: aged 20 ‐ 50 years, ASA I, scheduled to undergo elective abdominal surgery (laparotomic gynaecological surgery, laparotomic correction of laparocele, pancreatoduodenectomy, hepatectomy) requiring at least a 10‐cm‐long skin incision Excluded criteria: Patients undergoing laparoscopic procedures, obese patients (BMI > 30 kg/m²), and patients with hypertension or a history of cardiac, pulmonary, or renal diseases, drug or alcohol abuse, or current use of any medications that might affect the cardiovascular system or block adrenergic responses to surgical incision |
|
| Interventions |
Intervention Characteristics Nitrous oxide‐based – A Name: N3 Desflurane plus N₂O plus remifentanil 3 ng/ml Induction: IV propofol (2 mg/kg) and TCI remifentanil 3 ng/ml for tracheal intubation, which was facilitated by cisatracurium besilate (0.2 mg/kg) Maintenance: desflurane plus 60% N₂O in oxygen TCI remifentanil 3 ng/ml. Up/down procedure starting at desflurane 4% Recovery: NR Other drugs used: NR Premedication: None Duration of anaesthesia (min): NR Nitrous oxide‐based – B Name: N1 Desflurane plus N₂O plus remifentanil 1 ng/ml Induction: IV propofol (2 mg/kg) and TCI remifentanil 3 ng/ml for tracheal intubation, which was facilitated by cisatracurium besilate (0.2 mg/kg). Maintenance: Desflurane plus 60% N₂O in oxygen TCI remifentanil 1 ng/ml. Up/down procedure starting at desflurane 4% Recovery: NR Other drugs used: NR Premedication: None Duration of anaesthesia (min): NR Nitrous oxide‐free – A Name: A3 Desflurane plus remifentanil 3 ng/ml Induction: IV propofol (2 mg/kg) and TCI remifentanil 3 ng/ml for tracheal intubation, which was facilitated by cisatracurium besilate (0.2 mg/kg) Maintenance: Desflurane plus 40% oxygen in air. TCI remifentanil 3 ng/ml. Up/down procedure starting at desflurane 4% Recovery: NR Other drugs used: NR Premedication: None Duration of anaesthesia (min): NR Nitrous oxide‐free – B Name: A1 Desflurane plus remifentanil 1 ng/ml Induction: IV propofol (2 mg/kg) and TCI remifentanil 3 ng/ml for tracheal intubation, which was facilitated by cisatracurium besilate (0.2 mg/kg) Maintenance: Desflurane plus 40% oxygen in air. TCI remifentanil 1 ng/ml. Up/down procedure starting at desflurane 5% Recovery: NR Other drugs used: NR Premedication: None Duration of anaesthesia (min): NR Monitoring: Desflurane monitored to designated MAC and remifentanil to designated TCI conc. Adjustments made for next participant. Similar to previous investigations, the MACBAR of desflurane was determined using an up/down sequential‐allocation technique. A participant’s response determined the concentration of desflurane given to the following participants in each group. Arbitrarily started in the nitrous‐free‐remi‐3 ng/ml group with an end‐tidal concentration of desflurane of 5% (0.83 MAC according to the age of the studied population). Other groups started with an end‐tidal concentration of desflurane of 4% (0.6 MAC according to the age of the studied population). If the response was positive (increase of either heart rate or MAP 15% above the mean of the values measured during the 2 mins before skin incision), the end‐tidal concentration given to the next participant was increased by 0.5% (0.083 MAC). If the response was negative, the end‐tidal concentration of desflurane given to the next participant was decreased by the same amount |
|
| Outcomes | Accidental awareness The day after surgery, all participants were interviewed to evaluate the presence of explicit recall of any intraoperative event. At the postoperative visit 24 hrs after surgery, no participant reported explicit recall of any intraoperative event |
|
| Identification |
Country: Italy Setting: University hospital Author's name: A. Albertin Institution: University of Milan and IRCCS Multimedica Email: albertinsimone@yahoo.it Address: A. Albertin, Department of Anesthesiology, IRCCS Multimedica, Via Milanese 300, 20099 Sesto S. Giovanni, Milan, Ital |
|
| Aim of study | To determine the effect of nitrous oxide on the desflurane requirement for blunting sympathetic response following surgical incision (MACBAR) when desflurane was combined with 2 different target‐controlled concentrations of remifentanil (1 and 3 ng/ml) | |
| Notes | Very similar design to Albertin 2005 Lower proportion of men in nitrous oxide‐free arm Numbers in Table 1 do not add up to column total "A total of 98 patients completed the study. Two patients in the A1 group, 1 patient in the N1 group and 2 patients in the N3 group were excluded from the investigation because of a significant reduction in MAP (<50 mmHg) before skin incision requiring administration of vasoconstrictors" Sponsorship source: This study was supported by the Vita‐Salute University of Milan |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a computer‐generated sequence of numbers, patients were randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Comment: No details given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The anesthesiologist recording cardiovascular parameters, BIS values and determining the positive ‐ negative response to surgical incision was blinded to patient grouping"
Quote: "double‐blind study" Comment: Does not state who is blinded as well as this anaesthetist. Anaesthetist delivering anaesthetic presumably not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: No details about participants but study described as double‐blind. Does not specify who asked about recall and whether they were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 5% lost, due to need for vasoactive response |
| Selective reporting (reporting bias) | Low risk | Comment: All relevant outcomes described in Methods reported |
| Other bias | Low risk | Comment: None identified |
ENIGMA.
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group |
|
| Participants |
Baseline Characteristics 19 participating centres of the ENIGMA trial group recruited participants between April 2003 and November 2004. Nitrous oxide‐free – A Number randomized: 1020 Number analysed: 997 Age (mean): 55.8 (± 17) % male: 54 Type of surgery: Various ‐ No cardiac/obstetric Other information: ASA III 23%, IV 1.0% Nitrous oxide‐based – A Number randomized: 1030 Number analysed: 1015 Age (mean): 54.6 (± 16) % male: 51 Type of surgery: Various ‐ No cardiac/obstetric Other information: ASA III 24%, IV 1.1% Included criteria: aged 18 yrs or older, scheduled to undergo general anaesthesia for surgery that included a skin incision and that was anticipated to exceed 2 hrs, and were expected to be in the hospital for at least 3 days after surgery Excluded criteria: Patients undergoing cardiac surgery, or thoracic surgery requiring one‐lung ventilation if the anaesthesiologist considered that N₂O was contraindicated (e.g. a history of postoperative emesis or if the anaesthesiologist wanted to use supplemental oxygen for colorectal surgery) |
|
| Interventions |
Intervention Characteristics Nitrous oxide‐free – A Induction: Standard anaesthetic care and monitoring. Choice of anaesthetic drugs and IV fluids at the discretion of the attending anaesthesiologist. Anaesthetic depth was adjusted according to clinical judgement and, if available, Bispectral Index monitoring. Combined regional and general anaesthetic techniques could be included. Anaesthesiologists were advised to avoid intraoperative hypothermia (35.5°C). Inspired oxygen concentration could be increased to 100% in both groups at the conclusion of anaesthetic administration. All other perioperative clinical care was conducted according to local practice. Maintenance: 80% oxygen with 20% nitrogen recommended (but range of FiO₂ 25 – 100% accepted according to clinical indication or anaesthetist preference Recovery: NR Other drugs used: NR Premedication: NR Duration of surgery: mean hrs 3.3 (± 2.0) Nitrous oxide‐based – A Induction: Standard anaesthetic care and monitoring. Choice of anaesthetic drugs and IV fluids at the discretion of the attending anaesthesiologist. Anaesthetic depth was adjusted according to clinical judgement and, if available, Bispectral Index monitoring. Combined regional and general anaesthetic techniques could be included. Anaesthesiologists were advised to avoid intraoperative hypothermia (35.5°C). Inspired oxygen concentration could be increased to 100% in both groups at the conclusion of anaesthetic administration. All other perioperative clinical care was conducted according to local practice. Maintenance: 70% N₂O with 30% oxygen, after induction of anaesthesia, and until completion of surgery. If haemoglobin oxygen saturation was inadequate, any airway and ventilatory manoeuvres deemed necessary, including an increase in inspired oxygen concentration, could be used Recovery: NR Other drugs used: NR Premedication: NR Duration of surgery: mean hr 3.3 (± 2.0) Monitoring: Anaesthetic depth was adjusted according to clinical judgement and, if available, Bispectral Index monitoring. Combined regional and general anaesthetic techniques could be included. Bispectral monitoring used In nitrous oxide‐free – A = 26%, nitrous oxide‐based – A = 16% |
|
| Outcomes | Accidental awareness Awareness: Postoperative recollection of intraoperative events, identified using a structured questionnaire, at 24 hrs and 30 days after surgery. Used Brice 1970 protocol. |
|
| Identification |
Country: Multicentre international study. 19 trial centres Setting: Australia, New Zealand, Hong Kong, Singapore, Saudi, UK Authors name: Paul S Myles Institution: Alfred Hospital, Melbourne Email: p.myles@alfred.org.au Address: Department of Anaesthesia and Perioperative Medicine, Alfred Hospital, Commercial Road, Melbourne, Victoria, 3004, Australia |
|
| Aim of study | To evaluate whether avoidance of nitrous oxide in the gas mixture for anaesthesia, an intervention that avoids potential nitrous oxide toxicity and in addition allows an increase in the inspired oxygen fraction, could decrease the duration of hospital stay after surgery and reduce postoperative complications, compared with a nitrous oxide–based anaesthetic regimen, in adults presenting for major surgery | |
| Notes | Different FiO₂ in each gp (nitrous‐free 80% O₂, nitrous gp 30% O₂) This study differs from others because the type of anaesthesia used was determined by the anaesthetist Details given in Table 2. Differences include: (N₂O‐free, N₂O respectively) Bispectral Index monitoring, n (%) 259 (26) vs 160 (16), P = 0.001 Propofol maintenance anaesthesia, n (%) 191 (19) vs 132 (13), P = 0.001 End‐tidal volatile concentration, median (IQR) MAC equivalents, 0.87 (0.61 – 1.06) vs 0.67 (0.52 – 0.83), P = 0.001 Sponsorship source: Supported by grants from the Australian National Health and Medical Research Council, Australian and New Zealand College of Anaesthetists and the Health and Health Services Research Fund (project 02030051), Hong Kong, People’s Republic of China and a direct grant for research from the Chinese University of Hong Kong (project #2041315) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using a computer generated code, accessed via an automated telephone voice recognition service" |
| Allocation concealment (selection bias) | Low risk | Quote: "using a computer generated code, accessed via an automated telephone voice recognition service" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Attending anesthesiologists were required to have knowledge of group identity for the safe administration of anaesthesia, but group identity was concealed from the surgeon using drapes or cardboard to screen the anaesthesia machine. At the end of the procedure, the intra‐operative case report form and documentation of group identity were faxed to the data management center and then placed in an opaque envelope by the anaesthesiologist. The envelope was then sealed to ensure blinding of research staff conducting the postoperative follow‐ups. The trial data management center checked each completed record for missing or illogical items within 24 – 48 h, with corrections verified via e‐mail contact to the site coordinator and local study investigator. The anaesthesia record was not concealed or removed from the patient’s medical record, because it is our experience that the anaesthetic record is not perused by surgical staff. The patient and surgical staff were not informed of the patient’s group identity. All research staff, including those responsible for postoperative data collection and outcome assessment, were precluded by protocol from accessing the anaesthetic record and so were blinded to group identity Patients and observers were blind to group identity" Comment: Anaesthetist not blinded and able to chose different anaesthetic agents and monitoring. Large difference in % with bispectral monitoring in 2 groups Participants blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Attending anaesthesiologists were required to have knowledge of group identity for the safe administration of anesthesia, but group identity was concealed from the surgeon using drapes or cardboard to screen the anesthesia machine. At the end of the procedure, the intra‐ operative case report form and documentation of group identity were faxed to the data management center and then placed in an opaque envelope by the anaesthesiologist. The envelope was then sealed to ensure blinding of research staff conducting the postoperative follow‐ups. The trial data management center checked each completed record for missing or illogical items within 24 – 48 h, with corrections verified via e‐mail contact to the site coordinator and local study investigator. The anesthesia record was not concealed or removed from the patient’s medical record, because it is our experience that the anaesthetic record is not perused by surgical staff. The patient and surgical staff were not informed of the patient’s group identity. All research staff, including those responsible for postoperative data collection and out‐ come assessment, were precluded by protocol from accessing the anaesthetic record and so were blinded to group identity" Comment: Participants and research staff assessing outcomes were blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "intention‐to‐ treat population for all primary and secondary analyses" Comment: 2.3% loss vs 1.5% loss. Both low |
| Selective reporting (reporting bias) | Low risk | Comment: All relevant outcomes specified in Methods were reported |
| Other bias | Low risk | Comment: None identified |
Girardi 1994.
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group |
|
| Participants |
Baseline Characteristics Nitrous oxide‐free ‐ A Number randomized: 26 Number analysed: 26 Age (mean): 40 ‐ 54 % male: 0 Type of surgery: Varicose veins Nitrous oxide‐based ‐ A Number randomized: 25 Number analysed: 25 Age (mean): 40 ‐ 54 % male: 0 Type of surgery: Varicose veins Included criteria: Women, ASA class I, surgery for venous disease of the lower limbs Excluded criteria: Previous neurological or psychiatric disease |
|
| Interventions |
Intervention Characteristics Nitrous oxide‐free ‐ A Name: Thiopental + air + isoflurane 5% ‐ 2% Induction: Thipoental 3.5 mg/kg. Until intubation, isoflurane 5% in air and oxygen (40% O₂) Maintenance: Isoflurane 2% in air (40% O₂) Recovery: NR Other drugs used: Atracurium 0.6 mgkg‐1 Premedication: 45 mins before induction: Atropine 0.007 mg/kg, pethidine 1 mg/kg Duration of anaesthesia (min): ‐ 86.2 (± 12.3) Nitrous oxide‐based ‐ A Name: Thiopental + N₂O 60% + Isoflurane 3% ‐ 1.2% Induction: Thipoental 3.5 mg/kg. Until intubation isoflurane 3% in 60% N₂O and 40% oxygen Maintenance: Isoflurane 1.2% in 60% N₂O and 40% oxygen Recovery: NR Other drugs used: Atracurium 0.6 mg/kg Premedication: 45 mins before induction: Atropine 0.007 mg/kg, pethidine 1 mg/kg Duration of anaesthesia (min): 89.5 (± 10.3) Monitoring: Depth of anaesthesia assessed using Evans score for clinical signs depth, plus ECG monitoring (Compressed Spectral Array. Brain Surveyor) |
|
| Outcomes | Accidental awareness Collected 60 mins and 24 hrs after surgery, through a structured questionnaire. Participants were asked: The last thing they remembered before going to sleep The first thing they remembered when they woke up Does the patient believe they remembered anything about the operation, if yes, can it be related to actual events? Did the patient experience pain during the operation? Did the patient dream during the operation? If yes, what? Emotional tone of the dream? Final judgement on the experience |
|
| Identification |
Country: Italy Setting: University clinic Authors name: G Della Rocca Institution: Instituto do Anestesiologia e Riaanimazione, Universita Degli di Firenze Email: Not reported Address: Istituto do anestesiologia e Rianimazione, Policlinico di Careggi, Viale Morgangni, 85 50123 Firenze |
|
| Aim of study | To control the depth, the quality of recovery of total inhalation isoflurane anaesthesia with or without nitrous oxide | |
| Notes | Sponsorship source: No details given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up reported |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes specified in Methods reported |
| Other bias | Low risk | None identified |
Handa 2010.
| Methods |
Study design: Randomized control trial Study grouping: Parallel group |
|
| Participants |
Baseline Characteristics 118 participants (men n = 38, women n = 80) Nitrous oxide‐free ‐ A Number randomized: 58 Number analysed: 58 Age (mean): NR % male: 18/58 = 31.0%* Type of surgery: Saggital split ramus osteotomy Nitrous oxide‐based ‐ A Number randomized: 60 Number analysed: 60 Age (mean): NR % male: 20/60 = 33.3%* Type of surgery: Saggital split ramus osteotomy Included criteria: Patients undergoing saggital split ramus osteotomy between August 2008 ‐ April 2009 Excluded criteria: History of alcoholism, substance misuse, psychiatric disorders, disorders which may affect metabolism of anaesthetic drugs |
|
| Interventions |
Intervention Characteristics Nitrous oxide‐free ‐ A Name: Air ‐ Oxygen ‐ Propofol group Induction: Fentanyl 2 mcg/kg, vecuronium/rocuronium, TCI propofol 3.5 mcg/ml plasma target concentration Maintenance: TCI propofol 3.0 ‐ 4.0 mcg/ml plasma target concentration. Additional fentanyl bolus as required based on haemodynamics, surgical stimulation, FiO₂ 0.33, Local anaesthetic infiltration by surgeons (lignocaine/adrenaline), Atropine/neostigmine neuro‐muscular blockade reversal Recovery: PCA fentanyl with droperidol Other drugs used: Muscle relaxant = vecuronium/rocuronium (dose unspecified) for all participants Premedication: None Duration of anaesthesia (mins) = NR Nitrous oxide‐based ‐ A Name: Nitrous oxide‐Oxygen‐Propofol group Induction: Fentanyl 2 mcg/kg, vecuronium/rocuronium, TCI propofol 3.5 mcg/ml plasma target concentration Maintenance: TCI propofol 3.0 ‐ 4.0 mcg/ml plasma target concentration, additional fentanyl bolus as required based on haemodynamics, surgical stimulation, FiO₂ 0.33, local anaesthetic infiltration by surgeons (lignocaine/adrenaline), atropine/neostigmine neuro‐muscular blockade reversal Recovery: PCA fentanyl with droperidol Other drugs used: Muscle relaxant = vecuronium/rocuronium (dose unspecified) for all participants Premedication: None Duration of anaesthesia (mins) = NR Monitoring: Haemodynamics |
|
| Outcomes | Accidental awareness Details: Participants interviewed once able to obey commands and answer questions verbally in recovery room with regards to presence of dreams during anaesthesia. Furthermore, participants requested to fill in a paper questionnaire 24 hrs post‐op Questionnaire asked ‘Do you have memories of the surgery’ and ‘Did you dream something’ If answered ‘yes’ to awareness participant asked to state whether they: 1. Heard something 2. Felt pain 3. Felt vibration 4. Felt paralysed 5. Other No participants reported awareness |
|
| Identification |
Country: Japan Setting: Operating theatre in single centre Authors name: Handa Institution: Tokyo Dental College Email: Not stated Address: Not stated |
|
| Aim of study | We investigated the frequency and the content of dreams during propofol anaesthesia in patients undergoing mandibular sagittal split ramus osteotomy (SSRO) | |
| Notes | *Error in table 2 stating number of men/women in this subdivision – i.e. Table 2 states 40 men to 18 women in this branch of study group which contradicts the total number of men/women recruited and also what is stated in the main body of text Sponsorship source: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: No method stated for randomization |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: No information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No attrition |
| Selective reporting (reporting bias) | Low risk | Comment: Main aim of study to ascertain incidence and nature of dreaming during anaesthesia, however awareness stated as the other measured outcome in study methodology |
| Other bias | Low risk | Comment: None identified |
Handa Tsutsui 2007.
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group |
|
| Participants |
Baseline Characteristics Nitrous oxide‐free – A Number randomized: 23 Number analysed: 23 Age (mean): 35 (± 3.3) % male: 0 Type of surgery: Transvaginal US guided oocyte retrieval for in‐vitro fertilization Nitrous oxide‐based – A Number randomized: 24 Number analysed: 24 Age (mean): 36 (± 7.8) % male: 0 Type of surgery: Transvaginal US guided oocyte retrieval for in‐vitro fertilization Included criteria: Women ASA class I ‐ II, unpremedicated and undergoing scheduled transvaginal ultrasound‐guided oocyte retrieval were recruited Excluded criteria: NR |
|
| Interventions |
Intervention Characteristics Nitrous oxide‐free – A Name: Propofol + air Induction: Propofol was started at target concentration using a Diprifusor™ anaesthesia pump Maintenance: Oxygen‐enriched air (FiO₂ 0.5). Participants experiencing movement immediately had their propofol plasma‐site concentration increased to 6 ‐ 10 mcg/ml. Target concentration of propofol was started at 4 mcg/ml for the first participant. Subsequent participants received target concentration 0.5 mcg/ml higher or lower using up/down sequential allocation. If the response of the previous woman was movement, the target concentration for the next participant was increased by 0.5 mcg/ml. If the response was no movement, the next target concentration was reduced Recovery: Recovery time (mins) 11 (± 6.2) Other drugs used: NR Premedication: To reduce vascular pain, 2% lidocaine 1 mg/kg was administered IV before propofol induction Duration of anaesthesia (mins): NR Nitrous oxide‐based – A Name: Propofol + nitrous oxide Induction: Propofol was started at target concentration using a Diprifusor™ anaesthesia pump Maintenance: After induction of anaesthesia, mask ventilation was maintained with 50% N₂O and 50% oxygen. Participants experiencing movement immediately had their propofol plasma‐site concentration increased to 6 ‐ 10 mcg/ml.Target concentration of propofol was started at 4 mcg/ml for the first participant. Subsequent participants received target concentration 0.5 mcg/ml higher or lower using up/down sequential allocation. If the response of the previous woman was movement, the target concentration for the next participant was increased by 0.5 mcg/ml. If the response was no movement, the next target concentration was reduced Recovery: NR. Recovery time (mins)12 (± 4.7) Other drugs used: NR Premedication: To reduce vascular pain, 2% lidocaine 1 mg/kg was administered iv before propofol induction Duration of anaesthesia (min): NR Monitoring: as described above, up/down dosing method used. Depth of anaesthesia in both groups should be equivalent |
|
| Outcomes | Accidental awareness "All women were interviewed about memory recall and post‐procedure pain in the recovery room." "Direct questioning in the recovery room yielded no complaint of recall of the procedure or anaesthesia" |
|
| Identification |
Country: Japan Setting: NR Authors name: F. Handa‐Tsutsui Institution: Department of Anesthesiology, Saitama Medical Center, Kamoda Email: PXN01110@nifty.com Address: Dept. of Cardiac Surgery, Saitama Medical School, Moroyama, Saitama, 350‐0495 Japan |
|
| Aim of study | Determine the target concentration of propofol required to prevent movement in 50% (Cp50) and 95% (Cp95) of women during oocyte retrieval, and investigated whether supplemental N₂O modified the Cp50 and Cp95 | |
| Notes | Sponsorship source: Saitama MedicalCenter. Neither author has corporate support or any relationship with commercial companies | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned into two groups using random table:" Comment: No further details |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomly assigned into two groups using random table:" Comment: No further details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Presume anaesthetists not blinded. No mention of participant blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Not clear if same investigators asked about recall. No mention of participant blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No attrition |
| Selective reporting (reporting bias) | Low risk | Comment: All relevant outcomes described in Methods reported |
| Other bias | Low risk | Comment: None identified |
Heath 1996.
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group |
|
| Participants |
Baseline Characteristics Nitrous oxide‐free – A Number randomized: NR Number analysed: NR Age (mean): NR % male: 0 Type of surgery: Routine gynaecological surgery Nitrous oxide‐based – A Number randomized: NR Number analysed: NR Age (mean): NR % male: 0 Type of surgery: Routine gynaecological surgery Included criteria: Women, ASA I & II, undergoing routine gynaecological surgery Excluded criteria: NR |
|
| Interventions |
Intervention Characteristics Nitrous oxide‐free – A Name: Propofol + air Induction: Propofol 10 mg/ml + alfentanil 30 mcg/ml in same 50 ml syringe. Initial bolus of 0.2 ml/kg at 800 ml/hrIf required for intubation or during surgery ‐ vecuronium 0.1 mg/kg initial bolus Maintenance: Maintained using same mixture at initial rate of 1 ml/kg/hr. Ventilated with oxygen and air FiO₂ 30%. Experienced anaesthetists adjusted as required to maintain depth. Volume of propofol/alfentanil = 49.6 ml. Further boluses of 0.025 mg/kg vecuronium as required Recovery: NR Other drugs used: Intraoperative analgesia by lumbar or caudal epidural injection of 20 ml 0.25% bupivacaine. When epidural analgesia was not indicated participants received IV morphine during the operation. All participants received diclofenac 100 mg per rectum, unless contraindicated, after induction of an anaesthesia Premedication: Temazepam 20 mg orally Duration of anaesthesia (mins): NR Nitrous oxide‐based – A Name: Propofol + N₂O Induction: Propofol 10 mg/ml + alfentanil 30 mcg/ml in same 50 ml syringe. Initial bolus of 0.2 ml/kg at 800 ml/hrIf required for intubation or during surgery ‐ vecuronium 0.1 mg/kg initial bolus Maintenance: Maintained using same mixture at initial rate of 1 ml/kg/hr. Ventilated with oxygen and nitrous oxide, FiO₂ 67%. Experienced anaesthetists adjusted as required to maintain depth.Volume of propofol/alfentanil = 39.3 ml. Further boluses of 0.025 mg/kg vecuronium as required Recovery: NR Other drugs used: Intraoperative analgesia by lumbar or caudal epidural injection of 20 ml 0.25% bupivacaine. When epidural analgesia was not indicated participants received IV morphine during the operation. All participants received diclofenac 100 mg per rectum, unless contraindicated, after induction of an anaesthesia Premedication: Temazepam 20 mg orally Duration of anaesthesia (mins): NR Monitoring: Experienced anaesthetists adjusted as required to maintain depth. No details given of what was monitored Intraoperative stimulation: no additional measures |
|
| Outcomes | Accidental awareness Participants asked about any evidence that awareness occurred during anaesthesia at 4 hrs and 24 hrs post‐op. No reports of awareness |
|
| Identification |
Country: UK Setting: Military hospital Authors name: K.J. Heath Institution: Cambridge Military Hospital Email: NR Address: Anaesthetic Department, Addenbrookes Hospital, Cambridge, UK |
|
| Aim of study | To calculate the cost of an IV anaesthetic technique using a mixture of propofol and alfentanil when nitrous oxide and oxygen were used instead of oxygen‐enriched air and to assess the postoperative complications of the 2 different techniques | |
| Notes | Numbers in individual groups not given (total = 101) Sponsorship source: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients allocated randomly into two groups" No further details |
| Allocation concealment (selection bias) | Unclear risk | Comment: No details given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Presume anaesthetist not blinded and the depth of anaesthetic adjusted by anaesthetist. No mention of participant blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: Participants were visited by an anaesthetist who was unaware of the anaesthetic technique used. Not clear whether participants blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: No details given and no numbers in each group |
| Selective reporting (reporting bias) | Low risk | Comment: All relevant outcomes described in Methods were reported |
| Other bias | Low risk | Comment: None identified |
Lindekaer 1995.
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group |
|
| Participants |
Baseline Characteristics Nitrous oxide‐free – A Number randomized: 21 Number analysed: 21 Age (mean): 44 (± 12.2) % male: 90.5 Type of surgery: Inguinal herniotomy Nitrous oxide‐based – A Number randomized: 21 Number analysed: 21 Age (mean): 47 (± 10.6) % male: 95.2 Type of surgery: Inguinal herniotomy Included criteria: aged 18 ‐ 60 years, ASA 1 or 2, scheduled for day‐case inguinal herniotomy Excluded criteria: NR |
|
| Interventions |
Intervention Characteristics Nitrous oxide‐free – A Name: Propofol + air Induction: Alfentanil 15 mcg/kg and propofol 2 mg/kg IV followed by alfentanil 45 mcg/kg/hr and propofol 10 mg/kg/hr. Tracheal intubation was facilitated by vecuronium 85 mcg/kg Maintenance: Separate infusions of alfentanil 45 mcg/kg/hr and propofol 10 mg/kg/hr participant's lungs were manually ventilated with air/O₂. FiO₂ 30%. Propofol infusion was continued for 5 mins then reduced to a minimum rate judged clinically on the signs of ‘light’ anaesthesia: movement, lacrimation, sweating, arrhythmia,tachycardia, increasing arterial blood pressure compared to baseline measurements. If necessary, boluses of propofol (20 mg) could be administered Recovery: Alfentanil and propofol infusions were stopped at fascia and skin closure respectively. After skin closure the participant's lungs were ventilated with oxygen only and muscle relaxation was reversed with atropine and neostigmine Other drugs used: Premedication: Diazepam 0.15 mg/kg by mouth and naproxen 1 g per rectum 30 mins before operation Duration of infusion: Min 68 (± 19.1), mean maintenance propofol 0.088 mg/kg/min Nitrous oxide‐based – A Name: Propofol + N₂O Induction: Alfentanil 15 mcg/kg and propofol 2 mg/kg/hr IV followed by alfentanil 45 mcg/kg/hr and propofol 10 mg/kg/hr. Tracheal intubation was facilitated by vecuronium 85 mcg/kg Maintenance: separate infusions of alfentanil 45 mcg/kg/hr and propofol 10 mg/kg/hr participant's lungs were manually ventilated with N₂O/O₂ with a FiO₂ of 0.30. Propofol infusion was continued for 5 mins then reduced to a minimum rate judged clinically on the signs of ‘light’ anaesthesia: movement, lacrimation, sweating, arrhythmia, tachycardia, increasing arterial blood pressure compared to baseline measurements. If necessary, boluses of propofol (20 mg) could be administered Recovery: The alfentanil and propofol infusions were stopped at fascia and skin closure respectively. After skin closure the participant's lungs were ventilated with oxygen only and muscle relaxation was reversed with atropine and neostigmine Other drugs used: Premedication: Diazepam 0.15 mg/kg‐1 by mouth and naproxen 1g per rectum 30 mins before operation Duration of infusion: Min 66 (± 19.3) mean maintenance propofol 0.084 mg/kg/min Monitoring: The propofol infusion was continued for 5 mins at this rate; it was then reduced to a minimum rate judged clinically on the signs of ‘light’ anaesthesia: movement, lacrimation, sweating, arrhythmia, tachycardia, increasing arterial blood pressure compared to baseline measurements. If necessary, boluses of propofol (20 mg) could be administered and these were recorded |
|
| Outcomes | Accidental awareness "Before discharge from hospital the anaesthetist questioned the patients about possible awareness during the operation or any dreams." "Two hours after propofol all the patients felt well; none had any unpleasant recollection of events during anaesthesia but one patient reported pleasant dreams" |
|
| Identification |
Country: Denmark Setting: University hospital Authors name: AL Lindekaer Institution: University of Copenhagen Email: NR Address: AL Lindekrer, Virumvej 104 B, 2830 Virum, Denmark |
|
| Aim of study | To evaluate the influence of N₂O on the infusion rate of propofol, allowing anaesthetic depth, as evaluated clinically, to determine the infusion rate | |
| Notes | Sponsorship source: "We thank AGA for their support of the study" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "by random allocation" Comment: No further details |
| Allocation concealment (selection bias) | Unclear risk | Quote: "by random allocation" Comment: No further details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "nurse, who was not involved in adjusting the propofol infusion rate, adjusted the flowmeters for both groups to give an inspired oxygen fraction (FiO₂) of 0.30." Quote: "double blind design" Comment: States double‐blind but does not say who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double blind design" Quote: "anaesthetist questioned the patients about possible awareness during the operation or any dreams" Comment: Described as double‐bind but no details of who was blinded. Anaesthetist, who presumably was not blinded, asked about awareness |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No apparent attrition |
| Selective reporting (reporting bias) | Low risk | Comment: All relevant outcomes described in Methods reported |
| Other bias | Low risk | Comment: None identified |
Ngan Kee 2002.
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group |
|
| Participants |
Baseline Characteristics Nitrous oxide‐free – A Number randomized: 20 Number analysed: 20 Age (mean): 35 (range 27 ‐ 43) % male: 0 Type of surgery: Caesarean section Nitrous oxide‐based – A Number randomized: 20 Number analysed: 20 Age (mean): 34 (range 26 ‐ 41) % male: 0 Type of surgery: Caesarean section Nitrous oxide‐based – B Number randomized: 20 Number analysed: 20 Age (mean): 34 (range 27 ‐ 41) % male: 0 Type of surgery: Caesarean section Included criteria: ASA I and II women with term singleton pregnancies having elective Caesarean section under GA Excluded criteria: Pre‐existing or pregnancy‐induced hypertension, cardiovascular or cerebrovascular disease or known foetal abnormalities |
|
| Interventions |
Intervention Characteristics Nitrous oxide‐free – A Name: Sevoflurane Induction: Pre‐oxygenation rapid sequence induction using thiopental 4 mg/kg and succinylcholine 1.5 mg/kg atracurium as required for further muscle relaxation as indicated by results of peripheral nerve stimulation Maintenance: Lungs ventilated to maintain end‐tidal CO₂ concentration of 4.3 kPa. FiO₂ 1.0 with end‐tidal sevoflurane 2.0%. Circle circuit with a fresh gas flow of 6 l/min was used and for all participants sevoflurane vaporiser was originally set to 6% for the first 60 secs and then adjusted to the required end‐tidal concentration Recovery: Neostigmine and atropine Other drugs used: Ranitidine 150 mg night before surgery 30 ml 0.3 M sodium citrate on arrival at theatre Premedication: NR Duration of anaesthetic (mins): NR Nitrous oxide‐based – A Name: Sevoflurane + Fi N₂O 0.5 Induction: Pre‐oxygenation rapid sequence induction using thiopental 4 mg/kg and succinylcholine 1.5 mg/kg atracurium as required for further muscle relaxation as indicated by results of peripheral nerve stimulation Maintenance: Lungs ventilated to maintain end‐tidal CO₂ concentration of 4.3 kPa. Inspired fractions chosen to give approximately equivalent MAC values in all groups. FiO₂ 0.5 + FiN₂O 0.5 with end‐tidal sevoflurane 1.0%. Circle circuit with a fresh gas flow of 6 l/min was used and for all participants sevoflurane vaporiser was originally set to 6% for the first 60 secs and then adjusted to the required end‐tidal concentration Recovery: Neostigmine and atropine Other drugs used: Ranitidine 150 mg night before surgery 30 ml 0.3 M sodium citrate on arrival at theatre Premedication: NR Duration of anaesthetic (mins): NR Nitrous oxide‐based – B Name: Sevoflurane +Fi N₂O 0.7 Induction: Pre‐oxygenation rapid sequence induction using thiopental 4 mg/kg and succinylcholine 1.5 mg/kg atracurium as required for further muscle relaxation as indicated by results of peripheral nerve stimulation Maintenance: Lungs ventilated to maintain end‐tidal CO₂ conc of 4.3 kPa. FiO₂ 0.3 Fi N₂O 0.7 with end‐tidal sevoflurane 0.6%. Circle circuit with a fresh gas flow of 6 l/min was used and for all participants sevoflurane vaporiser was originally set to 6% for the first 60 secs and then adjusted to the required end‐tidal concentration Recovery: Neostigmine and atropine Other drugs used: Ranitidine 150 mg night before surgery 30 ml 0.3 M sodium citrate on arrival at theatre Premedication: NR Duration of anaesthetic (mins): NR Monitoring: Anaesthetics in different groups aimed to give same overall MAC. Anaesthetic concentration adjusted to maintain allocated end‐tidal concentration. No mention of adjusting anaesthetic concentration according to participant's response or haemodynamic variables |
|
| Outcomes | Accidental awareness "Each patients was visited by a research nurse on the first day after the operation who asked if the patient was able to recall any intra‐operative events or remembered any dreams during the operation" "No patient reported recall of intraoperative events" |
|
| Identification |
Country: Hong Kong Setting: University Hospital Authors name: WD Ngan Kee Institution: Prince of Wales Hospital, Shatin, Hong Kong Email: NR Address: NR |
|
| Aim of study | To compare the effect of FiO₂ of 0.3, 0.5 and 1.0 on umbilical cord blood oxygen content in participants having elective Caesarean section under anaesthesia | |
| Notes | Obstetric patients. Anaesthetics in different groups aimed to give same overall MAC Sponsorship source: Direct Grant for research from the Chinese University of Hong Kong |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were then randomly allocated to one of three groups by drawing of sequentially numbered sealed envelopes that each contained a computer‐generated randomization code" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomly allocated to one of three groups by drawing of sequentially numbered sealed envelopes that each contained a computer‐generated randomization code." Comment: Probably was concealed allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Patients were not informed of the group allocation. One anaesthetist was responsible for controlling the delivery of the anaesthetic. Separate investigators were responsible for the blood sampling and analysis. To mask these investigators and the surgeon to the treatment, the anaesthesia machine was turned away so the monitors were not visible to them" Comment: Anaesthetists not blinded but anaesthetic inspired concentrations set |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Each patient was visited on the first day after operation by a research nurse, who asked the patient if she was able to recall any intraoperative events" Comment: Not clear whether these research nurses were blinded. Participants were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No reported attrition |
| Selective reporting (reporting bias) | Low risk | Comment: All relevant outcomes described in Methods were reported |
| Other bias | Low risk | Comment: None identified |
Singh 2011.
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group |
|
| Participants |
Baseline Characteristics Nitrous oxide‐free – A Number randomized: 60 Number analysed: 46 Age (mean): 36.6 (± 9.6) % male: 58.7 Type of surgery: elective supratentorial tumour surgery Other information: mean tumour vol cm³ 164.2 (± 280.7) Nitrous oxide‐based – A Number randomized: 56 Number analysed: 41 Age (mean): 36.1 (± 11.6) % male: 65.9 Type of surgery: elective supratentorial tumour surgery Other information: mean tumour vol cm³ 159.7 (± 278.3) Included criteria: patients between 18 and 60 years of age, either gender, ASA I and II, scheduled for elective supratentorial tumour surgery, with anticipated duration of anaesthesia more than 4 hours Excluded criteria: history of smoking, patients with history of megaloblastic anaemia, those requiring postoperative mechanical ventilation, patients receiving vitamin B12/folic acid supplementation, history of exposure to general anaesthesia in the last month, history of motion sickness/postoperative emesis, evidence of pneumothorax/pneumocephalus, and bleeding disorders |
|
| Interventions |
Intervention Characteristics Nitrous oxide‐free – A Name: Isoflurane + air +O₂ Induction: fentanyl 2 mcg/kg and thiopentone 4 to 6 mg/kg and tracheal intubation facilitated with rocuronium 1 mg/kg. Additional dose of thiopentone 1 ‐ 2 mg/kg was given before laryngoscopy and intubation to prevent the pressor response Maintenance: Isoflurane (end‐tidal concentration: 1.2%). Oxygen and air (FiO₂) Intermittent doses of fentanyl (1 mcg/kg) and vecuronium (0.01 mg/kg) repeated as required. Use of other drugs and IV fluids was at the discretion of the attending anaesthesiologist. Anaesthetic depth was adjusted according to clinical judgement Recovery: At the end of the surgery, anaesthetic agent (isoflurane) was discontinued at the beginning of skin closure and the medical air switched off at the time of dressing of the surgical site. Residual neuromuscular block was reversed with neostigmine 0.05 mg/kg and glycopyrrolate 0.01 mg/kg, and trachea extubated after neurologic assessment Other drugs used: Mannitol (1 gm/kg) was given to all participants over a period of 20 to 30 mins, starting at the time of skin incision Premedication: Glycopyrrolate 0.2 mg intramuscularly was given 1 hour before the scheduled surgery Duration of anaesthesia (mins): NR Nitrous oxide‐based – A Name: Isoflurane + N₂O + O₂ Induction: Fentanyl 2 mcg/kg and thiopentone 4 to 6 mg/kg and tracheal intubation facilitated with rocuronium 1 mg/kg. Additional dose of thiopentone 1 to 2 mg/kg was given before laryngoscopy and intubation to prevent the pressor response Maintenance: Isoflurane (end‐tidal concentration: 0.7%) oxygen and N₂O (FiO₂). Intermittent doses of fentanyl (1 mcg/kg) and vecuronium (0.01 mg/kg) repeated as required. Use of other drugs and IV fluids was at the discretion of the attending anaesthesiologist. Anaesthetic depth was adjusted according to clinical judgement Recovery: At the end of the surgery, anaesthetic agent (isoflurane) was discontinued at the beginning of skin closure and the N₂O switched off at the time of dressing of the surgical site. Residual neuromuscular block was reversed with neostigmine 0.05 mg/kg and glycopyrrolate 0.01 mg/kg, and trachea extubated after neurologic assessment Other drugs used: Mannitol (1 gm/kg) was given to all participants over a period of 20 to 30 mins, starting at the time of skin incision Premedication: Glycopyrrolate 0.2 mg intramuscularly was given 1 hour before the scheduled surgery Duration of anaesthesia (min): NR Monitoring: Anaesthetic gases kept at set flow rate. Intermittent doses of fentanyl (1 mcg/kg) and vecuronium (0.01 mg/ kg) repeated as required. Anaesthetic depth was adjusted according to clinical judgement |
|
| Outcomes | Accidental awareness Awareness: Postoperative recollection of intraoperative events identified using a structured questionnaire at 24 hrs after surgery. a) What was the last thing you remembered happening before you went to sleep? b) What is the last thing you remembered happening on awakening? c) Did you dream or have any other experience whilst you were asleep? d) What was the worst thing about your operation? e) What was the next worst? No participant in either group reported awareness of intraoperative events |
|
| Identification |
Country: India Setting: University hospital Authors name: Dr. Hemanshu Prabhakar Institution: All India Institute of Medical Sciences Email: prabhakarhemanshu@rediffmail.com Address: Department of Neuroanesthesiology, Neurosciences Center, 7th Floor, All India Institute of Medical Sciences,New Delhi ‐ 110 029, India |
|
| Aim of study | To evaluate if avoidance of nitrous oxide could decrease the duration of Intensive Care Unit (ICU) and hospital stay after elective surgery for supratentorial tumours | |
| Notes | 29 participants could not be tracheally extubated at the end of surgery (15 in nitrous oxide‐based group and 14 in nitrous oxide‐free group) Sponsorship source: Source of support ‐ nil; Conflict of interest ‐ none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly divided into two groups by a computer‐generated randomization chart" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomly divided into two groups by a computer‐generated randomization chart" Comment: No details of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Attending anesthesiologist was aware of the group identity (for safe administration of anesthesia), but it was concealed from the surgeons (using drapes to cover the anesthesia machine)" Quote: "double blinded" Comment: Described as double‐blind. No explicit mention of participant blinding but unlikely to know. Anaesthetist was unblinded and depth of anaesthesia at discretion |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double blinded" Comment: Assessors blinded Quote: "Staff conducting the postoperative follow‐ups (i.e., those responsible for postoperative data collection and outcome assessment) was blinded to the group identity" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Of the 116 patients, 29 patients could not be tracheally extubated at the end of surgery (15 patients in group I and 14 in group II), and so the data of these patients were excluded from final analysis" Comment: 23.3% in N₂O‐free group and 26.8% in N₂O‐based group excluded |
| Selective reporting (reporting bias) | Low risk | Comment: All relevant outcomes described in Methods were reported |
| Other bias | Low risk | Comment: None identified |
Sukhani 1994.
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group |
|
| Participants |
Baseline Characteristics Nitrous oxide‐free – A Number randomized: 36 Number analysed: 36 Age (mean): 30.1 (± 4.9) % male: 0 Type of surgery: gynaecological laparoscopy Nitrous oxide‐based – A Number randomized: 34 Number analysed: 34 Age (mean): 34.6 (± 5.6) % male: 0 Type of surgery: gynaecological laparoscopy Included criteria: Adult non‐pregnant women aged 19 ‐ 40 yrs scheduled for ambulatory gynaecological laparoscopy Excluded criteria: Weight > 150% of ideal body weight or had predisposing factors for delayed gastric emptying such as diabetes, chronic cholecystectitis, scleroderma, neuropathies, and neuromuscular disorders. Women who demonstrated significant anxiety and who, in the anaesthesiologist's judgement required pre‐operative anxiolytic therapy were also excluded |
|
| Interventions |
Intervention Characteristics Nitrous oxide‐free – A Name: Propofol + air +O₂ Induction: Lidocaine /kg given IV propofol infusion started 200 mcg/kg/min Induction dose of propofol IV 2 ‐ 2.5 mg/kg over 1 min until loss of eyelash reflex. Tracheal intubation facilitated with atracurum 0.5 mg/kg. Ventilation controlled and minute ventilation adjusted to maintain end‐tidal CO₂ at 35 (± 5) mm‐Hg Maintenance: Infusion rate of propofol was adjusted to maintain adequate depth of anaesthesia as indicated by clinical signs and haemodynamic changes. Ventilated with mixture of air and O₂ ‐ FiO₂ 30% Recovery: 10 mins before expected conclusion of surgery propofol infusion discontinued and 10 mg boluses given as clinically indicated. Gas mixture switched to 100% oxygen when skin suturing complete. Muscle relaxant reversed with neostigmine 50 mcg/kg and glycopyrolate 10 mcg/kg Other drugs used: Additional doses of atracurium were used if clinically indicated Premedication: None Duration of surgery (mins): 51.0 (± 17.1) Duration of Anaesthesia (mins): 82.7 (± 24.5) Nitrous oxide‐based – A Name: Propofol +N₂O+O₂ Induction: Lidocaine 1mg/kg given IV propofol infusion started 200 mcg/kg/min Induction dose of propofol IV 2 ‐ 2.5 mg/kg over 1 min until loss of eyelash reflex. Tracheal intubation facilitated with atracurum 0.5 mg/kg. Ventilation controlled and minute ventilation adjusted to maintain end‐tidal CO₂ at 35 (±5) mm‐Hg Maintenance: Infusion rate of propofol was adjusted to maintain adequate depth of anaesthesia as indicated by clinical signs and haemodynamic changes.Ventilated with mixture of N₂O and O₂ ‐ FiO₂ 30% Recovery: 10 mins before expected conclusion of surgery propofol infusion discontinued and 10 mg boluses given as clinically indicated. Gas mixture switched to 100% oxygen when skin suturing complete. Muscle relaxant reversed with neostigmine 50 mcg/kg and glycopyrolate 10 mcg/kg Other drugs used: Additional doses of atracurium were used if clinically indicated Premedication: None Duration of surgery (mins): 52.8 (± 17.2) Duration of Anaesthesia (mins): 79.0 (± 19.3) Monitoring: After intubation, the infusion rate of propofol was adjusted to maintain adequate depth of anaesthesia, as indicated by clinical signs and haemodynamic changes |
|
| Outcomes | Accidental awareness Awareness of recall obtained in recovery room. No details of method used Not reported in Results section, only in Discussion "Although none of the patients in the study reported any awareness, awareness can be a risk in patients who receive total IV anaesthesia with propofol in the event of an infusion pump or IV malfunction" |
|
| Identification |
Country: USA Setting: University Hospital Authors name: R Sukhani Institution: Loyola University Medical Center Email: NR Address: Department of Anesthesiology, Loyola University Medical Center,2160 South First Avenue, Maywood, IL 60153 |
|
| Aim of study | To compare the emetic sequelae and quality of recovery between a group of participants anaesthetized with propofol alone and a group anaesthetized with propofol plus nitrous oxide | |
| Notes | Sponsorship source: NR. No statement of conflicts | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the patients were assigned randomly to one of the two treatment groups using a non‐blinded study design" Comment: No details of method of sequence generation or allocation concealment |
| Allocation concealment (selection bias) | Unclear risk | Comment: No details of method of sequence generation or allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Study described as unblinded. Depth of anaesthesia at discretion of anaesthetist |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Intermediate recovery variables were recorded by recovery room nurses and the attending anesthesiologist blinded to anesthetic technique" Comment: Assume different anaesthesiologist. Study described as unblinded but not clear if women were aware of allocation ‐ but questions asked in recovery room so unlikely to be important |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No attrition reported |
| Selective reporting (reporting bias) | Unclear risk | Quote: "Although none of the patients in the study reported any awareness, awareness can be a risk in patients who receive total IV anesthesia with propofol in the event of an infusion pump or IV malfunction." Comment: Relevant outcome not reported in the Results section, only mentioned in the Discussion |
| Other bias | Low risk | Comment: None identified |
Vanacker 1999.
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group |
|
| Participants |
Baseline Characteristics Nitrous oxide‐free – A Number randomized: 30 Number analysed: 30 Age (mean): 50.2 (± 1.7) % male: 0 Type of surgery: Breast surgery Other information: Fentanyl given (mcg/kg/hr): 2.31 (±0.2) Nitrous oxide‐based – A Number randomized: 30 Number analysed: 30 Age (mean): 48.3 (± 1.9) % male: 0 Type of surgery: Breast surgery Other information: Fentanyl given (mcg/kg/hr): 2.65 (± 0.6) Included criteria: Women scheduled for breast surgery with a duration of 1 ‐ 3 hrs. 18 ‐ 65 yrs, ASA I or II Excluded criteria: Body weight ≥ 20% outside normal, history of motion sickness or of PONV, pregnant or breastfeeding, history of alcohol or drug abuse, sensitivity to narcotics, impaired renal or hepatic function, recent (< 30 days) participation in another study |
|
| Interventions |
Intervention Characteristics Nitrous oxide‐free – A Name: Desflurane + air and oxygen FiO₂ 0.33 Induction: Propofol 2 mg/kg vecorunium 0.1 mg/kg Maintenance: Desflurane + air and oxygen FiO₂ 0.33. Concentration of anaesthetic given to participants was based on previously determined MAC values and adjusted to participant needs as clinically indicated with the objective to maintain heart rate and blood pressure within 20% of baseline values. Mean end‐tidal desflurane concentration 5.65 (0.09)%. Additional fentanyl given if signs of inadequate anaesthesia (i.e. movement, swallowing, tearing or salivation) despite changes in inhalation concentration. Ventilatory settings were adjusted to achieve normocapnea Recovery: Desflurane was discontinued and participants received 100% oxygen (7 l/min fresh gas flow) Other drugs used: Tenoxicam 40 mg IV, administered 5 mins after skin incision Premedication: Alprazolam 0.5 mg orally 1 ‐ 2 hrs before surgery at discretion of investigator (anxious or worried participant). Pre‐induction dose of fentanyl 2 mcg/kg. Premed given: 13 (43.3%) Duration of anaesthetic (mins): 111.5 Nitrous oxide‐based – A Name: Desflurane + N₂O and oxygen FiO₂ 0.33 Induction: Propofol 2 mg/kg vecorunium 0.1 mg/kg Maintenance: Desflurane + N₂O and oxygen FiO₂ 0.33. Concentration of anaesthetic based on previously determined MAC values and adjusted to participant needs as clinically indicated with the objective to maintain heart rate and blood pressure within 20% of baseline values. Mean end‐tidal desflurane concentration 3.18 (0.07)%. Additional fentanyl given if signs of inadequate anaesthesia (i.e. movement, swallowing, tearing or salivation) despite changes in inhalation concentration. Ventilatory settings were adjusted to achieve normocapnea Recovery: At end of surgery, desflurane and N₂O were discontinued and participants received 100% oxygen (7 l/min fresh gas flow) Other drugs used: Tenoxicam 40 mg IV administered 5 mins after skin incision Premedication: Alprazolam 0.5 mg orally 1 ‐ 2 hrs before surgery at discretion of investigator (anxious or worried participant). Pre‐induction dose of fentanyl 2 mcg/kg. Premed given: 12 (40%) Duration of anaesthetic (mins): 109.7 Monitoring: Concentration of anaesthetic given to participants was based on previously determined MAC values and adjusted to participant needs as clinically indicated with the objective to maintain heart rate and blood pressure within 20% of baseline values. Additional fentanyl given if there were signs of inadequate anaesthesia (i.e. movement, swallowing, tearing or salivation) despite changes in inhalation concentration |
|
| Outcomes | Accidental awareness Assessed 0 ‐ 2 hrs after surgery ‐ asked about recall of intra‐operative events or dreams during anaesthesia. No recall was reported in either group |
|
| Identification |
Country: Belgium Setting: University Hospital Authors name: BF Vanacker Institution: University Hospitals K.U. Leuven Email: NR Address: University Hospitals K.U. Leuven, Department of Anaesthesiology, Herestraat, 49, B‐3000, Leuven, Belgium |
|
| Aim of study | To evaluate the effect of the combination of desflurane with nitrous oxide versus desflurane alone on postoperative nausea and vomiting in a subgroup of female inpatients | |
| Notes | Sponsorship source: NR. No statement re conflict of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: "randomized (performed using the sealed envelope technique) ". No details given of sequence generation |
| Allocation concealment (selection bias) | Low risk | Comment: "randomized (performed using the sealed envelope technique) ". Allocation probably concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: No mention of blinding. Anaesthetist varied inhalation concentration and fentanyl doses |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: No mention of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No reported attrition |
| Selective reporting (reporting bias) | Low risk | Comment: All relevant outcomes described in Methods reported |
| Other bias | Low risk | Comment: None identified |
Anaes ‐ anaesthetic ASA ‐ American Society of Anesthesiologists Physical Status Classification System BMI ‐ body mass index CNS ‐ central nervous system FiO₂ ‐ fraction of inspired oxygen hr ‐ hour HR ‐ heart rate IV ‐ intravenous kg ‐ kilogram KPa ‐ kilopascal MAC ‐ minimum alveolar concentration mcg ‐ microgram mg ‐ milligram mins ‐ minutes ml ‐ millilitre ML‐AERS ‐ Midlatence Auditor evoked Responses N₂O ‐ Nitrous oxide ng ‐ nanogram NR ‐ not reported O₂ ‐ oxygen gas PACU ‐ paediatric acute care unit PONV ‐ postoperative nausea and vomiting surg ‐ surgery TCI ‐ target controlled infusion US ‐ ultrasound yrs ‐ years
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chowdhury 2014 | Different depth of anaesthesia in study groups ‐ full paper |
| ENIGMA‐II | Does not measure AAGA |
| Goto 1997 | Wrong intervention ‐ Xenon versus nitrous oxide‐ sevoflurane Vs nitrous oxide‐isoflurane |
| Goto 1997a | Wrong intervention ‐ Xenon versus nitrous oxide‐ sevoflurane Vs nitrous oxide‐isoflurane |
| Inada 1999 | Different depth of anaesthesia in study groups |
| Kang 2013 | Different depth of anaesthesia in study groups (abstract) |
| Liu 2014 | Different depth of anaesthesia in study groups |
| Luginbuhl 2005 | Wrong intervention ‐Xenon versus nitrous oxide + desflurane |
| Nakata 1999 | Wrong intervention ‐ sevoflurane with one of three anaesthetics; 1 MAC xenon, 0.7 MAC xenon and 0.7 MAC nitrous oxide |
| Ochiai 1999 | Wrong intervention ‐ all participants were maintained with nitrous oxide |
| Rocca 2000 | Different depth of anaesthesia in study groups |
| Ropcke 2001 | Wrong intervention ‐ each participant had 2 concentrations of nitrous oxide so those who had none at 1 time also had another concentration |
AAGA ‐ accidental awareness during general anaesthesia MAC ‐ minimum alveolar concentration
Differences between protocol and review
We made the following changes to the protocol (Nicholson 2014):
Authorship:
The authorship of the review changed, with two additional authors (JH and JG) joining the team. JH became the lead author.
We added further information regarding the 5th National Audit Project (NAP5) and updated references
We deleted details of other endpoints due to the removal of the secondary objectives of this review (see later for details)
We added information on the ENIGMA II study (Myles 2014a)
We have reworded the primary objective from “unintentional intraoperative explicit awareness” to AAGA, as this term is now more widely used
We removed the secondary objectives from the review
The secondary objectives were to assess the effect of general anaesthesia including nitrous oxide on the risk of wakefulness without recall during anaesthesia and on the results of depth of anaesthesia brain monitoring during surgery. However brain monitoring results are not equivalent to awareness (this relationship is not established) and we wanted to ensure that the review focused on the clinically relevant endpoint of accidental awareness.
We excluded studies where the depth of anaesthesia were reported as differing between study arms
Since depth of anaesthesia will affect the likelihood of accidental awareness, we excluded studies where we judged the two arms to have had different depths of anaesthetic. In order to assess this we used the reported minimum alveolar concentration (MAC) in the published reports. MAC is the concentration of the vapour in the lungs that is needed to prevent visible movement in 50% of people in response to a standard surgical stimulus. MAC is used to compare the strengths, or potency, of anaesthetic agents. We assumed that, broadly, MACs are additive and used this to determine whether the two intervention arms had 'similar depth anaesthetics'.
We removed secondary outcomes from the review
The secondary outcomes outlined in the protocol included wakefulness and results from instruments used to monitor brain activity. However, as the secondary objectives of the review were removed the secondary outcomes were not relevant
We included studies that reported AAGA as a secondary outcome
The inclusion criteria for studies in the published protocol stated that studies must have any of our outcomes as a primary outcome. During the initial stages of the review we found no studies that reported AAGA as a primary outcome. We did, however, identify studies that recorded AAGA as one of a number of secondary outcomes, and the quality of these appeared to be adequate. In consultation with the content editor we amended the protocol so that we included studies which included AAGA as a prespecified outcome, provided all participants were asked postoperatively about awareness and that the study did not rely on volunteered self report. Awareness did not need to be the main/primary aim of the study.
This meant that many studies had to be reviewed in full text to ensure that awareness had not been included as an outcome.
Search methods for identification of studies
The above changes meant that the searches we ran were modified, dropping the requirement for awareness or other outcomes. In order to limit the number of studies to be reviewed in full text, we restricted the search to studies published in or after 1994. We did not search CINAHL or ISI Web of Science.
Due to changes in authorship, different authors were involved in the data collection and analysis
Due to the inclusion of AAGA as a study's secondary outcome, many studies could not be excluded at screening. We therefore reviewed all trials with eligible design, population, intervention and comparison groups in full text
Instead of using a paper extraction form we used Covidence for review of full‐text articles and for data extraction and quality assessment. Forms used as in Appendix 2; Appendix 3
We did not contact any authors for further information, due to the volume of full texts reviewed
Assessment of risk of bias in included studies
We expanded on details of the criteria for assessing risk of bias
We removed details of risk of bias for brain monitoring studies
We restricted the assessment of detection bias to the blinding of outcome assessors and participants, as there were insufficient details on the method of ascertainment of outcome on which to assess studies
As the review now included only one outcome (AAGA) which is dichotomous, we deleted details of other possible reporting of outcomes
We did not expect eligible studies to include more than one comparison; however, four studies did so and we therefore added details on how we managed this
We did not contact any authors for further information due to the volume of full texts reviewed
Assessment of reporting biases
We did not contact any authors for further information due to the volume of full texts reviewed
Summary of findings
As there were no secondary outcomes included in the review, we applied the GRADE criteria only to AAGA
Due to changes in authorship, different authors were involved in the application of GRADE
Many of these changes to the protocol will not have biased the review process; however, several may have and should be discussed.
The most substantial changes to the protocol were the removal of the secondary objectives and therefore the secondary outcomes, and the inclusion of studies reporting AAGA as a secondary outcome. This decision was based on our findings at the start of the review process and on discussion with clinicians and the content editor. We found no studies on wakefulness as a primary outcome and the clinical assertion was that brain monitoring results are not equivalent to awareness. These changes may have had an impact on the bias in the review, as these decisions were made after the initial searches. The inclusion of studies where AAGA was a secondary outcome may also have impacted on the risk of bias of the review. To accurately identify all studies which included AAGA as a secondary outcome, we would have needed to contact all study authors. However the sheer volume of studies meant this was impractical and we relied upon the reporting of secondary outcomes in the Methods section of papers, which we reviewed in full. Therefore some studies that did measure AAGA as a predetermined secondary outcome may have been excluded from the review if the outcome was not stated in the study publication.
Finally in order to limit the number of studies to be reviewed in full text, we restricted the search to studies published in or after 1994, and did not search CINAHL or ISI Web of Science. This may have biased the review process. However, as clinical practices have changed significantly in the last 20 years we judged this to be a reasonable cut‐off.
Contributions of authors
Juliet Hockenhull (JH) Amanda Nicholson (AN), Janette Greenhalgh (JG) Tim M Cook (TC), Andrew F Smith (AS), Sharon R Lewis (SL)
Conceiving the review: TC, AS
Co‐ordinating the review: AN
Undertaking manual searches: AN
Screening search results: JH, JG, AN.
Organizing retrieval of papers: JH
Screening retrieved papers against inclusion criteria: JH, JG, AN.
Appraising quality of papers: JH, AN
Abstracting data from papers: JH, AN
Data management for the review: JH
Entering data into RevMan (RevMan 5.3): AN, SL, JH
RevMan statistical data: N/A
Other statistical analysis not using RevMan: N/A
Interpretation of data: JH, AN, TC, AS
Statistical inferences: JH, AN, TC, AS
Writing the review: AN, TC, AS, JH
Securing funding for the review: AS
Performing previous work that was the foundation of the present study: TC
Guarantor for the review (one author): JH
Person responsible for reading and checking review before submission: JH
Sources of support
Internal sources
No sources of support supplied
External sources
-
NIHR Cochrane Collaboration Programme Grant. Enhancing the safety, quality and productivity of perioperative care. Project Ref: 10/4001/04., UK, Other.
This grant funds the work of AN, AS & SL on this review
Declarations of interest
Juliet Hockenhull: none known
Amanda Nicholson: from March to August 2011, AN worked for the Cardiff Research Consortium, which provided research and consultancy services to the pharmaceutical industry. Cardiff Research Consortium has no connection with AN's work with Cochrane. AN's husband has small direct holdings in several drug and biotech companies as part of a wider balanced share portfolio. All AN's contributions to this review were prior to her work with Q Medical Technologies Limited, she conducted the work on this review whilst employed by Liverpool University.
From June 2015 AN has worked for Q Medical Technologies Limited, a firm which markets and distributes a range of medical devices. Q Medical Technologies do not sell any products that promote, or are specific to, nitrous‐oxide based anaesthesia. See Sources of support.
Janette Greenhalgh: none known
Tim M Cook: none known.
Sharon R Lewis: none known.
Andrew F Smith: none known.
New
References
References to studies included in this review
Aceto 2002 {published data only}
- Aceto P, Valente A, Adducci E, Gorgoglione M, Cosmo G. Implicit memory, dream and auditory evoked responses during anaesthesia. Acta Medica Romana 2002;40(1):21‐9. [Google Scholar]
- Aceto P, Valente A, Gorgoglione M, Adducci E, Cosmo G. Relationship between awareness and middle latency auditory evoked responses during surgical anaesthesia. British Journal of Anaesthesia 2003;90(5):630‐5. [PUBMED: 12697591] [DOI] [PubMed] [Google Scholar]
Albertin 2005 {published data only}
- Albertin A, Casati A, Bergonzi PC, Moizo E, Lombardo F Torri G. The effect of adding nitrous oxide on MAC of sevoflurane combined with two target‐controlled concentrations of remifentanil in women. European Journal of Anaesthesiology 2005;22(6):431‐7. [PUBMED: 15991505] [DOI] [PubMed] [Google Scholar]
Arellano 2000 {published data only}
- Arellano RJ, Pole ML, Rafuse SE, Fletcher M, Saad YG, Friedlander M, et al. Omission of nitrous oxide from a propofol‐based anesthetic does not affect the recovery of women undergoing outpatient gynecologic surgery. Anesthesiology 2000;93(2):332‐9. [PUBMED: 10910478] [DOI] [PubMed] [Google Scholar]
Crawford 1998 {published data only}
- Crawford MW, Lerman J, Sloan MH, Sikich N, Halpern L, Bissonnette B. Recovery characteristics of propofol anaesthesia, with and without nitrous oxide: a comparison with halothane/nitrous oxide anaesthesia in children. Paediatric Anaesthesia 1998;8(1):49‐54. [PUBMED: 9483598] [DOI] [PubMed] [Google Scholar]
Dedola 2008 {published data only}
- Dedola E, Albertin A, Poli D, Colla L, Gandolfi A, Martani C, et al. Effect of nitrous oxide on desflurane MACBAR at two target‐controlled concentrations of remifentanil. Minerva Anestesiologica 2008;74(5):165‐72. [PUBMED: 18414359] [PubMed] [Google Scholar]
ENIGMA {published data only}
- Chan MT, Gin T. Perioperative outcome after nitrous oxide anaesthesia: an ENIGMA trial. Hong Kong Medical Journal 2014;20(S3):S34‐5. [PUBMED: 25001034] [PubMed] [Google Scholar]
- Graham AM, Myles PS, Leslie K, Chan MTV, Paech MJ, Peyton P, et al. A cost‐benefit analysis of the ENIGMA Trial. Anesthesiology 2011;115(2):265‐72. [PUBMED: 21681081] [DOI] [PubMed] [Google Scholar]
- Leslie K, Myles PS, Chan MTV, Forbes A, Paech MJ, Peyton P, et al. Nitrous oxide and long‐term morbidity and mortality in the ENIGMA trial. Anesthesia and Analgesia 2011;112(2):387‐93. [PUBMED: 20861416] [DOI] [PubMed] [Google Scholar]
- Leslie K, Myles PS, Chan MTV, Paech MJ, Peyton P, Forbes A, et al. Risk factors for severe postoperative nausea and vomiting in a randomized trial of nitrous oxide‐based vs nitrous oxide‐free anaesthesia. British Journal of Anaesthesia 2008;101:498‐505. [PUBMED: 18682411] [DOI] [PubMed] [Google Scholar]
- Myles PS, Chan MT, Leslie K, Peyton, P, Paech M, Forbes AA. Effect of nitrous oxide on plasma homocysteine and folate in patients undergoing major surgery. British Journal of Anaesthesia 2008;100(6):780‐6. [DOI: ; PUBMED: 18400808] [DOI] [PubMed] [Google Scholar]
- Myles PS, Leslie K, Chan MT, Forbes A, Paech MJ, Peyton P, et al. Avoidance of nitrous oxide for patients undergoing major surgery: a randomized controlled trial. Anesthesiology 2007;107(2):221‐31. [DOI: 10.1097/01.anes.0000270723.30772.da; PUBMED: 17667565] [DOI] [PubMed] [Google Scholar]
Girardi 1994 {published data only}
- Girardi G, Rossi R, Cellai MP, Pieraccioli E, Novelli GP. Anesthesia with isoflurane in air and with isoflurane and nitrous oxide [Italian]. Minerva Anestesiologica 1994;60:321‐8. [PUBMED: 7984314] [PubMed] [Google Scholar]
Handa 2010 {published data only}
- Handa T, Koike S, Kohkita Y, Ihara Y, Fukuda KI, Ichinohe T, et al. Frequency and content of dreams during propofol anesthesia in patients undergoing mandibular sagittal split ramus osteotomy. [Japanese]. Journal of Japanese Dental Society of Anesthesiology 2010;38(1):29‐34. [Google Scholar]
Handa Tsutsui 2007 {published data only}
- Handa‐Tsutsui F, Kodaka M. Effect of nitrous oxide on propofol requirement during target‐controlled infusion for oocyte retrieval. International Journal of Obstetric Anesthesia 2007;16(1):13‐6. [PUBMED: 17125991] [DOI] [PubMed] [Google Scholar]
Heath 1996 {published data only}
- Heath KJ, Sadler P, Winn J H, McFadzean WA. Nitrous oxide reduces the cost of intravenous anaesthesia. European Journal of Anaesthesiology 1996;13(4):369‐72. [PUBMED: 8842658] [DOI] [PubMed] [Google Scholar]
Lindekaer 1995 {published data only}
- Lindekaer AL, Skielboe M, Guldager H, Jensen EW. The influence of nitrous oxide on propofol dosage and recovery after total intravenous anaesthesia for day‐case surgery. Anaesthesia 1995;50(5):397‐9. [PUBMED: 7793542] [DOI] [PubMed] [Google Scholar]
Ngan Kee 2002 {published data only}
- Ngan Kee WD, Khaw KS, Ma KC, Wong ASY, Lee BB. Randomized, double‐blind comparison of different inspired oxygen fractions during general anaesthesia for Caesarean section. British Journal of Anaesthesia 2002;89(4):556‐61. [PUBMED: 12393355] [DOI] [PubMed] [Google Scholar]
Singh 2011 {published data only}
- Singh GP, Prabhakar H, Bithal PK, Dash HH. A comparative evaluation of nitrous oxide‐isoflurane vs isoflurane anesthesia in patients undergoing craniotomy for supratentorial tumors: A preliminary study. Neurology India 2011;59(1):18‐24. [PUBMED: 21339653] [DOI] [PubMed] [Google Scholar]
Sukhani 1994 {published data only}
- Sukhani R, Lurie J, Jabamoni R. Propofol for ambulatory gynecologic laparoscopy: Does omission of nitrous oxide alter postoperative emetic sequelae and recovery?. Anesthesia and Analgesia 1994;78(5):831‐5. [PUBMED: 8160978] [DOI] [PubMed] [Google Scholar]
Vanacker 1999 {published data only}
- Vanacker B. Comparative study of the effect of desflurane with nitrous oxide vs desflurane without nitrous oxide on postoperative nausea and vomiting in female patients undergoing breast surgery [abstract]. British Journal of Anaesthesia 1998;80 Suppl 1:134. [Google Scholar]
- Vanacker BF. The impact of nitrous oxide on postoperative nausea and vomiting after desflurane anesthesia for breast surgery. Acta Anaesthesiologica Belgica 1999;50(2):77‐81. [PUBMED: 10418646] [PubMed] [Google Scholar]
References to studies excluded from this review
Chowdhury 2014 {published data only}
- Chowdhury T, Prabhakar H, Bithal P K, Schaller B, Dash H H. Early cognitive decline in pituitary surgery: Is nitrous oxide the culprit?. Future Neurology 2014;9:579‐85. [Google Scholar]
- Chowdhury T, Prabhakar H, Kumar Bithal P, Dash HH. To assess the effects of two anesthetic techniques (with and without nitrous oxide) on cognitive function in patients undergoing transsphenoidal pituitary surgery. Journal of Neurosurgical Anesthesiology 2011;23(4):436. [Google Scholar]
ENIGMA‐II {published data only}
- Leslie K, Myles PS, Kasza J, Forbes A, Peyton PJ, Chan MTV, et al. Nitrous oxide and serious long‐term morbidity and mortality in the evaluation of nitrous oxide in the gas mixture for anaesthesia (ENIGMA)‐II trial. Anesthesiology 2015;123(6):1267‐80. [PUBMED: 26501387] [DOI] [PubMed] [Google Scholar]
- Myles PS, Leslie K, Chan MT, Forbes A, Peyton PJ, Paech MJ, et al. The safety of addition of nitrous oxide to general anaesthesia in at‐risk patients having major non‐cardiac surgery (ENIGMA‐II): a randomised, single‐blind trial. Lancet 2014;384(9952):1446‐54. [PUBMED: 25142708] [DOI] [PubMed] [Google Scholar]
Goto 1997 {published data only}
- Goto T, Saito H, Shinkai M, Nakata Y, Ichinose F, Morita S. Xenon provides faster emergence from anesthesia than does nitrous oxide‐ sevoflurane or nitrous oxide‐isoflurane. Anesthesiology 1997;86(6):1273‐8. [PUBMED: 9197295] [DOI] [PubMed] [Google Scholar]
Goto 1997a {published data only}
- GotoT, Saito H, Nakata Y, Uezono S, Ichinose F, Morita S. Emergence times from xenon anaesthesia are independent of the duration of anaesthesia. British Journal of Anaesthesia 1997;79(5):595‐9. [PUBMED: 9422897] [DOI] [PubMed] [Google Scholar]
Inada 1999 {published data only}
- Inada T, Shingu K, Nakao S, Nagata A. Effects of nitrous oxide on haemodynamic and electroencephalographic responses induced by tetanic electrical stimulation during propofol anaesthesia. Anaesthesia 1999;54(5):423‐6. [PUBMED: 10995137] [DOI] [PubMed] [Google Scholar]
Kang 2013 {published data only}
- Kang DH, Lee SH, Kim SJ, Choi JI, Jeong CW, Jeong SW, et al. Anesthetic requirements and stress hormone responses in chronic spinal cord‐injured patients undergoing surgery below the level of injury: nitrous oxide vs remifentanil. Korean Journal of Anesthesiology 2013;65(6):531‐8. [DOI: 10.4097/kjae.2013.65.6.531; PUBMED: 24427459] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2014 {published data only}
- Liu N, Guen M, Boichut N, Genty A, Herail T, Schmartz D, et al. Nitrous oxide does not produce a clinically important sparing effect during closed‐loop delivered propofol‐remifentanil anaesthesia guided by the bispectral index: a randomized multicentre study. British Journal of Anaesthesia 2014;112(5):842‐51. [DOI: 10.1093/bja/aet479; PUBMED: 24486835] [DOI] [PubMed] [Google Scholar]
Luginbuhl 2005 {published data only}
- Luginbuhl M, Petersen‐Felix S, Zbinden AM, Schnider TW. Xenon does not reduce opioid requirement for orthopedic surgery. Canadian Journal of Anesthesia 2005;52(1):38‐44. [PUBMED: 15625254] [DOI] [PubMed] [Google Scholar]
Nakata 1999 {published data only}
- Nakata Y, Goto T, Ishiguro Y, Terui K, Niimi Y, Morita S. Xenon suppresses the hypnotic arousal in response to surgical stimulation. Journal of Clinical Anesthesia 1999;11(4):305‐9. [PUBMED: 10470632 ] [DOI] [PubMed] [Google Scholar]
Ochiai 1999 {published data only}
- Ochiai N, Tashiro C, Okutani R, Murakawa K, Kinouchi K, Kitamura S. Improved oxygen delivery to the fetus during cesarean section under sevoflurane anesthesia with 100% oxygen. Journal of Anesthesia 1999;13(2):65‐70. [PUBMED: 14530942] [DOI] [PubMed] [Google Scholar]
Rocca 2000 {published data only}
- Rocca G, Montecchi C, Baisi F, Monaco S, Romboli D, Gasparetto A. N2O‐free sevoflurane anesthesia. Clinical evaluation. [Italian]. Minerva Anestesiologica 2000;66(9):611‐9. [PUBMED: 11070960] [PubMed] [Google Scholar]
Ropcke 2001 {published data only}
- Ropcke H, Wirz S, Bouillon T, Bruhn J, Hoeft A. Pharmacodynamic interaction of nitrous oxide with sevoflurane, desflurane, isoflurane and enflurane in surgical patients: measurements by effects on EEG median power frequency. European Journal of Anaesthesiology 2001;18(7):440‐9. [PUBMED: 11070960] [DOI] [PubMed] [Google Scholar]
Additional references
Alkire 2004
- Alkire MT, Gorski LA. Relative amnesic potency of five inhalational anesthetics follows the Meyer‐Overton rule. Anesthesiology 2004;101(2):417‐29. [PUBMED: 15277925] [DOI] [PubMed] [Google Scholar]
ASA 2006
- American Society of Anesthesiologists Task Force on Intraoperative Awareness. Practice advisory for intraoperative awareness and brain function monitoring. Anesthesiology 2006;104(4):847‐64. [PUBMED: 16571982] [DOI] [PubMed] [Google Scholar]
Avidan 2008
Avidan 2011
- Avidan MS, Jacobsohn E, Glick D, Burnside BA, Zhang L, Villafranca A, et al. Prevention of intraoperative awareness in a high‐risk surgical population. The New England Journal of Medicine 2011;365(7):591‐600. [PUBMED: 21848460] [DOI] [PubMed] [Google Scholar]
Brice 1970
Cook 2008
- Cook TM. Anesthesia awareness and the bispectral index. The New England Journal of Medicine 2008;359(4):430; author reply 430‐1. [PUBMED: 18655251] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. www.covidence.org. Covidence, 2014.
Davidson 2011
- Davidson AJ, Smith KR, Blusse van Oud‐Alblas HJ, Lopez U, Malviya S, Bannister CF, et al. Awareness in children: a secondary analysis of five cohort studies. Anaesthesia 2011;66(6):446‐54. [PUBMED: 21501128] [DOI] [PubMed] [Google Scholar]
De Vasconcellos 2013
- Vasconcellos K, Sneyd JR. Nitrous oxide: are we still in equipoise? A qualitative review of current controversies. British Journal of Anaesthesia 2013;111(6):877‐85. [PUBMED: 23801743] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Errando 2008
- Errando CL, Sigl JC, Robles M, Calabuig E, Garcia J, Arocas F, et al. Awareness with recall during general anaesthesia: a prospective observational evaluation of 4001 patients. British Journal of Anaesthesia 2008;101(2):178‐85. [PUBMED: 18515816] [DOI] [PubMed] [Google Scholar]
Ghoneim 2009
- Ghoneim MM, Block RI, Haffarnan M, Mathews MJ. Awareness during anesthesia: risk factors, causes and sequelae: a review of reported cases in the literature. Anesthesia and Analgesia 2009;108(2):527‐35. [PUBMED: 19151283] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schünemann HJ. What is “quality of evidence” and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [PUBMED: 22008217] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011b
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hopkins 2005
- Hopkins PM. Nitrous oxide: a unique drug of continuing importance for anaesthesia. Best Practice & Research. Clinical Anaesthesiology 2005;19(3):381‐9. [PUBMED: 16013688] [DOI] [PubMed] [Google Scholar]
Imberger 2014
- Imberger G, Orr A, Thorlund K, Wetterslev J, Myles P, Möller AM. Does anaesthesia with nitrous oxide affect mortality or cardiovascular morbidity? A systematic review with meta‐analysis and trial sequential analysis. British Journal of Anaesthesiology 2014; Vol. 112, issue 3:410‐26. [PUBMED: 24408738] [DOI] [PubMed]
Isik 2007
- Isik B, Tuzuner T, Tezkirecioglu M, Oztas N. Nitrous oxide sedation and bispectral index. European Journal of Dentistry 2007;1(4):240‐5. [PUBMED: 19212474] [PMC free article] [PubMed] [Google Scholar]
Lampe 1990
- Lampe GH, Wauk LZ, Donegan JH, Pitts LH, Jackler RK, Litt LL, et al. Effect on outcome of prolonged exposure of patients to nitrous oxide. Anesthesia and Analgesia 1990;71(6):586‐90. [PUBMED: 2240628] [PubMed] [Google Scholar]
Lennmarken 2002
- Lennmarken C, Bildfors K, Enlund G, Samuelsson P, Sandin R. Victims of awareness. Acta Anaesthesiologica Scandinavica 2002;46(3):229‐31. [PUBMED: 11939910] [DOI] [PubMed] [Google Scholar]
Leslie 2010
- Leslie K, Chan MT, Myles PS, Forbes A, McCulloch TJ. Posttraumatic stress disorder in aware patients from the B‐aware trial. Anesthesia and Analgesia 2010;110(3):823‐8. [PUBMED: 19861364] [DOI] [PubMed] [Google Scholar]
Leslie 2011
- Leslie K, Myles PS, Chan MT, Forbes A, Paech MJ, Peyton P, et al. Nitrous oxide and long‐term morbidity and mortality in the ENIGMA trial. Anesthesia and Analgesia 2011;112(2):387‐93. [PUBMED: 20861416] [DOI] [PubMed] [Google Scholar]
Leslie 2013b
- Leslie K, Myles P, Devereaux PJ, Forbes A, Rao‐Melancini P, Williamson E, et al. Nitrous oxide and serious morbidity and mortality in the POISE trial. Anesthesia and Analgesia 2013;116(5):1034‐40. [PUBMED: 23337413] [DOI] [PubMed] [Google Scholar]
Mashour 2009
- Mashour GA, Tremper KK, Avidan MS. Protocol for the "Michigan Awareness Control Study": A prospective, randomized, controlled trial comparing electronic alerts based on bispectral index monitoring or minimum alveolar concentration for the prevention of intraoperative awareness. BMC Anesthesiology 2009;9:7. [PUBMED: 19891771] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mashour 2010
- Mashour GA, Esaki RK, Tremper KK, Glick DB, O'Connor M, Avidan MS. A novel classification instrument for intraoperative awareness events. Anesthesia and Analgesia 2010;110(3):813‐5. [PUBMED: 19713251] [DOI] [PubMed] [Google Scholar]
Mashour 2011
- Mashour GA, Orser BA, Avidan MS. Intraoperative awareness: from neurobiology to clinical practice. Anesthesiology 2011;114(5):1218‐33. [PUBMED: 21464699] [DOI] [PubMed] [Google Scholar]
Mashour 2012
- Mashour GA, Shanks A, Tremper KK, Kheterpal S, Turner CR, Ramachandran SK, et al. Prevention of intraoperative awareness with explicit recall in an unselected surgical population: a randomized comparative effectiveness trial. Anesthesiology 2012;117(4):717‐25. [PUBMED: 22990178] [DOI] [PMC free article] [PubMed] [Google Scholar]
Messina 2008
- Messina AG, Ward MJ, Pace NL. Anaesthetic interventions for prevention of awareness during surgery. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD007272] [DOI] [PMC free article] [PubMed] [Google Scholar]
Myles 2004
- Myles PS, Leslie K, McNeil J, Forbes A, Chan MT. Bispectral index monitoring to prevent awareness during anaesthesia: the B‐Aware randomised controlled trial. Lancet 2004;363(9423):1757‐63. [PUBMED: 15172773] [DOI] [PubMed] [Google Scholar]
Myles 2007
- Myles PS, Leslie K, Chan MT, Forbes A, Paech MJ, Peyton P, et al. Avoidance of nitrous oxide for patients undergoing major surgery: a randomized controlled trial. Anesthesiology 2007;107(2):221‐31. [PUBMED: 17667565] [DOI] [PubMed] [Google Scholar]
Myles 2014a
- Myles, PS, Leslie K, Chan MTV, Forbes A, Peyton PJ, Paech MJ. The safety of addition of nitrous oxide to general anaesthesia in at‐risk patients having major non‐cardiac surgery (ENIGMA‐II): a randomised, single‐blind trial. The Lancet 2014;384(9952):1446‐54. [PUBMED: 25142708] [DOI] [PubMed] [Google Scholar]
NICE 2012
- National Institute of Health and Clinical Excellence. Depth of anaesthesia monitors – Bispectral Index (BIS), E‐Entropy and Narcotrend‐Compact M [NICE diagnostics guidance 6]. www.nice.org.uk/nicemedia/live/13965/61547/61547.pdf (Accessed 13 January 2014).
Paech 2008
- Paech MJ, Scott KL, Clavisi O, Chua S, McDonnell N. A prospective study of awareness and recall associated with general anaesthesia for caesarean section. International Journal of Obstetric Anesthesia 2008;17(4):298‐303. [PUBMED: 18617387] [DOI] [PubMed] [Google Scholar]
Pandit 2013
- Pandit JJ, Cook TM, Jonker WR, O'Sullivan E, 5th National Audit Project of the Royal College of Anaesthetists and the Association of Anaesthetists of Great Britain and Ireland. A national survey of anaesthetists (NAP5 Baseline) to estimate an annual incidence of accidental awareness during general anaesthesia in the UK. Anaesthesia 2013; Vol. 68, issue 4:343‐53. [PUBMED: 23488832] [DOI] [PubMed]
Pandit 2014a
- Pandit JJ, Andrade J, Bogod DG, Hitchman JM, Jonker WR, Lucas N, et al. 5th National Audit Project (NAP5) on accidental awareness during general anaesthesia: protocol, methods, and analysis of data. British journal of anaesthesia 2014;113(4):540‐8. [PUBMED: 25204695] [DOI] [PubMed] [Google Scholar]
Pandit 2014b
- Pandit JJ, Andrade J, Bogod DG, Hitchman JM, Jonker WR, Lucas N, et al. 5th National Audit Project (NAP5) on accidental awareness during general anaesthesia: summary of main findings and risk factors. British Journal of Anaesthesia 2014;113(4):549‐59. [PUBMED: 25204697] [DOI] [PubMed] [Google Scholar]
Pollard 2007
Punjasawadwong 2014
- Punjasawadwong Y, Phongchiewboon A, Bunchungmongkol N. Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Database of Systematic Reviews 2014, issue 6. [DOI: 10.1002/14651858.CD003843.pub3.] [DOI] [PMC free article] [PubMed]
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rungreungvanich 2007
- Rungreungvanich M, Thienthong S, Charuluxananan S, Lekprasert V, Pulnitiporn A, Prakanrattana U. Predictors of intra‐operative recall of awareness: Thai Anesthesia Incidents Study (THAI Study): a case‐control study. Journal of the Medical Association of Thailand 2007;90(8):1551‐7. [PUBMED: 17926984] [PubMed] [Google Scholar]
Samuelsson 2007
- Samuelsson P, Brudin L, Sandin RH. Late psychological symptoms after awareness among consecutively included surgical patients. Anesthesiology 2007;106(1):26‐32. [PUBMED: 17197842] [DOI] [PubMed] [Google Scholar]
Sanders 2010
- Sanders RD, Avidan MS. Evidence is lacking for interventions proposed to prevent unintended awareness during general anesthesia for cesarean delivery. Anesthesia and Analgesia 2010;110(3):972‐3; author reply 973‐4. [PUBMED: 20185675] [DOI] [PubMed] [Google Scholar]
Sandin 2000
- Sandin RH, Enlund G, Samuelsson P, Lennmarken C. Awareness during anaesthesia: a prospective case study. Lancet 2000;355(9205):707‐11. [PUBMED: 10703802] [DOI] [PubMed] [Google Scholar]
Schallner 2013
- Schallner N, Goebel U. The perioperative use of nitrous oxide: renaissance of an old gas or funeral of an ancient relict?. Current Opinion in Anaesthesiology 2013;26:354‐60. [DOI] [PubMed] [Google Scholar]
Sebel 2004
- Sebel PS, Bowdle TA, Ghoneim MM, Rampil IJ, Padilla RE, Gan TJ, et al. The incidence of awareness during anesthesia: a multicenter United States study. Anesthesia and Analgesia 2004;99(3):833‐9, table of contents. [PUBMED: 15333419] [DOI] [PubMed] [Google Scholar]
Sengupta 1988
- Sengupta P, Plantevin OM. Nitrous oxide and day‐case laparoscopy: effects on nausea, vomiting and return to normal activity. British Journal of Anaesthesia 1988;60(5):570‐3. [PUBMED: 2967712] [DOI] [PubMed] [Google Scholar]
Shepherd 2013
- Shepherd J, Jones J, Frampton G, Bryant J, Baxter L, Cooper K. Clinical effectiveness and cost‐effectiveness of depth of anaesthesia monitoring (E‐Entropy, Bispectral Index and Narcotrend): a systematic review and economic evaluation. Health Technology Assessment (Winchester, England) 2013;17(34):1‐264. [PUBMED: 23962378] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 2015
- Sun R, Jia WQ, Zhang P, Yang K, Tian JH, Ma B, et al. Nitrous oxide‐based techniques versus nitrous oxide‐free techniques for general anaesthesia. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD008984.pub2; PUBMED: 26545294] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sury 2014
- Sury MJR, Palmer JH, Cook TM, Pandit JJ. The state of UK anaesthesia: a survey of National Health Service Activity in 2013. British Journal of Anaesthesia 2014;113:575‐84. [PUBMED: 26372493] [DOI] [PubMed] [Google Scholar]
Tramer 1996
- Tramer M, Moore A, McQuay H. Omitting nitrous oxide in general anaesthesia: meta‐analysis of intraoperative awareness and postoperative emesis in randomized controlled trials. British Journal of Anaesthesia 1996;76(2):186‐93. [PUBMED: 8777095] [DOI] [PubMed] [Google Scholar]
Turan 2013
- Turan A, Mascha EJ, You J, Kurz A, Shiba A, Saager L, et al. The association between nitrous oxide and postoperative mortality and morbidity after noncardiac surgery. Anesthesia and Analgesia 2013;116(5):1026‐33. [PUBMED: 22822187] [DOI] [PubMed] [Google Scholar]
Wennervirta 2002
- Wennervirta J, Ranta SO, Hynynen M. Awareness and recall in outpatient anesthesia. Anesthesia and Analgesia 2002;95(1):72‐7, table of contents. [PUBMED: 12088946] [DOI] [PubMed] [Google Scholar]
Wrigley 1991
- Wrigley SR, Fairfield JE, Jones RM, Black AE. Induction and recovery characteristics of desflurane in day case patients: a comparison with propofol. Anaesthesia 1991;46(8):615‐22. [PUBMED: 1887965] [DOI] [PubMed] [Google Scholar]
Zhang 2011
- Zhang C, Xu L, Ma YQ, Sun YX, Li YH, Zhang L, et al. Bispectral index monitoring prevent awareness during total intravenous anesthesia: a prospective, randomized, double‐blinded, multi‐center controlled trial. Chinese Medical Journal 2011;124(22):3664‐9. [PUBMED: 22340221] [PubMed] [Google Scholar]
References to other published versions of this review
Nicholson 2014
- Nicholson A, Cook TM, Smith AF, Lewis SR. Nitrous oxide‐based versus nitrous oxide‐free general anaesthesia and accidental awareness during general anaesthesia in surgical patients. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD011052] [DOI] [PMC free article] [PubMed] [Google Scholar]


