Summary
Background
Accelerated aging leads to increasing burdens of chronic diseases in late life, posing a huge challenge to the society. With two well-developed aging measures (i.e., physiological dysregulation [PD] and frailty index [FI]), this study aimed to evaluate the relative contributions of life course circumstances (e.g., childhood and adulthood socioeconomic status) to variance in aging.
Methods
We assembled data for 6224 middle-aged and older adults in China from the 2014 life course survey (June to December 2014), the 2015 biomarker collection (July 2015 to January 2016), and the 2015 main survey (July 2015 to January 2016) of the China Health and Retirement Longitudinal Study. Two aging measures (PD and FI) were calculated, with a higher value indicating more accelerated aging. Life course circumstances included childhood (i.e., socioeconomic status, war, health, trauma, relationship, and parents’ health) and adulthood circumstances (i.e., socioeconomic status, adversity, and social support), demographics, and behaviours. The Shapley value decomposition, hierarchical clustering, and general linear regression models were performed.
Findings
The Shapley value decomposition revealed that all included life course circumstances accounted for about 6·3% and 29·7% of variance in PD and FI, respectively. We identified six subpopulations who shared similar patterns in terms of childhood and adulthood circumstances. The most disadvantaged subpopulation (i.e., subpopulation 6 [more childhood trauma and adulthood adversity]) consistently exhibited accelerated aging indicated by the two aging measures. Relative to the most advantaged subpopulation (i.e., subpopulation 1 [less childhood trauma and adulthood adversity]), PD and FI in the most disadvantaged subpopulation were increased by an average of 0·14 (i.e., coefficient, by one-standard deviation, 95% confidence interval [CI] 0·06–0·21; p < 0·0001) and 0·10 (by one-point, 95% CI 0·09–0·11; p < 0·0001), respectively.
Interpretation
Our findings highlight the different contributions of life course circumstances to phenotypic and functional aging. Special attention should be given to promoting health for the disadvantaged subpopulation and narrowing their health gap with advantaged counterparts.
Funding
National Natural Science Foundation of China, Milstein Medical Asian American Partnership Foundation, Natural Science Foundation of Zhejiang Province, Fundamental Research Funds for the Central Universities, National Institute on Aging, National Centre for Advancing Translational Sciences, and Yale Alzheimer's Disease Research Centre.
Keywords: Life course perspective, Aging, Frailty index, Physiological dysregulation
Research in context.
Evidence before this study
We searched PubMed and Web of Science for articles published from database inception to July 15, 2021, using the following search terms: “life course”, “whole life”, “childhood”, “adulthood”, “circumstance”, “socioeconomic”, “social economic”, “environment”, “adversity”, “health”, “behavior”, “aging measure”, “phenotypic aging”, “functional aging”, “frailty index”, and “physiological dysregulation”. No restrictions on study type or language were implemented. We found that life course circumstances contribute to aging-related adverse outcomes among individuals, highlighting the importance of adopting a life course approach in aging research. However, the large numbers of life course circumstances pose challenges to the statistical analysis. To our knowledge, only one of our recently published works simultaneously assessed the effect of nearly 100 life course circumstances on phenotypic aging in the US population, using a series of novel statistical approaches.
Added value of this study
With two well-validated aging measures (i.e., physiological dysregulation [PD, phenotypic level] and frailty index [FI, functional level]), we performed this study to simultaneously evaluate the contribution of over 70 life course circumstances to phenotypic and functional aging among the middle-aged and older adults in China using unique data from the China Health and Retirement Longitudinal Study (CHARLS). The current study contributes to the literature in several aspects. First, we used two aging measures, resulting in novel findings, such as that life course circumstances contributed differently to the acceleration of phenotypic and functional aging. Second, we showed that the observations in white people and Black people did not appreciably apply to the Chinese. Third, we draw attention to many unique factors such as being born in the war era, and rural residence, which were not mentioned in our earlier analysis. Also, as a country with the largest number of aging population, the findings of life course circumstances contributed to aging among the Chinese provide clues for slowing aging and further reducing public health burden.
Implications of all the available evidence
The new evidence from this study highlights the important but different role of life course circumstances in phenotypic and functional aging in late life. The findings provide important clues for prevention and interventive programs aiming at slowing aging and thus ameliorating health inequalities through targeting multiple exposures (e.g., childhood and adulthood adversity) across the life course. Furthermore, special attention should be given to promoting health for the disadvantaged subpopulation and narrowing their health gap with advantaged counterparts.
Alt-text: Unlabelled box
Introduction
Although everyone gets older, individuals may have different aging rates. Accelerated aging indicates that a person is physiologically older than those of the same chronological age. Accelerated aging leads to increasing burdens of chronic diseases in late life, posing a huge challenge to the society. Geroscience investigators hypothesize that therapies or preventative programmes targeting aging would delay the severity or occurrence of most chronic diseases.1 Aging is a multi-dimensional process and has been quantified at hierarchical levels, including biological/molecular, phenotypic, and functional levels.2 Typical examples of summary aging measures include DNA methylation clocks (biological/molecular),3 physiological dysregulation (PD, phenotypic),4,5 and frailty index (FI, functional),6,7 respectively. Aging measures can provide reliable endpoints for which they could be evaluated in preventive and intervention programmes of aging.8 To date, studies have shown that these aging measures are different but also share similarities, and might be complementary.9 For instance, PD is a phenotypic aging measure based on routine clinical biomarkers, reflecting simultaneous dysregulation in multiple systems;4,5 while FI is a functional aging measure incorporating multiple health dimensions (e.g., physical function, cognitive function, comorbidity, etc.), and reflects cumulative health deficits.6,7 Both PD and FI perform well in predicting aging-related outcomes.5,7 Overall, these aging measures provide us with an opportunity to evaluate the effect of exposures on the aging process, and further propose potential therapies or preventative programmes against aging.
Social-environmental factors, from early life to adulthood, contribute to health inequalities among individuals,10,11 highlighting the importance of adopting a life course approach in aging research. Instead of concentrating on one or a few exposures in certain life stages, a life course approach provides a more complete picture showing how these numerous factors aggregate and synergistically work over a long period. Furthermore, it is well known that childhood and adulthood exposures (e.g., socioeconomic status [SES]) may lead to an increased risk of adverse health outcomes in late life through cumulative effects.12 However, the large numbers of life course circumstances pose challenges to statistical analyses that aim to model the effects of individual factors and cumulative effects, and address the high correlation between early life and adulthood factors. The Shapley value decomposition (hereafter, the Shapley method, see details in appendix p 3-5) can simultaneously estimate the relative contributions of various life course circumstances to aging variance, and hierarchical clustering analysis (HCA) can cluster individuals sharing similar circumstances into one subpopulation. In our recently published work,10 we used the above two approaches, and simultaneously assessed the effect of nearly 100 life course circumstances on a measure of phenotypic aging in 2339 US adults aged 51+ years from the US Health and Retirement Study. However, due to the cultural and genetic differences, the results may be not generalisable to other populations.
Facing the rapid population aging in China, we have recently developed a formula to calculate PD and demonstrated that it is robustly predictive of lifespan in the Chinese population and various subpopulations.5 Given that FI has been successfully calculated in the Chinese population,13 it seems that both PD and FI are significantly associated with an array of health indicators in the Chinese population, including subjective and objective physical functions, and short-term mortality, while the strength differs (appendix p 19).
With the unique data from the China Health and Retirement Longitudinal Study (CHARLS), in this study, we extended our earlier analysis of the US population10 to the Chinese population. We first assembled over 70 available life course circumstances characterising childhood and adulthood circumstances, and behaviours in Chinese middle-aged and older adults. Second, we evaluated the relative contributions of these life course circumstances to variance in the two well-validated aging measures, i.e., PD and FI. The findings will provide clues for policy programmes aimed at alleviating health inequalities and disparities in China, the country with the largest number of older adults, through targeting multiple exposures across the life course.
Methods
Study population
CHARLS is an ongoing nationally representative and longitudinal survey of Chinese community-dwelling adults aged ≥45 years, with a response rate of 80.5%.14 CHARLS used a multistage sampling strategy covering 28 provinces, 150 counties/districts, and 450 villages/urban communities across the country. Adults were recruited to the first wave in 2011/2012, and three follow-up waves biennially up to 2017/2018. Details of the CHARLS survey have been described in previous studies.14,15 In this study, we used data from the 2014 life course survey (June to December 2014), the 2015 biomarker collection (July 2015 to January 2016),15 and the 2015 main survey of CHARLS (July 2015 to January 2016). As shown in appendix p 20, 12,560 adults participated in all surveys; we excluded those with missing data on each of the biomarkers (N = 290) and each of the life course circumstances (N = 6046), leaving an analytic sample of 6224 adults. Ethical approval for collecting data on human individuals was received at the Peking University by their institutional review board (IRB00001052-11015), and all adults provided informed consent.
Measures
Childhood and adulthood circumstances
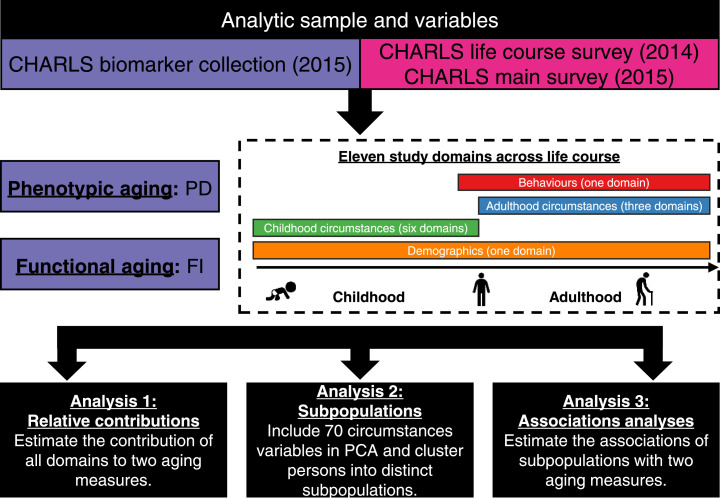
In this study, we considered a comprehensive set of factors across the life course (i.e., life course circumstances), which have been associated with adverse health outcomes. The details of questions and responses for these factors are provided in appendix p 7-9. Briefly, we defined six domains of childhood circumstances (i.e., childhood SES, childhood war, childhood health, childhood trauma, childhood relationship, and childhood parents’ health) and three adulthood circumstances (i.e., adulthood SES, adulthood adversity, and adulthood social support) (Figure 1).
Figure 1.
Roadmap for evaluating the association of life course circumstances with two aging measures.
The roadmap depicts our analytical procedures. We assembled analytic samples and a large array of variables from the 2014 life course survey, the 2015 biomarker collection, and the 2015 main survey of CHARLS. We categorized a large array of variables across the life course into 11 study domains, including six childhood circumstances domains, three adulthood circumstances domains, one behaviours domain, and one demographic domain. We then performed three analyses to evaluate the association of these domains (particularly the childhood and adulthood circumstances domains) with two aging measures. CHARLS=the China Health and Retirement Longitudinal Study. PD=physiological dysregulation. FI=frailty index. PCA=principal component analysis.
Demographics
We considered one domain for demographics, including chronological age, gender, and ethnicity. Ethnicity was classified as Han, and others.
Behaviours
We considered one domain for behaviours, including obesity, smoking status, and drinking status. For obesity, we defined a variable called “proportion of obesity” as the previous study did,10 representing the proportion (range 0–1) of waves at which a respondent had a body mass index (BMI) over 28 kg/m2 (i.e., obesity defined using the Chinese criteria).16 Smoking status was classified as non-smoker, ever smoker, and current smoker. Drinking status was classified as non-drinker, and drinker.
Phenotypic and functional aging measures
We considered two aging measures including PD and FI.
Following the procedure we previously described,5 we calculated PD (derived from Mahalanobis distance). In brief, we used eight biomarkers (i.e., total cholesterol, triglyceride, glycated hemoglobin, urea, creatinine, high-sensitivity C-reactive protein, platelet count, and systolic blood pressure) in the 2015 biomarker collection of CHARLS to calculate Mahalanobis distance, which was then logarithmically transformed (termed as PD in this study) for analysis.4 As done in previous studies,4,5 PD was standardized, with no unit. A larger value of PD indicates acceleration of aging and thus a higher risk of mortality.4,5
The FI was based on the degree of accumulation of health deficits and represented an alternative measurement of frailty that incorporates health dimensions (e.g., cognition, comorbidities, and disabilities).6,7 The FI was calculated as the ratio of the number of deficits (variables) out of the total possible deficits (variables) considered,6,7 with a range of 0 to 1. A larger value of FI indicates acceleration of aging. A 39-item version of self-report FI was constructed in CHARLS.13 The list of items included in the FI is provided in appendix p 10. Any adult who had missing data on 20% or less of the variables was retained. Of 6224 adults included in this study, more than 65% had complete data on FI items. Since median imputation has been proved to be non-inferior to complex approaches for imputing missing data,17 we imputed the missing values with the median for simplicity.
Statistical analysis
Characteristics of the total population were presented using mean ± standard deviation (SD) for continuous variables or numbers (percentages) for categorical variables. Figure 1 briefly summarises the analytic plan of this study. First, we used the Shapley method to estimate the overall and relative contributions of all life course variables including demographics, childhood and adulthood circumstances, and behaviours to the variance in PD and FI, respectively (see details in appendix p 3-5).
Second, we used several analyses to cluster adults into distinct subpopulations (see details in appendix p 5). We performed principal component analysis (PCA) for 70 circumstances variables (except demographics, and behaviours). According to the proportional variance explained (16.6%) (appendix p 21) and eigenvalues (>2), we chose the top four principal components and took them as inputs in an HCA, in which we clustered adults into six distinct subpopulations: subpopulation 1 (less childhood trauma and adulthood adversity) to subpopulation 6 (more childhood trauma and adulthood adversity), with an entropy value18 of 0.716. Adults in the same subpopulation shared similar life course circumstances. Then, we compared the two aging measures between different subpopulations to estimate whether accelerated aging occurred in some subpopulations. Finally, we estimated adults’ cluster membership for each subpopulation using a continuous measure that ranged from -1 to 1. To determine the characteristics of each subpopulation, we estimated the correlation between these cluster membership values and circumstances variables.
Third, we used general linear regression models to estimate the associations of identified subpopulations with two aging measures. We documented coefficients and corresponding 95% confidence intervals (CIs) from two models. Model 1 was unadjusted. Model 2 adjusted for chronological age, gender, proportion of obesity, smoking status, and drinking status.
All analyses were performed using SAS version 9·4 (SAS Institute, Cary, NC), R version 3·6·3 (2020-02-29), and Stata version 14·0 software (Stata Corporation, College Station, TX). A p value <0·05 (2-tailed) was considered to be statistically significant.
Role of the funding source
The funder had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
The characteristics of the study population
The mean chronological age of 6224 adults was 61·6 (SD=8·5) years. About 53% (N = 3313) were women, and the majority were Han (93·0%). The mean values of PD, and FI were 1·8 (SD=0·8), and 0·2 (SD=0·1), respectively. Details of the characteristics of the study population are presented in appendix p 6 and 11-13.
Potential contributions of life course circumstances to phenotypic and functional aging
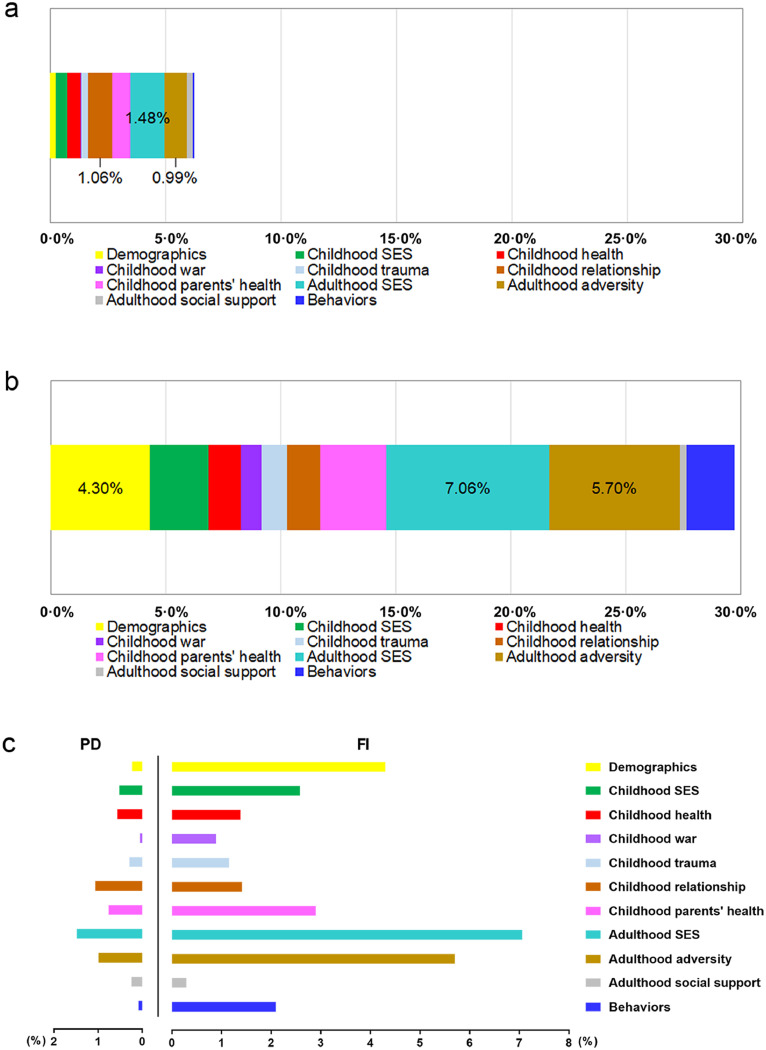
The Shapley method suggested that the 11 study domains contributed 6·3% (bootstrap standard error [SE]=-0·0004) and 29·7% (bootstrap SE=-0·006) of the variance in PD (Figure 2A) and FI (Figure 2B), respectively. All three domains characterizing adulthood circumstances consistently contributed relatively large proportions of variance (PD: 2·7%; FI: 13·1%), with the largest contributor being adulthood SES (PD: 1·5%; FI: 7·1%, rank the first of all 11 study domains, Figure 2C). Among the 6 childhood circumstances domains, childhood relationship was the largest contributor (1·1%) for PD, while childhood parent's health was the largest contributor (2·9%) for FI. Behaviours accounted for 0·1% (rank the tenth), and 2·1% (rank the sixth) of variance in PD, and FI, respectively.
Figure 2.
The contribution of all 11 study domains to PD and FI.
(a) The stacked bar chart shows the contribution of 11 study domains to PD. (b) The stacked bar chart shows the contribution of 11 study domains to FI. The contribution values of the top three contributors were presented in (a) and (b). (c) The clustered bar chart shows the contribution of 11 study domains to PD and FI simultaneously. The 11 domains include six childhood circumstances domains, three adulthood circumstances domains, one behaviours domain, and one demographic domain. Overall, the 11 study domains contributed 6·3% (bootstrap standard error=-0·0005) and 29·7% (bootstrap standard error=-0·006) of the variance in PD and FI, respectively. SES=socioeconomic status. PD=physiological dysregulation. FI=frailty index.
Profiles of life course circumstances and subpopulations
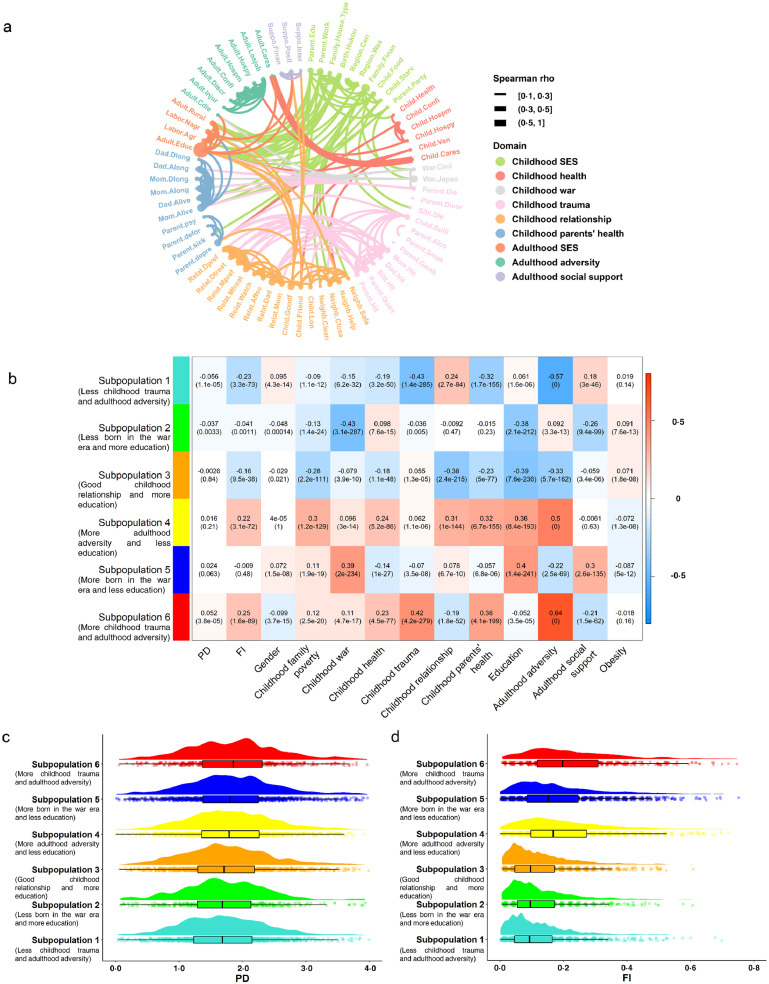
Correlations for 70 life course circumstances variables are visualized in Figure 3A. We found that childhood SES was mainly correlated with adult SES (evidenced by correlations between education, occupation, and rural residence), but also correlated with factors including being born during the war era and childhood neighborhood quality. Childhood trauma was mainly correlated with childhood parents’ health, as well as childhood relationships with friends and parents. We also observed that childhood health was correlated with factors including parents’ depression, childhood relationships with friends and neighborhood, and adulthood adversity.
Figure 3.
Life course circumstances correlations, cluster membership-trait correlations, and aging measures across six subpopulations.
(a) Chord chart of the correlations for each of the life course circumstances. The thickness of the ribbon reflects the spearman correlation. (b) Cluster membership-trait correlations and p values. To determine what each subpopulation/cluster represents, we calculated a continuous measure (cluster membership) for each cluster (between -1 and 1) that denotes how strongly an adult belongs to that given cluster — for example, someone may have a score of 0·9 for the subpopulation 2 (less born in the war era and more education) and -0·7 for the subpopulation 6 (more childhood trauma and adulthood adversity), suggesting that she/he is very similar to the profile represented by the subpopulation 2 (less born in the war era and more education), but not the subpopulation 6 (more childhood trauma and adulthood adversity). Each cell presents the correlation (and p value) resulting from correlating cluster membership (rows) to traits (columns, including PD, FI, and summarized measures of several circumstances). The table is color-coded by correlation according to the color legend. (c) The raincloud plot shows PD across the six subpopulations. (d) The raincloud plot shows FI across the six subpopulations. SES=socioeconomic status. PD=physiological dysregulation. FI=frailty index.
The correlations of these life course circumstances motivated us to identify six distinct subpopulations from PCA and HCA, each of which was characterized by similar childhood and adulthood circumstances (appendix p 22). Figure 3B shows the profiles of life course circumstances for each subpopulation. Adults in the subpopulation 6 (more childhood trauma and adulthood adversity) were characterized by experiencing more trauma during childhood and more adversity during adulthood, and having parents with more health problems. The subpopulation 5 (more born in the war era and less education) included adults who were more likely to be born in the war era, had a lower level of education, and acquired less social support during adulthood. The subpopulation 4 (more adulthood adversity and less education) included adults who had poor financial situations, had terrible relationships with others, had parents with more health problems during childhood, had a lower level of education, and experienced more adversity during adulthood. In contrast, those in the subpopulation 3 (good childhood relationship and more education) were characterized by having a higher level of education, living in great harmony with others, and experiencing less adversity during adulthood. Those in the subpopulation 2 (less born in the war era and more education) were not born in the war era, and were more likely to have a higher level of education. Finally, those in the subpopulation 1 (less childhood trauma and adulthood adversity) experienced less trauma during childhood and less adversity during adulthood, and had parents with fewer health problems. Additionally, we showed the characteristics by subpopulations (appendix p 14-17), which were in line with the profiles of life course circumstances for each subpopulation shown in Figure 3B. For instance, the subpopulation 6 (more childhood trauma and adulthood adversity) consisted of 536 adults, 57.3% of whom were male. Of the adults in the most disadvantaged subpopulation, in childhood, 82.8% ever experienced food deficiency, 40.5% were ever hit by the female guardian, 32.1% were ever hit by the male guardian, and 34.0% had parents being sick on bed for a long time; and in adulthood, 25.9% ever experienced lifetime discrimination, and 22.6% ever received a physical injury. The correlations among the six subpopulations are presented in appendix p 23. We observed an inverse relationship between the subpopulations 1 (less childhood trauma and adulthood adversity) and 6 (more childhood trauma and adulthood adversity) (r=-0·98). Similarly, the subpopulations 2 (less born in the war era and more education) and 5 (more born in the war era and less education) (r=-0·97), the subpopulations 3 (good childhood relationship and more education) and 4 (more adulthood adversity and less education) (r=-0·96) were also found to have different life course circumstances.
Associations of subpopulations with two aging measures
The distributions of PD and FI across the six subpopulations are shown in Figure 3 C-D. The subpopulation 6 (more childhood trauma and adulthood adversity) was identified as disadvantaged, with a consistently higher average level of PD and FI. In contrast, the subpopulation 1 (less childhood trauma and adulthood adversity) was identified as advantaged relative to the other five subpopulations.
Table 1 presents the formal associations of the six subpopulations with the two aging measures. After adjusting for chronological age, gender, and behaviours, compared with the subpopulation 1, the levels of PD and FI in the subpopulations 4 (more adulthood adversity and less education), 5 (more born in the war era and less education), and 6 (more childhood trauma and adulthood adversity) were significantly higher. For instance, relative to the subpopulation 1 (less childhood trauma and adulthood adversity), PD and FI in the subpopulation 6 (more childhood trauma and adulthood adversity) were increased by an average of 0·14 (by one-SD, 95% CI=0·06, 0·21; p < 0·0001) and 0·10 (by one-point, 95% CI=0·09, 0.11; p < 0·0001), respectively. Obesity was found to be consistently associated with two aging measures, such that proportion of obesity was associated with average increases of 0·07 (by one-SD, 95% CI=0·01, 0·13; p = 0·02) and 0·05 (by one-point, 95% CI=0·04, 0·06; p < 0·0001) in PD and FI, respectively. Compared with non-smokers, FI of ever smokers was increased by an average of 0·03 (by one-point, 95% CI=0·02, 0·04; p < 0·0001).
Table 1.
Associations of childhood and adulthood circumstances with two aging measures.
| Model 1a |
Model 2b |
|||
|---|---|---|---|---|
| Coef. (95% CI) | p value | Coef. (95% CI) | p value | |
| PD (by one-SD) | ||||
| Subpopulations for childhood and adulthood circumstances | ||||
| Subpopulation 1 (less childhood trauma and adulthood adversity) | ref | — | ref | — |
| Subpopulation 2 (less born in the war era and more education) | -0·01 (-0·07, 0·07) | 0·89 | 0·01 (-0·06, 0·08) | 0·76 |
| Subpopulation 3 (good childhood relationship and more education) | 0·08 (0·02, 0·14) | 0·01 | 0·07 (0·02, 0·13) | 0·01 |
| Subpopulation 4 (more adulthood adversity and less education) | 0·12 (0·06, 0·18) | <0·0001 | 0·10 (0·05, 0·16) | <0·0001 |
| Subpopulation 5 (more born in the war era and less education) | 0·16 (0·10, 0·22) | <0·0001 | 0·11 (0·05, 0·17) | <0·0001 |
| Subpopulation 6 (more childhood trauma and adulthood adversity) | 0·17 (0·09, 0·24) | <0·0001 | 0·14 (0·06, 0·21) | <0·0001 |
| Proportion of obesity | 0·07 (0·01, 0·13) | 0·02 | ||
| Smoking | ||||
| Non-smoker | ref | |||
| Ever smoker | 0·04 (-0·02, 0·11) | 0·22 | ||
| Current smoker | -0·02 (-0·08, 0·04) | 0·55 | ||
| Drinking | ||||
| Non-drinker | ref | |||
| Drinker | -0·01 (-0·05, 0·04) | 0·80 | ||
| FI (by one-point) | ||||
| Subpopulations for childhood and adulthood circumstances | ||||
| Subpopulation 1 (less childhood trauma and adulthood adversity) | ref | — | ref | — |
| Subpopulation 2 (less born in the war era and more education) | 0·01 (-0·00, 0·02) | 0·16 | 0·02 (0·01, 0·03) | <0·0001 |
| Subpopulation 3 (good childhood relationship and more education) | 0·007 (-0·002, 0·016) | 0·14 | 0·01 (-0·00, 0·02) | 0·14 |
| Subpopulation 4 (more adulthood adversity and less education) | 0·08 (0·07, 0·09) | <0·0001 | 0·074 (0·066, 0·083) | <0·0001 |
| Subpopulation 5 (more born in the war era and less education) | 0·06 (0·05, 0·07) | <0·0001 | 0·04 (0·03, 0·05) | <0·0001 |
| Subpopulation 6 (more childhood trauma and adulthood adversity) | 0·10 (0·09, 0·12) | <0·0001 | 0·10 (0·09, 0·11) | <0·0001 |
| Proportion of obesity | 0·05 (0·04, 0·06) | <0·0001 | ||
| Smoking | ||||
| Non-smoker | ref | — | ||
| Ever smoker | 0·03 (0·02, 0·04) | <0·0001 | ||
| Current smoker | 0·008 (-0·001, 0·018) | 0·08 | ||
| Drinking | ||||
| Non-drinker | ref | — | ||
| Drinker | 0·004 (-0·003, 0·010) | 0·29 | ||
CI = confidence interval. PD = physiological dysregulation. SD=standard deviation. FI=frailty index.
Model 1 was unadjusted.
Model 2 was adjusted for chronological age, gender, proportion of obesity, smoking status, and drinking status.
Discussion
In a sample of Chinese middle-aged and older adults, we showed that childhood and adulthood circumstances, and behaviours were consistently and significantly associated with phenotypic and functional aging, although to a different extent. Overall, the life course circumstances we evaluated accounted for 6·3% and 29·7% of the variance in PD and FI, respectively. Using variables characterizing childhood and adulthood circumstances, we identified six subpopulations, of which the most disadvantaged subpopulation (subpopulation 6 [more childhood trauma and adulthood adversity]) and the most advantaged subpopulation (subpopulation 1 [less childhood trauma and adulthood adversity]) sharing completely different life course circumstances. Moreover, subpopulation 6 (more childhood trauma and adulthood adversity) consistently exhibited the highest levels of PD and FI, while subpopulation 1 (less childhood trauma and adulthood adversity) had the lowest levels of PD and FI. The findings promote the understanding of aging etiology surrounding life course circumstances and provide important clues for preventive and interventive programmes aiming at slowing the pace of aging through targeting multiple exposures (e.g., those shared by the most disadvantaged subpopulation) across the life course. In addition, the results observed across the two aging measures confirmed that aging measures shared both similarities and differences, suggesting that they may complement each other.
Our results draw attention to several childhood circumstances including being born in the war era, traumatic experience, poor social interrelationship, and parents’ health problems. These childhood circumstances represent the defining features of the disadvantaged subpopulations (i.e., the subpopulations 5 [more born in the war era and less education] and 6 [more childhood trauma and adulthood adversity]). While those who had harmonious relationships with others were typical of the advantaged subpopulations (i.e., the subpopulation 3 [good childhood relationship and more education]). Prior studies have found that adults who live in hostile neighborhoods have relatively shorter telomere length, which is associated with accelerated cellular aging.19 Childhood negative events may have become biologically embedded in the disruption of functioning across body systems, including immunity, inflammation, and metabolism.20 In both human and animal studies, early life adversity has been linked to immune dysregulation over the life course.21,22 Adults who experienced adversity in childhood have enhanced stress sensitivity and fewer available psychological and social resources, thereby exhibiting greater physiological deterioration in the immune system.21 Despite plasticity during early life making it easier to attain functional capacity,23 adversity in adulthood still influences the acceleration of aging in this study. Our findings suggest preventing or reducing the severity of these negative events over the life course may help reshape the trajectory of the population towards healthy aging.
Childhood SES served as an important contributor to aging in this study, consistent with previous studies.24 The lasting influence of childhood SES on health in late life has been documented.25 The poor economic condition in childhood could induce biological damages that increase susceptibility to disease.26,27 Low SES could trigger high levels of stress hormones (e.g., catecholamines, and cortisol),26 which may accelerate the aging process.27 Also, childhood SES may be linked to accelerated aging through exposure to malnutrition, infection disease, and unavailability of medical care. Our results highlight the need for special attention to children from low SES families to diminish health inequality in late life.
As with childhood SES, numerous studies have focused on the influence of adulthood SES on aging.28,29 However, a recent study found that the positive influence of childhood SES on late life health trajectory was indirectly established through its influence on adulthood SES.29 Adults with poor childhood economic conditions may subsequently experience more adversities in adulthood. Thus, the associations of circumstances in childhood and adulthood with aging are difficult to be disentangled with traditional statistical methods. In this study, we were able to assess the relative contribution of life course circumstances using the Shapley method, so as to provide a relatively accurate estimate for the above associations. We found that both childhood and adulthood circumstances appear to contribute to variance in aging, suggesting that we should be aware of circumstances over the life course, and highlighting the importance of life course preventative programmes and policy.
Consistent with an earlier study in the US population,10 we demonstrated that behaviours contribute to accelerated aging, highlighting the importance of adherence to healthy behaviours to promote healthy aging. Particularly, BMI, as an indicator of overall adiposity, has been found to be positively associated with faster epigenetic aging as measured by DNA methylation.30 This is similar to our observations of proportion of obesity in this study. Although BMI is extremely arguable to define obesity in older adults and in the Asian population,31,32 a recent intervention trial found that lifestyle weight loss may attenuate DNA methylation age.33 Hence, it is highly recommended to propose policy programmes targeting behaviours (modifiable factors) to improve population health.
Although our results consistently demonstrated the impact of life course circumstances on aging, we should also note the differences. First, the total contribution of these factors varies greatly with respect to the different aging measures (29·7% for FI, but only 6·3% for PD). This is not surprising. PD was calculated based on eight routine clinical biomarkers, while FI incorporated various health dimensions (e.g., physical function, cognitive function, comorbidities) with 39 items. Obviously, FI captures more health information. Second, the same factor contributes differentially to the variance for each of the two aging measures. For instance, the behaviours ranked the sixth in 11 contributors for FI, while it was the penultimate contributor for PD. These differences could be explained by the heterogeneous information that aging measures covered (multisystem clinical biomarkers vs. function aspects) and algorithms used to develop aging measures (Mahalanobis distance vs cumulative approach). Our findings suggest that these aging measures share both differences and similarities.9 In moving forward, broader research should leverage the potential of multiple aging measures, such as DNA methylation age,3 and intrinsic capacity, to explore the reasons and consequences of the aging process.
Compared to our earlier analysis in the US population,10 the present study in the Chinese population contributes to the literature in several aspects. First, only one aging measure at the phenotypic level was considered in the earlier analysis, limiting our understanding of the comprehensive and hierarchical aging process. We used two aging measures in this study, resulting in novel findings as mentioned above. Second, since white and Black adults were included in the earlier analysis, it remains unclear whether the findings apply to other ethnic groups, e.g., the Chinese, with completely different social-environmental circumstances and genes. In this study, we showed that the observations in white and Black people did not appreciably apply to this group of Chinese adults. This was evidenced by the different magnitude of contributions of factors (e.g., behaviours) to phenotypic aging in the US and China. Third, in this study, we draw attention to many unique factors such as being born in the war era, and rural residence, which were not mentioned in our earlier analysis. Also, as a country with the largest number of aging population, the findings in this study provide important clues for slowing aging and further reducing public health burden among the Chinese. Last, because of the genetic differences in race/ethnicity, we should have estimated the influence of genes on aging, which was completely due to the unavailability of data in CHARLS.
A major strength of this study is the availability of the rich life course data from a large sample of the middle-aged and older Chinese, the world's largest aging population. Therefore, our results offer important evidence for life course management to promote healthy aging in developing countries like China. The second strength of the study is the novel approaches (e.g., the Shapley method) we used to address challenges raised by large numbers of life course circumstances. Finally, we simultaneously considered two aging measures in the same population and examined their associations with life course circumstances, which has not been done in prior research.
This study also has limitations. First, many circumstances variables were based on self-reports, and thus, our results may be influenced by recall bias. Regarding the alcohol classification, CHARLS only assessed drinking status, but not the amount of alcohol consumption. Thus, we were unable to evaluate the effect of alcohol consumption amount on aging, which needs further research. Second, the study included Chinese middle-aged and older adults, those born in that period with high mortality, which may introduce survival bias. Third, we did not have the specific timing of childhood and adulthood circumstances, which may affect the effect of circumstances on aging. Fourth, we were unable to cover all life course circumstances variables and confounding variables, leading to possible underestimations of the overall contribution to aging. Fifth, the cross-sectional measurements of aging impeded us to track differences in rates of aging, which could be addressed in the future with longitudinal data. Finally, a large proportion of adults were excluded due to missingness, partially affecting the representativeness of the study population. Future research among nationally representative samples is required to confirm our findings.
In summary, despite the varying contributions, life course circumstances were associated with accelerations of phenotypic and functional aging. We estimated that demographics, behaviours, and circumstances over the life course account for about 6%-30% of the variance in aging. Furthermore, we identified a vulnerable subpopulation who experienced childhood trauma and adulthood adversity and exhibited accelerated aging. The findings highlight the role of life course management in slowing aging and thus in ameliorating health inequalities in late life. In moving forward, studies should leverage aggregated and concurrent life course circumstances to develop potential therapies or preventative programmes of aging. Meanwhile, special attention should be given to promoting health for the disadvantaged subpopulation and narrowing their health gap with advantaged counterparts.
Contributors
ZL contributed to the conception and design of the work. XC and CM performed the analysis. All authors contributed to the interpretation of data. XC, CM, and ZL accessed and verified the underlying data reported. XC wrote the initial draft of the manuscript. All authors critically revised the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data sharing statement
The data that support the findings of this study are available from the website of China Health and Retirement Longitudinal Study at http://charls.pku.edu.cn/en. The analysis code for this study can be obtained upon request from the corresponding author (Prof. ZL, Zuyun.liu@outlook.com).
Declaration of interests
We declare no competing interests.
Acknowledgments
This research was supported by a grant from the National Natural Science Foundation of China (82171584), the 2020 Irma and Paul Milstein Program for Senior Health project award (Milstein Medical Asian American Partnership Foundation), a project from the Natural Science Foundation of Zhejiang Province (LQ21H260003), the Fundamental Research Funds for the Central Universities (Dr. Liu), the Key Laboratory of Intelligent Preventive Medicine of Zhejiang Province (2020E10004), and Zhejiang University Global Partnership Fund (188170-11103). Dr. Gill is supported by the Claude D. Pepper Older Americans Independence Center at Yale School of Medicine (P30AG021342) from the National Institute on Aging, and the National Center for Advancing Translational Sciences (UL1TR001863). Dr. Chen is supported by the Career Development Award (K01AG053408) from the National Institute on Aging, and Yale Alzheimer's Disease Research Center (P30AG066508). We appreciate all adults who participated in the China Health and Retirement Longitudinal Study. We thank two health librarians (Dr. Xiaojun Hu and Xiaoqing Chen) for their help with revising search strategies. We thank Chenxi Li for his help with the statistical analysis.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101548.
Appendix. Supplementary materials
References
- 1.Sierra F, Kohanski R. Geroscience and the trans-NIH Geroscience Interest Group, GSIG. Geroscience. 2017;39(1):1–5. doi: 10.1007/s11357-016-9954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrucci L, Levine ME, Kuo PL, Simonsick EM. Time and the metrics of aging. Circ Res. 2018;123(7):740–744. doi: 10.1161/CIRCRESAHA.118.312816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horvath S, Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet. 2018;19(6):371–384. doi: 10.1038/s41576-018-0004-3. [DOI] [PubMed] [Google Scholar]
- 4.Cohen AA, Milot E, Yong J, et al. A novel statistical approach shows evidence for multi-system physiological dysregulation during aging. Mech Ageing Dev. 2013;134(3-4):110–117. doi: 10.1016/j.mad.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Z. Development and validation of 2 composite aging measures using routine clinical biomarkers in the chinese population: analyses from 2 prospective cohort studies. J Gerontol A Biol Sci Med Sci. 2021;76(9):1627–1632. doi: 10.1093/gerona/glaa238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–336. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rebelo-Marques A, De Sousa Lages A, Andrade R, et al. Aging hallmarks: the benefits of physical exercise. Front Endocrinol. 2018;9:258. doi: 10.3389/fendo.2018.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Ploner A, Wang Y, et al. Longitudinal trajectories, correlations and mortality associations of nine biological ages across 20-years follow-up. Elife. 2020;9:e51507. doi: 10.7554/eLife.51507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Z, Chen X, Gill TM, Ma C, Crimmins EM, Levine ME. Associations of genetics, behaviors, and life course circumstances with a novel aging and healthspan measure: evidence from the health and retirement study. PLoS Med. 2019;16(6) doi: 10.1371/journal.pmed.1002827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDade TW, Ryan C, Jones MJ, et al. Social and physical environments early in development predict DNA methylation of inflammatory genes in young adulthood. Proc Natl Acad Sci USA. 2017;114(29):7611–7616. doi: 10.1073/pnas.1620661114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crimmins EM, Kim JK, Seeman TE. Poverty and biological risk: the earlier “aging” of the poor. J Gerontol A Biol Sci Med Sci. 2009;64(2):286–292. doi: 10.1093/gerona/gln010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin JH, Zeng YB, Zhou Z, Fang Y. Study on the status of frailty and related determinants among the elderly in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2018;39(9):1244–1248. doi: 10.3760/cma.j.issn.0254-6450.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS) Int J Epidemiol. 2014;43(1):61–68. doi: 10.1093/ije/dys203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Crimmins E, Hu PP, et al. Venous blood-based biomarkers in the China health and retirement longitudinal study: rationale, design, and results from the 2015 wave. Am J Epidemiol. 2019;188(11):1871–1877. doi: 10.1093/aje/kwz170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou BF. Cooperative Meta-Analysis Group of the Working Group on Obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15(1):83–96. [PubMed] [Google Scholar]
- 17.Berkelmans GFN, Read SH, Gudbjornsdottir S, et al. Population median imputation was noninferior to complex approaches for imputing missing values in cardiovascular prediction models in clinical practice. J Clin Epidemiol. 2022;145:70–80. doi: 10.1016/j.jclinepi.2022.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Wang M-C, Deng Q, Bi X, Ye H, Yang W. Performance of the entropy as an index of classification accuracy in latent profile analysis: a Monte Carlo simulation study. Acta Psychologica Sinica. 2017;49(11):1473–1482. [Google Scholar]
- 19.Needham BL, Carroll JE, Diez Roux AV, Fitzpatrick AL, Moore K, Seeman TE. Neighborhood characteristics and leukocyte telomere length: the multi-ethnic study of atherosclerosis. Health Place. 2014;28:167–172. doi: 10.1016/j.healthplace.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berens AE, Jensen SKG, Nelson CA., 3rd Biological embedding of childhood adversity: from physiological mechanisms to clinical implications. BMC Med. 2017;15(1):135. doi: 10.1186/s12916-017-0895-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fagundes CP, Glaser R, Kiecolt-Glaser JK. Stressful early life experiences and immune dysregulation across the lifespan. Brain Behav Immun. 2013;27(1):8–12. doi: 10.1016/j.bbi.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shirtcliff EA, Coe CL, Pollak SD. Early childhood stress is associated with elevated antibody levels to herpes simplex virus type 1. Proc Natl Acad Sci USA. 2009;106(8):2963–2967. doi: 10.1073/pnas.0806660106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azzolino D, Spolidoro GCI, Saporiti E, Luchetti C, Agostoni C, Cesari M. Musculoskeletal changes across the lifespan: nutrition and the life-course approach to prevention. Front Med. 2021;8 doi: 10.3389/fmed.2021.697954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers NT, Blodgett JM, Searle SD, Cooper R, Davis DHJ, Pinto Pereira SM. Early-life socioeconomic position and the accumulation of health-related deficits by midlife in the 1958 British Birth Cohort Study. Am J Epidemiol. 2021;190(8):1550–1560. doi: 10.1093/aje/kwab038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Angelini V, Howdon DDH, Mierau JO. Childhood socioeconomic status and late-adulthood mental health: results from the survey on health, ageing and retirement in Europe. J Gerontol B Psychol Sci Soc Sci. 2019;74(1):95–104. doi: 10.1093/geronb/gby028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen S, Doyle WJ, Baum A. Socioeconomic status is associated with stress hormones. Psychosom Med. 2006;68(3):414–420. doi: 10.1097/01.psy.0000221236.37158.b9. [DOI] [PubMed] [Google Scholar]
- 27.Gassen NC, Chrousos GP, Binder EB, Zannas AS. Life stress, glucocorticoid signaling, and the aging epigenome: implications for aging-related diseases. Neurosci Biobehav Rev. 2017;74(Pt B):356–365. doi: 10.1016/j.neubiorev.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Van der Linden BWA, Cheval B, Sieber S, et al. Life course socioeconomic conditions and frailty at older ages. J Gerontol B Psychol Sci Soc Sci. 2020;75(6):1348–1357. doi: 10.1093/geronb/gbz018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhatta TR, Albert JM, Kahana E, Lekhak N. Early origins of later life psychological well-being? A novel application of causal mediation analysis to life course research. J Gerontol B Psychol Sci Soc Sci. 2017;73(1):160–170. doi: 10.1093/geronb/gbx022. [DOI] [PubMed] [Google Scholar]
- 30.Kresovich JK, Garval EL, Martinez Lopez AM, et al. Associations of body composition and physical activity level with multiple measures of epigenetic age acceleration. Am J Epidemiol. 2021;190(6):984–993. doi: 10.1093/aje/kwaa251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, Thornton JC, Russell M, Burastero S, Heymsfield S, Pierson RN., Jr Asians have lower body mass index (BMI) but higher percent body fat than do whites: comparisons of anthropometric measurements. Am J Clin Nutr. 1994;60(1):23–28. doi: 10.1093/ajcn/60.1.23. [DOI] [PubMed] [Google Scholar]
- 32.Azzolino D, Cesari M. Obesity and COVID-19. Front Endocrinol. 2020;11 doi: 10.3389/fendo.2020.581356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yaskolka Meir A, Keller M, Bernhart SH, et al. Lifestyle weight-loss intervention may attenuate methylation aging: the CENTRAL MRI randomized controlled trial. Clin Epigenet. 2021;13(1):48. doi: 10.1186/s13148-021-01038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.